Abstract
Locomotor disorders caused by multidrug-resistant (MDR) bacterial pathogens denote one of the most detrimental issues that collectively threaten the poultry industry leading to pronounced economic losses across the world. Hence, searching for effective alternatives, especially those extracted from plant origins became of great priority targeting a partial or complete replacement of chemical antimicrobials to tackle their developing resistance. Therefore, we aimed to determine the prevalence and antimicrobial resistance of Staphylococcus aureus (S. aureus), Salmonella species, Mycoplasma synoviae (M. synoviae), and Escherichia coli (E. coli) recovered from 500 broilers and ducks (250 each) with locomotor disorders in various farms in Dakahlia and Sharkia Governorates, Egypt. Additionally, we assessed, for the first time, the in vitro antimicrobial effectiveness of marjoram, garlic, ginger and cinnamon essential oils (EOs) against MDR and multivirulent bacterial isolates as well as the in vivo efficiency of the most effective antibiotics and EOs either separately or in combination in the treatment of experimentally induced poultry leg disorders. The overall prevalence rates of S. aureus, E. coli, Salmonella species, and M. synoviae were 54, 48, 36, and 2%, respectively. Salmonella species and S. aureus prevailed among ducks and broilers (36 and 76%, respectively). Notably, MDR was observed in 100, 91.7, 81.1, and 78.5% of M. synoviae, E. coli, Salmonella, and S. aureus isolates, respectively. Our in vitro results displayed that marjoram was the most forceful EO against MDR and multivirulent chicken vancomycin-resistant S. aureus (VRSA) and duck S. Typhimurium isolates. The current in vivo results declared that marjoram in combination with florfenicol or amoxicillin/clavulanic acid succeeded in relieving the induced duck and chicken leg disorders caused by S. Typhimurium and VRSA, respectively. This was evidenced by improvement in the clinical and histopathological pictures with a reduction of bacterial loads in the experimental birds. Our encountered successful in vitro and in vivo synergistic effectiveness of marjoram combined with florfenicol or amoxicillin/clavulanic acid recommends their therapeutic application for leg disorders and offers opportunities for reducing the antibiotics usage in the poultry industry.
Key words: poultry, Salmonella, S. aureus, leg disorder, marjoram
INTRODUCTION
The locomotive disorders represent eminent challenges to the poultry industry causing great economic problems worldwide. These disorders usually occur at 14 to 70 days-old, but mostly at 35 days-old and they are implicated in tenosynovitis, arthritis, synovitis, osteomyelitis, femoral head necrosis, and bumble foot in broiler chickens (Itakura et al., 1976). Bacterial arthritis frequently occurs after localized infection to the joints or septicemic infections and it is associated with several bacterial pathogens including Staphylococcus aureus (S. aureus), Salmonella species, Mycoplasma synoviae (M. synoviae), and Escherichia coli (E. coli), which have direct associations with leg pathologies in poultry (Abd El-Hamid et al., 2019a). This disorder is mostly caused by S. aureus, which is a common bacterial pathogen of chickens and ducks causing up to 15% mortality rate and reduction in the production performance with a consequence of significant economic losses (Shiozawa et al., 1980). Moreover, E. coli has been reported as one of the major bacterial agents associated with musculoskeletal infections in poultry (Chansiripornchai, 2009). Salmonella species and M. synoviae had been also proposed to be prominent causes of arthritis and they have been implicated in joint lesions in poultry (Abd El-Hamid et al., 2019a). M. synoviae infection is mostly subclinical, but in cases in combination with secondary bacterial infections, it becomes systemic and results in acute and chronic cases of infectious synovitis (Landman and Feberwee, 2012). The success of any bacterial species as a pathogen is affected by its extraordinary aptitude to express an enormous repertoire of specialized virulence attributes, which aid in the occurrence of diseases with a consequence of detrimental toxic effects (Ahmed et al., 2019; Awad et al., 2019; Bendary et al., 2022c).
The identification of bacterial agents incriminated in the locomotor disorders simplifies control and preventive measures in poultry flocks, which consequently decreases the resulted economic losses (Costa et al., 2016). Owing to the lack of efficient vaccines for bacterial diseases, antibiotic agents are widely recommended for controlling these diseases in poultry flocks. The nonjudicious utilization of antibiotics causes increased resistance rates to the frequently used ones in the poultry field (Ahmed et al., 2021; Ammar et al., 2021b; Aljazzar et al., 2022). Currently, this problem is intensified by the rising emergence of multidrug-resistant (MDR) bacterial species, which is becoming an increasing concern in the poultry sector (Ammar et al., 2021a).
From this point, it is crucial to search for alternative therapies to fight the virulent and MDR bacterial strains (El-Sheikh et al., 2021; Abd El-Hamid et al., 2022a). There is an intensifying demand for new natural antimicrobials to improve the control of bacterial pathogens incriminated in leg affections in the poultry sector. In this direction, thousands of phytochemicals such as essential oils (EOs) have been used as effective and safe promising natural alternative agents and their potential antimicrobial effects against a broad range of Gram-positive and Gram-negative bacterial species are under comprehensive investigations (Aljazzar et al., 2022; Hashem et al., 2022). The benefits of EOs over antibiotics are their great antibacterial potency without inducing any bacterial resistance. This potency has been presented by various EOs like marjoram, garlic, ginger and cinnamon during previous in vitro studies against avian bacterial pathogens (Abd El-Hamid et al., 2019a; Elmowalid et al., 2019). Moreover, the combination of EOs with antibiotics offers an optimistic solution for combating MDR bacteria (Langeveld et al., 2014). Yet, the in vivo efficacy of the above-mentioned EOs either alone or in combination with antibiotics against bacterial pathogens incriminated in avian leg disorders needs further inquiry.
Insight of the above-mentioned threats, this study was undertaken to determine the occurrence and antimicrobial susceptibility patterns of some bacterial pathogens recovered from broiler chickens and ducks presenting locomotor disorders in Sharkia and Dakahlia Governorates, Egypt. Additionally, the most recovered MDR chicken and duck bacterial isolates were further characterized via virulence genes profiling. Moreover, we sought to explore the in vitro antimicrobial activities of marjoram, garlic, ginger and cinnamon EOs against MDR and multivirulent bacterial isolates. Finally, we targeted to evaluate the antibacterial efficiency of the most effective antibiotics and EOs either separately or in combination in broiler chicken and duck experimental models of leg disorders.
MATERIALS AND METHODS
Ethical Statement
All the experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC), Faculty of Veterinary Medicine, Zagazig University with the reference number of ZU-IACUC/2/F/386/2022.
Clinical Examination and Sampling
Five hundred diseased broiler chickens aged 20 to 35 d and ducks aged 20 to 60 d (250 each); 300 from 60 farms in Dakahlia Governorate (150 birds/30 farms for each species) and 200 from 40 farms in Sharkia Governorate (100 birds/20 farms for each species) were included in this study. The investigated birds were clinically examined and clinical signs and postmortem (PM) lesions of freshly dead and sacrificed birds were recorded. Descriptive data of the investigated birds in Sharkia and Dakahlia Governorates are listed in Supplementary Tables 1 to 4. Swabs from synovial fluid and joint lesions were collected, labeled, and transported aseptically in an ice box to the Reference laboratory for Veterinary Quality Control on Poultry Production, Sharkia, Egypt for further bacteriological examination.
Microbiological Characterization of Avian Bacterial Isolates
Isolation and identification of Salmonella species were carried out via standard bacteriological techniques adopting the International Organization for Standardization (ISO) 6579-1 (ISO 6579-1, 2017). In brief, the collected samples were transferred to sterile buffered peptone water before being inoculated into Rappaport-Vassiliadis broth and incubated at 42°C for 24 h. Subsequently, a loopful of Rappaport-Vassiliadis broth was streaked onto MacConkey's and xylose lysine desoxycholate agar plates and the plates were incubated at 37°C for 24 h. For E. coli isolation, all collected samples were suspended in buffered peptone water, then the enrichment broth was streaked onto MacConkey's and eosin methylene blue agar media. Subsequently, the suspected E. coli and Salmonella cultures were identified using standard conventional phenotypic techniques (Cruickshank et al., 1975; Ammar et al., 2015; Bendary et al., 2022a,b; Elfaky et al., 2022). Furthermore, presumptive isolates were confirmed via API20E identification system (BioMérieux, Marcy l'Etoile, France) following the manufacturer's directions. The preliminarily identified Salmonella isolates were serotyped according to Kauffman-white scheme (Kauffman, 1974) using polyvalent and monovalent O and H antisera (Denka-Seiken, Tokyo, Japan). Moreover, serotyping of E. coli isolates was conducted via slide agglutination test using commercial polyvalent and monovalent O antisera (Test Sera Enteroclon, Berlin, Germany). Isolation of S. aureus was firstly performed onto mannitol salt and Paired Parker agar media. Primary phenotypic identification of S. aureus was carried out through standard bacteriological methods (ISO 6888-1, 2003) based on mannitol fermentation, β hemolysis, Gram-positive grape-like cocci appearance and biochemical reactions including catalase and coagulase tests (Abd El-Hamid and Bendary, 2015; Ammar et al., 2016a; Abd El‐Hamid et al., 2019b; Abd El-Hamid et al., 2022b). Isolation of M. synoviae was conducted using Frey's broth and agar media under microaerophilic humid conditions (10% CO2) at 37°C. Preliminary identification of M. synoviae was achieved using digitonin and traditional biochemical tests and definitive characterization was performed serologically via growth inhibition test employing definite antisera (Ammar et al., 2022; Awad et al., 2022). All media used for the isolation and identification of investigated bacterial isolates were provided by Oxoid (Oxoid, England, UK).
Molecular Identification of Avian Bacterial Isolates
On the subject of molecular characterization of the isolated Salmonella species, all the recovered isolates were molecularly screened at the genus level via PCR amplification of invA (invasion A) gene, which is unique for genus Salmonella adopting the previously described PCR procedures (Oliveira et al., 2003; Ammar et al., 2016b). Moreover, the identities of E. coli isolates were confirmed by PCR assay based on alkaline phosphatase structural gene (phoA) specific primer and the conditions detailed previously (Hu et al., 2011). S. aureus isolates were molecularly identified through specific PCR amplification of the 23S ribosomal ribonucleic acid (23S rRNA) gene following the previously published PCR protocol (Bhati et al., 2016). Finally, all mycoplasma isolates were confirmed to be M. synoviae via PCR amplification using one primer set targeting variable lipoprotein hemagglutinin A (vlhA) gene according to the procedures previously reported (Jeffery et al., 2007; Abd El-Hamid et al., 2019a).
Antimicrobial Susceptibility Patterns of the Recovered Bacterial Isolates
Antimicrobial susceptibility rates of the recovered Salmonella, E. coli, and S. aureus isolates were detected by standard Kirby–Bauer disc diffusion approach as stated formerly (Bauer et al., 1966) utilizing commercial drug disks and Mueller Hinton agar medium (Oxoid); meanwhile, the in vitro susceptibility of all M. synoviae isolates to routine antimicrobials was determined via broth microdilution test (Hannan, 2000). The activities of amoxicillin/clavulanic acid (20/10 µg, AMC), ampicillin (10 µg, AMP), neomycin (30 µg, N), streptomycin (10 µg, S), oxytetracycline (30 µg, OTC), doxycycline (30 µg, DO), erythromycin (15 µg, E), trimethoprim/sulfamethoxazole (1.25/23.75 µg, SXT), florfenicol (30 µg, FF), and colistin sulfate (10 µg, CT) antimicrobials were tested against Salmonella and E. coli isolates. Meanwhile, amoxicillin/clavulanic acid (20/10 µg, AMC), ampicillin (10 µg, AMP), oxytetracycline (30 µg, OTC), oxacillin (1 μg, OX), vancomycin (30 μg, VA), streptomycin (10 µg, S), erythromycin (15 µg, E) and trimethoprim/sulfamethoxazole (1.25/23.75 µg, SXT) antimicrobials were evaluated against S. aureus isolates. The inhibition zone diameters were measured, in duplicate, and the tested isolates were categorized as sensitive or resistant in accordance with the interpretative criteria recommended by the Clinical and Laboratory Standards Institute (Clinical and Laboratory Standards Institute (CLSI), 2020). Based upon CLSI breakpoints, the disk diffusion test is not dependable for assessing vancomycin resistance, so the minimum inhibitory concentration (MIC) of vancomycin (Oxoid, England, UK) was further detected via broth microdilution test as endorsed by CLSI (Clinical and Laboratory Standards Institute (CLSI), 2020). Finally, all M. synoviae isolates were tested for a panel of 8 antimicrobials including erythromycin, doxycycline, oxytetracycline, spectinomycin, spiramycin, tilmicosin, tylosin, and tiamulin. The isolates being resistant to at least one agent in 3 or more various antimicrobial classes were categorized as MDR.
Molecular Characterization of Virulence Determinants of the Selected Bacterial Isolates
Genomic DNAs from the most isolated MDR S. aureus and S. Typhimurium from chickens and ducks were extracted using QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) adopting the manufacturer's instructions. Extracted DNAs were subsequently used for various PCR assays, in triplicate, to detect some virulence-associated genes; S. aureus staphylococcal surface protein A (spa), toxic shock syndrome toxin (tsst) and collagen adhesin gene (cna) and S. Typhimurium fimbrial gene (fimH), Salmonella plasmid virulence C (spvC), and Salmonella outer protein D2 (sopD2) using target primers and cycling protocols detailed elsewhere by several investigators (Mehrotra et al., 2000; Tristan et al., 2003, Huehn et al. 2010; Wada et al., 2010; Hojati et al., 2015 ; Elnady, 2019). The sequences of all oligonucleotide primers and their corresponding genes with PCR amplified products' sizes are illustrated in Table 1. The amplified PCR products were subjected to gel electrophoresis and visualized under UV illumination. For each PCR assay, applicable positive (DNAs from isolates previously affirmed to possess sequences for any of the target virulence genes) and negative (DNase/RNase free water) controls were involved.
Table 1.
Sequences of oligonucleotide primers and amplified PCR products of target genes.
| Bacterial species | Target gene | Primer sequence (5′-3′) | Amplified product (bp) | Reference |
|---|---|---|---|---|
| Staphylococcus aureus | 23S rRNA | F: ACGGAGTTACAAAGGACGAC R: AGCTCAGCCTTAACGAGTAC |
1250 | Bhati et al., 2016 |
| spa | F: TCAACAAAGAACAACAAAATGC R: GCTTTCGGTGCTTGAGATTC |
226 | Wada et al., 2010 | |
| tsst | F: ACCCCTGTTCCCTTATCATC R:TTTTCAGTATTTGTAACGCC |
326 | Mehrotra et al., 2000 | |
| cna | F: GTCAAGCAGTTATTAACACCAGAC R: AATCAGTAATTGCACTTTGTCCACTG |
423 | Tristan et al., 2003 | |
| Salmonella Typhimurium | invA | F: GTGAAATTATCGCCACGTTCGGGCAA R: TCATCGCACCGTCAAAGGAACC |
284 | Oliveira et al., 2003 |
| fimH | F: GTGCCAATTCCTCTTACCGTT R: TGGAATAATCGTACCGTTGCG |
164 | Hojati et al., 2015 | |
| spvC | F: ACCAGAGACATTGCCTTC C R: TTCTGATCGCCG CTATTCG |
467 | Huehn et al., 2010 | |
| sopD2 | F: ACCATGCGCTGGAAGTGTTA R: GCGGGACGCATCATCTCATA |
430 | Elnady, 2019 | |
| Escherichia coli | phoA | F: CGATTCTGGAAATGGCAAAAG R: CGTGATCAGCGGTGACTATGAC |
720 | Hu et al., 2011 |
| Mycoplasma synoviae | vlhA | F: TACTATTAGCAGCTAGTGC R: AGTAACCGATCCGCTTAAT |
350–400 | Jeffery et al., 2007 |
Herbal Essential Oils Antibacterial Activities
The antimicrobial properties of an array of marjoram, garlic, ginger and cinnamon EOs (Sigma, Taufkirchen, Germany) against the most common chicken and duck MDR and multivirulent isolates were evaluated using agar well diffusion and standard broth microdilution techniques (Elmowalid et al., 2022). Each assay was carried out in triplicate.
Essential Oils Interaction With Antibiotics via Checkerboard Assay
Evaluation of the interaction between the most effective antibiotics and EOs against the most dominant chicken and duck isolates showing MDR and multivirulence characteristics was achieved using a checkerboard method, in triplicate, following the method previously detailed (Hriouech et al., 2020). Analysis of the interaction between the screened antimicrobial compounds was carried out by calculating the fractional inhibitory concentration index (FICI) utilizing the formula described formerly (Hriouech et al., 2020). The FICI values were interpreted as following: FICI ≤ 0.5 (synergism), 0.5 < FICI ≤ 1 (additivity), 1 < FICI ≤ 4 (indifference) and FICI > 4 (antagonism).
In Vivo Antibacterial Activities
Experimental Birds and Design
Two hundred one-day-old broiler chicks (Ross 308) and Muscovy ducklings (100 each) were obtained from Dakahlia and El-Wafaa Companies, respectively and kept in separate cages at Animal Biosafety Level Two Facility at Animal Health Research Institute, Giza, Egypt. The birds were reared in a floor- based system in experimental partitions under optimal conditions of light, humidity and temperature and received antimicrobial-free balanced ration. The broiler chickens diet for starter, grower and finisher periods was formulated adopting Aviagen recommendations (Aviagen, 2018) as listed in Supplementary Table 5. Chemical analyses of feed ingredients were carried out following the standard protocols supplied by the Association of Official Analytical Chemists (AOAC) (AOAC, 2012). Moreover, starter and grower diets of Muscovy ducks were formulated in accordance with the guidelines of the National Research Council (National Research Council (NRC), 1994) as shown in Supplementary Table 6. All birds had ad libitum access to feed and water. The experimental chickens were vaccinated with Hitchiner B1 + IB vaccine at 3 days-old, Gumboro vaccine at 10 days-old and LaSota NDV vaccine at 7 and 21 days-old via intraocular route. The experimental ducks were vaccinated with AI vaccine at 3 and 21 days-old and the fowl cholera vaccine at 18 days-old via subcutaneous injection.
Two independent in vivo experimental models of leg disorders were carried out. Using standard bacteriological methods, chickens (Groups 1–5; 20 each) and ducklings (Groups 6–10; 20 each) were examined at their arrival and weekly until the time of experimental bacterial infection (21 days-old), to confirm that they were free from any bacterial infection. Birds in groups 1 and 6 were kept as negative controls (noninfected and nontreated). Chickens in groups 2 to 5 and ducks in groups 7 to 10 were experimentally infected with 107 colony-forming units (CFU)/mL of the most prevalent MDR and multivirulent isolates; vancomycin-resistant S. aureus (VRSA) via intra articular and S. Typhimurium via intra muscular route, respectively (Gu et al., 2013; Guo et al., 2019). The bacterial experimental infections were confirmed through clinical signs, PM findings and re-isolation and identification of the infecting pathogens. Additionally, establishment of the bacterial infection models were affirmed via re-investigating the antimicrobial susceptibility and virulence genes’ profiles of both chicken and duck bacterial pathogens. Following the clinical signs appearance, infected birds in groups 2 and 7; 3 and 8; 4 and 9 were treated for 5 consecutive days with the most effective antibiotics, EO and antibiotics/EO in combination, respectively using selected concentrations based on their in vitro MIC values.
Evaluation Parameters
Clinical Examination
All experimental birds were observed on a daily basis and clinical signs, the number of dead birds and PM lesions on freshly dead birds were recorded from the time of bacterial infection until the end of the experiment.
Bacteriological Examination
After the end of the treatment, the synovial fluids of both chickens and ducks were collected and then subjected to re-isolation and identification of the experimentally infecting bacterial isolates in addition to estimation of the numbers of their respective CFU/mL of synovial fluids.
Histopathological Examination
Specimens from the joints were collected from sacrificed chickens and ducks after the end of the treatment. Histopathological examination of these samples was performed after fixation, decalcification and paraffin embedding. The paraffin tissue sections of 5 to 7 µm thickness were cut by Histotome (Leica RM2135, Heidelberger, Germany) and stained with hematoxylin-eosin (H&E) stain (Suvarna et al., 2018).
Statistical Analyses
The chi-square and one-way ANOVA tests were carried out to define the statistically significant differences, which were determined at a P value < 0.05. A heatmap with hierarchical clustering was performed using the “heatmap” package in R software. The correlation between antimicrobial resistance phenotypes and virulence genes and visualization were done using the R packages ggcorrplot (version 4.0.2; https://www.r-project.org). The results of antimicrobial susceptibility testing were graphed as heatmaps through GraphPad Software (version 8.0.1, GraphPad Software Inc., La Jolla, CA).
RESULTS
Clinical Signs and Postmortem Findings
The clinical signs of the investigated naturally infected chickens and ducks were swollen footpads, swelling and inflammation of the hock joint, lameness, inability to stand and gradual emaciation. Some diseased birds showed respiratory signs in the form of sneezing and coughing. PM examination of the freshly dead and sacrificed birds revealed caseous exudates in the swollen joints, cartilage injury and synovial membrane thickening in addition to liver congestion, pneumonia and airsaculitis in some cases (Supplementary Tables 1–4).
Occurrence and Distribution of Avian Bacterial Species
Microbiological and molecular analysis revealed the occurrence of 4 major bacterial pathogens; Salmonella, E. coli, S. aureus and M. synoviae. Of all examined birds (n = 500), 270 (54%), 240 (48%) and 180 (36%) were positive for S. aureus, E. coli and Salmonella species, respectively and only 10 (2%) were infected by M. synoviae. Notably, S. aureus was the most prevalent (76%, 190/250) among the examined chickens, followed by E. coli (66%, 165/250) and Salmonella (36%, 90/250) isolates. Regarding the investigated ducks, Salmonella and S. aureus isolates were recovered with high frequencies (36%; 90/250 and 32%; 80/250, respectively). Obviously, M. synoviae displayed a low isolation percentage relative to the total number of diseased chickens (10/250, 4%) and it did not be isolated from diseased ducks. Altogether, S. aureus was highly distributed among the diseased birds in both Sharkia and Dakahlia Governorates (57.5 and 51.7%, respectively), followed by E. coli isolates (50 and 46.7%, respectively). In Sharkia and Dakahlia Governorates, S. aureus isolates recorded the highest isolation percentages from diseased chickens (80 and 73.3%, respectively); meanwhile, Salmonella species were the predominant bacterial isolates among the examined ducks (40 and 33.3%, respectively) (Figure 1). Notably, there were no statistically significant differences (P > 0.05) in the occurrence rates of all recovered chicken and duck isolates between Sharkia and Dakahlia Governorates. Meanwhile, the occurrence rates of recovered chicken and duck isolates exhibited statistically significant variations (P < 0.0001) in each investigated Governorate. Moreover, there were statistically significant differences (P < 0.0001) in the occurrence rates of the recovered E. coli and S. aureus isolates between chickens and ducks, but no statistically significant differences (P > 0.05) were recorded for the occurrence rates of Salmonella species between both avian hosts.
Figure 1.
Occurrence rates of Staphylococcus aureus (S. aureus), Salmonella species, Mycoplasma synoviae (M. synoviae), and Escherichia coli (E. coli) in diseased broilers and ducks from poultry farms in Sharkia (A) and Dakahlia (B) Governorates. Pink and blue colors denote broiler chickens and ducks, respectively.
Occurrence Rates of Single and Mixed Bacterial Isolates in Chickens and Ducks
Overall, S. aureus had the highest occurrence rate as a single bacterial isolate among the investigated birds with a percentage of 16.2%, while M. synoviae did not be recovered singly. Among the identified bacterial isolates, S. aureus and Salmonella species predominated singly in the investigated chickens and ducks (28.8 and 5.6%, respectively). Totally, the bacterial community profiling of mixed isolates revealed 6 different combinations (Table 2). The predominant mixed bacterial profiles were detected for E. coli and S. aureus in the diseased chickens (27.2%) and S. aureus plus E. coli and Salmonella species in the total examined birds and ducks (17 and 16%, respectively).
Table 2.
Occurrence of single and mixed bacterial infections in chickens and ducks.
| Single or mixed bacterial isolate(s) | No. of recovered isolates from avian source (%) |
||
|---|---|---|---|
| Chickens (250) |
Ducks (250) |
Total (500) |
|
| Staphylococcus aureus | 72 (28.8) | 9 (3.6) | 81 (16.2) |
| E. coli | 11 (4.4) | 0 (0) | 11 (2.2) |
| Salmonella species | 9 (3.6) | 14 (5.6) | 23 (4.6) |
| E. coli + Staphylococcus aureus | 68 (27.2) | 15 (6) | 83 (16.6) |
| E. coli + Salmonella species | 31 (12.4) | 20 (8) | 51 (10.2) |
| E. coli + M. synoviae | 5 (2) | 0 (0) | 5 (1) |
| Staphylococcus aureus + Salmonella species | 0 (0) | 16 (6.4) | 16 (3.2) |
| Staphylococcus aureus + E. coli + Salmonella species | 45 (18) | 40 (16) | 85 (17) |
| Staphylococcus aureus+ E. coli + Salmonella species + M. synoviae | 5 (2) | 0 (0) | 5 (1) |
Occurrence of E. coli and Salmonella Serotypes Among Examined Birds
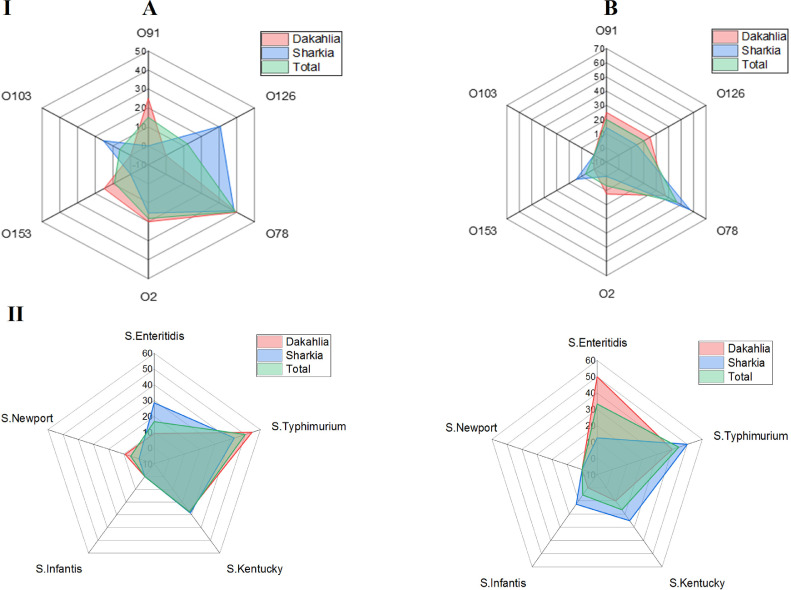
Serotyping of all recovered E. coli isolates revealed 6 different recognized serotypes; O91, O126, O78, O2, O153, and O103. Interestingly, O78 was the prevailing serotype among the typed E. coli isolates recovered from total birds, chickens and ducks accounting for 41.7, 39.4, and 46.7%, respectively (Figure 2I). Moreover, O78 was found to be the most predominant serotype identified among E. coli isolates recovered from both Sharkia and Dakahlia Governorates (45 and 39.3%, respectively).
Figure 2.
Total occurrence rates of various Escherichia coli (I) and Salmonella (II) serotypes in diseased broiler chickens (A) and ducks (B) and their distribution schemes in Sharkia and Dakahlia Governorates.
Five Salmonella serovars were characterized with S. Typhimurium, S. Enteritidis, and S. Kentucky being the most dominant ones among the total examined birds (47.2, 25, and 22.2%, respectively). Other 2 serotypes; S. Infantis and S. Newport, were recovered from duck and chicken origins, respectively with a total prevalence rate of 2.8% each. Totally, S. Typhimurium serotype displayed the highest occurrence rate among recovered isolates from chickens (50%) and ducks (44.4%) in both Sharkia (46.7%) and Dakahlia (47.6%) Governorates. Among chicken isolates, S. Typhimurium was the most predominant serotype in Sharkia (42.9%) and Dakahlia (54.6%) Governorates. Among duck isolates, S. Typhimurium serotype predominated in Sharkia Governorate (50%); meanwhile, S. Enteritidis was the most recovered serotype in Dakahlia Governorate (50%, Figure 2II). Notably, there were no statistically significant differences (P > 0.05) in the occurrence rates of E. coli and Salmonella serotypes between Sharkia and Dakahlia Governorates. Meanwhile, the occurrence rates of recovered E. coli and Salmonella serotypes exhibited statistically significant variations (P < 0.0001) in each studied Governorate. Moreover, there were statistically significant differences (P < 0.05) in the occurrence rates of all E. coli serotypes, S. Enteritidis S. Infantis and S. Newport between chickens and ducks, but no statistically significant differences (P > 0.05) were recorded for the occurrence rates of S. Typhimurium and S. Kentucky between both avian hosts.
Antimicrobial Susceptibility Patterns of the Recovered Avian Bacterial Isolates
All E. coli and Salmonella isolates recovered from chickens and ducks were examined for their susceptibilities against a spectrum of 10 selected antimicrobials of various classes as summarized in Table 3 and Supplementary Figures 1 to 4. The analyzed data demonstrated that all recovered E. coli isolates from chickens showed higher resistance rates towards the majority of the tested antimicrobials with absolute resistance (100%) to doxycycline, neomycin and oxyetracycline. On the other side, colistin sulfate was the most effective antibiotic against those isolates (100%). Antibiogram results of the recovered E. coli isolates from ducks revealed that the highest resistance levels were observed against amoxicillin/clavulanic acid, ampicillin and oxyetracycline (100% each). Meanwhile, streptomycin and colistin sulfate reported the maximum sensitivity rates against those isolates (86.7 and 80%, respectively).
Table 3.
Antimicrobial susceptibility patterns of E. coli and Salmonella isolates recovered from chickens and ducks.
| Antimicrobial agent | No. of avian E. coli isolates (%) showing antimicrobial susceptibility patterns |
No. of avian Salmonella species (%) showing antimicrobial susceptibility patterns |
||||||
|---|---|---|---|---|---|---|---|---|
| Chickens (165) |
Ducks (75) |
Chickens (90) |
Ducks (90) |
|||||
| Sensitivity | Resistance | Sensitivity | Resistance | Sensitivity | Resistance | Sensitivity | Resistance | |
| Doxycycline | 0 (0) | 165 (100) | 15 (20) | 60 (80) | 22 (24.4) | 68 (75.6) | 73 (81.1) | 17 (18.9) |
| Amoxicillin/clavulanic acid | 27 (16.4) | 138 (83.6) | 0 (0) | 75 (100) | 0 (0) | 90 (100) | 22 (24.4) | 68 (75.6) |
| Ampicillin | 29 (17.6) | 136 (82.4) | 0 (0) | 75 (100) | 0 (0) | 90 (100) | 24 (26.7) | 66 (73.3) |
| Streptomycin | 27 (16.4) | 138 (83.6) | 65 (86.7) | 10 (13.3) | 45 (50) | 45 (50) | 73 (81.1) | 17 (18.9) |
| Neomycin | 0 (0) | 165 (100) | 30 (40) | 45 (60) | 20 (22.2) | 70 (77.8) | 69 (76.7) | 21 (23.3) |
| Oxyetracycline | 0 (0) | 165 (100) | 0 (0) | 75 (100) | 10 (11.1) | 80 (88.9) | 60 (66.7) | 30 (33.3) |
| Erythromycin | 80 (48.5) | 85 (51.5) | 45 (60) | 30 (40) | 22 (24.4) | 68 (75.6) | 30 (33.3) | 60 (66.7) |
| Colistin sulfate | 165 (100) | 0 (0) | 60 (80) | 15 (20) | 0 (0) | 90 (100) | 35 (38.9) | 55 (61.1) |
| Trimethoprim/sulfamethoxazole | 108 (65.5) | 57 (34.5) | 45 (60) | 30 (40) | 90 (100) | 0 (0) | 90 (100) | 0 (0) |
| Florfenicol | 80 (48.5) | 85 (51.5) | 45 (60) | 30 (40) | 45 (50) | 45 (50) | 90 (100) | 0 (0) |
On the topic of antimicrobial susceptibility profiles of avian Salmonella isolates, the results revealed noticable high resistance rates of chicken isolates. The more precise interpretation of resistance data displayed that all chicken isolates were resistant to amoxicillin/clavulanic acid, ampicillin, and colistin sulfate (100% each) and a high proportion of the isolates was resistant to oxyetracycline 88.9%. However, trimethoprim/sulfamethoxazole was the most effective antimicrobial against these isolates (100%). The antimicrobial susceptibility profiles of duck Salmonella isolates demonstrated that high levels of resistance were documented for amoxicillin/clavulanic acid (75.6%). From the therapeutic viewpoint, trimethoprim/sulfamethoxazole, and florfenicol showed absolute sensitivity against those isolates (100%).
The in vitro antimicrobial susceptibility patterns of avian S. aureus isolates against antimicrobial agents of various classes are illustrated in Table 4 and Supplementary Figures 5 and 6. Chicken S. aureus isolates demonstrated high rates of susceptibility to amoxicillin/clavulanic acid (63.2%) and vancomycin (60.5%); however, they displayed high resistance rates against trimethoprim/sulfamethoxazole (84.2%) and oxyetracycline (73.7%). Among duck S. aureus isolates, the highest resistance rates were observed for oxacillin (87.5%), followed by trimethoprim/sulfamethoxazole (50%); meanwhile, they were highly susceptible to streptomycin, vancomycin and erythromycin (85, 80 and 80%, respectively). Notably, 75 chicken S. aureus isolates (39.5%) and 16 duck S. aureus isolates (20%) were resistant to vancomycin with MIC values ranging from 64 to 1024 μg/mL being classified as VRSA in agreement with CLSI breakpoints.
Table 4.
Antimicrobial susceptibility patterns of S. aureus isolates recovered from chickens and ducks.
| Antimicrobial agent | No. of avian S. aureus isolates (%) showing antimicrobial susceptibility patterns |
|||
|---|---|---|---|---|
| Chickens (190) |
Ducks (80) |
|||
| Sensitivity | Resistance | Sensitivity | Resistance | |
| Amoxicillin/clavulanic acid | 120 (63.2) | 70 (36.8) | 45 (56.3) | 35 (43.8) |
| Ampicillin | 110 (57.9) | 80 (42.1) | 41 (51.3) | 39 (48.8) |
| Oxyetracycline | 50 (26.3) | 140 (73.7) | 45 (56.3) | 35 (43.8) |
| Oxacillin | 90 (47.4) | 100 (52.6) | 10 (12.5) | 70 (87.5) |
| Vancomycin | 115 (60.5) | 75 (39.5) | 64 (80) | 16 (20) |
| Streptomycin | 85 (44.7) | 105 (55.3) | 68 (85) | 12 (15) |
| Erythromycin | 90 (47.4) | 100 (52.6) | 64 (80) | 16 (20) |
| Trimethoprim/sulfamethoxazole | 30 (15.8) | 160 (84.2) | 40 (50) | 40 (50) |
Another striking feature was that all M. synoviae isolates from chickens were resistant to erythromycin, doxycycline, oxytetracycline, tilmicosin and tylosin and sensitive to spectinomycin, spiramycin and tiamulin (Supplementary Figure 7).
Regarding the resistance patterns of various chicken E. coli serotypes, high levels of resistance to amoxicillin/clavulinc acid were displayed by O91, O126, O2, and O78 serotypes (92, 90, 86.7, and 83.1%, respectively). Moreover, O103 and O153 serotypes recorded high resistance rates to streptomycin (80 and 73.3%, respectively). Antibiogram of duck E. coli O126, O78, and O91 serotypes demonstrated high rates of resistance to doxycycline (86.7, 82.9, and 73.3%, respectively). Moreover, E. coli O153, and O2 serotypes exhibited high resistance levels to doxycycline (80 and 60%, respectively) and neomycin (80 and 60%, respectively).
Concerning the resistance profiles of investigated chicken Salmonella serovars, it was found that S. Newport displayed full resistance to erythromycin and oxyetracycline (100% each). Moreover, S. Typhimurium and S. Kentucky demonstrated high resistance rates to oxyetracycline (93.3 and 88%, respectively) and neomycin (82.2 and 92%, respectively). Additionally, S. Enteritidis showed high resistance percentages to erythromycin and doxycycline (80% each). More detailed interpretation of antimicrobial resistance patterns of duck Salmonella serovars revealed that the highest levels of resistance of S. Typhimurium and S. Enteritidis were obtained against amoxicillin/clavulinc acid (77.5 and 73.3%, respectively). Furthermore, a high proportion of S. Kentucky and S. Infantis serovars was resistant to erythromycin (80% each).
On overall, MDR was notably observed in 91.7, 81.1, and 78.5% of E. coli, Salmonella, and S. aureus isolates, respectively. Moreover, the MDR patterns of the recovered isolates of chicken and duck origins were highly variable. Salmonella and S. aureus isolates of chicken origin exhibited high MDR levels (89.9 and 91.1%, respectively) as compared to those recovered from duck origin (63.3 and 48.8%, respectively). Additionally, duck and chicken E. coli isolates displayed high MDR percentages (96 and 89.7%, respectively). It is worth noting that all chicken E. coli O2 and duck O91 and O153 serotypes were MDR. Moreover, all chicken E. coli serotypes O2, O126 and O91 and duck E. coli serotypes O126 and O91 were resistant to at least 5 of the 10 examined antimicrobials (Figures 3 and 4). There were great variations in the antimicrobial resistance patterns among different Salmonella serovars from duck and chicken origins with high levels of MDR displayed by chicken Salmonella serovars, unlike the duck ones. Analysis of the results revealed that all chicken S. Enteritidis, S. Kentucky and S. Newport were MDR. Among duck Salmonella serovars, S. Kentucky and S. Enteritidis exhibited higher MDR patterns with percentages of 66.67 and 63.3%, respectively. Regarding S. aureus antimicrobial resistance patterns, it was found that 91.1% of chicken isolates were MDR with 65.8% of them having phenotypic resistance to at least 4 different antimicrobial classes. Furthermore, 48.8% of duck S. aureus isolates expressed MDR with no isolates presented resistance to more than 6 antimicrobials (Figure 5). Notably, all chicken M. synoviae isolates (100%) had phenotypic resistance to 5 antimicrobial drugs of 3 different classes being MDR.
Figure 3.
In vitro antimicrobials resistance profiling of chicken isolates considering the percentages of Escherichia coli (I) and Salmonella (II) serotypes resistant to various antimicrobials (A) and antimicrobial classes (B).
Figure 4.
In vitro antimicrobials resistance profiling of duck isolates considering the percentages of Escherichia coli (I) and Salmonella (II) serotypes resistant to various antimicrobials (A) and antimicrobial classes (B).
Figure 5.
In vitro antimicrobials resistance profiling of chicken (I) and duck (II) isolates considering the percentages of Escherichia coli (E. coli), Salmonella species and Staphylococcus aureus (S. aureus) resistant to various antimicrobials (A) and antimicrobial classes (B).
Antibiotyping, Genotyping, and Virulence Gene Profiling of the Selected Avian Isolates
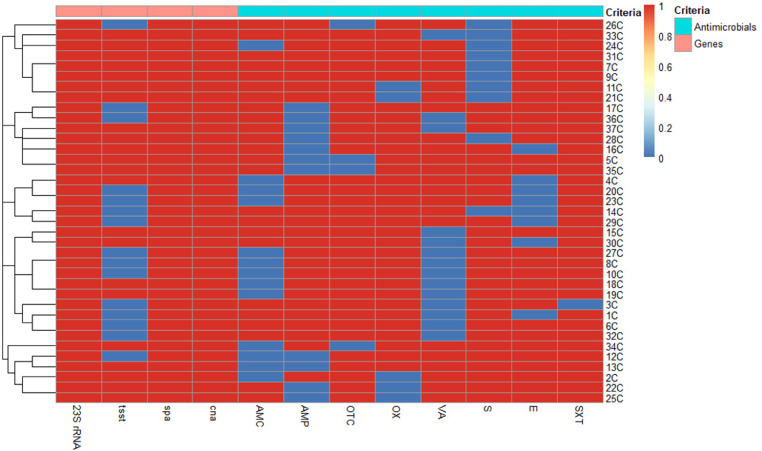
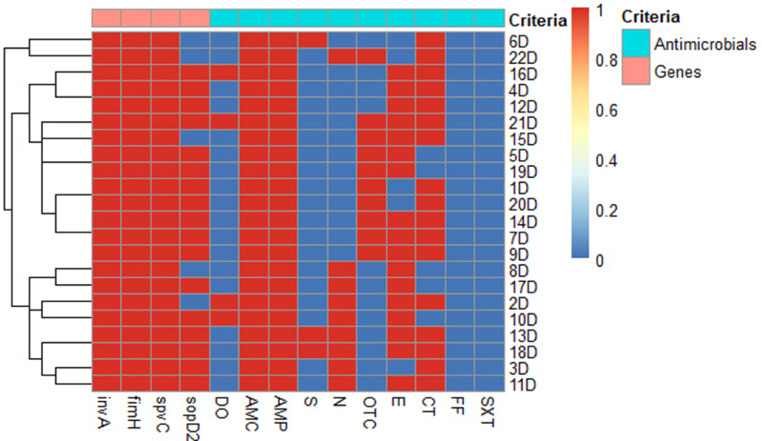
Basing on antimicrobial resistance data, 37 S. aureus and 22 S. Typhimurium isolates that were resistant to at least 6 and 4 antimicrobial drugs, respectively were selected for further investigations. Overall, 24 and 15 different antimicrobial resistance patterns of the investigated S. aureus (Figure 6) and S. Typhimurium (Figure 7) isolates were recognized. The most common pattern displayed by 5 S. aureus isolates (13.5%) included resistance to AMP, OTC, OX, S, E, and SXT antimicrobials and that demonstrated by 4 S. Typhimurium isolates (18.2%) comprised resistance to AMC, AMP, OTC, E, and CT antibiotics.
Figure 6.
Heat map and hierarchical clustering of the examined 37 chicken Staphylococcus aureus isolates based on the occurrence of antimicrobial resistance and target genes. In the heat map, red and blue colors denote the resistance/sensitivity to an antimicrobial agent and the presence/absence of the target genes, respectively. The code numbers on the right of the heat map infer the chicken (C) isolates. Abbreviations: AMC, amoxicillin/clavulanic acid; AMP, ampicillin; cna, collagen adhesin gene; E, erythromycin; OTC, oxytetracycline; OX, oxacillin; S, streptomycin; spa, staphylococcal surface protein A; SXT, trimethoprim/sulfamethoxazole; tsst, toxic shock syndrome toxin and 23S rRNA: 23S ribosomal ribonucleic acid; VA, vancomycin.
Figure 7.
Heat map and hierarchical clustering of the examined 22 duck Salmonella Typhimurium isolates based on the occurrence of antimicrobial resistance and target genes. In the heat map, red and blue colors denote the resistance/sensitivity to an antimicrobial agent and the presence/absence of the target genes, respectively. The code numbers on the right of the heat map infer the duck (D) isolates. Abbreviations: AMC, amoxicillin/clavulanic acid; AMP, ampicillin; CT, colistin sulfate; DO, doxycycline; E, erythromycin; FF, florfenicol; fimH, fimbrial gene; invA, invasion A gene; N, neomycin; OTC, oxytetracycline; S, streptomycin; sopD2, salmonella outer protein D2; spvC, salmonella plasmid virulence C; SXT, trimethoprim/sulfamethoxazole.
The distribution of examined virulence genes among the selected isolates revealed that all examined chicken S. aureus isolates (100%) harbored spa and cna genes and only 59.5% of them (22/37) were positive for tsst gene. Moreover, all duck S. Typhimurium isolates (100%) possessed fimH and spvC genes, while sopD2 gene was detected in only 77.3% (17/22) of these isolates (Figure 8). Various combinations of 3 tested virulence genes among the investigated S. aureus and S. Typhimurium isolates demonstrated that each of them exhibited 2 virulence genes’ profiles. It is of interest to reveal that the most dominant profile was that of S. aureus and S. Typhimurium isolates possessing all examined virulence genes (59.5 and 77.3%, respectively) being multivirulent isolates. Interestingly, out of 22 chicken multivirulent S. aureus isolates, 16 (72.7%) were confirmed to be VRSA.
Figure 8.
Distribution of target genes among selected 37 chicken Staphylococcus aureus and 22 duck Salmonella Typhimurium isolates. Abbreviations: cna, collagen adhesin gene; fimH, fimbrial gene; invA, invasion A gene; spa, staphylococcal surface protein A; 23S rRNA, 23S ribosomal ribonucleic acid; sopD2, salmonella outer protein D2; spvC: salmonella plasmid virulence C; tsst, toxic shock syndrome toxin.
Correlation Analyses Between Phenotypic Antimicrobial Resistances and Virulence Genes and Among Resistances to Antimicrobials
As illustrated in Figure 9A, S. Typhimurium sopD2 gene was positively correlated with resistances to erythromycin (r = 0.22) and oxytetracycline (r = 0.06), while it was negatively correlated with the other examined antibiotics with r values ranging from - 0.03 to - 0.21. Moreover, we noted positive correlations between resistances to colistin sulfate and both streptomycin (r = 0.22) and oxytetracycline (r = 0.06), erythromycin and both doxycycline (r = 0.26) and neomycin (r = 0.01), neomycin and both streptomycin (r = 0.21) and doxycycline (r = 0.09).
Figure 9.
Correlation (r) analyses among resistances to antimicrobials and between phenotypic antimicrobial resistance and virulence genes of duck Salmonella Typhimurium (A) and chicken Staphylococcus aureus (B) isolates. Red and blue colors indicate positive and negative correlations, respectively. The color key denotes the correlation coefficient (r). Abbreviations: AMC, amoxicillin/clavulanic acid; AMP, ampicillin; CT, colistin sulfate; DO, doxycycline; E, erythromycin; N, neomycin; OTC, oxytetracycline; OX, oxacillin; S, streptomycin; sopD2, salmonella outer protein D2; SXT, trimethoprim/sulfamethoxazole; tsst, toxic shock syndrome toxin; VA, vancomycin.
According to Figure 9B, S. aureus tsst gene was positively correlated with resistance to erythromycin (r = 0.23), vancomycin (r = 0.22), trimethoprim/sulfamethoxazole (r = 0.20) and amoxicillin/clavulanic acid (r = 0.04), while it was negatively correlated with the other tested antibiotics with r values ranging from - 0.04 to - 0.33. Furthermore, we recorded positive correlations between resistance to trimethoprim/sulfamethoxazole and vancomycin (r = 0.20), streptomycin and both oxacillin (r = 0.12) and oxytetracycline (r = 0.04), oxacillin and both ampicillin (r = 0.09) and amoxicillin/clavulanic acid (r = 0.02) and oxytetracycline and ampicillin (r = 0.02).
In Vitro Antibacterial Activities of Herbal Essential Oils
The antibacterial potentials of 4 herbal EOs against MDR and multivirulent chicken VRSA (n = 16) and duck S. Typhimurium (n = 17) isolates were studied in terms of assessing the bacterial growth inhibition zones and MIC values. All used EOs showed excellent antibacterial activities against all investigated bacterial isolates. Concerning the susceptibility of chicken VRSA to the tested EOs, they were all sensitive with relevant inhibition zones of diameters ranging from 16 to 31 mm and resultant MIC concentrations in the range from 64 to 8 ug/mL. The sensitivity to examined EOs was superior for duck S. Typhimurium isolates with estimated inhibition zones' diameters and MIC values ranging from 22 to 38 mm and 16 to 4 ug/mL, respectively. Among the 4 tested EOs, marjoram was the most potent one against chicken VRSA and duck S. Typhimurium isolates with maximum inhibition zones' diameters (up to 31 and 38 mm, respectively) and this effectiveness was reflected with recording very low MIC values (up to 8 and 4 μg/mL, respectively).
Assessment of Interaction Between Essential Oils and Antibiotics
According to the results of in vitro antimicrobial susceptibility patterns alongside the EOs antibacterial activities, the combination between the most effective antibiotics (florfenicol and amoxicillin/clavulanic acid) and EO (marjoram) against the most dominant MDR and multivirulent isolates; chickens VRSA (n = 16) and ducks S. Typhimurium (n = 17) exhibited synergistic interactions with FICI values ≤ 0.5.
In Vivo Efficacy of Antimicrobials in Chickens and Ducks
Clinical Signs, Mortalities, and Postmortem Lesions
None of chickens or ducks in groups 1 and 6 (negative controls) showed any clinical signs, mortality rates or PM lesions through the entire experimental period. Meanwhile, the experimentally VRSA-infected chickens via intra articular route in group 5 (positive control) showed numerous clinical signs in the form of gradual loss of appetite, weakness, swollen hock and toe joints, arthritis (Figure 10A and B), lameness, reluctance to move and emaciation with a mortality percentage of 20%. The PM examination of freshly dead and sacrificed chickens exhibited that the affected joints and their cavities were surrounded with yellowish-white caseous exudate. Moreover, the experimentally S. Typhimurium infected ducks via intra muscular route in group 10 (positive control) displayed anorexia, depression, diarrhea, pasty vent, swollen hock and toe joints, arthritis (Figure 10C and D), inability to stand, lameness and panophthalmitis in some ducks with a mortality percentage of 40%. The PM findings revealed fibrinous exudates in the affected joints, white cecal cores and generalized septicemia in freshly dead and sacrificed ducks. All the treatment regimens succeeded in reliving the clinical signs and PM lesions in all experimentally infected and treated chickens and ducks. Birds treated with amoxicillin/clavulanic acid (intramuscularly) or florfenicol (orally) in combination with marjoram (orally) presented the most pronounced improvement in the clinical pictures of the established experimental bacterial leg infections in chickens and ducks.
Figure 10.
Histopathological pictures of joint samples obtained from broiler chickens (I) and ducks (II) in negative control (A and F), positive control (B and G) and infected and marjoram (C and H), antibiotic (D and I) and marjoram/antibiotic (E and J) treated experimental groups (H&E stain, magnification = ×400, Scale bar = 100 µm). A and F: negative controls (noninfected and nontreated birds); normal dermis, epidermis and connective tissue layers. B and G: positive controls (chickens and ducks experimentally infected with vancomycin resistant S. aureus (VRSA) and S. Typhimurium, respectively); partial necrosis of chondrocytes (arrows) and chondromalacia (arrows' heads). C and H: VRSA and S. Typhimurium infected and oral marjoram treated chickens and ducks, respectively; poor ossification, erosions and ulceration (C, arrows) and focal area of hyperosification (H, arrow head). D and I: VRSA and S. Typhimurium infected and intramuscular amoxicillin/clavulanic acid and oral florfenicol treated chickens and ducks, respectively; focal areas of hypokeratinization (D, arrow) and regular joint ossification (I, arrow head). E and J: chickens and ducks experimentally infected with VRSA and S. Typhimurium and treated with intramuscular amoxicillin/clavulanic acid or oral florfenicol in combination with oral marjoram, respectively; regular progressive ossification with normal cartilage.
Bacteriological Studies
After the end of the treatment period, all treatment regimens displayed reductions in the viable bacterial counts in the examined synovial fluids. Interestingly, treatment of chicken VRSA and duck S. Typhimurium experimental infections using marjoram and amoxicillin/clavulanic acid or florfenicol in combination demonstrated the lowest bacterial counts (up to 1 × 101 CFU/mL of synovial fluids). Moreover, up to 103 and 104 CFU/mL were observed in the synovial fluids of experimentally infected birds following treatments with amoxicillin/clavulanic acid or florfenicol and marjoram, respectively.
Histopathological Findings
The histopathological pictures of joint samples obtained from chickens and ducks in all experimental groups are illustrated in Figure 10. Histopathological examination of chicken joint samples in the negative control group revealed no noticeable microscopic alterations with normal dermis, epidermis and connective tissue layers. Meanwhile, the positive control group displayed the most apparent changes in the examined joint tissues in the form of partial necrosis of chondrocytes and poor ossification of bony materials. All the applied treatments in the present study thrived in reliving the histopathological lesions in all VRSA-infected treated groups. Following marjoram and amoxicillin/clavulanic acid treatments, the joint histopathological photographs were improved with poor ossification, erosions and ulceration and focal areas of hypokeratinization, respectively. Interestingly, treatment with marjoram and amoxicillin/clavulanic acid in combination caused no histopathological indication of joint deterioration as the joint architecture showed normal cartilage with regular ossification.
With regard to the histopathological investigation of duck joint samples in the noninfected and nontreated group, normal dermis, epidermis and connective tissue layers were evident. Meanwhile, S. Typhimurium infected group revealed chondromalacia with poor ossification. The treatment with marjoram led to ameliorating the joint histopathological findings in infected treated ducks with only a focal area of hyperosification. Moreover, florfenicol treatment resulted also in a promotion in the joint structure in infected treated ducks in the form of apparently normal layers with regular ossification. Notably, a better improvement of the joint histopathological image was attained in infected ducks following treatment with marjoram and florfenicol in combination till exhibiting regular progressive ossification with normal cartilage.
DISCUSSION
Leg disorders are symptoms of a condition caused by a variety of etiological agents including several bacterial pathogens. They have a negative impact on poultry performance resulting in significant economic losses in poultry flocks. The most common clinical forms of this condition in chickens and ducks are arthritis, synovitis, tenosynovitis, osteomyelitis, bumble foot and femoral head necrosis with subsequent lameness. Antibiotic treatment of leg affection still represents a challenge in the poultry field and it is threatened with increasing the prevalence of MDR bacterial pathogens (Hriouech et al., 2020). Therefore, there is an urgent need to find novel plant-derived alternatives for overcoming this developing antibiotic resistance. From this point, the present study aimed to identify some bacterial pathogens incriminated in leg affections in chickens and ducks and their antimicrobial susceptibility patterns with emphasis on evaluating the in vivo antibacterial effectiveness of the most effective antibiotics and EOs against the highly prevalent MDR and multivirulent recovered bacterial isolates.
Herein, the observable clinical signs of the investigated naturally infected chickens and ducks were swollen footpad and hock joint, lameness, reluctance to move and gradual emaciation. The PM examination of the freshly dead and sacrificed birds revealed caseous exudates in the swollen joints in addition to congestion of internal organs, pneumonia and airsaculitis in some cases. The previous clinical signs and PM lesions are consistent with those observed in naturally infected birds suffering from leg affections in previous researches (Bakheet, 2011; Youssef et al., 2019).
Several bacterial pathogens are involved in leg dysfunction leading to great economic losses in poultry farms. According to the bacteriological and molecular investigation results of the current study, Salmonella, E. coli, S. aureus and M. synoviae were recovered from chickens with leg affections. Similar to our findings, S .aureus, E. coli and Salmonella isolates were previously recovered from chickens suffering from arthritis and bumble foot (Lebdah et al., 2015; Tawfik et al., 2016). Interestingly, S. aureus isolates were the most frequently bacterial species encountered in the present study among diseased chicken cases either singly or mixed with E. coli. The overall incidence level of S. aureus from chickens was close to those stated earlier in previous studies investigating chicken cases with leg affections; 66% (Abd EL-Tawab et al., 2017) and 62% (Bakheet, 2011). Differently, higher prevalence rates of S. aureus were recorded from broilers with septic arthritis in Pakistan; up to 81% (Nazia et al., 2015) and Iran; 85.7% (Feizi et al., 2012) and lower isolation percentages of S. aureus were documented from broilers suffering from bumble foot lesions; 45.8% (Youssef et al., 2019) and arthritis; 36.6% (Omayma, 2005) in Egypt. In line with our findings regarding the predominant mixed bacterial profile (S. aureus with E. coli) in the naturally diseased chickens, a previous study also recorded that S. aureus was recovered in mixed form with E. coli (26.7%) from bumble foot diseased chicken cases (Youssef et al., 2019). Observations of the current study illustrated a low isolation percentage of M. synoviae from diseased chickens, which is consistent with a previous study conducted in Egypt recording the same occurrence rate of this pathogen in chickens (Abdanaser et al., 2019). However, this isolation rate is higher than that reported in another study carried out in Egypt; 1.6% (Emam et al., 2020) and it is lower than those recorded in previous studies in Egypt; 22.7% (Abd-El-Tawab et al., 2020) and Turkey; 12.9% (Yilmaz et al., 2011). Analyzing the phenotypic and genotypic identification results of the current study, Salmonella, E. coli, and S. aureus were isolated from ducks with leg disorders. Similar findings were noticed in an earlier study conducted in Denmark (Bisgaard, 1981), where Salmonella, E. coli and S. aureus were recovered from ducks with arthritis. Another study in India isolated S. aureus and E. coli from a duck with a bumble foot (Choudhury, 2019). The variations in the occurrence rates of the incriminated avian bacterial pathogens among various studies in different countries might reflect substantial discrepancies in geographic location, sample types, identification protocols, infection control practices, antibiotic usage and seasonal and environmental factors. Notably, M. synoviae did not be isolated from the investigated ducks in the present study. This could be attributed to the explanation suggested previously (Bencina et al., 1988), where Mycoplasma species could infect ducks when they were reared in close contact with Mycoplasma infected chickens. Meanwhile, the examined ducks in the current study were obtained from farms rearing ducks only.
Herein, serotyping of all investigated E. coli isolates revealed 6 different serotypes with O78 being the most prevalent one in chickens and ducks. These findings are consistent with previous studies conducted in Egypt, where O78 was the most dominant serogroup identified from chickens with leg affections; 26.7% (Lebdah et al., 2015) and 31.3% (Youssef et al., 2019). Moreover, this result is supported by another study carried out earlier in Denmark, where O78 was the most prevalent serotype from ducks with arthritis (Bisgaard, 1981). Comparably, other E. coli serotypes (O88, O25, O12, and O45) were recovered from broilers with osteomyelitis and arthritis in Brazil (Braga et al., 2016). Regarding Salmonella serotyping, S. Typhimurium serotype exhibited the highest occurrence rates among the investigated chickens (50%) and ducks (44.4%). Another study in Mexico reported an outbreak in chickens with lameness caused by S. Typhimurium (Padron, 1990). In Denmark, S. Typhimurium dominated among the isolated Salmonella serogroups identified from ducks with arthritis (Bisgaard, 1981). Conversely, Guo et al., (2019) identified S. Pullorum serotype from broilers with lameness and arthritis. Serotyping results reflect that E. coli O78 and S. Typhimurium serogroups are frequently accompanied with avian leg affections confirming their contribution in extra intestinal infections. Meanwhile, other serotypes identified in previous studies could be related to variations in geographical and period aspects.
In the current study, E. coli isolates recovered from chickens and ducks displayed high resistance levels to oxytetracycline, amoxicillin/clavulanic acid, doxycycline and ampicillin. These resistance rates are comparable to those formerly reported for E. coli isolates originating from chickens with leg affections (Lebdah et al., 2015), where the recovered isolates were resistant to amoxicillin/clavulanic acid, doxycycline and ampicillin. Likewise, a high resistance rate to amoxicillin (73.3%) has been reported for E. coli isolates recovered from broilers with osteomyelitis and arthritis in Brazil (Braga et al., 2016). Another investigation conducted in Egypt documented that duck E. coli isolates were resistant to ampicillin and penicillin (Eid et al., 2019). However, contrary to our results, E. coli isolates recovered from chickens with leg affections in earlier studies conducted in Brazil (Braga et al., 2016) and Egypt (Youssef et al., 2019) were highly sensitive to tetracycline. Herein, the high sensitivity rates of duck E. coli isolates to streptomycin and chicken E. coli ones to trimethoprim/sulfamethoxazole are in contradiction with those recorded in former studies in Egypt (Eid et al., 2019; Youssef et al., 2019).
Analyzing the antibiogram results of chicken Salmonella isolates in our study demonstrated that they showed high resistance rates to amoxicillin/clavulanic acid, ampicillin, erythromycin and doxycycline. A similar trend in the occurrence of ampicillin and doxycycline-resistant chicken Salmonella isolates was observed in previous Chinese (Guo et al., 2019) and Egyptian (Lebdah et al., 2015) reports, but in contrast to our research, Salmonella isolates recovered previously from chickens with leg affections in Egypt were sensitive to amoxicillin (Lebdah et al., 2015). With regard to the antimicrobial susceptibility results of duck Salmonella isolates in the current study, high resistance rates of amoxicillin/clavulanic acid, ampicillin and erythromycin and excellent activities of trimethoprim/sulfamethoxazole, florfenicol and neomycin were observed. This is comparable with a previous study performed in South Korea (Cha et al., 2013), where Pekin duck Salmonella isolates were resistant to amoxicillin/clavulanic acid, ampicillin and trimethoprim/sulfamethoxazole. Another previous investigation in Taiwan (Tsai and Hsiang, 2005) reported sensitivity patterns of duck Salmonella isolates to trimethoprim/sulfamethoxazole, florfenicol, neomycin, and amoxicillin/clavulanic acid.
Our antimicrobial susceptibility findings of chicken S. aureus isolates revealed high susceptibility rates to amoxicillin/clavulanic acid as previously documented for S. aureus isolates recovered from chickens with arthritis (Abd El Tawab et al., 2018) and bumble foot (Youssef et al., 2019). On contrary, a higher resistance rate was observed for amoxicillin/clavulanic acid (87.7%) in an earlier study carried out in Egypt (Bakheet et al., 2018). Herein, the high resistance rate observed for oxacillin (52.6%) is in harmony with those reported in previous studies in Egypt (Bakheet et al., 2018; Youssef et al., 2019). Moreover, higher resistance rates of our S. aureus isolates were recorded against trimethoprim/sulfamethoxazole (84.2%) and oxyetracycline (73.7%). In another study carried out in Egypt (Lebdah et al., 2015), S. aureus isolates from chickens with leg affections were sensitive to sulfamethoxazole/trimethoprim and resistant to doxycycline. Regarding duck S. aureus isolates, the resistance was mostly observed for oxacillin and trimethoprim/sulfamethoxazole; meanwhile, they were highly susceptible to streptomycin and erythromycin. An investigation on antimicrobial resistance profiles of S. aureus isolates recovered from ducks revealed higher resistance rates to ampicillin, amoxicillin, and penicillin (100% each), streptomycin (90%) and erythromycin (80%) (Eid et al., 2019). Moreover, higher cloxacillin sensitivity and sulfamethizole resistance rates of ducklings S. aureus isolates were previously reported in India (Mondal and Sahoo, 2014). Notably, higher occurrence rates of VRSA isolates were observed among chickens and ducks. Comparably, a previous research carried out in Egypt demonstrated vancomycin resistance among S. aureus isolates recovered from chickens with arthritis with a percentage of 10.5% (Abd El Tawab et al., 2018). A remarkable observation in the current study was that all chicken M. synoviae isolates were resistant to erythromycin, doxycycline, oxytetracycline, tilmicosin, and tylosin and sensitive to spectinomycin, spiramycin, and tiamulin. Another recent investigation from Egypt found similar results, where a higher resistance rate of M. synoviae isolated from diseased chickens was recorded to tetracycline (Abd-El-Tawab et al., 2020). Moreover, several previous studies in Egypt demonstrated higher sensitivity levels among chicken M. synoviae isolates to spiramycin (Emam et al., 2020) and tiamulin (Abdanaser et al., 2019; Abd El-Hamid et al., 2019a). Seemingly, the great discrepancies in the antimicrobial resistance rates of the recovered isolates among various studies may be well contributed to the type and dose of antimicrobials prescribed for treating avian leg bacterial infections in various geographical areas, management practices in avian production and variances in legislation controlling the use of antimicrobial agents.
Notably, the present study demonstrated alarming MDR frequencies for chicken S. aureus, Salmonella, and E. coli and duck E. coli and Salmonella isolates as they exhibited resistance to at least one drug in 3 or more different antimicrobial classes. Of interest, MDR among chicken and duck isolates causing leg disorders constituted a major subject of concern worldwide, where 26.3% of chicken S. aureus isolates in Egypt (Abd El Tawab et al., 2018), 73% of chicken E. coli strains in Brazil (Braga et al., 2016), 80% of chicken Salmonella isolates in China (Guo et al., 2019) and 50.5% of duck Salmonella isolates in South Korea (Cha et al., 2013) showed MDR phenotypes. Totally, the outcome, which is common between our research and previous studies (Ahmed et al., 2021; Ammar et al., 2021a) is the increasing trend of MDR among avian isolates. This could be attributable to the uncontrollable usage of antimicrobial agents for controlling bacterial pathogens in the poultry industry in many geographical localities because of their availability. Therefore, appropriate legislation and judicious usage of antimicrobials in poultry production must be adopted to overcome this serious situation.
Regarding the distribution of particular virulence genes among the selected isolates, our results demonstrated full occurrence of spa and cna genes among chicken S. aureus isolates and fimH and spvC genes among duck S. Typhimurium isolates. Likewise, a wide distribution of spa and cna genes (100%) had also been documented earlier among chicken S. aureus isolates associated with arthritis (Abd El Tawab et al., 2018) and bumble foot (Tawfik et al., 2016). This strengthens the findings of the current study confirming their roles in the pathogenesis of S. aureus arthritis. Both genes are notable members of the microbial surface components recognizing adhesive matrix molecules that are anchored on the cell wall peptidoglycan, where spa is a vital virulence factor important for adherence and invasion of host cells and also enables S. aureus to evade the host immune response and cna mediates S. aureus collagen adhesion (Foster et al., 2014; Abd El-Hamid and Bendary, 2015). The high incidence rates of fimH and spvC genes among duck S. Typhimurium isolates were also reported by other authors in South Korea (Cha et al., 2013) and Egypt (Nasser et al., 2018) emphasizing their ability to promote the persistence of S. Typhimurium at extra-intestinal sites, where fimH encodes an adhesive protein that plays important roles in S. Typhimurium adhesion and spvC has a phosphothreonine lyase activity and inhibits mitogen-activated protein kinase signaling (Ammar et al., 2016b).
Considering the previous scenario for the high prevalence of MDR and multivirulent bacterial isolates, there is a dire necessity for developing alternative strategies to fight the infections caused by MDR and multivirulent strains (Ammar et al., 2021b; Ibrahim et al., 2021a,b, 2022a,b,c). Plant-derived compounds are gaining rekindled attention as reasonable novel antimicrobials. So, we evaluated the effects of marjoram, garlic, ginger and cinnamon EOs against MDR and multivirulent chicken VRSA and duck S. Typhimurium isolates. Critical analysis of our results demonstrated promising in vitro antibacterial activities for the used EOs with prominent potential for marjoram. Indeed, a previous study had focused on the potent antibacterial aspects of garlic and ginger against both Salmonella and S. aureus strains (Chand, 2013). Other studies carried out in Egypt (Elmowalid et al., 2019) and USA (Robinson et al., 2022) confirmed that garlic and ginger had effective antibacterial properties against S. aureus and Salmonella strains. Like the findings of the current study, the considerable antibacterial activity of cinnamon EO had been proved against S. Typhimurium (Angienda et al., 2010) and S. aureus (Liu et al., 2017). Another previous evidence (Indu et al., 2006) supported our antibacterial findings of garlic and ginger EOs against Salmonella strains. Notably, the promising in vitro antibacterial efficacy of marjoram against the investigated isolates is in a link with an earlier study conducted in Iran (Moghadam and Ahanjan, 2015), where marjoram exhibited strong antibacterial effects against S. aureus and S. enterica strains. We speculate reasonable various explanations by which the EOs mediated their favorable antibacterial effects such as alteration of cell wall permeability, interference with cell wall synthesis and membrane functions, interaction with membrane proteins and functional alteration in DNA synthesis. Other anticipated scenarios clarifying the antimicrobial actions of EOs might be related to the inactivation of extracellular enzymes or the generation of free toxic radicals. Finally, the inhibition of bacterial efflux pumps could not be excluded (Abd El-Hamid et al., 2019c; Elmowalid et al., 2019).
The in vitro findings attained in the current study encouraged us to implement 2 independent in vivo trials to investigate, for the first time, the therapeutic efficacy of marjoram EO in combination with florfenicol or amoxicillin/clavulanic acid compared with marjoram, florfenicol or amoxicillin/clavulanic acid alone in chicken and duck bacterial arthritis models. Our successful experimental models of VRSA and S. Typhimurium arthritis in experimental birds were supported by previous comparable studies (Gu et al., 2013; Guo et al., 2019), where arthritis was established after intra-articular injection of S. aureus and intra-muscular injection of S. Pullorum in chickens. Regarding the efficacy of treatment regimens, amoxicillin/clavulanic acid or florfenicol in combination with marjoram EO treatments were able to reserve the arthiritis signs, reduce the mortalities, joint macroscopic lesions and viable VRSA and S. Typhimurium counts and improve the joints histopathological pictures rendering these protocols as potent approaches for experimental avian VRSA and S. Typhimurium arthritis. Similarly, a previous study carried out in Iran demonstrated that birds treated with florfenicol had better outcomes in the histopathological picture of the tibiotarsal joint compared with the positive control group, especially relating to the thickness of reparative cartilage (Mosleh et al., 2016). With regard to the effectiveness of intramuscular administration of amoxicillin/clavulanic acid in chickens, it was reported that antibiotics administration intramuscularly could lead to ultimate serum concentrations within minutes (Soranoglou et al., 2017). Additionally, the bioavailability of amoxicillin-clavulanic acid after the intramuscular injection was high in chickens (Carceles et al., 1995). Concerning the effectiveness of florfenicol in ducks, it was proved that the oral route was efficient for its administration in the field owing to rapid absorption and bioavailability. The concurrent administration of 2 antibiotics is among the probable policies for controlling the bacterial related diseases in poultry, but this leads to the appearance of numerous MDR strains (Rondevaldova et al., 2018). Therefore, there is a growing attention in replacing one of the 2 antibiotics by natural antimicrobials. This replacement leads to a reduction in the effective doses of drugs with a consequence of diminishing their potential side effects, fighting the resistance phenomenon and decreasing the treatment costs (Fadli et al., 2012). In the current study, we emphasized, for the first time, the augmented antibacterial effects of amoxicillin/clavulanic acid or florfenicol upon the combination with marjoram EO in the treatment of induced VRSA and S. Typhimurium experimental leg infections in chickens and ducks, respectively. Only one previous study in Morocco stated that 1,8-cineole EO showed a synergistic effect when combined with amoxicillin/clavulanic acid for the treatment of S. aureus osteomyelitis in experimental rabbits (Hriouech et al., 2020). The synergistic activity of amoxicillin and 1,8-cineole EO considerably reinforced the antibacterial effect of amoxicillin. This could be attributed to the protecting role of 1,8- cineole EO for the antibiotic against β-lactamases action in resistant bacterial pathogens and its substantial capacity to disrupt the bacterial cell membrane or affect its respiration (Chaves et al., 2018). Notably, reviewing the accessible preceding literature, we found that there were no in vivo prospective applications of florfenicol and EOs alone or in combination as therapeutic agents against avian bacterial arthritis. These outcomes should guide future in vivo studies for further assessing the efficacy of EOs alone or in combination with antibiotics in treating bacterial pathogens included in leg disorders in poultry.
In conclusion, based upon the findings of the current investigation, we could conclude that S. aureus, Salmonella species, E. coli and M. synoviae are major pathogens of chickens and ducks with leg disorders in Egypt. Moreover, our outcomes offer further evidence of the emergence of MDR and multivirulent avian bacterial isolates calling for crucial approaches to successfully treat these worrisome pathogens. To our knowledge, it is striking to highlight that this is the first in-depth report assessing the in vitro and in vivo synergistic antibacterial effects of marjoram in combination with florfenicol or amoxicillin/clavulanic acid in controlling the induced experimental S. Typhimurium or VRSA leg infections. In light of the data described herein, enhancing the antimicrobial influence of antibiotics using marjoram seems to be a certainly promising approach to explore novel pathways in developing new antimicrobial drugs for the treatment of bacterial pathogens associated with leg infections in the field alongside navigating successful opportunities for antimicrobials usage reduction and/or antibiotics-free poultry production.
ACKNOWLEDGMENTS
We want to acknowledge Princess Nourah bint Abdulrahman University Researchers Supporting Project number PNURSP2023R84, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
DISCLOSURES
The authors have no conflicts of interest to declare.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2023.102889.
Appendix. Supplementary materials
REFERENCES
- Abdanaser A.H.T., Zyan K.A.M., Hashem Y.H.M., Nouh M.S.A. Molecular and histopathological investigation of Mycoplasma gallisepticum and Mycoplasma synoviae isolates from chickens. Benha Vet. Med. J. 2019;36:342–350. [Google Scholar]
- Abd El-Hamid M.I., Awad N.F.S., Hashem Y.M., Abdel-Rahman M.A., Abdelaziz A.M., Mohammed I.A.A., Abo-Shama U.H. In vitro evaluation of various antimicrobials against field Mycoplasma gallisepticum and Mycoplasma synoviae isolates in Egypt. Poult. Sci. 2019;98:6281–6288. doi: 10.3382/ps/pez576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hamid M.I., Bendary M.M. Comparative phenotypic and genotypic discrimination of methicillin resistant and susceptible Staphylococcus aureus in Egypt. Cell. Mol. Biol. 2015;61:101–112. [PubMed] [Google Scholar]
- Abd El-Hamid M.I., Bendary M.M., Merwad A.M.A., Elsohaby I., Ghaith D.M., Alshareef W.A. What is behind phylogenetic analysis of hospital-, community and livestock-associated methicillin-resistant Staphylococcus aureus? Transbound. Emerg. Dis. 2019;66:1506–1517. doi: 10.1111/tbed.13170. [DOI] [PubMed] [Google Scholar]
- Abd El-Hamid M.I., El-Sayed M.E., Aisha R.A., Abdallah H.M., Marwa I.A., El-mowalid G.A. Marjoram extract down-regulates the expression of Pasteurella multocida adhesion, colonization and toxin genes: A potential mechanism for its antimicrobial activity. Comp. Immunol. Microbiol. Infect. Dis. 2019;62:101–108. doi: 10.1016/j.cimid.2018.11.007. [DOI] [PubMed] [Google Scholar]
- Abd El-Hamid M.I., Ibrahim D., Hamed R.I., Nossieur H.H., Elbanna M.H., Baz H., Abd-Allah E.M., El Oksh A.S.A., Ibrahim G.A., Khalifa E., Ismail T.A., Awad N.F.S. Modulatory impacts of multi-strain probiotics on rabbits’ growth, nutrient transporters, tight junctions and immune system to fight against Listeria monocytogenes infection. Animals. 2022;12:2082. doi: 10.3390/ani12162082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hamid M.I., Sewid A.H., Samir M., Hegazy W.A.H., Bahnass M.M., Mosbah R.A., Ghaith D.M., khalifa E., Ramadan H., Alshareef W.A., Alshareef H.M., Ghoneim M.M., Al-Sanea M.M., Bendary M.M. Clonal diversity and epidemiological characteristics of ST239-MRSA strains. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.782045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd-El-Tawab A., Hassan W.M., El-ordy M.S. Detection of virulence factors of Mycoplasma species isolated from chicken by multiplex PCR. Benha Vet. Med. J. 2020;38:61–65. [Google Scholar]
- Abd El Tawab A., Hofy F.I., Mohamed S.R., Amin S.H. Characterization of methicillin resistance Staphylococcus aureus isolated from chicken and human. Benha Univ. Med. J. 2017;32:132–137. [Google Scholar]
- Abd El Tawab A.A., Alekhnawy K.I., Talaie A.T. Virulence and resistant genes detection of Staphylococcus aureus associated with arthritis in chickens. Benha Vet. Med. J. 2018;35:96–106. [Google Scholar]
- Ahmed H.A., Awad N.F.S., Abd El-Hamid M.I., Shaker A., Mohamed R.E., Elsohaby I. Pet birds as potential reservoirs of virulent and antibiotic resistant zoonotic bacteria. Comp. Immunol. Microbiol. Infect. Dis. 2021;75 doi: 10.1016/j.cimid.2020.101606. [DOI] [PubMed] [Google Scholar]
- Ahmed H.A, Tahoun A.B.M.B., Abou Elez R.M.M., Abd El-Hamid M.I., Abd Ellatif S.S. Prevalence of Yersinia enterocolitica in milk and dairy products and the effects of storage temperatures on survival and virulence gene expression. Int. Dairy J. 2019;94:16–21. [Google Scholar]
- Aljazzar A., Abd El-Hamid M.I., El-Malt R.M.S., El-Gharreb W.R., Abdel-Raheem S.M., Ibrahim A.M., Abdelaziz A.M., Ibrahim D. Prevalence and antimicrobial susceptibility of Campylobacter species with particular focus on the growth promoting, immunostimulant and anti-Campylobacter jejuni activities of eugenol and trans-cinnamaldehyde mixture in broiler chickens. Animals. 2022;12:905. doi: 10.3390/ani12070905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar A.M., Abd El-Hamid M.I., Eid S.E.A., El Oksh A.S. Insights into antimicrobial resistance and virulence genes of emergent multidrug resistant avian pathogenic Escherichia coli in Egypt: how closely related are they? Rev. Méd. Vét. 2015;166:304–314. [Google Scholar]
- Ammar A.M., Abd El-Hamid M.I., El-Malt R.M.S., Azab D.S., Albogami S., Al-Sanea M.M., Soliman W.E., Ghoneim M.M., Bendary M.M. Molecular detection of fluoroquinolone resistance among multidrug-, extensively drug-, and pan-drug-resistant Campylobacter species in Egypt. Antibiotics. 2021;10:1342. doi: 10.3390/antibiotics10111342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar A.M., Abd El-Hamid M.I., Mohamed Y.H., Mohamed H.M., Al-khalifah D.H.M., Hozzein W.N., Selim S., El-Neshwy W.M., El-Malt R.M.S. Prevalence and antimicrobial susceptibility of bovine Mycoplasma species in Egypt. Biology. 2022;11:1083. doi: 10.3390/biology11071083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar A.M., Attia A.M, Abd El-Hamid M.I, El-Shorbagy I.M., Abd El-Kader S.A. Genetic basis of resistance waves among methicillin resistant Staphylococcus aureus isolates recovered from milk and meat products in Egypt. Cell. Mol. Biol. 2016;62:7–15. [PubMed] [Google Scholar]
- Ammar A.M., El-Naenaeey E.Y., El-Malt R.M.S., El-Gedawy A.A., Khalifa E., Elnahriry S.S., Abd El-Hamid M.I. Prevalence, antimicrobial susceptibility, virulence and genotyping of Campylobacter jejuni with a special reference to the anti-virulence potential of eugenol and beta-resorcylic acid on some multi-drug resistant isolates in Egypt. Animals. 2021;11:3. doi: 10.3390/ani11010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar A.M., Mohamed A.A., Abd El-Hamid M.I., El-Azzouny M.M. Virulence genotypes of clinical Salmonella serovars from broilers in Egypt. J. Infect. Dev. Ctries. 2016;10:337–346. doi: 10.3855/jidc.7437. [DOI] [PubMed] [Google Scholar]
- Angienda P.O., Onyango D.M., Hill D.J. Potential application of plant essential oils at sub-lethal concentrations under extrinsic conditions that enhance their antimicrobial effectiveness against pathogenic bacteria. Afr. J. Microbiol. Res. 2010;4:1678–1684. [Google Scholar]
- AOAC . Association of Official Analytical Chemists; Washington, DC: 2012. Official Methods of Analysis of AOAC International. [Google Scholar]
- Aviagen W. AOAC International Aviagen Inc.; Huntsville, AL, USA: 2018. Ross 308: Broiler’s Management and Nutrition Specification. [Google Scholar]
- Awad N.F.S., Abd El-Hamid M.I., Hashem Y.M., Erfan A.M., Abdelrahman B.A., Mahmoud H.I. Impact of single and mixed infections with Escherichia coli and Mycoplasma gallisepticum on Newcastle disease virus vaccine performance in broiler chickens: an in vivo perspective. J. Appl. Microbiol. 2019;127:396–405. doi: 10.1111/jam.14303. [DOI] [PubMed] [Google Scholar]
- Awad N.F.S., Hashem Y., Elshater N., Khalifa E., Hamed R., Nossieur H., Abd-Allah E., Elazab S.T., Nassan M., Abd El-Hamid M.I. Therapeutic potentials of aivlosin and/or zinc oxide nanoparticles against Mycoplasma gallisepticum and/or Ornithobacterium rhinotracheale with a special reference to the effect of zinc oxide nanoparticles on aivlosin tissue residues: an in vivo approach. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakheet A.A. Bacterial aspects of arthritis in broiler chickens. Zagazig Vet. J. 2011;39:22–32. [Google Scholar]
- Bakheet A.A., Amen O., Habaty S., Darwish S.F. Prevalence of Staphylococcus aureus in broiler chickens with special reference to beta-lactam resistance genes in the isolated strains. Alex. J. Vet. Sci. 2018;57:25–33. [Google Scholar]
- Bauer A.W., Kirby W.M.M., Sherris J.C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- Bencina D., Tadina T., Dorrer D. Natural infection of ducks with Mycoplasma synoviae and Mycoplasma gallisepticum and mycoplasma egg transmission. Avian Pathol. 1988;17:441–449. doi: 10.1080/03079458808436462. [DOI] [PubMed] [Google Scholar]
- Bendary M.M., Abd El-Hamid M.I., Alhomrani M., Alamri A.S., Elshimy R., Mosbah R.A., Bahnass M.M., Omar N.N., Al-Sanea M.M., Elmanakhly A.R., Safwat N.A., Alshareef W.A. What is behind the correlation analysis of diarrheagenic E. coli pathotypes? Biology. 2022;11:1004. doi: 10.3390/biology11071004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendary M.M., Abdel-Hamid M.I., Alshareef W.A., Alshareef H.M., Mosbah R.A., Omar N.N., Al-Sanea M.M., Alhomrani M., Alamri A.S., Moustafa W.H. Comparative analysis of human and animal E. coli: serotyping, antimicrobial resistance, and virulence gene profiling. Antibiotics. 2022;11:552. doi: 10.3390/antibiotics11050552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendary M.M., Abd El-Hamid M.I., El-Tarabili R.M., Hefny A.A., Algendy R.M., Elzohairy N.A., Ghoneim M.M., Al-Sanea M.M., Nahari M.H., Moustafa W.H. Clostridium perfringens associated with foodborne infections of animal origins: insights into prevalence, antimicrobial resistance, toxin genes profiles, and toxinotypes. Biology. 2022;11:551. doi: 10.3390/biology11040551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhati T., Nathawat P., Sharma S.K., Yadav R., Bishnoi J., Kataria A.K. Polymorphism in spa gene of Staphylococcus aureus from bovine subclinical mastitis. Vet. World. 2016;9:421–424. doi: 10.14202/vetworld.2016.421-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgaard M. Arthritis in ducks I. Aetiology and public health aspects. Avain Pathol. 1981;10:11–21. doi: 10.1080/03079458108418454. [DOI] [PubMed] [Google Scholar]
- Braga J.F.V., Chanteloup N.K., Trotereau A., Baucheron S., Guabiraba R., Ecco R., Schouler C. Diversity of Escherichia coli strains involved in vertebral osteomyelitis and arthritis in broilers in Brazil. BMC Vet. Res. 2016;12:140. doi: 10.1186/s12917-016-0762-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carceles C.M., Soledad Vicente M., Escudero E. Pharrnacokinetics of amoxicillin-clavulanic acid cornbination after intravenous and intrarnuscular adrninistration to turkeys and chickens. Avian Pathol. 1995;24:643–652. doi: 10.1080/03079459508419104. [DOI] [PubMed] [Google Scholar]
- Cha S.Y., Kang M., Yoon R.H., Park C.K., Moon O.K., Jang H.K. Prevalence and antimicrobial susceptibility of Salmonella isolates in Pekin ducks from South Korea. Comp. Immunol. Microbiol. Infect. Dis. 2013;36:473–479. doi: 10.1016/j.cimid.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Chand B. Anti-bacterial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against Staphylococcus aureus, Salmonella typhi, Escherichia coli and Bacillus cereus. J. of Microbiol. Biotechnol. Food Sci. 2013;2:2481–2491. [Google Scholar]
- Chansiripornchai V.R. The efficacy of Escherichia coli Aro A-live vaccine in broilers against avian E. coli serotype O78 Infection. Thai J. Vet. Med. 2009;39:337–342. [Google Scholar]
- Chaves T.P., Pinheiro R.E.E., Melo E.S., Soares M.S., Souza J.N., de Andrade T.B., de Lemos T.L.G., Coutinho H.D.M. Essential oil of Eucalyptus camaldulensis Dehn potentiates β-lactam activity against Staphylococcus aureus and Escherichia coli resistant strains. Ind. Crops Prod. 2018;112:70–74. [Google Scholar]
- Choudhury D. Management of bumble foot in duck. Int. J. Curr. Microbiol. App. Sci. 2019;8:12–15. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI) 30th ed. Clinical and Laboratory Standards Institute; Wayne, PA: 2020. Performance Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100. [Google Scholar]
- Costa D.R., Santana E.S., Coelho K.O. Infectious arthritis in broiler chickens. Enciclopéd. Biosfera. 2016;13:87. [Google Scholar]
- Cruickshank R., Duguid I.P., Marmion B.P., Swain R.H.A. 12th ed. Churchill Livingstone Edinburgh; London and New York: 1975. Medical Microbiology; pp. 126–128. [Google Scholar]
- Eid H.M., Algammal A.M., Elfeil W.K., Youssef F.M., Harb S.M., Abd-Allah E.M. Prevalence, molecular typing, and antimicrobial resistance of bacterial pathogens isolated from ducks. Vet. World. 2019;12:677–683. doi: 10.14202/vetworld.2019.677-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfaky M.A., Abdel-Hamid M.I., Khalifa E., Alshareef W.A., Mosbah R.A., Elazab S.T., Ghoneim M.M., Al-Sanea M.M., Bendary M.M. Innovative next-generation therapies in combating multi-drug-resistant and multi-virulent Escherichia coli isolates: insights from in vitro, in vivo, and molecular docking studies. Appl. Microbiol. Biotechnol. 2022;106:1691–1703. doi: 10.1007/s00253-022-11781-w. [DOI] [PubMed] [Google Scholar]
- Elmowalid G.A., Abd El-Hamid M.I., Abd El-Wahab A.M., Atta M., Abd El-Naser G., Attia A.M. Garlic and ginger extracts modulated broiler chicks innate immune responses and enhanced multidrug resistant Escherichia coli O78 clearance. Comp. Immunol. Microbiol. Infect. Dis. 2019;66 doi: 10.1016/j.cimid.2019.101334. [DOI] [PubMed] [Google Scholar]
- Elmowalid G.A.E., Adel Attia M.A., Abd El-Hamid M.I., Ibrahim D., Wahdan A., El Oksh A.S.A., Yonis A.E., Elkady M.A., Ismail T.A., Alkhedaide A.Q., Elnahriry S.S. Nigella sativa extract potentially inhibited methicillin resistant Staphylococcus aureus induced infection in rabbits: potential immunomodulatory and growth promoting properties. Animals. 2022;12:2635. doi: 10.3390/ani12192635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnady M.R.A. Univ. Zagazig; Egypt: 2019. Molecular Detection of Non Culturable Enteric Pathogenic Bacteria. PhD Thesis. [Google Scholar]
- El-Sheikh S.M.A., Abd El-Alim A.F., Ibrahim H.A., Mobarez E.A., El-Sayed W.A., Galal A.A.A., Awad N.F.S. Chitosan propolis nanocomposite alone or in combination with apramycin: an alternative therapy for multidrug-resistant Salmonella Typhimurium in rabbits: in vitro and in vivo study. J. Med. Microbiol. 2021;70 doi: 10.1099/jmm.0.001412. [DOI] [PubMed] [Google Scholar]
- Emam M., Hashem Y.M., El-Hariri M., El-Jakee J. Detection and antibiotic resistance of Mycoplasma gallisepticum and Mycoplasma synoviae among chicken flocks in Egypt. Vet. World. 2020;13:1410–1416. doi: 10.14202/vetworld.2020.1410-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadli M., Saad A., Sayadi S., Chevalier J., Mezrioui N.E., Pagès J.M., Hassani L. Antibacterial activity of Thymus maroccanus and Thymus broussonetii essential oils against nosocomial infection - bacteria and their synergistic potential with antibiotics. Int. J. Phytomed. 2012;19:464–471. doi: 10.1016/j.phymed.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Feizi A., Nazeri M., Pilevar A. Isolation of Staphylococcus spp. Genera from broiler breeder flocks in East Azerbaijan Province of Iran: prevalence and antimicrobial susceptibility. Afr. J. Microbiol. Res. 2012;6:5819–5823. [Google Scholar]
- Foster T.J., Geoghegan J.A., Ganesh V.K., Höök M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014;12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C.Q, Hu X.Y., Xie C.Q., Zhang W.P., Wang D.H., Zhou Q., Cheng G.F. Observations on arthritis in broiler breeder chickens experimentally infected with Staphylococcus aureus. Pak. Vet. J. 2013;33:195–199. [Google Scholar]
- Guo R., Li Z., Zhou X., Huang C., Hu U., Geng S., Chen X., Li Q., Pan Z., Jiao X. Induction of arthritis in chickens by infection with novel virulent Salmonella Pullorum strains. Vet. Microbiol. 2019;228:165–172. doi: 10.1016/j.vetmic.2018.11.032. [DOI] [PubMed] [Google Scholar]
- Hannan P.C. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary Mycoplasma species. International Research Programme on Comparative Mycoplasmology. Vet. Res. 2000;31:373–395. doi: 10.1051/vetres:2000100. [DOI] [PubMed] [Google Scholar]
- Hashem Y.M., Abd El-Hamid M.I., Awad N.F.S., Ibrahim D., Elshater N.S., El-Malt R.M.S., Hassan W.H., Abo-Shama U.H., Nassan M.A., El-Bahy S.M., Samy Omima M., El Sharkawy R.B., Algabri N., Elnahriry S.S. Insights into growth-promoting, anti-inflammatory, immunostimulant, and antibacterial activities of Toldin CRD as a novel phytobiotic in broiler chickens experimentally infected with Mycoplasma gallisepticum. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojati Z., Zamanzad B., Hashemzadeh M., Molaie R., Gholipour A. The FimH gene in uropathogenic Escherichia coli strains isolated from patients with urinary tract infection. Jundishapur J. Microbiol. 2015;8:e17520. doi: 10.5812/jjm.17520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hriouech S., Akhmouch A.A., Mzabi A., Chefchaou H., Tanghort M., Oumokhtar B., Chami N., Remmal A. The antistaphylococcal activity of amoxicillin/clavinic acid, gentamycin, and 1,8-cineol alone or in combination and their efficacy through rabbit model of methicillin-resistant Staphylococcus aureus osteomyelitis. Evid. Based Complement. Altern. Med. 2020;2020 doi: 10.1155/2020/4271017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q., Tu J., Han X., Zhu Y., Ding C., Yu S. Development of multiplex PCR assay for rapid detection of Riemerella anatipestifer, Escherichia coli and Salmonella enterica simultaneously from ducks. J. Microbiol. Methods. 2011;87:64–69. doi: 10.1016/j.mimet.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Huehn S., La Ragione R.M., Anjum M., Saunders M., Woodward M.J., Bunge C., Helmuth R., Hauser E., Guerra B., Beutlich J., Brisabois A., Peters T., Svensson L., Madajczak G., Litrup E., Imre A., Herrera-Leon S., Mevius D., Newell D.G., Malorny B. Virulotyping and antimicrobial resistance typing of Salmonella enterica serovars relevant to human health in Europe. Foodborne Pathog. Dis. 2010;7:523–535. doi: 10.1089/fpd.2009.0447. [DOI] [PubMed] [Google Scholar]
- Ibrahim D., Abd El-Hamid M.I., Al-zaban M.I., ElHady M., El-Azzouny M.M., ElFeky T.M., Al Sadik G.M., Samy O.M., Hamed T.A., Albalwe F.M., Alenezi M.A., Omar A.E. Impacts of fortifying Nile tilapia (Oreochromis niloticus) diet with different strains of microalgae on its performance, fillet quality and disease resistance to Aeromonas hydrophila considering the interplay between antioxidant and inflammatory response. Antioxidants. 2022;11:2181. doi: 10.3390/antiox11112181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim D., Eldemery F., Sh. Metwally A., Abd-Allah E.M., Mohamed D.T., Ismail T.A., Hamed T.A., Al Sadik G.M., Neamat-Allah A.N.F, Abd El-Hamid M.I. Dietary eugenol nanoemulsion potentiated performance of broilers chickens: orchestration of digestive enzymes, intestinal barrier functions and cytokines related genes expression with a consequence of attenuating the severity of E. coli O78 infection. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.847580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim D., Ismail T.A., Khalifa E., Abd El-Kader S.A., Mohamed D.I., Mohamed D.T., Shahin S.E., Abd El-Hamid M.I. Supplementing garlic nanohydrogel optimized growth, gastrointestinal integrity and economics and ameliorated necrotic enteritis in broiler chickens using a Clostridium perfringens challenge model. Animals. 2021;11:2027. doi: 10.3390/ani11072027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim D., Kishawy A.T.Y., Khater S.I., Khalifa E., Ismail T.A., Mohammed H.A., Elnahriry S.S., Tolba H.A., Sherief W.R.I.A., Farag M.F.M., Abd El-Hamid M.I. Interactive effects of dietary quercetin nanoparticles on growth, flesh antioxidant capacity and transcription of cytokines and Aeromonas hydrophila quorum sensing orchestrating genes in Nile tilapia (Oreochromis niloticus) Fish Shellfish Immunol. 2021;119:478–489. doi: 10.1016/j.fsi.2021.10.034. [DOI] [PubMed] [Google Scholar]
- Ibrahim D., Shahin S.E., Alqahtani L.S., Hassan Z., Althobaiti F., Albogami S., Soliman M.M., El-Malt R.M.S., Al-Harthi H.F., Alqadri N., Elabbasy M.T., Abd El-Hamid M.I. Exploring the interactive effects of thymol and thymoquinone: moving towards an enhanced performance, gross margin, immunity and Aeromonas sobria resistance of Nile tilapia (Oreochromis niloticus) Animals. 2022;12:3034. doi: 10.3390/ani12213034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indu M.N., Hatha A.A.M., Abirosh C., Harsha1 U., Vivekanandan G. Antimicrobial activity of some of the south-Indian spices against serotypes of Escherichia coli, Salmonella, Listeria monocytogenes and Aeromonas hydrophila. Braz. J. Microbiol. 2006;37:153–158. [Google Scholar]
- ISO 6579-1. 2017. Microbiology of food and animal feeding stuff- horizontal method for detection of enumeration and serotyping Salmonella spp. International standard. (First ed., 02-2017).
- ISO 6888-1.2003. Microbiology of food and animal feeding stuff-horizontal method for enumeration of coagulase positive Staphylococci, EN ISO 6888-1 (Staphylococci and other species).
- Itakura C., Kurisu K., Goto M. Histopathology of purulent arthritis of chickens. Jpn. J. Vet. Sci. 1976;38:451–459. doi: 10.1292/jvms1939.38.451. [DOI] [PubMed] [Google Scholar]
- Jeffery N., Gasser R.B., Steer P.A., Noormohammadi A.H. Classification of Mycoplasma synoviae strains using single-strand conformation polymorphism and high-resolution melting-curve analysis of the vlhA gene single copy region. Microbiology. 2007;153:2679–2688. doi: 10.1099/mic.0.2006/005140-0. [DOI] [PubMed] [Google Scholar]
- Kauffman F. Kauffman White Scheme Minkagaord; Copenhagen, Denmark: 1974. Serological Diagnosis of Salmonella Species. [Google Scholar]
- Landman W.J.M., Feberwee A. Longitudinal field study on the occurrence of Mycoplasma synoviae in Dutch turkey flocks with lameness and experimental induction of the condition. Avian Pathol. 2012;41:141–149. doi: 10.1080/03079457.2011.652595. [DOI] [PubMed] [Google Scholar]
- Langeveld W.T., Veldhuizen E.J.A., Burt S.A. Synergy between essential oil components and antibiotics: a review. Crit. Rev. Microbiol. 2014;40:76–94. doi: 10.3109/1040841X.2013.763219. [DOI] [PubMed] [Google Scholar]
- Lebdah M.A., Youssef F.M., Elwan E.A. Bacterial leg infections in broiler chickens. Zagazig Vet. J. 2015;43:179–188. [Google Scholar]
- Liu Q., Meng X., Li Y., Zhao C.N., Tang G.Y., Li H.B. Antibacterial and antifungal activities of spices. Int. J. Mol. Sci. 2017;18:1283. doi: 10.3390/ijms18061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra M., Wang G., Johnson W.M. Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J. Clin. Microbiol. 2000;38:1032–1035. doi: 10.1128/jcm.38.3.1032-1035.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadam M.R., Ahanjan M. The germicidal effect of marjoram alcoholic extract on Staphylococcus aureus, E. coli, and Salmonella enterica. J. Mazandaran Univ. Med. Sci. 2015;25:119–123. [Google Scholar]
- Mondal D., Sahoo S.K. Omphalitis in ducklings with Staphylococcus aureus infection. J. Anim. Res. 2014;4:217–222. [Google Scholar]
- Mosleh N., Shomali T., Namazi F., Marzban M., Mohammadi M., Boroojeni A.M. Comparative evaluation of therapeutic efficacy of sulfadiazine-trimethoprim, oxytetracycline, enrofloxacin and florfenicol on Staphylococcus aureus-induced arthritis in broilers. Br. Poult. Sci. 2016;57:179–184. doi: 10.1080/00071668.2016.1148263. [DOI] [PubMed] [Google Scholar]
- Nasser M., El-Gohary A., Mohamed A. Role of domestic birds in transmission of Escherichia coli and Salmonella species as a zoonotic pathogens. Mansoura Vet. Med. J. 2018;19:355–369. [Google Scholar]
- National Research Council (NRC) 9th ed. National Academy Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Nazia K.K.Malhi, Durrani N.U., Kamboh A.A., Lakho S.A., Rind R., Abro S.H., Soomro N.M. Prevalence of septic arthritis caused by Staphylococcus aureus in poultry birds at Tandojam. Pak. J. Anim. Health Prod. 2015;3:73–77. [Google Scholar]
- Oliveira S.D., Rodenbusch C.R., Ce′ M.C., Rocha S.L.S., Canal C.W. Evaluation of selective and non-selective enrichment PCR procedures for Salmonella detection. Lett. Appl. Microbiol. 2003;36:217–221. doi: 10.1046/j.1472-765x.2003.01294.x. [DOI] [PubMed] [Google Scholar]
- Omayma K.I. Isolation of some bacterial agents associated with lesions in chickens in Sharkia Province of Egypt. Zag. Vet. J. 2005;33:100–107. [Google Scholar]
- Padron M. S. Typhimurium outbreak in broiler chicken flocks in Mexico. Avian Dis. 1990;34:221–223. [PubMed] [Google Scholar]
- Robinson K., Assumpcao A.L.F.V., Arsi K., Donoghue A., Jesudhasan P.R.R. Ability of garlic and ginger oil to reduce Salmonella in post-harvest poultry. Animals. 2022;12:2974. doi: 10.3390/ani12212974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondevaldova J., Hummelova J., Tauchen J., Kokoska L. In vitro antistaphylococcal synergistic effect of isoflavone metabolite demethyltexasin with amoxicillin and oxacillin. Microb. Drug. Resist. 2018;24:24–29. doi: 10.1089/mdr.2017.0033. [DOI] [PubMed] [Google Scholar]
- Shiozawa K., Kato E., Shimizu A. Enterotoxigenicity of Staphylococcus aureus strains isolated from chickens. J. Food Prot. 1980;43:683–685. doi: 10.4315/0362-028X-43.9.683. [DOI] [PubMed] [Google Scholar]
- Soranoglou V., Galanopoulous I., Giamarellos-Bourboulis E.I. Efficacy of intramuscular moxifloxacin in the treatment of experimental osteomyelites caused by methicillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents. 2017;50:186–190. doi: 10.1016/j.ijantimicag.2017.01.041. [DOI] [PubMed] [Google Scholar]
- Suvarna K.S., Layton C., Bancroft J.D. Bancroft’s Theory and Practice of Histological Techniques. 8th edition. Churchill Livingstone; Edinburg, London: 2018. [Google Scholar]
- Tawfik R.G., Khalil S.A., Ellakany H.F., Torky H.A. Mycoplasma synoviae and other associated bacteria causing arthritis in chicken. Alex. J. Vet. Sci. 2016;49:163–169. [Google Scholar]
- Tristan A., Ying L., Bes M., Etienne J., Vandenesch F., Lina G. Use of multiplex PCR to identify Staphylococcus aureus adhesins involved in human hematogenous infections. J. Clin. Microbiol. 2003;41:4465–4467. doi: 10.1128/JCM.41.9.4465-4467.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H.J., Hsiang P. The Prevalence and antimicrobial susceptibilities of Salmonella and Campylobacter in ducks in Taiwan. J. Vet. Med. Sci. 2005;67:7–12. doi: 10.1292/jvms.67.7. [DOI] [PubMed] [Google Scholar]
- Wada M., Lkhagvadorj E., Bian L., Wang C., Chiba Y., Nagata S., Shimizu T., Yamashiro Y., Asahara T., Nomoto K. Quantitative reverse transcription-PCR assay for the rapid detection of methicillin-resistant Staphylococcus aureus. J. Appl. Microbiol. 2010;108:779–788. doi: 10.1111/j.1365-2672.2009.04476.x. [DOI] [PubMed] [Google Scholar]
- Yilmaz F., Timurkaan N., Kiliç A., Kalender H., Kilinç Ü. Detection of Mycoplasma synoviae and Mycoplasma gallisepticum in chickens by immunohistochemical, PCR and culture methods. Rev. Méd. Vét. 2011;162:79–86. [Google Scholar]
- Youssef F.M., Soliman A.A., Ibrahim G.A., Salah H.A. Advanced bacteriological studies on bumble foot infections in broiler chicken with some clinicopathological alteration. Vet. Sci. Res. J. 2019;1:1–10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.