Abstract
Background and Objectives
Patients with classic locked-in syndrome (LIS), typically caused by ventral pontine stroke, present with quadriplegia, mutism, intact consciousness, and communication skills limited to vertical gazing and/or blinking. Clinical presentations and definitions of LIS differ, especially regarding incomplete LIS. In our study, we explored the functional diversity of LIS, its outcomes, and the complexity of its course along with variations in the location of lesions and their potential significance for outcomes.
Methods
A national cohort of patients with vascular LIS who remained in the LIS state for at least 6 weeks according to a functional definition of LIS was studied. Demographic, medical, and follow-up data, collected between 2012 and 2022, were obtained from the quality register of the Norwegian National Unit for Rehabilitation of Locked-In Syndrome. Outcomes in verbal communication, motor function, and dependency were evaluated according to criteria for being in or not in the LIS state. The modified Rankin scale and LIS motor recovery scale were applied. Descriptive analysis was performed. The relationship between lesion location and functional outcome was investigated.
Results
The sample included 51 patients (median age: 55.7 years, 36 male individuals), 43 of whom had follow-up data. Ischemic stroke was the most common etiology (n = 35). Twenty-three patients had emerged from the LIS state, mostly within 2 years after onset. All but 1 patient achieved some motor improvement, whereas only 3 achieved full motor recovery, and 88% had a persistently high level of dependence. The 3-year survival rate was 87%. Five patients had an isolated pontine lesion, whereas 80% showed various lesions outside the brain stem. Patients who emerged from the LIS state had a significantly lower prevalence of lesions outside the brain stem than patients who remained in the LIS state did.
Discussion
Investigating an unselected population-based sample of patients with vascular LIS offers important insights into the functional diversity of LIS. Although most patients remained severely disabled, even small improvements in function can substantially increase the potential for activity and participation. Additional lesions outside the brain stem seem to be common in long-lasting LIS and might be prognostic for remaining in the LIS state.
Introduction
Patients with locked-in syndrome (LIS) have severe paralysis and communication difficulties1 but function well cognitively2,3 and can communicate to varying degrees using eye movements.4,5 The most common cause of LIS is vascular,6-9 while more rare etiologies include trauma, infection, severe polyneuropathy, myelinolysis, and inflammatory demyelination.5,6
LIS can be divided into 3 categories based on clinical presentation.10 Classic LIS refers to a condition characterized by intact consciousness, mutism, and quadriplegia, with communication abilities limited to vertical gazing and/or blinking.1 Patients with total LIS, by contrast, cannot communicate even with their eyes, while ones with incomplete LIS have regained voluntary motion beyond upper eyelid and vertical eye movement. LIS can be temporary.10-12 The term chronic LIS has primarily been used to describe patients remaining in the LIS state for more than a year.8,13-15
In general, the categories of classic LIS and total LIS seem to be applied consistently in the literature. However, studies on incomplete LIS have used different criteria that are sometimes imprecisely described,6-8,13,14,16 including the ability to move any limb against gravity,8,15,17 the ability to walk independently,18 and the ability to walk a few steps with assistance but otherwise be completely dependent in activities of daily living (ADL).16 Some studies have excluded patients who regained the ability to make voluntary sounds,9,12,17 whereas others have included patients who regained oral communication with vowels, occasional words,16 or even sentences.10,11
Regarding consciousness, the American Congress of Rehabilitation Medicine's definition includes that basic cognitive abilities have to be evident on examination without providing further detail.19 Frequently, related literature does not state the cognitively related inclusion criteria used or how they were examined.4,7,13,14 In a recent study, several patients who failed to gesture “yes” or “no” and lacked orientation to time and place were included; a level of functioning not in accordance with the requirement of “basic cognitive abilities.”20 In those ways, LIS continues to be variously defined and usually poorly described, especially regarding incomplete LIS.
Different lesions within the brain stem have been characterized in postmortem examinations in earlier studies on LIS.21-23 Although vascular LIS typically occurs when an ischemic incident affects the ventral pons,6,10 LIS may also be caused by any extensive bilateral destruction of corticospinal or corticobulbar tracts,6,10 sometimes by injury to the mesencephalon alone (e.g., bilateral injury to cerebral peduncles).11,23,24 However, even in reports on newer larger studies, the specific location of lesions in the brain stem and any additional lesions elsewhere are not described.7,8,13,14,25
Despite high mortality rates in the acute phase of LIS,6,10,26 mortality among patients who have received rehabilitation has been low.16,27 Long-term survival was also found to be high among patients who survived the first year after LIS onset,15,17 some of whom have lived for decades.8
Because LIS is a rare condition, few studies have examined large groups of patients. Although large studies have been performed by reviewing cases in the literature6 or by recruiting patients through user organizations,4,7,13,28,29 the only population-based study regards a smaller national cohort from Sweden.16 Moreover, the mixed etiologies for LIS among patients included in such research has rendered the results less accurate for translation to populations with vascular LIS.6-8 In response, large studies are needed to investigate different etiologies separately, preferably using unselected population-based samples and following a strict, well-described method. The typical rehabilitation trajectory for patients with LIS in Norway includes inpatient high-specialized services, community-based rehabilitation after discharge, and long-term nursing and care services. In Norway, all citizens have equal access to specialized and community rehabilitation services covered by the national public insurance system along with long-term care services in institutions or at home. All hospitals in Norway are required to refer patients with LIS who need rehabilitation to the Norwegian National Unit for Rehabilitation of Locked-In Syndrome (NoRLIS). This system of mandatory referrals to a national unit enabled us to examine a relatively large national cohort of unselected patients with long-lasting LIS due to vascular etiology.
Despite the difficulties of interpreting the literature, LIS can be reasonably hypothesized to not be a homogeneous condition as earlier assumed. We investigated outcomes of long-lasting LIS and the complexity of its course. To answer our primary research question of how the functional status of patients with LIS develops over time, we investigated the functional diversity of LIS, its outcomes, and the complexity of its course in a population with long-lasting LIS. A secondary aim was to examine variations in the location of lesions within and outside the brain stem and their potential significance for outcomes.
Method
This cohort study used data from the quality register of the NoRLIS. All patients in Norway with LIS referred due to an acquired brain injury are included in the register as long as they stay in the LIS state, whether total, classic, or incomplete, for more than 6 weeks after onset and meet all the following 4 criteria (Figure 1): severe communication impairment, paralysis or paresis in all 4 extremities, complete dependence on help in participating in ADL, and normal or close-to-normal cognition (i.e., orientation to time, place, and situation at a minimum).
Figure 1. Definition of Locked-In Syndrome (LIS) Used by the Norwegian National Unit for Rehabilitation of LIS.
We further distinguished total and classic LIS from incomplete LIS (Figure 1). If patients no longer fulfilled 1 or more of the 4 criteria, they were defined as not being in the LIS state by having regained partial independence in ADL, independent walking with or without aids, speech using primarily verbal communication instead of movement or aids, or from worsening of the condition due to no longer being able to meet the cognitive criteria.
Data, including demographic data from before LIS onset and medical data from the acute phase, were collected from the records of patients during acute inpatient rehabilitation. Comorbidity was identified using ICD-10 codes, while LIS onset was the date when the patient deteriorated into the LIS state as documented on their patient record. The location of brain lesions was determined based on findings from MRI and CT. Only 5 individuals have ever entered data into the quality register of the NoRLIS in its decade of existence. We confirmed the register data by reviewing patient records for our study, including radiologic reports. The time span of the LIS state, in years, was defined for each patient who no longer fulfilled the criteria for LIS by identifying the earliest date of a written description of regained function or deterioration on their patient record.
Data from all patients in the register as of December 31, 2021, with LIS due to a cerebrovascular cause were included in our study. To our knowledge, they represent the entire population of patients with LIS in Norway (Norwegian population: 5,425,270 as of December 202130).
Follow-up Data
As part of the commitment of the NORLIS to lifelong services, annual follow-up visits at patients' homes are performed. Thus, the quality register of the NoRLIS also contains follow-up data comprising demographic data, current function, LIS status, and medical data. Information is retrieved by the unit in direct communication with the patient; in some cases, follow-up is conducted through telephone, videoconference, or letter. The most recent follow-up was performed in May 2022.
The LIS motor recovery scale by Patterson et al.6 grossly quantifies functional motor status in 5 categories, ranging from “no recovery” to “no neurologic deficit.” The scale was scored for all patients at the point of last-registered follow-up based on information in the quality register.
The modified Rankin scale (mRS) measures the degree of disability or dependence in daily activities. For each patient, the mRS score before LIS onset was based on information retrieved from their patient record. For patients who received follow-up, mRS scores are also reported from the most recent follow-up available.
Statistics
Demographic and clinical data are presented as medians with ranges or as counts and percentages. Outcome measures included mortality and functional outcome at 3 months, 6 months, 1 year, 2 years, and more than 2 years after LIS onset, presented in a Sankey diagram (Sankeymatic.com). Differences in continuous variables between groups were tested with the Mann-Whitney U test. The Pearson χ2 test or Fisher exact test for contingency tables was used to detect differences in categorical variables. Binomial proportion CIs were calculated using the Clopper-Pearson method. Cumulative survival rates were computed using the Kaplan-Meier method, and follow-up time was calculated as person-years from the onset of LIS until the date of emigration, death from any cause, or the end of follow-up on December 31, 2021. All tests were 2-sided, and a significance level of 5% was adopted. Statistical analyses were performed in IBM SPSS, version 28.0.
Standard Protocol Approvals, Registrations, and Patient Consents
All patients were included in the quality register of the NoRLIS. Consent is not required; however, they have the right to withdraw and to require that their registered data are deleted. Following Norwegian law, the register was approved by the hospital and its data protection officer. At our request, the Regional Committee for Medical Research Ethics confirmed that the publication of anonymized data from the register does not require further approval (Ref. No. 207625).
Data Availability
Data in the register are available only for the purpose of assuring quality work and will generally not be shared, for they have been collected without the patients' consent and may be identifying, given the small number of patients. In some cases, data supporting our findings may be available upon reasonable request.
Results
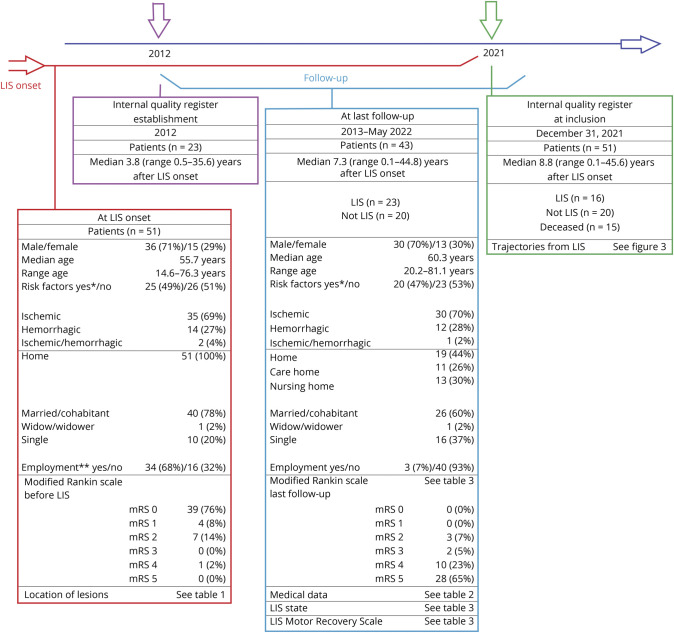
Fifty-one patients from the database met the inclusion criteria—15 female and 36 male individuals—with a median age of 55.7 years (range: 14.6–76.3) at LIS onset. Male individuals had a nonsignificantly higher median age at LIS onset (56.0 years, range: 14.6–76.3) than female individuals (47.4 years, range: 23.4–76.3, p = 0.426). Before LIS onset, all patients were living at home, and two-thirds (n = 34) were employed full-time or part-time or were students. Most of the patients (n = 39) had no symptomatic diseases or disabilities and thus an mRS score of 0 before LIS onset. Seven patients were unable to work due to previous stroke or other chronic illness (i.e., mRS 2). One patient was dependent on daily care after a previous stroke (i.e., mRS 4). Three patients had preexisting drug abuse documented in their records. A schematic overview of the data collected at LIS onset and at the final follow-up is presented in Figure 2.
Figure 2. Overview of Data Collection and Sample.
Some patients had acquired locked-in syndrome (LIS) before the quality register's establishment in 2012 (n = 23). The time point for inclusion in our study was December 31, 2021 (n = 51). Follow-up was conducted between 2012 and 2022 (n = 43). *Nineteen hypertension, 8 preexisting atherosclerotic disease (4 heart disease and/or 5 stroke sequela), 8 hypercholesterolemia, 8 diabetes, and/or 4 atrial fibrillation. Some had multiple risk factors **(n = 50, 1 unknown).
Occurring in 35 patients, ischemic stroke was the most common etiology and in 5 cases was associated with dissection and in another 3 with ongoing infection. In 14 patients, the etiology was hemorrhagic stroke, including 6 cases of aneurysms or arteriovenous malformation. In one case, brain stem hemorrhaging occurred after invasive treatment of an ischemic stroke, and in another, septic infarctions and hemorrhaging had caused LIS.
Only approximately half of the patients had preexisting risk factors of atherosclerotic disease including nonsignificantly more men (n = 20/36) than women (n = 5/15, p = 0.148). Hypertension was the most prevalent risk factor (n = 19/51). Beyond that, 14 patients had no risk factors or any preexisting chronic diagnosis.
Location of Lesions
Radiologic reports were available for 49 patients but nonexistent or irretrievable for the 2 patients who experienced LIS onset more than 30 years ago. Written MRI reports were available for most of the patients (n = 44), and their data were combined with data from CT reports retrieved from patient records.
Imaging data revealed numerous combinations of lesions within and outside the brain stem (Table 1). Isolated pontine lesion without any other lesions in the brain or brain stem was found only in 5 patients. Seven patients had lesions only in the mesencephalon and/or medulla oblongata that had caused their LIS state without injuring the pons.
Table 1.
Distribution of Lesions in Patients With Available Radiologic Reports

As much as 39 patients had 1 or more lesions outside the brain stem, most commonly in the cerebellum (n = 30) and occipital lobe (n = 8). All patients with an etiology of dissection had cerebellar lesions.
Fifteen patients had only 1 lesion located outside the brain stem, all in the cerebellum, in 5 of those cases isolated to the cerebellar peduncle/s. Six patients had primary cerebellar/posterior cranial fossa hemorrhaging, thus with brain stem lesions probably occurring as secondary lesions.
Survival and Emergence From the LIS State
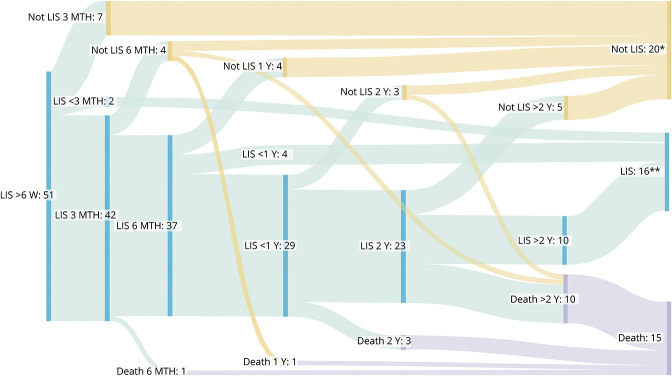
The last registered status for the 51 patients included was dead in 15 cases and emerged from LIS in 20 cases. All 16 patients who remained in LIS were in an incomplete LIS state. Nearly half of the patients (n = 23/51) had emerged from the LIS state, including 3 patients who emerged from LIS before death. The course in which patients had either emerged from an LIS state and/or died is presented in Figure 3. No patients deteriorated from the LIS state by no longer being oriented. Most of the patients who had emerged from the LIS state (n = 18/23) did so between 6 weeks and 2 years after LIS onset. Few patients (n = 2/15) had died from 6 weeks to 1 year after LIS onset.
Figure 3. Trajectories of Long-lasting Locked-in Syndrome (LIS).
LIS state trajectories for 5 periods after LIS onset (n = 51): >6 weeks (6 W) to within 3 months (3 MTH), 3–6 months (6 MTH), 6 months–1 year (1 Y), 1–2 years (2 Y), and >2 years to inclusion date (>2 Y). Sixteen patients remained in an LIS state. Most of the 15 deceased patients died >2 years after LIS onset (n = 10, range: 2.2–30.6 years). Some patients emerged from LIS later than 2 years after onset (n = 5, range: 2.9–9.7 years). Twenty patients had emerged from the LIS state and were alive. *One patient emerged from the LIS state and moved abroad. Registered in the figure as emerged; however, it is unknown whether the patient is still alive. **Two patients had LIS for less than 3 months, 4 patients had LIS for 6–12 months. The remaining 10 patients had remained in an incomplete LIS state for median 8.5 years (range 5.8–45.6). Two patients had not received follow-up after 2019, but were registered as LIS because they had remained in a stable LIS state for >10 years and were known to be alive.
In total, 15 patients had died, at a median of 3.5 years (range: 0.3–30.6) after LIS onset and at a median of 65.4 years of age (range: 52.2–83.7). The patients who had died were older at LIS onset than the survivors (p = 0.019). Three patients had emerged from an LIS state before death, whereas 12 had remained in LIS—1 with classic LIS and 11 with incomplete LIS—until death. No significant difference emerged in the occurrence of lesions outside the brain stem between patients who had died and survivors.
Survival rates for patients alive at least a year after LIS onset were calculated for 42 patients; as for the others, 2 had died in less than a year, 6 had had LIS for less than a year, and 1 had moved abroad. The cumulative 3-year, 5-year, and 10-year survival rates were 87% (95% CI 72.4–94.6), 79% (95% CI 62.8–89.1), and 73% (95% CI 55.8–84.7), respectively.
Outcomes at Follow-up
Follow-up data were available for 43 patients; as for the others, 4 patients had died before the first follow-up, 1 had moved abroad, and 3 had not yet received follow-up. Follow-up information was retrieved during home visits for 22 patients, during inpatient stays for 3 patients, through videoconferencing for 11 patients, and by letter for 7 patients. Nine patients provided the information independently, whereas 34 patients provided it together with healthcare personnel and/or a family member. Latest follow-up was performed at a median of 7.3 years after LIS onset, ranging from 0.1 to 44.8 years (including 11 patients who later died). Medical data and outcomes are summarized in Tables 2 and 3, respectively.
Table 2.
Medical Data at Final Follow-up (n = 43)

Table 3.
Improvement in Motor Function in Relation to Locked-in Syndrome (LIS) State and Level of Disability at Final Follow-up (n = 43)
Of the 43 patients with follow-up data, 23 were in an LIS state at the most recent follow-up, whereas 20 had emerged from LIS. Only 1 patient remained in a classic LIS state, whereas the rest exhibited incomplete LIS.
An analysis of the 41 patients with descriptions of their location of injury available showed that lesions outside the brain stem were less prevalent (n = 14/20, that is, 70% (95% CI 46–88)) among patients who emerged from the LIS state than among patients who did not (n = 20/21, that is, 95% (95% CI 76–100), p = 0.045). More patients who remained in an LIS state had multiple vs single lesions outside the brain stem (n = 15/20, that is, 75% (95% CI 51–91)) than patients who emerged from the LIS state (n = 5/14, that is, 36% (95% CI 13–65), p = 0.035).
As for motor function at the latest follow-up, most of the patients (n = 27) had achieved “minimum recovery” according to Patterson,6 being entirely dependent in their care, equivalent to an mRS score of 5, which includes 5 patients who regained the ability to speak and thus were no longer in the LIS state. Only 3 patients had achieved “full recovery” at the latest follow-up being independent, but with neurologic deficits and not having returned to work, scoring mRS 2 (see Table 3).
Seven patients had no verbal response at the latest follow-up, whereas 12 were able to make sounds. Several patients could occasionally or consistently pronounce words and/or sentences; 16 exhibited dysarthric speech and 8 functional speech without dysarthria.
Discussion
Our study of an unselected, population-based sample of patients with vascular LIS offers important insights into the heterogeneity within both the functional entity of the LIS state and trajectories of long-lasting LIS. Although having had LIS for at least 6 weeks, nearly half of the patients eventually emerged from the LIS state due to regaining at least some motor or communication function; however, most of them remained severely dependent on help. The few patients who achieved full motor recovery had all emerged from the LIS state fairly quickly, namely between 6 weeks and 6 months. Only 10% of the patients had an isolated pontine lesion, and though 80% of patients had various lesions outside the brain stem, they all met the criteria of LIS according to our function-based definition. However, such lesions were significantly less prevalent among patients who emerged from the LIS state than ones who did not.
In line with the past research, we found that LIS occurred more frequently among men than women,6-8,27 mainly being due to ischemic stroke and with hypertension being the most common preexisting diagnosis.6,26 The age of the current sample (median 55.7 years) is substantially lower than of the Norwegian stroke population as a whole (76 years31). Only relatively younger patients might survive the first 6 weeks due to the natural course of the syndrome or treatment limitation. Another explanation could be limited access to the national rehabilitation service for relatively older patients. The service has no age limit, and all referred patients are served regardless of age, but some older patients might not have been referred.
Previous studies6-8,27 found a lower mean or median age (33.6–52.0 years) than that observed in our study (median 55.7 years). The difference might be explained by those studies including also patients with LIS due to traumatic brain injury and some including other nonvascular etiologies.6-8 In a review by Patterson,6 patients with LIS due to stroke (i.e., 105 of 139 cases) had a mean age of 56 years, similar to that observed in our result. Meanwhile, among patients with exclusively ischemic etiology in another study,26 the mean age was somewhat higher (i.e., 62 years) than that observed in our study.
A previous follow-up study8 found a 10-year survival rate of 83% in patients with LIS who survived the first year after LIS onset, compared with this result of 73%. The difference may be due to the previous study's younger sample of patients (mean age: 33.6 years) with mixed etiology, which aligns with previous results suggesting better prognosis among patients with LIS with nonvascular etiology.6 When the NoRLIS register was established in 2012, some patients who had already had LIS for many years were included, whereas patients who had both acquired LIS and died before 2012 were not. Because that circumstance may have affected the long-term survival rate that we found, we consider the 3-year survival rate of 87% and the 5-year rate of 79% as being the most reliable.
Using our function-based definition of LIS helps to identify patients with LIS who do not necessarily have classic LIS due to a ventral pons lesion and provides a relatively clear description of when a patient should be identified as being in an incomplete LIS state vs emerged from LIS.
The term chronic LIS has previously been used to describe patients who remain in the LIS state for more than a year. Our results indicate that most patients emerge from the LIS state within the first 2 years after LIS onset but can also emerge much later. One patient emerged from the LIS state nearly 10 years after onset due to regaining oral communication, thereby indicating that late emergence is possible. Although emergence from the LIS state more than a year after onset is rare, it has been reported.15 It should be noted, however, that pinpointing the exact date of emergence is difficult, especially in later stages of LIS.
There is considerable variation in how patients can emerge from the LIS state. A patient may regain functional speech and the ability to walk a few steps with assistance but remain unable to participate in ADL due to ataxia and/or a lack of distal motor control (e.g., due to severe cerebellar damage). Another patient may be wheelchair bound, communicate with eye movements, but able to partly participate in ADL due to having regained unilateral hand function that permits independence in some ADL tasks (e.g., using an electric toothbrush). Neither patient is considered to have LIS by definition, however; though their outcomes differ, they share a higher level of functioning and independence. Those examples showcase the complexity of the trajectories of LIS concerning outcomes in motor function, dependence, and verbal communication.
Several studies have revealed various degrees of motor improvement in patients with LIS (e.g.,8,18,27,32). One study6 observed larger motor improvement than that observed in this study, with some patients making complete recovery classified as “no neurologic deficit” (3.5%) and one-third making “full recovery” (31%). Whereas that study included patients also from the acute phase, our study included only patients who remained in an LIS state for at least 6 weeks. Our results correspond better with those of another study27 that included patients with LIS who received rehabilitation and described no patients as achieving “no neurologic deficit” and only 7% achieving “full recovery.” By contrast, only 1 patient in our study was designated as achieving “no recovery” and remained in classic LIS, which is a considerably lower rate than that found by both previously mentioned studies reporting 31% and 21%, respectively.6,27 Based on our functional criteria, even small voluntary movements (e.g., horizontal eye movement and facial, jaw, head, or neck movements) were registered as improvements and excluded patients from the “no recovery” category. Repeated clinical examinations are probably necessary to detect such minor improvements that patients themselves or their proxies might not report, which can lead to miscategorizations as “no motor recovery.” Small improvements can be functionally important because they may enable patients to use, or more efficiently use, technological aids for communication, environmental control, or achieve independent wheelchair mobility. By contrast, some patients may regain more major voluntary movements without increasing their level of independence. In our study, most of the patients (i.e., 88%) had a persistently high level of dependence, given mRS scores of 4 or 5.
Based on our clinical experience, most patients with LIS initially communicate through eye movements and/or blinking, while some communicate using other voluntary motor responses when in an incomplete LIS state from onset. However, information about the first form of communication in the acute phase is not provided in the NoRLIS register. Our results nevertheless show that most of the patients (84%) regained some verbal response function (i.e., sound or speech) and that improvement of verbal speech occurred even in patients making only minimum progress in motor function.
All patients in our study were conscious and could communicate that they were oriented to time, place, and situation. It is well known that cognitive disabilities can occur in LIS, in both patients with isolated pontine lesions and patients with additional brain lesions.3,33 Therefore, we did not exclude patients with various lesions outside the brain stem but rather examined the locations of any additional lesions.
Lesions outside the brain stem in patients with LIS do not constitute a new finding.3,21,22,26 One study reported lesions outside the pons in 50% of patients with LIS,26 which is a lower rate than in our study. However, the same study26 also revealed that those lesions most commonly occurred in the cerebellum and occipital lobe, which, similar to the brain stem, receive blood supply from the posterior cerebral circulation. In our study, 14% of patients had primary cerebellar or posterior cranial fossa hemorrhaging, presumably with secondary injury to the brain stem. That mechanism has been referred to as “pseudo-LIS.”9 Our findings show that LIS also can be mesencephalic (n = 3) or due to isolated injury to the medulla oblongata (n = 3) without any other lesions in the brain stem.
In patients who had emerged from the LIS state at follow-up, the occurrence of lesions outside the brain stem, especially multiple lesions, was significantly lower than among the other patients, which implies that the factor may be prognostic for emergence from LIS. Notably, that group also included older and incidental lesions. Lesions outside the brain stem thus include lesions having occurred before LIS onset and extrapontine lesions co-occurring with the acute event resulting in LIS, thereby making patients with such lesions a heterogeneous group. However, those differences were not shown to be associated with age. When analyzing changes in oral communication (i.e., “yes” or “no”), motor function (i.e., with the LIS Motor Recovery Scale), and independence (i.e., with the mRS) separately, we observed no significant differences between patients with or without lesions outside the brain stem.
Our findings, though based on a substantial number of patients compared with that observed in past studies, are limited by our small sample size. Moreover, the present results are based on register data, which might be considered of less quality than data collected for specific research purposes. However, the register data that we used were collected in only 1 center by only a few specially trained employees, all specializing in LIS. Last, MRI and CT images were not directly reviewed for our study; instead, data regarding the location of lesions stemmed from radiology reports primarily elaborated for clinical purposes.
Overall, our findings indicate that patients affected by vascular incidents to the brain stem might have various lesions outside the brain stem but, nevertheless, clinically present as having LIS with preserved consciousness and orientation. Using more distinct functional criteria to define LIS can help to differentiate patients who have emerged from the LIS state from ones still in an incomplete LIS state. Overall, although most patients remained severely disabled and dependent on others over time, small improvements in function (e.g., regarding communication) can be essential to an individual patient's quality of life.
Even patients who remain in the LIS state for more than 6 weeks were found to emerge from it, most commonly within 2 years from onset. In some cases, late emergence from the LIS state occurred even after several years. Those findings do not align with the term chronic LIS, when referring to patients who have remained in the LIS state for a year only. In conclusion, long-lasting LIS should be regarded as a chronic condition with various trajectories and outcomes. Future research should explore how the functional diversity and different trajectories revealed by our study relate to patients' everyday lives, participation, and quality of life. To allow better prognostic considerations, larger studies that include patients from LIS onset are needed.
Acknowledgment
The authors thank all the patients for their valuable contributions to the study. All authors thank Kjersti Falch and Jane Svartskuren at Sunnaas Rehabilitation Hospital for collecting register data. The authors thank the stroke user organization NFS (Norsk forening for Slagrammede) for collaboration and the user representative of NoRLIS, Wenche Løseth.
Glossary
- ADL
activities of daily living
- LIS
locked-in syndrome
- mRS
modified Rankin scale
- NoRLIS
Norwegian National Unit for Rehabilitation of Locked-In Syndrome
Appendix. Authors

Footnotes
CME Course: NPub.org/cmelist
Study Funding
The project is funded by Foundation Dam (grant number 2021/FOS371669).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Plum F, Posner JB. The Diagnosis of Stupor and Coma. Contemporary Neurology Series, 1. F. A. Davis Co; 1966. [PubMed] [Google Scholar]
- 2.Allain P, Joseph PA, Isambert JL, Gall DL, Emile J. Cognitive functions in chronic locked-in syndrome: a report of two cases. Cortex. 1998;34(4):629-634. doi: 10.1016/S0010-9452(08)70520-3 [DOI] [PubMed] [Google Scholar]
- 3.Schnakers C, Majerus S, Goldman S, et al. Cognitive function in the locked-in syndrome. J Neurol. 2008;255(3):323-330. doi: 10.1007/s00415-008-0544-0 [DOI] [PubMed] [Google Scholar]
- 4.Lugo ZR, Bruno MA, Gosseries O, et al. Beyond the gaze: communicating in chronic locked-in syndrome. Brain Inj. 2015;29(9):1056-1061. doi: 10.3109/02699052.2015.1004750 [DOI] [PubMed] [Google Scholar]
- 5.Farr E, Altonji K, Harvey RL. Locked-in syndrome: practical rehabilitation management. PM R. 2021;13(12):1418-1428. doi: 10.1002/pmrj.12555 [DOI] [PubMed] [Google Scholar]
- 6.Patterson JR, Grabois M. Locked-in syndrome: a review of 139 cases. Stroke. 1986;17(4):758-764. doi: 10.1161/01.STR.17.4.758 [DOI] [PubMed] [Google Scholar]
- 7.Rousseau MC, Baumstarck K, Alessandrini M, Blandin V, Billette de Villemeur T, Auquier P. Quality of life in patients with locked-in syndrome: evolution over a 6-year period. Orphanet J Rare Dis. 2015;10(1):88. doi: 10.1186/s13023-015-0304-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doble JE, Haig AJ, Anderson C, Katz R. Impairment, activity, participation, life satisfaction, and survival in persons with locked-in syndrome for over a decade: follow-up on a previously reported cohort. J Head Trauma Rehabil. 2003;18(5):435-444. doi: 10.1097/00001199-200309000-00005 [DOI] [PubMed] [Google Scholar]
- 9.León-Carrión J, van Eeckhout P, Domínguez-Morales Mdel R. The locked-in syndrome: a syndrome looking for a therapy. Brain Inj. 2002;16(7):555-569. doi: 10.1080/02699050110119466 [DOI] [PubMed] [Google Scholar]
- 10.Bauer G, Gerstenbrand F, Rumpl E. Varieties of the locked-in syndrome. J Neurol. 1979;221(2):77-91. doi: 10.1007/BF00313105 [DOI] [PubMed] [Google Scholar]
- 11.Fujimoto Y, Ohnishi Y, Wakayama A, Yoshimine T. Transient total mesencephalic locked-in syndrome after bilateral ptosis due to basilar artery thrombosis. J Stroke Cerebrovasc Dis. 2012;21(8):909.e7-909.e8. doi: 10.1016/j.jstrokecerebrovasdis.2011.10.016 [DOI] [PubMed] [Google Scholar]
- 12.Khurana RK, Genut AA, Yannakakis GD. Locked-in syndrome with recovery. Ann Neurol. 1980;8(4):439-441. doi: 10.1002/ana.410080418 [DOI] [PubMed] [Google Scholar]
- 13.Bruno MA, Bernheim JL, Ledoux D, Pellas F, Demertzi A, Laureys S. A survey on self-assessed well-being in a cohort of chronic locked-in syndrome patients: happy majority, miserable minority. BMJ Open. 2011;1(1):e000039. doi: 10.1136/bmjopen-2010-000039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snoeys L, Vanhoof G, Manders E. Living with locked-in syndrome: an explorative study on health care situation, communication and quality of life. Disabil Rehabil. 2013;35(9):713-718. doi: 10.3109/09638288.2012.705950 [DOI] [PubMed] [Google Scholar]
- 15.Katz RT, Haig AJ, Clark BB, DiPaola RJ. Long-term survival, prognosis, and life-care planning for 29 patients with chronic locked-in syndrome. Arch Phys Med Rehabil. 1992;73(5):403-408. [PubMed] [Google Scholar]
- 16.Svernling K, Törnbom M, Nordin Å, Sunnerhagen KS. Locked-in syndrome in Sweden, an explorative study of persons who underwent rehabilitation: a cohort study. BMJ Open. 2019;9(4):e023185. doi: 10.1136/bmjopen-2018-023185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haig AJ, Katz RT, Vinod S. Mortality and complications of the locked-in syndrome. Arch Phys Med Rehabil. 1987;68(1):24-27. [PubMed] [Google Scholar]
- 18.Richard I, Pereon Y, Guiheneu P, Nogues B, Perrouin-Verbe B, Mathe JF. Persistence of distal motor control in the locked in syndrome. Review of 11 patients. Paraplegia. 1995;33(11):640-646. doi: 10.1038/sc.1995.135 [DOI] [PubMed] [Google Scholar]
- 19.American Congress of Rehabilitation M. Recommendations for use of uniform nomenclature pertinent to patients with severe alterations in consciousness. Arch Phys Med Rehabil. 1995;76(2):205-209. doi: 10.1016/S0003-9993(95)80031-X [DOI] [PubMed] [Google Scholar]
- 20.Kumral E, Dorukoglu M, Uzunoglu C, Cetin FE. The clinical and cognitive spectrum of locked-in syndrome: 1-year follow-up of 100 patients. Acta Neurol Belg. 2022;122(1):113-121. doi: 10.1007/s13760-021-01675-5 [DOI] [PubMed] [Google Scholar]
- 21.Reznik M. Neuropathology in seven cases of locked-in syndrome. J Neurol Sci. 1983;60(1):67-78. doi: 10.1016/0022-510X(83)90127-2 [DOI] [PubMed] [Google Scholar]
- 22.Nordgren RE, Markesbery WR, Fukuda K, Reeves AG. Seven cases of cerebromedullospinal disconnection: the "locked-in" syndrome. Neurology 1971;21(11):1140-1148. doi: 10.1212/WNL.21.11.1140 [DOI] [PubMed] [Google Scholar]
- 23.Dehaene I, Dom R. A mesencephalic locked-in syndrome. J Neurol. 1982;227(4):255-259. doi: 10.1007/BF00313393 [DOI] [PubMed] [Google Scholar]
- 24.Chia LG. Locked-in syndrome with bilateral ventral midbrain infarcts. Neurology. 1991;41(3):445-446. doi: 10.1212/wnl.41.3.445 [DOI] [PubMed] [Google Scholar]
- 25.Kohnen RF, Lavrijsen JC, Bor JH, Koopmans RT. The prevalence and characteristics of patients with classic locked-in syndrome in Dutch nursing homes. J Neurol. 2013;260(6):1527-1534. doi: 10.1007/s00415-012-6821-y [DOI] [PubMed] [Google Scholar]
- 26.Nikić PM, Jovanović D, Paspalj D, Georgievski-Brkić B, Savić M. Clinical characteristics and outcome in the acute phase of ischemic locked-in syndrome: case series of twenty patients with ischemic LIS. Eur Neurol. 2013;69(4):207-212. doi: 10.1159/000345272 [DOI] [PubMed] [Google Scholar]
- 27.Casanova E, Lazzari RE, Lotta S, Mazzucchi A. Locked-in syndrome: improvement in the prognosis after an early intensive multidisciplinary rehabilitation. Arch Phys Med Rehabil. 2003;84(6):862-867. doi: 10.1016/S0003-9993(03)00008-X [DOI] [PubMed] [Google Scholar]
- 28.León-Carrión J, Eeckhout Pv, Domínguez-Morales MdR, Pérez-Santamaría FJ. Survey: the locked-in syndrome: a syndrome looking for a therapy. Brain Inj. 2002;16(7):571-582. doi: 10.1080/02699050110119781 [DOI] [PubMed] [Google Scholar]
- 29.Laureys S, Pellas F, Van Eeckhout P, et al. The locked-in syndrome: what is it like to be conscious but paralyzed and voiceless? Prog Brain Res. 2005;150:495-511. doi: 10.1016/S0079-6123(05)50034-7 [DOI] [PubMed] [Google Scholar]
- 30.Statistics Norway. Population. Updated May 16, 2023. Accessed March 30, 2023. ssb.no/befolkning/folketall/statistikk/befolkning
- 31.Fjærtoft H, Skogseth-Stephani R, Indredavik B, et al. Norsk Hjerneslagregister: Årsrapport for 2021; 2022. stolav.no/Documents/%C3%85rsrapport%20Norsk%20hjerneslagregister%202021.pdf. Accessed March 30, 2023. [Google Scholar]
- 32.Høyer E, Normann B, Sørsdal R, Strand LI. Rehabilitation including treadmill therapy for patients with incomplete locked-in syndrome after stroke; a case series study of motor recovery. Brain Inj. 2010;24(1):34-45. doi: 10.3109/02699050903471805 [DOI] [PubMed] [Google Scholar]
- 33.Rousseaux M, Castelnot E, Rigaux P, Kozlowski O, Danzé F. Evidence of persisting cognitive impairment in a case series of patients with locked-in syndrome. J Neurol Neurosurg Psychiatry. 2009;80(2):165-170. doi: 10.1136/jnnp.2007.128686 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data in the register are available only for the purpose of assuring quality work and will generally not be shared, for they have been collected without the patients' consent and may be identifying, given the small number of patients. In some cases, data supporting our findings may be available upon reasonable request.