Abstract
Background and Objectives
Odor identification deficits are associated with transition to dementia, whereas intact odor identification and global cognition test performance may identify lack of transition. The purpose of this study was to examine intact odor identification and global cognition as prognostic indicators of lack of transition to dementia in a biracial (Black and White) cohort.
Methods
In a community-dwelling sample of older adults from the Health, Aging, and Body Composition study, odor identification was measured using the Brief Smell Identification Test (BSIT), and global cognition was measured using the Teng Modified Mini-Mental State Examination (3MS). Survival analyses for dementia transition over 4 and 8 years of follow-up used Cox proportional hazards models.
Results
A total of 2,240 participants had an average age of 75.5 years (SD 2.8). Approximately 52.7% were female individuals. Approximately 36.7% were Black and 63.3% were White individuals. Impaired odor identification (hazard ratio [HR] 2.29, 95% CI 1.79–2.94, p < 0.001) and global cognition (HR 3.31, 95% CI 2.26–4.84, p < 0.001) were each independently associated with transition to dementia (n = 281). Odor identification remained robustly associated with transition to dementia for Black (HR 2.02, 95% CI 1.36–3.00, p < 0.001, n = 821) and White participants (HR 2.45, 95% CI 1.77–3.38, p < 0.001, n = 1,419), whereas global cognition was associated with transition among Black participants only (HR 5.06, 95% CI 3.18–8.07, p < 0.001). ApoE genotype was consistently associated with transition among White participants only (HR 1.75, 95% CI 1.20–2.54, p < 0.01). Among participants with intact performance on both odor identification (BSIT ≥9/12 correct) and global cognition (3MS ≥ 78/100 correct), 8.8% transitioned to dementia over 8 years. Intact performance on both measures had high positive predictive value for identifying individuals who did not transition to dementia over 4 years (0.98 for ages 70–75 years with only 2.3% transitioning, 0.94 for ages 76–82 years with only 5.8% transitioning).
Discussion
Odor identification testing paired with a global cognitive screening test identified individuals at low risk of transition to dementia in a biracial community cohort with a pronounced effect in the eighth decade of life. Identification of such individuals can reduce the need for extensive investigation to establish a diagnosis. Odor identification deficits showed utility in both Black and White participants, unlike the race-dependent utility of a global cognitive test and ApoE genotype.
Introduction
During the course of Alzheimer disease (AD), neuropathology develops early in the regions responsible for olfactory processing. Neurofibrillary tangles and volume loss are observed within the olfactory epithelium, olfactory bulb, piriform cortex, entorhinal cortex, and hippocampus.1 Such pathology is clinically manifest through impaired performance on tests of olfactory functioning, chiefly tests of odor identification.2 Several longitudinal studies have shown that impaired odor identification ability is associated with an increased likelihood of cognitive decline3 and conversion to dementia.4-6 Similarly, impairment on brief cognitive screening tests is also associated with an increased likelihood of conversion to dementia.7 Conversely, the negative predictive value of smell tests is high for clinical AD dementia because for this diagnosis, zero odor identification errors are virtually nonexistent.8-10
The question that remains is if both odor identification ability and brief cognitive test performance are intact, how strongly does this combination indicate the lack of likelihood of conversion to dementia during long-term follow-up? If intact odor identification and intact brief cognitive test performance are associated with cognitive stability over long-term follow-up, this approach can be used to inform patients about their likely prognosis and prevent unnecessary extensive investigation for the diagnosis of dementia and to rapidly screen in or screen out participants for the prevention and treatment trials of cognitive-enhancing therapies.
As previously reported, in the Washington Heights/Inwood Columbia Aging Project (WHICAP) community-based cohort, 749 older adults aged 70–83 years were followed up for 4 years. Participants with intact olfactory and cognitive ability transitioned to dementia at a low rate (3.4% compared with 14.6% for the full sample), with no transitions in the youngest 70–75 years and in the 81–83 years age group quartiles.11 This supports the notion that odor identification tests used in conjunction with brief cognitive screening tests offer strong negative predictive value for conversion to dementia. This finding, however, requires replication and extension in other diverse cohorts because of the known influence of social determinants of health and structural racism on differential predictive value of measures and access to participation in clinical research. Indeed, despite greater risk of AD, Black and Hispanic/Latino populations are underrepresented in observational research and randomized clinical trials for AD.12,13
The Health, Aging, and Body Composition (Health ABC) study involved 3,075 participants aged 70–79 years at baseline. This biracial cohort was followed up for more than a decade. Studies published on the use of the Brief Smell Identification Test (BSIT) from the Health ABC cohort have focused on association with dementia and cognitive decline in Black and White participants6 and on mortality as an outcome.14 In the Health ABC database, we evaluated whether intact performance on both a cognitive and an olfaction screening test were associated with lack of conversion to dementia. We hypothesized that intact performance on these measures will each independently be associated with lower frequency of conversion to dementia during follow-up compared with the rest of the sample, with intact performance on both measures being associated with the lowest frequency of conversion. We additionally hypothesized that this effect will be manifest in both Black and White participants and will remain robust after adjusting for covariates. We also explored the impact of ApoE e4 genotype on the effects of brief olfactory and cognitive tests because of the known association of ApoE e4 with olfactory impairment15,16 and racial differences in ApoE e4 risk of dementia.17,18
Methods
Participants
Health ABC is a longitudinal cohort study consisting of 3,075 men and women who were aged 70–79 years at baseline, with 45% of the women and 33% of the men identifying as Black; comparison of health characteristics over time of White and Black participants was a primary goal of the study. Race was self-identified by participants. Participants were recruited in 1997–1998 from a random sample of Medicare-eligible adults living within zip codes in Pittsburgh, PA, and Memphis, TN. To be eligible at baseline, participants were required to walk ¼ mile and climb up 10 steps without difficulty. In addition, participants must have reported no difficulties in activities of daily living, been free from life-threatening illnesses, and planned to remain in the study area for at least 3 years.
Standard Protocol Approvals, Registrations, and Patient Consents
All participants signed informed consent forms, and the study was approved by the institutional review boards at both sites.
Sample Selection
The BSIT was administered only in year 3, which was used as a baseline to establish the utility of odor identification ability over the course of 8 years through year 11. We excluded participants who were missing either the year 3 BSIT (n = 538) or the year 3 Modified Mini-Mental State Examination (3MS) (n = 539), which led to a total of 541 excluded participants. Next, 7 participants were excluded because they met criteria for dementia at year 3, and a subsequent 211 participants who had no follow-up after year 3 were excluded. Finally, 76 participants were excluded because of anosmia (BSIT ≤3/12; olfactory performance at or below statistical chance). The final sample comprised 2,240 participants (Figure 1).
Figure 1. Flowchart of Participant Selection.
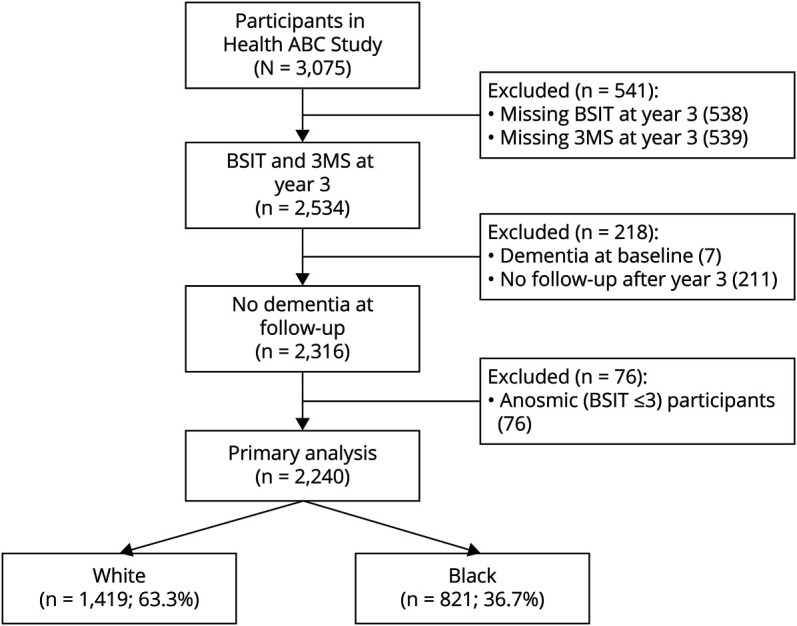
3MS = Modified Mini-Mental State Examination; BSIT = Brief Smell Identification Test; Health ABC = Health, Aging, and Body Composition.
Measures
Olfaction was measured during year 3 with the 12-item BSIT (Sensonics International, Haddon Heights, NJ).19 The participant scratches an odorant strip containing a microcapsule, sniffs the emanated odor, and identifies the odor from 4 choices. The BSIT score ranges from 0 to 12 with 0 indicating all odors incorrectly identified and 12 indicating all 12 odors correctly identified. “Intact” BSIT performance was defined as scores 9–12, and “impaired” BSIT performance was defined as scores 0–8. There is no consensus in the field on the optimal BSIT cutoff. This cutoff was selected because it has been previously used in another community cohort of older adults.11
Global cognition was measured using the Teng 3MS.20 The 3MS is an expanded version of the original Folstein Mini-Mental State Examination (MMSE) and includes tests of orientation, attention, registration, verbal recall, calculation, and visuospatial ability. The 3MS was collected during years 1, 3, 5, 7, 9, 10, and 11. Scores range from 1 to 100, with higher scores indicating better cognitive performance. “Intact” 3MS performance was defined as scores 78–100, and “impaired” 3MS performance was defined as scores 1–77 as previously reported in other publications from this cohort.21
To be classified as having developed dementia, participants must have had either (1) dementia diagnosis from adjudicated hospital records or (2) records of prescribed antidementia medication (galantamine, rivastigmine, memantine, donepezil, or tacrine) from hospital records or study records. To identify and exclude individuals who had already developed dementia by year 3, the same definition was used. This dementia definition is based on definitions used in previous Health ABC publications,6,22,23 except that cognitive decline on the 3MS alone was not categorized as dementia because such decline is not specific to dementia and is insufficient to make the diagnosis in the absence of functional impairment. Furthermore, this approach is consistent with the criteria used for similar measures published from the WHICAP study, and it avoids the confound of using the 3MS both as a variable of interest (year 3 performance) and as an outcome measure (if used to define dementia).11
Statistical Analyses
Distributions and group differences in demographic and clinical variables were examined by χ2 and 2-sample t tests as appropriate. Survival analyses for dementia transition between year 3 and year 11 were conducted using Cox proportional hazards models.
The primary outcome was time until dementia from year 3. The censoring time was determined as the years from year 3 to the last observed year or year 11. The proportional hazard assumption was checked using statistical tests based on the correlation between time and scaled Schoenfeld residuals. The proportional hazard assumption is supported by a nonsignificant relationship between residuals and time (p > 0.05). All model assumptions were met. Variables of primary interest were the BSIT and 3MS, each dichotomized by their respective cutoffs. A secondary variable of interest was the ApoE genotype. Because we tested 3 variables of interest sequentially, we did not perform multiple comparison corrections. Models were first adjusted for demographics (age, sex, education, and site) and then for expanded covariates evaluated in prior publications from the Health ABC cohort including medical comorbidities (age, sex, education, site, race, literacy [defined as at or above ninth grade reading level on the Rapid Estimate of Adult Literacy in Medicine], depressive symptoms [Center for Epidemiologic Studies–Depression scale], ApoE genotype, smoking status [never, former, current], current alcohol consumption, body mass index, walking speed [m/sec over 20 m], history of diabetes, hypertension, transient ischemic attack, cerebrovascular accident, head injury, and self-reported smell problems) (identified by a single question as part of the BSIT). We performed the same analyses, stratified by race. Mortality was examined as a competing risk by modeling the subdistribution hazard function of a specific cause as recommended when estimating incidence or predicting prognosis in the presence of competing risks.24 The analysis was performed using the comp.risk function with proportional hazard models and Cox censoring models as implemented in timereg R package.25
All survival analyses modeled transition to dementia. Prediction of lack of transition to dementia was examined by contingency table and binary diagnostic tests with fixed time points of 4 years and 8 years of follow-up. In these analyses, for the predictors of BSIT, 3MS, and their combinations, we assessed sensitivity, specificity, positive predictive value, and negative predictive value for identifying lack of transition to dementia. Analyses were conducted using R version 4.0.1.
Results
Baseline Demographics
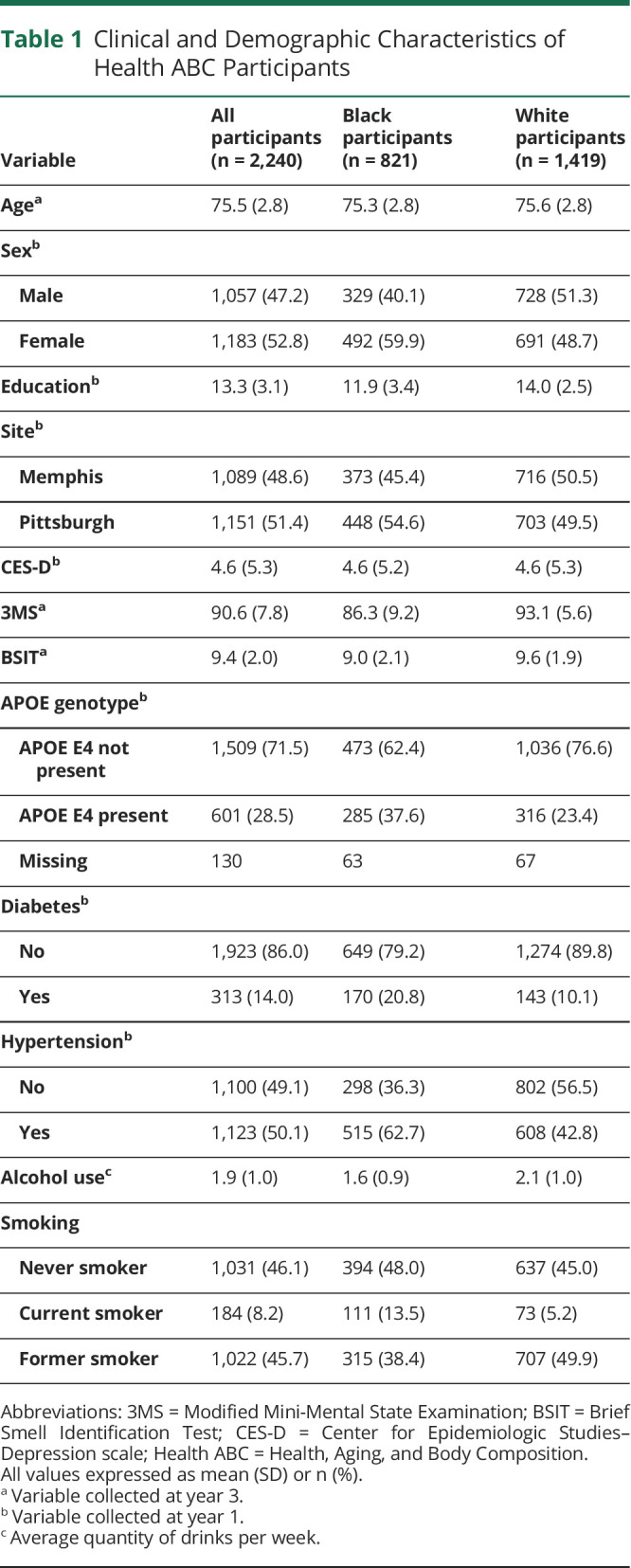
Participant demographic and clinical characteristics at year 3 are summarized in Table 1. The average age of the 2,240 participants in the analytic sample was 75.5 (SD 2.8) years. Approximately 47.3% of the sample was male individuals, and 52.7% was female individuals. Black participants comprised 36.7% of the sample, whereas White participants constituted 63.3%. The mean BSIT at year 3 was 9.4 (SD 2.0). Approximately 29.2% of participants fell within the “impaired” range (<9), and 70.8% fell within the “intact” range (≥9). BSIT scores were significantly lower in Black participants (mean 9.0, SD 2.1) than in White participants (mean 9.6, SD 1.9). Approximately 34.5% of Black participants fell within the “impaired” range (<9) compared with 26.1% of White participants. The mean 3MS at year 3 was 90.6 (SD 7.8). Approximately 7.0% of participants fell within the “impaired” range (<78), whereas 93.0% were within the “intact” range (≥78). 3MS scores were significantly lower in Black participants (mean 86.3, SD 9.2) than in White participants (mean 93.1, SD 5.6). Approximately 16.3% of Black participants fell within the “impaired range” (<78) compared with 1.6% of White participants. Compared with participants with intact BSIT performance, participants with impaired BSIT performance were more likely to be older, male, Black, enrolled at Pittsburgh, have a history of diabetes, current smokers, report a subjective history of smell/taste problems, and have lower performance on the 3MS (all p values < 0.05).
Table 1.
Clinical and Demographic Characteristics of Health ABC Participants
Proportions Transitioning to Dementia
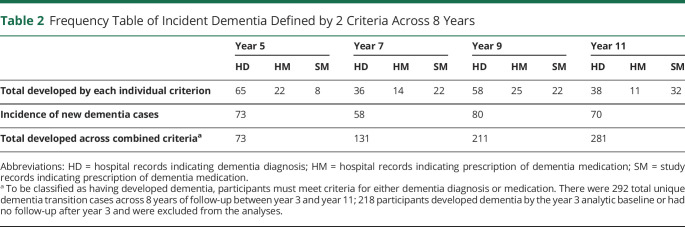
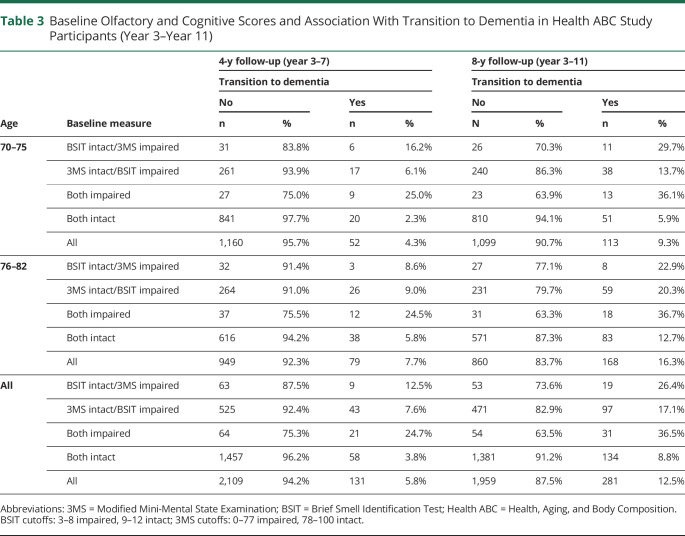
Across 4 years of follow-up between year 3 and year 7, 131 participants (5.8%) converted to dementia (Table 2). During the 4 years of follow-up, participants who had both intact BSIT and 3MS exhibited a conversion rate of 3.8%, with the younger half of the sample (70–75 years) converting at only 2.3% (20/861) (Table 3). By contrast, impairment on both the BSIT and 3MS was associated with a conversion rate of 25.0% in the 70–75 years age range and 24.7% across the entire sample (Table 3).
Table 2.
Frequency Table of Incident Dementia Defined by 2 Criteria Across 8 Years
Table 3.
Baseline Olfactory and Cognitive Scores and Association With Transition to Dementia in Health ABC Study Participants (Year 3–Year 11)
Across 8 years of follow-up between year 3 and year 11, 281 participants (12.5%) converted to dementia. Participants who had intact performance on both BSIT and 3MS had a low likelihood of conversion to dementia over 8 years of follow-up (8.8%), with the younger half of the sample (age 70–75 years) converting at a rate of 5.9%. Impairment on both the BSIT and 3MS was associated with a conversion rate of 36.1% in the 70–75 years age range and 36.5% across the entire sample. Across 8 years of follow-up, there were 73 new cases between years 3 and 5, 58 new cases between years 5 and 7, 80 new cases between years 7 and 9, and 70 new cases between years 9 and 11.
To rule out the possibility that small sample size of certain strata was responsible for the differences in dementia transition between strata in Table 3, we conducted 3 complementary permutation analyses that provided the null distribution of each cell when the BSIT and 3MS are not correlated with dementia transition. In all scenarios, the high dementia transition rate in the “both impaired” group and low dementia transition rate in the “both intact” group were beyond the random quantity generated by random permutations and in turn unlikely due to the cell size (eTables 1–3 and eFigures 1–3, links.lww.com/WNL/C965).
Association With Lack of Transition to Dementia: Sensitivity, Specificity, and Predictive Values
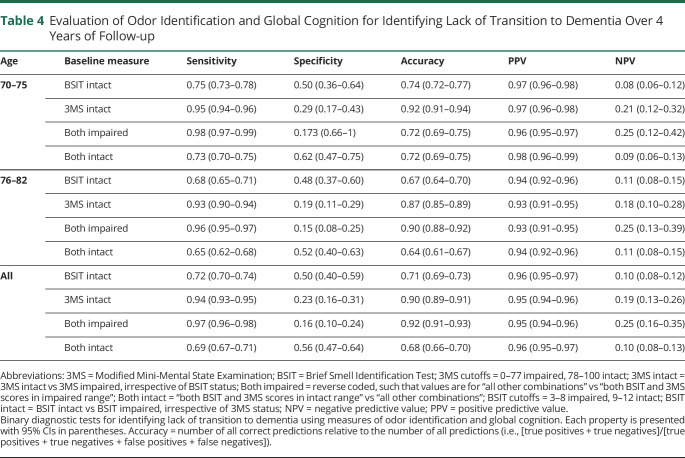
To determine the clinical utility of the measures for identifying lack of transition to dementia, sensitivity, specificity, accuracy, positive predictive value, and negative predictive value were determined across 4 years of follow-up at year 7 (Table 4). The BSIT and 3MS were evaluated both alone at their respective cutoffs and jointly in the forms of “both impaired” (vs all other combinations) and “both intact” (vs all other combinations). For identifying lack of transition to dementia, the sensitivity of these measures was greater than their specificity (0.72 vs 0.50 for BSIT Intact; 0.94 vs 0.23 for 3MS Intact). Similarly, the positive predictive value was greater than the negative predictive value (0.96 vs 0.10 for BSIT intact; 0.95 vs 0.19 for 3MS intact). When performance on these measures was evaluated jointly, the “both intact” (vs all other combinations) category yielded a more balanced distribution of sensitivity vs specificity (0.69 vs 0.56), with a similar positive predictive value vs negative predictive value distribution (0.96 vs 0.10).
Table 4.
Evaluation of Odor Identification and Global Cognition for Identifying Lack of Transition to Dementia Over 4 Years of Follow-up
Association With Transition to Dementia: Survival Analyses
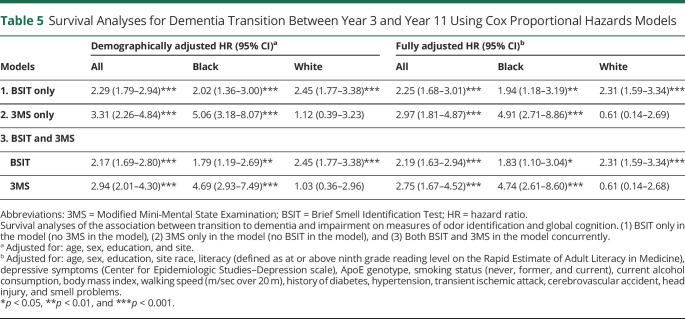
Survival analyses for dementia transition between year 3 and year 11 are summarized in Table 5. In the BSIT-only model, demographically adjusted, low BSIT performance was associated with transition to dementia (hazard ratio [HR] 2.29, 95% CI 1.79–2.94, p < 0.001). In the fully adjusted model, older age, ApoE e4 genotype, history of diabetes, history of hypertension, and body mass index were also associated with transition to dementia (all p values < 0.05). In the 3MS-only model, demographically adjusted, low 3MS performance was associated with transition to dementia (HR 3.31, 95% CI 2.26–4.84, p < 0.001). As in the previous model, older age, ApoE e4 genotype, history of diabetes, history of hypertension, and body mass index were also associated with transition to dementia in the fully adjusted model (all p values < 0.05).
Table 5.
Survival Analyses for Dementia Transition Between Year 3 and Year 11 Using Cox Proportional Hazards Models
In the models examining BSIT and 3MS concurrently, both measures were independently and significantly associated with transition to dementia in the demographically adjusted model (BSIT: HR 2.17, 95% CI 1.69–2.80, p < 0.001; 3MS: HR 2.94, 95% CI 2.01–4.30, p < 0.001) (Figure 2). In the fully adjusted model, the BSIT (HR 2.19, 95% CI 1.63–2.94, p < 0.001) and the 3MS (HR 2.75, 95% CI 1.67–4.52, p = 0.001) remained significantly associated with transition to dementia. Older age, ApoE e4 genotype, and a history of diabetes were also associated with transition to dementia (all p < 0.05).
Figure 2. Survival Function for Dementia Transition Over 8 Years of Follow-up.
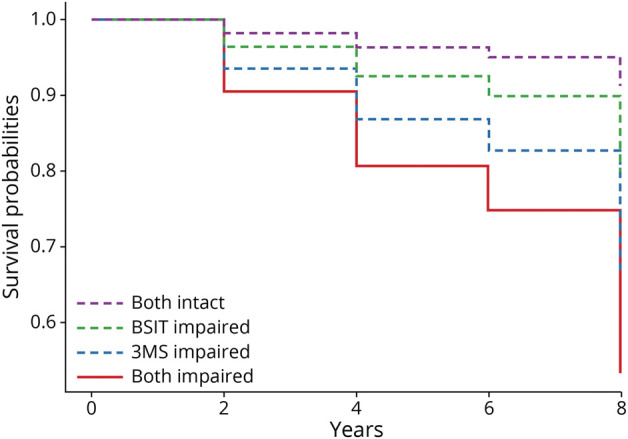
3MS = Modified Mini-Mental State Examination; BSIT = Brief Smell Identification Test.
To test for interactions, a BSIT × 3MS interaction term was added to the BSIT and 3MS models, although this was not associated with transition to dementia (p = 0.666). Similarly, when ApoE and a BSIT × ApoE interaction term were added to the BSIT model, the interaction was also not associated with transition (p = 0.499).
Two hundred twenty-nine participants died across 11 years of follow-up. To determine whether death was a competing risk, mortality was examined as a variable alongside the main variables of interest in competing risk regression models. Measures within the BSIT-only (BSIT: HR 2.22, 95% CI 1.73–2.87, z = 6.16, p < 0.0001), 3MS-only (3MS: HR 2.98, 95% CI 2.13–4.16, z = 6.38, p < 0.0001), and BSIT plus 3MS models (BSIT: HR 2.08, 95% CI 1.60–2.72, z = −5.39, p < 0.0001; 3MS: HR 2.60, 95% CI 1.83–3.66, z = 5.45, p < 0.0001) remained significantly associated with transition to dementia after accounting for the competing risk of mortality. Across all fully adjusted models, the following covariates were not associated with transition to dementia (all p values > 0.05): education, race, literacy, current alcohol consumption, depressive symptoms, walking speed, transient ischemic attack, cerebrovascular accident, head injury, and subjective smell problems.
Association With Transition to Dementia by Race
To clarify the effect of each variable within each race group, models were rerun with Black participants only (n = 821) and with White participants only (n = 1,419). Both BSIT-only models remain unchanged compared with the models with all participants. However, in the 3MS-only models, the 3MS was significantly associated with transition to dementia for the Black participants though not for the White participants. This was the case for both the demographically (Black, HR 5.06, 95% CI 3.18–8.07, p < 0.001; White, HR 1.12, 95% CI 0.39–3.23, p = 0.837) and fully adjusted models (Black, HR 4.91, 95% CI 2.71–8.86, p < 0.001; White, HR 0.61, 95% CI 0.14–2.69, p = 0.513). Notably, ApoE was significantly associated with transition to dementia in the fully adjusted 3MS model for White participants (HR 1.75, 95% CI 1.20–2.54, p = 0.004) though not for Black participants (HR 1.45, 95% CI 0.90–2.34, p = 0.125).
This pattern continued in the models examining BSIT and 3MS concurrently, where 3MS was associated with transition for Black participants (HR 4.69, 95% CI 2.93–7.49, p < 0.001) but not White participants (HR 1.03, 95% CI 0.36–2.96, p = 0.993). The BSIT remained significantly associated with transition for both Black participants (HR 1.79, 95% CI 1.19–2.69, p = 0.005) and White participants (HR 2.45, 95% CI 1.77–3.38, p < 0.001). These effects were consistent across the demographically and fully adjusted models. ApoE e4 genotype was significantly associated with transition to dementia in the fully adjusted model for White participants (HR 1.69, 95% CI 1.15–2.46, p = 0.007) though not for Black participants (HR 1.44, 95% CI 0.89–2.32, p = 0.140).
The BSIT × 3MS interaction was tested in Black participants and remained not significantly associated with transition to dementia (p = 0.761). We did not run the BSIT × 3MS interaction models in White participants due to instability of the model fit from small sample sizes in select categories. The BSIT × ApoE interaction was also tested, though this interaction remained not significantly associated with transition to dementia for both Black participants (p = 0.210) and White participants (p = 0.621).
Discussion
Odor identification and brief global cognitive screening performance were found to be associated with dementia incidence over both 4 and 8 years of follow-up. The lowest risk of dementia was found for participants with intact performance on both the BSIT and 3MS. Intact performance on both measures has high positive predictive value (0.98 for ages 70–75 years, 0.94 for ages 76–82 years) for identifying individuals who will not transition to dementia. The measures performed better at predicting outcomes at 4 years of follow-up than at 8 years, likely owing to the development of new deficits or intervening medical comorbidities across longer intervals.
These results are very similar to those obtained in the independent WHICAP cohort, average age 80 years, where combined intact BSIT and global cognition measured by the Blessed Orientation Memory Concentration Test were associated with 3.4% conversion to dementia over 4 years of follow-up with no conversions in the 70–75 years age group. The consistency of the findings in different cohorts with large White (Health ABC and WHICAP), Black (Health ABC and WHICAP), and Latino (WHICAP) representation can be translated broadly to clinical settings. The 2 instruments found in Health ABC to be associated with lack of transition to dementia are simple, quick to complete (<20 minutes combined), and can be taught to research and clinical personnel of all levels. The clearly defined cutoffs (<9 for BSIT, <78 for 3MS) also aid in ease of interpretability of an individual's risk level. It is likely that the original Folstein MMSE, which is widely used and was the basis for the development of the 3MS, will show similar effects.
Impaired odor identification, impaired global cognition, and ApoE e4 genotype were each independently associated with increased transition to dementia in survival models comprising the full sample. When the sample was subdivided by race, a more nuanced pattern emerged. Odor identification was robustly associated with transition, unlike the global cognitive screen and ApoE genotype, which were influenced by race. Impaired odor identification was associated with transition to dementia in both White and Black participants, while global cognition was associated with transition for Black but not White participants. The inconsistent findings for the global cognitive screener may have been driven partly by the distinctive distributions of scores for this measure across these groups, where scores among Black participants fell within the “impaired” range if they were 1 SD below the mean, though scores among White participants fell within the impaired range only if they were approximately 3 SDs below the mean. These comparisons must be interpreted in the established context that cognitive screeners tend to disadvantage Black individuals, contributing to misdiagnosis most frequently in the form of false positives for the diagnosis of dementia.26 Collectively, these findings suggest that odor identification deficits are less influenced by race than cognitive measures, and this improves its practical relevance across broad diverse populations.
Impaired odor identification and ApoE genotype each contributed independent value for modeling association with transition to dementia. Odor identification deficits are linked with extent of tau pathology,27,28 more than with β-amyloid,27,29 while ApoE e4 confers greater risk for amyloid plaque accumulation.30 The presence of both biomarker abnormalities is associated with worse cognitive trajectories, which may explain the findings of complementary value from both impaired BSIT performance and ApoE e4 genotype.31 Furthermore, patients with AD who are ApoE e4 carriers tend to exhibit cortical atrophy primarily in medial temporal areas (which themselves are linked to olfactory functioning), whereas patients with AD who are noncarriers display greater atrophy of frontoparietal areas (which have less pronounced roles in olfaction).32
ApoE e4 is more of a risk factor of AD in White individuals than in Black individuals.17,18,33,34 The odor identification/ApoE interaction was not significant for participants in this sample. This type of interaction has been found in some prior studies examining odor identification as a predictor of cognitive decline,3,35 though null findings have also been reported.4,36 The studies reporting an interaction between odor identification and ApoE on cognitive decline, that is, the Swedish National Study on Aging and Care in Kungsholmen 33 and the Betula Study35 were both conducted in Sweden and were comprised predominantly by individuals identifying as White/European. The studies reporting no interaction, that is, the WHICAP 111 and the Rush Memory and Aging Project36 were conducted in the United States and included a higher proportion of individuals identifying as Black and or Hispanic. However, this difference in racial identification proportions between the studies does not necessarily serve as a proxy for ancestry because the average African American genome is 24.0% European, and 3.5% of European Americans have 1% or more African ancestry.37
Results of our current study align with the previous Health ABC findings of BSIT being associated with dementia incidence, though our design differs from prior Health ABC olfaction/mortality/dementia studies6,22,23 in several crucial ways. First, we considered the added value of odor identification performance to a global cognitive screening measure compared with either measure in isolation. Second, our definition of dementia required participants to have either (1) hospital records indicating dementia diagnosis or (2) prescription records of dementia medications. This definition is different from the definition previously used, where a 1.5 SD decline on the 3MS was a diagnostic criterion for dementia. Using a global cognitive screener as a single criterion is problematic because a report from the Canadian Study on Health and Aging determined that measured impairment on the 3MS has a low false-negative rate (0.64%) but has an exceedingly high false-positive rate for detecting dementia (71%) with less than one-third of those impaired on the 3MS actually having dementia.21 By using a stricter definition than prior Health ABC studies, we reduced the chances that participants are misclassified by exhibiting mild cognitive impairment alone or by exhibiting temporary fluctuations in cognitive performance due to a variety of possible nondementing causes. Third, we excluded anosmic participants, who had a level of smell performance below even what is common for individuals in moderate stages of dementia. This approach excluded individuals who could have had congenital anosmia or low odor identification performance due to causes unrelated to dementia.
There are several limitations to this study. The Health ABC study did not distinguish between subtypes of dementia, and no plasma, CSF, or neuroimaging biomarkers were studied. Dementia was not diagnosed clinically, and dementia etiologic subtypes could not be identified. Instead, we used multiple criteria that have been established for large epidemiologic datasets, which nonetheless may have misdiagnosed some participants. We additionally used dementia diagnosis carried forward, such that we could not account accurately for possible reversion in dementia status. For example, if a participant had records in year 7 indicating dementia medications, though at year 9 they had records omitting any mention of dementia medications, this was not considered to constitute reversion because omission or discontinuation of a medication may have occurred for reasons other than reversion to cognitively normal status. Because participants in the Health ABC study lived within large urban area zip codes, results may not generalize to rural populations. Last, because of sample size, select strata formed by age group, BSIT, and 3MS categories are sparse, thereby limiting our ability to detect interactions and precisely estimate dementia incidence for each category. While it would have been advantageous to have larger sample sizes in each stratum, the random permutation analyses suggest it is unlikely the results are due to sampling error.
In conclusion, odor identification testing paired with global cognitive testing identified individuals at low risk of transition to dementia in a biracial community cohort. In this study, the utility of odor identification deficits is independent of race, unlike a brief cognitive screener (3MS) and ApoE e4 genotype. The extremely low conversion rate for intact BSIT combined with intact global cognitive performance across 2 large independent cohorts suggests that these measures have the potential to be used in tandem for brief, cost-effective “umbrella” screening (screening out or screening in, as needed) of participants for a broad range of dementia prevention and therapeutic trials. Clinically, identification of such individuals can also obviate the need for extensive investigation to establish a diagnosis, for example, intact olfaction and cognition can lead to a decision that biomarker-based diagnostic workup will be conducted only if there is persistent cognitive or functional decline on long-term follow-up.
Glossary
- 3MS
Modified Mini-Mental State Examination
- AD
Alzheimer disease
- BSIT
Brief Smell Identification Test
- Health ABC
Health, Aging, and Body Composition
- HR
hazard ratio
- MMSE
Mini-Mental State Examination
- WHICAP
Washington Heights/Inwood Columbia Aging Project
Appendix. Authors
Study Funding
This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, NIA grant R01-AG028050; and NINR grant R01-NR012459. This research was funded partly by the Intramural Research Program of the NIH, National Institute on Aging. This work was also supported by the National Institute of Mental Health (NIMH) grant 2T32MH020004-21.
Disclosure
J.N. Motter, J. Choi, S. Lee, T.E. Goldberg, and S.M. Albert report no disclosures relevant to the manuscript. D.P. Devanand has been a scientific adviser to Acadia, Eisai, Genentech, Jazz, TauRx, Novo Nordisk, and Biogen. Go to Neurology.org/N for full disclosures.
References
- 1.Kovacs T. Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders. Ageing Res Rev. 2004;3(2):215-232. doi: 10.1016/j.arr.2003.10.003 [DOI] [PubMed] [Google Scholar]
- 2.Vassilaki M, Christianson TJ, Mielke MM, et al. Neuroimaging biomarkers and impaired olfaction in cognitively normal individuals. Ann Neurol. 2017;81(6):871-882. doi: 10.1002/ana.24960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olofsson JK, Larsson M, Roa C, Wilson DA, Jonsson Laukka E. Interaction between odor identification deficit and APOE4 predicts 6-year cognitive decline in elderly individuals. Behav Genet. 2020;50(1):3-13. doi: 10.1007/s10519-019-09980-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devanand DP, Lee S, Manly J, et al. Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology. 2015;84(2):182-189. doi: 10.1212/wnl.0000000000001132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts RO, Christianson TJ, Kremers WK, et al. Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol. 2016;73(1):93-101. doi: 10.1001/jamaneurol.2015.2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaffe K, Freimer D, Chen H, et al. Olfaction and risk of dementia in a biracial cohort of older adults. Neurology. 2017;88(5):456-462. doi: 10.1212/wnl.0000000000003558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakata E, Kasai M, Kasuya M, et al. Combined memory and executive function tests can screen mild cognitive impairment and converters to dementia in a community: the Osaki-Tajiri project. Neuroepidemiology. 2009;33(2):103-110. doi: 10.1159/000222092 [DOI] [PubMed] [Google Scholar]
- 8.Christensen IT, Larsson EM, Holm IE, Nielsen OBF, Andersen S. Olfactory testing in consecutive patients referred with suspected dementia. BMC Geriatr. 2017;17(1):129. doi: 10.1186/s12877-017-0516-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makowska I, Kloszewska I, Grabowska A, Szatkowska I, Rymarczyk K. Olfactory deficits in normal aging and Alzheimer's disease in the polish elderly population. Arch Clin Neuropsychol. 2011;26(3):270-279. doi: 10.1093/arclin/acr011 [DOI] [PubMed] [Google Scholar]
- 10.Kjelvik G, Sando SB, Aasly J, Engedal KA, White LR. Use of the Brief Smell Identification Test for olfactory deficit in a Norwegian population with Alzheimer's disease. Int J Geriatr Psychiatry. 2007;22(10):1020-1024. doi: 10.1002/gps.1783 [DOI] [PubMed] [Google Scholar]
- 11.Devanand DP, Lee S, Luchsinger JA, et al. Intact global cognitive and olfactory ability predicts lack of transition to dementia. Alzheimers Dement. 2020;16(2):326-334. doi: 10.1016/j.jalz.2019.08.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raman R, Aisen P, Carillo MC, et al. ; CTAD Task Force. Tackling a major deficiency of diversity in Alzheimer's disease therapeutic trials: an CTAD Task force report. J Prevent Alzheimers Dis. 2022;9(3):388-392. doi: 10.14283/jpad.2022.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kornblith E, Bahorik A, Boscardin WJ, Xia F, Barnes DE, Yaffe K. Association of race and ethnicity with incidence of dementia among older adults. JAMA. 2022;327(15):1488-1495. doi: 10.1001/jama.2022.3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu B, Luo Z, Pinto JM, et al. Relationship between poor olfaction and mortality among community-dwelling older adults: a cohort study. Ann Intern Med. 2019;170(10):673-681. doi: 10.7326/m18-0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olofsson JK, Nordin S, Wiens S, Hedner M, Nilsson LG, Larsson M. Odor identification impairment in carriers of ApoE-ɛ4 is independent of clinical dementia. Neurobiol Aging. 2010;31(4):567-577. doi: 10.1016/j.neurobiolaging.2008.05.019 [DOI] [PubMed] [Google Scholar]
- 16.Oleson S, Murphy C. Olfactory dysfunction in ApoE ɛ4/4 homozygotes with Alzheimer's disease. J Alzheimers Dis. 2015;46(3):791-803. doi: 10.3233/jad-150089 [DOI] [PubMed] [Google Scholar]
- 17.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349-1356. doi: 10.1001/jama.278.16.1349 [DOI] [PubMed] [Google Scholar]
- 18.Rajabli F, Feliciano BE, Celis K, et al. Ancestral origin of ApoE ε4 Alzheimer disease risk in Puerto Rican and African American populations. PLoS Genet. 2018;14(12):e1007791. doi: 10.1371/journal.pgen.1007791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doty RL, Marcus A, William Lee W. Development of the 12-Item Cross-Cultural Smell Identification Test (CC-SIT). Laryngoscope. 1996;106(3):353-356. doi: 10.1097/00005537-199603000-00021 [DOI] [PubMed] [Google Scholar]
- 20.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314-318. [PubMed] [Google Scholar]
- 21.Bland RC, Newman SC. Mild dementia or cognitive impairment: the Modified Mini-Mental State Examination (3MS) as a screen for dementia. Can J Psychiatry. 2001;46(6):506-510. doi: 10.1177/070674370104600604 [DOI] [PubMed] [Google Scholar]
- 22.Brenowitz WD, Kaup AR, Lin FR, Yaffe K. Multiple sensory impairment is associated with increased risk of dementia among black and white older adults. J Gerontol A Biol Sci Med Sci. 2019;74(6):890-896. doi: 10.1093/gerona/gly264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deal JA, Betz J, Yaffe K, et al. ; Health ABC Study Group. Hearing impairment and incident dementia and cognitive decline in older adults: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2017;72(5):703-709. doi: 10.1093/gerona/glw069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601-609. doi: 10.1161/circulationaha.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheike TH, Zhang MJ. Analyzing competing risk data using the R timereg package. J Stat Soft. 2011;38(2):1-15. doi: 10.18637/jss.v038.i02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranson JM, Kuźma E, Hamilton W, Muniz-Terrera G, Langa KM, Llewellyn DJ. Predictors of dementia misclassification when using brief cognitive assessments. Neurol Clin Pract. 2019;9(2):109-117. doi: 10.1212/cpj.0000000000000566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Risacher SL, Tallman EF, West JD, et al. Olfactory identification in subjective cognitive decline and mild cognitive impairment: association with tau but not amyloid positron emission tomography. Alzheimers Dement (Amst). 2017;9(1):57-66. doi: 10.1016/j.dadm.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein J, Yan X, Johnson A, et al. Olfactory impairment is related to tau pathology and neuroinflammation in Alzheimer's disease. J Alzheimers Dis. 2021;80(3):1051-1065. doi: 10.3233/jad-201149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lafaille-Magnan ME, Poirier J, Etienne P, et al. Odor identification as a biomarker of preclinical AD in older adults at risk. Neurology. 2017;89(4):327-335. doi: 10.1212/wnl.0000000000004159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belloy ME, Napolioni V, Greicius MD. A quarter century of APOE and Alzheimer's disease: progress to date and the path forward. Neuron. 2019;101(5):820-838. doi: 10.1016/j.neuron.2019.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burnham SC, Coloma PM, Li QX, et al. Application of the NIA-AA Research Framework: towards a biological definition of Alzheimer's disease using cerebrospinal fluid Biomarkers in the AIBL Study. J Prevent Alzheimers Dis. 2019;6(4):248-255. doi: 10.14283/jpad.2019.25 [DOI] [PubMed] [Google Scholar]
- 32.Wolk DA, Dickerson BC; Alzheimer's Disease Neuroimaging Initiative. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer's disease. Proc Natl Acad Sci USA. 2010;107(22):10256-10261. doi: 10.1073/pnas.1001412107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maestre G, Ottman R, Stern Y, et al. Apolipoprotein E and Alzheimer's disease: ethnic variation in genotypic risks. Ann Neurol. 1995;37(2):254-259. doi: 10.1002/ana.410370217 [DOI] [PubMed] [Google Scholar]
- 34.Tang MX, Maestre G, Tsai WY, et al. Relative risk of Alzheimer disease and age-at-onset distributions, based on APOE genotypes among elderly African Americans, Caucasians, and Hispanics in New York City. Am J Hum Genet. 1996;58(3):574-584. [PMC free article] [PubMed] [Google Scholar]
- 35.Olofsson JK, Rönnlund M, Nordin S, Nyberg L, Nilsson LG, Larsson M. Odor identification deficit as a predictor of five-year global cognitive change: interactive effects with age and ApoE-ε4. Behav Genet. 2009;39(5):496-503. doi: 10.1007/s10519-009-9289-5 [DOI] [PubMed] [Google Scholar]
- 36.Dintica CS, Marseglia A, Rizzuto D, et al. Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology. 2019;92(7):e700-e709. doi: 10.1212/wnl.0000000000006919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bryc K, Durand EY, Macpherson JM, Reich D, Mountain JL. The genetic ancestry of African Americans, Latinos, and European Americans across the United States. Am J Hum Genet. 2015;96(1):37-53. doi: 10.1016/j.ajhg.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]