Summary
Quantitative assessment of endogenously synthesized and released bilirubin from brain tissue remains a challenge. Here, we present a sensitive and reproducible experimental paradigm to quantify, in real time, unconjugated bilirubin (UCB) from isolated murine brain tissue during oxygen-glucose deprivation (OGD). We describe steps for perfusion, brain dissection, brain slice preparation and incubation, glucose depletion, and OGD processing. We then detail procedures for standard calibration plotting and sample UCB measurement.
For complete details on the use and execution of this protocol, please refer to Liu et al.1
Subject areas: Cell Biology, Cell-based Assays, Neuroscience, Molecular/Chemical Probes
Graphical abstract

Highlights
-
•
Protocol to assess time-dependent UCB levels from isolated brain slices
-
•
Step-by-step guide to OGD processing of ex vivo brain slices
-
•
Detailed description of assay of UCB by using the sensitive fluorescent UnaG protein
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Quantitative assessment of endogenously synthesized and released bilirubin from brain tissue remains a challenge. Here, we present a sensitive and reproducible experimental paradigm to quantify, in real time, unconjugated bilirubin (UCB) from isolated murine brain tissue during oxygen-glucose deprivation (OGD). We describe steps for perfusion, brain dissection, brain slice preparation and incubation, glucose depletion, and OGD processing. We then detail procedures for standard calibration plotting and sample UCB measurement.
Before you begin
The following protocol describes the specific steps for brain slice preparation, OGD processing, and UCB measurement. These procedures have been optimized for monitoring dynamic bilirubin levels released from acutely isolated mouse brain slices. This protocol not only offers the method for detecting endogenous bilirubin level, but could also provide an effective model for assaying other neuroactive metabolites in brain injuries.
Institutional permissions
All the procedures relating to the caring and sacrificing of the animals were conducted in conformity with the institutional guidelines for the Institutional Animal Care and Use Committee (IACUC), and experimental protocols were approved by the Ethics Committee of the Sixth People’s Hospital of Shanghai and Shanghai Jiao Tong University School of Medicine. Readers should acquire permissions from the relevant institutions before performing animal work.
Solution preparation
Timing: 1–2 h
-
1.
Prepare 1% sodium pentobarbital solution 10 mL.
-
2.
Prepare 500 mL cutting solution for brain slices.
-
3.
Prepare 500 mL of aCSF (Artificial Cerebrospinal Fluid Solution), pH 7.35–7.45.
-
4.
Prepare 100 mL of glucose-free aCSF, pH 7.35–7.45.
-
5.
Prepare 0.1 mol/L PBS 50 mL.
-
6.
Prepare standard bilirubin solution with concentration gradient (0.09 μM, 0.18 μM, 0.36 μM, 0.71 μM, 1.43 μM, and 2.86 μM).
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Healthy adult mice brain tissue | Collected in the lab | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| NaCl | Sigma-Aldrich | Cat# S5886 |
| Sucrose | Sangon Biotech | Cat# A502792 |
| KCl | Sigma-Aldrich | Cat# P5405 |
| Glucose | Sigma-Aldrich | Cat# G8270 |
| NaH2PO4 | Sigma-Aldrich | Cat# 71505 |
| Na-pyruvate | Sigma-Aldrich | Cat# P8574 |
| Myo-inositol | Sigma-Aldrich | Cat# I525 |
| Ascorbic acid | Sigma-Aldrich | Cat# 1043003 |
| NaHCO3 | Sigma-Aldrich | Cat# S5761 |
| MgCl2 | Sigma-Aldrich | Cat# M8266 |
| CaCl2 | Sigma-Aldrich | Cat# C4901 |
| KH2PO4 | Sigma-Aldrich | Cat# P5655 |
| Na2HPO4·7H2O | Sangon Biotech | Cat# A501288 |
| Bilirubin | Sigma-Aldrich | Cat# B4126 |
| DMSO | Sigma-Aldrich | Cat# V900090 |
| UnaG | Gifted by Yu-Zheng Zhao | Shanghai Key Laboratory of New Drug Design, School of Pharmacy, East China University of Science and Technology, 130 Mei Long Road, Shanghai 200237, China |
| Enoxaparin sodium injection | Shanghai Sixth People’s Hospital | N/A |
| Sodium pentobarbital | Animal Laboratory of Shanghai Sixth People’s Hospital | N/A |
| Experimental models: Organisms/strains | ||
| C57BL/6 mice, age 6–8 weeks, either gender | Animal Laboratory of Shanghai Sixth People’s Hospital | N/A |
| Software and algorithms | ||
| OxyCycler | BioSpherix | https://biospherix.com |
| Gen5 CHS 3.05 | Agilent | https://www.agilent.com.cn/en/support/biotek-software-releases |
| Microsoft Excel | Microsoft | https://www.microsoft.com |
| GraphPad Prism 9.0 | GraphPad Software | https://www.graphpad.com |
| Adobe Illustration 2020 | Adobe System Inc. | https://www.adobe.com/ |
| Other | ||
| 3M Vetbond | 3M Science | Cat# 1469SB |
| Pasteur pipette | Sangon Biotech | Cat# F621003 |
| 24-well plate | Falcon | Cat# 353047 |
| Light-shield 96-well plate | Corning | Cat# 3925 |
| Dynamic O2 and CO2 Subchamber Controller | BioSpherix | OxyCycler C42 |
| CelMate CO2 Incubator | ESCO | CLM-170B-8-NF |
| Microplate reader | Agilent | BioTek Synergy H1 |
| Leica vibrating blade microtome | Leica Biosystems | VT1200S |
Materials and equipment
1% sodium pentobarbital solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Pentobarbital | 1% | 0.1 g |
| 5% saline | N/A | 10 mL |
| Total | N/A | 10 mL |
Note: This pentobarbital stock solution can be stored at 20°C–25°C for 3 months.
Cutting solution
| Reagent | Final concentration | Amount |
|---|---|---|
| NaCl | 87 mM | 2.542 g |
| Sucrose | 75 mM | 12.847 g |
| KCl | 2.5 mM | 0.093 g |
| Glucose | 10 mM | 0.901 g |
| NaH2PO4 | 1.25 mM | 0.075 g |
| Na-pyruvate | 2 mM | 0.110 g |
| Myo-inositol | 3 mM | 0.270 g |
| Ascorbic acid | 0.5 mM | 0.044 g |
| NaHCO3 | 26 mM | 1.092 g |
| MgCl2 | 7 mM | 0.333 g |
| CaCl2 | 0.1 mM | 0.006 g |
| miliQ H2O | N/A | 500 mL |
| Total | N/A | 500 mL |
Note: Bubble with 95% O2, 5% CO2 for 30 min after adding the glucose and CaCl2 in order to maintain aCSF stability and prevent calcium carbonate precipitation. Keep bubbling continuously.
Note: Prepare and use fresh cutting solution on the day of the experiment; and do not store it for later use.
Note: Final pH is maintained at 7.35–7.45 by the gas mixture and titration with HCl (3 M) or NaOH (1 M). The osmolality is adjusted to 290–320 mOsm.
CRITICAL: HCl is a corrosive agent and inhalation/exposure may cause eye, skin and respiratory tract irritation. It should be manipulated with gloves and under a fume hood. Contact with skin, eyes and mucous membrane must be avoided.
aCSF solution
| Reagent | Final concentration | Amount |
|---|---|---|
| NaCl | 124 mM | 3.623 g |
| KCl | 5 mM | 0.186 g |
| Glucose | 10 mM | 0.901 g |
| KH2PO4 | 1.2 mM | 0.082 g |
| NaHCO3 | 24 mM | 1.008 g |
| MgCl2 | 1.3 mM | 0.048 g |
| CaCl2 | 2.4 mM | 0.133 g |
| miliQ H2O | N/A | 500 mL |
| Total | N/A | 500 mL |
Note: Bubble with 95% O2, 5% CO2 for 30 min before adding the glucose and CaCl2 in order to maintain aCSF stability and prevent calcium carbonate precipitation. Keep bubbling continuously.
Note: Prepare and use fresh aCSF on the day of experiment. Do not store it for later use.
Note: Final pH is maintained at 7.35–7.45 by the gas mixture and titration with HCl (3 M) or NaOH (1 M). The osmolality is adjusted to 290–320 mOsm.
Glucose-free aCSF solution
| Reagent | Final concentration | Amount |
|---|---|---|
| NaCl | 124 mM | 0.725 g |
| KCl | 5 mM | 0.037 g |
| Sucrose | 10 mM | 0.342 g |
| KH2PO4 | 1.2 mM | 0.016 g |
| NaHCO3 | 24 mM | 0.180 g |
| MgCl2 | 1.3 mM | 0.010 g |
| CaCl2 | 2.4 mM | 0.027 g |
| miliQ H2O | N/A | 100 mL |
| Total | N/A | 100 mL |
Note: Final pH is adjusted to 7.35–7.45 by using HCl (3 M) or NaOH (1 M), accordingly. The osmolality is adjusted to 290–320 mOsm.
Note: The Glucose-free aCSF solution can be stored at 4°C for 1 week.
0.1 M Phosphate buffered saline (PBS) (pH 7.4)
| Reagent | Final concentration | Amount |
|---|---|---|
| NaCl | 1.37 M | 4 g |
| KCl | 27 mM | 0.1 g |
| Na2HPO4·7H2O | 81 mM | 1.09 g |
| KH2PO4 | 15 mM | 0.13 g |
| ddH2O | N/A | 50 mL |
| Total | N/A | 50 mL |
Note: PBS solution can be stored at 20°C–25°C for 6 months.
5 mM bilirubin solution
| Reagent | Final concentration | Amount |
|---|---|---|
| bilirubin | 5 mM | 2.92 mg |
| DMSO | N/A | 1 mL |
| Total | N/A | 1 mL |
Note: Bilirubin is easily decomposed by light, the bilirubin solution is prepared in a light-sheltered reservoir before diluting to the required concentration to prevent degradation and denaturation.
Note: The bilirubin stock solution should be prepared and used immediately. Do not store it for later use.
CRITICAL: Bilirubin is not considered a hazardous substance or mixture by the Regulation (EC) No 1272/2008; however, due to its powder form and strong colorant characteristics, it is recommended to wear respiratory masks and protective gloves while preparing bilirubin-based solutions or mixtures. If skin contact occurs, please wash off with soap and plenty of water. Dispose the waste bilirubin solution safely and not in the drains.
10 μM bilirubin solution
| Reagent | Final concentration | Amount |
|---|---|---|
| 5 mM bilirubin | 10 μM | 2 μL |
| 0.1 M PBS | N/A | 1 mL |
| Total | N/A | 1 mL |
Note: The bilirubin solution should be prepared and used immediately. Do not store it for later use.
2.86 μM bilirubin solution
| Reagent | Final concentration | Amount |
|---|---|---|
| 10 μM bilirubin | 2.86 μM | 114 μL |
| 0.1 M PBS | N/A | 286 μL |
| Total | N/A | 400 μL |
Note: The bilirubin solution should be prepared and used immediately. Do not store it for later use.
1.43 μM bilirubin solution
| Reagent | Final concentration | Amount |
|---|---|---|
| 2.86 μM bilirubin | 1.43 μM | 200 μL |
| 0.1 M PBS | N/A | 200 μL |
| Total | N/A | 400 μL |
Note: The bilirubin solution should be prepared and used immediately. Do not store it for later use.
0.71 μM bilirubin solution
| Reagent | Final concentration | Amount |
|---|---|---|
| 1.43 μM bilirubin | 0.71 μM | 200 μL |
| 0.1 M PBS | N/A | 200 μL |
| Total | N/A | 400 μL |
Note: The bilirubin solution should be prepared and used immediately. Do not store it for later use.
0.36 μM bilirubin solution
| Reagent | Final concentration | Amount |
|---|---|---|
| 0.71 μM bilirubin | 0.36 μM | 200 μL |
| 0.1 M PBS | N/A | 200 μL |
| Total | N/A | 400 μL |
Note: The bilirubin solution should be prepared and used immediately. Do not store it for later use.
0.18 μM bilirubin solution
| Reagent | Final concentration | Amount |
|---|---|---|
| 0.36 μM bilirubin | 0.18 μM | 200 μL |
| 0.1 M PBS | N/A | 200 μL |
| Total | N/A | 400 μL |
Note: The bilirubin solution should be prepared and used immediately. Do not store it for later use.
0.09 μM bilirubin solution
| Reagent | Final concentration | Amount |
|---|---|---|
| 0.18 μM bilirubin | 0.09 μM | 200 μL |
| 0.1 M PBS | N/A | 200 μL |
| Total | N/A | 400 μL |
Note: The bilirubin solution should be prepared and used immediately. Do not store it for later use.
Step-by-step method details
Brain slice preparation
Timing: 2 h
Bilirubin is primarily produced from heme catabolism. In order to minimize interference of endogenous release of bilirubin from neurons and glia by residual blood in brain tissue, cardiac perfusion is first performed to completely flush the brain vessels. This step describes how to perfusion and prepare mouse brain slices. The main purpose of this process is to ensure optimal bioactivity of brain slices.
-
1.Perfusion (Figure 1).
-
a.Anesthetize mice with 1% sodium pentobarbital (55 mg/kg).
-
b.Open the thoracic cavity with surgical scissors to expose the beating heart.
-
c.Make an incision to the right atrial appendage with surgical scissors.
-
d.Insert a 22-gauge needle into the left ventricle of heart.
-
e.Perfuse with 20 mL pre-oxygenated ice-cold aCSF with enoxaparin sodium Injection over 10 min by using a 20 mL syringe.
-
a.
Note: The aCSF is perfused until the tail, limbs and liver of the mice turned into pale (during which the heart remains beating) before sacrifice.
Note: The pentobarbital sodium can be replaced with ketamine or gas anesthetic (e.g., isoflurane), etc., but the mechanism and effect of different anesthetic reagents are different, and researchers should carefully choose according to their own experimental needs.
-
2.Brain slice preparation (Figure 2).
-
a.After perfusion, the skin and skull from the base of the neck up to in between the eyes was cut open with surgical scissors to expose the brain tissue.
-
b.The brain tissue is isolated and the forebrain is dissected and attached onto a specimen tray with 3M Biogel.
-
c.Attach the tray to the vibratome bath and fill the bath with crushed ice.
-
d.Pour pre-oxygenated ice-cold cutting solution to the specimen tray and continuously bubbled with oxygen.2
-
e.Set the sectioning speed to 0.08 mm/s with 1.0 mm amplitude.
-
f.Set the vibratome frequency to 75 Hz–77 Hz.
-
g.Cut 300-μm-thickness coronal brain slices.
-
h.Collect the slices and place into a 50 mL beaker containing 10 mL aCSF and continuously bubbled with oxygen.
-
i.Put the beaker in the thermostat water bath at 37°C and incubate for 30 min for recovery.
-
a.
Note: In order to reduce tissue damage during sectioning, the mouse brain is kept immersed in oxygenated sectioning solution throughout the procedure.
Note: The 3M Biogel can be substituted with other biological glue that does not damage tissue viability.
Figure 1.
Cardiac perfusion with ice-cold oxygenated aCSF to flush blood in systemic circulation
Blood in the general systemic circulation is largely flushed out after cardiac perfusion (from A to B), and successful perfusion is marked by the liver, perioral area, limbs, and tail turn ischemic paleness. (related to step 1).
Figure 2.
Isolation, section and incubation of perfused brain (related to step 2)
(A) Mouse brain is dissected after successful perfusion to remove general circulation blood. Compared with a brain without prior perfusion (left), successfully perfused brain exhibits obvious paleness and absence of visible blood vessels (right).
(B) Setup panel of the vibratome (Leica Vibrating blade microtome VT1200S), speed: 0.08 mm/s, amplitude: 1.0 mm, and thickness: 300 μm.
(C) The 300-μm-thick brain slices are incubated with continuously oxygenated aCSF solution in 37°C thermostat water bath for 30 min.
Oxygen-glucose deprivation model in brain slices
Timing: 7–8 h
This section describes the oxygen-glucose deprivation (OGD) steps. The main purpose of this process is to simulate the ischemia state ex-vivo as much as possible.
-
3.
After incubation, brain slices are transferred to continuously oxygenated glucose-free aCSF (room temperature, RT) with trimmed pasteur pipette and washed for 10 min to deplete the remaining glucose from the extracellular space RT).
Optional: A hook made of bent needle can also be used to pick up the brain slice for transfer.
-
4.
The glucose-depleted brain slices are transferred with a bent syringe needle to a foil wrapped, light-shielded 24-well plate, in which 500 μL unoxygenated glucose-free aCSF (RT) and 3 brain slices are added to each well.
Note: The specific volume of solution and the number of brain slices added in each well can be adjusted depending on the desired purposes and bioactive substances to be assayed.
-
5.Place the 24-well plate in a hypoxia incubator for OGD processing (Figure 3).
-
a.Turn on the hypoxic incubator and set the temperature to 37°C.
-
b.Setting the control software to execute the hypoxia program (95% N2, 5% CO2), which consists of two stages.
-
i.Stage 1: Oxygen decent stage: Continuous N2 infusion, increasing N2 concentration to 95% and transpiring O2 within 10 min.
-
ii.Stage 2: Hypoxia sustain Stage: Maintain hypoxia for up to 6 h.
-
i.
-
a.
-
6.
During the hypoxia process, 5 μL of glucose-free aCSF is collected into 200 μL PCR tubes at different time points (15 min, 30 min, 1 h, 2 h, 3 h, 4 h, 5 h and 6 h) for the measurements of unconjugated bilirubin (UCB) concentration.
Note: Prior to the glucose-free aCSF collection, gently stir the brain slices to ensure that UCB spreads evenly into the external fluid.
Note: Eppendorf tubes are temporarily stored at 4°C until use. Avoid light exposure during aCSF’s sample collection and storage.
Figure 3.
OGD processing after glucose depletion (related to step 4 and 5)
(A) Samples 1–3 are placed in the 500 μL glucose-free aCSF containing 24-well plate after glucose depletion.
(B) The Dynamic O2 and CO2 Sub-chamber Controller, which replaces O2 with N2.
(C and D) The Celmate CO2 Incubator and 2 steps OGD protocol.
(E) The real-time variation curve of O2 concentration in OGD processing.
Unconjugated bilirubin measurement
Timing: 1–2 h
This section describes a highly sensitive fluorescent protein-based (UnaG) assay of UCB.3
-
7.Making a standard calibration curve of fluorescence intensity using the artificial UCB solutions.
-
a.50 μL 2 μM UnaG solution and 50 μL the bilirubin concentration gradient solutions (0.09 μM, 0.18 μM, 0.36 μM, 0.71 μM, 1.43 μM, and 2.86 μM) are mixed into 100 μL reaction system which are add into light-shield black 96-well plate (final UnaG concentration 1 μM).
-
b.Add 100 μL 0.1 M PBS solution without bilirubin as blank control.
-
c.React in darkness for 10–20 min at room temperature.
-
d.Use microplate reader to measure the fluorescence intensity with filters for excitation and emission wavelengths at 485 and 528 nm, respectively.
-
e.The standard calibration curve is drawn with bilirubin concentration against fluorescence intensity (Figure 4A).
-
a.
Note: The actual fluorescence intensity is reported as the difference between the fluorescence intensity in each reaction well minus the basal (blank) fluorescence intensity of the control well.
-
8.Detect UCB concentrations in samples at different time points.
-
a.The 5 μL sample is added to 45 μL 0.1 M PBS solution and diluted 10-fold.
-
b.The 100 μL reaction mixture containing 50 μL UnaG solution (2 μM) and 50 μL diluted UCB-containing samples (for a final UnaG concentration of 1 μM and samples are diluted 20-fold).
-
c.Allow reaction in darkness for 10–20 min at room temperature.
-
d.Use microplate reader to detect the fluorescence intensity with filters for excitation and emission wavelengths at 485 and 528 nm, respectively.
-
a.
Note: The actual fluorescence intensity is recorded as the difference between the fluorescence intensity in each reaction well minus the basal (blank) fluorescence intensity of the control well.
-
9.
The specific concentration of UCB in samples at different time points is extrapolated from the standard calibration curve.
Note: The concentration calculated from the standard curve should be multiplied by 20 to get the actual UCB concentration of the sample.
-
10.
Finally, plot the time-dependent curve of endogenous generated UCB during OGD (Figure 4B).
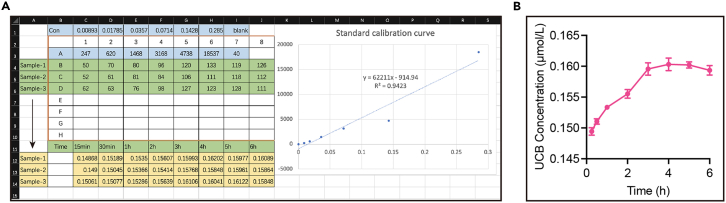
Figure 4.
Time-dependent increase of UCB concentration during OGD treatment of the isolated brain slices (related to step 7 to 10)
(A) The fluorescence intensity of standard bilirubin solution is labeled with blue background and the standard calibration curve is plotted on the right. The fluorescence intensity of samples 1 to 3 is labeled with green background, while the actual time-dependent UCB concentration in samples 1 to 3 is calculated and labeled with yellow background.
(B) The time-dependent curve of mean UCB concentration of samples 1 to 3 during 6 h of OGD processing in isolated brain slices is plotted.
Expected outcomes
This protocol uses ex vivo brain slices to simulate the pathological onset and progression of the ischemia during stroke by performing OGD. Contamination by peripheral sources of neuroactive substances such as bilirubin from blood circulation should be ruled out. One can further separate parts of the brains (the cortex, hippocampus, cerebellum…) to optimize and focus on the synthesis or release of other metabolites in specific brain regions. In this protocol, along with OGD, the brain injury is continuously aggravated, and the harmful metabolic product, bilirubin, will also be continuously generated and released. As shown by our experimental results, pharmacological blockade of or molecular perturbation to bilirubin binding with the TRPM2 channel can effectively inhibit the release of bilirubin from ischemic brain regions.1 This protocol may provide a potential methodology for measuring other endogenous neurotransmitters, peptides, toxins and even inflammatory markers (e.g., glutamate, LDH, IL, etc.) from acute slices as an in vitro model of ischemic insults.
Limitations
As this protocol is simple and reliable, it could be applied to the ischemic-hypoxia study of cranial nerves, cell lines and even other acute isolated organs or cultured organoid. Our protocol with a pulse-chase approach can follow the release of endogenous bilirubin from brain slices upon OGD challenge for several hours (up to 6 h). However, ex-vivo brain slices OGD processing cannot perfectly simulate the true pathological situation of ischemic regions in the brain in vivo. At the same time, the UCB concentration of the samples cannot accurately reflect the exact local levels around the injured neurons/glial cells. Finally, although this protocol excludes the influence of peripheral factors, it is still challenging to determine whether the harmful products (i.e., UCB) come from different cell types included trapped red blood cells in the brain slice. In summary, optimization of this protocol by combining gene manipulation technique (e.g., knock-out or knock-in mice) and chemical biology can further improve the detection of endogenous metabolites and validate their effects in the ischemic core.
Troubleshooting
Problem 1
Reduced viability of brain slices. (related to Step 2).
Potential solution
Brain is easily damaged if the extraction process is too slow. Try to use a high sucrose aCSF solution or reduce the speed of the vibratome and increase the vibration frequency of the blade. If this is not enough, be sure to prepare fresh aCSF the day before you start and oxygenated with CO2 (5%) and O2 (95%). And just as important, regularly check the pH and osmolality. Cell viability can be quantitatively assayed with PI and Calcein-am colabeling.1 Note that the superficial layer of slices contains significant higher number of dead cells due to slicing itself and thus viability assessment should be done for cells in deeper layers.
Problem 2
Weak fluorescence intensity. (related to Step 8).
Potential solution
Use of light resistant 96 well black plates may effectively improve the efficiency of fluorescence detection and avoid interference between adjacent sample-containing wells.
Problem 3
Low concentration of the substance to be detected. (related to Step 8).
Potential solution
Increasing the number of brain’s slices in each well of the 96-well plate or reducing the volume of external aCSF may elevate the threshold concentration of the substance to be detected in the external solution.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Lu-Yang Wang (luyang.wang@utoronto.ca).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81720108010 to S.-K.Y. and 81870722 and 82020108008 to H.-B.S.), CIHR (PJT-156034 and PJT-156439), NSERC (RGPIN-2017-06665), and Canada Research Chair Program (to L.-Y.W.). We thank Dr. Yu-Zheng Zhao for the UnaG protein.
Author contributions
H.-W.L. optimized and carried out experiments and wrote the manuscript draft. K.L. and L.-N.G. participated in figure generation. H.-B.S., S.-K.Y., and L.-Y.W. proofread the manuscript, provided guidance in writing, and participated in figure assembly. All authors discussed and commented on the manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Han-Wei Liu, Email: melody.l@alumni.sjtu.edu.cn.
Lu-Yang Wang, Email: luyang.wang@utoronto.ca.
Data and code availability
This study did not generate any datasets and code.
References
- 1.Liu H.W., Gong L.N., Lai K., Yu X.F., Liu Z.Q., Li M.X., Yin X.L., Liang M., Shi H.S., Jiang L.H., et al. Bilirubin gates the TRPM2 channel as a direct agonist to exacerbate ischemic brain damage. Neuron. 2023;111:1609–1625.e6. doi: 10.1016/j.neuron.2023.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carriere C.H., Wang W.X., Sing A.D., Fekete A., Jones B.E., Yee Y., Ellegood J., Maganti H., Awofala L., Marocha J., et al. The gamma-Protocadherins Regulate the Survival of GABAergic Interneurons during Developmental Cell Death. J. Neurosci. 2020;40:8652–8668. doi: 10.1523/JNEUROSCI.1636-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumagai A., Ando R., Miyatake H., Greimel P., Kobayashi T., Hirabayashi Y., Shimogori T., Miyawaki A. A bilirubin-inducible fluorescent protein from eel muscle. Cell. 2013;153:1602–1611. doi: 10.1016/j.cell.2013.05.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate any datasets and code.