Abstract
Immune checkpoint inhibitors (ICIs) are monoclonal antibody antagonists, which can block cytotoxic T lymphocyte antigen-4 (CTLA-4), programmed death-1/ligand-1 (PD-1/PD-L1) pathways, and other molecules exploited by tumor cells to evade T cell-mediated immune response. ICIs have transformed the treatment landscape for various cancers due to their amazing efficacy. Many anti-tumor therapies, including targeted therapy, radiotherapy, and chemotherapy, combine ICIs to make the treatment more effective. However, the off-target immune activation caused by ICIs may lead to a broad spectrum of immune-related adverse events (irAEs) affecting multiple organ systems. Among irAEs, cardiotoxicity induced by ICIs, uncommon but fatal, has greatly offset survival benefits from ICIs, which is heartbreaking for both patients and clinicians. Consequently, such cardiotoxicity requires special vigilance, and it has become a common challenge both for patients and clinicians. This article reviewed the clinical manifestations and influence of cardiotoxicity from the view of patients and clinicians, elaborated on the underlying mechanisms in conjunction with animal studies, and then attempted to propose management strategies from a cardio-immuno-oncology multidisciplinary perspective.
Keywords: Cancer management, Cardio-immuno-oncology multidisciplinary, Cardiotoxicity, Immune checkpoint inhibitors, Immune tolerance
Introduction
Generally, cancer, cardiac disorders, and autoimmune disease are three different diseases. However, as far as immune checkpoint inhibitors (ICIs)-induced cardiotoxicity is concerned, the above three diseases are intricately linked to each other.1 With the expanded application of ICIs in tumor therapy,2, 3, 4 the occurrence of immune-related adverse events (irAEs) keeps growing.5 Among all irAEs, cardiotoxicity, uncommon but fatal, with a significant risk of mortality, substantially limits the efficiency of tumor treatment.6
ICIs-induced cardiotoxicity refers to a series of cardiac disorders such as myocarditis, pericarditis, arrhythmias, myocardial infarction (MI), etc.7 Among these events, myocarditis has been reported to have the highest morbidity and mortality.8 Judging from the currently reported pathological results of endomyocardial biopsy (EMB), ICIs-induced myocarditis belongs to lymphocytic myocarditis, and T cell-mediated immune response is deemed to be the main culprit in this process.9
In the heart, T-cell responses are regulated by multiple mechanisms of central and peripheral tolerance10 to maintain cardiac immune homeostasis.11,12 Cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed death-1/ligand-1 (PD-1/PD-L1) are crucial to central and peripheral tolerance.13 Hence, ICIs-induced cardiotoxicity must be inseparable from the destruction of immune tolerance. Therefore, a holistic understanding of the underlying mechanisms behind cardiotoxicity shall be helpful to seek optimal strategies for cancer management.1,14
In recent years, it has been of great concern to clinicians that potential short- and long-term ICIs-induced cardiotoxicity is ever-increasing. It is an emerging challenge for oncologists to manage such “heartbreaking” cardiotoxicity. In this dilemma, it is urgent to explore a new multidisciplinary approach for the cardio-immuno-oncology research concerning pre-treatment screening, prevention, detection, monitoring, and treatment of cardiotoxicity, in order to minimize the impact on heart health and ensure the steady development of novel immunotherapies in the future.
Overall, based on the above issues, this review aims to give insights into the clinical manifestations and mechanism of the topic by the current findings. In addition, we attempt to propose a 5 S management strategy from the cardio-immuno-oncology multidisciplinary perspective, with an expectation to minimize the morbidity and mortality of cancer patients undergoing ICI therapy.1,15
Immune checkpoint inhibitors make both patients and clinicians heartbreaking
ICIs-induced cardiotoxicity does damage the entire heart of patients, involving myocardial tissue, pericardium, conduction systems, and interstitial components, thus leading to a host of heart disorders, such as myocarditis, pericarditis, arrhythmias, myocardial infarction (MI), Takotsubo cardiomyopathy, etc.16,17 Cardiotoxicity can occur at any time during the process of ICIs therapy or even a few months after ICIs discontinuation and is deeply hidden without clinical characteristic manifestations.16 Most of the initial symptoms are non-specific and easily overlooked, such as fatigue and palpitations. Hence, it is hard for clinicians to diagnose and deal with it in time. If left unchecked, it is likely to result in serious life-threatening consequences such as heart failure and even sudden death.18
The treatment regimens vary depending on the specific case of cardiotoxicity. Generally, suspending ICIs therapy is the first step, and then corticosteroids are referred to as the first-line treatment. In addition to glucocorticoids, other immunomodulatory treatments are available, such as mycophenolate mofetil (MMF), anti-thymocyte globulin (ATG), infliximab, etc.19,20 For cases of critically-ill cardiotoxicity, it is recommended to enter the intensive care unit (ICU) to receive the respiratory and circulatory support containing ventilator, extracorporeal membrane oxygenation, etc.21,22 For cancer patients and clinicians, no matter which of the above measures was taken, it means that: i) the curative effect may be hindered for the interruption of the anti-tumor treatment23; ii) the quality of patients' life is decreased, or even life is threatened24; iii) hospitalization and even intensive care are required,20 which must increase medical costs for the patients. Obviously, ICIs may break the patient's anatomical heart and make the patients and clinicians feel “heartbreaking".
The “heartbreaking” clinical features of ICIs-induced cardiotoxicity
By and large, ICIs-induced cardiotoxicity has the following clinical features based on current literature: i) low incidence but high mortality; ii) undefined risk factors; iii) heterogeneity in onset time, clinical presentation, treatment regimen, and clinical outcome.
Low incidence but high mortality
Compared to irAEs involving other organs, ICIs-induced cardiotoxicity is rare. Based on relevant case reports and the World Health Organization (WHO) VigiBase pharmacovigilance database, the incidence of cardiotoxicity ranges from 0.06% to 4.2%,24, 25, 26 but the mortality is around 35%–50%.25,27,28 In the real world, the frequency of cardiotoxicity may be underestimated due to the absence of standardized definitions, non-specific symptoms, or low clinical awareness.9 The actual incidence of both early and delayed cardiotoxicity induced by ICIs is still unknown. Rubio-Infante et al summarized rules as follows: i) The incidence of cardiotoxicity is about 3.1% for ICIs monotherapies, 5.8% for dual ICIs therapies, and 3.7% for ICIs plus chemotherapy; iii) Patients treated with anti-PD-1/PD-L1 antibodies present more cardiotoxicity than patients treated with anti-CTLA-4 antibodies (69.4% vs. 20%); iii) Myocarditis is the most frequent symptom (50.8%), with the highest case fatality rate (24.6%).8
Undefined risk factors
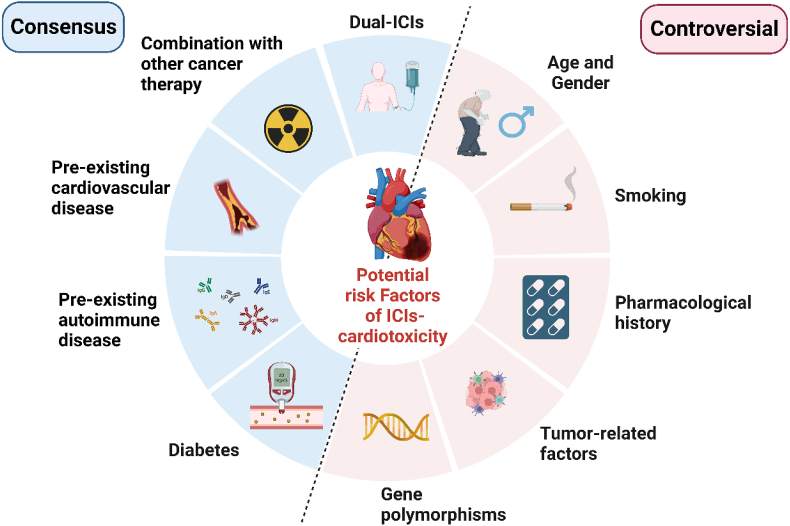
Unlike traditional cardiotoxicity caused by chemicals, pathogens, or rays, there are no big-scale epidemiological or clinical studies to identify the risk factors of ICIs-induced cardiotoxicity.18,29,30 Views on potential risk factors of ICIs-induced cardiotoxicity vary between literature, with certain consensus and some controversies (Fig. 1). Here, we categorize most potential risk factors, hoping to help clinicians assess the cardiac risk factors of their patients before initiating ICIs therapy.
Figure 1.
The potential risk factors of ICIs-induced cardiotoxicity.
Dual ICIs therapy is the most defined risk factor, which increases the severity and mortality of ICIs-induced myocarditis.24,25,31 According to relevant case reports and meta-analyses, pre-existing cardiovascular16,25 and autoimmune32 diseases, diabetes,33,34 and combinations with other cardiotoxic therapies35, 36, 37, 38 are also potential risk factors. Besides, there are some controversial risk factors. Age greater than 80 years may be a risk factor,39 but complications occur in a broad age range. Present studies show males are more likely to suffer from cardiotoxicity than women,40 but this does not indicate a predisposition of males compared to females, given that males have a higher proportion of both ICIs use and clinical trial enrollment.26 It is reported that smoking may cause cardiac dysfunctions.39 The previous treatment potentially damages the heart and increases the risk.25,41,42 A study discovered that lung cancer and PD-L1 usage are significantly correlated with a high incidence of pericardial diseases.26 Common antigens between tumors and the heart may be related to cardiotoxicity.24,43 CTLA-4 single nucleotide polymorphisms (rs4553808, rs11571317, rs231775)44 were found to be associated with irAEs in ipilimumab-treated cases. Comparative transcriptomics of ICIs-induced myocarditis identifies the dysregulation of guanylate-binding proteins 5 and 6.45
Heterogeneity in onset time, clinical manifestations, treatment regimens, and clinical outcomes
The existing heterogeneity and complexity of ICIs-induced cardiotoxicity in onset time, clinical manifestations, treatment regimens, and clinical outcomes have become a huge challenge for clinicians to diagnose and manage conditions. Myocarditis is the most common cardiotoxicity induced by ICIs (45%), with a rate of 15% for fulminant myocarditis, and pericardial involvement is the second.26 The mortality of myocarditis is up to 50%, ranking first among all irAEs, while the mortality of pericardial disease or vasculitis is 21% and 6%, respectively.23 Based on the available literature, we focus on myocarditis and pericardial disease in this section.
Heterogeneity in onset time
Most irAEs occur within the first 4 months of ICIs therapy, but cardiotoxicity can occur at any time during the therapy or even a few months after discontinuation of the therapy.17 The onset time varies depending on the medical history, type of ICIs, combination therapy, and duration of usage.46 Myocarditis most commonly presents within a median time of 17–34 days after ICIs initiation,25,31 and some are much later, even several months after ICIs initiation.25,47 The median time for the occurrence of pericardial disease is 30 days after the first administration of ICIs, which is more common in patients taking PD-1/PD-L1 therapy than those taking anti-CTLA-4 therapy.26
Heterogeneity in clinical manifestations
The wide manifestations spectrum of cardiotoxicity can range from asymptomatic to severe (cardiogenic shock, acute heart failure, ventricular arrhythmias), and even death.16,48, 49, 50 The external clinical phenotype of ICIs-associated myocarditis may be one or more of the following cardiac disorders such as heart failure (HF), various arrhythmias (atrial fibrillation, atrioventricular block, bundle branch block, ventricular tachycardia, etc.), myocarditis–pericarditis, cardiomyopathy, and sudden cardiac death.19,31,51,52 Typical syndromes of myocarditis include palpitations, chest pain, acute or chronic heart failure, pericarditis, and pericardial effusion.53,54 The above manifestations may be related to ICIs-induced myocarditis, and may also be related to the patient's general status and nutritional level, or may be caused by primary cardiovascular disease, tumor progression with complications, or cardiovascular complications induced by other anticancer therapies. If the symptoms can be merely attributed to one non-ICIs-induced myocarditis disease, the exact reasons resulting in the myocarditis cannot be easily determined.6,9,53,54
About 90% of myocarditis cases have electrocardiogram (ECG) abnormalities, which can manifest as various types of arrhythmias, QT interval prolongation, ST-segment elevation, T-wave inversion, R wave amplitude reduction, abnormal Q wave, or low voltage.25,31,55 The level of cardiac troponin (cTn) is increased in 90% of patients with myocarditis, usually markedly in patients with clinical symptoms, and the level of brain natriuretic peptide (BNP) or N-terminal (NT)-proBNP (NT-proBNP) is elevated in about 70% myocarditis patients. If the patients develop concomitant myositis, their creatine kinase (CK) may be elevated as well.25,31,48,51,56 Echocardiography is usually used as a first-line imaging modality for the evaluation of myocarditis. However, decreased left ventricular ejection fraction (LVEF) occurs in less than 50% of the patients.25,31,57 A reduction of global longitudinal strain (GLS) in speckle-tracking echocardiography (STE) examination may present in an early stage of myocarditis.58,59 Cardiac magnetic resonance (CMR) imaging, a gold standard for noninvasive diagnosis of myocarditis, may demonstrate myocardial inflammation, edema, and necrosis in T1 and T2 sequences, and characteristic late gadolinium enhancement.23,58,60,61 EMB is the gold standard to provide a definite diagnosis, however, it is not often performed due to its invasiveness and potential complications. The pathological reports show predominant lymphocytic infiltration.31 Despite various adjunctive tests, there are still no specific diagnostic criteria for ICIs-induced myocarditis. In clinical trials, the 2013 European Society of Cardiology (ESC) guidelines for the clinical diagnosis of myocarditis have been widely used currently.62
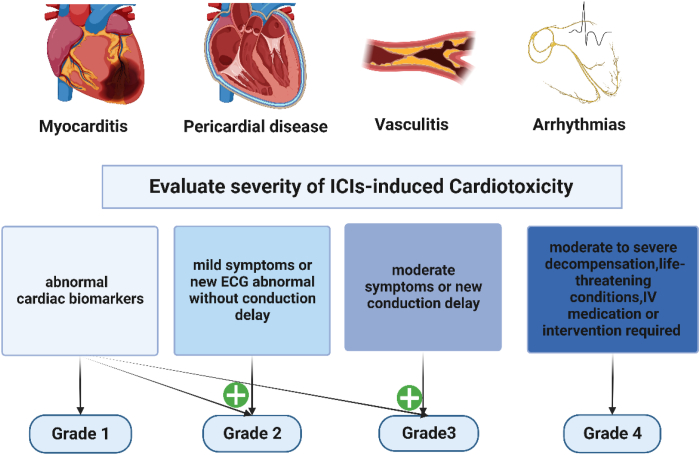
ICIs-induced pericardial diseases, including pericarditis (or myopericarditis), pericardial effusion, or tamponade,31,54,63 are often seen in conjunction with myocarditis.26 Notably, the majority (56%) of pericardial diseases developed in patients with lung cancer.26 It might be asymptomatic in most cases, while shortness of breath and pleuritic pain are the most common symptoms.64 A systematic review reveals that the majority is severe and life-threatening, identifying 28 cases of ICIs-associated pericardial involvement.65 In some critical cases, dyspnea, chest pain, and hemodynamic instability may be happened due to the tamponade caused by large pericardial effusion.64 Physical examination may reveal typical pericardial chest pain and sometimes the presence of a friction rub may be found during cardiac auscultation. In many cases, it should not be underestimated that the occurrence of the pericardial effusion may represent the malignant involvement of the pericardium but not merely one of the cardiac irAEs.64,66 ECG may range from normal to typical PR-segment depression or diffused ST-T changes. When cardiac biomarkers are elevated, concomitant myocardial involvement should be considered. CMR is helpful to determine whether myocarditis is also involved.65 Therefore, cardiotoxicity has been classified into four grades based on the elevation level of cardiac biomarkers, echocardiographic findings, and symptoms23,67,68 (Fig. 2).
Figure 2.
Common ICIs-induced cardiotoxicity and grading. Grading criteria vary slightly in different guidelines or expert consensus, and the grading criteria used in this article originate from the 2021 edition of the ASCO update.68 ASCO, American Society of Clinical Oncology; ECG, electrocardiogram; IV, intravenous injection.
Heterogeneity in treatment regimens
Because of low incidence, non-specific clinical features, and the horrendous fatality rate, ICIs-induced cardiotoxicity should be highly aware by clinicians. Discontinuing ICIs therapy and early initiating glucocorticoids are the mainstays of treatment for ICIs-induced cardiotoxicity.68,69 The management of abnormal electrical conduction, pain, and dyspnea, and even life support are also required.70 Nevertheless, not every cardiotoxicity patient can be treated in the same way. It is necessary to develop a corresponding treatment plan according to the specific clinical manifestations. For example, in the case of hemodynamic instability caused by large pericardial effusion, urgent pericardiocentesis should be performed. In addition to achieving the therapeutic purpose, pericardiocentesis can also provide the possibility of pathologic examination of pericardial fluid/pericardial biopsy in order to assist in the differential diagnosis between ICI-related and cancer-related pericardial involvements.54,66,71
Heterogeneity in clinical outcomes
Clinical outcomes and grades of cardiotoxicity are closely related. Stable subclinical heart injury may return to baseline heart function, but for some more serious ones, for instance, high-grade irAEs are life-threatening and often cause a such severe decline in performance status that patients do not qualify for any further anticancer treatments. Myocarditis is the most frequent (50.8%) with the highest case fatality rate (24.6%),8 which has become an important cause of patient death in the short term.9 In an evaluation based on VigiBase, Salem et al included 95 cases of pericardial diseases associated with ICIs, of which 60% were severe and 21% were fatal.26 However, prompt recognition and suspending of ICIs and immunosuppressive therapy can improve cardiac contractility and conduction abnormalities in patients.55
How do ICIs break the heart?
The primary function of the heart is to pump blood to supply oxygen and nutrients to the entire body. This function depends on cardiomyocytes, the extracellular matrix (ECM), fibroblasts, endothelial cells, arteries, veins, valves, and an electrical conduction system that allows the heart to sequentially coordinate contractions.72 In the heart, there are complex interactions between various types of immune cells that either inhabit or infiltrate heart tissue and the resident cardiac cells, including cardiomyocytes, fibroblasts, and endothelial cells.73, 74, 75 The mechanism of ICIs-induced cardiotoxicity has not been entirely elucidated, and there are multiple theories established on animal models.76 Experimental autoimmune myocarditis (EAM) murine models have immunologic features and male gender bias resembling the human so they are suitable for being used to explore pathophysiologic cognitions on myocarditis.77,78 In this section, we employ ICIs-induced myocarditis as an example to discuss the underlying mechanisms in conjunction with animal studies from the cardio-immuno-oncology perspective.
Heart: an immunologically privileged organ, not an immunodeficient organ
An immune balance is needed to protect normal cells and organs, moreover, such critical balance differs within different organs in the body. Certain organs, such as the eyes, brain, and heart, whose continuous work is essential for the survival of people, have regulatory mechanisms to depress the low-level immune responses that are continuously taking place. These organs are known as immune-privileged sites.79,80 It is worth noting that the heart in a state of relative immune privilege is not equal to an immunodeficient organ.81,82 Although there are very few T cells found in a healthy heart, there are numbers of tissue-resident macrophages and dendritic cells (DCs) throughout the healthy heart and lodged between cardiomyocytes to form an immune network to participate in initiating effector T cell responses, tissue repairment, immune regulation, and tolerance.81,83 In the pathophysiology of ICIs-induced myocarditis, the changes in the abundance, distribution, polarization, and diversity of immune cells reveal that adaptive immunity plays a critical role in such kinds of irAEs67,84 (Fig. 3).
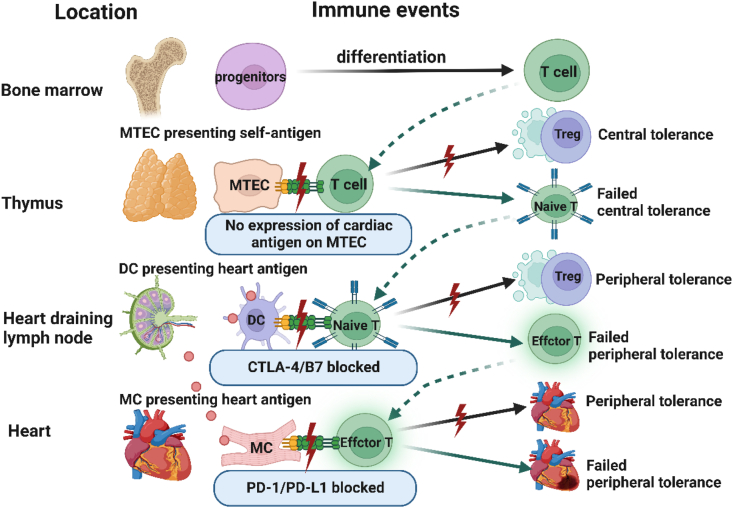
Figure 3.
The failed T cell tolerance to heart antigens. T cells in the bone marrow are differentiated from progenitor cells and then enter the thymus. Central T tolerance to self-antigens occurs during thymic development of T cells, which causes deletion of self-reactive clones and/or development of Treg specific for self-antigens. There are no cardiac antigens expressed on METCs, thus cardiac antigen-specific T cells are not deleted and mature into naive cardiac antigen-specific T cells, which recirculate through SLOs. If the naive cardiac antigen-specific T cells encounter heart antigens presented by DCs in heart-draining lymph nodes, their activation may be blocked by peripheral tolerance mechanisms, such as Treg and/or CTLA-4-B7. However, when CTLA-4/B7 is blocked, naive cardiac antigen-specific T cells can escape SLO-based tolerance and can be activated to clonally expand and differentiate into effector T cells. The effector T cells can enter the heart, where their activation by antigens and their capacity to cause damage can be blocked by tissue-based tolerance mechanisms, such as PD-L1 expression on myocytes and endothelium. When PD-1/PD-L1 is blocked, the peripheral tolerance is failed and autoreactive T-cell myocarditis occurs. METCs, medullary thymic epithelial cells; DC, dendritic cells; Treg, regulatory T cells; MC, myocardium cell.
T cells: the main labor of the cardiac immune response
T-cell infiltration into the heart has been widely reported in patients with ICIs-induced myocarditis,85 which reflects that T-cell immune response is the main driving force in the development of myocarditis.86,87 Each step of T-cell development, activation, and recruitment is responsive to different stimuli in the heart, and it is precisely regulated to protect the heart from T-cell attack.
During the development of T cells in the thymus, the central immune tolerance to self-antigens occurs through the expression of an autoimmune regulator (AIRE) of tissue-restricted antigens by medullary thymic epithelial cells (mTECs) and their presentation to immature T cells,88 which leads to the deletion of self-reactive T-cell clones (called T-cell thymic negative selection) and/or the development of regulatory T cells (Treg) specific for these antigens.89, 90, 91, 92, 93, 94 However, the central immune tolerance to cardiac-specific antigens fails. Cardiac myosin (especially α-cardiac myosin heavy chain, α-MyHC) is one of the most important self-antigens to induce myocarditis.95 Myh6, the gene encoding α-MyHC, is not expressed in either mTECs or peripheral lymphoid stromal cells.78 Physiologically, specific self-reactive α-MyHC T cell clones escape from thymic negative selection and mature into naive cardiac-specific T cells in the thymus. Then the cardiac-specific T cells can recirculate through secondary lymphoid organs (SLOs).
Risk events, either infection, toxins, ischemia, aging, or other insults can lead to myocardial damage and subsequent exposure to intracellular proteins (cryptic epitopes).96 In a context of a proper microenvironment, cardiac antigens can be released and circulated to SLOs, for instance, heart-draining lymph nodes.97 Then the adaptive immune response leading to myocarditis will be triggered.98, 99, 100
Johnson et al performed T cell receptor (TCR) sequencing on biopsies from the tumor, heart, and skeletal muscles and put focus on the highly variable complementarity determining region 3 (CDR3). Elevated expression of muscle-specific transcripts in tumor samples and high-frequency TCR sequences shared between the tumor, heart, and skeletal muscles suggested that the activated T cells might be responding to a common antigen, thereby possibly resulting in the development of autoimmune myocarditis and myositis.24
In preclinical studies, EAM can be induced by immunizing susceptible mice strains with cardiac myosin or with a myocarditogenic peptide derived from α-MyHC.78,101,102 In contrast, transgenic mice, expressing Myh6 in mTECs, are protected from myocarditis induction, which indicates impaired thymic tolerance to alpha-myosin directs autoimmunity to the heart in mice.92,93,103
CTLA-4 and PD-1/PD-L1 pathways maintain cardiac immune homeostasis
Numerous studies have suggested that blocking the CTLA-4 and PD-1/PD-L1 pathways can enhance T cell responses. Anti-CTLA-4 mAb (ipilimumab, tremelimumab), anti-PD-1 mAbs (pembrolizumab, nivolumab, cemiplimab), and anti-PD-L1 (avelumab, atezolimab, durvalumab) have been successively approved for cancer therapy. Leaving aside tumorigenesis, CTLA-4 and PD-1 serve as “gate-keepers” that negatively regulate T cell activity to counter autoimmunity104,105 and maintain immune homeostasis in the heart.11,12 Blocking these pathways means the loss of immune regulatory functions, which may lead to hyperactive or autoimmune events.
In SLOs, CD28 is ubiquitously expressed on naive T cells, and B7 proteins are highly expressed on DCs when exposed to immune stimuli. Once B7 proteins bind to CD28, B7-CD28 signaling will synergize with antigen-TCRs generated signals to initiate T cell activation.106 Unlike CD28, CTLA-4 inhibits T-cell activation. In conditions of chronic antigen exposure, CTLA-4 is expressed on the surface of most naive T cells soon after activation and is constitutively expressed on Treg and memory T cells with an exhausted phenotype.107 CTLA-4 can bind both B7 proteins with significantly higher affinity than CD28, thus competitively inhibiting B7-CD28-dependent T cell co-stimulation.108 If naive cardiac-specific T cells encounter cardiac antigens presented by DCs in lymph nodes draining the heart, their activation may be blocked by Treg and/or CTLA-4-B7 mediated block in co-stimulation. Once co-stimulators on DCs are up-regulated and/or deficient in regulatory pathways, naive heart antigen-specific T cells may escape SLOs-based tolerance mechanisms and are activated to clonally expand and differentiate into effector T cells, which can enter the heart.
PD-1 mainly acts to inhibit T cell activation in the peripheral blood, where effector T cells recognize antigens again to activate their functional responses in the injured or infected sites.109 Antigens can induce PD-1 expression in T cells, especially in exhausted T cells. PD-L1 is expressed in many cell types, including DCs, macrophages, epithelial parenchymal cells, endothelial cells (EC), etc. Once PD-1 binds with PD-L1 expression on myocytes and endothelium, biochemical events are launched to inhibit T cell immune response89, 90, 91, 92, 93,110(Fig. 3).
CTLA-4-B7 and PD-1/PD-L1 can both suppress the activation of T cells. However, their mechanisms of action are distinct, and their main physiological roles act at different stages of a T cell response. CTLA-4-B7 is most critical for naive T cell activation in SLOs, and this is when and where the effects of CTLA-4 exert mostly. PD-L1 is expressed in many non-lymphoid tissues. Thus, PD-1/PD-L1 is important for inhibiting the activation of effector T cells in the tissues to mediate peripheral tolerance.111
Data from animal models suggest that: i) Disruption of the PD-1/PD-L1 pathway leads to autoimmune diseases of the heart, primarily in the form of autoimmune myocarditis.112 For example, knockout of the PD-1 receptor in mice causes severe dilated cardiomyopathy characterized by high levels of immunoglobulin G (IgG) autoantibodies that react specifically to cardiac troponin113. In the hearts of MRL PD-1 deleted mice, massive infiltration of CD4+- and CD8+-T cells and myeloid cells were found, as well as the production of high-titer autoantibodies against cardiac myosin. These data suggest that myocarditis in PD-1 deficiency may be mediated by an antigen-specific autoimmune response.114,115 Studies on mice genetically deficient in PD-L1 showed that PD-L1 can protect the myocardium from T cell-mediated damage, which is dependent on PD-L1 expression by endothelium or myocytes.116 ii) Absence of CTLA-4 in mice causes autoimmunity by nonspecifically activating the CD4+-T cells, leading to lethal multi-organ lymphocytic infiltration and tissue destruction,117 including T cell-mediated myocarditis.118 iii) Induction of the tolerance and up-regulation of Treg could be a pharmacologic approach to preventing autoimmune myocarditis.86
Let us overcome ICIs-induced cardiotoxicity: 5 S management
Up to date, no randomized controlled trials have determined the optimal treatment strategy for ICIs-induced cardiotoxicity, and current recommendations are based on expert opinion from the European Society of Medical Oncology (ESMO),119 National Comprehensive Cancer Network (NCCN), Society for Immunotherapy of Cancer Toxicity Management Working Group, and Chinese Society of Clinical Oncology (CSCO). Moreover, almost all of them do not provide detailed recommendations for treating ICIs-induced cardiotoxicity.
Managing patients undergoing ICIs therapy requires an interdisciplinary collaboration among the departments of cardiology, oncology, immunology, and even intensive care medicine. The multidisciplinary team should adopt a balanced approach to minimizing cardiotoxicity meanwhile limiting the reduction or discontinuation of anticancer therapy. Therefore, in this review, combined with existing guidelines, literature, and clinical practice, the management of cardiotoxicity can be summarized as a “5 S″ approach: Screen, Surveil, Suspend, Suppress, and Support19, 20, 21,120 (Fig. 4).
Figure 4.
5 S management strategy for ICIs-induced cardiotoxicity. ASCO, American Society of Clinical Oncology; ESMO, European Society for Medical Oncology; ECG, electrocardiogram; CMR, Cardiac magnetic resonance imaging; ATG, anti-thymocyte globulin; MMF, mycophenolate mofetil.
Screen: comprehensive screening before initiating ICIs therapy
Screening before initiating ICIs therapy aims primarily to assess the likelihood of secondary cardiotoxicity during ICIs therapy and manage cancer patients hierarchically according to the potential risk factors.
It is recommended that the patients should undergo a comprehensive physical examination and a systematic review of their demographic characteristics (age, gender, race, smoking, etc.), medical history (pre-existing cardiovascular and autoimmune diseases), and pharmacological history (previous cardiotoxic therapies like chemotherapy, thoracic radiotherapy, anti-ErbB2, VEGF/MEK/RAF inhibitors, etc.). The following items should be detected as baseline controls: i) blood test and inflammatory biomarkers; ii) ECG, echocardiography (LVEF measured by Simpson method); iii) cTn, BNP, Mb or CK, CK-MB, D-dimer; iv) autoimmune antibodies. Those with a history of cardiovascular or autoimmune disease, or abnormal baseline examinations require the assistance of a cardiologist or immunologist for evaluation and standardized management.
The following conditions are regarded as temporary contraindications: acute coronary syndrome (ACS) within 4 weeks, acute decompensated heart failure or chronic heart failure with grade IV cardiac function, acute pulmonary embolism, etc. The above-mentioned diseases need to be managed in a standardized manner. After the condition is stable, ICIs therapy can be started under close surveillance. A rigorous monitoring strategy is required during ICIs therapy. In addition, it is also necessary to educate patients on drug adverse reactions so that they can self-identify the symptoms and signs of cardiotoxicity at an early stage.
Surveil: throughout the entire therapy
Patients should be inquired in detail about their symptoms and undergo a physical examination before each administration. Seven days after the first ICIs administration, it is recommended to follow up on symptoms and signs, review ECG, and detect cTn and if necessary, other markers BNP, Mb, and CK. During the 2nd to 9th cycles for patients receiving ICIs biweekly regimen (in the 2nd to 6th cycles for a three-week regimen), ECG and cTn should be detected, and combined detection of BNP, Mb, or CK should be considered. In the subsequent treatment, the conditions of the symptoms, physical examination, and ECG of patients are supposed to be inquired about before each medication. When there is a positive manifestation, not only cTn, BNP, Mb, and CK, but also cardiac color Doppler ultrasound and even CMR ought to be performed if necessary to help early diagnose the cardiotoxicity of patients. In addition to a comprehensive cardiovascular evaluation, patients still need to be evaluated for concomitant irAEs affecting other organ systems.31
Suspend: as soon as possible
When cardiotoxicity is suspected, such as elevated cTn (abnormal natriuretic peptide, Mb, or CK) or any new ECG changes, ICIs should be suspended until the diagnosis of cardiotoxicity is ruled out. Detailed inquiries about symptoms and physical examination should be performed at once. It is suggested to complete the screening items of ECG, echocardiography, Mb or CK, CK-MB, cTn, BNP, and D-dimer, which are helpful for initial diagnosis and differential diagnosis (e.g., ACS, pulmonary embolism, other causes-related myocarditis, arrhythmias, cardiac insufficiency, or heart failure). Meanwhile, patients should be evaluated for whether concomitant irAEs are affecting other organ systems. Seek the assistance of a cardiologist or immunologist, if necessary.
Suppress: limit the hyperreactive immune response
To date, glucocorticoids are the first choice and core regimen for treating ICIs-related cardiotoxicity. Early and sufficient glucocorticoids can improve the prognosis of myocarditis without destroying the anti-tumor effect of ICIs.19,22,69 For severe and refractory cardiotoxicity, when the level of glucocorticoids fails to improve the condition within 24 h, it can be taken into account to add anti-thymocyte globulin (ATG) and infliximab. While, it should be noted that infliximab may result in heart failure, and high-dose infliximab is contraindicated in patients with moderate to severe heart failure. In addition to the above drugs, there are other alternative immunomodulatory drugs, such as mycophenolate mofetil (MMF), tacrolimus, and immunoglobulin. Furthermore, there are non-drug treatments such as plasma exchange and lymphocyte depletion.121
Blood glucose levels should be monitored during glucocorticoid therapy, and appropriate measures should be taken to prevent deep vein thrombosis, osteoporosis, peptic ulcer, secondary bacteria, fungi, and Pneumocystis pneumonia.
Support: more than just life support
Life support is a key link in the treatment of critically ill patients with cardiotoxicity, including circulatory support, respiratory support, and renal replacement.42 Through life support treatment, the heart can take rest, which can gain time for other treatments.
In addition to life support, tailoring treatment according to cardiac function, hemodynamic indices, and symptoms of the patient is also part of support treatment, such as pericardiocentesis and drainage during tamponade, placement of a pacemaker for malignant abnormal heart rate, and relief for severe pain.
Conclusions
Cancer incidence increases with age, and as life expectancy increases, the number of elderly patients with cancer keeps rising. In such an aging population with traditional cardiovascular risk factors,122 it is estimated that the incidence of cardiovascular complications associated with cancer will continue to increase and anti-tumor therapy has been gradually intertwined with cardiovascular diseases.123
Inadequate management of cardiovascular risk factors increases the risk of cardiotoxicity of cancer therapies and reduces the probability of cancer cure. Therefore, strategies to improve their prevention and treatment are global priorities. The interdisciplinary cooperation among oncologists, cardiologists, immunologists, and other medical professionals should be emphasized in order to provide professional pre-therapy assessment, recognition, diagnosis, monitoring, and treatment for cardiotoxicity. Such cooperation is essential, with the goals of eliminating the potential adverse influence of ICIs therapy on the cardiovascular system, implementing strategies to reduce the incidence of cardiotoxicity, and ensuring the smooth running of ICIs therapy.20,26,124
Furthermore, additional studies are needed to incorporate several tests, measures, and actions before, during, and long after ICIs administration for cardiotoxicity monitoring. Molecular and cellular changes in both the T-cells themselves, as well as the local cardiac immune environment, play a role in the development of autoimmunity in the setting of ICIs administration and cardiotoxicity. In order to study the relationships between these cellular populations and signaling pathways, it is necessary to explore a comprehensive and multi-modal approach concerning both translational studies in humans as well as rigorous and mechanistic studies in mice, thereby paving the way to novel therapies for combatting the ICIs-induced cardiotoxicity with minimal side effects.
Author contributions
YH analyzed the data and wrote the manuscript. HY and SD provided helpful discussion and drew the schematic diagram. ML, ZX, and MH provided helpful discussion. FL and LW reviewed and proofread the manuscript. All authors reviewed the manuscript.
Conflict of interests
There is neither financial support nor other benefits from commercial sources to be reported for the work presented in the manuscript, nor any other financial interests that any of the authors may have, which could create a potential conflict of interest or the appearance of a conflict of interest concerning the work.
Funding
This work was supported by a fund from Deyang Science and Technology Foundation (Sichuan, China) (No. 2019SZ120).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Feng Luo, Email: hxluofeng@163.com.
Li Wang, Email: wangli37029192@sina.com.
References
- 1.Campochiaro C., De Luca G., Dagna L. Cardiac immune-related adverse events: an immune-cardio-oncology puzzle. Eur J Heart Fail. 2021;23(10):1748–1749. doi: 10.1002/ejhf.2329. [DOI] [PubMed] [Google Scholar]
- 2.Wang J., Yang T., Xu J. Therapeutic development of immune checkpoint inhibitors. Adv Exp Med Biol. 2020;1248:619–649. doi: 10.1007/978-981-15-3266-5_23. [DOI] [PubMed] [Google Scholar]
- 3.van den Bulk J., Verdegaal E.M., de Miranda N.F. Cancer immunotherapy: broadening the scope of targetable tumours. Open Biol. 2018;8(6) doi: 10.1098/rsob.180037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y., Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807–821. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan S., Gerber D.E. Autoimmunity, checkpoint inhibitor therapy and immune-related adverse events: a review. Semin Cancer Biol. 2020;64:93–101. doi: 10.1016/j.semcancer.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel R.P., Parikh R., Gunturu K.S., et al. Cardiotoxicity of immune checkpoint inhibitors. Curr Oncol Rep. 2021;23(7):79. doi: 10.1007/s11912-021-01070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarifa A., Lopez-Mattei J., Palaskas N., et al. Immune checkpoint inhibitors (ICIs)-related cardiotoxicity. Adv Exp Med Biol. 2020;1244:277–285. doi: 10.1007/978-3-030-41008-7_15. [DOI] [PubMed] [Google Scholar]
- 8.Rubio-Infante N., Ramírez-Flores Y.A., Castillo E.C., et al. Cardiotoxicity associated with immune checkpoint inhibitor therapy: a meta-analysis. Eur J Heart Fail. 2021;23(10):1739–1747. doi: 10.1002/ejhf.2289. [DOI] [PubMed] [Google Scholar]
- 9.Moslehi J., Lichtman A.H., Sharpe A.H., et al. Immune checkpoint inhibitor-associated myocarditis: manifestations and mechanisms. J Clin Invest. 2021;131(5) doi: 10.1172/JCI145186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kino T., Khan M., Mohsin S. The regulatory role of T cell responses in cardiac remodeling following myocardial infarction. Int J Mol Sci. 2020;21(14):5013. doi: 10.3390/ijms21145013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.January C.T., Wann L.S., Alpert J.S., et al. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on practice guidelines and the heart rhythm society. Circulation. 2014;130(23):e199–e267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarrio M.L., Grabie N., Bu D.X., et al. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol. 2012;188(10):4876–4884. doi: 10.4049/jimmunol.1200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagchi S., Yuan R., Engleman E.G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–249. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 14.Zaha V.G., Meijers W.C., Moslehi J. Cardio-immuno-oncology. Circulation. 2020;141(2):87–89. doi: 10.1161/CIRCULATIONAHA.119.042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kounis N.G., Koniari I., Hahalis G. Cardio-oncology, immuno-oncology, onco-cardiology and onco-immunology. Int J Cardiol. 2016;223:254–257. doi: 10.1016/j.ijcard.2016.08.219. [DOI] [PubMed] [Google Scholar]
- 16.Lyon A.R., Yousaf N., Battisti N.M.L., et al. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19(9):e447–e458. doi: 10.1016/S1470-2045(18)30457-1. [DOI] [PubMed] [Google Scholar]
- 17.Dolladille C., Akroun J., Morice P.M., et al. Cardiovascular immunotoxicities associated with immune checkpoint inhibitors: a safety meta-analysis. Eur Heart J. 2021;42(48):4964–4977. doi: 10.1093/eurheartj/ehab618. [DOI] [PubMed] [Google Scholar]
- 18.Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol. 2020;17(8):474–502. doi: 10.1038/s41569-020-0348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brahmer J.R., Lacchetti C., Schneider B.J., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brahmer J.R., Abu-Sbeih H., Ascierto P.A., et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9(6) doi: 10.1136/jitc-2021-002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson J.A., Schneider B.J., Brahmer J., et al. Management of immunotherapy-related toxicities, version 1.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(4):387–405. doi: 10.6004/jnccn.2022.0020. [DOI] [PubMed] [Google Scholar]
- 22.Thompson J.A., Schneider B.J., Brahmer J., et al. NCCN guidelines insights: management of immunotherapy-related toxicities, version 1.2020. J Natl Compr Canc Netw. 2020;18(3):230–241. doi: 10.6004/jnccn.2020.0012. [DOI] [PubMed] [Google Scholar]
- 23.Ball S., Ghosh R.K., Wongsaengsak S., et al. Cardiovascular toxicities of immune checkpoint inhibitors: JACC review topic of the week. J Am Coll Cardiol. 2019;74(13):1714–1727. doi: 10.1016/j.jacc.2019.07.079. [DOI] [PubMed] [Google Scholar]
- 24.Johnson D.B., Balko J.M., Compton M.L., et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmood S.S., Fradley M.G., Cohen J.V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salem J.E., Manouchehri A., Moey M., et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mir H., Alhussein M., Alrashidi S., et al. Cardiac complications associated with checkpoint inhibition: a systematic review of the literature in an important emerging area. Can J Cardiol. 2018;34(8):1059–1068. doi: 10.1016/j.cjca.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Wang D.Y., Okoye G.D., Neilan T.G., et al. Cardiovascular toxicities associated with cancer immunotherapies. Curr Cardiol Rep. 2017;19(3):21. doi: 10.1007/s11886-017-0835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain D., Aronow W. Cardiotoxicity of cancer chemotherapy in clinical practice. Hosp Pract. 2019;47(1):6–15. doi: 10.1080/21548331.2018.1530831. 1995. [DOI] [PubMed] [Google Scholar]
- 30.Nicolazzi M.A., Carnicelli A., Fuorlo M., et al. Anthracycline and trastuzumab-induced cardiotoxicity in breast cancer. Eur Rev Med Pharmacol Sci. 2018;22(7):2175–2185. doi: 10.26355/eurrev_201804_14752. [DOI] [PubMed] [Google Scholar]
- 31.Escudier M., Cautela J., Malissen N., et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136(21):2085–2087. doi: 10.1161/CIRCULATIONAHA.117.030571. [DOI] [PubMed] [Google Scholar]
- 32.Alexander S., Swami U., Kaur A., et al. Safety of immune checkpoint inhibitors in patients with cancer and pre-existing autoimmune disease. Ann Transl Med. 2021;9(12):1033. doi: 10.21037/atm-20-8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mladosievičová B., Országhová Z., Jr., Jablonická M., et al. Challenges and solutions in management of cardiotoxicity induced by checkpoint inhibitors. Klin Onkol. 2020;33(5):350–355. doi: 10.14735/amko2020350. [DOI] [PubMed] [Google Scholar]
- 34.Chen X., Jiang A., Zhang R., et al. Immune checkpoint inhibitor-associated cardiotoxicity in solid tumors: real-world incidence, risk factors, and prognostic analysis. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.882167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berner A.M., Sharma A., Agarwal S., et al. Fatal autoimmune myocarditis with anti-PD-L1 and tyrosine kinase inhibitor therapy for renal cell cancer. Eur J Cancer. 2018;101:287–290. doi: 10.1016/j.ejca.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 36.Guo C.W., Alexander M., Dib Y., et al. A closer look at immune-mediated myocarditis in the era of combined checkpoint blockade and targeted therapies. Eur J Cancer. 2020;124:15–24. doi: 10.1016/j.ejca.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Balanescu D.V., Donisan T., Palaskas N., et al. Immunomodulatory treatment of immune checkpoint inhibitor-induced myocarditis: pathway toward precision-based therapy. Cardiovasc Pathol. 2020;47 doi: 10.1016/j.carpath.2020.107211. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y., Xu L., Wang D., et al. Anti-PD-1 and regorafenib induce severe multisystem adverse events in microsatellite stability metastatic colorectal cancer: a case report. Immunotherapy. 2021;13(16):1317–1323. doi: 10.2217/imt-2020-0327. [DOI] [PubMed] [Google Scholar]
- 39.Oren O., Yang E.H., Molina J.R., et al. Cardiovascular health and outcomes in cancer patients receiving immune checkpoint inhibitors. Am J Cardiol. 2020;125(12):1920–1926. doi: 10.1016/j.amjcard.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 40.Triggianese P., Novelli L., Galdiero M.R., et al. Immune checkpoint inhibitors-induced autoimmunity: the impact of gender. Autoimmun Rev. 2020;19(8) doi: 10.1016/j.autrev.2020.102590. [DOI] [PubMed] [Google Scholar]
- 41.Pirozzi F., Poto R., Aran L., et al. Cardiovascular toxicity of immune checkpoint inhibitors: clinical risk factors. Curr Oncol Rep. 2021;23(2):13. doi: 10.1007/s11912-020-01002-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agrawal N., Khunger A., Vachhani P., et al. Cardiac toxicity associated with immune checkpoint inhibitors: case series and review of the literature. Case Rep Oncol. 2019;12(1):260–276. doi: 10.1159/000498985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez-Calle N., Rodriguez-Otero P., Villar S., et al. Anti-PD1 associated fulminant myocarditis after a single pembrolizumab dose: the role of occult pre-existing autoimmunity. Haematologica. 2018;103(7):e318–e321. doi: 10.3324/haematol.2017.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shek D., Read S.A., Ahlenstiel G., et al. Pharmacogenetics of anticancer monoclonal antibodies. Cancer Drug Resist. 2019;2(1):69–81. doi: 10.20517/cdr.2018.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finke D., Heckmann M.B., Salatzki J., et al. Comparative transcriptomics of immune checkpoint inhibitor myocarditis identifies guanylate binding protein 5 and 6 dysregulation. Cancers. 2021;13(10):2498. doi: 10.3390/cancers13102498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moreno Diaz R., Cazalla Garcia M., Bautista Sanz M.P., et al. What about cardiovascular toxicities of immune checkpoint inhibitors? Eur J Hosp Pharm. 2021;28(1):2–3. doi: 10.1136/ejhpharm-2020-002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamaguchi S., Morimoto R., Okumura T., et al. Late-onset fulminant myocarditis with immune checkpoint inhibitor nivolumab. Can J Cardiol. 2018;34(6):812.e1–812.e3. doi: 10.1016/j.cjca.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Läubli H., Balmelli C., Bossard M., et al. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer. 2015;3:11. doi: 10.1186/s40425-015-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yun S., Vincelette N.D., Mansour I., et al. Late onset ipilimumab-induced pericarditis and pericardial effusion: a rare but life threatening complication. Case Rep Oncol Med. 2015;2015 doi: 10.1155/2015/794842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gibson R., Delaune J., Szady A., et al. Suspected autoimmune myocarditis and cardiac conduction abnormalities with nivolumab therapy for non-small cell lung cancer. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2016-216228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ederhy S., Voisin A.L., Champiat S. Myocarditis with immune checkpoint blockade. N Engl J Med. 2017;376(3):290–291. doi: 10.1056/NEJMc1615251. [DOI] [PubMed] [Google Scholar]
- 52.McDowall L.M., Fernando S.L., Ange N., et al. Immune checkpoint inhibitor-mediated myocarditis and ventricular tachycardia storm. HeartRhythm Case Rep. 2019;5(10):497–500. doi: 10.1016/j.hrcr.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonaca M.P., Olenchock B.A., Salem J.E., et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. 2019;140(2):80–91. doi: 10.1161/CIRCULATIONAHA.118.034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu J.R., Florido R., Lipson E.J., et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. 2019;115(5):854–868. doi: 10.1093/cvr/cvz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reddy N., Moudgil R., Lopez-Mattei J.C., et al. Progressive and reversible conduction disease with checkpoint inhibitors. Can J Cardiol. 2017;33(10):1335. doi: 10.1016/j.cjca.2017.05.026. e13-1335.e15. [DOI] [PubMed] [Google Scholar]
- 56.Heinzerling L., Ott P.A., Hodi F.S., et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer. 2016;4:50. doi: 10.1186/s40425-016-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang L., Jones-O'Connor M., Awadalla M., et al. Cardiotoxicity of immune checkpoint inhibitors. Curr Treat Options Cardiovasc Med. 2019;21(7):32. doi: 10.1007/s11936-019-0731-6. [DOI] [PubMed] [Google Scholar]
- 58.Čelutkienė J., Pudil R., López-Fernández T., et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the heart failure association (HFA), the European association of cardiovascular imaging (EACVI) and the cardio-oncology council of the European society of cardiology (ESC) Eur J Heart Fail. 2020;22(9):1504–1524. doi: 10.1002/ejhf.1957. [DOI] [PubMed] [Google Scholar]
- 59.Awadalla M., Mahmood S.S., Groarke J.D., et al. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis. J Am Coll Cardiol. 2020;75(5):467–478. doi: 10.1016/j.jacc.2019.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferreira V.M., Schulz-Menger J., Holmvang G., et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation. J Am Coll Cardiol. 2018;72(24):3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 61.Gräni C., Eichhorn C., Bière L., et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. 2017;70(16):1964–1976. doi: 10.1016/j.jacc.2017.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caforio A.L.P., Pankuweit S., Arbustini E., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33):2636–2648. doi: 10.1093/eurheartj/eht210. 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 63.Altan M., Toki M.I., Gettinger S.N., et al. Immune checkpoint inhibitor-associated pericarditis. J Thorac Oncol. 2019;14(6):1102–1108. doi: 10.1016/j.jtho.2019.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Upadhrasta S., Elias H., Patel K., et al. Managing cardiotoxicity associated with immune checkpoint inhibitors. Chronic Dis Transl Med. 2019;5(1):6–14. doi: 10.1016/j.cdtm.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Inno A., Maurea N., Metro G., et al. Immune checkpoint inhibitors-associated pericardial disease: a systematic review of case reports. Cancer Immunol Immunother. 2021;70(10):3041–3053. doi: 10.1007/s00262-021-02938-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adler Y., Charron P., Imazio M., et al. ESC guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European society of cardiology (ESC) endorsed by: the European association for cardio-thoracic surgery (EACTS) Eur Heart J. 2015;36(42):2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palaskas N., Lopez-Mattei J., Durand J.B., et al. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. 2020;9(2) doi: 10.1161/JAHA.119.013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schneider B.J., Naidoo J., Santomasso B.D., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39(36):4073–4126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 69.Puzanov I., Diab A., Abdallah K., et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the society for immunotherapy of cancer (SITC) toxicity management working Group. J Immunother Cancer. 2017;5(1):95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gong J., Drobni Z.D., Zafar A., et al. Pericardial disease in patients treated with immune checkpoint inhibitors. J Immunother Cancer. 2021;9(6) doi: 10.1136/jitc-2021-002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Michel L., Rassaf T., Totzeck M. Cardiotoxicity from immune checkpoint inhibitors. Int J Cardiol Heart Vasc. 2019;25 doi: 10.1016/j.ijcha.2019.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anderson P.A. The heart and development. Semin Perinatol. 1996;20(6):482–509. doi: 10.1016/s0146-0005(96)80064-4. [DOI] [PubMed] [Google Scholar]
- 73.Swirski F.K., Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol. 2018;18(12):733–744. doi: 10.1038/s41577-018-0065-8. [DOI] [PubMed] [Google Scholar]
- 74.Ramos G.C., van den Berg A., Nunes-Silva V., et al. Myocardial aging as a T-cell-mediated phenomenon. Proc Natl Acad Sci U S A. 2017;114(12):E2420–E2429. doi: 10.1073/pnas.1621047114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steffens S., Nahrendorf M., Madonna R. Immune cells in cardiac homeostasis and disease: emerging insights from novel technologies. Eur Heart J. 2022;43(16):1533–1541. doi: 10.1093/eurheartj/ehab842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tajiri K., Yasutomi Y., Aonuma K. Recent advances in the management of autoimmune myocarditis: insights from animal studies. Curr Pharmaceut Des. 2016;22(4):427–439. doi: 10.2174/1381612822666151222160702. [DOI] [PubMed] [Google Scholar]
- 77.Ciháková D., Sharma R.B., Fairweather D., et al. Animal models for autoimmune myocarditis and autoimmune thyroiditis. Methods Mol Med. 2004;102:175–193. doi: 10.1385/1-59259-805-6:175. [DOI] [PubMed] [Google Scholar]
- 78.Bracamonte-Baran W., Čiháková D. Cardiac autoimmunity: myocarditis. Adv Exp Med Biol. 2017;1003:187–221. doi: 10.1007/978-3-319-57613-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carrillo-Salinas F.J., Ngwenyama N., Anastasiou M., et al. Heart inflammation: immune cell roles and roads to the heart. Am J Pathol. 2019;189(8):1482–1494. doi: 10.1016/j.ajpath.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stevenson P.G., Bangham C.R., Hawke S. Recruitment, activation and proliferation of CD8+ memory T cells in an immunoprivileged site. Eur J Immunol. 1997;27(12):3259–3268. doi: 10.1002/eji.1830271225. [DOI] [PubMed] [Google Scholar]
- 81.Koc A., Cagavi E. Cardiac immunology: a new era for immune cells in the heart. Adv Exp Med Biol. 2021;1312:75–95. doi: 10.1007/5584_2020_576. [DOI] [PubMed] [Google Scholar]
- 82.Hill M.A., Kwon J.H., Gerry B., et al. Immune privilege of heart valves. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.731361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nicolás-Ávila J.A., Lechuga-Vieco A.V., Esteban-Martínez L., et al. A network of macrophages supports mitochondrial homeostasis in the heart. Cell. 2020;183(1):94–109. doi: 10.1016/j.cell.2020.08.031. [DOI] [PubMed] [Google Scholar]
- 84.Zou W., Lu J., Hao Y. Myocarditis induced by immune checkpoint inhibitors: mechanisms and therapeutic prospects. J Inflamm Res. 2021;14:3077–3088. doi: 10.2147/JIR.S311616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ammirati E., Veronese G., Bottiroli M., et al. Update on acute myocarditis. Trends Cardiovasc Med. 2021;31(6):370–379. doi: 10.1016/j.tcm.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lichtman A.H. The heart of the matter: protection of the myocardium from T cells. J Autoimmun. 2013;45:90–96. doi: 10.1016/j.jaut.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barin J.G., Čiháková D. Control of inflammatory heart disease by CD4+ T cells. Ann N Y Acad Sci. 2013;1285:80–96. doi: 10.1111/nyas.12134. [DOI] [PubMed] [Google Scholar]
- 88.Alpdogan O., van den Brink M.R.M. Immune tolerance and transplantation. Semin Oncol. 2012;39(6):629–642. doi: 10.1053/j.seminoncol.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Derbinski J., Kyewski B. How thymic antigen presenting cells sample the body's self-antigens. Curr Opin Immunol. 2010;22(5):592–600. doi: 10.1016/j.coi.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 90.Gallegos A.M., Bevan M.J. Central tolerance: good but imperfect. Immunol Rev. 2006;209:290–296. doi: 10.1111/j.0105-2896.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 91.Koble C., Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med. 2009;206(7):1505–1513. doi: 10.1084/jem.20082449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Metzger T.C., Anderson M.S. Myocarditis: a defect in central immune tolerance? J Clin Invest. 2011;121(4):1251–1253. doi: 10.1172/JCI57211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lv H., Lipes M.A. Role of impaired central tolerance to α-myosin in inflammatory heart disease. Trends Cardiovasc Med. 2012;22(5):113–117. doi: 10.1016/j.tcm.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matsumoto M., Tsuneyama K., Morimoto J., et al. Tissue-specific autoimmunity controlled by Aire in thymic and peripheral tolerance mechanisms. Int Immunol. 2020;32(2):117–131. doi: 10.1093/intimm/dxz066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Caforio A.L.P., Mahon N.J., McKenna W.J. Cardiac autoantibodies to myosin and other heart-specific autoantigens in myocarditis and dilated cardiomyopathy. Autoimmunity. 2001;34(3):199–204. doi: 10.3109/08916930109007385. [DOI] [PubMed] [Google Scholar]
- 96.Reddy J., Massilamany C., Buskiewicz I., et al. Autoimmunity in viral myocarditis. Curr Opin Rheumatol. 2013;25(4):502–508. doi: 10.1097/BOR.0b013e3283620036. [DOI] [PubMed] [Google Scholar]
- 97.Müller A.M., Bockstahler M., Hristov G., et al. Identification of novel antigens contributing to autoimmunity in cardiovascular diseases. Clin Immunol. 2016;173:64–75. doi: 10.1016/j.clim.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 98.Pollack A., Kontorovich A.R., Fuster V., et al. Viral myocarditis: diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015;12(11):670–680. doi: 10.1038/nrcardio.2015.108. [DOI] [PubMed] [Google Scholar]
- 99.Lasrado N., Reddy J. An overview of the immune mechanisms of viral myocarditis. Rev Med Virol. 2020;30(6):1–14. doi: 10.1002/rmv.2131. [DOI] [PubMed] [Google Scholar]
- 100.Massilamany C., Gangaplara A., Steffen D., et al. Identification of novel mimicry epitopes for cardiac myosin heavy chain-α that induce autoimmune myocarditis in A/J mice. Cell Immunol. 2011;271(2):438–449. doi: 10.1016/j.cellimm.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 101.Donermeyer D.L., Beisel K.W., Allen P.M., et al. Myocarditis-inducing epitope of myosin binds constitutively and stably to I-Ak on antigen-presenting cells in the heart. J Exp Med. 1995;182(5):1291–1300. doi: 10.1084/jem.182.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pummerer C.L., Luze K., Grässl G., et al. Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB/c mice. J Clin Invest. 1996;97(9):2057–2062. doi: 10.1172/JCI118642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lv H., Havari E., Pinto S., et al. Impaired thymic tolerance to α-myosin directs autoimmunity to the heart in mice and humans. J Clin Invest. 2011;121(4):1561–1573. doi: 10.1172/JCI44583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Walker L.S.K., Sansom D.M. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11(12):852–863. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- 105.Jin H.T., Ahmed R., Okazaki T. Role of PD-1 in regulating T-cell immunity. Curr Top Microbiol Immunol. 2011;350:17–37. doi: 10.1007/82_2010_116. [DOI] [PubMed] [Google Scholar]
- 106.Nagai S., Azuma M. The CD28-B7 family of co-signaling molecules. Adv Exp Med Biol. 2019;1189:25–51. doi: 10.1007/978-981-32-9717-3_2. [DOI] [PubMed] [Google Scholar]
- 107.Tekguc M., Wing J.B., Osaki M., et al. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc Natl Acad Sci U S A. 2021;118(30) doi: 10.1073/pnas.2023739118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hosseini A., Gharibi T., Marofi F., et al. CTLA-4: from mechanism to autoimmune therapy. Int Immunopharm. 2020;80 doi: 10.1016/j.intimp.2020.106221. [DOI] [PubMed] [Google Scholar]
- 109.Kuol N., Stojanovska L., Nurgali K., et al. PD-1/PD-L1 in disease. Immunotherapy. 2018;10(2):149–160. doi: 10.2217/imt-2017-0120. [DOI] [PubMed] [Google Scholar]
- 110.Keir M.E., Butte M.J., Freeman G.J., et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Keir M.E., Liang S.C., Guleria I., et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu S.Y., Huang W.C., Yeh H.I., et al. Sequential blockade of PD-1 and PD-L1 causes fulminant cardiotoxicity-from case report to mouse model validation. Cancers. 2019;11(4):580. doi: 10.3390/cancers11040580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nishimura H., Okazaki T., Tanaka Y., et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 114.Wang J., Okazaki I.M., Yoshida T., et al. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol. 2010;22(6):443–452. doi: 10.1093/intimm/dxq026. [DOI] [PubMed] [Google Scholar]
- 115.Lucas J.A., Menke J., Rabacal W.A., et al. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J Immunol. 2008;181(4):2513–2521. doi: 10.4049/jimmunol.181.4.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grabie N., Gotsman I., DaCosta R., et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation. 2007;116(18):2062–2071. doi: 10.1161/CIRCULATIONAHA.107.709360. [DOI] [PubMed] [Google Scholar]
- 117.Lo B., Abdel-Motal U.M. Lessons from CTLA-4 deficiency and checkpoint inhibition. Curr Opin Immunol. 2017;49:14–19. doi: 10.1016/j.coi.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 118.Mitsuiki N., Schwab C., Grimbacher B. What did we learn from CTLA-4 insufficiency on the human immune system? Immunol Rev. 2019;287(1):33–49. doi: 10.1111/imr.12721. [DOI] [PubMed] [Google Scholar]
- 119.Curigliano G., Lenihan D., Fradley M., et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31(2):171–190. doi: 10.1016/j.annonc.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Haanen J.B.A.G., Carbonnel F., Robert C., et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv264–iv266. doi: 10.1093/annonc/mdy162. [DOI] [PubMed] [Google Scholar]
- 121.Yogasundaram H., Alhumaid W., Chen J.W., et al. Plasma exchange for immune checkpoint inhibitor-induced myocarditis. CJC Open. 2020;3(3):379–382. doi: 10.1016/j.cjco.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 123.Sueta D., Hokimoto S. Onco-cardiology: present and future. Int J Cardiol. 2016;215:38–40. doi: 10.1016/j.ijcard.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 124.Lyon A.R., Dent S., Stanway S., et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22(11):1945–1960. doi: 10.1002/ejhf.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]