Abstract
Background and Objectives
Antiseizure medications (ASMs) are among the most commonly prescribed teratogenic drugs in women of childbearing age. Limited data exist on utilization patterns across different indications for therapy and for the newer-generation ASMs in this population. Thus, we assessed the pattern of ASM use in women of childbearing age with epilepsy and nonepilepsy indications (pain and psychiatric disorders).
Methods
We conducted a retrospective analysis of deidentified administrative data submitted to the Optum Clinformatics database. Eligible participants included women aged 12–50 years who filled ASMs between year 2011 and 2017. Participants were followed from date of index prescription filled to study end or insurance disenrollment, whichever came first. For the overall cohort and potential therapy indications, we assessed the type and frequency of ASMs filled; proportion of participants on monotherapy, polytherapy, or treatment switching; and duration of continuous use. Trends were characterized using annual percent change from study start to study end.
Results
Our analysis included 465,131 participants who filled 603,916 distinct ASM prescriptions. At baseline, most of the participants had chronic pain (51.0%) and psychiatric disorders (32.7%), with epilepsy the least common (0.9%). The most frequently dispensed were diazepam (24.3%), lorazepam (20.1%), gabapentin (17.4%), clonazepam (12.7%), topiramate (11.3%), and lamotrigine (4.6%). Significant linear increase in trends were observed with gabapentin (annual percent change [95% CI]: 8.4 [7.3–9.4]; p < 0.001) and levetiracetam (3.4 [0.7–6.2]; p = 0.022) and decreasing trends for diazepam (−3.5 [−2.4 to 4.5]; p < 0.001) and clonazepam (−3.4 [−2.3 to 4.5]; p = 0.001). No significant change in trend was observed with valproate (−0.4 [−2.7 to 1.9]; p = 0.651), while nonlinear changes in trends were observed with lorazepam, topiramate, lamotrigine, and pregabalin.
Discussion
Decreasing trends were observed with older ASMs in the overall cohort and across the potential indications for therapy. Conversely, increasing trends were seen with the newer ASMs. Considering the risk of teratogenicity associated with the newer medications largely unknown, counseling and education in addition to a careful consideration of the benefits vs potential risks should remain pivotal when prescribing ASMs for women of childbearing age.
Antiseizure medications (ASMs) are commonly prescribed to manage epilepsy. More than 3 million adult individuals in the United States are treated for epilepsy,1 but the total population exposed to ASMs is considerably higher as more than half of ASMs are prescribed to manage nonepilepsy indications such as mood disorders and pain.2
ASMs are one of the most prescribed teratogenic drugs in women of childbearing age. This complicates treatment decisions and drug selection.3 A ≥2-fold increased risk of major congenital malformation (MCMs) have been reported among children exposed to several ASMs during pregnancy,4,5 with the greatest risks for valproate.4–6 Notably, the association of most ASMs, particularly the second-generation and third-generation ASMs, on the incidence of fetal anatomical and neurocognitive development remains ill-defined.2,7 Increased neurodevelopmental disorders (e.g., cognitive impairments and autism) have also been reported with fetal ASM exposures.2,8–10 Although preconception care has been increasingly adopted in the care of women of reproductive age, approximately half of all pregnancies are unplanned.11,12 Therefore, fetal ASM exposure may occur before the woman is aware of pregnancy. Moreover, several ASMs such as topiramate, phenytoin, and carbamazepine may interact with oral contraceptives leading to hormonal contraception failure.13
Over the past 2 decades, several studies have assessed treatment patterns of the older-generation and newer-generation ASMs.14–17 Changes in prescription patterns surrounding ASMs have been reported; specifically, reduced valproate use driven in part by Food and Drug Administration (FDA) label changes in 2011 and 2013 noting increased risk of MCMs and cognitive impairments with prenatal exposure, which has contributed to increased prescription for newer-generation ASMs.15 These findings have largely accrued from small sample size registry-based studies (which are not representative of the general population) or large representative, population-based studies assessing ASM use during pregnancy or limited only to women with epilepsy.4,14–17 The only study evaluating ASM use regardless of indication in women of childbearing age, albeit limited to Danish women.17 Here, we present analysis from a large, the US claims database on the pattern of utilization of ASMs for epilepsy and nonepilepsy indications in women of childbearing age. Given that neurologists frequently treat epilepsy and pain as well as commonly have patients with psychiatric comorbidities, patterns in the change ASM use for a variety of indications in women of childbearing potential are important for neurologists to understand.
Methods
Data Source
This retrospective cohort study was conducted using administrative claims data submitted to the Optum Clinformatics Data Mart (“Optum”). The Optum database contains longitudinal, deidentified information on demographics, medical visits, and outpatient prescription services for patients enrolled in specific commercial and Medicare Advantage health insurance plans across 50 US states. Diagnoses were recorded using International Classification of Disease, Ninth or Tenth Revision, Clinical Modification (ICD-9-CM or ICD-10-CM) diagnoses codes.
Eligibility Criteria
The analysis cohort included women who (1) filled ≥1 day supply of eligible ASMs between January 1, 2011, and December 31, 2017; (2) had ≥365 days of continuous health insurance enrollment with <30 days gap before first ASM prescription (i.e., index date); and (3) were aged 12–50 years at index date. The 12–50 years age range has been used in previous pregnancy safety studies and was selected to facilitate comparisons with previous studies assessing the utilization of medication use in women of childbearing age.14,16,18 Moreover, this age range largely covers a period between menarche and menopause among women. The 1-year period before the index date was considered as the baseline period.
A complete list of ASMs, including benzodiazepines assessed in this study, is provided in eTable 1 (links.lww.com/WNL/C951). We regarded nonbenzodiazepine ASMs approved before 1990 as older-generation or first-generation ASMs (e.g., phenytoin, carbamazepine) and those approved 1990 or thereafter as second-generation or third-generation ASMs. ASMs approved between years 1990 and 2005 in the United States were regarded as second-generation ASMs (e.g., lamotrigine, gabapentin, pregabalin, topiramate, and levetiracetam), while those approved after year 2005 as third-generation ASMs19 (eTable 1).
Eligible participants were followed from the index date to treatment discontinuation, study end date (December 31, 2017), or insurance disenrollment, whichever came first. Our analysis was restricted to the first eligible episode after treatment initiation. Dose regimen is unavailable in our data; therefore, this study does not include analyses on changes in dosing regimen during the follow-up period or differences in prescribed dose by potential indication for ASMs.
Case Identification
Potential indications for ASMs were assessed from inpatient and outpatient visits during the 1-year baseline period. These 3 potentially overlapping indications for ASMs were evaluated in the cohort of women of childbearing age: chronic pain, psychiatric disorders, and epilepsy.
Consistent with the International League Against Epilepsy Epidemiology Commission and previous studies,14,20–22 epilepsy cases were defined by (1) ≥2 medical encounters on separate dates with ICD-9-CM or ICD-10-CM codes 345.xx or G40.xx, respectively; (2) ≥1 medical encounter with ICD-9-CM 345.xx or ICD-10-CM code G40.xx and ≥1 ICD-9-CM code 780.39 or ICD-10-CM code R56.xx; (3) ≥1 medical encounter with ICD-9-CM or ICD-10-CM codes 345.xx or G40.xx, respectively, and ≥1 ASM prescription; or (4) ≥2 medical encounters on separate dates with ICD-9-CM or ICD-10-CM codes 780.39 or R56.8, respectively, and ≥1 antiepileptic drug prescription (Table 1).22
Table 1.
Algorithms for Identifying Indications for ASMsa
Individuals with chronic pain were identified by the presence of ≥2 diagnosis codes during the baseline period for relevant chronic pain conditions such as migraine and back and neck pain. Cases of psychiatric disorders were identified by the presence of ≥2 diagnosis codes for any of the following medical conditions during the 1-year baseline period: schizophrenia, bipolar disorder, depression, or mood or anxiety disorders (Table 1). A complete list of diagnosis codes used to identify relevant indications for ASMs is presented in Table 1.
An eligible woman may have 2 or more potential indications for ASMs, for example, chronic pain and epilepsy. Therefore, included individuals were considered to have overlapping indications for ASMS if they had medical encounters for any 2 or all 3 potential indications for ASMs during the baseline period. The groups for potentially overlapping categories for ASM included women with (1) chronic pain and epilepsy; (2) chronic pain and psychiatric disorders; (3) epilepsy and psychiatric disorders; and (4) chronic pain, epilepsy, and psychiatric disorders.
Pattern of Utilization of ASMs
Yearly and overall ASM use were defined at the participant level, categorized based on treatment following the index prescription and evaluated by (1) type and frequency of ASM filled; (2) proportion of participants on monotherapy, polytherapy, or treatment switching; and (3) measures of medication retention. Participants were considered receiving ASM monotherapy if the index ASM remained unchanged during follow-up. Participants filling ≥1 ASM different from the index ASM with ≥30 days of overlapping supply between prescriptions were considered receiving ASM polytherapy or to have switched therapy when ≥2 distinct ASMs were concurrently filled but with <30 days of overlapping supply. Medication retention on ASM treatment was assessed by duration of continuous use,23–25 computed as time from index date till absence of a refill, or a gap between fill dates of consecutive ASM prescriptions ≥30 days (i.e., treatment discontinuation). When participants refilled ASMs before exhausting the previous fill, we calculated duration of use while allowing the overlapping days' supply to accumulate.
In addition, we assessed prescribing patterns by geographic regions in the United States. Based on the US Census Bureau classification,26 US states were broadly categorized into (1) Northeast, (2) Midwest, (3) South, (4) West, and (5) Puerto Rico. An unknown category was added to account for individuals with missing or unknown geographic information.
Pregnancy Status and Maternal Exposure to ASMs
We used validated algorithms to identify women who filled ASMs and had service records for pregnancy outcomes of interest, live birth or mixed birth and whether live or nonlive outcomes.27,28 The start of pregnancy was estimated to be 245 and 273 days before the date of service for preterm and term births, respectively.29 Maternal use of ASMs was also assessed and defined as fill(s) for eligible ASMs anytime between the estimated pregnancy start and a pregnancy outcome.
Contraceptive Use
In additional analyses, we adapted an algorithm previously reported by the US Office of Population Affairs to determine baseline use of contraceptive methods in the analysis cohort.30,31 Individuals who received contraceptive care were identified by the presence of ≥1 procedure code or National Drug Codes indicative for permanent or reversible, FDA-approved moderately or highly effective contraceptive method anytime during the 1-year baseline period. Eligible contraceptive methods were limited to long-acting reversible contraceptives (i.e., intrauterine devices or systems and implants), short-acting hormonal contraceptives (i.e., patch, ring, oral pills, and injectables), and sterilization.30,31
Statistical Analyses
We performed descriptive analyses on use, duration, frequency and type of ASM filled, and potential indication annually and overall. Descriptive analyses were performed using SAS 9.4 version (SAS Institute, Cary, NC). Joinpoint Regression Program version 4.9.0.0 (National Cancer Institute, Silver Spring, MD) was used to assess temporal changes in ASM use. Detailed explanation of this regression software methodology is available in a study32 and surveillance.cancer.gov/joinpoint/. The Joinpoint software fits a series of annual data using the simplest model possible on a single line, while simultaneously estimating annual percent change (APC) and corresponding 95% CI to quantitatively characterize the magnitude of the trend. In computation of APC, entries for any given year are assumed to change at a constant rate linearly on a log scale. When this assumption is true, the model yields a single straight line and computes a single APC; otherwise, multiple lines are plotted, and different APCs are computed to indicate trend changes over the study period. In addition, we computed the average APC (AAPC)—defined as the ratio of sum of APCs from the resulting model(s) and length of the APC interval—to provide a single summary measure of the trend over the study period. The univariate linear regression slope (β) of each estimated APC was tested under H0: β = 0. For all tests, significance level was set at p < 0.05.
Standard Protocol Approvals
The study was approved by the University of Rhode Island Institutional Review Board.
Data Availability
The Optum Clinformatics Data Mart is available by the data vendor under license to the University of Rhode Island College of Pharmacy. Other researchers may obtain access under licensing agreements with the data vendor.
Results
Baseline Patient Characteristics
The analysis cohort included 465,131 participants with mean (SD) age of 35.5 (10.2) years (Table 2). Approximately 30% of participants concomitantly filled opioids (28.3%) during the baseline period. The median (interquartile quartile) follow-up was 526 days (219–1,046), with 60.9% (n = 283,464) having ≥12 months of follow-up. At baseline, 215,698 (46.4%) of participants included in our analysis had residence in a state in the South, 110,342 (23.7%) in the Midwest, 93,897 (20.2%) in the West, and 44,186 (9.5%) in the Northeastern (9.5%) region of the United States (Table 2).
Table 2.
Baseline Characteristics of Women of Childbearing Age Who Filled ASMs
During the baseline period, 27.3% women of childbearing age (n = 126,925) had ≥1 medical visit or prescription fill for relevant contraceptives (Table 2). Among the 126,925 women on relevant contraceptives, oral pills were the most frequent (n = 117,362; 25.2%) (Table 2). The median days of supply of oral contraceptives was 230 (86–370) days. None of the 465,131 included women had a procedure for sterilization, an irreversible contraceptive method (Table 2).
There were 20,141 women with ≥1 relevant pregnancy outcome (Table 2). Overall, 18,288 participants (n = 90.8%) had a singleton or multiple gestation birth, while the remaining 1,853 participants had ≥2 deliveries during the study period. A total of 3,059 (0.7%) women filled ≥1 prescription of ASM during the estimated pregnancy window.
Among the 3 potential indications for ASM, chronic pain was the most common at 51.0% (n = 237,392); 32.7% participants had psychiatric disorders (n = 151,919), and 0.9% had epilepsy (n = 4,295) (Table 2). An individual could have overlapping (≥2) or distinct indication for ASM therapy. In those with nonoverlapping indications for ASMs, 161,834 participants (34.8%) had chronic pain, 16.6% (n = 77,148) had psychiatric disorders, and 0.3% (n = 1,356) had epilepsy. Among individuals with overlapping conditions for ASMs, psychiatric disorders and chronic pain conditions were the most frequent (15.7%; n = 73,216); 0.3% (n = 1,384) had epilepsy and chronic pain disorders, and 0.13% (n = 597) had epilepsy and psychiatric disorders. Notably, 67.1% of the total participants included in our analyses had chronic pain and/or psychiatric disorders, while 0.2% (n = 958) had diagnosis codes indicative of all 3 indications (Table 2). There were 148,638 participants (32%) with no medical records for any of 3 potential indications for ASM.
Pattern of Utilization
A total of 603,915 ASM prescriptions were filled and 2,345,578 refills of ASM during the follow-up period. The mean (SD) number of filled prescriptions was 5 (9.9), with 52% of enrollees (n = 241,806) filling ≥2 prescriptions (eTable 2, links.lww.com/WNL/C951). The most frequently used ASMs were the benzodiazepines diazepam (24.3%), lorazepam (20.1%), and clonazepam (12.7%) and the nonbenzodiazepine second-generation ASMs: gabapentin (17.4%), topiramate (11.3%), and lamotrigine (4.6%) (Figure 1 and eTable 3). Median cumulative days' supply for commonly filled nonbenzodiazepine ASMs were often longer than benzodiazepines (eTable 4). Valproate, comprising 1.5% of distinct prescriptions filled, had a median cumulative days' supply of 60 (30–165) days and 30 (30–107) days of continuous use. Of the 11,130 prescriptions of ASMs filled during pregnancy, gabapentin, lamotrigine, topiramate, levetiracetam, and valproate were the most common nonbenzodiazepines (eTable 5).
Figure 1. Overall ASM Use in Women of Childbearing Age Who Filled a Prescription for ASM.
ASM = antiseizure medication.
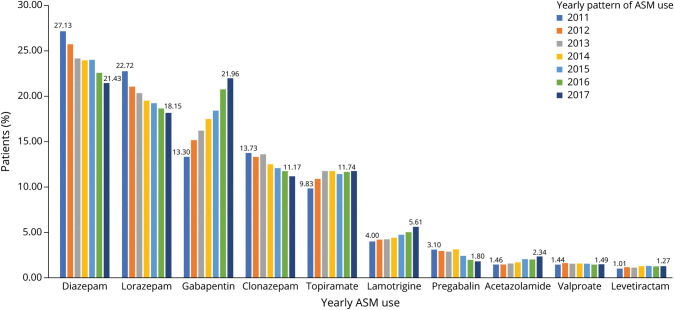
The yearly pattern for commonly dispensed ASMs in the overall cohort is presented in Figure 2 and eTable 6 (links.lww.com/WNL/C951) and trends in Table 3. From 2011 to 2017, significant linear increasing trends, evidenced by increasing APC, were observed with gabapentin (APC [95% CI] 8.4 [7.3–9.4]; p < 0.001), acetazolamide (8.8 [6.1–11.6]; p < 0.001), and levetiracetam (3.4 [0.7–6.2]; p = 0.022) (Table 3). Significantly decreasing trends were observed for diazepam (−3.5 [−2.4 to 4.5]; p < 0.001) and clonazepam (−3.4 [−2.3 to 4.5]; p = 0.001). No significant change in trend was observed with valproate during the study period (−0.4 [−2.7 to 1.9]; p = 0.99). Nonlinear changes in trends were observed between years 2011–2013 and then 2013–2017 with lorazepam and topiramate and between years 2011–2014 and 2014–2017 for lamotrigine and pregabalin (Table 3).
Figure 2. Temporal Patterns of Commonly Used ASM Use by Year in Overall Cohort.
ASM = antiseizure medication.
Table 3.
APC and AAPC for Commonly Prescribed ASMs in the Overall Cohort
From 2011 to 2017, the amount of ASMs dispensed to individuals with epilepsy diagnoses increased (9.1 [0.0–19.0]; p = 0.05). Nonlinear trends were observed with pain disorders, a decrease in 2011–2015 (−0.1 [−3.7 to 3.7]; p = 0.928) and increase afterward (8.7 [−3.2 to 21.9]; p = 0.09) although the slopes were not statistically significant. ASMs dispensed to individuals with diagnosed psychiatric disorders decreased from 2011 to 2014 (−0.8 [−28.7 to 38.0]; p = 0.922) but increased from 2014 to 2017 (22.4 [−10.7 to 67.7]; p = 0.110). These changes were not statistically significant. Overall, the AAPC suggests significantly increasing trend for pain (2.7 [0.6–4.9], p = 0.01) but a nonsignificant increase for psychiatric disorders (10.2 [−0.7 to 22.2]; p = 0.1).
Assessing prescribing patterns by potential indication, the benzodiazepines lorazepam (24.4%), clonazepam (19.6%), and diazepam (14.5%)—totaling a frequency of 58.5%—were the most frequently prescribed ASMs in individuals with psychiatric disorders. Other commonly dispensed ASMs in these individuals were gabapentin (13.9%), lamotrigine (9.1%), and topiramate (8.9%) (Figure 3A). Among individuals with epilepsy, levetiracetam (27.0%), lamotrigine (12.0%), and topiramate (9.3%) were the most dispensed ASMs (Figure 3B). These ASMs were the most prescribed for both generalized and focal seizures (eFigures 1 and 2, links.lww.com/WNL/C951). Diazepam (22.7%), gabapentin (22.5%), lorazepam (16.0%), and topiramate (14.4%) were the ASMs most frequently dispensed to individuals with pain diagnoses (Figure 3C).
Figure 3. ASM Use in Women of Childbearing Age by Presence of Epilepsy and Nonepilepsy Indications.
ASM = antiseizure medication.
Differences in trends were observed for commonly dispensed ASMs across the 3 indications (eTables 7–9, links.lww.com/WNL/C951). Significantly decreasing trends were observed for lorazepam, clonazepam, and diazepam in chronic pain (eTable 8) and lorazepam and clonazepam for psychiatric disorders (eTable 7); however, increasing trends albeit nonsignificantly were observed for these 3 specific ASMs in individuals with epilepsy (eTable 9). Significant increases were consistently observed for gabapentin across the 3 indications, while use of topiramate and lamotrigine decreased in epilepsy but increased in psychiatric and chronic pain disorders. Decreasing trends were observed with valproate across the 3 potential indications, although not significant for chronic pain (eTables 7–9).
Most participants received ASM monotherapy (n = 366,860; 78.9%), with others switching from their index therapy to a different ASM (n = 93,626; 20.1%) or receiving ASM polytherapy (n = 4,645; 1.0%). Across the 3 indications, gabapentin, diazepam, lorazepam, clonazepam, topiramate, and lamotrigine were consistently the most frequently dispensed ASMs, representing 90% of total number of ASMs filled (eFigures 3–5, links.lww.com/WNL/C951). For monotherapy, significant linear increases were observed with gabapentin, topiramate, lamotrigine, acetazolamide, and levetiracetam. By contrast, significant linear decreases were seen with diazepam, lorazepam, clonazepam, and pregabalin (eTable 10). Additional information on ASM polytherapy is presented in eTable 11 and for switching in eTable 12.
Assessing ASM retention overall, individuals receiving nonbenzodiazepine ASMs more commonly remained on therapy for longer (average ≥90 consecutive days or ≥30 median days) before discontinuation, whereas shorter continuous use was observed with benzodiazepines except for clonazepam (eTable 13, links.lww.com/WNL/C951). The duration of continuous use for ASM polytherapy was consistently more than double that for monotherapy (eTables 14–16).
The magnitude and direction of trends for commonly dispensed ASMs were similar between individuals that filled ≥1 relevant contraceptive method vs women with no contraceptive use (eTables 17 and 18, respectively, links.lww.com/WNL/C951). The exception was women dispensed valproate; increasing trend was seen among women on relevant contraceptives (1.9 [−1.1 to 5.0], p = 0.167) and conversely in those without contraceptive use (−1.2 [−3.5 to 1.1], p = 0.225).
Across the various US geographic regions, increasing trends were consistently observed with gabapentin, lamotrigine, and levetiracetam, whereas decreasing trend was seen with pregabalin (eTable 19, links.lww.com/WNL/C951). A decreasing trend was observed with topiramate in the South and conversely in the Midwest, West, and Northeastern regions of the United States. Decreasing trends were seen with valproate across the different geographic regions of the United States, except the Midwest.
Discussion
This study provides much-needed information on the patterns of ASM use in women of childbearing age without epilepsy, data largely absent in the literature. In addition, it provides updated data that complement previous analyses characterizing the use of ASMs in pregnant women or women of childbearing age with epilepsy.14–17,33
Our analyses indicate that two-thirds of included women had chronic pain and/or psychiatric disorders at baseline, but less than 1% had epilepsy diagnoses, a finding that aligns with previous studies reporting that more than half of prescribed ASMs are to manage nonepilepsy indications.2 ASMs are the cornerstone for seizure management; however, several alternative medications exist to manage other nonepilepsy indications that may otherwise be treated with ASMs. Considering an estimated half of pregnancies in the United States are unplanned,11,12,34 and more than half of women in our analysis cohort had no medical record or prescription fill for ≥1 moderately or highly effective contraceptive methods prior to initiation of ASM therapy, the finding of a relatively high frequency of ASM use in women of childbearing age with nonepilepsy indications raises the question as to whether these individuals were adequately counseled on the potential risks of teratogenicity associated with ASM exposure. Importantly, this finding highlights the need for careful consideration of relative teratogenic risks when selecting ASMs and for prescribing alternative effective medications with little-to-no known risk of teratogenicity where possible in women of childbearing age with other indications for ASM.
Furthermore, differences in trend patterns for valproate were seen in the overall cohort, by potential therapy indication, between geographic regions in the United States, and also by the use of relevant contraceptive methods during the baseline period. The differing pattern of valproate use is noteworthy as in our previous study comprising pregnant women enrolled in Florida Medicaid, we observed significantly decreasing trends with valproate in women with epilepsy from year 2000–2009, but not among those with nonepilepsy indications.16 Previous studies reporting pattern of use associated with valproate were limited to epilepsy, pregnant women, or involved data before label changes by the FDA to valproate in 2011 and 2013.14–16,33 Given the more recent data used in this study compared with the previous,16 the absence of consistent changes in valproate use across indications for ASM, including by baseline use of ≥1 contraceptive method is contrary to the well-known risks of anatomical and behavioral defects from fetal valproate exposure. In addition, the increase in use of valproate in women with contraceptive use has public health ramifications and raises questions whether these women were being adequately counseled on the risk of contraceptive failure and consequently increased risk of major malformations associated with maternal valproate use.
Similar to previous epidemiologic studies,14–16,33 our results indicate a shift in prescribing patterns from older generation including benzodiazepines toward ASMs approved after 1990. Specifically, upward trends were observed with several of the second-generation ASMs such as gabapentin, lamotrigine, and levetiracetam in the overall cohort, patterns that remained largely unchanged across the 3 evaluated indications for ASM therapy and various US geographic regions Northeastern regions. The increasing trend with several of the commonly dispensed second-generation ASMs may, in part, be attributable to regulatory approaches to mitigate overprescribing of opioids,35,36 dovetailed with increased off-label use of nonopioids such as gabapentin for the pharmacologic management of chronic pain.37,38 The use of gabapentin in the United States, the most frequently dispensed nonbenzodiazepine ASM in our cohort, has been estimated to have doubled from 2009 to 2016.38 In addition, in 2019, gabapentin was reported to be the seventh most prescribed drug in the United States.39 Notable exceptions of the increasing trend seen with ASMs approved after 1990 were observed with pregabalin and topiramate. Although an increasing trend was observed with topiramate between years 2011 and 2013 in the general cohort and specifically among individuals with pain disorders, the use of topiramate decreased afterward. The changes observed with topiramate may be partly driven by evidence of teratogenicity2 and the FDA black-box warnings in 2011 and 2013 indicating increased risk of anatomical malformations in children exposed to fetal topiramate.40 Nonetheless, it is worth mentioning that despite its well-known risks, over the entire study period, topiramate use still significantly increased in the overall cohort and among individuals with psychiatric disorders or chronic pain.
Decreasing trends were observed with the most frequently dispensed benzodiazepines (diazepam, lorazepam, and clonazepam) in the overall cohort, women with psychiatric disorders and chronic pain. This observed trend might be related to (1) concerns of potential neurodevelopmental effects of prenatal exposure which remain uncertain41,42 and (2) the opioid overdose epidemic, increased awareness, and evidence indicating that the combination of opioids with benzodiazepines can lead to synergistic increase in the risk of respiratory depression and death.43
Unfortunately, the teratogenic risks of many of the new-generation ASMs which are now increasingly prescribed to manage epilepsy or nonepilepsy indications in women of childbearing ages remained inadequate or absent.2,7,33,44 More specifically, of more than 30 ASMs available in the United States, the teratogenic risks for anatomical malformations are well-known for only approximately 8 ASMs, while the risk for fetal exposure to cognitive/behavioral impairments are known for only 8 ASMs.2,7,33,45 However, several of these ASMs approved after 1990, particularly the most prescribed (i.e., gabapentin, lamotrigine, and levetiracetam), were used continuously for ≥12 weeks on average. Considering ASMs can be teratogenic at lower doses or even with short duration of utero exposure, and neuronal apoptosis and cognitive deficits occurring in animal models at blood levels similar to those used in humans,46 further research is urgently needed to delineate the individual teratogenic risks associated with the second-generation and third-generation ASMs. The most prescribed used ASMs were benzodiazepines, gabapentin, and topiramate. Benzodiazepines produce neuronal apoptosis in the immature brain similar to fetal alcohol exposure.47 Gabapentin antagonizes thrombospondin binding to α2δ–1, and powerfully inhibits new excitatory synapse formation in vitro and in vivo, which could potentially affect brain neurodevelopment.48 Topiramate has known increased risks for malformations40 and possible neurodevelopmental risks.49,50
Taken together, our study findings indicate that ASM use in women of childbearing age is prominent for nonepilepsy conditions and although somewhat minimal, a significant number of women of childbearing age are still exposed to well-known teratogens such as valproate without dispensing of effective contraceptive methods. Women in childbearing ages should receive education and counseling on the potential risks regarding interaction with birth control methods, teratogenicity risks to the fetus, and long-developmental risks to the infant. Further research is also needed to better understand factors moderating the differential prescribing of certain ASMs by geographic location, to inform resource allocation and implementation of strategies to reduce unnecessary fetal exposure to well-known teratogens.
First, because our analysis is based on administrative claims data, inferences may be limited by several forms of systematic errors prevalent in observational studies, particularly measurement errors. For example, exposure misclassification may have occurred in individuals who did not consume dispensed medications and as such not at risk of teratogenicity attributable to ASM exposure. Second, the direct indications for ASM therapy are unavailable in our data. Although we relied on validated algorithms to extrapolate the potential indications for prescribed ASMs, diagnosis codes in claims data are not always captured with fidelity; therefore, some degree of misclassification is possible. Third, because our analysis cohort comprised only individuals who filled ASMs, we are unable to determine the prevalence of use of ASMs in the source population. In addition, our findings may not be generalizable to women with a low socioeconomic status and no access to the commercial insurance plan.
Fourth, dosing regimens are unavailable in our data. Consequently, we were unable to differentiate between benzodiazepines prescribed as epilepsy rescue vs daily use or as-needed use. Fifth, while we assessed for the presence of moderately or highly effective contraceptive methods during the baseline period, we cannot empirically confirm the effectiveness of these approaches in preventing pregnancies. More so, our results may be underestimated by women who had initiated certain contraceptive methods (e.g., implants, intrauterine devices, patches) before the start of the 1-year baseline period. Finally, considering information available in our data, we are unable to ascertain to what extent education and counseling about fetal risks associated with prenatal use of ASMs have been given.
Because all ASMs are potentially teratogenic and may be used for many neurologic and psychiatric diagnoses, the common prescription of ASMs to women of childbearing potential poses a societal and public health concern. Our results suggest that strides have been made over the past decades in reducing the prescriptions of well-known teratogenic ASMs in women of childbearing age. However, it remains unclear how many women received adequate informed consent before pregnancy on the risks of fetal ASM exposures and potential of failed contraception. Furthermore, the risks of teratogenicity for many ASMs remain uncertain. As such, mothers receiving ASMs with unknown risk to manage epilepsy or other indications may needlessly be exposing their children to increased risk of anatomical and neurobehavioral abnormalities. Concerted efforts are urgently needed to address this evidence gap2; adequate counseling and a careful consideration of the benefits vs potential risks of teratogenicity should remain pivotal when initiating, augmenting, or switching ASM therapy for any indication in women of childbearing age.
Glossary
- AAPC
annual APC
- APC
annual percent change
- ASM
antiseizure medication
- FDA
Food and Drug Administration
- ICD-9-CM
International Classification of Disease, Ninth Revision, Clinical Modification
- ICD-10-CM
International Classification of Disease, Tenth Revision, Clinical Modification
- MCM
major congenital malformation
Appendix. Authors

Study Funding
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the NIH under Award Number R15HD097588. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Principal investigator: X. Wen.
Disclosure
O.D. Lawal reports no disclosures relevant to the manuscript. K.J. Meador has received research support from the NIH and Sunovion Pharmaceuticals and travel support from Eisai and the Epilepsy Study Consortium pays his university for his research consultant time related to Eisai, GW Pharmaceuticals, NeuroPace, Novartis, Supernus, Upsher-Smith Laboratories, and UCB Pharma. A.L. Hume and X. Wen report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy: United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(31):821-825. doi: 10.15585/mmwr.mm6631a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meador KJ, Loring DW. Developmental effects of antiepileptic drugs and the need for improved regulations. Neurology. 2016;86(3):297-306. doi: 10.1212/WNL.0000000000002119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomson T, Battino D, Bromley R, et al. Management of epilepsy in pregnancy: a report from the International League Against Epilepsy Task Force on Women and Pregnancy. Epileptic Disord. 2019;21(6):497-517. doi: 10.1684/epd.2019.1105 [DOI] [PubMed] [Google Scholar]
- 4.Tomson T, Battino D, Bonizzoni E, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol. 2011;10(7):609-617. doi: 10.1016/S1474-4422(11)70107-7 [DOI] [PubMed] [Google Scholar]
- 5.Hernández-Díaz S, Smith CR, Shen A, et al. Comparative safety of antiepileptic drugs during pregnancy. Neurology. 2012;78(21):1692-1699. doi: 10.1212/WNL.0b013e3182574f39 [DOI] [PubMed] [Google Scholar]
- 6.Veroniki AA, Cogo E, Rios P, et al. Comparative safety of anti-epileptic drugs during pregnancy: a systematic review and network meta-analysis of congenital malformations and prenatal outcomes. BMC Med. 2017;15(1):95. doi: 10.1186/s12916-017-0845-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight R, Wittkowski A, Bromley RL. Neurodevelopmental outcomes in children exposed to newer antiseizure medications: a systematic review. Epilepsia. 2021;62(8):1765-1779. doi: 10.1111/epi.16953 [DOI] [PubMed] [Google Scholar]
- 8.Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360(16):1597-1605. doi: 10.1056/NEJMoa0803531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meador KJ, Baker GA, Browning N, et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12(3):244-252. doi: 10.1016/S1474-4422(12)70323-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bromley RL, Calderbank R, Cheyne CP, et al. Cognition in school-age children exposed to levetiracetam, topiramate, or sodium valproate. Neurology. 2016;87(18):1943-1953. doi: 10.1212/WNL.0000000000003157 [DOI] [PubMed] [Google Scholar]
- 11.Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001-2008. Am J Public Health. 2014;104(suppl 1):S43-S48. doi: 10.2105/AJPH.2013.301416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843-852. doi: 10.1056/NEJMsa1506575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reimers A, Brodtkorb E, Sabers A. Interactions between hormonal contraception and antiepileptic drugs: clinical and mechanistic considerations. Seizure. 2015;28:66-70. doi: 10.1016/j.seizure.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 14.Kim H, Faught E, Thurman DJ, Fishman J, Kalilani L. Antiepileptic drug treatment patterns in women of childbearing age with epilepsy. JAMA Neurol. 2019;76(7):783-790. doi: 10.1001/jamaneurol.2019.0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meador KJ, Pennell PB, May RC, et al. Changes in antiepileptic drug-prescribing patterns in pregnant women with epilepsy. Epilepsy Behav. 2018;84:10-14. doi: 10.1016/j.yebeh.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen X, Meador KJ, Hartzema A. Antiepileptic drug use by pregnant women enrolled in Florida Medicaid. Neurology. 2015;84(9):944-950. doi: 10.1212/WNL.0000000000001304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daugaard CA, Sun Y, Dreier JW, Christensen J. Use of antiepileptic drugs in women of fertile age. Dan Med J. 2019;66(8):A5563. [PubMed] [Google Scholar]
- 18.Wen X, Lawal OD, Belviso N, et al. Association between prenatal opioid exposure and neurodevelopmental outcomes in early childhood: a retrospective cohort study. Drug Saf. 2021;44(8):863-875. doi: 10.1007/s40264-021-01080-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knezevic CE, Marzinke MA. Clinical use and monitoring of antiepileptic drugs. J Appl Lab Med. 2018;3(1):115-127. doi: 10.1373/jalm.2017.023689 [DOI] [PubMed] [Google Scholar]
- 20.Glauser T, Ben-Menachem E, Bourgeois B, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013;54(3):551-563. doi: 10.1111/epi.12074 [DOI] [PubMed] [Google Scholar]
- 21.Helmers SL, Thurman DJ, Durgin TL, Pai AK, Faught E. Descriptive epidemiology of epilepsy in the U.S. population: a different approach. Epilepsia. 2015;56(6):942-948. doi: 10.1111/epi.13001 [DOI] [PubMed] [Google Scholar]
- 22.Thurman DJ, Beghi E, Begley CE, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52(suppl 7):2-26. doi: 10.1111/j.1528-1167.2011.03121.x [DOI] [PubMed] [Google Scholar]
- 23.Hudson M, Rahme E, Richard H, Pilote L. Comparison of measures of medication persistency using a prescription drug database. Am Heart J. 2007;153(1):59-65. doi: 10.1016/j.ahj.2006.10.018 [DOI] [PubMed] [Google Scholar]
- 24.Bourgeois FT, Olson KL, Poduri A, Mandl KD. Comparison of drug utilization patterns in observational data: antiepileptic drugs in pediatric patients. Paediatr Drugs. 2015;17(5):401-410. doi: 10.1007/s40272-015-0139-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol. 2018;75(3):279-286. doi: 10.1001/jamaneurol.2017.3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Census Bureau. 2010 Census Regions and Divisions of the United States. Accessed December 4, 2021. census.gov/geographies/reference-maps/2010/geo/2010-census-regions-and-divisions-of-the-united-states.html. [Google Scholar]
- 27.Ailes EC, Simeone RM, Dawson AL, Petersen EE, Gilboa SM. Using insurance claims data to identify and estimate critical periods in pregnancy: an application to antidepressants. Birth Defects Res A Clin Mol Teratol. 2016;106(11):927-934. doi: 10.1002/bdra.23573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarayani A, Wang X, Thai TN, Albogami Y, Jeon N, Winterstein AG. Impact of the transition from ICD-9-CM to ICD-10-CM on the identification of pregnancy episodes in US health insurance claims data. Clin Epidemiol. 2020;12:1129-1138. doi: 10.2147/CLEP.S269400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margulis AV, Setoguchi S, Mittleman MA, Glynn RJ, Dormuth CR, Hernández-Díaz S. Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiol Drug Saf. 2013;22(1):16-24. doi: 10.1002/pds.3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. Department of Health & Human Services. Contraceptive Provision Measures | HHS Office of Population Affairs. Accessed July 6, 2022. opa.hhs.gov/claims-data-sas-program-instructions. [Google Scholar]
- 31.Rodriguez MI, Skye M, Lindner S, et al. Analysis of contraceptive use among immigrant women following expansion of Medicaid coverage for postpartum care. JAMA Netw Open. 2021;4(12):e2138983. doi: 10.1001/jamanetworkopen.2021.38983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335-351. doi: [DOI] [PubMed] [Google Scholar]
- 33.Meador KJ, Penovich P, Baker GA, et al. Antiepileptic drug use in women of childbearing age. Epilepsy Behav. 2009;15(3):339-343. doi: 10.1016/j.yebeh.2009.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herzog AG, Mandle HB, Cahill KE, Fowler KM, Hauser WA. Predictors of unintended pregnancy in women with epilepsy. Neurology. 2017;88(8):728-733. doi: 10.1212/WNL.0000000000003637 [DOI] [PubMed] [Google Scholar]
- 35.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645. doi: 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raji MA, Kuo YF, Adhikari D, Baillargeon J, Goodwin JS. Decline in opioid prescribing after federal rescheduling of hydrocodone products. Pharmacoepidemiol Drug Saf. 2018;27(5):513-519. doi: 10.1002/pds.4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peckham AM, Ananickal MJ, Sclar DA. Gabapentin use, abuse, and the US opioid epidemic: the case for reclassification as a controlled substance and the need for pharmacovigilance. Risk Manag Healthc Policy. 2018;11:109-116. doi: 10.2147/RMHP.S168504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pauly NJ, Delcher C, Slavova S, Lindahl E, Talbert J, Freeman PR. Trends in gabapentin prescribing in a commercially insured U.S. adult population, 2009-2016. JMCP. 2020;26(3):246-252. doi: 10.18553/jmcp.2020.26.3.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The IQVIA Institute. Medicine Spending and Affordability in the U.S | Understanding Patients' Costs for Medicines. Accessed July 8, 2022. iqvia.com/insights/the-iqvia-institute/reports/medicine-spending-and-affordability-in-the-us. [Google Scholar]
- 40.Center for Drug Evaluation and Research. FDA Drug Safety Communication: Risk of Oral Clefts in Children Born to Mothers Taking Topamax (Topiramate). FDA. Published online June 18, 2019. Accessed September 21, 2021. fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-risk-oral-clefts-children-born-mothers-taking-topamax-topiramate. [Google Scholar]
- 41.Wang X, Zhang T, Ekheden I, et al. Prenatal exposure to benzodiazepines and Z-drugs in humans and risk of adverse neurodevelopmental outcomes in offspring: a systematic review. Neurosci Biobehav Rev. 2022;137:104647. doi: 10.1016/j.neubiorev.2022.104647 [DOI] [PubMed] [Google Scholar]
- 42.Jensen AG, Knudsen SS, Bech BH. Prenatal exposure to benzodiazepines and the development of the offspring: a systematic review. Neurotoxicol Teratol. 2022;91:107078. doi: 10.1016/j.ntt.2022.107078 [DOI] [PubMed] [Google Scholar]
- 43.Gladden RM. Changes in opioid-involved overdose deaths by opioid type and presence of benzodiazepines, cocaine, and methamphetamine—25 States, July-December 2017 to January-June 2018. MMWR Morb Mortal Wkly Rep. 2019;68(34):737-744. doi: 10.15585/mmwr.mm6834a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol. 2012;11(9):803-813. doi: 10.1016/S1474-4422(12)70103-5 [DOI] [PubMed] [Google Scholar]
- 45.Meador K. Teratogenicity and antiseizure medications. Epilepsy Curr. 2020;20(6 suppl):15S-17S. doi: 10.1177/1535759720945298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gedzelman ER, Meador KJ. Neurological and psychiatric sequelae of developmental exposure to antiepileptic drugs. Front Neurol. 2012;3:182. doi: 10.3389/fneur.2012.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bittigau P, Sifringer M, Ikonomidou C. Antiepileptic drugs and apoptosis in the developing brain. Ann NY Acad Sci. 2003;993:103-114; discussion 123-124. doi: 10.1111/j.1749-6632.2003.tb07517.x [DOI] [PubMed] [Google Scholar]
- 48.Eroglu C, Allen NJ, Susman MW, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139(2):380-392. doi: 10.1016/j.cell.2009.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blotière PO, Miranda S, Weill A, et al. Risk of early neurodevelopmental outcomes associated with prenatal exposure to the antiepileptic drugs most commonly used during pregnancy: a French nationwide population-based cohort study. BMJ Open. 2020;10(6):e034829. doi: 10.1136/bmjopen-2019-034829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bjørk MH, Zoega H, Leinonen MK, et al. Association of prenatal exposure to antiseizure medication with risk of autism and intellectual disability. JAMA Neurol. 2022;79(7):672-681. doi: 10.1001/jamaneurol.2022.1269 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Optum Clinformatics Data Mart is available by the data vendor under license to the University of Rhode Island College of Pharmacy. Other researchers may obtain access under licensing agreements with the data vendor.