Abstract
Usher syndrome is the most common cause of deafness-blindness in the world. Usher syndrome type 1B (USH1B) is associated with mutations in MYO7A. Patients with USH1B experience deafness, blindness, and vestibular dysfunction. In this study, we applied adeno-associated virus (AAV)-mediated gene therapy to the shaker-1 (Myo7a4626SB/4626SB) mouse, a model of USH1B. The shaker-1 mouse has a nonsense mutation in Myo7a, is profoundly deaf throughout life, and has significant vestibular dysfunction. Because of the ∼6.7-kb size of the MYO7A cDNA, a dual-AAV approach was used for gene delivery, which involves splitting human MYO7A cDNA into 5′ and 3′ halves and cloning them into two separate AAV8(Y733F) vectors. When MYO7A cDNA was delivered to shaker-1 inner ears using the dual-AAV approach, cochlear hair cell survival was improved. However, stereocilium organization and auditory function were not improved. In contrast, in the vestibular system, dual-AAV-mediated MYO7A delivery significantly rescued hair cell stereocilium morphology and improved vestibular function, as reflected in a reduction of circling behavior and improved vestibular sensory-evoked potential (VsEP) thresholds. Our data indicate that dual-AAV-mediated MYO7A expression improves vestibular function in shaker-1 mice and supports further development of this approach for the treatment of disabling dizziness from vestibular dysfunction in USH1B patients.
Keywords: gene therapy, hearing loss, Usher syndrome, USH1B, MYO7A, AAV, dual-AAV, vestibular dysfunction
Graphical abstract
Mutations of MYO7A encoding myosin 7A are associated with Usher syndrome 1B (USH1B), characterized by vision, hearing, and vestibular deficits. In this study, we showed that delivery of dual-AAV vectors containing human MYO7A cDNA to the inner ear led to restoration of vestibular function in a mouse model of USH1B.
Introduction
Usher syndrome (USH) is the most common cause of inherited deafness-blindness.1 Its prevalence ranges from 3.2–6.5 per 100,000 people depending on the population.2,3 USH is inherited in an autosomal-recessive pattern and is classified into three major clinical subtypes (types 1–3), with type 1 patients having the most clinically severe phenotype.4 USH type 1B (USH1B) is the most common form of USH type 15 and is associated with variants in MYO7A (MIM: 276900).4,6 Patients with USH1B are born with profound hearing loss and vestibular dysfunction and develop progressive loss of vision because of retinitis pigmentosa during the first decade of life.7 The MYO7A gene encodes the unconventional MYO7A,6,8 and it is expressed in mechanosensory hair cells in the inner ear, photoreceptors, and retinal pigment epithelium cells.8,9 Recent studies have shown that adeno-associated virus (AAV)-mediated gene replacement therapy can be used to improve the auditory and vestibular functions in several mouse models of USH.10 However, the challenge of applying AAV-mediated gene replacement therapy to mouse models of USH1B is the fact that the cDNA of MYO7A is ∼6.7 kb in length, which exceeds the ∼4.7-kb carrying capacity of AAV.11,12
One way of overcoming this challenge is to use a dual-AAV vector-based approach, which divides a large cDNA into two halves and utilizes two separate AAV expression cassettes to deliver these cDNA fragments.13,14 The first vector contains the promoter and 5′ half of the cDNA, whereas the second vector contains the 3′ half of the cDNA along with the polyadenylation signal. When both capsids co-infect the same cell, these 5′ and 3′ cDNA halves recombine to form the full-length coding sequence and generate full-length protein. There are two major dual vector strategies that promote recombination and generation of full-length protein: overlapping and hybrid.13,14 “Overlap” dual vectors contain cDNA fragments with complementary genetic sequences that drive homologous recombination. “Hybrid” dual vectors rely on a recombinogenic sequence from the human placental ALPL gene (which encodes alkaline phosphatase [AP]) to promote recombination15 and splice donor and acceptor sites for excision of the intervening sequence from the resulting transcript. In a study by Dyka et al.,13 these dual-AAV strategies were compared for their ability to drive full-length MYO7A expression in vitro and in vivo. They found that the hybrid strategy resulted in the highest level of full-length MYO7A expression. They also showed that subretinal injection of the hybrid dual-AAV vectors carrying human MYO7A cDNA was capable of producing human MYO7A expression in the C57BL/6J mouse retina.13 Similarly, in another study by Trapani et al.,14 these different dual-AAV strategies were tested, and it was found that the hybrid strategy was highly effective at delivering human MYO7A cDNA and promoting expression of full-length MYO7A in vitro. In addition, sub-retinal injection of the hybrid dual-AAV vectors carrying human MYO7A cDNA into a Myo7a mutant mouse (shaker-1, Myo7a4626SB/4626SB) resulted in expression of full-length human MYO7A and improvement of melanosome and rhodopsin localization in the mouse retina.14
In this study, we asked whether hybrid dual-AAV vectors could mediate sufficient levels of full-length MYO7A expression in the inner ear of the shaker-1 mouse model for USH1B. The deaf shaker-1 mouse has a nonsense mutation in Myo7a and has significant vestibular dysfunction throughout life.6,16 We injected hybrid dual-AAV vectors carrying the human MYO7A cDNA into neonatal shaker-1 mice through the posterior semicircular canal (PSC) and evaluated whether gene replacement therapy could improve auditory and vestibular function in these mutant mice.
Results
Dual-AAV8(Y733F) vectors restore MYO7A expression in cochlear hair cells
The shaker-1 (Myo7a4626SB/4626SB) mutant mouse has a nonsense mutation in Myo7a and is considered a null mutant.17,18 To restore MYO7A expression in shaker-1 mice, we utilized the hybrid dual-AAV vectors packaged in AAV8(Y733F).13 The 5′ vector contains the ubiquitous CMV/chicken β-actin (smCBA) promoter, the 5′ portion of human MYO7A cDNA (2,904 bp), a splice donor, and a recombinogenic sequence derived from a coding sequence for AP (287 bp, labeled “AP”). The 3′ vector contains a complementary AP coding sequence, a splice acceptor, the 3′ portion of human MYO7A cDNA (3,627 bp), a hemagglutinin (HA) tag, and a polyadenylation signal (Figure S1A). We call this set of dual-AAV vectors “dual-AAV8(Y733F)-MYO7A.” The human MYO7A cDNA was used in this study instead of the mouse Myo7a cDNA because it is the human MYO7A cDNA that will be used for clinical translation in USH1B patients in the future. In addition, the human MYO7A cDNA has been successfully delivered into the mouse retina using the dual-AAV approach.13,14 Subretinal injection of these dual-AAV8(Y733F)-MYO7A vectors in Myo7a−/− mice results in full-length MYO7A protein expression.19 Infection of these vectors in COS-7 cells resulted in the detection of MYO7A expression with anti-MYO7A or anti-HA-tag antibodies directed against the N-terminal, middle, and C-terminal (HA) portions of the protein sequence (Figure S1B). In addition, the antibody targeting the middle portion of MYO7A, which was used for the rest of the study, only detected MYO7A expression when the 5′ and 3′ vectors were used to infect COS-7 cells (Figure S1C). These results confirm that dual-AAV8(Y733F)-MYO7A vectors can produce full-length MYO7A expression in infected cells.
Using the PSC approach, we microinjected dual-AAV8(Y733F)-MYO7A (0.5 μL of the 5′ vector and 0.5 μL of the 3′ vector) into the inner ears of live neonatal (post-natal day 0 [P0]–P4) shaker-1 mouse pups. The cochleae of these animals were then dissected at P30–P37 and examined using immunofluorescence and confocal microscopy. In untreated wild-type and heterozygous shaker-1 mice, we found that MYO7A was highly expressed in the inner hair cells (IHCs) and outer hair cells (OHCs) of the cochlea. In contrast, we were unable to detect MYO7A expression in the cochlea of shaker-1 mutant mice (Figure 1A). When dual-AAV8(Y733F)-MYO7A vectors were injected into shaker-1 inner ears, MYO7A expression was restored in the transduced IHCs and OHCs (Figure 1A). The IHC transduction rate was 77% ± 12% at the apex, 77% ± 11% at the middle turn, and 47% ± 12% at the base of the cochlea (Figure 1B; n = 6 mice). The OHC transduction rate was 82% ± 16% at the apex, 45% ± 18% at the middle turn, and 22% ± 9.5% at the base of the cochlea (Figure 1C; n = 6 mice). MYO7A expression in the IHCs and OHCs of the injected shaker-1 mice also co-localized with HA expression, indicating that the MYO7A expression seen in the injected shaker-1 mice was a result of the dual-AAV8(Y733F)-MYO7A treatment (Figure S2). Our results indicate that dual-AAV8(Y733F)-MYO7A treatment restored MYO7A protein expression in the transduced shaker-1 IHCs and OHCs.
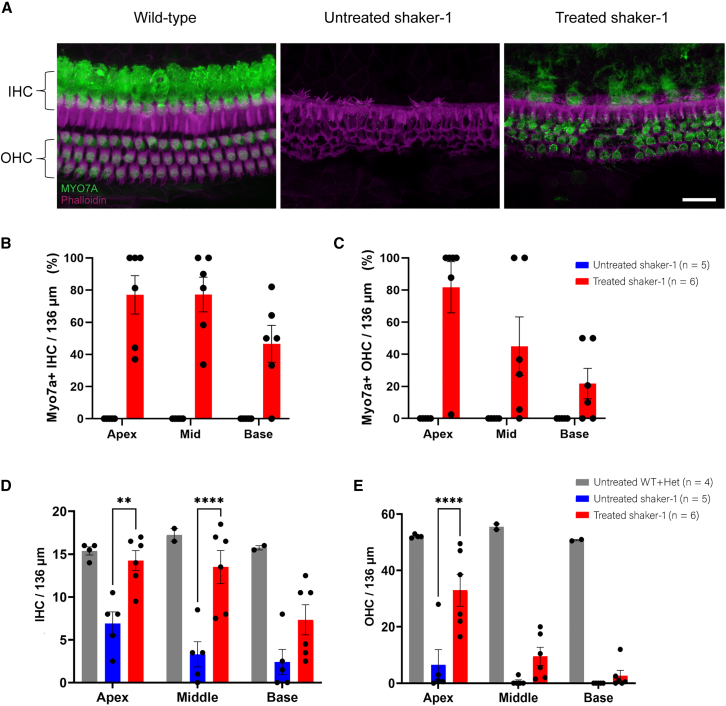
Figure 1.
Dual-AAV8(Y733F)-MYO7A vectors restore MYO7A expression and prolong the survival of shaker-1 cochlear hair cells
(A) Representative confocal images of inner hair cells (IHCs) and outer hair cells (OHCs) of the cochlear middle turn. In an untreated wild-type mouse, MYO7A expression (green) is found in the IHCs and OHCs. The shaker-1 mouse does not express MYO7A. Shaker-1 mice that were treated with dual-AAV8(Y733F)-MYO7A vectors exhibit restoration of MYO7A in transduced IHCs and OHCs. Images were taken at P30–P37. The scale bar represents 20 μm. (B and C) Quantification of IHC (B) and OHC (C) transduction rates in shaker-1 mice that were treated with dual-AAV8(Y733F)-MYO7A vectors. (D and E) Quantification of IHC (D) and OHC (E) survival in shaker-1 mice that were treated with dual-AAV8(Y733F)-MYO7A vectors. Shaker-1 mice that were treated with dual-AAV8(Y733F)-MYO7A vectors showed significant improvement in hair cell survival compared with untreated shaker-1 mice. n = the number of animals used. Error bars represent standard errors. ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
Dual-AAV8(Y733F)-MYO7A vectors improve IHC and OHC survival in the shaker-1 cochlea
IHCs and OHCs in the shaker-1 mouse undergo significant degeneration (Figure 1A). Therefore, we wanted to examine whether dual-AAV vector treatment could improve IHC and OHC survival in the treated shaker-1 mouse. At ∼P30, the average IHC count for the wild-type mouse was 15 ± 0.47 cells/136 μm at the apex, 17 ± 0.75 cells/136 μm at the middle turn, and 16 ± 0.25 cells/136 μm at the base of the cochlea (Figure 1D; n = 4 mice). The average IHC count for the untreated shaker-1 mouse was 6.9 ± 1.4 cells/136 μm at the apex, 3.3 ± 1.5 cells/136 μm at the middle turn, and 2.4 ± 1.5 cells/136 μm at the base of the cochlea (Figure 1D; n = 5 mice). When dual-AAV8(Y733F)-MYO7A vectors were injected into the shaker-1 mouse, we noticed a significantly higher number of surviving IHCs in treated shaker-1 mice compared with untreated homozygous mutants (p < 0.0001, two-way ANOVA). For shaker-1 mice that were treated with dual-AAV8(Y733F)-MYO7A, the average IHC count was 14 ± 1.2 cells/136 μm at the apex (p = 0.003 compared with the untreated mutants), 14 ± 1.9 cells/136 μm at the middle turn (p < 0.0001 compared with the untreated mutants), and 7.3 ± 1.8 cells/136 μm at the base of the cochlea (p = 0.06 compared with the untreated mutants), respectively (Figure 1D; n = 6 mice, ANOVA with Tukey’s multiple-comparisons test). The magnitude of preservation was consistent with the relative levels of transduction in these different regions by our dual vectors (i.e., apex > middle turn > base).
Similar results were observed for OHC survival. The average OHC count in wild-type mice was 52 ± 0.32 cells/136 μm at the apex, 56 ± 1.0 cells/136 μm at the middle turn, and 51 ± 0.25 cells/136 μm at the base of the cochlea (Figure 1E; n = 4 mice). The average OHC count for the untreated shaker-1 mice was 6.5 ± 5.4 cells/136 μm at the apex, 0.60 ± 0.60 cells/136 μm at the middle turn, and 0.0 ± 0.0 cells/136 μm at the base of the cochlea (Figure 1E; n = 5 mice). Injection with dual-AAV8(Y733F)-MYO7A vectors resulted in significant preservation of OHCs in the shaker-1 mice (p < 0.0001, two-way ANOVA). For shaker-1 mice that were treated with dual-AAV8(Y733F)-MYO7A, the average OHC count was 33 ± 5.7 cells/136 μm at the apex (p < 0.0001 compared with the untreated mutants), 9.5 ± 3.2 cells/136 μm at the middle turn (p = 0.2 compared with the untreated mutants), and 2.7 ± 1.9 cells/136 μm at the base of the cochlea (p = 0.8 compared with the untreated mutants), respectively (Figure 1E; n = 6 mice, ANOVA with Tukey’s multiple-comparisons test). As seen for IHCs, the magnitude of structural preservation observed for OHCs was consistent with the relative levels of transduction in these different regions by our dual vectors (i.e., apex > middle turn > base). Our results indicate that dual-AAV8(Y733F)-MYO7A treatment improved IHC and OHC survival in shaker-1 mice.
Dual-AAV8(Y733F)-MYO7A vectors did not improve cochlear stereocilium organization or auditory function in shaker-1 mice
Stereocilium bundles on the apical surface of hair cells play a critical role in mechanotransduction of auditory information. In wild-type mice, stereocilium bundles are organized in a cohesive staircase-like pattern of stereocilium rows (Figure 2A). In shaker-1 mice, IHCs and OHC stereocilium bundles are highly disorganized from birth, because of the loss of MYO7A that is required for the organization and stabilization of several stereocilium sub-compartmental links (Figure 2A).20,21 We examined the effect of dual-AAV8(Y733F)-MYO7A vectors on the stereocilium bundle organization in the cochlear hair cells of treated shaker-1 mice and observed that stereocilium bundles remained highly disorganized in IHCs and OHCs, similar to those in untreated shaker-1 homozygous mutant mice (Figure 2A).
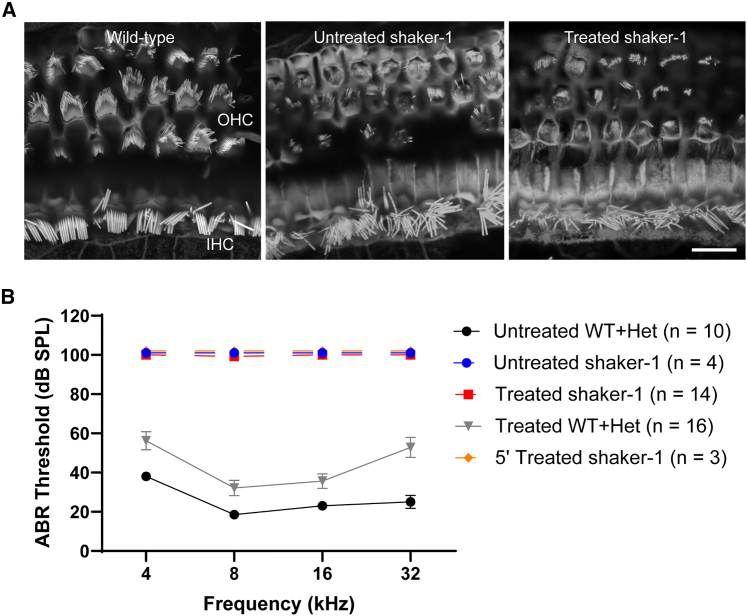
Figure 2.
Dual-AAV8(Y733F)-MYO7A vectors did not improve stereocilium organization of cochlear hair cells or auditory function in shaker-1 mice
(A) Representative confocal images showing stereocilium organization in IHCs and OHCs. Shaker-1 mice have highly disorganized stereocilium bundles compared to wild-type animals. Dual-AAV8(Y733F)-MYO7A vectors did not improve stereocilium organization in shaker-1 cochlear hair cells, as revealed by phalloidin staining to visualize F-actin. The scale bar represents 10 μm. (B) Auditory brain stem response (ABR) thresholds at the four measured frequencies (4, 8, 16, and 32 kHz). An ABR threshold of 100 dB SPL represents no response. ABR testing was done at ∼P30. Dual-AAV8(Y733F)-MYO7A vectors did not improve ABR in shaker-1 mice. Error bars represent standard errors.
We further examined auditory function in treated shaker-1 mice using tone-burst auditory brainstem response (ABR) testing at ∼P30 (Figure 2B). In wild-type and heterozygous mice, the average ABR thresholds were 18.5–38 dB from 4–32 kHz (Figure 2B; n = 10 mice). In untreated shaker-1 mutant mice, no measurable ABR waveforms were detected even at the maximal stimulus level of 90 dB (Figure 2B; n = 4 mice). In shaker-1 mutant mice that were treated with dual-AAV8(Y733F)-MYO7A vectors, no measurable ABR waveforms were detected, comparable with responses obtained in untreated shaker-1 mutant mice (Figure 2B; n = 14 mice). Some shaker-1 mutant mice were treated with the single AAV8(Y733F) vector carrying the 5′ MYO7A cDNA (5′ AAV8(Y733F), 3.3 × 109 GC) as a control, and no measurable ABR waveforms were observed in these animals (Figure 2B; n = 3 mice). Interestingly, in wild-type and heterozygous littermates that were treated with dual-AAV8(Y733F)-MYO7A vectors, a ∼10–30 dB ABR threshold elevation was observed compared with untreated wild-type and heterozygous littermates (Figure 2B; n = 16 mice, p < 0.0001, ANOVA with Tukey’s multiple-comparisons test). A similar ABR threshold elevation was observed in wild-type and heterozygous littermates that were treated with the 5′ AAV8(Y733F) vector alone (n = 4 mice) or AAV8(Y733F)-GFP (7.7 × 109 GC, n = 3 mice) compared with untreated wild-type and heterozygous littermates (Figure S3; p = 0.0007 and 0.002, respectively; ANOVA with Tukey’s multiple-comparisons test). In contrast, wild-type and heterozygous littermates that were treated with vehicle alone (PBS with 5% glycerol) showed no statistically significant ABR threshold elevation compared with untreated wild-type and heterozygous littermates (Figure S3; n = 5 mice, p = 0.3, ANOVA with Tukey’s multiple-comparisons test). These results suggest that the ABR threshold elevation observed in wild-type and heterozygous littermates that were treated with dual-AAV8(Y733F)-MYO7A vectors is not due to surgical trauma to the inner ear but, rather, the dual-AAV8(Y733F)-MYO7A vectors. In summary, our results suggest that dual-AAV8(Y733F)-MYO7A vectors were unable to restore cochlear stereocilium organization and auditory function in the shaker-1 homozygous mutant mouse.
Dual-AAV8(Y733F)-MYO7A vectors restore MYO7A expression in vestibular hair cells
One of the hallmarks of the shaker-1 mouse phenotype is vestibular dysfunction manifesting as repetitive circling, which is due to the lack of MYO7A expression in vestibular hair cells.22 We have reported previously that inner ear gene delivery via the PSC approach can effectively transduce hair cells in the cochlea and vestibular organs simultaneously.23,24 Therefore, we examined the transduction efficiency and physiological effects of dual-AAV8(Y733F)-MYO7A vectors in the vestibular system of shaker-1 mice. In the wild-type mouse, MYO7A expression is detected in all vestibular hair cells (Figure 3A). In contrast, the homozygous shaker-1 mutant mouse does not express MYO7A in the vestibular organs (Figure 3A). When dual-AAV8(Y733F)-MYO7A vectors were delivered into the shaker-1 inner ears, MYO7A expression was restored in the transduced vestibular hair cells (Figure 3A). The vestibular hair cell transduction rate was 77% ± 9.2% in the utricle (Figure 3B; n = 6 mice).
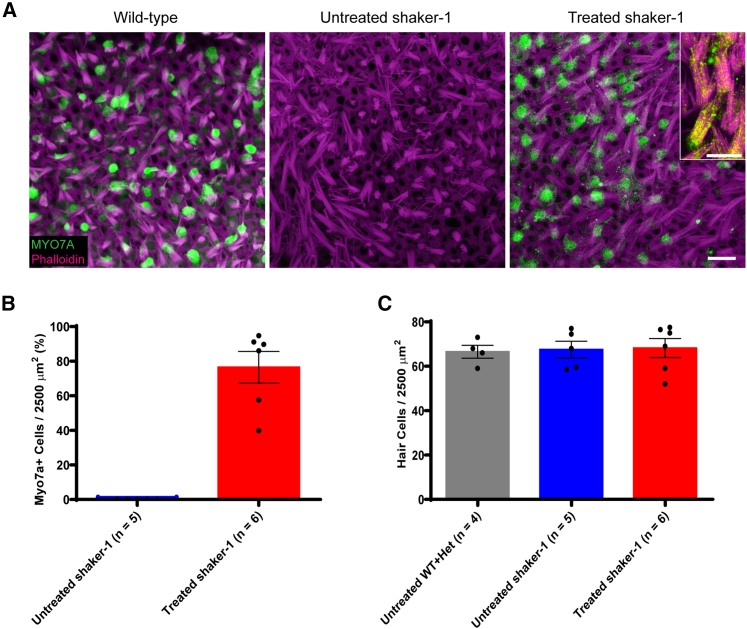
Figure 3.
Dual-AV8(Y733F)-MYO7A vectors restore MYO7A expression in transduced vestibular hair cells
(A) Representative confocal images of vestibular hair cells from mouse utricles. Images were taken at P30–P37. The shaker-1 mice that were treated with dual-AAV8(Y733F)-MYO7A showed robust MYO7A expression (green), whereas the untreated shaker-1 mice showed no MYO7A expression. A higher-magnification view of the treated shaker-1 stereocilia revealed MYO7A expression along the stereocilia bundles (inset). Scale bars represent 10 μm. (B) Quantification of vestibular hair cell transduction rates. (C) Quantification of hair cell survival in mouse utricles. In contrast to the shaker-1 cochlear hair cells, the vestibular hair cells do not undergo degeneration in shaker-1 mice. Error bars represent standard errors.
In the shaker-1 cochlea, IHCs and OHCs show signs of severe defects postnatally and undergo rapid degeneration (Figure 1A). Therefore, we wanted to see whether similar hair cell degeneration also occurs in the vestibular system. In wild-type mice, the average vestibular hair cell count was 67 ± 2.9 cells/2,500 μm2 (Figure 3C; n = 4 mice). Surprisingly, at 1 month of age, the vestibular hair cell count in untreated shaker-1 mice was similar to the wild-type mouse, with the average vestibular hair cell count of 68 ± 3.8 cells/2,500 μm2 (n = 5 mice, p > 0.9, ANOVA with Tukey’s multiple-comparisons test) (Figure 3C). In shaker-1 mice that were treated with dual-AAV8(Y733F)-MYO7A vectors, the average vestibular hair cell count was 68 ± 4.3 cells/2,500 μm2 (n = 6 mice, p > 0.9, compared with wild-type mice, ANOVA with Tukey’s multiple comparisons test) (Figure 3C). The fact that the vestibular hair cells in shaker-1 mice do not undergo degeneration at this age suggests that the vestibular system in these animals may be more amenable to inner ear gene therapy treatment.
Dual-AAV8(Y733F)-MYO7A vectors improve vestibular hair cell stereocilium morphology in shaker-1 mice
Next, we asked whether, in vestibular hair cells, these dual vectors can improve stereocilium bundle organization. Vestibular hair cell stereocilium bundles in wild-type mice are organized in rows of increasing height (Figure 4A), whereas vestibular hair cell stereocilium bundles in shaker-1 mice are highly disorganized (Figure 4A). After injection with dual-AAV8(Y733F)-MYO7A vectors, the stereocilium bundles of treated shaker-1 vestibular hair cells became much more organized compared with untreated shaker-1 mice (Figure 4A). The fact that dual-AAV8(Y733F)-MYO7A vectors improve stereocilium organization in the vestibular system suggests that the vestibular system is amenable to gene therapy in this mouse model.
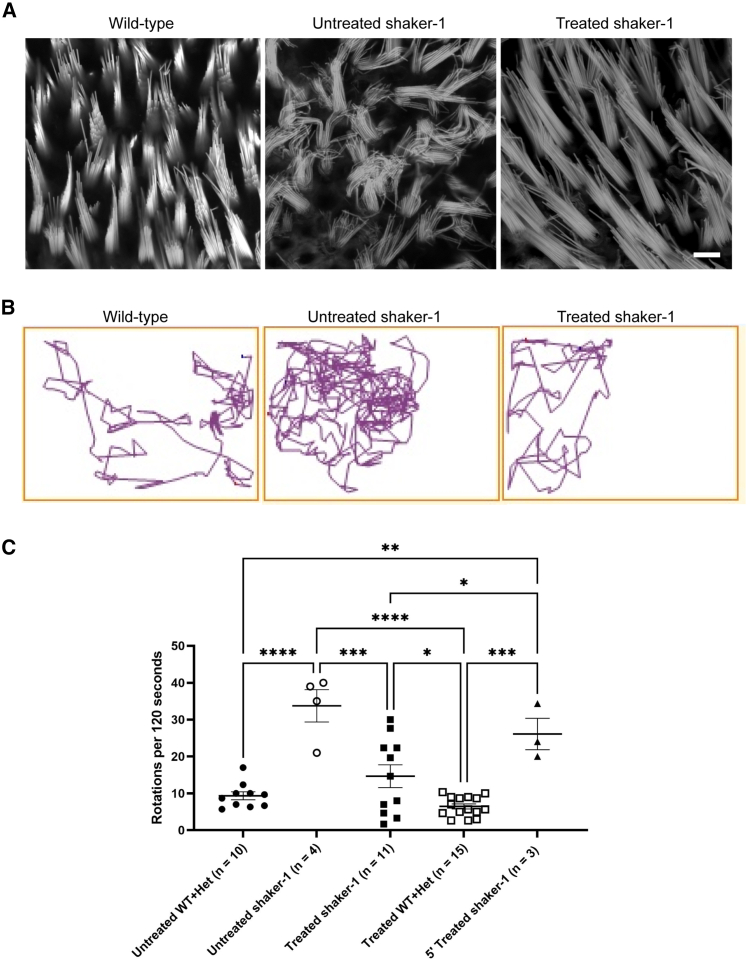
Figure 4.
Dual-AAV8(Y733F)-MYO7A vectors improve vestibular stereocilium organization and circling behavior in shaker-1 mice
(A) Representative confocal images of stereocilium bundles of vestibular hair cells from mouse utricles stained by phalloidin to visualize F-actin. The shaker-1 vestibular hair cells have highly disorganized stereocilium bundles compared with wild-type hair cells. Shaker-1 mice treated with dual-AAV8(Y733F)-MYO7A vectors showed significant improvement in vestibular stereocilium organization compared with untreated shaker-1 vestibular hair cells. The scale bar represents 10 μm. (B) Representative track plots from a wild-type mouse, an untreated shaker-1 mouse, and a shaker-1 mouse treated with dual-AAV8(Y733F)-MYO7A vectors. Dual-AAV8(Y733F)-MYO7A vectors decreased circling behavior in treated shaker-1 mice. (C) Quantification of circling behavior was performed at ∼P30. Shaker-1 mice that were treated with dual-AAV8(Y733F)-MYO7A showed significant reduction in circling compared with untreated shaker-1 mice. Error bars represent standard errors. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Dual-AAV8(Y733F)-MYO7A vectors reduce circling behavior in shaker-1 mice
Shaker-1 mice exhibit vestibular dysfunction throughout life. One of the manifestations of vestibular dysfunction in these animals is circling behavior. Wild-type mice typically walk in a straight line, and they like to explore the periphery of their housing cage (Figure 4B). In contrast, shaker-1 mice tend to walk in circles, likely because of significant vestibular dysfunction (Figure 4B). When shaker-1 mice were treated with dual-AAV8(Y733F)-MYO7A vectors, we saw a significant reduction in circling behavior (Figure 4B). The circling behavior was quantified by counting the number of circles these animals made in 120 s using a video tracking system (Figure 4C; ANY-Maze video tracking system; Stoelting, Wood Dale, IL, USA). The untreated wild-type and heterozygous littermates made 9.3 ± 1.1 rotations/120 s (n = 10 mice), whereas the untreated shaker-1 mice made 34 ± 4.4 rotations/120 s (n = 4, p = <0.0001 compared with wild-type/heterozygous mice, ANOVA with Tukey’s multiple-comparisons test). When shaker-1 mice were treated with dual-AAV8(Y733F)-MYO7A vectors, a significant reduction of circling behavior was observed. Shaker-1 mice that were injected with dual-AAV8(Y733F)-MYO7A vectors made 15 ± 3.1 rotations/120 s, which is significantly reduced compared with untreated shaker-1 mice (n = 11 mice, p = 0.0004 compared with untreated shaker-1 mice, ANOVA with Tukey’s multiple-comparisons test). The wild-type and heterozygous littermates that were injected with dual-AAV8(Y733F)-MYO7A vectors made 6.5 ± 0.69 rotations/120 s (n = 15 mice). This is similar to untreated wild-type and heterozygous littermates (p = 0.8, ANOVA with Tukey’s multiple-comparisons test). No reduction in circling behavior was observed in shaker-1 mice that were treated with 5′ AAV8(Y733F)-MYO7A vector alone (26 ± 4.3 rotations/120 s, n = 3 mice, p = 0.7 compared with untreated shaker-1 mice and p ≤ 0.05 compared with dual-AAV8(Y733F)-MYO7A-treated shaker-1 mice, ANOVA with Tukey’s multiple-comparisons test). These data show that dual-AAV8(Y733F)-MYO7A vectors are capable of reducing circling behavior in shaker-1 mice, which is an indication of improvement of vestibular function.
Dual-AAV8(Y733F)-MYO7A vectors improve vestibular sensory-evoked potential (VsEP) in shaker-1 mice
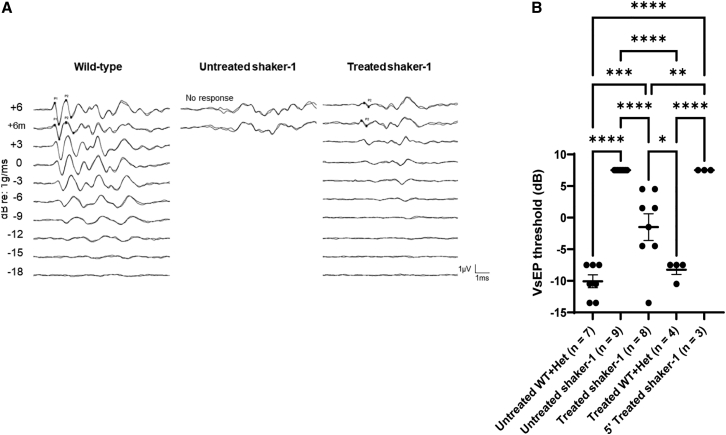
While circling behavior is often used as a measure for vestibular dysfunction, it is not specific to the vestibular system. To directly assess the impact of dual-AAV8(Y733F)-MYO7A gene therapy on the vestibular system in shaker-1 mice, we tested the VsEPs in these animals (Figure 5). VsEPs measure the neural activities of the vestibular system in response to a linear head motion that stimulates the gravity receptor organs (utricle and saccule).25 In wild-type mice, the average VsEP threshold was −10 ± 1.0 dB (n = 7 mice), whereas in untreated shaker-1 mice, VsEP waveforms were not detected even at the maximal stimulus level of +6 dB, and therefore were designated to have a threshold of +7.5 dB (n = 9 mice, p < 0.0001 compared with untreated wild-type/heterozygous littermates, ANOVA with Tukey’s multiple-comparisons test). When shaker-1 mice were treated with dual-AAV8(Y733F)-MYO7A, VsEP waveforms were detected in many animals (Figure 5A). In shaker-1 mice that were treated with dual-AAV8(Y733F)-MYO7A vectors (n = 13 mice), eight animals had measurable VsEP thresholds, and the average VsEP threshold of all 13 mice was +2.0 ± 1.8 dB (p = 0.04 compared with untreated shaker-1 mice, ANOVA with Tukey’s multiple-comparisons test, Figure 5B). The wild-type and heterozygous littermates that were injected with dual-AAV8(Y733F)-MYO7A vectors showed similar VsEP thresholds as untreated wild-type and heterozygous littermates, with the average VsEP threshold being −8.3 ± 0.75 dB (n = 4 mice, p > 0.9 compared with untreated wild-type/heterozygous littermates, ANOVA with Tukey’s multiple-comparisons test). This suggests that dual-AAV8(Y733F)-MYO7A vectors did not cause any toxicity to the vestibular system in shaker-1 mice. The shaker-1 mice that received the 5′ vector alone did not show any measurable VsEP thresholds (+7.5 dB, n = 3 mice), similar to untreated shaker-1 mice. These data indicate that dual-AAV8(Y733F)-MYO7A vectors can improve vestibular function in shaker-1 mice.
Figure 5.
Dual-AAV8(Y733F)-MYO7A vectors improved VsEP thresholds in shaker-1 mice
(A) Representative vestibular sensory-evoked potential (VsEP) recordings from a wild-type mouse, an untreated shaker-1 mouse, and a shaker-1 mouse treated with dual-AAV8(Y733F)-MYO7A vectors. The untreated shaker-1 mouse had no measurable VsEP responses, whereas the treated shaker-1 mouse had measurable VsEP responses. (B) VsEP threshold measurements. Shaker-1 mice that were treated with dual-AAV8(Y733F)-MYO7A vectors showed improvement in VsEP thresholds compared with untreated shaker-1 mice. Error bars represent standard errors. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Discussion
USH is a genetic disease that has a profound impact on a patient’s quality of life, particularly those who have type 1 USH, such as USH1B. While patients with USH1B often have profound hearing loss and visual loss, they also experience vestibular dysfunction, which can significantly increase their risk for fall-related injuries. Therefore, our study investigated the effect of gene replacement therapy as a treatment for auditory and vestibular dysfunction in a mouse model of USH1B, the shaker-1 mouse. The shaker-1 mouse (Myo7a4626SB/4626SB) was generated using N-ethyl-N-nitrosourea (ENU) mutagenesis, which resulted in a premature stop codon (NM_001256081).17 In mice, the 4626SB mutation in Myo7a has been shown to cause abnormal hair cell stereocilium bundle morphology.26,27 Consequently, shaker-1 mice have profound deafness and vestibular dysfunction. Therefore, the inner ear of the shaker-1 mouse is a good model for USH1B because patients with USH1B also demonstrate profound hearing loss and vestibular dysfunction.
The dual-AAV vector-based approach has been successfully applied in the mouse inner ear. Previously, two studies have shown that the dual-AAV vectors are capable of delivering the otoferlin protein in a DFNB9 mouse model.12,28 Otoferlin is involved in calcium ion binding activity and synaptic vesicle trafficking.29,30 Dual-AAV vectors can produce a recombinant full-length otoferlin protein in cells co-infected with 5′ and 3′ vectors, thus restoring otoferlin expression in the inner ear.12 Similarly, it has been found that injection of dual-AAV-otoferlin vectors partially restores synaptic function in mutant mice.28 These studies also demonstrated that the dual-AAV vector-based approach improves hearing in a DFNB9 mouse model and corrects profound deafness.12,28 Furthermore, it has been shown that dual-AAV vectors can prolong hearing recovery over a sustained time period. There was improved auditory function for at least 20 weeks post injection.12 More recently, the dual-AAV vector-based approach has been shown to successfully improve auditory function in a mouse model of DFNB16.31 In this case, the dual-AAV vectors employ intein-mediated protein recombination rather than recombination at the DNA level.31 Taken together, these studies suggest that dual-AAV gene delivery can be successfully applied to mouse models of human hereditary hearing loss to improve their auditory function.
In this study, we applied the dual-AAV vector-based approach to deliver human MYO7A cDNA into the shaker-1 mouse inner ear. The dual-AAV vectors we used in this study have been shown to restore MYO7A expression in the mouse retina following sub-retinal injection.19 We injected the dual-AAV8(Y733F)-MYO7A vectors into the inner ear of neonatal shaker-1 mice using the PSC approach. This resulted in successful transduction of IHCs and OHCs in the cochlea. We observed that shaker-1 mice treated with these dual-AAV vectors had improved cochlear hair cell survival, and many of the surviving hair cells expressed MYO7A. This contrasts with the untreated shaker-1 mice that have severe cochlear hair cell degeneration. We also quantified the transduction rates of IHCs and OHCs in the treated animals and found that the transduction rate was higher at the apex than at the base of the cochlea. The lower transduction rate at the cochlear base may in part be due to the fact that cochlear hair cell degeneration is most profound at the cochlear base. Taken together, these results show that dual-AAV8(Y733F)-MYO7A vectors have a protective effect on cochlear hair cells and can prolong their survival.
Stereocilium bundles of inner ear hair cells play an important role in hair cell mechanotransduction. Shaker-1 mice have highly disorganized stereocilium bundles, likely because of the absence of MYO7A. While there was significant improvement in cochlear hair cell survival in shaker-1 mice treated with dual-AAV8(Y733F)-MYO7A vectors, we did not see improvements in stereocilium organization in the treated shaker-1 cochlea. The shaker-1 mice treated with dual-AAV8(Y733F)-MYO7A vectors had disorganized stereocilium bundles comparable with the stereocilia bundles seen in the untreated shaker-1 mice. These persistent structural deficits in stereocilium bundles correlate with the lack of hearing rescue seen in the treated shaker-1 mice. However, it is important to note that the cochlear hair cells in this mouse model undergo degeneration as early as embryonic day 17.5 (E17.5), and it may be necessary to deliver gene therapy in utero to rescue auditory function in these animals.27 We also observed a 10- to 30-dB ABR threshold elevation in wild-type and heterozygous littermates that were treated with dual-AAV8(Y733F)-MYO7A vectors, while wild-type and heterozygous littermates that were treated with virus vehicle did not show significant ABR threshold elevation compared with untreated wild-type and heterozygous animals. In addition, similar ABR threshold elevation was observed in wild-type and heterozygous littermates that were treated with 5′ vector alone or with AAV8(Y733F)-GFP. This suggests that there may be some toxicity associated with the dual-AAV8(Y733F)-MYO7A vectors in the cochlea. Interestingly, these were the same hybrid dual-AAV(Y733F) vectors used by Dyka et al.13 These vectors, used at similar doses as those employed in the current study, led to partial retinal degeneration following subretinal injection. Both results may be attributed to the deleterious effects of truncated protein arising from the 5′ vector or to the AAV8(Y733F) vector itself. Notably, these vectors have since been optimized to prevent production of this truncated protein. Future studies will evaluate their performance in the inner ear.
The vestibular system is integral for maintaining balance and positioning. In contrast to the cochlea, the vestibular hair cells in shaker-1 mice do not undergo degeneration. Their structural maintenance relative to cochlear hair cells likely explains why the vestibular system was more amenable to the beneficial effects of inner ear gene therapy in this mouse model. Shaker-1 mice treated with the dual-AAV8(Y733F)-MYO7A vectors show high virus transduction (70%–80%) of vestibular hair cells throughout the utricle. In addition, we observed significant improvement in the organization and cohesion of the vestibular stereocilium bundles in the shaker-1 mice treated with the dual-AAV8(Y733F)-MYO7A vectors compared with that seen in the untreated shaker-1 mice.
We examined circling behavior as a measure of vestibular function in shaker-1 mice treated with the dual-AAV8(Y733F)-MYO7A vectors. We observed that treated shaker-1 mice had a significant reduction in circling behavior compared with untreated shaker-1 mice. Several shaker-1 mice treated with dual-AAV8(Y733F)-MYO7A vectors had a significantly reduced amount of circling behavior comparable with that seen in the wild-type and heterozygous animals. The abnormally high level of circling behavior in shaker-1 mice was not improved with injection of the 5′ vector alone, indicating that the 5′ and 3′ AAV8(Y733F) vectors are necessary for a reduction of circling behavior in shaker-1 mice.
Because circling behavior is an indirect measurement of vestibular function, we assessed VsEPs as a direct test of vestibular function. We found that dual-AAV8(Y733F)-MYO7A vectors improved VsEP thresholds in shaker-1 mice. In contrast, the untreated shaker-1 mice did not have measurable VsEP waveforms, even at the highest stimulus level. However, many of the treated shaker-1 mice had measurable VsEP waveforms. In fact, in one treated shaker-1 mouse, the VsEP thresholds were similar to those in the wild type. No VsEP responses were observed in the shaker-1 mice that received 5′ vector alone. In addition, the wild-type and heterozygous littermates that were treated with dual-AAV8(Y733F)-MYO7A vectors did not show any increase in circling or elevation of VsEP thresholds, in contrast to the 10- to 30-dB ABR threshold elevation observed in these animals. This suggests that the vestibular system in the shaker-1 mice may be more resistant to the potential toxicity of these dual vectors.
Our study showed that delivery of MYO7A cDNA via the dual-AAV8(Y733F) vectors improved cochlear hair cell survival and vestibular function in shaker-1 mice. In a study by Wafa et al.,32 a cohort of 90 USH patients who had a molecular genetic diagnosis underwent a battery of vestibular tests to characterize their vestibular dysfunction. They found that all patients with type 1 USH had measurable vestibular abnormality in at least one of the vestibular tests that were performed. In addition, results from sensory organization test showed that many of the type 1 USH patients were at risk for falling,32 which is the leading cause of injury-related death in US adults older than 65 years of age.33,34 Furthermore, it has been shown that vestibular hair cells are still present in a human temporal bone specimen from an elderly patient with USH1B.35 This demonstrates that the mature vestibular system in USH1B patients may still be viable and amenable to the therapeutic effects of inner ear gene therapy. Our results show that gene replacement therapy with dual-AAV8(Y733F)-MYO7A vectors has great translational potential for improving vestibular function in USH1B patients, reducing their risk of falling. It is our hope that the results from this study will serve as a foundation for future clinical translation of gene replacement therapy to help patients with USH1B.
Materials and methods
AAV vector construction
The production of dual-AAV vectors has been described previously.13 The dual-AAV vectors were produced by the Boye Lab at the University of Florida. They were designed to ultimately deliver the full-length coding sequence of MYO7A (human isoform 2; NM_001127180, with an alternatively spliced GTGAAG sequence at the end of exon 46 encoding two additional residues) under control of the ubiquitous smCBA promoter. The 5′ vector contained the smCBA promoter, the 5′ segment of MYO7A cDNA (2,904 bp), a highly recombinogenic sequence derived from the AP coding sequence, and a splice donor. The 3′ vector contained a splice acceptor, a complementary AP coding sequence, the 3′ segment (3,627 bp) of MYO7A cDNA (with an HA tag), and a polyadenylation signal. Vectors were packaged, purified, and titered as described previously.36 The viral titer was 6.5 × 1012 GC/mL for the 5′ vector and 1.1 × 1013 GC/mL for the 3′ vector. The AAV8(Y733F)-smCBA-GFP virus was produced in the same facility using a similar protocol. Its viral titer was 7.7 × 1012 GC/mL.
Animals
Mice were housed at the National Institute on Deafness and Other Communication Disorders (NIDCD) animal facility and cared for by NIDCD animal facility staff. The shaker-1 mice used in this study were acquired from Jackson Laboratory and backcrossed with C57BL/6J mice.
AAV infection of COS-7 cells
COS-7 cells were plated on 24 × 24 mm glass coverslips and maintained at 37°C and 5% CO2 in DMEM supplemented with 5% fetal bovine serum (FBS) until 40%–50% confluence. Cultures were transduced with 2 μL of either 5′ AAV (1.3 × 1010 GC) or 3′ AAV (2.2 × 1010 GC) or a combination of 2 μL of each of 5′ AAV and 3′ AAV diluted in 0.5 mL of culture medium and pre-incubated for 12 h. 1.5 mL of culture medium was added, and cells were incubated for up to 4 days. Cells were then fixed for 20 min in 4% paraformaldehyde in PBS, permeabilized for 10 min in 0.5% Triton X-100 in PBS, and blocked with 5% BSA in PBS for 2 h. Then primary antibodies were applied for 3 h, coverslips were washed 3 times with PBS, secondary antibodies (Thermo Fisher Scientific) were applied for 1 h, and coverslips were washed 3 times with PBS and then mounted on a microscope slide with ProLong Diamond Antifade Mountant (Thermo Fisher Scientific).37 The primary mouse monoclonal anti-MYO7A antibody directed against amino acids 11–70 was purchased from Santa Cruz Biotechnology (Dallas, TX, USA; catalog number sc-74516, dilution 1/200 in PBS). The immunopurified rabbit anti-MYO7A antibody directed against amino acids 880–1,077 was purchased from Proteus Biosciences (Ramona, CA, USA; catalog number 25-6790, dilution 1/400 in PBS). The immunopurified rabbit anti-HA tag antibody was purchased from Cell Signaling Technology (Danvers, MA, USA; catalog number 3724S, dilution 1/400 in PBS). Cells were counterstained with phalloidin and DAPI. Preparations were scanned in either super-resolution mode or confocal mode using an LSM 880 Airyscan confocal microscope (Carl Zeiss, White Plains, NY, USA).
Animal surgery
All animals used in this study were approved by the animal care and use committee at the NIDCD (protocol number ASP1378). The surgical procedures were performed in accordance with the ethical guidelines of the animal care and use committee at the NIDCD. Hypothermia was used to induce and maintain anesthesia in neonatal mice (P0–P5). Surgery was performed only on the left ear of each animal. To deliver AAV vectors, the PSC approach was used.23 Briefly, a post-auricular incision was made, and the tissue was dissected to expose the PSC. Care was taken to avoid facial nerve injury during dissection. A Nanoliter Microinjection System (Nanoliter2000; World Precision Instruments, Sarasota, FL, USA) was used to load the dual-AAV vectors into a glass micropipette. The dual-AAV virus was injected over approximately 40 s (3.3 × 109 GC for the 5′ vector and 5.5 × 109 GC for the 3′ vector; total volume, 1 μL). The incision was closed with 5-0 Vicryl sutures and tissue glue (Ethicon, Somerville, NJ, USA).
Auditory and VsEP testing
All mice were tested in the Mouse Auditory Testing Core Facility at the NIDCD. Animals were anesthetized with ketamine (50–75 mg/kg; MWI Animal Health, Boise, ID, USA) and dexdomitor (0.5 mg/kg, MWI Animal Health) via intraperitoneal injections. Puralube ointment was applied to the eyes of mice to prevent corneal damage while the animals remained anesthetized. Stimulus generation and physiological recordings were completed using Tucker-Davis Technologies (Gainesville, FL, USA) hardware (RZ6 Multi I/O Processor) and software (BioSigRx, v.5.1). The electrophysiological activity was amplified 200,000×, filtered (300–3,000 Hz), and digitized (125 kHz).
ABR testing was performed to evaluate hearing sensitivity at ∼P30. Following anesthesia, the animals were placed on a warming pad in a sound booth (ETS-Lindgren Acoustic Systems, Cedar Park, TX, USA). Sub-dermal needle electrodes were inserted at the vertex (+) and test ear mastoid (−) with a ground electrode inserted under the contralateral ear. ABR thresholds were measured at 4, 8, 16, and 32 kHz using 3-ms, Blackman-gated tone pips presented at 29.9/s with alternating stimulus polarity. At each stimulus level, 512–1,024 responses were averaged. Thresholds were determined by visual inspection of the waveforms and defined as the lowest stimulus level at which any wave could be reliably detected. A minimum of two waveforms were obtained at the threshold level to ensure repeatability of the responses. Physiological results were analyzed for individual frequencies and then averaged for each of these frequencies from 4–32 kHz.
VsEPs were obtained as described previously.38 Subcutaneous electrodes were placed at the nuchal crest, at the left ear, and at the right hip. Mice were placed in the supine position on the heating pad with the head placed in a custom, non-invasive head clip attached to an electromechanical shaker (ET 132-2; Labworks, Costa Mesa, CA, USA). The shaker delivered linear pulses (2-ms duration, 17 pulses per second) to the head in the naso-occipital axis. An accelerometer attached to the shaker was used to calibrate the stimulus in jerk (i.e., first derivative of acceleration) at the start of each test session. Stimuli were delivered in 3-dB steps from +6 dB to −18 dB re:1.0 g/ms (1.0 g = 9.8 m/s2). Four waveforms were measured at each stimulus level: two in the positive (occipital toward nasal) and two in the negative (nasal toward occipital) direction (256 responses per waveform). Pairs of the positive and negative waveforms were averaged together to produce two averaged waveforms for analyses. The two averaged waveforms were superimposed and compared to ensure reproducibility. To ensure no contribution of an auditory response to the waveform, testing began with presentation of the highest stimulus level (+6 dB) under quiet conditions and in the presence of a masking noise (50–50,000 Hz, 96-dB SPL). If the unmasked response peak amplitude changed by more than 50% in the presence of the masker, then the test was performed with the masker on. We defined the threshold measured as the stimulus level (dB) halfway between the lowest stimulus level producing a response and the next stimulus level at which no response was measured.
Circling behavior
Circling behavior was characterized and quantified using optical tracking and the ANY-Maze tracking software (Stoelting). The mice were placed in a 38 × 58 cm box with a video camera (Fujinon YV5X2.7R4B-2 1/3-inch 2.7–13.5 mm F1.3 Day/Night Aspherical Vari-Focal Lens, Fujifilm) directly above. The ANY-Maze software was set to track the movements of the mice placed in the box. We placed each mouse into the box and allowed 2 min for the mouse to become acclimatized to the new environment. The number of rotations, speed, and distance traveled were recorded over a 2-min period for each trial with a 1-min “cool-down” period between trials where no data was recorded. Three trials were performed for each mouse, and the average was calculated. Testing was performed at approximately P30.
Immunohistochemistry
After auditory testing, the mice were euthanized by CO2 asphyxiation followed by decapitation. Temporal bones were harvested and fixed overnight with formaldehyde (4% paraformaldehyde solution in 1× PBS) followed by decalcification in 120 mM disodium EDTA for 4 days. The vestibular organs and cochlear sensory epithelia were micro-dissected, blocked with normal goat serum in PBS, and labeled with the mouse anti-MYO7A antibody to label hair cells (1:200, product 25-6790, Proteus Biosciences), and with chicken anti-GFP antibody (1:1,000, product ab13970; Abcam, Cambridge, MA, USA). Primary and secondary antibodies were diluted in PBS.
Quantification and transduction efficiency measurements
Z stacks of images were obtained using a Carl Zeiss LSM880 confocal microscope using 10× and 63× objectives. Images were taken for quantification of hair cells and calculation of the viral transduction efficiency. Two images were taken with a 63×, 1.4 N.A. objective at the three turns of the cochlea: apex, middle, and base. To ensure consistency between quantification of images, the pictures were taken at opposite ends of each cochlear turn. The total IHCs and OHCs were counted in a 136-μm segment of each turn and reported on an Excel sheet. Because shaker-1 mice do not express MYO7A, the total number of surviving hair cells was calculated by counting the hair cells with stereocilium bundles, labeled by phalloidin. The transduction efficiency was calculated by dividing the number of hair cells expressing MYO7A, indicating virus transduction, by the total number of hair cells in a 136-μm segment. The total number of hair cells and infection rates were calculated separately for IHCs and OHCs.
For each animal, two images were taken from the utricle in the extra-striolar region using a 63×, 1.4 N.A. objective. The imaging location was consistent across all specimens. To quantify the number of vestibular hair cells, a 50 × 50 μm box was placed within each z-stacked image. The location of these boxes was consistent across all specimens. All hair cells in the box were counted and quantified. The total number of surviving hair cells was calculated by counting the hair cells with stereocilium bundles, labeled by phalloidin. To measure the transduction efficiency of vestibular hair cells, we divided the number of infected cells that expressed MYO7A by the total number of hair cells in a 50 × 50 μm area of interest. After calculating the total number of utricular hair cells and infection rate for the two images, the quantification and transduction rates were averaged.
Statistics
ANOVA and Tukey’s multiple-comparisons test were used to determine the statistical significance when comparing measurements for hair cell quantification and transduction rates. ANOVA and Tukey’s multiple-comparisons test were also used to analyze the statistical significance in ABR thresholds, circling behavior, and VsEP thresholds. A p value of less than 0.05 indicates statistical significance.
Acknowledgments
This work is supported by NIDCD Division of Intramural Research grants DC000082-02 (to W.W.C.), DC000039 (to T.B.F.), DC000080 (to the Mouse Auditory Testing Core), and National Eye Institute grant R01EY024280 (to S.E.B.). This research was made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the NIH from the Doris Duke Charitable Foundation (DDCF grant 2014194), the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, Elsevier, and other private donors. We are also grateful for the NIDCD animal facility staff for caring for our animals.
Author contributions
W.W.C., S.E.B., S.L.B., I.A.B., and T.B.F. conceived and designed the study. S.C.L., M.G., K.I., M.S., K.R.C., J.L.Z., Y.I., Z.O., T.W., T.F., and W.W.C. performed the experiment and analyzed the data. W.W.C. and M.G. supervised the work. S.C.L., M.G., and W.W.C. wrote the manuscript with participation of all authors.
Declaration of interests
S.E.B. and S.L.B. are founders and consultants for Atsena Therapeutics.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtm.2023.08.012.
Supplemental information
Data and code availability
Data will be made available upon request.
References
- 1.Vernon M. Usher's syndrome--deafness and progressive blindness. Clinical cases, prevention, theory and literature survey. J. Chron. Dis. 1969;22:133–151. doi: 10.1016/0021-9681(69)90055-1. [DOI] [PubMed] [Google Scholar]
- 2.Saihan Z., Webster A.R., Luxon L., Bitner-Glindzicz M. Update on Usher syndrome. Curr. Opin. Neurol. 2009;22:19–27. doi: 10.1097/wco.0b013e3283218807. [DOI] [PubMed] [Google Scholar]
- 3.Galbis-Martínez L., Blanco-Kelly F., García-García G., Ávila-Fernández A., Jaijo T., Fuster-García C., Perea-Romero I., Zurita-Muñoz O., Jimenez-Rolando B., Carreño E., et al. Genotype-phenotype correlation in patients with Usher syndrome and pathogenic variants in MYO7A: implications for future clinical trials. Acta Ophthalmol. 2021;99:922–930. doi: 10.1111/aos.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toms M., Pagarkar W., Moosajee M. Usher syndrome: clinical features, molecular genetics and advancing therapeutics. Ther. Adv. Ophthalmol. 2020;12 doi: 10.1177/2515841420952194. 2515841420952194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jouret G., Poirsier C., Spodenkiewicz M., Jaquin C., Gouy E., Arndt C., Labrousse M., Gaillard D., Doco-Fenzy M., Lebre A.S. Genetics of Usher Syndrome: New Insights From a Meta-analysis. Otol. Neurotol. 2019;40:121–129. doi: 10.1097/MAO.0000000000002054. [DOI] [PubMed] [Google Scholar]
- 6.Petit C. Usher syndrome: from genetics to pathogenesis. Annu. Rev. Genom. Hum. Genet. 2001;2:271–297. doi: 10.1146/annurev.genom.2.1.271. [DOI] [PubMed] [Google Scholar]
- 7.Ouyang X.M., Yan D., Du L.L., Hejtmancik J.F., Jacobson S.G., Nance W.E., Li A.R., Angeli S., Kaiser M., Newton V., et al. Characterization of Usher syndrome type I gene mutations in an Usher syndrome patient population. Hum. Genet. 2005;116:292–299. doi: 10.1007/s00439-004-1227-2. [DOI] [PubMed] [Google Scholar]
- 8.Castiglione A., Möller C. Usher Syndrome. Audiol. Res. 2022;12:42–65. doi: 10.3390/audiolres12010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Y., Zhou D., King R., Zhu S., Simpson C.L., Jones B.C., Zhang W., Geisert E.E., Lu L. The genetic dissection of Myo7a gene expression in the retinas of BXD mice. Mol. Vis. 2018;24:115–126. [PMC free article] [PubMed] [Google Scholar]
- 10.Géléoc G.G.S., El-Amraoui A. Disease mechanisms and gene therapy for Usher syndrome. Hear. Res. 2020;394:107932. doi: 10.1016/j.heares.2020.107932. [DOI] [PubMed] [Google Scholar]
- 11.Lopes V.S., Boye S.E., Louie C.M., Boye S., Dyka F., Chiodo V., Fofo H., Hauswirth W.W., Williams D.S. Retinal gene therapy with a large MYO7A cDNA using adeno-associated virus. Gene Ther. 2013;20:824–833. doi: 10.1038/gt.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akil O., Dyka F., Calvet C., Emptoz A., Lahlou G., Nouaille S., Boutet de Monvel J., Hardelin J.P., Hauswirth W.W., Avan P., et al. Dual AAV-mediated gene therapy restores hearing in a DFNB9 mouse model. Proc. Natl. Acad. Sci. USA. 2019;116:4496–4501. doi: 10.1073/pnas.1817537116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyka F.M., Boye S.L., Chiodo V.A., Hauswirth W.W., Boye S.E. Dual adeno-associated virus vectors result in efficient in vitro and in vivo expression of an oversized gene, MYO7A. Hum. Gene Ther. Methods. 2014;25:166–177. doi: 10.1089/hgtb.2013.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trapani I., Colella P., Sommella A., Iodice C., Cesi G., de Simone S., Marrocco E., Rossi S., Giunti M., Palfi A., et al. Effective delivery of large genes to the retina by dual AAV vectors. EMBO Mol. Med. 2014;6:194–211. doi: 10.1002/emmm.201302948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh A., Yue Y., Duan D. Efficient transgene reconstitution with hybrid dual AAV vectors carrying the minimized bridging sequences. Hum. Gene Ther. 2011;22:77–83. doi: 10.1089/hum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson F., Walsh J., Mburu P., Varela A., Brown K.A., Antonio M., Beisel K.W., Steel K.P., Brown S.D. A type VII myosin encoded by the mouse deafness gene shaker-1. Nature. 1995;374:62–64. doi: 10.1038/374062a0. [DOI] [PubMed] [Google Scholar]
- 17.Hasson T., Walsh J., Cable J., Mooseker M.S., Brown S.D., Steel K.P. Effects of shaker-1 mutations on myosin-VIIa protein and mRNA expression. Cell Motil Cytoskeleton. 1997;37:127–138. doi: 10.1002/(SICI)1097-0169(1997)37:2<127::AID-CM5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Mburu P., Liu X.Z., Walsh J., Saw D., Jr., Cope M.J., Gibson F., Kendrick-Jones J., Steel K.P., Brown S.D. Mutation analysis of the mouse myosin VIIA deafness gene. Gene Funct. 1997;1:191–203. doi: 10.1046/j.1365-4624.1997.00020.x. [DOI] [PubMed] [Google Scholar]
- 19.Calabro K.R., Boye S., Choudhury S., McCullough K.T., Fajardo D., Strang C.E., Chakraborty D., Witherspoon C.D., Gamlin P., Boye S. Investigating Dual AAV-Based Treatments for MYO7A Usher Syndrome in Myo7a-/- Mice and Macaque. Mol. Ther. 2019;27:316–317. [Google Scholar]
- 20.Morgan C.P., Krey J.F., Grati M., Zhao B., Fallen S., Kannan-Sundhari A., Liu X.Z., Choi D., Müller U., Barr-Gillespie P.G. PDZD7-MYO7A complex identified in enriched stereocilia membranes. Elife. 2016;5:e18312. doi: 10.7554/eLife.18312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grati M., Shin J.B., Weston M.D., Green J., Bhat M.A., Gillespie P.G., Kachar B. Localization of PDZD7 to the stereocilia ankle-link associates this scaffolding protein with the Usher syndrome protein network. J. Neurosci. 2012;32:14288–14293. doi: 10.1523/JNEUROSCI.3071-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shnerson A., Lenoir M., van de Water T.R., Pujol R. The pattern of sensorineural degeneration in the cochlea of the deaf shaker-1 mouse: ultrastructural observations. Brain Res. 1983;285:305–315. doi: 10.1016/0165-3806(83)90028-7. [DOI] [PubMed] [Google Scholar]
- 23.Isgrig K., Chien W.W. Posterior Semicircular Canal Approach for Inner Ear Gene Delivery in Neonatal Mouse. J. Vis. Exp. 2018 doi: 10.103791/56648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isgrig K., Shteamer J.W., Belyantseva I.A., Drummond M.C., Fitzgerald T.S., Vijayakumar S., Jones S.M., Griffith A.J., Friedman T.B., Cunningham L.L., Chien W.W. Gene Therapy Restores Balance and Auditory Functions in a Mouse Model of Usher Syndrome. Mol. Ther. 2017;25:780–791. doi: 10.1016/j.ymthe.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones S.M., Subramanian G., Avniel W., Guo Y., Burkard R.F., Jones T.A. Stimulus and recording variables and their effects on mammalian vestibular evoked potentials. J. Neurosci. Methods. 2002;118:23–31. doi: 10.1016/s0165-0270(02)00125-5. [DOI] [PubMed] [Google Scholar]
- 26.Prosser H.M., Rzadzinska A.K., Steel K.P., Bradley A. Mosaic complementation demonstrates a regulatory role for myosin VIIa in actin dynamics of stereocilia. Mol. Cell Biol. 2008;28:1702–1712. doi: 10.1128/mcb.01282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lefèvre G., Michel V., Weil D., Lepelletier L., Bizard E., Wolfrum U., Hardelin J.P., Petit C. A core cochlear phenotype in USH1 mouse mutants implicates fibrous links of the hair bundle in its cohesion, orientation and differential growth. Development. 2008;135:1427–1437. doi: 10.1242/dev.012922. [DOI] [PubMed] [Google Scholar]
- 28.Al-Moyed H., Cepeda A.P., Jung S., Moser T., Kügler S., Reisinger E. A dual-AAV approach restores fast exocytosis and partially rescues auditory function in deaf otoferlin knock-out mice. EMBO Mol. Med. 2019;11:e9396. doi: 10.15252/emmm.201809396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roux I., Safieddine S., Nouvian R., Grati M., Simmler M.-C., Bahloul A., Perfettini I., Le Gall M., Rostaing P., Hamard G., et al. Otoferlin, Defective in a Human Deafness Form, Is Essential for Exocytosis at the Auditory Ribbon Synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 30.Yasunaga S., Grati M., Cohen-Salmon M., El-Amraoui A., Mustapha M., Salem N., El-Zir E., Loiselet J., Petit C. A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nat. Genet. 1999;21:363–369. doi: 10.1038/7693. [DOI] [PubMed] [Google Scholar]
- 31.Shubina-Oleinik O., Nist-Lund C., French C., Rockowitz S., Shearer A.E., Holt J.R. Dual-vector gene therapy restores cochlear amplification and auditory sensitivity in a mouse model of DFNB16 hearing loss. Sci. Adv. 2021;7:eabi7629. doi: 10.1126/sciadv.abi7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wafa T.T., Faridi R., King K.A., Zalewski C., Yousaf R., Schultz J.M., Morell R.J., Muskett J., Turriff A., Tsilou E., et al. Vestibular phenotype-genotype correlation in a cohort of 90 patients with Usher syndrome. Clin. Genet. 2021;99:226–235. doi: 10.1111/cge.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens J.A., Corso P.S., Finkelstein E.A., Miller T.R. The costs of fatal and non-fatal falls among older adults. Inj. Prev. 2006;12:290–295. doi: 10.1136/ip.2005.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gelbard R., Inaba K., Okoye O.T., Morrell M., Saadi Z., Lam L., Talving P., Demetriades D. Falls in the elderly: a modern look at an old problem. Am. J. Surg. 2014;208:249–253. doi: 10.1016/j.amjsurg.2013.12.034. [DOI] [PubMed] [Google Scholar]
- 35.Osgood R.T., Zhu M.Y., Jennifer T., O'Malley, Chien W., Cremers C., Nadol J.B., Jr., Charles Liberman M., Quesnel A.M., Indzhykulian A.A. 2022. Vestibular Hair Cell Survival and Stereocilia Bundle Morphology in Usher Syndrome Type 1 Patients. The Association for Research in Otolaryngology (ARO) - the 45th Annual MidWinter Meeting. [Google Scholar]
- 36.Zolotukhin S., Potter M., Zolotukhin I., Sakai Y., Loiler S., Fraites T.J., Jr., Chiodo V.A., Phillipsberg T., Muzyczka N., Hauswirth W.W., et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–167. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]
- 37.Grati M., Kachar B. Myosin VIIa and sans localization at stereocilia upper tip-link density implicates these Usher syndrome proteins in mechanotransduction. Proc. Natl. Acad. Sci. USA. 2011;108:11476–11481. doi: 10.1073/pnas.1104161108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tona R., Chen W., Nakano Y., Reyes L.D., Petralia R.S., Wang Y.-X., Starost M.F., Wafa T.T., Morell R.J., Cravedi K.D., et al. The phenotypic landscape of a Tbc1d24 mutant mouse includes convulsive seizures resembling human early infantile epileptic encephalopathy. Hum. Mol. Genet. 2019;28:1530–1547. doi: 10.1093/hmg/ddy445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon request.