Abstract
Transient visual loss (TVL) is a common complaint in the emergency department, with numerous possible etiologies. Prompt evaluation and management of TVL can potentially prevent progression to permanent visual loss. In this case, a 62-year-old woman presented with acute, painless, unilateral TVL. Two weeks before presentation, the patient reported bitemporal headaches and paresthesia of the distal extremities. A review of systems revealed chronic fatigue, cough, diffuse arthralgias, and decreased appetite for the previous 6 months. This case highlights the diagnostic approach to patients with TVL. Some common and rare causes associated with this clinical manifestation are briefly reviewed.
Section 1
A 62-year-old woman with a medical history of asthma presented to the emergency department with painless transient vision loss (TVL) of the left eye lasting 15 minutes with complete resolution. Similar symptoms were reported in the contralateral eye 6 days before presentation. In the past 2 weeks, she complained of persistent dull bitemporal headaches associated with photophobia and worsening numbness of bilateral hands and feet. A review of systems revealed fatigue, cough, arthralgias and decreased appetite for the past 6 months. She denied any focal weakness, weight loss, preceding infectious illness, or trauma. Her medication regimen included inhaled ipratropium, intranasal fluticasone, and oral montelukast.
Neurologic examination showed an awake and oriented woman with prosodic and fluent speech. Pupils were symmetric and reactive to bright light. No afferent pupillary defect, ophthalmoplegia, or nystagmus were detected, visual fields were full to confrontation, visual acuity was 20/20, intraocular pressure was 23, and extraocular movements were full without pain or diplopia on the far gaze, bilaterally. On dilated fundoscopic examination, vitreous, macula, vessels, and periphery in both eyes were within normal limits. The disk had sharp margins and normal color, without edema, and a cup-to-disc ratio of 0.8 with a cupped appearance. Pinprick, vibration, and proprioception were mildly decreased at the toes and hands bilaterally. Manual motor testing revealed grade 5/5 strength in proximal and distal upper and lower extremities. Finger-to-nose and heel-to-shin revealed no ataxia or dysmetria. Reflexes were preserved.
Skin examination revealed erythematous maculopapular lesions with central purple involving the right dorsal foot and ankle. There were scattered erythematous papules on the bilateral lower extremities and right arm.
Questions for Consideration:
What is the differential diagnosis?
What diagnostic workup should be conducted?
Section 2
The patient in this vignette presented with painless unilateral TVL and bitemporal headache. Causes of TVL can be divided into ischemic or nonischemic. The most common cause of TVL is ischemic optic neuropathy (arteritic or nonarteritic). Therefore, patients presenting with these symptoms require urgent evaluation because delay can quickly lead to infarction. Risk factors of nonarteritic optic nerve ischemia include older age, hypertension, and diabetes. Carotid imaging duplex ultrasound, magnetic resonance angiography, or computed tomographic angiography) is required, especially if vascular risk factors are present.1 Computed tomographic angiography of the head and neck showed no evidence of large vessel arteritis, normal superficial temporal artery bilaterally, and no atherosclerotic disease.
Optic nerve ischemia can be attributed to embolism, coagulopathy, vasospasm, or vasculitis. A detailed funduscopic examination could reveal the implicated mechanism and is part of the diagnostic workup for TVL. In patients older than 50 years, it is recommended to obtain erythrocyte sedimentation rate (ESR) and C-reactive protein to screen for giant cell arteritis (GCA), the most common cause of arteritic ischemic optic neuropathy, and a treatable cause of otherwise permanent visual loss. Risk factors of arteritic ischemic optic neuropathy include female sex and older age.2 Headache is more common in GCA than in carotid vascular disease and could help differentiate these 2 conditions; temporal artery biopsy can be diagnostic.3
Nonischemic etiologies for optic neuropathies include infectious (West Nile virus and syphilis), inflammatory (postviral optic neuritis, sarcoidosis, and autoimmune disease), toxins (methanol and ethambutol), or nutritional deficiency (vitamin B1 and vitamin B12). Brain lesions can also cause compression of the optic nerve, typically identified in neuroimaging and usually presenting as chronic, progressive vision loss.4 Transient visual loss and headache, particularly positional headaches, could be a manifestation of idiopathic intracranial hypertension. Common MRI findings include optic nerve tortuosity and bulging, empty sella turcica, posterior globe flattening, and transverse sinus stenosis. Lumbar puncture with opening pressure can be both diagnostic and therapeutic, although not pathognomonic.5
Demyelinating etiologies for optic neuropathy causing optic neuritis are multiple sclerosis (MS), myelin oligodendrocyte glycoprotein antibody–associated disease (MOGAD), or neuromyelitis optic spectrum disorder (NMOSD). The vision loss in these disorders is painful and not transient, lasting days to weeks. MRI findings and associated antibody testing are diagnostic for these conditions.6
Initial workup included an MRI brain (Figure 1), CSF, autoimmune panel, inflammatory markers, urinalysis, and vitamin B12 and B9 levels. The MRI revealed retrobulbar and perioptic nerve sheath enhancement associated with mild leptomeningeal enhancement and extensive sinus disease. She underwent lumbar puncture with normal opening pressure, and CSF analysis showed increased IgG (3.6) and white blood cell count (9/mm3), with increased eosinophils (11% neutrophils, 33% lymphocytes, 22% monocytes, and 33% eosinophils), normal protein (42 mg/dL), and normal glucose (56 mg/dL). Immunologic workup showed positive results for antinuclear antibodies (1:160) and myeloperoxidase antineutrophil cytoplasmic antibody (MPO-ANCA). ANCA-PR3 showed negative results. Her ESR (46 mm/hr) was mildly elevated. Peripheral cell blood count demonstrated significant eosinophilia (6,440/mm3). Urinalysis showed microhematuria (6 RBC/HPF). Vitamin B12 and B9 levels were within normal limits.
Figure 1. Brain MRI.
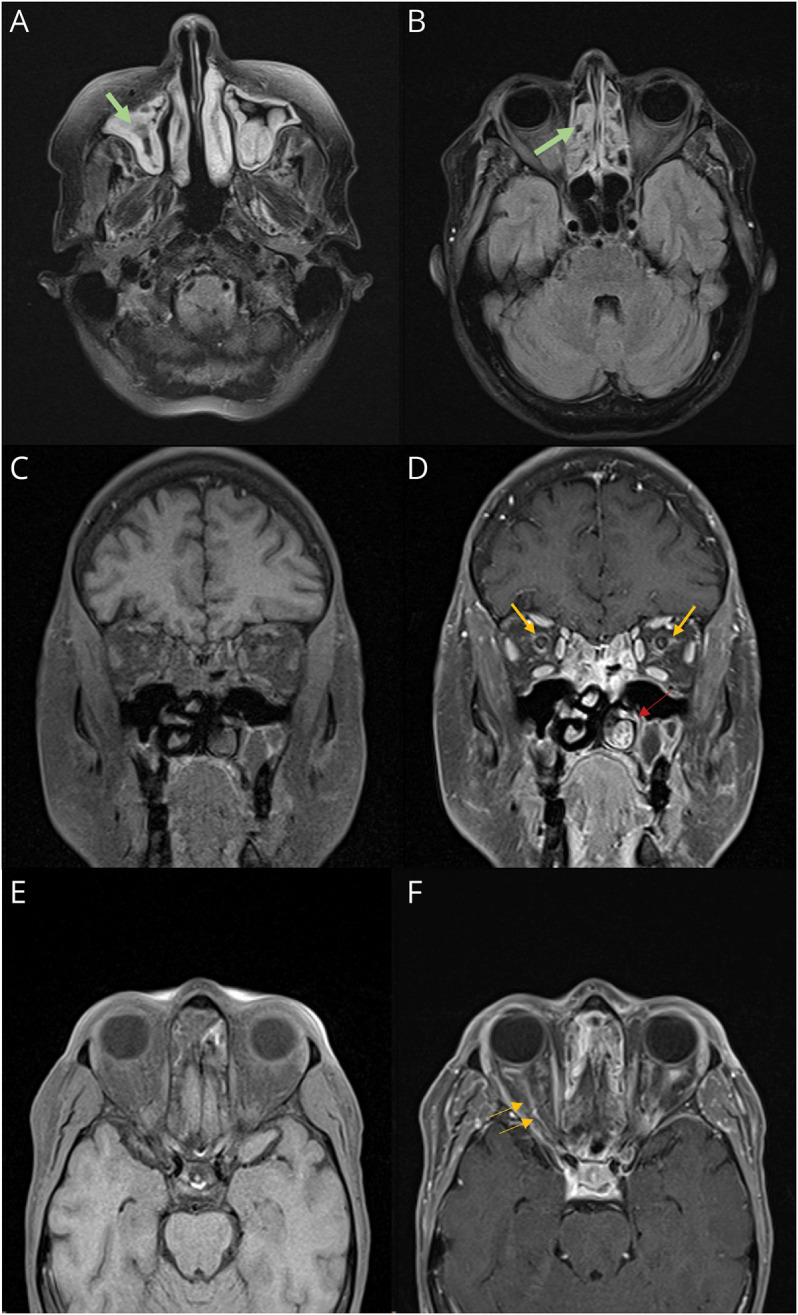
(A and B) Axial FLAIR brain MRI demonstrates hyperintensity of the periorbital soft tissue, temporalis muscle, and extensive ethmoid and maxillary sinus disease (green arrows). (C) Coronal T1 without contrast. (D) Coronal T1 sequence with contrast showing optic perineuritis and fat stranding (yellow arrow). Note the enlarged inferior turbinate (red arrow). (E) Axial T1 without contrast. (F) Axial T1 with contrast showing perioptic nerve enhancement (yellow arrows). FLAIR, fluid-attenuated inversion recovery.
Questions for Consideration:
What additional test can narrow the differential diagnosis?
How would you treat the patient?
Section 3
The inflammation was confined to the optic nerve sheath, conferring optic perineuritis as opposed to optic neuritis that presents with inflammation of the optic nerve axons. Some etiologies for optic perineuritis include Graves disease, sarcoidosis, rheumatoid arthritis, scleroderma, gout, tuberculosis, sparganosis, ocular toxoplasmosis, bacterial and fungal meningitis, IgG4-related diseases, CMV neuroretinitis, Behcet disease, and granulomatosis with polyangiitis. Most cases of optic perineuritis are associated with systemic autoimmune diseases, in contrast to optic neuritis that is more commonly associated with central nervous system (CNS) autoimmune disorders such as MS, MOGAD, or NMOSD.7
Optic perineuritis and systemic symptoms with sinuses/lung involvement suggest an autoimmune/inflammatory or infectious etiology. Workup included blood test showing negative results for syphilis, HIV, hepatitis, tuberculosis, strongyloidiasis, parasites, and meningoencephalitis panel and CSF Gram stain/cultures showing negative results. Serum levels of angiotensin-converting enzyme, copper, methylmalonic acid, and vitamin B6 were normal. Nerve conduction studies revealed peripheral sensorimotor axonal neuropathy affecting lower extremities more than upper extremities.
Considering the patient's eosinophilia, neurologic symptoms (TVL and peripheral neuropathy), sinus abnormalities, optic perineuritis, a history of asthma, cutaneous palpable purpura, arthralgias, and MPO-ANCA positivity, a diagnosis of eosinophilic granulomatosis with polyangiitis (EGPA) was made. Intravenous methylprednisolone was started and continued for 3 days followed by a daily oral maintenance dose of prednisone. On the second day of treatment, the patient denied new episodes of visual loss, headaches, paresthesias, or other neurologic symptoms. Her eosinophil count reduced to 44/mm3 after treatment. The rheumatology team recommended a course of cyclophosphamide. Montelukast was discontinued because there were reported cases of an association with EGPA.8
The purpuric rash on the ankle (Figure 2A) was biopsied, showing eosinophilic and neutrophilic infiltration and vasculitis (Figure 2, B–D). Biopsy results were compatible with bullous eosinophilic vasculitis. The patient was discharged with 35 mg of oral prednisone daily, which was tapered off as outpatient. After clinical remission, methotrexate was started as maintenance therapy. The patient continues to be in clinical remission to the publication of this case, approximately 17 months after her admission.
Figure 2. Histopathologic Features.
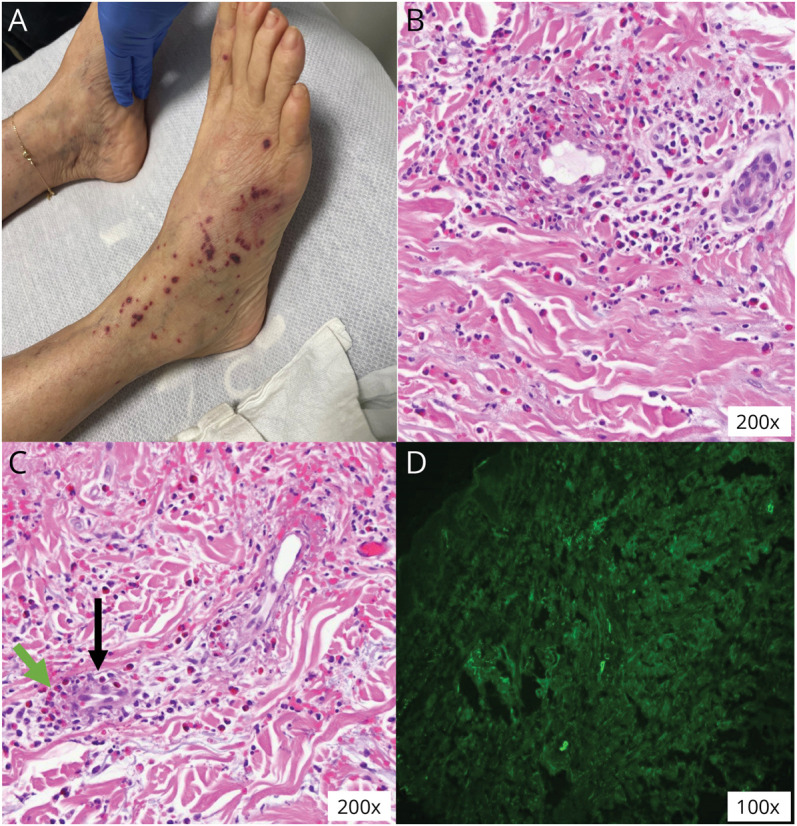
(A) Palpable nonblanching lesions suggestive of cutaneous vasculitis. (B and C) Skin biopsy showing eosinophilic infiltration (green arrow) around vessels (black arrow). (D) On direct immunofluorescence, skin vessels stained positive for antibodies against IgG, IgM, C3, and fibrinogen; intercellular basement membrane and superficial dermis stained negative for antibodies.
Discussion
This case illustrates TVL as a possible, but unusual, CNS manifestation of EGPA. EGPA is a rare necrotizing vasculitis of small-sized and medium-sized vessels, which involves multiple organs. Clinical clues included (1) the systemic and constitutional symptoms in combination with upper and lower airway dysfunction and (2) palpable purpura, a common dermatologic finding in many small-vessel vasculitis.
The American College of Rheumatology established a classification criterion for EGPA, after a diagnosis of small or medium vessel vasculitis has been made: maximum eosinophil count ≥1 × 109/L (+5), obstructive airway disease (+3), nasal polyps (+3), cytoplasmic antineutrophil cytoplasmic antibody (c-ANCA) or antiproteinase 3-ANCA positivity (−3), extravascular eosinophilic-predominant inflammation (+2), mononeuritis multiplex/motor neuropathy not due to radiculopathy (+1), and hematuria (−1). A total risk score ≥6, as seen in this case, yielded a sensitivity of 84.9% and specificity of 99.1% for EGPA.9
In a review of 46 cases of the ophthalmic manifestations of EGPA,10 central retinal artery or vein occlusion, ischemic optic neuropathy, conjunctival nodules, orbital myositis, proptosis, dacryoadenitis, retinal vasculitis/infarcts/edema, cranial nerve palsy, and amaurosis were reported. On imaging, the most common findings include orbital mass or infiltration, enhancement or enlargement of rectus muscles, lacrimal gland involvement, and enhancement along the optic nerve sheath. The ophthalmic presentations were divided into 2 groups: idiopathic orbital inflammation-like or ischemic vasculitis. The most common presenting symptoms in the idiopathic orbital inflammation-like group were redness, periocular swelling, and diplopia. By contrast, patients in the ischemic vasculitis group presented most frequently with acute-onset loss of vision. At least 6 patients with the ischemic vasculitis type reported episodes of unilateral TVL before permanent visual loss. Of the 8 patients with ischemic vasculitis, 3 had improvement in visual outcomes after intravenous steroid therapy, reinforcing the importance of correct identification and treatment of the ophthalmic manifestations of EGPA.
In this case, the patient presented with a chief complaint of TVL and clinical findings compatible with EGPA. Although rare, TVL is among the ophthalmic manifestations of EGPA. Knowledge of the ophthalmic manifestations of EGPA may yield prompt diagnosis and treatment, decreasing patient morbidity and mortality.7,10,11
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
L.M. Tedesco Silva, J.C. Reategui, L. Tornes, E. Marulanda, and K. Detyniecki report no disclosures relevant to the manuscript. A. Alkhachroum is supported by an institutional KL2 Career Development Award from the Miami CTSI NCATS UL1TR002736 and by the National Institute of Neurologic Disorders and Stroke of the National Institutes of Health under Award Number K23NS126577 & R21NS128326. J. Margolesky and M. Melo Bicchi report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Volkers EJ, Donders RC, Koudstaal PJ, van Gijn J, Algra A, Jaap Kappelle L. Transient monocular blindness and the risk of vascular complications according to subtype: a prospective cohort study. J Neurol. 2016;263(9):1771-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machado EB, Michet CJ, Ballard DJ, et al. Trends in incidence and clinical presentation of temporal arteritis in Olmsted County, Minnesota, 1950-1985. Arthritis Rheum. 1988;31(6):745-749. [DOI] [PubMed] [Google Scholar]
- 3.Vodopivec I, Rizzo JF III. Ophthalmic manifestations of giant cell arteritis. Rheumatology (Oxford). 2018;57(suppl_2):ii63-ii72. [DOI] [PubMed] [Google Scholar]
- 4.Van Stavern GP. Metabolic, hereditary, traumatic, and neoplastic optic neuropathies. Continuum (Minneap Minn). 2014;20(4 Neuro-Ophthalmology):877-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81(13):1159-1165. [DOI] [PubMed] [Google Scholar]
- 6.Sechi E, Cacciaguerra L, Chen JJ, et al. Myelin Oligodendrocyte glycoprotein antibody-associated disease (MOGAD): a review of clinical and MRI features, diagnosis, and management. Front Neurol. 2022;13:885218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Zhou H, Sun J, et al. Optic perineuritis and its association with autoimmune diseases. Front Neurol. 2021;11:627077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giusti Del Giardino L, Cavallaro T, Anzola GP, Lombardi C, Ferrari S. Neuropathy in eosinophilic granulomatosis with polyangiitis: a comparison study of 24 cases with or without prior leukotriene antagonist exposure. Eur Ann Allergy Clin Immunol. 2014;46(6):201-209. [PubMed] [Google Scholar]
- 9.Grayson PC, Ponte C, Suppiah R, et al. 2022 American College of rheumatology/European alliance of associations for rheumatology classification criteria for eosinophilic granulomatosis with polyangiitis. Ann Rheum Dis. 2022;81(3):309-314. [DOI] [PubMed] [Google Scholar]
- 10.Akella SS, Schlachter DM, Black EH, Barmettler A. Ophthalmic eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome): a systematic review of the literature. Ophthalmic Plast Reconstr Surg. 2019;35(1):7-16. [DOI] [PubMed] [Google Scholar]
- 11.André R, Cottin V, Saraux JL, et al. Central nervous system involvement in eosinophilic granulomatosis with polyangiitis (Churg-Strauss): report of 26 patients and review of the literature. Autoimmun Rev. 2017;16(9):963-969. [DOI] [PubMed] [Google Scholar]


