Abstract
In bilaterian central nervous systems, coordination of neurotransmission by glial cells enables highly sophisticated neural functions. The diversity of transcription factors (TFs) involved in gliogenesis suggests multiple evolutionary origins of various glial cell types of bilaterians. Many of these TFs including the glial cells missing (Gcm) are also present in genomes of Cnidaria, the closest outgroup to Bilateria, but their function remains to be elucidated. In this study, we analyzed the function of Gcm, a multifunctional TF involved in development of glial and non-glial cell types, in the sea anemone, Nematostella vectensis. siRNA-mediated knockdown of Nematostella Gcm altered expression of cell adhesion proteins, glutamate and GABA transporters, ion channels, metabolic enzymes, and zinc finger and Ets-related TFs. NvGcm and mRNAs of downstream genes are expressed in broad neural cell clusters. However, immunostaining of a NvGcm target protein, the glutamate transporter, NvEAAT1, visualized a novel class of cells with flat cell bodies and no clear processes. Together with the finding of unique morphological features of NvEAAT1-functioning cells, these data suggest that extracellular glutamate metabolism, one of major glial functions, is deployed downstream of Gcm in specific neural cell types in Cnidaria.
Subject terms: Cell biology, Computational biology and bioinformatics, Evolution, Genetics, Molecular biology
Introduction
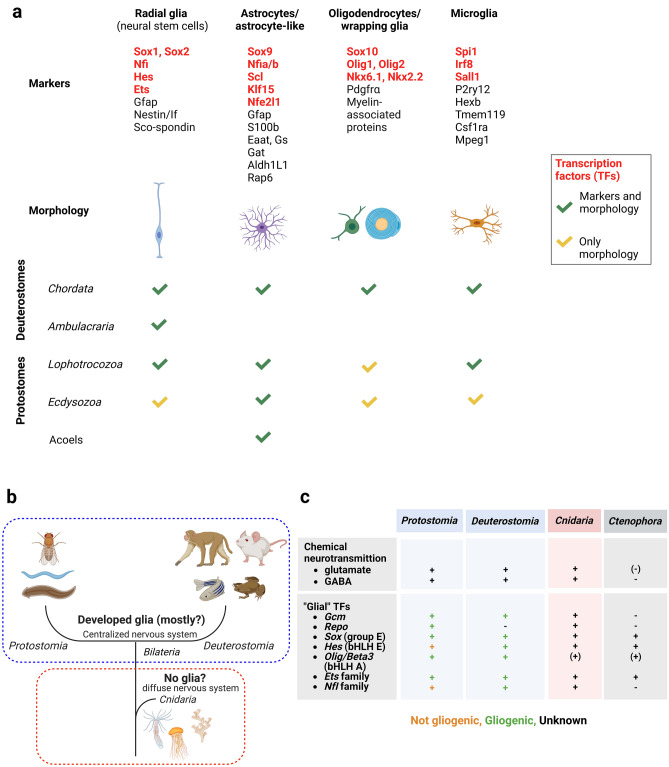
Nervous systems (NS) of bilaterian animals are generally composed of neurons and glial cells. Neurons are the electrically active primary functional units of the NS. As support cells, glial cells participate in almost every process in bilaterian nervous systems, including neurotransmission, homeostasis, and development1,2. In the course of bilaterian evolution, glial cells have acquired greater morphological and functional diversity2, including distinct types, even within individual glial cell classes (Fig. 1a)3. Although the emergence of glial cells is one of the key novelties in evolution of the nervous system, extensive glial studies have been mostly confined to a few bilaterian model organisms, such as nematodes, fruit flies, zebrafish, rodents, and humans. Evolutionary origins of glia remain obscure.
Figure 1.
Prerequisites for glial origins in the Metazoa (a) Glial cell type conservation in bilaterian phyla. Molecular markers and morphological features of major glial cell types are shown. (b) The current view of glial evolution assumes that glia emerged with centralization of the nervous system, i.e., after the last common bilaterian ancestor diverged from the Cnidaria. (c) Nervous system features accompanying glial presence are conserved in Cnidaria, including all bilaterian gliogenic TFs.
Where did glial cells originate, and what were their first functions? Glial cells are hypothesized to have evolved with the appearance of a centralized nervous system, i.e., after the common bilaterian ancestor branched off from the Cnidaria (Fig. 1b)2,4. Gliogenic program conservation has not been investigated to date in Cnidaria or any other non-bilaterians.
Cnidaria and Ctenophora are the neuron-bearing phyla among non-bilaterians. Neurons of both phyla are organized in nerve nets with regional condensation sites and are neuropeptide-rich5–8. The Cnidaria is thought to share key features of the bilaterian NS, including a diverse repertoire of neurons and neurotransmitters, in addition to a complete set of bilaterian gene homologs driving neurogenesis and neuron specification9–11. Neuronal expression of glutamate and GABA/glycine vesicular transporters in N. vectensis suggests that chemical transmitters in secretory vesicles bearing these transporters are used as neurotransmitters in Cnidaria (Fig. 1c)5. Glutamate has been suggested as a candidate chemical messenger in ctenophores12. However, vesicular transporter expression is abundant in epithelial cells, and it is unclear whether glutamate functions as a neurotransmitter in this basal lineage5.
Incidentally, glutamate signaling modulation is one of the most prominent and conserved features of glial cells from Caenorhabditis elegans13 and Drosophila melanogaster14 to mammals15. Moreover, excitatory amino acid transporters (EAATs) are expressed in glia or glia-like cells of non-model invertebrates, including lancets16 and planarians17. To understand still enigmatic early evolution of glial system in neural functions, especially regulatory mechanisms of glutamate signaling, it is useful to analyze in cnidarians the function of gliogenic transcription factors (TFs) , endogenous drivers of glial cell identity in bilaterians. There are several TFs that drive generation of glial cells in the bilaterian nervous systems. In Drosophila, Gcm is a master regulator of gliogenesis. A mutation in this gene turns presumptive glia into neurons18. The glial program is activated by a downstream target of Gcm, Repo, which drives expression of glial-specific markers, including an Ets protein family member Pnt (pointed) (Fig. 1c)19. In vertebrates, gliogenetic mechanisms are more complicated, with multiple TFs, including not only Gcm, but also bHLHs, Sox group E, Ets, and NFI family proteins (Fig. 1c)20–22. Although vertebrate Gcm genes have diverse functions, Gcm1, a vertebrate Gcm ortholog, has neuro/gliogenic functions. For example, Gcm1 drives neuronal differentiation in chickens23,24. Rodent Gcm1 has the capacity to induce gliogenesis and to drive astrocyte differentiation25,26. The neural potential of Gcm homologs was also shown in other metazoans. For instance, Gcm expression confined to neurosecretory-like cells was demonstrated in freshwater crayfish27. Neuronal expression/function of Gcm homologues in various bilaterian lineages is of interest in regards to what the symplesiomorphy of Gcm function is.
In Cnidaria, a Gcm homolog is expressed in a subset of probable neuronal cells in Nematostella vectensis7, one of the most widely used cnidarian model organisms to address questions about evolution of the nervous system, thanks to the ease with which it can be cultured and to numerous established gene manipulation techniques28,29. Cell cluster enrichment analysis in a single-cell transcriptome of N. vectensis supports assumed neuronal functions of NvGcm9–11; however, the authors performed no functional exploration of its NvGcm-dependent gene regulatory network or characterization of NvGcm-expressing cells. This prompted us to investigate NvGcm and its candidate targets more carefully.
We performed NvGcm knockdown (KD) experiments using gene-specific siRNA and genome-wide screening of genes affected by Gcm depletion in N. vectensis. By characterizing transcripts affected by NvGcm KD, we identified genes involved in neurotransmission, in lipid and glucose metabolism, and cell adhesion molecules, suggesting a neural nature of the NvGcm-controlled program. Surprisingly, a previously undescribed cell type was revealed by immunostaining for the glutamate transporter EAAT1, a conserved target of Gcm. Morphologically these cells do not resemble any described N. vectensis neurons; therefore, they are a novel neural cell type. We use the term “neural” to denote cells including neurons and supporting cells like glial cells, whereas “neuronal” is used to denote neurons specifically. Therefore, we refer to Nematostella Eaat1-expressing cells as neural, but not necessarily neurons. These cells may represent “protoglia”, functioning primarily as glutamate-quenching cells for pepti-glutamatergic neurons that use both glutamate and neuropeptides as neurotrasnmitters.
Results
Homologs of all bilaterian glial TFs, including Gcm, are found in a cnidarian model organism, Nematostella vectensis, but not in ctenophores (Fig. 1c). Moreover, among non-bilaterians, the Gcm domain is conserved only in the Cnidaria (Supplementary Fig. 1).
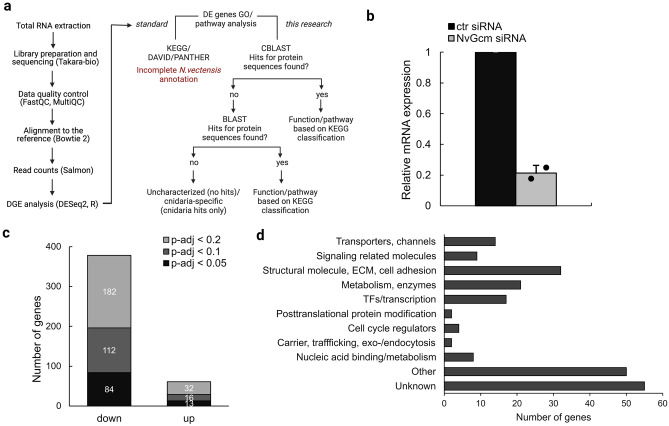
To develop an unbiased view of NvGcm targets in N. vectensis, we knocked it down using NvGcm-specific siRNA30 and performed transcriptomic analysis (Fig. 2a). KD efficiency of NvGcm-targeted siRNA was ~ 75% for samples selected for RNA-seq analysis (Fig. 2b). To identify genes affected by NvGcm KD, we performed differential gene expression (DE) analysis and identified genes that were downregulated or upregulated in NvGcm-depleted larvae (Fig. 2c).
Figure 2.
Transcriptomic analysis of Gcm KG in N. vectensis. (a) RNA-seq workflow used in this study (b) NvGcm KD efficiency of siRNA in experimental batches selected for RNA-seq analysis. (c) Total number of DE genes in NvGcm-depleted larvae depending on p-adj. (d) Functional classification of NvGcm candidate target genes.
We next analyzed the composition of all DE genes (p-adj < 0.2, 214 genes) with respect to their pathway groups, as defined in the KEGG PATHWAY Database. All candidate genes were grouped into the same eleven functional categories to characterize NvGcm candidate target genes in Drosophila (Fig. 2d)31. Functions of about a fourth of the identified genes are unknown. As in Drosophila, transcription factors and genes encoding ion transporters and channels constitute 8% and 7% of affected genes respectively, suggesting a possible function in cell fate specification and homeostatic control of NvGcm. A large fraction (15%) of NvGcm candidate target genes are structural and cell adhesion molecules, whereas 4% are signaling-related molecules. Another 10% of these genes are enzymes involved in general metabolism. All DE genes and their functional classifications are listed in Supplementary Table 2.
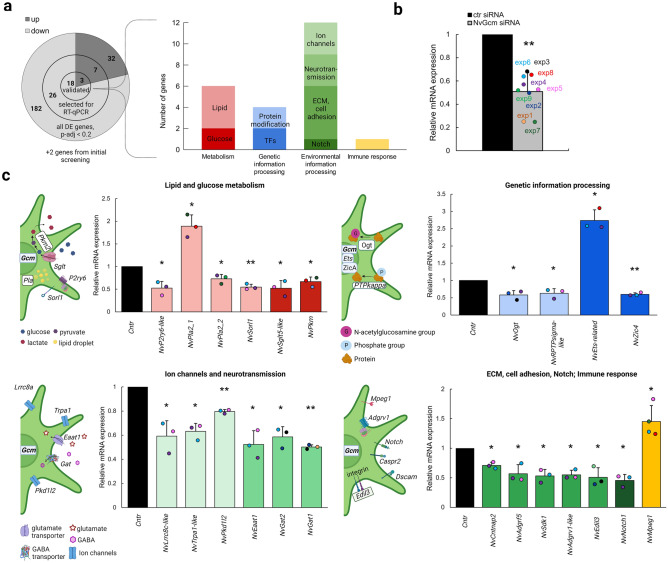
To further validate NvGcm candidate target genes, we selected several genes from each of the major GO groups for RT-qPCR analysis (Fig. 3a). We ignored those with functional groups that would not contribute to understanding the NvGcm-regulated program (unknown function, ncRNA/miscRNA, RNA splicing, ribosome, RT/retrotransposons, etc.). NvGcm KD efficiency was assessed with RT-qPCR for each experimental batch (Fig. 3b). We selected 33 genes for RT-qPCR validation and confirmed that 21 of them are up/downregulated in NvGcm-depleted larvae (Fig. 3a). In addition to these 21 genes identified in RNA-seq data, we confirmed that two genes encoding GABA transporters (Gats) are affected by NvGcm KD. We grouped validated NvGcm candidate target genes into nine functional categories, described below (Fig. 3a). All RT-qPCR validated candidate genes, their functional classifications, and RNA-seq attributes are listed in Supplementary Table 3.
Figure 3.
RT-qPCR validation of DE genes in NvGcm-depleted larvae. (a) Total number of DE genes identified in RNA-seq at p-adj < 0.2, numbers of DE genes selected for RT-qPCR validation, and numbers of genes validated by RT-qPCR. Functional classification of RT-qPCR validated DE genes (b) NvGcm KD efficiency in experimental batches used for RT-qPCR validation. Each experiment is color-coded. (c) Relative mRNA expression of validated candidate genes in NvGcm-depleted larvae compared to controls. Some up-regulated DE genes (NvPla2.1, NvEts-related, and NvMpeg1) are also shown. Each data point is color-coded and corresponds to the experiment in b. Simplified cartoons to the left of the bar graphs in c demonstrate cellular processes in which validated candidate genes are involved.
Interestingly, NvGcm downstream targets in Nematostella included genes essential in bilaterian neuronal and glial functions. First, are genes encoding proteins involved in glucose and lipid metabolism: Sorl1 (sortilin-related receptor 1), Pla2s (phospholipases A2), P2Y6-like (purinoceptor P2y6-like), Sglt5-like (sodium-dependent glucose cotransporter 5-like), and Pkm (pyruvate kinase) (Fig. 3c). Second, there are post-translational protein modification enzymes required for axonogenesis and axonal support: RPTPsigma-like (receptor-type tyrosine-protein phosphatase S-like) and Ogt (UDP-N-acetylglucosamine–peptide N-acetylglucosaminyltransferase) (Fig. 3c)32. Third, there are TFs involved in development of the nervous system and in driving glio/neurogenesis: Ets-related and Zic4 (zinc finger) (Fig. 3c). Fourth, several ion channels and chemical neurotransmitter transporters are among the candidate genes: Eaat1 (glutamate transporter), Lrrc8a (volume-regulated anion channel), Gats (GABA transporters), Pkd1l2 (transient receptor potential polycystic (TRPP) channel), and Trpa1 (transient receptor potential cation channel) (Fig. 3c). Finally, candidate genes include cell adhesion and signaling molecules involved in organizing synapses and promoting glio-neuronal interactions: Caspr2 (contactin-associated protein-like 2), Adgrf5 (adhesion G protein-coupled receptor F5), Sdk1 (sidekick 1), ADGRV1-like (adhesion G protein-coupled receptor V1-like), an integrin ligand Edil3 (EGF-like repeat and discoidin I-like domain-containing protein, Notch1, and Mpeg1 (Fig. 3c).
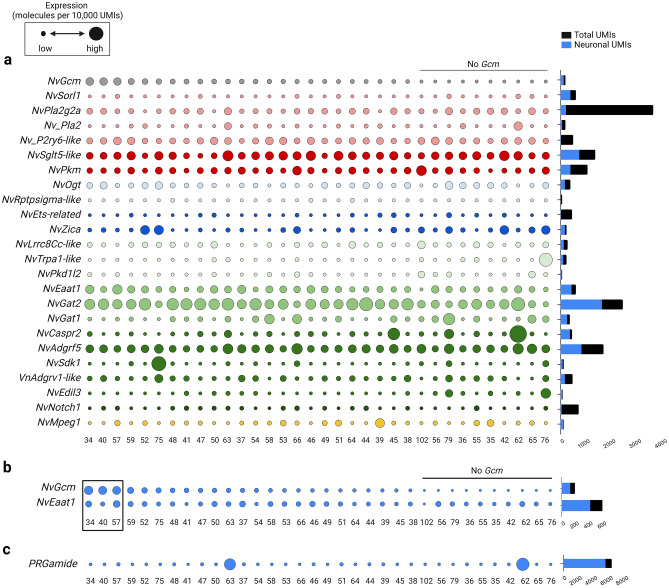
We next analyzed expression profiles of NvGcm and its downstream genes using single-cell transcriptomic (SCT) data from adult N. vectensis to furnish an overview of functionally fully differentiated cell repertoires10. We found that NvGcm and most candidate genes are predominantly expressed in neuronal cell clusters (Fig. 4a, Supplementary Fig. 3a,b). NvGcm is broadly expressed across neuronal cell clusters, with the highest expression in C34, C40, and C57 (Fig. 4b, Supplementary Fig. 3c). Expression of NvGcm mRNA in a relatively broad range of cell types suggests several possible explanations. If it indicates the breadth of cell types in which this transcription factor actually functions, NvGcm may be involved in regulating expression of these downstream genes in various neurons, rather than deciding the fate of specific neuronal cell types, such as glia. Alternatively, alteration of gene expression by NvGcm depletion may be due, at least in part, to non-cell-autonomous effects. Among candidate genes, a glutamate transporter, NvEaat1, is of particular interest, as the highest expression of NvEaat1 mRNA is found in C34 and C57, clusters enriched with NvGcm (Fig. 4b, Supplementary Fig. 3c). This is in sharp contrast to neuropeptides, e.g., PRGamide, that tends to be expressed only in specific neuronal clusters (Fig. 4c). In Drosophila, expression of excitatory amino acid transporters, Eaat1 and Eaat2, depends on Gcm33,34, suggesting that Eaat1 is a conserved Gcm target. Although these data reveal NvGCM-dependent gene expression, it is helpful to confirm protein expression to gain further insight into cells in which downstream genes function. Transcriptomes and proteomes tend to be correlated overall, but there are known exceptions35,36.
Figure 4.
Genetic signature of NvGcm-expressing cells. Gene expression is based on scRNA-seq data (10). The dot plot shows normalized expression values (molecules per 10,000 UMIs) scaled by the gene. Smallest and largest dots represent the lowest and highest expression, respectively. Cell clusters are organized left to right in descending order of NvGcm expression. (a) Expression of NvGcm and its candidate target genes across neuronal cell clusters in adult N. vectensis. Dots are colored in accordance with gene functional groups as presented in Fig. 3. (b) Expression of NvGcm and NvEaat1 across adult neuronal cell clusters. Cell clusters with the highest expression of NvGcm are framed (c) Expression of PRGamide neuropeptide gene across adult neuronal cell clusters.
To obtain information on morphological and physiological features of cells expressing glial genes, we first tried to identify cells expressing NvGCM protein using a specific antibody. However, our NvGCM antibody identified multiple bands in western blotting (Supplementary Fig. 4) and no clear signal in immunostaining, probably due to the low expression level of NvGcm mRNA (Fig. 4a). We next tried commercially available EAAT1 antibodies. The antibodies were selected based on the sequence similarity of the NvEAAT1 sequences and the epitopes declared to be recognized by the antibodies (Supplementary Fig. 5). An antibody from German Research Products gave a strong, specific signal in western blotting, while an antibody from Bio-Techne recognized multiple proteins of various molecular sizes (Supplementary Fig. 6a). Nevertheless, both EAAT1 antibodies detected a band with the expected size of NvEAAT1 (65 kDa) and identical staining patterns in immunostaining (Fig. 5a).
Figure 5.
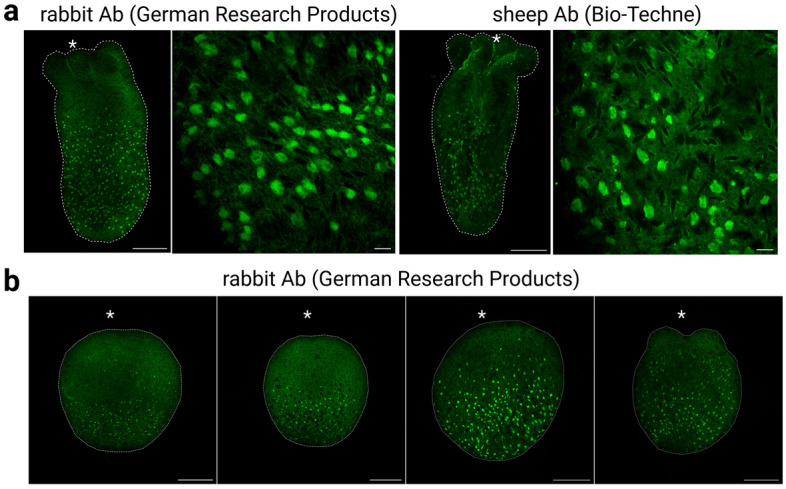
Morphology of NvEAAT1-positive cells. (a) EAAT1-expressing cells stained with German Research Products (rabbit) and Bio-Techne (sheep) anti-EAAT1 antibodies are broadly detected throughout the aboral region of the body column during the juvenile polyp stage (9dpf). Cells have flat morphology and lack processes. (b) Expression of NvEAAT1 at different developmental stages revealed by immunostaining with German Research Products anti-EAAT1 antibody. EAAT1-expressing cells became visible during larval stages. Side views of (from left to right): late gastrula stage (3dpf), early planula stage (4dpf), planula stage (5dpf), tentacle bud stage (6dpf). Asterisks indicate oral positions. Large and small scale bars are 100 μm and 10 μm, respectively.
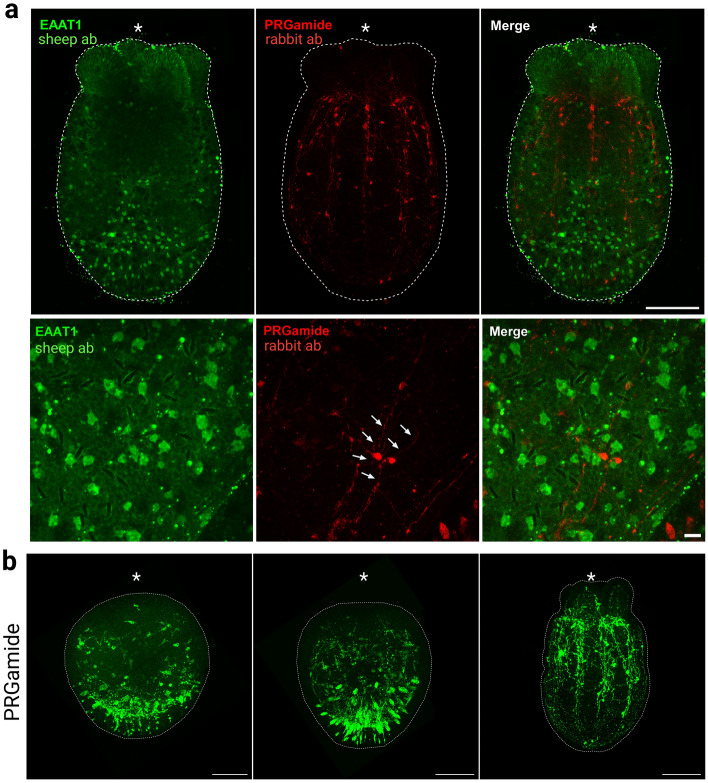
EAAT1-positive cells began to be detected at late gastrula stage in outer ectoderm of the aboral region (Fig. 5b). At late gastrula and early planula stages, only a few cells were detected. Numerous EAAT1-positive cells became visible in a broad aboral region from planula through primary polyp stages. The salt-and-pepper-like distribution of NvEAAT1-expressing cells in aboral epithelium is reminiscent of expression patterns of other genes expressed in neurons37,38. However, EAAT1-positive cells exhibited flat morphology without clear long neurites, a characteristic different from that of cnidarian interneurons with spherical or flask-shaped cell bodies (Fig. 6a, Supplementary Fig. 7). In fact, immunostaining for PRGamide, a neuropeptide most abundantly expressed in the NvGcm-enriched neuronal cluster during the larval stage (Supplementary Fig. 8), demonstrated typical morphology of neuropeptide-positive neurons at the aboral side of planula larvae (Fig. 6b).
Figure 6.
Non-overlapping expression of PRGamide and EAAT1 protein in the N. vectensis nervous system. (a) Immunofluorescence co-staining using anti-EAAT1 antibody (Bio-Techne) and anti-PRGamide antibody during the primary polyp stage (7dpf). EAAT1 staining and PRGamide staining do not overlap. EAAT1-positive cells lack processes, unlike PRGamide-positive neurons, which extend neurites (arrows). (b) PRGamide-expressing neurons detected at different developmental stages. Side views of (from left to right): early planula stage (4dpf), planula stage (5dpf), juvenile polyp stage (7dpf). Asterisks indicate oral positions. Large and small scale bars are 100 μm and 10 μm, respectively.
Taken together, our functional analysis of NvGcm identified a number of Gcm downstream genes including neuronal/glial genes involved in glutamate quenching in N. vectensis. Although mRNAs of NvGcm and many of its downstream genes, including EAAT1, were widely expressed in various neuronal clusters, EAAT1 protein was visualized mainly in a population of cells with non-neuronal morphology.
Discussion
The glial cell system is highly developed in the central nervous system of various bilaterian lineages. This suggests that glial cell function is required for sophisticated nervous systems. To understand evolution of the neuro-glial system, it is necessary to understand how glial cells became functionally deployed in early stages of evolution. However, glial function in the nervous system in pre-bilaterians is still unknown. In this work, for the first time, we analyzed Gcm function in non-bilaterian animals.
Although it is not clear what functions NvGcm-target genes have, they can be assumed from what is known about bilaterian homologs of these genes. NvGcm-dependent genes include a TF homolog of the zinc finger protein family, Zic4, which is required for development of the nervous system in mice, frogs, and humans39,40, as well as homologs of post-translational protein modification enzymes, RPTPsigma and Ogt. RPTPsigma is indispensable for axon guidance and axonogenesis in bilaterians32. Ogt is an important metabolic sensor involved in glycolysis regulation, which mediates glia-neuron interaction by promoting glial axonal support41. Surprisingly, we found that expression of an Ets domain-containing TF increased in NvGcm-deficient larvae. Conversely, in Drosophila, Gcm induces expression of a glia-specific Ets protein family member, Pnt (pointed domain) via a homeobox TF, Repo. Ets family genes are a large group of TFs, some of which also drive gliogenesis in vertebrates42–44. In N. vectensis, 12 Ets genes were found, with NvGcm being a member of an apical pole gene regulatory network of a Pointed-containing Ets gene, NvErg45,46. An Ets gene we identified as a NvGcm candidate target in this study, does not contain a pointed domain and is not an obvious homolog of a specific bilaterian Ets protein. Its function in N. vectensis is unknown.
Future studies in N. vectensis should clarify the extent to which the Gcm-Ets axis is functionally conserved in non-bilaterians. In Drosophila, Repo is an important downstream target of Gcm, driving gliogenesis. However, in N. vectensis expression patterns of NvRepo and NvGcm do not overlap7, and our RNA-seq data did not contain NvRepo TF as a DE gene in NvGcm-deficient larvae. Moreover, the two TFs are most abundant in different cell clusters at both adult and larval stages (Supplementary Fig. 9). In adult animals, the neuronal cell cluster with the highest expression of NvRepo is devoid of NvGcm. Similarly, during the larval stage, NvRepo is most abundant in NvGcm-negative cell clusters. At both adult and larval stages, gland/secretory cell clusters demonstrate the highest expression of NvRepo, unlike NvGcm, indicating a non-neuronal function of Repo. Given that Repo is not reported to have a gliogenic role in animals other than insects, with vertebrates lacking the gene altogether, the Gcm-Repo axis may have been established during arthropod evolution.
As with Drosophila Gcm, NvGcm candidate target genes include genes with known metabolic functions in bilaterians. These include sodium/glucose co-transporter, NvSglt5-like, and a pyruvate kinase, NvPkm, mediating the last step of glucose conversion to pyruvate, as well as receptors, NvSorl1 and NvP2ry6, and enzymes, NvPlas, involved in lipid metabolism. This suggests that N. vectensis Gcm-expressing cells are metabolically active in ways resembling astrocytes and Drosophila glia, which store energy in the form of glycogen and lipid droplets for subsequent nutrient supply to neurons47–50. On the other hand, an increased expression of inflammatory marker homologs in NvGcm KD animals, a phospholypase PLA251 and an immune effector, Mpeg152,53, may indicate unknown involvement in the anti-inflammatory function of NvGcm. This is consistent with the recent finding of a conserved anti-inflammatory transcriptional cascade of Gcm from Drosophila to vertebrates54.
We identified homologs of several cell adhesion molecules, G-protein-coupled receptor and signaling proteins as NvGcm candidate targets abundant in neuronal cell clusters (Fig. 4a). This suggests that NvGcm-expressing cells actively participate in organizing the nervous system during N. vectensis development. Reduced expression of ion channels and neurotransmitter transporters in NvGcm-depleted larvae indicates functional involvement of these cells in chemical neurotransmission. Although in our RNA-seq data no plasmalemmal GABA transporters were affected by NvGcm KD, we analyzed expression of three NvGats in NvGcm-depleted larvae and found a reduction of two NvGats (Fig. 3c). This suggests possible involvement of Gcm-expressing cells in GABA uptake following neurotransmission in N. vectensis. However, it is still debated whether GABA is used as a neurotransmitter in cnidarians5,11. For instance, most expression of glutamic acid decarboxylase (GAD), the enzyme that catalyzes conversion of glutamate to GABA, is confined to non-neural cells, such as gastrodermis. Glutamate, on the other hand, is likely released via vesicular packaging from neurons, based on the gene expression profile of glutamate neurotransmission machinery in N. vectensis5. It is interesting to note that the emergence of the Gcm gene coincides with the appearance of neurons that use glutamate as a chemical transmitter.
Immunostaining of NvEAAT1 revealed a previously undescribed cell type, scattered throughout the aboral ectoderm during both larval and polyp stages of N. vectensis (Fig. 5). Although cell clusters with abundant expression of NvGcm/NvEaat1 in the adult SCT are classified as neuronal because of their enrichment with classical neuronal markers, in at least some of those cells, the function of quenching extracellular glutamate is under control of Gcm.
Due to the lack of successful visualization of glutamate-releasing neurons, further investigation is needed to determine how cells expressing EAAT1 protein are spatially and physiologically associated with glutamatergic neurons. The extent of functional diversity of neurons in the Cnidaria is still unclear. However, the diversity of genetic signatures exhibited by neuronal clusters of N. vectensis at the mRNA level, taken together with the possible presence of additional regulatory mechanisms at the protein translation level, implies potential richness of neuronal functional variation. For example, we currently do not know the reason for the discrepancy between the broad expression profile of NvEAAT1 mRNA seen on dot plots and the restricted expression pattern in immunostaining of NvEAAT1 protein. Perhaps NvEAAT1 protein expression is also regulated at the translational as well as transcriptional level. In addition, it is not yet clear whether the cells visualized by the EAAT1 antibodies are a type of neural cell. However, the fact that the Gcm and Eaat1 genes are predominantly expressed in neuronal clusters in cnidarians suggests that cell types with developed glial functions have an evolutionary origin as “protoglia”, which had both neuronal and glial features. It is assumed that a new cell type emerges by a modification of a pre-existing cell type55,56. It is possible that the last common ancestor of Bilateria and Cnidaria possessed cells combining characteristics of both neurons and glia, which were inherited by both animal groups and subsequently diverged. Later through the “division of labor” various glial cell types emerged in the Bilateria.
Methods
Phylogenetic analysis of GCM
Bidirectional BLAST57,58 searches using the Drosophila melanogaster GCM1 protein sequence were performed using databases of representative bilaterians and non-bilaterians (Supplementary Table 1). Fungal protein sequences with the highest similarity to animal GCM were used as an outgroup. Sequences were aligned using MUSCLE (Mega7) and trimmed by eye to include only the domain. Trees were constructed with the maximum likelihood (ML) method using PhyML (SeaView). Bootstrap support is based on 2000 replicates.
Nematostella vectensis culture
N. vectensis was cultured as described previously28.
RNA interference
For NvGcm and NvEaat1 RNA interference by transfection, N. vectensis embryos were electroporated as described in the previous report59 with the following modifications. No Ficoll was added to the brackish water medium because it decreased embryo survival. siRNA was used instead of shRNA due to the simplicity of siRNA manufacturing. Egg masses were collected, de-gelled, and fertilized prior to transfection. Electroporation was carried out using a Gene Pulser Xcell electroporation system (BIO-RAD).
NvGcm-specific and NvEaat1-specific siRNAs sequences were designed to knock down those genes (Supplementary Table 4). Updated N. vectensis genome assembly (NCBI, 20 Mar 2021) distinguishes two Gcm genes: 5,495,825 and 5,504,408 (IDs). Because of the high similarity of the sequences (98%), it is technically difficult to separate the two genes and to analyze their protein functions independently. The two NvGcms are not distinguished in single-cell data10. In our study we do not separate them either. Instead, NvGcm-targeted siRNAs and NvGcm primers recognize both paralogs. Control embryos were electroporated with a negative control siRNA that was not complementary to any part of the genome (Supplementary Table 4). An siRNA concentration of 500 ng/uL was used. 4dpf N. vectensis planulae were collected for RNA extraction and knock-down efficiency assessment employed RT-qPCR.
RNA extraction
Total RNA was extracted from N. vectensis planula larvae 4 days post-fertilization (dpf) using an RNeasy Mini Kit per manufacturer's guidelines (QIAGEN). Total RNA was dissolved in 30 μL RNase-free water and used immediately for, or stored at − 80 °C prior to cDNA synthesis. A NanoDropTM 1000 Spectrophotometer (Thermo Fisher Scientific) was used to check RNA concentrations and purity. Samples with absorbance ratios OD260/OD280 and OD260/OD230 higher than 1.6 were used. Extracted RNA for RNA-seq experiments was quality-checked using an Agilent TapeStation (Takara Bio facility). RIN (RNA integrity number) values of all samples were confirmed to be 10, i.e., sufficient for high-quality sequencing.
Next-generation sequencing
Total RNA was extracted from five biological replicates in each of the three groups: Gcm KD siRNA1, Gcm KD siRNA2, and control siRNA. RNA extracted from all 15 samples was placed on dry ice and sent to Takara for sequencing. Library preparation (TruSeq RNA Library Prep Kit) and sequencing (NovaSeq6000) were also done by Takara bio (paired-end: 150 bp × 2; depth: 50–80 ml). Gcm KD efficiency in RNA samples used for sequencing was assessed with RT-qPCR analysis. RNA from several knock-down experiments was used to confirm DE genes identified by RNA-seq data analysis.
RNA-seq data quality control and read mapping
Read quality was assessed using FastQC (Andrews, 2010) and visualized with the MultiQC python package60. These data were of good quality and no trimming was required. Paired reads were aligned to the most updated N. vectensis gene models available on Figshare using Bowtie 2 aligner61 with prior filtering using CD-HIT-EST (similarity threshold set to 94%) to remove isoforms62. The overall mean alignment rate was 76% (Supplementary Table S5). SAMtools was used to convert files from SAM to BAM format. Salmon63 was used to quantify transcript abundances. Salmon-generated pseudo counts, represented as normalized TPM (transcripts per million), were converted into non-normalized count estimates for differential gene expression analysis. RNA-seq profiles of 23,913 transcripts were obtained.
Differential gene expression analysis
To identify genes differentially expressed (DE) in Gcm KD and control groups, Bioconductor DESeq2 was used64. Sample-level quality control (QC) was performed using Principal Component Analysis (PCA) and hierarchical clustering methods. Two of five experimental batches were selected to ensure that experimental conditions constituted the primary source of variation. Gcm KD for both siRNA1 and siRNA2 was most efficient in these batches (Supplementary Fig. 10). Experiment dates represented another source of variation, which was controlled during DEseq analysis. Gcm KD siRNA1 and siRNA2 groups were compared to the control group separately. The P-adj (false discovery rate) was set to 0.05, 01, and 0.2. Results for siRNA2 vs. control are shown because this siRNA was consistently more efficient and yielded more DE genes. DE genes for siRNA1 vs control are presented in Supplementary Table 6.
Functional analysis of DE genes
Available databases used for gene ontology (GO)/pathway analysis such as KEGG, PANTHER, and DAVID have incomplete N. vectensis annotations. Therefore, in order to categorize DE genes into functional groups and explore their molecular pathways, each protein function was inferred from its similarity to bilaterian proteins using CBLAST (protein data bank)/BLAST57,58. Genes were then assigned to pathway groups as defined in the KEGG PATHWAY Database65.
Real-time qPCR
Single-stranded cDNA was synthesized from 300 to 500 ng of total RNA in a final volume of 20 µL using oligo(dT)20 and SuperScript IV Reverse Transcriptase (Invitrogen), according to manufacturer’s instructions. cDNA was stored at − 20 °C for future use. Agarose gels of PCR products obtained using primers for a gene not affected by Gcm KD as confirmed by RT-qPCR were run to check for genomic DNA contamination of cDNA. Primers were designed to yield PCR products of different lengths for cDNA and gDNA (Supplementary Table 7). RT-qPCR was performed using a StepOne Plus™ Real-Time PCR System (Thermo Fisher Scientific). The amplification program was set as follows (fast mode ~ 40 min): holding stage at 95 °C for 20 s followed by 40 cycles of 3 s at 95 °C and 30 s at 60 °C. After amplification, a denaturing cycle (15 s at 95° followed by 1 min at 60 °C and 15 s at 95 °C) was performed to obtain melting curves and to verify amplification specificity. Samples for RT-qPCR were prepared using PowerUp SYBR® Green Master Mix, as per the manufacturer’s instructions. Primers were designed using Geneious 10.2.4 and Primer-BLAST66. Primer sequences and product sizes are listed in Supplementary Table 7. Primer efficiencies were assessed using cDNA fivefold serial dilutions through generation of a standard curve. Primers with 80–110% efficiency were chosen for qPCR analysis. Four samples were run for each gene. Triplicates were used for analysis. In qPCR data normalization, Norm-Finder67 was used to assess variability of three candidate housekeeping genes, Gapdh, Ef1a, and 18S, and how much they are affected by experimental conditions, which are important factors to consider when choosing normalization genes68. Ef1a was consistently the best candidate among the three with the lowest intra- and intergroup variation (3 experiments, 4–8 samples per group, 3 groups: siRNA1, siRNA2, control siRNA). As a result, expression of genes of interest was normalized to Ef1a. The comparative Ct (△△Ct) value method was used for relative quantification69. RT-qPCR data are presented as expression fold change (± SD) normalized to one endogenous control (Ef1a) and relative to control siRNA. Means of different groups were compared (siRNA2 and siRNA control) and analyzed using Student’s unpaired t-test. Results from three independent experiments were used for the analysis unless stated otherwise. Differences were considered statistically significant when the p-value was < 0.05.
Immunostaining and western blotting
To detect NvEAAT1 two polyclonal antibodies were used: rabbit anti-EAAT1 antibody (German Research Products, 1:400 for IF and 1:1000 for WB), sheep anti-EAAT1/GLAST-1 antibody (Bio-Techne, Cat. #AF6048, 10 μg/mL for IF and 5 μg/mL for WB). To detect PRGamide, an affinity-purified rabbit IgG raised against amidated Cys-PRGamide (1:300 for IF)5. The following secondary antibodies were used: Alexa Fluor 488-conjugated anti-rabbit and anti-sheep antibodies (goat, Jackson ImmunoResearch, 1:500) for IF; Alexa Fluor 647-conjugated anti-rabbit antibodies (goat, Jackson ImmunoResearch, 1:500) for IF; anti-rabbit and anti-sheep peroxidase-conjugated antibodies (goat, Jackson ImmunoResearch, 1:10,000) for WB. For indirect immunostaining, bud/polyp stage Nematostella were anesthetized using 2.43% MgCl2 for 10 min. Planulae and anesthetized bud/polyps were fixed in ice-cold 4% PFA/PBS + 0.1% Triton X-100 (PBSTx) for 1 h at RT on a rotator. Samples were washed 3 times (10 min each) with PBS + 0.1%Tween (PBT). Samples were then incubated in blocking solution (PBT/1%BSA/5% Normal goat serum/0.01%NaN3) for 1 h at RT, followed by o/n incubation with primary antibodies at 4 °C on the rotator. For double immunostaining, samples were incubated with sheep anti-EAAT1 antibody (Bio-Techne) and rabbit anti-PRGamide antibody simultaneously. Samples were then washed 2 times (10 min each) with PBSTx and incubated in the blocking solution for 1 h at RT on the rotator. Samples were incubated in secondary antibodies for 1 h at RT on the rotator. Samples were then washed 3 times with PBSTx (15 min each) at RT. Samples were then incubated for 30 min in DAPI (1:1000) and mounted with SlowFade Gold Antifade Mountant (Invitrogen). Western blotting was carried out as described elsewhere70.
Microscopy
Images were captured on a Zeiss LSM780 confocal microscope system using × 40 and × 63 objectives. Image manipulation was performed with ZEISS ZEN microscope software and subsequently with ImageJ.
Supplementary Information
Acknowledgements
We thank J. Higuchi and A. Tanimoto for maintaining N. vectensis culture; C. Guzman and H. Wang for their help with retrieving scRNA-seq data, and Steven D. Aird for editing and proof-reading the manuscript. This study was supported by Okinawa Institute of Science and Technology Graduate University.
Author contributions
H.W. and L.S. designed the research and wrote the paper. LS performed experiments and analyzed data.
Data availability
RNA sequencing results were deposited in DNA Data Bank of Japan Sequence Read Archive under submission number DRA016214. Code used for RNA-seq analysis is available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-42046-9.
References
- 1.Araque A, Navarrete M. Glial cells in neuronal network function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010;365:2375–2381. doi: 10.1098/rstb.2009.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verkhratsky A, Butt A. Glial Physiology and Pathophysiology. John Wiley & Sons Ltd; 2013. pp. 73–104. [Google Scholar]
- 3.Zhang Y, Barres BA. Astrocyte heterogeneity: An underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Freeman MR, Rowitch DH. Evolving concepts of gliogenesis: A look way back and ahead to the next 25 years. Neuron. 2013;80:613–623. doi: 10.1016/j.neuron.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayakawa E, Guzman C, Horiguchi O, et al. Mass spectrometry of short peptides reveals common features of metazoan peptidergic neurons. Nat. Ecol. Evol. 2022;6:1438–1448. doi: 10.1038/s41559-022-01835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sachkova MY, et al. Neuropeptide repertoire and 3D anatomy of the ctenophore nervous system. Curr. Biol. 2021;31:5274–5285.e6. doi: 10.1016/j.cub.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Marlow HQ, Srivastava M, Matus DQ, Rokhsar D, Martindale MQ. Anatomy and development of the nervous system of Nematostella vectensis, an anthozoan cnidarian. Dev. Neurobiol. 2009;69:235–254. doi: 10.1002/dneu.20698. [DOI] [PubMed] [Google Scholar]
- 8.Havrilak JA, Al-Shaer L, Baban N, Akinci N, Layden MJ. Characterization of the dynamics and variability of neuronal subtype responses during growth, degrowth, and regeneration of Nematostella vectensis. BMC Biol. 2021;19:104. doi: 10.1186/s12915-021-01038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe H. Brain Evolution by Design. Springer; 2017. pp. 45–75. [Google Scholar]
- 10.Sebé-Pedrós A, et al. Cnidarian cell type diversity and regulation revealed by whole-organism single-cell RNA-Seq. Cell. 2018;173:1520–1534.e20. doi: 10.1016/j.cell.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Oren M, Brickner I, Brikner I, Appelbaum L, Levy O. Fast neurotransmission related genes are expressed in non nervous endoderm in the sea anemone Nematostella vectensis. PLoS ONE. 2014;9:e93832. doi: 10.1371/journal.pone.0093832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moroz LL, et al. The ctenophore genome and the evolutionary origins of neural systems. Nature. 2014;510:109–114. doi: 10.1038/nature13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrew Hardaway J, et al. Glial expression of the Caenorhabditis elegans gene swip-10 supports glutamate dependent control of extrasynaptic dopamine signaling. J. Neurosci. 2015;35:9409–9423. doi: 10.1523/JNEUROSCI.0800-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stacey SM, et al. Drosophila glial glutamate transporter Eaat1 is regulated by fringe-mediated notch signaling and is essential for larval locomotion. J. Neurosci. 2010;30:14446–14457. doi: 10.1523/JNEUROSCI.1021-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- 16.Bozzo M, et al. Amphioxus neuroglia: Molecular characterization and evidence for early compartmentalization of the developing nerve cord. Glia. 2021;69:1654–1678. doi: 10.1002/glia.23982. [DOI] [PubMed] [Google Scholar]
- 17.Wang IE, Lapan SW, Scimone ML, Clandinin TR, Reddien PW. Hedgehog signaling regulates gene expression in planarian glia. Elife. 2016;5:e16996. doi: 10.7554/eLife.16996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosoya T, Takizawa K, Nitta K, Hotta Y. glial cells missing: a binary switch between neuronal and glial determination in Drosophila. Cell. 1995;82:1025–1036. doi: 10.1016/0092-8674(95)90281-3. [DOI] [PubMed] [Google Scholar]
- 19.Yuasa Y, et al. Drosophila homeodomain protein REPO controls glial differentiation by cooperating with ETS and BTB transcription factors. Development. 2003;130:2419–2428. doi: 10.1242/dev.00468. [DOI] [PubMed] [Google Scholar]
- 20.Stolt CC, et al. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17:1677–1689. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molofsky AV, et al. Expression profiling of Aldh1l1-precursors in the developing spinal cord reveals glial lineage-specific genes and direct Sox9-Nfe2l1 interactions. Glia. 2013;61:1518–1532. doi: 10.1002/glia.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glasgow SM, et al. Mutual antagonism between Sox10 and NFIA regulates diversification of glial lineages and glioma subtypes. Nat. Neurosci. 2014;17:1322–1329. doi: 10.1038/nn.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soustelle L, et al. Neurogenic role of Gcm transcription factors is conserved in chicken spinal cord. Development. 2007;134:625–634. doi: 10.1242/dev.02750. [DOI] [PubMed] [Google Scholar]
- 24.Hitoshi S, et al. Mammalian Gcm genes induce Hes5 expression by active DNA demethylation and induce neural stem cells. Nat. Neurosci. 2011;14:957–964. doi: 10.1038/nn.2875. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki Y, et al. The potential to induce glial differentiation is conserved between Drosophila and mammalian glial cells missing genes. Development. 2003;130:6027–6035. doi: 10.1242/dev.00822. [DOI] [PubMed] [Google Scholar]
- 26.Saito A, et al. Unfolded protein response, activated by OASIS family transcription factors, promotes astrocyte differentiation. Nat. Commun. 2012;3:967. doi: 10.1038/ncomms1971. [DOI] [PubMed] [Google Scholar]
- 27.Junkunlo K, Söderhäll K, Söderhäll I. A transcription factor glial cell missing (Gcm) in the freshwater crayfish Pacifastacus leniusculus. Dev. Comp. Immunol. 2020;113:103782. doi: 10.1016/j.dci.2020.103782. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe H, et al. Sequential actions of β-catenin and Bmp pattern the oral nerve net in Nematostella vectensis. Nat. Commun. 2014;5:5536. doi: 10.1038/ncomms6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Layden MJ, Rentzsch F, Röttinger E. The rise of the starlet sea anemone Nematostella vectensis as a model system to investigate development and regeneration. Wiley Interdiscip. Rev. Dev. Biol. 2016;5:408–428. doi: 10.1002/wdev.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masuda-Ozawa T, et al. siRNA-mediated gene knockdown via electroporation in hydrozoan jellyfish embryos. Sci. Rep. 2022;12:16049. doi: 10.1038/s41598-022-20476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altenhein B, et al. Expression profiling of glial genes during Drosophila embryogenesis. Dev. Biol. 2006;296:545–560. doi: 10.1016/j.ydbio.2006.04.460. [DOI] [PubMed] [Google Scholar]
- 32.Johnson KG, Van Vactor D. Receptor protein tyrosine phosphatases in nervous system development. Physiol. Rev. 2003;83:1–24. doi: 10.1152/physrev.00016.2002. [DOI] [PubMed] [Google Scholar]
- 33.Soustelle L, Besson M-T, Rival T, Birman S. Terminal glial differentiation involves regulated expression of the excitatory amino acid transporters in the Drosophila embryonic CNS. Dev. Biol. 2002;248:294–306. doi: 10.1006/dbio.2002.0742. [DOI] [PubMed] [Google Scholar]
- 34.Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38:567–580. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Beyer A, Aebersold R. On the dependency of cellular protein levels on mRNA abundance. Cell. 2016;165:535–550. doi: 10.1016/j.cell.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Buccitelli C, Selbach M. mRNAs, proteins and the emerging principles of gene expression control. Nat. Rev. Genet. 2020;21:630–644. doi: 10.1038/s41576-020-0258-4. [DOI] [PubMed] [Google Scholar]
- 37.Havrilak JA, Faltine-Gonzalez D, Wen Y, Fodera D, Simpson AC, Magie CR, et al. Characterization of NvLWamide-like neurons reveals stereotypy in Nematostella nerve net development. Dev Biol. 2017;431:336–346. doi: 10.1016/j.ydbio.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 38.Tournière O, et al. NvPOU4/Brain3 functions as a terminal selector gene in the nervous system of the cnidarian Nematostella vectensis. Cell Rep. 2020;30:4473–4489.e5. doi: 10.1016/j.celrep.2020.03.031. [DOI] [PubMed] [Google Scholar]
- 39.Gaston-Massuet C, Henderson DJ, Greene NDE, Copp AJ. Zic4, a zinc-finger transcription factor, is expressed in the developing mouse nervous system. Dev. Dyn. 2005;233:1110–1115. doi: 10.1002/dvdy.20417. [DOI] [PubMed] [Google Scholar]
- 40.Fujimi TJ, Mikoshiba K, Aruga J. Xenopus Zic4: Conservation and diversification of expression profiles and protein function among the Xenopus Zic family. Dev. Dyn. 2006;235:3379–3386. doi: 10.1002/dvdy.20906. [DOI] [PubMed] [Google Scholar]
- 41.Kim S, et al. Schwann cell O-GlcNAc glycosylation is required for myelin maintenance and axon integrity. J. Neurosci. 2016;36:9633–9646. doi: 10.1523/JNEUROSCI.1235-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiyota T, Kato A, Kato Y. Ets-1 regulates radial glia formation during vertebrate embryogenesis. Organogenesis. 2007;3:93–101. doi: 10.4161/org.3.2.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hagedorn L, et al. The Ets domain transcription factor Erm distinguishes rat satellite glia from Schwann cells and is regulated in satellite cells by neuregulin signaling. Dev. Biol. 2000;219:44–58. doi: 10.1006/dbio.1999.9595. [DOI] [PubMed] [Google Scholar]
- 44.Ahmad ST, et al. Capicua regulates neural stem cell proliferation and lineage specification through control of Ets factors. Nat. Commun. 2019;10:2000. doi: 10.1038/s41467-019-09949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larroux C, et al. Developmental expression of transcription factor genes in a demosponge: Insights into the origin of metazoan multicellularity. Evol. Dev. 2006;8:150–173. doi: 10.1111/j.1525-142X.2006.00086.x. [DOI] [PubMed] [Google Scholar]
- 46.Amiel AR, et al. A bipolar role of the transcription factor ERG for cnidarian germ layer formation and apical domain patterning. Dev. Biol. 2017;430:346–361. doi: 10.1016/j.ydbio.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown AM, Ransom BR. Astrocyte glycogen and brain energy metabolism. Glia. 2007;55:1263–1271. doi: 10.1002/glia.20557. [DOI] [PubMed] [Google Scholar]
- 48.Freeman MR, Doherty J. Glial cell biology in Drosophila and vertebrates. Trends Neurosci. 2006;29:82–90. doi: 10.1016/j.tins.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Kis V, Barti B, Lippai M, Sass M. Specialized cortex glial cells accumulate lipid droplets in Drosophila melanogaster. PLoS ONE. 2015;10:e0131250. doi: 10.1371/journal.pone.0131250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ioannou MS, et al. Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell. 2019;177:1522–1535.e14. doi: 10.1016/j.cell.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Sun GY, et al. Phospholipase A2 in astrocytes: Responses to oxidative stress, inflammation, and G protein-coupled receptor agonists. Mol. Neurobiol. 2005;31:27–41. doi: 10.1385/MN:31:1-3:027. [DOI] [PubMed] [Google Scholar]
- 52.Benard EL, et al. Macrophage-expressed perforins mpeg1 and mpeg1.2 have an anti-bacterial function in zebrafish. J. Innate Immun. 2015;7:136–152. doi: 10.1159/000366103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pang SS, et al. The structure of Macrophage Expressed Gene-1, a phagolysosome immune effector that is activated upon acidification. bioRxiv. 2019 doi: 10.1101/580712. [DOI] [Google Scholar]
- 54.Pavlidaki A, Panic R, Monticelli S, Riet C, Yuasa Y, Cattenoz PB, et al. An anti-inflammatory transcriptional cascade conserved from flies to humans. Cell Rep. 2022;41:111506. doi: 10.1016/j.celrep.2022.111506. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt-Rhaesa A, Harzsch S, Purschke G. Structure and Evolution of Invertebrate Nervous Systems. Oxford University Press; 2015. [Google Scholar]
- 56.Arendt D, Bertucci PY, Achim K, Musser JM. Evolution of neuronal types and families. Curr. Opin. Neurobiol. 2019;56:144–152. doi: 10.1016/j.conb.2019.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 58.States DJ, Gish W. Combined use of sequence similarity and codon bias for coding region identification. J. Comput. Biol. 1994;1:39–50. doi: 10.1089/cmb.1994.1.39. [DOI] [PubMed] [Google Scholar]
- 59.Karabulut A, He S, Chen C-Y, McKinney SA, Gibson MC. Electroporation of short hairpin RNAs for rapid and efficient gene knockdown in the starlet sea anemone Nematostella vectensis. Dev Biol. 2019;448:7–15. doi: 10.1016/j.ydbio.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 60.Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32:3047–3048. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li W, Fu L, Niu B, Wu S, Wooley J. Ultrafast clustering algorithms for metagenomic sequence analysis. Brief. Bioinform. 2012;13:656–668. doi: 10.1093/bib/bbs035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods. 2017;14:417–419. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ye J, et al. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 68.Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005;6:279–284. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- 69.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 70.Gilmore, T. D., Wolenski, F. S. & Finnerty, J. R. Preparation of antiserum and detection of proteins by Western blotting using the starlet sea anemone, Nematostellavectensis. Sci. Protoc. (2012).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA sequencing results were deposited in DNA Data Bank of Japan Sequence Read Archive under submission number DRA016214. Code used for RNA-seq analysis is available upon request.