Abstract
Virilization is a rare condition in postmenopausal women, usually attributed to androgen excess of ovarian or adrenal origin. A 62-year-old woman presented with excessive hair loss of 3 months' duration and was investigated for an endocrine cause of alopecia. The hormonal evaluation revealed increased testosterone but normal levels of androstenedione and dehydroepiandrosterone sulfate, while the results of transvaginal ultrasonography and abdominal computed tomography were unremarkable. Based on these findings, the possibility of an adrenal androgen-secreting tumor was ruled out and suspicion of Leydig cell hyperplasia was raised. A bilateral laparoscopic salpingo-oophorectomy was performed due to the age of the patient and the diagnosis of Leydig cell hyperplasia was confirmed by histopathological examination. The postoperative course of the patient was uneventful and a repeat hormonal evaluation after the operation showed a normalization of androgen levels. In conclusion, Leydig cell hyperplasia should be considered as a likely cause of hyperandrogenism of ovarian origin in women who develop virilization. In postmenopausal women, bilateral oophorectomy will treat the disorder and provide a conclusive diagnosis via histopathological examination.
Keywords: Ovary, Leydig cell hyperplasia, Hilus cell hyperplasia, Virilization, Case report
Highlights
-
•
Postmenopausal virilization is a rare condition, usually attributed to androgen excess of ovarian or adrenal origin.
-
•
Leydig cell hyperplasia should be considered as a cause of hyperandrogenism of ovarian origin in women with virilization.
-
•
Bilateral oophorectomy will treat Leydig cell hyperplasia and provide a conclusive diagnosis in postmenopausal women.
1. Introduction
Virilization, which is defined as the development of male secondary sexual characteristics in a female due to excessive exposure of tissues to androgen action, is a rare condition in postmenopausal women, usually attributed to abnormal androgen secretion by benign or malignant lesions of the ovaries or adrenal glands. In some cases, virilization can be the result of androgen overproduction by ovarian Leydig (or hilus) cells in the context of hyperplasia or tumor [1].
Hyperplasia of Leydig cells was initially described by Scully. More than half of his series of patients presented evidence of androgen excess [2]. Expansion of the Leydig cell population in the form of hyperplasia can be the result of hormonal stimulation by either human chorionic gonadotropin (hCG) in pregnancy or luteinizing hormone (LH) in menopause and it is part of a generalized response of the ovarian stroma. Leydig cell hyperplasia constitutes a benign entity and is not grossly visible, whereas Leydig cell tumor is distinguished by the formation of a mass [3]. Diagnosis of these conditions can be challenging and is usually aided by hormone levels rather than imaging. A systematic approach to diagnosis is mandatory.
Herein, we present the case of a postmenopausal woman who developed rapidly progressive androgenetic alopecia owing to androgen overproduction by foci of Leydig cell hyperplasia in both ovaries.
2. Case Presentation
A 62-year-old woman, gravida 3, para 2, presented with excessive hair loss of 3 months' duration and was investigated for an endocrine cause of alopecia. She had stopped menstruating at the age of 52 years and mentioned no gynecological or general health issues except for well-controlled hypertension. Physical examination revealed areas of baldness (Fig. 1), a body mass index (BMI) of 28 kg/m2 and a Ferriman-Gallwey score of 6 [4]. Neither Cushingoid features nor symptoms and signs suggestive of diabetes mellitus were present. Systemic examination, which included a bimanual pelvic examination, was unremarkable.
Fig. 1.
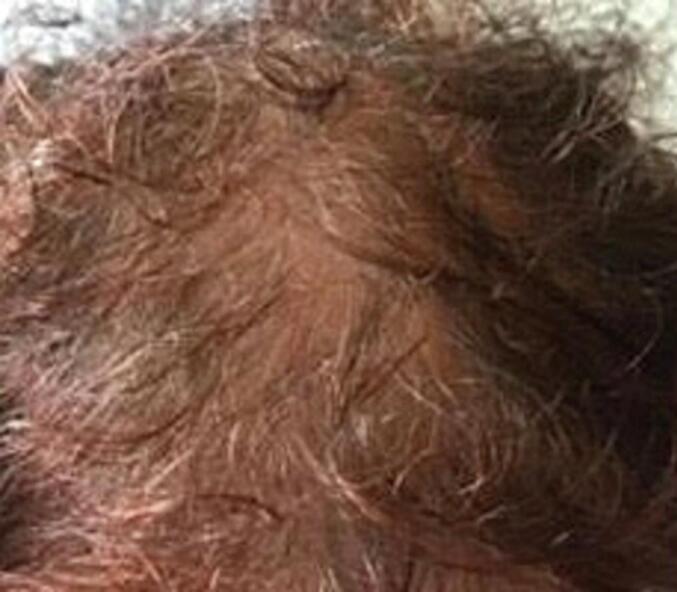
The patient's scalp with apparent hair loss.
Laboratory evaluation included hormone tests and tumor marker measurements to investigate for a possible ovarian sex cord-stromal tumor (Table 1). Testosterone levels were elevated, while androstenedione and dehydroepiandrosterone sulfate (DHEAS) levels were within normal limits.
Table 1.
Main preoperative laboratory findings.
| Test | Measured value | Reference range |
|---|---|---|
| Total testosterone | 1.75 μg/L | 0.10–0.75 μg/L |
| Free testosterone | 21.00 ng/L | 0.20–6.40 ng/L |
| DHEAS | 1.10 mg/L | 0.09–2.46 mg/L |
| Estradiol | 6.20 pg/mL | <20.00 pg/mL |
| Inhibin B | 14.90 ng/L | <37.00 ng/L |
DHEAS, Dehydroepiandrosterone sulfate.
Transvaginal ultrasonography (US) and computed tomography (CT) of the abdomen were also performed. On US, both ovaries were of normal morphology and size (volume of 2.8 mL and 3.5 mL for the left and right ovary, respectively), while the endometrium was represented as a thin stripe. The abdominal CT did not reveal any abnormality in either the ovaries or the adrenal glands.
In view of these findings, the possibility of an adrenal androgen-secreting tumor was ruled out and suspicion of Leydig cell hyperplasia was raised. A bilateral laparoscopic salpingo-oophorectomy and a dilatation and curettage (D&C) were performed due to the age of the patient. The peritoneal and pelvic cavities appeared normal. The right ovary had a yellowish color and was slightly larger than the left one (Fig. 2).
Fig. 2.
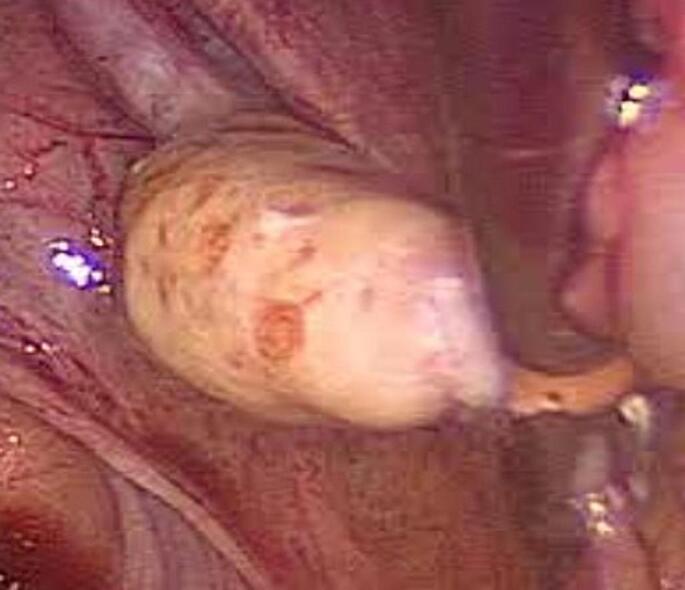
Macroscopic view of the right ovary during laparoscopy.
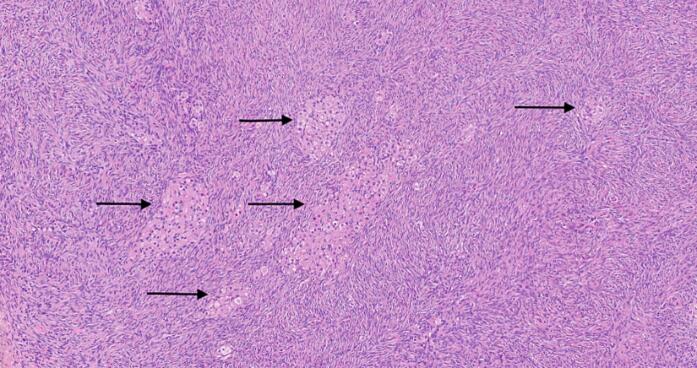
Histopathological examination of the ovaries confirmed the diagnosis. It revealed scattered aggregates of Leydig cells <1 mm in size mainly in the cortical and less in the medullar area of both ovaries. Their nuclei were normal and surrounded by granular eosinophilic cytoplasm (Fig. 3). These findings are consistent with Leydig cell hyperplasia. Endometrium was atrophic upon histopathological examination.
Fig. 3.
Histopathological findings with hematoxylin & eosin staining and 100× magnification. Several small irregular islands of hyperplastic Leydig cells (arrows) with abundant eosinophilic cytoplasm and bland nuclei and without atypia or mitoses are observed within the ovarian stroma.
The postoperative recovery of the patient was uneventful. A repeat hormonal evaluation showed a normalization of androgen levels after surgery.
3. Discussion
Ovarian Leydig cells, which resemble testicular Leydig cells and produce androgens, are located in the ovarian hilum and adjacent mesovarium, often next to nerves [5]. They are initially seen in the fetal ovary and identifiable during the first year of life, disappear after infancy and reappear at puberty [6]. Excessive stimulation of these cells by increased LH levels can result in hyperplasia and overproduction of testosterone.
Scalp hair thinning is an uncommon manifestation of androgen excess and is to some degree a normal consequence of aging after 50 years. However, an investigation for hyperandrogenism is appropriate when it is observed. In a series of 109 younger women with alopecia, two-thirds had no underlying abnormality, only 38.5% had elevated serum androgens, two had non-classic congenital adrenal hyperplasia and two had pituitary adenomas [7].
Serum total testosterone is the primary measure of androgen production and values above 150 ng/dL are suggestive of an androgen-secreting tumor, although lower levels should not detain evaluation in a woman with rapidly progressive symptoms [8]. In postmenopausal women, levels above 100 ng/dL should raise suspicion. Free testosterone values are less accurate because methods of measurement are not always consistent between laboratories and precise (equilibrium dialysis is considered the reference method) [9]. Free testosterone is more useful in patients who do not respond to treatment. DHEAS levels are important for the identification of women with an adrenal androgen-secreting tumor only in cases of pronounced elevation (>700 μg/dL) [9,10]. Mildly increased DHEAS can be found in polycystic ovary syndrome (PCOS) [11].
Androgen-secreting tumors are usually of ovarian and rarely of adrenal origin. Such tumors that derive from the ovaries represent mainly sex cord-stromal tumors and can usually be depicted with transvaginal US, although very small Leydig cell tumors and hyperplastic foci, as in the present case, cannot be visualized [8,12]. Ovarian causes of androgen overproduction also include endocrine abnormalities, such as PCOS, stromal hyperthecosis and hyperandrogenism, insulin resistance and acanthosis nigricans (HAIR-AN) syndrome. The last two are both associated with severe insulin resistance that is diagnosed with glucose tolerance test (GTT) and elevated insulin levels (Table 2) [13].
Table 2.
Ovarian causes of androgen overproduction and associated diagnostic features.
| Cause | Diagnosis |
|---|---|
| Tumors | |
| Sertoli-Leydig cell tumor | High testosterone levels Rapidly progressive hirsutism Usually identification of an ovarian lesion by imaging Absence of adrenal pathology Normal DHEAS levels |
| Sertoli cell tumor | |
| Leydig cell tumor | |
| Leydig cell hyperplasia | |
| Steroid cell tumor | |
| Granulosa cell tumor | |
| Thecoma | |
| Gonadoblastoma | |
| Brenner tumor | |
| Metastases from NETs | |
| Endocrine abnormalities | |
| PCOS | US of the ovaries Oligomenorrhea |
| Stromal hyperthecosis | Histopathological examination Insulin resistance |
| HAIR-AN syndrome | Acanthosis nigricans Insulin resistance |
NETs, Neuroendocrine tumors; DHEAS, Dehydroepiandrosterone sulfate; PCOS, Polycystic ovary syndrome; US, Ultrasonography; HAIR-AN, Hyperandrogenism, insulin resistance and acanthosis nigricans.
In cases of virilization, where the presence of an androgen-secreting tumor is suspected, imaging of the adrenal glands by either CT or magnetic resonance imaging (MRI) is suggested, especially in patients with increased DHEAS [1]. Selective ovarian venous catheterization has been proposed for cases where imaging is not diagnostic, but the results have been inconsistent [14]. Dynamic endocrine testing is not recommended when an androgen-secreting tumor is suspected because it is unreliable and misleading, although all ovarian causes of androgen overproduction are sensitive to LH suppression by gonadotropin-releasing hormone (GnRH) analogs [15].
The appropriate treatment for hirsutism and virilization depends on the cause. Treatments for hirsutism aim at reducing the production, increasing the protein-bound proportion, and/or blocking the action of androgens. Tumors should be resected and, if malignant, adjuvant therapy may be required. As in this case, Leydig cell hyperplasia in postmenopausal women should be treated by bilateral oophorectomy as imaging does not consistently show enlargement of the ovaries and the condition may involve both ovaries. In premenopausal women with a presumptive diagnosis of Leydig cell hyperplasia, cyproterone acetate can be administered as it can benefit patients by blocking androgen receptors [1]. As previously mentioned, GnRH agonists effectively suppress ovarian androgen production and can be combined with oral contraceptive therapy for the treatment of hirsute women [15]. GnRH agonists have effectively lowered testosterone levels in a case of Leydig cell tumor temporarily before surgery [16]. Nevertheless, laparoscopic bilateral oophorectomy should be recommended in all patients unless they have not completed their family as it is easily tolerated, provides a specimen for absolute diagnosis, eliminates the cause and requires minimal follow-up.
4. Conclusion
Leydig cell hyperplasia should be considered as a cause of hyperandrogenism of ovarian origin in women developing alopecia, hirsutism or other manifestations of virilization. Hormonal work-up should be supplemented by imaging, which may not always be diagnostic. Bilateral oophorectomy will treat the disorder and provide a conclusive diagnosis in postmenopausal women.
Acknowledgments
Contributors
Anastasia Vatopoulou was involved in patient care and contributed to the conception of the case report and drafting of the manuscript.
Fani Gkrozou contributed to the drafting and editing of the manuscript.
Effrosyni Birbas contributed to the editing and finalization of the manuscript.
Theofilos Kanavos contributed to the editing and finalization of the manuscript.
Chara Skentou contributed to the drafting of the manuscript.
Dimosthenis Miliaras was involved in patient care and contributed to the conception of the case report, drafting of the manuscript and supervision of the study.
All authors approved the final submitted manuscript.
Funding
No funding from an external source supported the publication of this case report.
Patient consent
Informed consent was obtained from the patient for the publication of this case report.
Provenance and peer review
This article was not commissioned and was peer reviewed.
Acknowledgments
Conflict of interest statement
The authors declare that they have no conflict of interest regarding the publication of this article.
Contributor Information
Anastasia Vatopoulou, Email: anastasiavat@uoi.gr.
Fani Gkrozou, Email: fani.gkrozou@uoi.gr.
Effrosyni Birbas, Email: faybirbas@gmail.com.
Theofilos Kanavos, Email: kanavosus@gmail.com.
Chara Skentou, Email: haraskentou@uoi.gr.
Dimosthenis Miliaras, Email: miliaras@auth.gr.
References
- 1.Hofland M., Cosyns S., De Sutter P., Bourgain C., Velkeniers B. Leydig cell hyperplasia and Leydig cell tumour in postmenopausal women: report of two cases. Gynecol. Endocrinol. 2013;29:213–215. doi: 10.3109/09513590.2012.705375. [DOI] [PubMed] [Google Scholar]
- 2.Rutgers J.L., Scully R.E. Functioning ovarian tumors with peripheral steroid cell proliferation: a report of twenty-four cases. Int. J. Gynecol. Pathol. 1986;5:319–337. doi: 10.1097/00004347-198612000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Zafrakas M., Venizelos I.D., Theodoridis T.D., Zepiridis L., Agorastos T., Bontis J.N. Virilizing ovarian hilus (Leydig) cell tumor with concurrent contralateral hilus cell hyperplasia: a rare diagnosis. Eur. J. Gynaecol. Oncol. 2009;30:338–340. [PubMed] [Google Scholar]
- 4.Ferriman D., Gallwey J.D. Clinical assessment of body hair growth in women. J. Clin. Endocrinol. Metab. 1961;21:1440–1447. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 5.He H.-L., Lee Y.-E., Chang C.-C. Hilus cell heterotopia accompanying bilateral ovarian serous cystadenomas: a case report and review of the literature. Int. J. Clin. Exp. Pathol. 2014;7:1246–1249. [PMC free article] [PubMed] [Google Scholar]
- 6.Sternberg W.H. The morphology, androgenic function, hyperplasia, and tumors of the human ovarian hilus cells. Am. J. Pathol. 1949;25:493–521. [PMC free article] [PubMed] [Google Scholar]
- 7.Futterweit W., Dunaif A., Yeh H.C., Kingsley P. The prevalence of hyperandrogenism in 109 consecutive female patients with diffuse alopecia. J. Am. Acad. Dermatol. 1988;19:831–836. doi: 10.1016/s0190-9622(88)70241-8. [DOI] [PubMed] [Google Scholar]
- 8.Shakir M.K.M., Snitchler A.N., Vietor N.O., Mai V.Q., Hoang T.D. Bilateral ovarian Leydig cell tumors in a postmenopausal woman causing hirsutism and Virilization. AACE Clin. Case Rep. 2021;7:26–28. doi: 10.1016/j.aace.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kacker R., Hornstein A., Morgentaler A. Free testosterone by direct and calculated measurement versus equilibrium dialysis in a clinical population. Aging Male. 2013;16:164–168. doi: 10.3109/13685538.2013.835800. [DOI] [PubMed] [Google Scholar]
- 10.Iliev D.I., Braun R., Sánchez-Guijo A., Hartmann M., Wudy S.A., Heckmann D., et al. Very high Dehydroepiandrosterone sulfate (DHEAS) in serum of an overweight female adolescent without a tumor. Front. Endocrinol. (Lausanne) 2020;11:240. doi: 10.3389/fendo.2020.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodarzi M.O., Carmina E., Azziz R. DHEA, DHEAS and PCOS. J. Steroid Biochem. Mol. Biol. 2015;145:213–225. doi: 10.1016/j.jsbmb.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Macut D., Ilić D., Mitrović Jovanović A., Bjekić-Macut J. Androgen-secreting ovarian tumors. Front. Horm. Res. 2019;53:100–107. doi: 10.1159/000494906. [DOI] [PubMed] [Google Scholar]
- 13.Cussen L., McDonnell T., Bennett G., Thompson C.J., Sherlock M., O’Reilly M.W. Approach to androgen excess in women: clinical and biochemical insights. Clin. Endocrinol. 2022;97:174–186. doi: 10.1111/cen.14710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levens E.D., Whitcomb B.W., Csokmay J.M., Nieman L.K. Selective venous sampling for androgen-producing ovarian pathology. Clin. Endocrinol. 2009;70:606–614. doi: 10.1111/j.1365-2265.2008.03389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elkind-Hirsch K.E., Anania C., Mack M., Malinak R. Combination gonadotropin-releasing hormone agonist and oral contraceptive therapy improves treatment of hirsute women with ovarian hyperandrogenism. Fertil. Steril. 1995;63:970–978. [PubMed] [Google Scholar]
- 16.Klotz R.K., Müller-Holzner E., Fessler S., Reimer D.U., Zervomanolakis I., Seeber B., et al. Leydig-cell-tumor of the ovary that responded to GnRH-analogue administration - case report and review of the literature. Exp. Clin. Endocrinol. Diabetes. 2010;118:291–297. doi: 10.1055/s-0029-1225351. [DOI] [PubMed] [Google Scholar]