Abstract
Background and Objectives
Ganglion cell + inner plexiform layer (GCIPL) thinning, measured by optical coherence tomography (OCT), reflects global neurodegeneration in multiple sclerosis (MS). Atrophy of the inner (INL) and outer nuclear layer (ONL) may also be prominent in progressive MS (PMS). The phase 2, SPRINT-MS trial found reduced brain atrophy with ibudilast therapy in PMS. In this post hoc analysis of the SPRINT-MS trial, we investigate (1) retinal atrophy (2) differences in response by subtype and (3) associations between OCT and MRI measures of neurodegeneration.
Methods
In the multicenter, double-blind SPRINT-MS trial, participants with secondary progressive MS (SPMS) or primary progressive MS (PPMS) were randomized to ibudilast or placebo. OCT and MRI data were collected every 24 weeks for 96 weeks. Extensive OCT quality control and algorithmic segmentation produced consistent results across Cirrus HD-OCT and Spectralis devices. Primary endpoints were GCIPL, INL, and ONL atrophy, assessed by linear mixed-effects regression. Secondary endpoints were associations of OCT measures, brain parenchymal fraction, and cortical thickness, assessed by partial Pearson correlations.
Results
One hundred thirty-four PPMS and 121 SPMS participants were included. GCIPL atrophy was 79% slower in the ibudilast (−0.07 ± 0.23 µm/y) vs placebo group (−0.32 ± 0.20 µm/y, p = 0.003). This effect predominated in the PPMS cohort (ibudilast: −0.08 ± 0.29 µm/y vs placebo: −0.60 ± 0.29 µm/y, a decrease of 87%, p < 0.001) and was not detected in the SPMS cohort (ibudilast: −0.21 ± 0.28 µm/y vs placebo: −0.14 ± 0.27 µm/y, p = 0.55). GCIPL, INL, and ONL atrophy rates correlated with whole brain atrophy rates across the cohort (r = 0.27, r = 0.26, and r = 0.20, respectively; p < 0.001). Power calculations from these data show future trials of similar size and design have ≥80% power to detect GCIPL atrophy effect sizes of approximately 40%.
Discussion
Ibudilast treatment decreased GCIPL atrophy in PMS, driven by the PPMS cohort, with no effect seen in SPMS. Modulated atrophy of retinal layers may be detectable in sample sizes smaller than the SPRINT-MS trial and correlate with whole brain atrophy in PMS, further highlighting their utility as outcomes in PMS.
Classification of Evidence
This study provides Class II evidence that ibudilast reduces composite ganglion cell + inner plexiform layer atrophy, without reduction of inner or outer nuclear layer atrophy, in patients with primary progressive MS but not those with secondary progressive MS.
Introduction
Optical coherence tomography (OCT) has emerged as a useful and precise tool to assess neurodegeneration in multiple sclerosis (MS) by assessing the retina. While overt optic neuritis (ON) occurs in approximately 50% of people with MS (PwMS) during their disease course, subclinical optic neuropathy is virtually ubiquitous, with 94%–99% of PwMS exhibiting demyelinating optic nerve plaques postmortem.1,2 Retrograde degeneration of optic nerve axons results in retinal nerve fiber layer (RNFL) and ganglion cell layer (estimated by composite ganglion cell and inner plexiform layer [GCIPL] thickness on OCT) atrophy. OCT-derived GCIPL measurements possess greater reliability and reproducibility than peripapillary RNFL (pRNFL) thickness, and GCIPL thickness in particular seems to provide insight into global MS disease processes, correlating with and predicting disability measures.3-7 Rates of GCIPL, whole brain, and particularly gray matter atrophy correlate over time in relapsing-remitting MS (RRMS),8 although this is less clear in progressive MS (PMS), for which there are conflicting results.9 Some studies show correlations between pRNFL or GCIPL and MRI changes in PMS,10,11 while others show no association.6 Even comparisons of retinal layers between MS subtypes have shown discrepancies. People with secondary progressive MS (PwSPMS) often have lower pRNFL and GCIPL thicknesses compared with primary progressive MS (PPMS) and RRMS.12,13 While on average, people with PMS (PwPMS) may have lower pRNFL and GCIPL thicknesses than those with RRMS,14,15 when people with PPMS (PwPPMS) are assessed separately, they can be seen to exhibit higher16 or lower pRNFL and GCIPL thicknesses than people with RRMS (PwRRMS),17 with such comparisons potentially driven by ON history in PwRRMS. All MS subtypes exhibit pRNFL and GCIPL thinning relative to healthy controls (HCs).18-21 To date, PMS OCT studies have varied greatly by sample size, OCT platform used, inclusion of eyes with previous ON, and the retinal layers assessed. While less-understood, inner (INL) and outer nuclear layer (ONL) atrophy have been suggested to occur in MS on the basis of numerous OCT and electroretinography studies.3,16,22,23 Moreover, neuronal dropout in the INL has been demonstrated in 40% of MS eyes postmortem.1 Interestingly, INL thickening may predict inflammatory disease activity and disability progression in RRMS, while INL atrophy may be associated with advanced disease in PMS.17,24,25 INL and ONL atrophy may be more prominent in PMS than RRMS, while GCIPL atrophy tends to be accelerated across all MS subtypes.26 While there have now been several studies27-30 demonstrating that conventional disease-modifying therapies (DMTs) differentially modulate retinal atrophy in RRMS in accordance with their potency, this has not been demonstrated in PwPMS treated with conventional DMTs, regardless of potency.17,31
The NeuroNEXT SPRINT-MS clinical trial was a phase 2, multicenter, randomized, double-blind, parallel-group, placebo-controlled study of ibudilast in primary (PPMS, n = 134) and secondary progressive MS (SPMS, n = 121). Ibudilast is a small molecule inhibitor that crosses the blood-brain barrier and acts on cyclic nucleotide phosphodiesterases PDE3A, PDE4, PDE10, PDE11, macrophage migration inhibitory factor, and toll-like receptor 4. It is licensed to treat asthma and poststroke vertigo in Japan and South Korea.32 In the SPRINT-MS trial, ibudilast was administered to 129 people with PMS, while 126 participants with PMS received placebo (eFigure 1, links.lww.com/WNL/C983). Participants were studied for 96 weeks, with MRI, OCT, and clinical assessments performed every 24 weeks33
The primary finding was a close to 50% reduction in whole brain atrophy (estimated by brain parenchymal fraction; BPF) in those treated with ibudilast, compared with placebo.34 Additional analyses revealed that this effect was primarily driven by the PPMS cohort.35 During the trial, participants underwent OCT either on Cirrus HD-OCT (n = 183, 75% of total) or Spectralis (n = 61, 25% of total) for the duration of the study. Previous preliminary OCT analyses found that total macular volume change measured on Spectralis was lower in those receiving ibudilast. However, owing to the lack of a consistent segmentation approach, analyses in this study could not assess total macular volume across pooled data acquired from both devices, did not have GCIPL data from Spectralis scans, and did not assess INL or ONL thickness changes.36
We have previously shown that our open-source random forest classifier algorithm developed at Johns Hopkins University (JHU) can segment OCT images from PwMS and HCs acquired across both the Cirrus HD-OCT and Spectralis OCT platforms. This allows the pooling of segmentation-derived measures from both devices for assessing outcomes cross-sectionally and longitudinally in clinical trials with high reproducibility and interscanner agreement.37 In this study, we sought to analyze all OCT data acquired in the SPRINT-MS trial (over 2000 scans), deriving GCIPL, INL, and ONL thickness measures using the JHU segmentation approach, while at the same time applying rigorous quality control presegmentation and postsegmentation. We have specifically selected GCIPL thickness as an outcome because of its association with functional measures in MS to a degree equal or exceeding that of other retinal measures4,38 and its ability to detect atrophy modulation that varies according to DMT potency.17 We selected both INL and ONL thicknesses for assessment because atrophy in these layers may be more prominent in PMS and less altered or modulated by conventional anti-inflammatory DMTs based on our previous work, thus potentially representing different pathobiologies than may be captured by GCIPL assessment alone.17 Our primary research questions were (1) what effect does ibudilast have on GCIPL, INL, and ONL atrophy in PMS; (2) how does PMS subtype affect treatment response; and (3) how are OCT and MRI measures of neurodegeneration correlated in PMS.
Methods
In the phase 2 SPRINT-MS study, PwSPMS (n = 121) or PwPPMS (n = 134) at 28 sites were allocated to the ibudilast (n = 129) or placebo (n = 126) groups, as described elsewhere.39 Participants were between ages 21 and 65 years, had Expanded Disability Status Scale (EDSS) scores between 3.0 and 6.5 inclusive, and demonstrated disease progression within the previous 2 years. Ibudilast started at 60 mg/d, raised to 100 mg/d as tolerated.
Participants were assessed by blinded evaluators every 24 weeks over 96 weeks with OCT, noncontrast MRI, and clinical assessments including EDSS and Multiple Sclerosis Functional Composite. MRI-derived BPF and gray matter volumetric determinations were performed at the Cleveland Clinic Foundation, while lesion analyses were performed by NeuroRx Research (Montreal, Canada).39,40 OCT imaging was acquired on either Cirrus HD-OCT (Carl Zeiss Meditec, California, n = 183 participants) or Spectralis (Heidelberg Engineering, Germany, n = 61 participants) devices. Cirrus macular scans were acquired using the Macular Cube Scan 512 × 128 protocol, as described in detail elsewhere.34 Spectralis macular scans were acquired using the Posterior Pole protocol, with 61 line scans 12 μm apart, with an ART of 9.31 Only Spectralis scans with a quality ≥25 dB and Cirrus HD-OCT scans with a signal strength ≥6 were included in analyses.34 For both Cirrus HD-OCT and Spectralis devices, scans were obtained twice per eye with care for image position and quality.
OCT images were reviewed by the JHU team (H.E., E.V., N.P., M.D., S.D., N.N., and A.Q.) in a blinded manner, confirming imaging quality parameters, including positioning, image definition, artifact, pathology, and illumination, in accordance with OSCAR-IB criteria.41 Because 2 scans per visit were acquired for each eye, the best available scan was used. Scans were segmented with our validated automated segmentation algorithm, which normalizes scan intensities, estimates retinal layer boundaries, flattens the image with respect to the lower retinal boundary to reduce the effect of retinal curvature, and detects features in the image.40 A random forest classifier that has been trained on manually delineated scans then uses the features to map out a set of boundary probabilities, which are refined to produce retinal layer boundaries so that GCIPL, INL, and ONL retinal layer thicknesses can be determined in the region of an annulus encompassing the area between 0.5 mm and 2.5 mm around the fovea.42 This approach produces high reproducibility and interscanner agreement across Cirrus HD-OCT and Spectralis devices, with interscanner agreements of 98.3 ± 1.5% for GCIPL, 96.2 ± 2.0% for INL, and 96.4 ± 2.1% for ONL thickness measurements when examined at a cohort level.35 After segmentation, reviewers evaluated scans, verifying the accuracy of the segmentation for each layer, the centering and quality of the image, and noting any retinal pathology. Any borderline cases were independently evaluated by a second blinded reviewer.
Sample size for the trial was based on pilot trial and RRMS BPF data for 80% power and an alpha of 10%. Inclusion in analyses was based on the trial's previously determined modified intent-to-treat (MITT) criterion: Participants were included if they had received at least one dose of ibudilast or placebo and one instance of clinical or imaging data beyond the baseline visit. Of 129 participants allocated to ibudilast, 123 met MITT (3 failed to receive ibudilast, 3 lacked any assessment after baseline). Of 126 participants allocated to placebo, 121 met MITT (1 didn't receive placebo, 4 lacked any assessment after baseline). Recruitment occurred from November 2013 through May 2015, and follow-up continued for the full duration until the defined end in May 2017.
Statistical analyses used RStudio 1.4.1717 (RStudio, Boston, MA) and STATA (StataCorp, College Station, TX) version 13. Demographic and clinical characteristics were compared using the t test (age, disease duration, OCT values, MRI values, T25FW, 9-HPT) or the χ2 test (sex, race, subtype). Changes in GCIPL, INL, and ONL thickness were modeled over time using mixed-effects regression models, incorporating patient and eye-specific intercepts and random slopes, with unstructured variance-covariance structure of the random effects. The addition of another nested level for device was also used for comparison as part of sensitivity analyses. The models included variables for age, sex, disease duration, and subtype because each can influence retinal atrophy.17,43 Since data regarding previous ON were not collected as part of the trial, this could not be specifically accounted for. However, the use of random intercepts in the mixed-effects models inherently accounts for differences in baseline thicknesses. To determine partial Pearson correlations for OCT and MRI rates of changes over time, the average rate of change for each OCT and MRI measure was first determined using ordinary least square regressions of the relevant OCT or MRI values. Next, partial Pearson correlations between the participants' average rates of change were computed, adjusting for age, sex, disease duration, and subtype (where applicable), in line with previous work from this group.11 Percentage change per year was estimated through log-linear transformation. Power estimates for future prospective studies were estimated from the observed GCIPL-based parameter for various sample sizes at a range of effect sizes.44 Statistical significance was defined as p ≤ 0.05. Additional sensitivity analyses were also performed to assess for the effects of concurrent DMT use, baseline GCIPL intereye differences ≥ 4 µm (as a surrogate of optic neuropathy/prior optic neuritis), and of active MS (as evidenced by the formation of new T2 MRI lesions during follow-up) affecting the differential effect of ibudilast vs placebo on rates of GCIPL atrophy during the study period.
Standard Protocol Approvals, Registrations, Patient Consents, Funding, and Data Availability
Please see eMethods (links.lww.com/WNL/C983) for further details regarding standard protocol approvals, registrations, patient consents, funding, and data availability.
Results
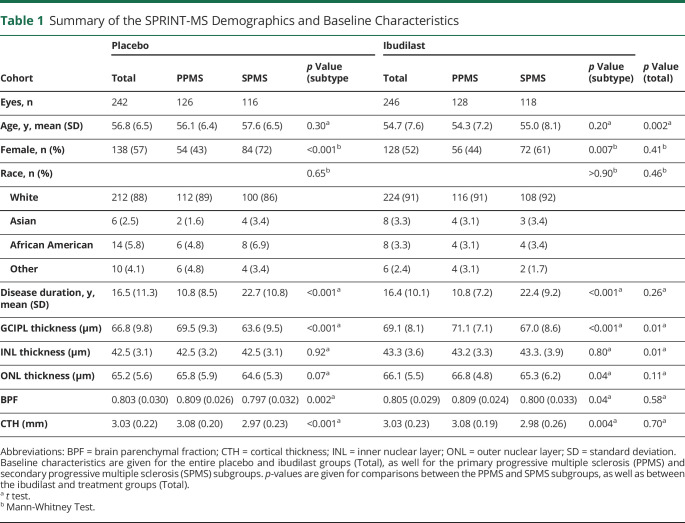
In the SPRINT-MS trial cohort, 244 people met the modified intent-to-treat criteria (ibudilast; n = 123, placebo; n = 121). Usable OCT imaging was available for 239 eyes in the ibudilast cohort and 234 eyes in the placebo cohort. A total of 2,220 OCT scans underwent segmentation, including quality control assessments before and after the segmentation of each scan (eTable 1, links.lww.com/WNL/C983). Eighty scans were unusable because of image quality, including 26 off-center scans and 16 with noticeable segmentation errors. An additional 71 scans contained pathology which interfered with the segmentation of the GCIPL, 68 with the INL, and 128 with the ONL. Overall, GCIPL data were usable in 81% of scans. 90% of study visits provided at least one usable OCT scan for each participant (eTable 2). Of note, 401 (18%) of the 2,220 scans had at least one form of intraretinal pathology, and of the 1805 scans with usable GCIPL segmentations, 256 (14%) had some instance of intraretinal pathology which did not affect segmentation (eTable 3). Sex, race, disease duration, baseline ONL thickness, BPF, and CTH did not differ between the placebo and ibudilast groups (Table 1). However, average age was lower in the ibudilast group (54.7 years vs 56.8 years, p = 0.002), and both GCIPL (69.1 μm vs 66.8 μm, p = 0.01) and INL (43.3 μm vs 42.5 μm, p = 0.01) thicknesses were higher. PwPPMS were less likely to be female than PwSPMS (43% vs 67%, p < 0.01) and had shorter average disease durations (11.1 vs 22.1 years, p < 0.01). Age and race did not differ between subtypes. PwPPMS had higher baseline GCIPL (overall 70.3 μm vs 65.3 μm, p < 0.01) and ONL (66.3 μm vs 65.0 μm, p = 0.01) thicknesses, and higher BPF (0.81 vs 0.80, p < 0.01) volumes, as compared with SPMS. Data regarding histories of ON were not collected as part of the trial. As part of sensitivity analyses for the effect of the OCT device, another nested level in the mixed-effects model for the type of OCT device type was added and the results were similar to the original model.
Table 1.
Summary of the SPRINT-MS Demographics and Baseline Characteristics
Differences in Retinal Atrophy Rates in People With PMS Receiving Treatment With Ibudilast as Compared With Placebo
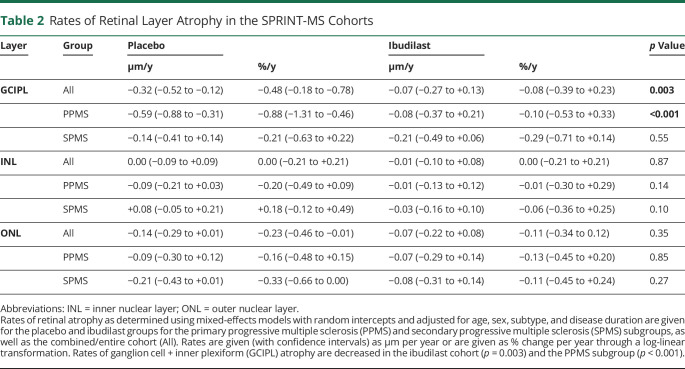
Retinal atrophy was modeled with linear mixed-effects models adjusted for age, sex, subtype, and disease duration (Table 2). Relative to the placebo cohort, the rate of GCIPL atrophy was lower in the ibudilast group (−0.07 ± 0.23 μm/y vs −0.32 ± 0.20 μm/y, a decrease of 79%, p = 0.003). Rates of change in other retinal layer thicknesses over the study duration were similar between the ibudilast and placebo cohorts. In PMS subtype analyses, PwPPMS taking ibudilast exhibited markedly slower GCIPL atrophy than those taking placebo (−0.08 ± 0.29 μm/y vs −0.60 ± 0.29 μm/y for a decrease of 87%, p < 0.001), while there was no difference in PwSPMS (ibudilast: −0.21 ± 0.28 µm/y vs placebo: −0.14 ± 0.27 µm/y, p = 0.55, Figure 1). The coefficient for the interaction of subtype and rate of GCIPL atrophy was −3.506 ± 2.567, p = 0.007.
Table 2.
Rates of Retinal Layer Atrophy in the SPRINT-MS Cohorts
Figure 1. Change in GCIPL Thickness Over Time, by Treatment Group and PMS Subgroup, in the SPRINT-MS Clinical Trial.
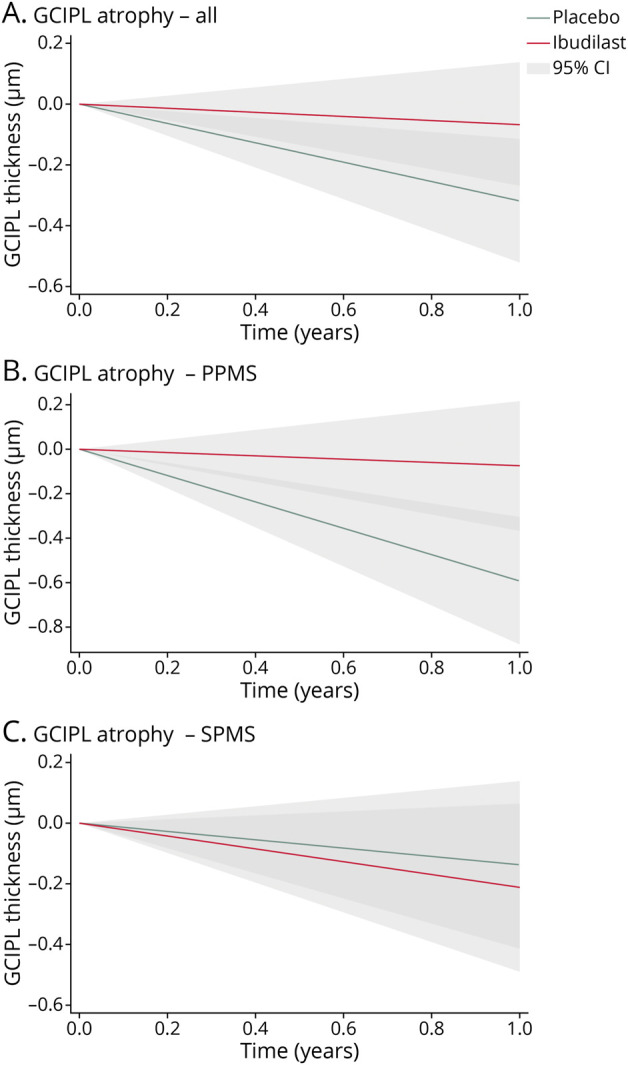
Lines represent estimated annual rates of ganglion cell + inner plexiform layer (GCIPL) change for the entire population (A), as well as the primary progressive multiple sclerosis (PPMS, B) and secondary progressive multiple sclerosis (SPMS, C) subgroups (placebo [green] and ibudilast [red] lines). Shaded areas represent the 95% confidence intervals (light gray or dark gray for overlapping confidence intervals). Rates were estimated using mixed-effects models with random intercepts and adjusted for age, sex, subtype, and disease duration. The lines are shown originating at 0 μm to show annual change from baseline. The x axis ranges from 0 to 1 to represent the estimated change over the course of 1 year.
Associations of Baseline GCIPL Thickness With GCIPL Atrophy Rate Differences
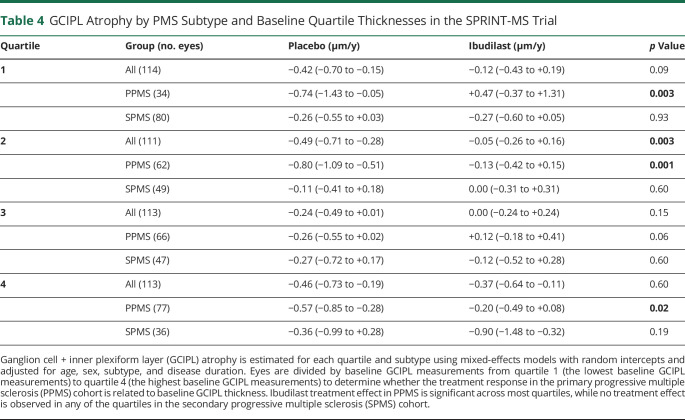
To investigate a potential effect of the higher baseline GCIPL thickness in PwPPMS compared with PwSPMS explaining the markedly differential modulation of GCIPL atrophy in the subtypes by ibudilast, each participant's eye was ranked by baseline GCIPL thickness into quartiles. Quartile 1 consisted of the eyes with the lowest 25% of baseline GCIPL thickness values, and quartile 4 consisted of those eyes with the highest 25% of baseline GCIPL thickness values. The composition of the quartiles by PMS subtype is illustrated in Table 3. In general, higher baseline GCIPL thickness values were observed among PwPPMS. The rate of GCIPL atrophy by treatment group was modeled with a linear mixed-effects model similar to the previous GCIPL atrophy analyses, with adjustments for age, sex, subtype, and disease duration (Table 4). Regardless of baseline GCIPL thickness quartiles, none of the SPMS quartile groups demonstrated differences in GCIPL atrophy between the ibudilast and placebo arms. On the other hand, in the PPMS subtype, slower GCIPL atrophy was observed with ibudilast treatment vs placebo when the baseline GCIPL thickness was in the first (+0.47 μm/y vs −0.74 μm/y, p = 0.003, n = 114 eyes), second (−0.13 μm/y vs −0.80 μm/y, p = 0.001, n = 113 eyes), or fourth (−0.20 μm/y vs −0.57 μm/y, p = 0.02, n = 113 eyes) quartiles. The difference in the rate of GCIPL atrophy between the ibudilast and placebo arms when baseline GCIPL thickness was in the third quartile approached but did not meet significance (+0.12 μm/y vs −0.26 μm/y, p = 0.058, n = 113 eyes).
Table 3.
Baseline GCIPL Thicknesses by Quartiles and Subtypes
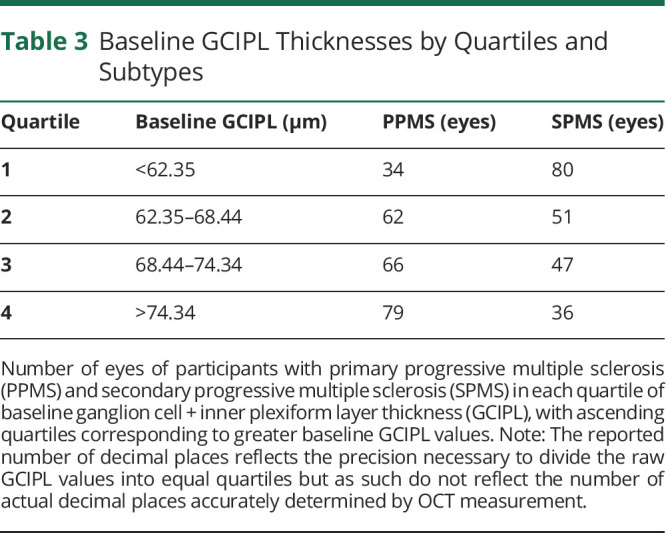
Table 4.
GCIPL Atrophy by PMS Subtype and Baseline Quartile Thicknesses in the SPRINT-MS Trial
Relationships Between Rates of Retinal and Brain Atrophy
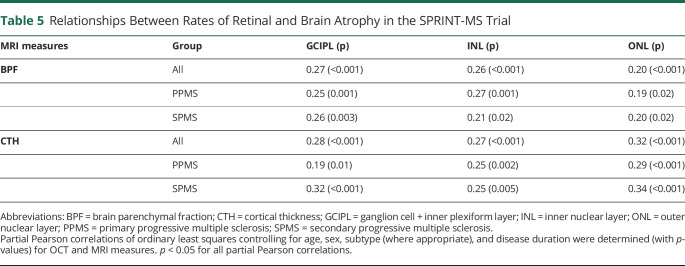
Partial Pearson correlations between the rates of change in OCT values, BPF and CTH measures, were calculated (Table 5), adjusting for age, sex, disease duration, and subtype (where appropriate). Scatter plots of these relationships are illustrated in the appendix (eFigures 2 and 3, links.lww.com/WNL/C983). Correlations between the OCT and MRI measures were statistically significant for all relationships and in both PPMS and SPMS. Across the cohort, change in GCIPL thickness had partial correlations of 0.270 and 0.279 with changes in BPF and CTH, respectively (p < 0.001 for both). Similar relationships were also observed between changes in INL and ONL thicknesses over time with changes in BPF and CTH across the cohort. In PwPPMS, change in GCIPL thickness had partial correlations of 0.249 with BPF (p < 0.001) and 0.192 with CTH (p = 0.01) changes. Similarly, in PwSPMS, change in GCIPL had partial correlations of 0.257 with BPF (p = 0.003) and 0.317 with CTH (p < 0.001). Comparable relationships between rates of INL and ONL change with BPF and CTH changes were also observed in both subtypes.
Table 5.
Relationships Between Rates of Retinal and Brain Atrophy in the SPRINT-MS Trial
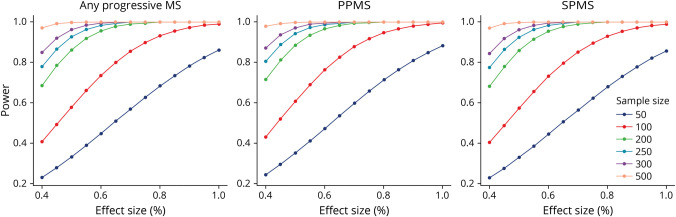
Power Calculations for Future Studies
GCIPL data from the study were used to determine the theoretical power for prospective studies using a variety of sample sizes over a range of effect sizes for both a combined cohort of participants with PPMS and participants with SPMS in a composition similar to this study or for cohorts of only PwPPMS or PwSPMS (Figure 2).
Figure 2. Sample Size Graphs for Power vs Effect Size in a Combined Cohort and Subtypes.
Models for sample size of future studies show the relationship between effect size and theoretical power in a combined cohort composed of participants with any form of progressive multiple sclerosis (MS) with a similar composition of the SPRINT-MS trial, of a cohort composed of participants with primary progressive multiple sclerosis (PPMS), and a cohort composed of participants with secondary progressive multiple sclerosis (SPMS).
Sensitivity Analyses
Sensitivity analyses were completed to test for the potential confounding effects of concurrent DMT use, baseline intereye GCIPL thickness differences ≥4 µm, and of active MS (as evidenced by new T2 MRI lesion formation during follow-up) affecting GCIPL atrophy or the treatment effect and did not alter the above findings (data not shown). Because only one participant in the entire cohort was deemed to have a relapse during follow-up, sensitivity analyses to account for the effect of relapses could not be performed.
Discussion
In our analysis, we observed a significant treatment effect of ibudilast wherein PwPMS treated with ibudilast exhibited slower GCIPL atrophy compared with those given placebo. The difference in GCIPL atrophy between the treatment and placebo groups observed might provide evidence not only for a retinal neuroprotection effect of ibudilast but possibly a more global neuroprotective effect, given the widely demonstrated association between GCIPL atrophy and global disease processes in MS. Because GCIPL atrophy has been shown to reflect clinical and radiologic disease progression in MS, our findings provide further support that ibudilast could be clinically beneficial for PwPMS, for whom there have been a paucity of treatments available to date and those are generally only of modest benefit. While previous analyses from the SPRINT-MS trial have not shown a difference in disease progression by treatment group when examining confirmed EDSS progression, they have shown slower rates of whole brain atrophy in those receiving ibudilast compared with placebo. Given the correlations between rates of GCIPL and brain atrophy in MS, and indeed even as corroborated within the current analysis of the SPRINT-MS PMS cohort, it may be unsurprising that we also found that ibudilast treatment in PMS was similarly associated with significantly slower rates of GCIPL atrophy. Previous OCT analyses have revealed significant decreases in macular volume atrophy among ibudilast-treated compared with placebo-treated participants assessed on Spectralis OCT. In the same previous analysis, trends toward decreased GCIPL atrophy with ibudilast on the Cirrus HD-OCT device were observed, but did not reach statistical significance. Of note, GCIPL data were not previously available for the Spectralis OCT images; however, our segmentation algorithm allowed us to include GCIPL data from both the Cirrus HD-OCT and Spectralis devices and also examine changes in INL and ONL thicknesses. Our detection of an effect of ibudilast on GCIPL atrophy using the entire cohort, where the previous study did not find an effect on pRNFL atrophy, may reflect the superior utility, possibly related to reliability, of GCIPL thickness in MS in general, or our methodology of image processing and quality control. Alternatively, this finding could less likely relate specifically to the mechanism of action of ibudilast. In addition, while we observed a significantly slower rate of GCIPL atrophy across the PMS cohort treated with ibudilast, it is noteworthy that this treatment effect was driven by a response only observed in PwPPMS and not PwSPMS.
Our findings in this study further corroborate previously published MRI results from the SPRINT-MS trial, both for the possible neuroprotective treatment effect of ibudilast (supported by slower rates of whole brain atrophy) and the treatment effect being primarily observed in people with PPMS.33 Previous analysis of BPF change by subtype revealed that the treatment effect of ibudilast was driven by the PPMS cohort and not the SPMS cohort, which was proposed to possibly reflect faster brain atrophy in the PPMS cohort, rendering this subtype more capable of capturing a treatment effect. A similar pattern was also seen in our study. There was a higher baseline GCIPL thickness and faster rate of GCIPL atrophy in the placebo PPMS group than the placebo SPMS group; so, similarly, the PPMS group may have been more capable of capturing a treatment effect. There was also a slightly higher baseline GCIPL thickness found in the ibudilast group as a whole despite the randomization of the participants and a similar split between subtypes. However, baseline GCIPL thickness is inherently accounted for in the statistical models used, and no floor affect that could create a bias based on the baseline GCIPL values is apparent in the analyses by quartiles. Additional sensitivity analyses were completed to test for the potential confounding effects of concurrent DMT use and active MS (as evidenced by new T2 MRI lesion development during follow-up) affecting the differential treatment effects on GCIPL atrophy across PMS subtypes. Our primary result of significantly slower GCIPL atrophy in those treated with ibudilast, primarily in the PPMS cohort, was unchanged, but this cannot rule out the contribution of these factors that might be detectable with increased power.
Unfortunately, data were not available on participants' ON history, so this could not be directly adjusted for in analyses. This is relevant because a reasonable proportion of SPMS eyes are likely to have a history of ON, while by definition none of the PPMS eyes should have had ON. However, sensitivity analyses using an intereye baseline GCIPL difference ≥ 4 µm as a surrogate for optic neuropathy or possible previous optic neuritis based on published literature did not change our findings. The difference in rates of GCIPL atrophy (between ibudilast and placebo treatment) across the quartiles of baseline GCIPL thickness values in PPMS, as well as the absence of any treatment differences in people with SPMS regardless of quartiles of baseline GCIPL thickness both persisted. This suggests that the difference in ibudilast treatment effect by subtype might not reflect an effect of the amount of GCIPL tissue available to atrophy. The differential effects of ibudilast by subtype seen in the current GCIPL results and previous MRI findings could relate to differing pathobiological underpinnings, such as the degree of innate immune system dysfunction, between PPMS and SPMS, possibly rendering PPMS more amenable to the mechanism of action of ibudilast in PPMS.45,46 Alternatively, the differential findings by subtype observed might relate to difficult-to-identify patient-specific and/or site-specific factors. Regardless, the mechanism(s) underlying differential modulation of retinal (and brain) atrophy by subtype requires elucidation, because it might have major implications for future study of PMS. Understanding these findings is crucial for determining whether SPMS and PPMS cohorts can be combined in future clinical trials, as has frequently been assumed to be the case in published literature. While the current analysis did not identify a treatment effect on INL or ONL atrophy, these OCT measures demonstrate significant correlations with changes in BPF and cortical thickness in PMS and remain exploratory outcomes of interest in PMS, especially because changes in these layers appear more prominent in advanced/progressive MS. Overall, our analyses should be considered exploratory and are not corrected for multiple comparisons. Further studies of ibudilast should confirm our findings and can expand on them by incorporating clinical ON history, examining more diverse patient populations, and testing the applicability of these initial findings to demographics outside of the inclusion criteria, including PwPMS with EDSS scores outside of the 3.0–6.5 range, and participants who may not have exhibited recent overt disease progression by conventional disability measures. More extended follow-up could help elucidate longer-term effects and determine whether the impact of ibudilast on OCT and MRI measures becomes detectable by clinical measures. Moreover, in the future, the relationship between OCT measures to distinct patterns of brain atrophy, such as thalamic atrophy, can be explored in greater detail than was possible in this study.
The agreement between OCT and MRI atrophy observed provides additional support for the use of OCT measures and GCIPL thickness in particular as an outcome in MS trials. The quality and precision of OCT data allow detection of significant differences even at lower sample sizes. Our sample size calculations demonstrate that prospective ibudilast studies using this approach may be powered to detect differences in combined or PPMS-only cohorts at sample sizes less than 100 participants or a future study of a similar size and setup because this trial has power ≥80% to detect an effect size of 41% using this approach under an alpha of 5%. Previous work examining longitudinal changes in GCIPL thickness related to different DMTs in RRMS26,28 have observed effect sizes upwards of 60% between DMTs of different potencies, so this approach seems eminently feasible for not only detection of effects from novel DMTs compared against controls but also in comparisons between different classes of DMTs.
In this study, we have demonstrated the feasibility of using a common segmentation algorithm to combine OCT images from Cirrus HD-OCT and Spectralis scanners in a large-scale, multicenter study. This example offers an approach to OCT acquisition and processing that can allow retinal layer atrophy to be used as an easily-acquired endpoint for trials and offer detection of treatment effects potentially before clinical effects become apparent. The speed and ease of use of OCT acquisition may be advantageous in clinical trials, and more widespread use of this modality will also contribute to the growing body of knowledge surrounding the INL and ONL and their role in PMS. The absence of a similar approach may be a limitation of some preceding multicenter studies using OCT.47
In summary, treatment with ibudilast resulted in decreased GCIPL atrophy in PPMS, supporting previous subtype analyses of the MRI data for the SPRINT-MS study. The difference in ibudilast treatment response between PPMS and SPMS groups was not explained by differences in baseline GCIPL thickness, age, sex, or disease duration, and this is an area that requires further exploration in future studies. Determining the reason for this effect may have major implications for our understandings of PMS subtype pathobiology, the mechanism(s) of action of ibudilast, and future PMS clinical trial design. Moreover, we found that rates of change in OCT measures correlate with changes in BPF and cortical thickness over time, providing further support that OCT measures reflect global CNS processes in PMS and in this case specifically have utility as outcome measures in PMS. Finally, our findings demonstrate the feasible role that OCT can play in multicenter clinical trials, using 2 commonly available OCT platforms. Future prospective studies using similar approaches may help to confirm these findings and expand on their implications for the MS field.
Glossary
- DMTs
disease-modifying therapies
- EDSS
Expanded Disability Status Scale
- GCIPL
ganglion cell + inner plexiform layer
- HC
healthy control
- JHU
Johns Hopkins University
- MITT
modified intent to treat
- MS
multiple sclerosis
- OCT
optical coherence tomography
- ON
optic neuritis
- ONL
outer nuclear layer
- PPMS
primary progressive MS
- pRNFL
peripapillary RNFL
- RNFL
retinal nerve fiber layer
- RRMS
relapsing-remitting MS
- SPMS
secondary progressive MS
Appendix. Authors
Footnotes
Editorial, page 420
Class of Evidence: NPub.org/coe
Study Funding
This work was supported by a grant from the US Department of Defense (MS180179 to Shiv Saidha).
Disclosure
Dr. Ehrhardt reports no disclosures; Dr. Lambe reports no disclosures; Dr. Murphy reports no disclosures; Dr. Vasileiou reports no disclosures; Dr. Kalaitzidis reports no disclosures; Dr. Moussa reports no disclosures; Dr. Filippatou reports no disclosures; Ms. Pellegrini reports no disclosures; Ms. Douglas reports no disclosures; Mr. Davis reports no disclosures; Ms. Nagy reports no disclosures; Ms. Quiroga reports no disclosures; Ms. Hu reports no disclosures; Ms. Zambriczki Lee reports no disclosures; Dr. Sotirchos has served on scientific advisory boards for Horizon Therapeutics, Viela Bio, Alexion, and Genentech and has received speaker fees from Alexion, Viela Bio, and Biogen; Dr. Prince is a founder of Sonovex, Inc. and serves on its Board of Directors and has received consulting fees from JuneBrain LLC and is PI on research grants to Johns Hopkins from Genentech and Biogen; Dr. Fitzgerald is funded by a Career Transition Fellowship from the National MS Society (NMSS); Dr. Calabresi has received consulting fees from Disarm, Nervgen, and Biogen and is PI on grants to JHU from Genentech, Principia, Biogen, and Annexon; Dr Bermel has served as a consultant for Biogen, Genzyme, Genentech, EMD Serono, Viela Bio, and Novartis and receives research support from Biogen, Genentech, and Novartis and shares rights to intellectual property underlying the Multiple Sclerosis Performance Test, currently licensed to Qr8 Health and Biogen; Dr. Saidha has received consulting fees from Medical Logix for the development of CME programs in neurology and has served on scientific advisory boards for Biogen, Novartis, Genentech Corporation, TG therapeutics, Rewind therapeutics, & Clene Pharmaceuticals. He has performed consulting for Novartis, Genentech Corporation, innocare pharma, Kiniksa pharmaceuticals, JuneBrain LLC, and Lapix therapeutics. He is the PI of investigator-initiated studies funded by Genentech Corporation, Novartis, and Biogen. He previously received support from the Race to Erase MS foundation. He has received equity compensation for consulting from JuneBrain LLC and Lapix therapeutics. He was also the site investigator of trials sponsored by MedDay Pharmaceuticals and Clene Pharmaceuticals and is the site investigator of a trial sponsored by Novartis and Lapix therapeutics. Go to Neurology.org/N for full disclosures.
References
- 1.Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133(6):1591-1601. doi: 10.1093/brain/awq080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toussaint D, Périer O, Verstappen A, Bervoets S. Clinicopathological study of the visual pathways, eyes, and cerebral hemispheres in 32 cases of disseminated sclerosis. J Clin Neuroophthalmol. 1983;3(3):211-220. [PubMed] [Google Scholar]
- 3.Saidha S, Syc SB, Ibrahim MA, et al. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain. 2011;134(2):518-533. doi: 10.1093/brain/awq346 [DOI] [PubMed] [Google Scholar]
- 4.Saidha S, Syc SB, Durbin MK, et al. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler. 2011;17(12):1449-1463. doi: 10.1177/1352458511418630 [DOI] [PubMed] [Google Scholar]
- 5.Nguyen J, Rothman A, Gonzalez N, et al. Macular ganglion cell and inner plexiform layer thickness is more strongly associated with visual function in multiple sclerosis than bruch membrane opening–minimum rim width or peripapillary retinal nerve fiber layer thicknesses. J Neuroophthalmol. 2019;39(4):444-450. doi: 10.1097/wno.0000000000000768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambe J, Fitzgerald KC, Murphy OC, et al. Association of spectral-domain OCT with long-term disability worsening in multiple sclerosis. Neurology. 2021;96(16):e2058-e2069. doi: 10.1212/wnl.0000000000011788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bsteh G, Hegen H, Altmann P, et al. Retinal layer thinning predicts treatment failure in relapsing multiple sclerosis. Eur J Neurol. 2021;28(6):2037-2045. doi: 10.1111/ene.14829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winges KM, Murchison CF, Bourdette DN, Spain RI. Longitudinal optical coherence tomography study of optic atrophy in secondary progressive multiple sclerosis: results from a clinical trial cohort. Mult Scler. 2017;25(1):55-62. doi: 10.1177/1352458517739136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerrieri S, Comi G, Leocani L. Optical coherence tomography and visual evoked potentials as prognostic and monitoring tools in progressive multiple sclerosis. Front Neurosci. 2021;15:692599. doi: 10.3389/fnins.2021.692599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petracca M, Cordano C, Cellerino M, et al. Retinal degeneration in primary-progressive multiple sclerosis: a role for cortical lesions? Mult Scler. 2016;23(1):43-50. doi: 10.1177/1352458516637679 [DOI] [PubMed] [Google Scholar]
- 11.Saidha S, Al-Louzi O, Ratchford JN, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol. 2015;78(5):801-813. doi: 10.1002/ana.24487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderson APD, Trip SA, Schlottmann PG, et al. An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain. 2008;131(Pt 1):277-287. doi: 10.1093/brain/awm285 [DOI] [PubMed] [Google Scholar]
- 13.Oberwahrenbrock T, Schippling S, Ringelstein M, et al. Retinal damage in multiple sclerosis disease subtypes measured by high-resolution optical coherence tomography. Mult Scler Int. 2012;2012:530305. doi: 10.1155/2012/530305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behbehani R, Abu Al-Hassan A, Al-Salahat A, Sriraman D, Oakley JD, Alroughani R. Optical coherence tomography segmentation analysis in relapsing remitting versus progressive multiple sclerosis. PLoS ONE. 2017;12(2):e0172120. doi: 10.1371/journal.pone.0172120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jankowska-Lech I, Wasyluk J, Palasik W, Terelak-Borys B, Grabska-Liberek I. Peripapillary retinal nerve fiber layer thickness measured by optical coherence tomography in different clinical subtypes of multiple sclerosis. Mult Scler Relat Disord. 2019;27:260-268. doi: 10.1016/j.msard.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 16.Balk L, Tewarie P, Killestein J, Polman C, Uitdehaag B, Petzold A. Disease course heterogeneity and OCT in multiple sclerosis. Mult Scler. 2014;20(9):1198-1206. doi: 10.1177/1352458513518626 [DOI] [PubMed] [Google Scholar]
- 17.Saidha S, Sotirchos ES, Ibrahim MA, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol. 2012;11(11):963-972. doi: 10.1016/s1474-4422(12)70213-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pulicken M, Gordon-Lipkin E, Balcer LJ, Frohman E, Cutter G, Calabresi PA. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology. 2007;69(22):2085-2092. doi: 10.1212/01.wnl.0000294876.49861.dc [DOI] [PubMed] [Google Scholar]
- 19.Albrecht P, Ringelstein M, Müller A, et al. Degeneration of retinal layers in multiple sclerosis subtypes quantified by optical coherence tomography. Mult Scler. 2012;18(10):1422-1429. doi: 10.1177/1352458512439237 [DOI] [PubMed] [Google Scholar]
- 20.Siepman TAM, Wefers Bettink-Remeijer M, Hintzen RQ. Retinal nerve fiber layer thickness in subgroups of multiple sclerosis, measured by optical coherence tomography and scanning laser polarimetry. J Neurol. 2010;257(10):1654-1660. doi: 10.1007/s00415-010-5589-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelfand JM, Goodin DS, Boscardin WJ, Nolan R, Cuneo A, Green AJ. Retinal axonal loss begins early in the course of multiple sclerosis and is similar between progressive phenotypes. PLoS ONE. 2012;7(5):e36847. doi: 10.1371/journal.pone.0036847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papakostopoulos D, Fotiou F, Hart JC, Banerji NK. The electroretinogram in multiple sclerosis and demyelinating optic neuritis. Electroencephalogr Clin Neurophysiol. 1989;74(1):1-10. [DOI] [PubMed] [Google Scholar]
- 23.Forooghian F, Sproule M, Westall C, et al. Electroretinographic abnormalities in multiple sclerosis: possible role for retinal autoantibodies. Doc Ophthalmol. 2006;113(2):123-132. [DOI] [PubMed] [Google Scholar]
- 24.Knier B, Schmidt P, Aly L, et al. Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis. Brain. 2016;139(11):2855-2863. doi: 10.1093/brain/aww219 [DOI] [PubMed] [Google Scholar]
- 25.Cordano C, Yiu HH, Oertel FC, et al. Retinal >INL thickness in multiple sclerosis: a mere marker of neurodegeneration? Ann Neurol. 2020;89(1):192-193. doi: 10.1002/ana.25933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sotirchos ES, Gonzalez Caldito N, Filippatou A, et al. Progressive multiple sclerosis is associated with faster and specific retinal layer atrophy. Ann Neurol. 2020;87(6):885-896. doi: 10.1002/ana.25738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratchford JN, Saidha S, Sotirchos ES, et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology. 2012;80(1):47-54. doi: 10.1212/wnl.0b013e31827b1a1c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Button J, Al-Louzi O, Lang A, et al. Disease-modifying therapies modulate retinal atrophy in multiple sclerosis. Neurology. 2017;88(6):525-532. doi: 10.1212/wnl.0000000000003582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zivadinov R, Tavazzi E, Hagemeier J, et al. The effect of glatiramer acetate on retinal nerve fiber layer thickness in patients with relapsing–remitting multiple sclerosis: a longitudinal optical coherence tomography study. CNS Drugs. 2018;32(8):763-770. doi: 10.1007/s40263-018-0521-9 [DOI] [PubMed] [Google Scholar]
- 30.Lambe J, Risher H, Filippatou AG, et al. Modulation of retinal atrophy with rituximab in multiple sclerosis. Neurology. 2021;96(20):e2525-e2533. doi: 10.1212/wnl.0000000000011933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakimovski D, Zivadinov R, Vaughn CB, Ozel O, Weinstock-Guttman B. Clinical effects associated with five-year retinal nerve fiber layer thinning in multiple sclerosis. J Neurol Sci. 2021;427:117552. doi: 10.1016/j.jns.2021.117552 [DOI] [PubMed] [Google Scholar]
- 32.Rolan P, Hutchinson M, Johnson K. Ibudilast: a review of its pharmacology, efficacy and safety in respiratory and neurological disease. Expert Opin Pharmacother. 2009;10(17):2897-2904. doi: 10.1517/14656560903426189 [DOI] [PubMed] [Google Scholar]
- 33.Fox RJ, Coffey CS, Cudkowicz ME, et al. Design, rationale, and baseline characteristics of the randomized double-blind phase II clinical trial of ibudilast in progressive multiple sclerosis. Contemp Clin Trials. 2016;50:166-177. doi: 10.1016/j.cct.2016.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naismith RT, Bermel RA, Coffey CS, et al. Effects of ibudilast on MRI measures in the phase 2 SPRINT-MS study. Neurology. 2020;96(4):e491-e500. doi: 10.1212/wnl.0000000000011314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman AD, Fedler JK, Yankey J, et al. Response to ibudilast treatment according to progressive multiple sclerosis disease phenotype. Ann Clin Transl Neurol. 2021;8(1):111-118. doi: 10.1002/acn3.51251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bermel RA, Fedler JK, Kaiser P, et al. Optical coherence tomography outcomes from SPRINT-MS, a multicenter, randomized, double-blind trial of ibudilast in progressive multiple sclerosis. Mult Scler. 2020;27(9):1384-1390. doi: 10.1177/1352458520964409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhargava P, Lang A, Al-Louzi O, et al. Applying an open-source segmentation algorithm to different OCT devices in multiple sclerosis patients and healthy controls: implications for clinical trials. Mult Scler Int. 2015;2015:1-10. doi: 10.1155/2015/136295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petracca M, Sumowski J, Fabian M, Miller A, Lublin F, Inglese M. Looking into cognitive impairment in primary-progressive multiple sclerosis. Eur J Neurol. 2017;25(1):192-195. doi: 10.1111/ene.13489 [DOI] [PubMed] [Google Scholar]
- 39.Fox RJ, Coffey CS, Conwit R, et al. Phase 2 trial of ibudilast in progressive multiple sclerosis. N Engl J Med. 2018;379(9):846-855. doi: 10.1056/nejmoa1803583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Safety, Tolerability and Activity Study of Ibudilast in Subjects With Progressive Multiple Sclerosis. ClinicalTrials.gov identifier: NCT01982942. Updated November 13, 2013. Accessed December 1, 2021. clinicaltrials.gov/ct2/show/NCT01982942 [Google Scholar]
- 41.Tewarie P, Balk L, Costello F, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS ONE. 2012;7(4):e34823. doi: 10.1371/journal.pone.0034823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lang A, Carass A, Hauser M, et al. Retinal layer segmentation of macular OCT images using boundary classification. Biomed Opt Express. 2013;4(7):1133. doi: 10.1364/boe.4.001133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caldito NG, Saidha S, Sotirchos ES, et al. Brain and retinal atrophy in African-Americans versus Caucasian-Americans with multiple sclerosis: a longitudinal study. Brain. 2018;141(11):3115-3129. doi: 10.1093/brain/awy245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu G, Liang KY. Sample size calculations for studies with correlated observations. Biometrics. 1997;53(3):937. doi: 10.2307/2533554 [DOI] [PubMed] [Google Scholar]
- 45.Font LF. A Molecular Characterization of Meningeal Inflammation in Progressive MS Brain [dissertation]. Imperial College London; 2020. [Google Scholar]
- 46.Ratzer R, Søndergaard H, Christensen JR, et al. Gene expression analysis of relapsing–remitting, primary progressive and secondary progressive multiple sclerosis. Mult Scler. 2013;19(14):1841-1848. doi: 10.1177/1352458513500553 [DOI] [PubMed] [Google Scholar]
- 47.Paul F, Calabresi PA, Barkhof F, et al. Optical coherence tomography in multiple sclerosis: a 3‐year prospective multicenter study. Ann Clin Transl Neurol. 2021;8(12):2235-2251. doi: 10.1002/acn3.51473 [DOI] [PMC free article] [PubMed] [Google Scholar]