Abstract
An increasing number of clinical trials are enrolling patients with myasthenia gravis (MG). A lack of standardization in the performance of outcome measures leads to confusion among site research teams and is a source of variability in clinical trial data. MGNet, the NIH-supported Rare Disease Clinical Research Network for MG, views standardization of MG outcome measures as a critical need. To address this issue, a group of experts summarized key outcome measures used in MG clinical trials and a symposium was convened to address issues contributing to outcome measure variability. Consensus recommendations resulted in changes to outcome measure instructions and, in some cases, modifications to specific instruments. Recommended changes were posted for public commentary before finalization. Changes to the MG-Activities of Daily Living, MG–Quality of Life–15r, and MG–Impairment Index were limited to adding details to the administration instructions. Recommendations for proper positioning of participants and how to score items that could not be performed because of non-MG reasons were provided for the MG Composite. The Quantitative MG (QMG) score required the most attention, and changes were made both to the instructions and the performance of certain items resulting in the QMG-Revised. The Postintervention Status was believed to have a limited role in clinical trials, except for the concept of minimal manifestation status. As a next step, training materials and revised source documents, which will be freely available to study teams, will be created and posted on the MGNet website. Further studies are needed to validate changes made to the QMG-Revised.
Several outcome measures are commonly used in myasthenia gravis (MG) clinical trials.1 Prior task forces provided consensus recommendations on the use of outcome measures in MG clinical research.2,3 For many years, the quantitative MG (QMG) score was the accepted key outcome measure for MG clinical trials. The phase 3 clinical trial of eculizumab in patients with refractory generalized MG with AChR antibodies marked a transition to a greater emphasis on patient-reported outcome measures in the field.4 In this study, the MG–Activities of Daily Living Scale (MG-ADL) score was the primary outcome measure, and the MG-ADL has served as a primary or key secondary efficacy endpoint for several subsequent phase 2 and 3 studies.5,6
There has been a steadily increasing number of therapeutics under development for patients with MG. An observation by site investigators participating in clinical trials, and a source of frustration for sponsors and sites alike, is a lack of standardization in the training and performance of MG outcome measures. This lack of standardization is a source of variability in clinical trial data. This also leads to confusion among site research teams and complicates comparability of results across studies. Variability in outcome measure administration could also lead to trials measuring aspects of the disease differently and in a way that is not transparent in publications or trial reports.
The NIH-supported Rare Disease Clinical Research Network (RDCRN) for MG (MGNet) views standardization of MG outcome measures as a critical need for clinical trials and convened a group of experts to address the issue. After an outcome measure symposium, MG outcome measures were refined with the specific goal of improving the clarity of instructions and scoring, thereby improving the consistency of how outcome measures are performed and reducing the variability in outcome measure data.
Methods
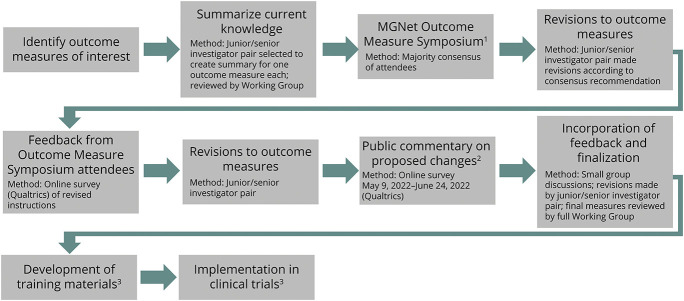
We identified the most frequently used MG-specific outcome measures in clinical trials. These included the MG-ADL,7 QMG Score,8 Myasthenia Gravis Foundation of America (MGFA) Postintervention Status (PIS),2 MG Composite,9 MG–Quality of Life–15 (MG-QOL-15)10 and MG-QOL-15 revised version (MG-QOL-15r),11 and MG Impairment Index (MGII).12 As an initial step, each outcome measure was summarized regarding administration, domains evaluated, psychometric properties, translations, and aspects contributing to a lack of standardization (Figure).
Figure. Overview of Methods for Standardizing MG Outcome Measures.
1Refer to eAppendix 1 (links.lww.com/WNL/C754) for list of attendees. 2Groups notified of public commentary: AANEM Connect, AAN Synapse, Alexion, Argenx, Biosensics, Cabaletta Bio, Clinical and Translational Science Award sites, Conquer MG, Horizon Therapeutics, Janssen, MGNet clinical sites, Muscular Dystrophy Association, Muscle Study Group, Myasthenia Gravis Foundation of America (posted on website), Rare Disease Clinical Research Networks (including NIH staff), Rick's Real Neuromuscular Friends, Signant, and UCB Pharma. 3Next steps of process, currently pending. AAN = American Academy of Neurology; AANEM = American Association of Neuromuscular & Electrodiagnostic Medicine; MG = myasthenia gravis.
Outcome measure summary findings were presented at an MGNet Symposium (December 2020). Attendees included MGNet investigators, patient advocacy groups, patients, the NIH, and representatives from the RDCRN Data Management and Coordinating Center, and industry (eAppendix 1, links.lww.com/WNL/C754). These attendees were broadly inclusive of stakeholders involved in MG clinical trials, including thought leaders in the field with experience in designing clinical trials and those who have developed and extensively used MG outcome measures. Issues related to variability and standardization of each outcome measure in a clinical trial setting were discussed by attendees and informal consensus (majority agreement) was achieved for the best approach to standardize each outcome measure. Modifications were made to each outcome measure to align with the informal consensus achieved at the outcome measure symposium. The revised outcome measures and/or their instructions for administration were then reviewed by symposium attendees and approved. In cases where consensus was not reached at the symposium, options for how to revise a measure were presented to attendees and voted on separately. A simple majority determined how these areas were addressed.
The approved outcome measures were then posted on the MGNet website for a 4-week public comment period. Relevant stakeholders were informed of the opportunity for public commentary through the MGFA 14th International Conference on Myasthenia Gravis and Related Disorders,13 posting on relevant professional communication platforms (e.g., American Association of Neuromuscular & Electrodiagnostic Medicine Connect and American Academy of Neurology Synapse) and direct email communication (Figure). Community neurologists with an interest in MG outcome measures would have had an opportunity to provide input at the MGFA Conference (if in attendance) and during the public commentary period. Comments from the public were reviewed and outcome measures were further modified, with additional input from MGNet Symposium attendees, experts in outcome measure development, and patients with MG. Patient-facing outcome measures were optimized for an eighth-grade reading level.14
Results
A summary of each outcome measure and available translations can be found in eAppendix 2 (links.lww.com/WNL/C755). There was consensus among symposium attendees that the general approach to modifying outcome measures was not to change the outcome measure itself, unless deemed absolutely necessary. No new items were to be added. The focus was on strengthening the outcome measure instructions/administration to enhance standardization and address situations that arise during clinical trials and are either not accounted for in current instructions or addressed in inconsistent ways by study sponsors.
One area of considerable discussion for patient-reported outcome measures (MG-ADL, MGII, and MG-QOL-15r) was whether they should capture only those symptoms/signs that are attributable to MG or to capture function/status “as is,” recognizing the potential (indeed, likelihood) that comorbidities might introduce some confounding, given that patients may struggle to determine what is attributable to MG and what is not. Consensus was reached that patients should try to respond with symptoms related to MG, largely because prior validation studies had used this approach.7,11 Future research, however, could explore whether patients should be instructed to answer questions “as they are” to minimize these potential confounding effects.
Other topics of emphasis with strong consensus included the following: (1) recording the time of day for assessments and maintaining consistent timing of assessments throughout a trial due to variability in the disease over the course of a day; (2) maintaining the same order of assessments and same raters throughout a study; (3) that MG trials should be as inclusive as possible and that patients with fixed deficits preventing completion of a specific item should be allowed to participate if an acceptable standardized method of handling these items can be determined; (4) the need to avoid missing data to the extent possible; (5) the need for trial statistical analysis plans to include instrument-specific approaches for handling missing data (e.g., how to handle a permanent injury that occurs during the course of a trial and prevents an assessment and for which recovery is not expected); (6) the importance of withholding pyridostigmine (or other cholinesterase inhibitor) for at least 12 hours for clinician-assessed outcome measures with the time, dose, and form (regular or long acting) of the last pyridostigmine dose clearly documented; and (7) the general principle that the primary outcome measure should be completed first at a study visit; it is recommended that instruments with muscle testing that can cause fatigue should also be performed early after arrival to study site.
Additional outcome measure–specific summaries and recommendations are discussed further below. The revised instructions for each outcome measure, which incorporate the changes recommended during the process described in the Methods, are found in eAppendix 3 (links.lww.com/WNL/C756). The revised outcome measures are also available on the MGNet website (mgnet.rarediseasesnetwork.org/resources/researchers-clinicians), which will remain the best source for the most up-to-date instructions.
MG-ADL
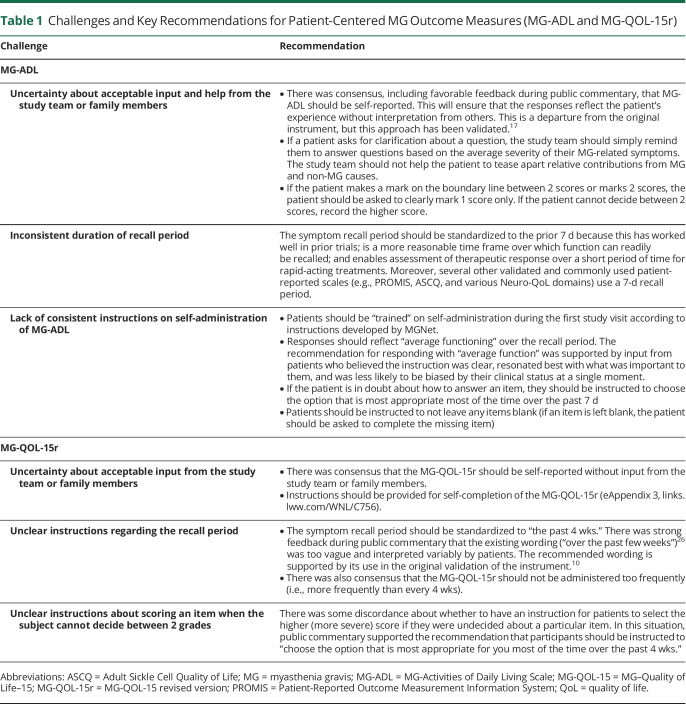
The MG-ADL is an 8-item patient-reported outcome measure that assesses MG-specific symptoms and their impact on daily activities.7 The MG-ADL is a common primary endpoint in recent clinical trials4,15 and may be more sensitive to clinical change than the QMG.16 In clinical trials to date, the MG-ADL has been administered with varying degrees of instruction and guidance, or patients complete the instrument without any study team interaction. Other areas contributing to a lack of standardization include the following: a variable time frame for recall of symptoms (e.g., 7 vs 14 days), inconsistent instruction as to whether only MG symptoms should be considered in their responses, and a lack of clarity about how patients summarize their function over the specified time frame (e.g., do they consider “worst” or “average” function). While there is no evidence to support one time frame vs another, we recommend consistency across all studies. Key recommendations are summarized in Table 1.
Table 1.
Challenges and Key Recommendations for Patient-Centered MG Outcome Measures (MG-ADL and MG-QOL-15r)
MG-QOL-15r
The MG-QOL-15r is a 15-item patient-reported outcome measure assessing physical, psychological, and social domains commonly affected by MG.10 A revised version, which reduces the number of responses for each item from 4 to 3, has been validated and is commonly used.11 Areas of inconsistency in the administration of the MG-QOL-15r include differences in recall time (past week vs 2 weeks, etc), self-administration or administration by study team, and whether to include an instruction for individuals having difficulty deciding between 2 scores on an item to score higher. Key recommendations are summarized in Table 1.
QMG
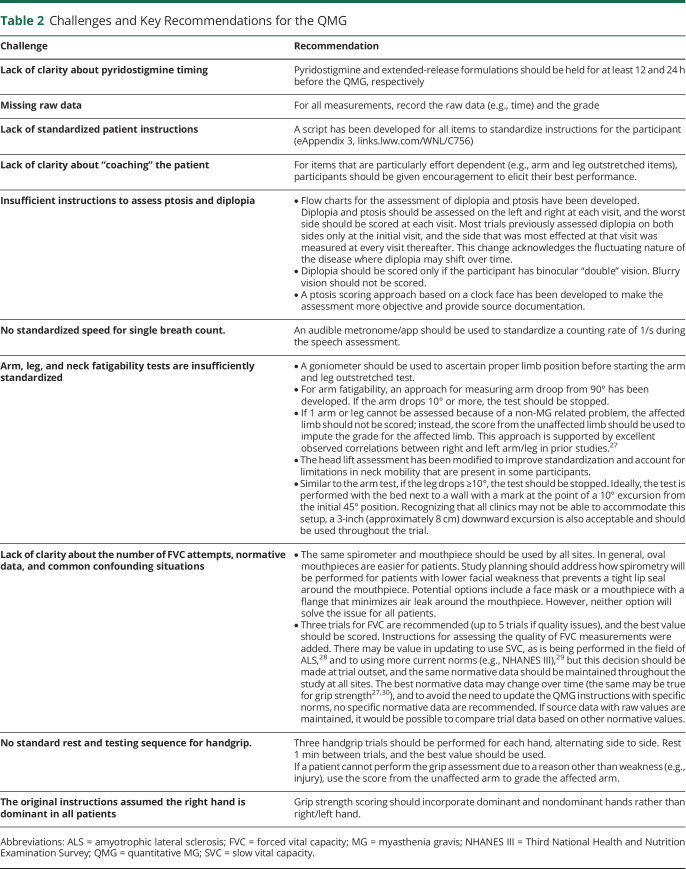
The QMG is a clinician-administered assessment of strength and fatigable weakness in the domains of ocular, bulbar, respiratory, and limb/axial muscles. It requires special equipment and takes approximately 30 minutes to complete.8,17 Of all the outcome measures addressed, the QMG had the most concerns related to variability in performance (Table 2). Challenging issues included positioning of individuals and standardizing instructions (particularly ocular items), how to score MG-related weakness for several items in specific situations, concerns about the number of trials to perform and the normative data used to assess forced vital capacity, and how to score items that cannot be completed because of non-MG factors. It was recognized that patient factors can sometimes limit performance of certain items, including factors that limit performance of items tested bilaterally (e.g., shoulder and leg items limited by cervical/lumbar spine pain). Because there is no simple solution for this, it is recommended that the patient perform to the best of their ability on that day. Recommended changes to the evaluation of ptosis were extensive. The changes to the QMG were sufficiently extensive to justify indicating “revised” in the name of the instrument (QMG-Revised) to avoid confusion. The case report form was updated to include the timing of last cholinesterase inhibitor, handedness of the participant, signature of the evaluator, and the specific cause if an arm or a leg was not tested because of a non-MG–related cause. Key recommendations are summarized in Table 2.
Table 2.
Challenges and Key Recommendations for the QMG
MG Composite
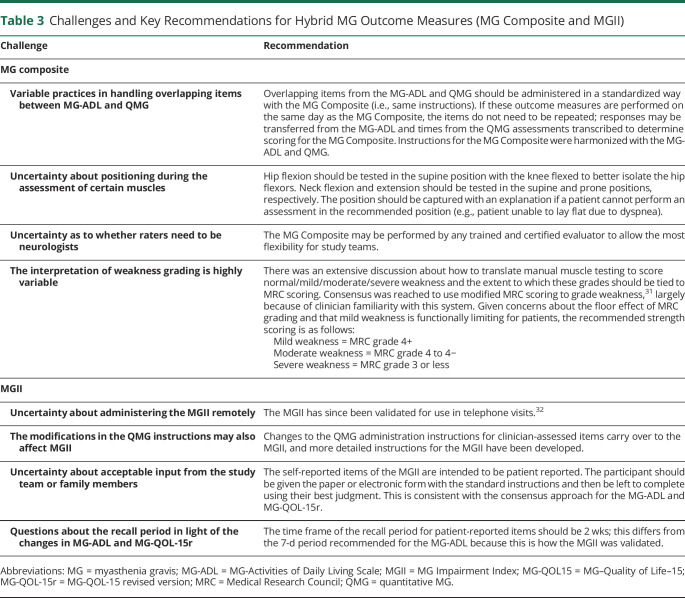
The MG Composite assesses disease-specific symptoms and examination findings derived from the MG-ADL (patient reported), QMG, and MG-Manual Muscle Test (clinician determined). It consists of 10 weighted items and takes approximately 5 minutes to complete.9 Areas of uncertainty for the MG Composite included proper positioning of individuals for clinician-assessed items and how to grade weakness. Additional areas of variability include the following: whether overlapping items that are assessed in other outcome measures and measured on the same day need to be repeated for the MG Composite (e.g., diplopia in the QMG); whether raters need to be neurologists; and how to score items that cannot be performed for MG or non-MG reasons. Key recommendations are summarized in Table 3.
Table 3.
Challenges and Key Recommendations for Hybrid MG Outcome Measures (MG Composite and MGII)
MG Impairment Index
The MGII assesses MG-specific impairments through patient self-report (22 items) and clinical examination (6 items).12 It assesses ocular, bulbar, respiratory, and limb domains and takes approximately 10 minutes to complete. Patients are instructed to consider only symptoms related to MG, and certain clinician-evaluated items (arm endurance, leg endurance, and neck endurance) follow the same instructions as the QMG, including patient positioning. However, scoring of items performed in the QMG and MGII differ. The MGII has been administered in the clinic, and during the symposium, it was uncertain whether it would be suitable to telemedicine assessments. In many cases, the QMG is administered at the same visit as the MGII; in light of the changes to the QMG, the instructions for carrying over QMG scores to the MGII needed to be addressed. Key recommendations are summarized in Table 3.
MGFA PIS
The MGFA PIS is a clinician-assessed instrument developed to measure the effects of a therapeutic intervention on disease status.2 It has 8 major categories and can be used both in clinical trials and in the clinic. Major considerations regarding the PIS were lack of definition for criteria when defining improvement or worsening (i.e., change in QMG or MG Composite score vs overall impression), its relevance for clinical trials, and a lack of category standardization across use in trials. The anchor time point for assessing categories (e.g., change from initial visit vs last visit vs worst ever) has also been variably assessed.
Key Recommendations for MGFA PIS
In the setting of interventional clinical trials, the full MGFA PIS is not recommended. Several categories defined in the PIS, such as Pharmacologic Remission that requires that a “patient has had no symptoms or signs of MG for at least 1 year,”2 are not relevant to most interventional studies that evaluate treatment effect over a shorter time frame. In addition, improved/worse status are usually redundant with other analyses performed on quantitative measures (e.g., change in QMG score). Of importance, there was clear consensus on the value of the PIS in other clinical research settings that were beyond the scope of these recommendations focused on outcome measures for clinical trials.
An alternative approach to the PIS categories of improved/worse is a clinician and patient global impression of change score. A 7-point Likert scale for clinician-reported global assessment of disease severity or change has been included in several trials to date and achieved consensus.18 Validated patient-reported global assessments, such as the Single Simple Question (which has been studied in MG19), might also be used to supplement physician-reported global assessments, but further research would be needed before doing so.
Minimal manifestation status (MMS) is widely accepted as a critical treatment goal for patients with MG20 and remains an important concept that is worth retaining for clinical trials. In addition to measuring MMS as an outcome, MMS can also serve as a guide for steroid tapering in clinical trials.15,21 It was noted during public commentary that the definition of MMS is a source of confusion. The original definition of MMS is “the patient has no symptoms or functional limitations from MG but has some weakness on examination…” for a duration of at least 1 year.2 In clinical trials using MMS, the time requirement has been dropped because trial durations are often less than 1 year. There was also significant disagreement about whether to preserve, discard, or modify the existing subgrades of MMS. It was noted that, outside of open-label extension studies, baseline treatments are usually not altered. Thus, the important concept is the clinical status, and the subcategories are less important and not recommended for use in clinical trials. Of note, a separate task force is currently addressing this issue for the clinic, and further recommendations for clinical use may be forthcoming.22
The anchor time point for assessing any category of the PIS in a clinical trial is the last assessment before initiation of the experimental therapeutic (typically the baseline or randomization visit).
Discussion
An increased number of therapeutics under development for MG in recent years has led to important observations about outcome measure training and performance in the clinical trial setting. The MGNet Clinical Trial Outcome Measure Working Group was convened to synthesize our collective experience and to use the cumulative expertise of the group to make recommendations that apply “lessons learned.” Of importance, the Working Group focused on issues related to standardization of MG-specific outcome measures specifically for clinical trials. The Working Group did not specify which outcome measures should be used or is “best”; this decision needs to be made independently for each trial depending on the goals and often with input from regulatory authorities. The scope did not include the use of these outcome measures in the clinic or other clinical research settings. The MGFA Clinical Classification was also considered outside the scope because it is not commonly used as a clinical trial outcome measure. In addition, we have not addressed the potential need for new outcome measures.
Improving standardization of MG outcome measures will have many benefits for future clinical trials. First, training will be consistent across trials and MGNet will develop a standardized set of training tools that will be freely available for future use. This should reduce costs previously borne by individual sponsors to develop their own training materials and training plans and potentially yield faster startup times. For example, MG outcome measure training completed for 1 trial should be valid for another trial within a specified time frame to reduce redundancy. To date, detailed training materials for MG outcome measures have been maintained by study sponsors and are not widely available. Because MGNet outcome measure instructions, case report forms, and training materials will be freely available, the enhanced accessibility should reduce this barrier to clinical trial implementation, potentially increasing the number of trained sites and facilitating participation among sites that have not traditionally been involved in MG clinical trials. This is an important need, given the competition for patients among a growing number of clinical trials in this rare disease and the potential for inexperienced sites to have more variability in their data.
Ultimately, implementing these recommendations is expected to lead to less noisy data and fewer errors at sites. This standardization should have benefits for study design, such as the potential for sample size reductions (e.g., smaller standard deviations), although this would need to be proven in future studies. Finally, standardization could also permit greater comparison across studies and, hopefully, eventually pooling of data in a repository akin to Pooled Resource Open-Access ALS Clinical Trials for amyotrophic lateral sclerosis.23 Implementation of these standardization recommendations may make comparisons with trials completed under historical outcome measure approaches more challenging.
Increasing outcome measure standardization necessitated adding clarifying language to the instructions. The Working Group was challenged with finding the right balance between providing appropriate guidance without being excessive. This was a particular concern for patient-reported outcomes where there is a risk that patients may not read the instructions if they are too long or they could become excessively burdensome. Specifically for the MG-ADL, it was decided to separate each item into its own block that contains the item followed by any instructions.
The public commentary period revealed several important themes. Several comments suggested adding or extensively modifying existing questions. Examples included the following: (1) adding items to assess vision in primary or downgaze; (2) developing a more patient-centric presentation of questions and responses for the MG-ADL; (3) shifting the focus of the MG-ADL from choking specifically to a more general assessment of swallowing; (4) increasing the focus of outcome measures on ocular symptom impact; and (5) assessing anxiety and mood specifically related to living with MG and/or an MG exacerbation. These comments suggest that there are residual issues with the current outcome measures used in clinical trials that cannot be easily resolved. Prior studies have highlighted other limitations with outcome measures currently in use.1,6,24 More holistic assessments may be needed that measure aspects of the disease not currently captured and that are developed with extensive patient input.25
A potential limitation of our approach was the lack of a formal consensus process. We did, however, take several steps to ensure the rigor of our process, which included multiple and overlapping opportunities for broad stakeholder input, both in the presence of peers (e.g., during symposium) and anonymously (e.g., online surveys). The incorporation of a public commentary provided an additional opportunity for broad feedback from a diverse set of stakeholders.
The next steps for the MGNet Clinical Trial Outcome Measure Working Group include the development of a comprehensive set of training materials and case report forms for each outcome measure that reflects the recommended changes. MGNet anticipates holding training sessions for study teams at future meetings and offering initial and renewal certifications for clinical trial raters. Wide dissemination of this information to study teams is expected to improve efficiency in MG clinical trial outcome measure training. Unlike most of the recommendations that were limited to additional instructions, rather than changes to the outcome measure itself, there were extensive changes to the QMG instrument. Thus, a study is needed to validate the impact of these changes on the performance of the QMG-Revised. In addition, there will be a need for official translations (including assessment of local dialects and cultural adaptations) and, in some cases, validation of these instructions for use in other countries. MGNet is willing and able to centrally host official translations that can then be made available to study teams.
Acknowledgment
The Working Group thanks the peer reviewers, whose thorough and thoughtful review increased the quality of this manuscript. The Working Group also thanks Helen Girma for administrative support and the Myasthenia Gravis Foundation of America for supporting this program.
Glossary
- MG
myasthenia gravis
- MG-ADL
MG-Activities of Daily Living Scale
- MGFA
Myasthenia Gravis Foundation of America
- MGII
MG Impairment Index
- MG-QOL-15
MG–Quality of Life–15
- MG-QOL-15r
MG-QOL-15 revised version
- MMS
minimal manifestation status
- PIS
Postintervention Status
- QMG
quantitative MG
- RDCRN
Rare Disease Clinical Research Network
Appendix 1. Authors
Appendix 2. Coinvestigators

Study Funding
The work was supported by MGNet (NIH U54 NS115054), a member of the Rare Disease Clinical Research Network Consortia (RDCRN), and the Data Management and Coordinating Center (NIH U2CTR002818). Funding support for the RDCRN is provided by the National Center for Advancing Translational Sciences (NCATS) and the National Institute of Neurological Disorders and Stroke.
Disclosure
J.T. Guptill has consulted for Immunovant, Alexion, Apellis, Momenta, Ra Pharma, Becton Dickinson, Cabaletta Bio, Regeneron, argenx, Sanofi, Janssen, UCB, Toleranzia, and Piedmont Pharmaceuticals. He received industry grant support from UCB pharma for a fellowship training grant. He has served as a site investigator for Alexion, Janssen, UCB Pharma, Argenx, and Takeda. He received grant/research support from NIH (NIAID, National Institute of Neurological Disorders and Stroke, NIMH), Myasthenia Gravis Foundation of America, and Centers for Disease Control and Prevention. He is currently an employee of argenx. M. Benatar has consulted for Alexion, Immunovant, Takeda, UCB, Ad Scientam, and Sanofi. He receives research funding from Alexion and Immunovant. He has served as the site principal investigator for MG trials sponsored by Alexion, UCB, and the NIH. V. Granit received honoraria as a consultant or advisory board member from Alexion Pharmaceuticals, Argenx, Immunovant Inc., and Amylyx Pharmaceuticals Inc. He is employed by Biohaven Pharmaceuticals. A.A. Habib reports no relevant disclosures. J.F. Howard reports research support (paid to his institution) from Alexion Pharmaceuticals, Argenx, Cartesian Therapeutics, the Centers for Disease Control and Prevention (Atlanta, GA), the Myasthenia Gravis Foundation of America, the Muscular Dystrophy Association, the NIH (including the National Institute of Neurological Disorders and Stroke and the National Institute of Arthritis and Musculoskeletal and Skin Diseases), PCORI, Ra Pharmaceuticals (now UCB Biosciences), and Millennium Pharmaceuticals/Takeda Pharmaceuticals; honoraria from Alexion Pharmaceuticals, Argenx, F. Hoffman-LaRoche Ltd., Immunovant Inc., Ra Pharmaceuticals (now UCB Biosciences), Regeneron Pharmaceuticals, and Sanofi US and nonfinancial support from Alexion Pharmaceuticals, Argenx, Ra Pharmaceuticals (now UCB Biosciences), and Toleranzia AB. C. Barnett-Tapia has received honoraria as consultant or member of advisory board from Alexion, Sanofi, and Argenx. She is the primary developer of the MGII and may receive royalties for its use. R.J. Nowak reports no relevant disclosures. I. Lee reports no relevant disclosures. K. Ruzhansky has served on advisory boards for Alexion, Argenx, Immunovant, and UCB/Ra, has served as a site PI for Alexion, Argenx, UCB, and Janssen, and has grant funding from MGFA. M.M. Dimachkie serves or recently served as a consultant for Abcuro, Amazentis, ArgenX, Astellas, Catalyst, Cello, Covance/Labcorp, CSL-Behring, EcoR1, Janssen, Kezar, MDA, Medlink, Momenta, NuFactor, Octapharma, Priovant, Ra Pharma/UCB, Roivant Sciences Inc, Sanofi Genzyme, Shire Takeda, Scholar Rock, Spark Therapeutics, Abata/Third Rock, UCB Biopharma, and UpToDate. He received research grants or contracts or educational grants from Alexion, Alnylam Pharmaceuticals, Amicus, Biomarin, Bristol-Myers Squibb, Catalyst, Corbus, CSL-Behring, FDA/OOPD, GlaxoSmithKline, Genentech, Grifols, Kezar, Mitsubishi Tanabe Pharma, MDA, NIH, Novartis, Octapharma, Orphazyme, Ra Pharma/UCB, Sanofi Genzyme, Sarepta Therapeutics, Shire Takeda, Spark Therapeutics, The Myositis Association, UCB Biopharma/RaPharma, Viromed/Healixmith, & TMA. G.R. Cutter reports no relevant disclosures. H.J. Kaminski is a consultant for Roche, Cabaletta Bio, and UCB Pharmaceuticals and is a CEO and CMO of ARC Biotechnology, LLC, based on US Patent 8,961,98. He is the principal investigator of the Rare Disease Network for Myasthenia Gravis (MGNet) National Institute of Neurological Disorders and Stroke, U54 NS115054, Targeted Therapy for Myasthenia Gravis. R41 NS110331 to ARC Biotechnology, and coinvestigator for R43NS124329 MV2C2 antibody as a new therapeutic for myasthenia gravis to Mimivax, LLC. Go to Neurology.org/N for full disclosures.
References
- 1.Thomsen JLS, Andersen H. Outcome measures in clinical trials of patients with myasthenia gravis. Front Neurol. 2020;11:596382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaretzki A III, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology. 2000;55(1):16-23. [DOI] [PubMed] [Google Scholar]
- 3.Benatar M, Sanders DB, Burns TM, et al. Recommendations for myasthenia gravis clinical trials. Muscle Nerve. 2012;45(6):909-917. [DOI] [PubMed] [Google Scholar]
- 4.Howard JF Jr, Utsugisawa K, Benatar M, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017;16(12):976-986. [DOI] [PubMed] [Google Scholar]
- 5.Howard JF Jr, Bril V, Vu T, et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021;20(7):526-536. [DOI] [PubMed] [Google Scholar]
- 6.Muppidi S, Silvestri NJ, Tan R, Riggs K, Leighton T, Phillips GA. Utilization of MG-ADL in myasthenia gravis clinical research and care. Muscle Nerve. 2022;65(6):630-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfe GI, Herbelin L, Nations SP, Foster B, Bryan WW, Barohn RJ. Myasthenia gravis activities of daily living profile. Neurology. 1999;52(7):1487-1489. [DOI] [PubMed] [Google Scholar]
- 8.Barohn RJ, McIntire D, Herbelin L, Wolfe GI, Nations S, Bryan WW. Reliability testing of the quantitative myasthenia gravis scorea. Ann NY Acad Sci. 1998;841:769-772. [DOI] [PubMed] [Google Scholar]
- 9.Burns TM, Conaway MR, Cutter GR, Sanders DB. Construction of an efficient evaluative instrument for myasthenia gravis: the MG composite. Muscle Nerve. 2008;38(6):1553-1562. [DOI] [PubMed] [Google Scholar]
- 10.Burns TM, Conaway MR, Cutter GR, Sanders DB, Muscle Study Group. Less is more, or almost as much: a 15-item quality-of-life instrument for myasthenia gravis. Muscle Nerve. 2008;38(2):957-963. [DOI] [PubMed] [Google Scholar]
- 11.Burns TM, Sadjadi R, Utsugisawa K, et al. International clinimetric evaluation of the MG-QOL15, resulting in slight revision and subsequent validation of the MG-QOL15r. Muscle Nerve. 2016;54(6):1015-1022. [DOI] [PubMed] [Google Scholar]
- 12.Barnett C, Bril V, Kapral M, Kulkarni A, Davis AM. Development and validation of the Myasthenia Gravis Impairment Index. Neurology. 2016;87(9):879-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guptill JT, Granit V, Habib A, et al. Addressing outcome measure variability in myasthenia gravis clinical trials. Muscle Nerve. 2022;65:S11-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Good Calculators. Flesch Kincaid Calculator [online]. Accessed August 28, 2022. goodcalculators.com/flesch-kincaid-calculator/. [Google Scholar]
- 15.Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med. 2016;375(6):511-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard JF Jr, Freimer M, O'Brien F, Wang JJ, Collins SR, Kissel JT. QMG and MG-ADL correlations: study of eculizumab treatment of myasthenia gravis. Muscle Nerve. 2017;56(2):328-330. [DOI] [PubMed] [Google Scholar]
- 17.Bedlack RS, Simel DL, Bosworth H, Samsa G, Tucker-Lipscomb B, Sanders DB. Quantitative myasthenia gravis score: assessment of responsiveness and longitudinal validity. Neurology. 2005;64(11):1968-1970. [DOI] [PubMed] [Google Scholar]
- 18.Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28-37. [PMC free article] [PubMed] [Google Scholar]
- 19.Abraham A, Breiner A, Barnett C, Katzberg HD, Bril V. The utility of a single simple question in the evaluation of patients with myasthenia gravis. Muscle Nerve. 2018;57(2):240-244. [DOI] [PubMed] [Google Scholar]
- 20.Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. 2016;87(4):419-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee I, Kuo HC, Aban IB, et al. Minimal manifestation status and prednisone withdrawal in the MGTX trial. Neurology. 2020;95(6):e755-e766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruzhansky K, Li Y, Wolfe G, et al. MGFA task force for standardization of myasthenia gravis outcome measures in clinical practice. Muscle Nerve. 2022;65:S15. [Google Scholar]
- 23.Atassi N, Berry J, Shui A, et al. The PRO-ACT database: design, initial analyses, and predictive features. Neurology. 2014;83(19):1719-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McPherson T, Aban I, Duda PW, et al. Correlation of quantitative myasthenia gravis and myasthenia gravis activities of daily living scales in the MGTX study. Muscle Nerve. 2020;62(2):261-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cleanthous S, Mork AC, Regnault A, Cano S, Kaminski HJ, Morel T. Development of the Myasthenia Gravis (MG) Symptoms PRO: a case study of a patient-centred outcome measure in rare disease. Orphanet J Rare Dis. 2021;16(1):457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burns TM, Grouse CK, Conaway MR, Sanders DB; Mg composite and Mg-qol15 study group. Construct and concurrent validation of the MG-QOL15 in the practice setting. Muscle Nerve. 2010;41(2):219-226. [DOI] [PubMed] [Google Scholar]
- 27.Barnett TC, Bril V, Davis AM. Performance of individual items of the quantitative myasthenia gravis score. Neuromuscul Disord. 2013;23(5):413-417. [DOI] [PubMed] [Google Scholar]
- 28.Lechtzin N, Cudkowicz ME, de Carvalho M, et al. Respiratory measures in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19(5-6):321-330. [DOI] [PubMed] [Google Scholar]
- 29.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179-187. [DOI] [PubMed] [Google Scholar]
- 30.Peters MJ, van Nes SI, Vanhoutte EK, et al. Revised normative values for grip strength with the Jamar dynamometer. J Peripher Nerv Syst. 2011;16(1):47-50. [DOI] [PubMed] [Google Scholar]
- 31.Medical Research Council. Aids to the Examination of the Peripheral Nervous System. The White Rose Press; 1976. Memordanum No. 45. [Google Scholar]
- 32.Menon D, Alnajjar S, Barnett C, et al. Telephone consultation for myasthenia gravis care during the COVID-19 pandemic: assessment of a novel virtual myasthenia gravis index. Muscle Nerve. 2021;63(6):831-836. [DOI] [PMC free article] [PubMed] [Google Scholar]