Abstract
An accumulation of previous work has established organoids as good preclinical models of human tumors, facilitating translation from basic research to clinical practice. They are changing the paradigm of preclinical cancer research because they can recapitulate the heterogeneity and pathophysiology of human cancers and more closely approximate the complex tissue environment and structure found in clinical tumors than in vitro cell lines and animal models. However, the potential applications of cancer organoids remain to be comprehensively summarized. In the review, we firstly describe what is currently known about cancer organoid culture and then discuss in depth the basic mechanisms, including tumorigenesis and tumor metastasis, and describe recent advances in patient-derived tumor organoids (PDOs) for drug screening and immunological studies. Finally, the present challenges faced by organoid technology in clinical practice and its prospects are discussed. This review highlights that organoids may offer a novel therapeutic strategy for cancer research.
Keywords: Drug assays, Immunotherapy, Mechanism research, Organoids, Precision medicine
Introduction
Cancer is a leading cause of global mortality with a huge impact on health, and social and economic development, necessitating progress toward preventive and treatment options. A total of 28.4 million cases are predicted by 2040 around the globe.1 Therefore, the establishment of high-fidelity preclinical tumor models is crucial to investigate cancer-related mechanistic studies as well as allow personalized anti-cancer therapy in clinical.
Maintenance of tumor heterogeneity and mimicry of the microenvironment are challenges in establishing preclinical models. Cancer cell lines are limited in applications such as high-throughput drug screening due to the lack of tumor architecture and microenvironment.2 Mouse models better mimic the in vivo situation but have low success rates, long generation cycles, high costs, and dubious clinical applications. Therefore, a three-dimensional (3D) cell culture technique producing “organoids” has been developed.3 Organoids are multicellular clusters with the in vitro capacity for self-renewal and proliferation while maintaining the physiological structure and function of the tissue from which they are derived.4 Compared with the traditional model, cancer organoids have their unique advantages, including a better ability to simulate the physiological and pathological state of tumor organs, moderate cost, and better combination with some emerging technologies. In addition, compared with traditional 2D cell culture, this 3D model maintains the mutation pattern without genetic changes during long-term culture, which makes it more suitable for studying dynamic processes, such as tumor development. These 3D constructs represent a promising, near-physiological model for human cancers, and exhibit enormous potential for translational studies of solid tumors.5,6 Thus, our review will discuss various cancer organoid culture strategies and summarize the recent advances in tumor organoids from the basic mechanism and clinical application. Finally, the limitations of this emerging technology and future developments are discussed.
Establishment of organoids
Organoids are miniaturized in vitro organ models developed from stem cells or tumor tissues extracted from patients and grown in a specific 3D in vitro microenvironment. Organoids mimic the characteristics of real organs in vivo and 3D culture systems may be stably expanded.7 Tissue may be enzymatically and/or mechanically digested and cultured on a basement membrane (BME) or at the air-liquid interface, using Transwell inserts.8, 9, 10 Organoids may be passaged every 1–2 weeks. Crucially, the media in which organoids are cultured varies, often depending on the tissue of origin. For example, small intestinal organoids cultured with epidermal growth factor (EGF), R-spondin, and noggin, while colonic organoids require nicotinamide, the p38 inhibitor SB202190, prostaglandin E2, and the TFG-β inhibitor A83-014.8
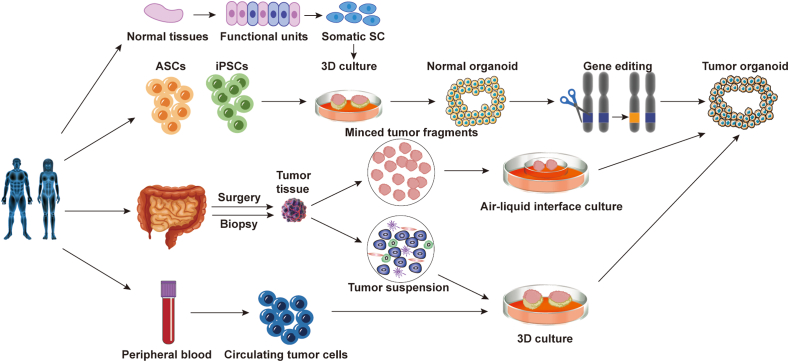
Cancer organoids (Fig. 1) may be generated through differentiation of iPSCs or directly derived from tumor tissues,5,11 such as patient surgical specimens or needle biopsies.12 Gao et al established metastatic prostate cancer 3D organoid cultures from patient biopsies and circulating tumor cells (CTCs).13 Cancer organoids derived from primary and/or metastatic samples of breast,14,15 bladder,16 colons,17,18 esophagus,19 stomachs,20 gallbladders,21,22 head and neck,23, 24, 25 kidney,26,27 liver,28,29 lung,30,31 ovary,32,33 pancreas,34,35 and prostate13,36 cancers have been described (Table 1). Cancer organoids vary in their growth rate and size.12 This depends on the culture system but is also influenced by the viability and amount of necrosis of the original tumor tissue, whether samples were taken pre- or posttreatment, and the sample processing time and technique. As with other organoids, PDOs retain many histological, transcriptional, and genetic characteristics of the original tumor, maintaining the high heterogeneity of tumor cells in a simple and time-efficient culture system.37,38 However, culture stability may vary depending on tumor type and culture conditions. Therefore, characterizing and tracking the genotype and phenotype of organoids over time is essential to avoid culturing only one or a few dominant clones from heterogeneous tumor tissue and to ensure that the model represents the original tumor.
Figure 1.
Schematic representation of patient-derived tumor organoids. Patient-derived tumor organoids from surgically resected/biopsied tissues and circulating tumor cells. Non-tumor organoids may be mutated into tumor organoids after gene editing.
Table 1.
Culture systems of different cancer organoid models.
| Tumoroid type | Extracellular matrix | culture components |
Rf. | ||
|---|---|---|---|---|---|
| Growth factors | Small Molecules | Molecule inhibitors | |||
| Breast cancer | Basement membrane extract | Advanced DMEM/F12 Wnt3A EGF FGF-7/FGF-10 Noggin R-spondin 1 | nicotinamide N-acetylcysteine B27 primocine neuregulin 1 | A83-01 SB202190 Y-27632 | 14,15 |
| Bladder cancer | Matrigel or Basement membrane extract | Advanced DMEM/F12 FGF-7/FGF-10/FGF-2 | nicotinamide N-acetylcysteine B27 | A83-01 Y-27632 | 16 |
| Colorectal cancer | Matrigel | Advanced DMEM/F12 Wnt3A EGF FGF-10 Noggin R-spondin 1 | nicotinamide PGE2 Gastrin N-acetylcysteine B27 | A83-01 SB202190 Y-27632 | 17,18 |
| Esophageal cancer | BME-2 | Advanced DMEM/F12 Wnt3A EGF FGF-10 Noggin R-spondin 1 | nicotinamide Gastrin N-acetylcysteine B27 | A83-01 SB202190 Y-27632 | 19 |
| Gastric cancer | Matrigel | Advanced DMEM/F12 Wnt3A EGF FGF-10 Noggin R-spondin 1 | nicotinamide Gastrin N-acetylcysteine B27 | A83-01 Y-27632 | 20 |
| Gallbladder cancer | Matrigel | Advanced DMEM/F12 EGF FGF-10 IGF HGF Noggin | nicotinamide Gastrin N-acetylcysteine B27 Forskolin N2 Dexamethasone Primocin | A83-01 Y-27632 | 21,22 |
| Glioblastoma | Matrigel | DMEM/F12 EGF/bEGF Noggin R-spondin 1 | nicotinamide PGE2 Gastrin | CHIR99021 A83-01 SB202190 Y-27632 | 23,24 |
| Head and neck squamous cell carcinoma | Matrigel | Advanced DMEM/F12 EGF FGF-10/FGF-2 Noggin R-spondin 1 |
nicotinamide PGE2 N-acetylcysteine B27 Forskolin |
CHIR99021 A83-01 |
25 |
| Kidney cancer | Growth factor-reduced Matrigel or Basement membrane extract | Advanced DMEM/F12 EGF FGF-10 R-spondin 1 |
N-acetylcysteine B27 Primocin |
A83-01 Y-27632 |
26,27 |
| Liver cancer | Matrigel or Basement membrane extract | Advanced DMEM/F12 Wnt3A EGF, HGF FGF-10 Noggin R-spondin 1 |
nicotinamide Gastrin N-acetylcysteine B27 Forskolin Dexamethasone |
A83-01 Y-27632 |
28,29 |
| Lung cancer | Matrigel | MBM Wnt3A EGF FGF-4/FGF-7/FGF-10 Noggin R-spondin 1 |
/ | A83-01 | 30,31 |
| Ovarian cancer | Matrigel | Advanced DMEM/F12 EGF FGF-10 Noggin R-spondin 1 BMP-4 |
nicotinamide N-acetylcysteine B27 forskolin primocine Heregulinβ-1 |
A83-01 Y-27632 |
32,33 |
| Pancreatic cancer | Matrigel | Advanced DMEM/F12 Wnt3A EGF FGF-10 Noggin R-spondin 1 |
nicotinamide PGE2 Gastrin N-acetylcysteine B27 |
A83-01 Y-27632 |
34,35 |
| Prostate cancer | Matrigel | Advanced DMEM/F12 EGF,HGF FGF-2/FGF-10 Noggin R-spondin 1 |
nicotinamide PGE2 N-acetylcysteine B27 Dihydrotestosterone |
A83-01 SB202190 Y-27632 |
13,36 |
A83–01: Transforming growth factor-beta inhibitor which suppresses organoid proliferation; BME-2: Soluble basement membrane purified from Engelbreth-Holm-Swarm (EHS) tumor; contains laminin, collagen IV, entactin, and heparin sulphate proteoglycan; EGF: Binds EGF receptor to induce cancer cell proliferation; FGFs: A group of pluripotent and promiscuous growth factors; Gastrin: Stimulates proliferation and suppresses apoptosis of cancer cells; HGF: HGF/Met signaling promotes oncogenesis, tumor angiogenesis, and invasion; Nicotinamide: Vitamin B3 required for the long-term culture of organoids; Noggin: Inhibits bone morphogenetic proteins and modulates cellular differentiation, proliferation, and apoptosis; promotes bone metastasis of some cancers and is associated with tumorigenesis of primary bone malignancies; PGE2: Promotes angiogenesis in gastric cancer through up-regulation of vascular endothelial growth factor; R-spondin 1: Binds Lgr5 and a niche factor required for self-renewal of stem cells and activates Wnt signaling; facilitates growth and metastasis of cancer cells; SB202190: p38 inhibitor which suppresses the proliferation and migration of cancer cells; Wnt: Master regulator of cell development, proliferation, differentiation, adhesion, and polarity; aberrant Wnt signaling promotes carcinogenesis and cancer progression; Y-27632: Rho kinase inhibitor that reduces anoikis of dissociated stem cells, improves culture media, and promotes proliferation of tumor epithelial cells.
Applications of organoids in various cancers
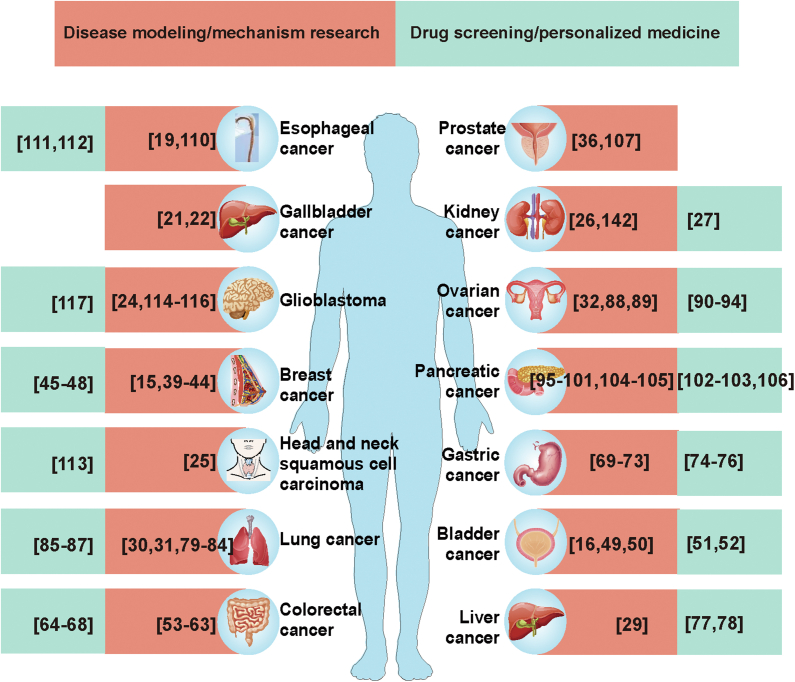
Organoids are suitable preclinical models due to their ability to recapitulate the genotype, phenotype, and cellular features of their parent tissues. Some applications of patient-isolated organoids are listed in Figure 2.
Figure 2.
Current applications of various cancer organoid models (references in brackets).
Breast cancer
Dekkers et al described a protocol for long-term culture of normal human breast and breast cancer organoids from clinical samples15 and a diverse biobank of normal and breast cancer PDOs with a focus on triple-negative breast cancer (TNBC) has been compiled.39 In addition, organoids have been used to study rare cancers, such as adenomyoepithelioma,40 papillary carcinomas,41 Paget's disease,42 and TNBC with malignant pleural effusion,43 and genes mimicking neoplasia have been knocked out by CRISPR/Cas9 in organoids for transplant into mice.44 Furthermore, patient-specific drug sensitivity may be evaluated in vitro organoids to guide treatment.45,46 Other applications include the identification of agents that reverse the epithelial–mesenchymal transition47 and response to oncolytic agents for testing of oncolytic viruses.48
Bladder cancer
The heterogeneity of bladder cancer causes a high mutation rate, high risk of recurrence, and poor prognosis. Culture systems for bladder cancer organoids have been mentioned in many studies.16,49,50 Mullonders et al created a live biobank of bladder cancer organoids from 53 patients in which common bladder cancer mutations were identified.16 In addition, Kong's team used organoid models to identify biomarkers that accurately predict responses to chemotherapy in 77 bladder cancer patients.51 Yu et al used organoids to assess chimeric antigen receptor (CAR) T cell-mediated cytotoxicity against bladder cancer.52
Colorectal cancer
Several groups have successfully established organoids for colorectal cancer (CRC).53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68 For example, Zhao et al used organoid models to show that cancer stem cells (CSCs) and differentiated cancer cells (non-CSCs) have different metabolic phenotypes. Lactate derived from non-CSCs promoted self-renewal of CSCs, thereby promoting CRC progression.55Another study modeled the progression of colorectal tumors and metastases using paired PDOs and found that organoids from metastatic sites exhibited more tumorigenic and metastatic abilities than those from primary lesions.54 Organoids have also been used to find more targeted therapies for CRC. Bruun et al developed an in vitro pharmacogenomic profiling platform using PDOs and found it useful for drug screening to find new therapeutic strategies for metastatic colorectal cancer.59 Furthermore, the use of locally advanced rectal cancer organoids has demonstrated a strong matching of response to chemoradiotherapy between patients and organoids with an accuracy of 84.43%, a sensitivity of 78.01%, and a specificity of 91.97%.64
Gastric cancer
Several studies have described procedures for creating gastric cancer organoids from clinical samples and have been successfully used for mechanistic studies of drug resistance.69, 70, 71, 72, 73, 74 Ukai et al69 established 5-FU-resistant gastric cancer organoids and Harada et al70 established oxaliplatin-resistant gastric cancer organoids to explore the mechanism of gastric cancer resistance. In addition, several groups have developed organoid models of H. pylori infection for mechanistic studies of H. pylori-induced gastric cancer.75 Organoids have also been used to study the more uncommon gastric cancers, such as Signet ring cell carcinoma.76
Liver cancer
Broutier et al first established primary liver cancer organoids from eight surgically resected hepatocellular carcinoma (HCC), cholangiocarcinoma (CC), and combined HCC-CC subtypes in 2017.29 Resistance to chemotherapy is common in all types of cancer while organoids of liver cancer have been shown to help identify mechanisms of resistance to anticancer drugs.77 Furthermore, Liu et al established 3D co-culture models of primary liver tumor-derived organoids with CAF to understand their interaction and response to chemotherapeutic agents.78
Lung cancer
Biobanks of lung cancer organoids and normal bronchial organoids have been established from primary lung cancer tissues, including adenocarcinoma,79, 80, 81, 82 squamous cell carcinoma,30 small cell carcinoma,83 large cell carcinoma,31 and adenosquamous carcinoma,30 as well as paired non-neoplastic airway tissues.84 In addition, Dijkstra et al found a 17% rate of pure non-small cell lung cancer (NSCLC) organoid formation by assessing samples from >70 NSCLC samples, indicating that the methods of establishing pure NSCLC organoids from intrapulmonary lesions require improvement.85 Lung cancer organoid models can also accurately select anticancer drugs based on drug sensitivity to provide a powerful supplement and validation for gene sequencing.86,87
Ovarian cancer
Kopper et al established 56 organoids from 32 patients representing all major subtypes of ovarian cancer, including serous borderline tumor (BT), mucinous BT, low-grade serous BT, mucinous carcinoma, endometrioid carcinoma, clear cell carcinoma, and high-grade serous subtype.32 In addition, high-grade serous ovarian cancer organoids require a low Wnt environment for long-term growth.88,89 Some studies have shown that PDOs are physiologically appropriate in vitro tumor drug screening models and can use effective personalized drugs to target ovarian cancer.90, 91, 92, 93, 94 De Witte et al assessed the ability of PDOs to predict functional consequences of clinical drug response and tumor heterogeneity and showed that PDO drug screening identified 88% of patients as highly responsive to at least one drug.92
Pancreatic cancer
Pancreatic cancer is characterized by elevated intra-tumoral and intertumoral heterogeneity and a highly malignant phenotype which makes it difficult to establish organoids. Pancreatic ductal organoids are an in vitro model of pancreatic ductal adenocarcinoma (PDAC) and can be investigated in patients with local, advanced, and malignant metastases by surgical, fine needle aspiration of ascites specimens.95, 96, 97, 98, 99, 100, 101, 102, 103 Furthermore, Huang et al104 and Beato et al105 established the intraductal papillary mucinous neoplasms (IPMNs) organoid biobank to investigate the genetic and biological mechanisms of IPMNs. Another study achieved rapid molecular profiling and drug detection by generating PDOs from free DNA which has implications for clinical practice.106 Briefly, PDOs can mimic the response of primary tumors to drug therapy and therefore have the potential for translational research and drug discovery.
Prostate cancer
Human prostate cancer organoids have also been created. Karkampouna et al developed prostate cancer models with specific biological and genetic landscapes that can be used to investigate tumor growth, metastasis, and drug resistance in the early stages of the disease and to assess response to chemotherapy.36 Servant et al developed a biobank that included prostate cancer organoids from 81 patients for the construction of stable cell lines.107 In addition, the use of other biotechnologies in combination with prostate cancer organoids has helped to better investigate the complexity of tumor pathogenesis as well as integrate the tumor microenvironment.108,109
Other cancer
In addition to the organoids described in detail above, other organoids have also developed well in recent years. For example, some studies have shown that specific, targeted therapy for individual patients can be guided by PDO produced in biopsies from patients with esophageal cancer, including esophageal adenocarcinoma and esophageal squamous cell carcinoma.110, 111, 112 The head and neck squamous cell carcinoma (HNSCC) organoids established by Driehuis et al have been used to assess the efficacy of targeted photodynamic therapy for HNSCC.113 Many methods for generating patient-derived glioblastoma organoids have been established.114, 115, 116, 117 Patient-derived glioblastoma organoids have been demonstrated to recapitulate key features of the tissue of origin, including their histological features, cellular diversity, gene expression patterns, and mutation profiles.24
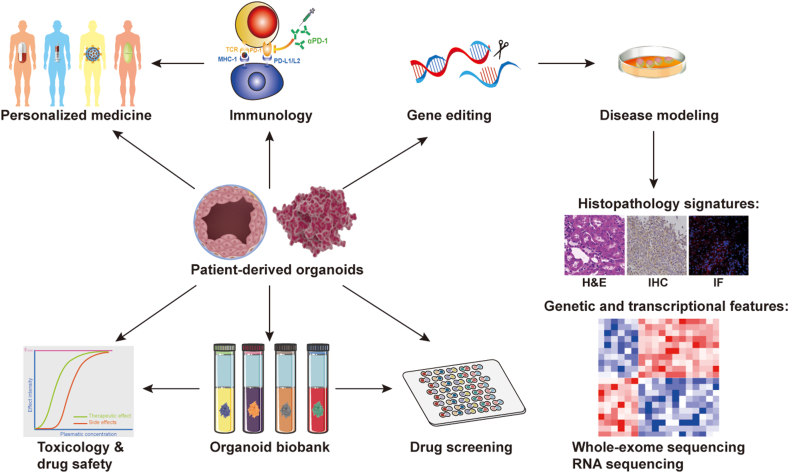
In brief, organoids have been applied (Fig. 3) to disease model construction, tumor mechanisms, drug screening, testing cancer immunotherapy, and developing new methods for personalized medicine.
Figure 3.
Application of organoids in cancer research. Organoid technology combined with CRISPR/Cas9 gene editing allows the impact of genetic changes on disease development to be studied. Organoid biobanks can be used for drug screening and validation. Patient-derived tumor organoids co-culture with immune cells mimics the mechanism of immunotherapy efficiency and drug resistance. In addition, tumor organoid models may become a tool for personalized medicine validation to optimize treatment selection.
Tumor mechanisms
Tumorigenesis
Tumorigenesis is characterized by multistep genetic alterations that lead to the inactivation of tumor suppressor genes and activation of oncogenes which together drive cancer growth.118,119 Introduction of targeted mutations by CRISPR-Cas9 allows tumorigenesis to be mimicked.120 Colorectal cancer (CRC) may be mimicked by the CRISPR-Cas9 introduced mutations of KRAS, Apc, p53, and Smad4.121,122 Organoids with activating mutations in KRAS (KRASG12D) and inactivating mutations in Apc, TP53, and Smad4 grow independently of the stem cell growth factors, EGF, WNT, R-spondin1, and noggin. Primary colonic organoids may be transformed into adenocarcinoid tumors via mutation of Apc, p53, KrasG12D, and Smad4, and gastric and pancreatic organoids transformed by p53 loss, KrasG12Dexpression, or both.10 Naruse et al used four genotoxic chemicals, ethylmethanesulfonate (EMS), acrylamide (AA), diethylnitrosamine (DEN), and 7,12-dimethylbenz [a] anthracene (DMBA) to study chemical carcinogenesis.123 DMBA-treated mammary tissue-derived organoids with a heterozygous knockout of Trp53 showed tumorigenicity while organoids with wild-type Trp53 did not, consistent with previous reports on corresponding mouse models. Pulmonary organoids with or without Trp53 knockout developed oncogenic histopathological features with oncogenic kinase activity in subcutaneous nodules when treated with EMS or AA and similar changes were found in hepatic organoids treated with DEN. Tumor organoid technology has also been used to investigate pathways involved during tumorigenesis. Mouse colonic organoids have been used to demonstrate that aryl hydrocarbon receptor (AhR) signaling regulates the IL22 response by regulating SOCS3 expression, validating a rationale for using AhR to reduce colon cancer risk.124 lnc-RP11-536 K7.3 was shown to promote colon cancer (CC) progression in CC-derived organoids through the SOX2/USP7/HIF-1α signaling axis, revealing regulation of chemosensitivity and exposing therapeutic targets related to lncRNAs.63 All these results demonstrate the utility of gene-editing organoid systems in validating driver pathway mutations in tumorigenesis, thus providing a flexible in vitro cancer model for the study of tumorigenesis.
Although CRISPR-Cas9 has been successfully used in organoids, precise integration of exogenous DNA sequences into organoids is lacking robust knock-in approaches. CRISPR-Cas9-mediated homology-independent organoid transgenesis (CRISPR-HOT) enables knock-in human organoids representing different tissues to be generated.125 CRISPR-HOT simplifies gene editing and has been used to introduce fluorescent labeling of reporter gene products for subcellular visualization in intestinal cells. Moreover, CRISPR-HOT has been used to generate human liver ductal organoids and human fetal hepatocyte organoids within 2–3 months.126 The application of CRISPR-HOT to organoid construction allows universal reproduction of the process of tumorigenesis.
Tumor metastasis
Cancer metastasis involves the spread of cancer cells from the primary site to other organs and is the leading cause of death in cancer patients.127 Metastasis is a complex molecular process and maintenance of tumor pathophysiological features by organoids renders this a suitable platform for study. For example, Fujii et al generated four independently matched sets of organoids for primary colorectal cancer and metastatic lesions. Exome sequencing confirmed that they share similar genetic profiles and niche factor requirements and metastasis-derived organoids exhibit higher metastatic capacity.18 Cancer organoids can also help identify critical targets that inhibit tumor metastasis. SOX4 has been shown to promote the maintenance of an undifferentiated and proliferative state in breast cancer organoids. Compared to SOX4-positive breast cancer organoids, SOX4-knockdown breast cancer organoids contained more differentiated cells, had intraluminal or basal gene expression patterns and lower levels of cell cycle genes, and showed the impaired ability of tumor metastasis and growth.128 Another study revealed that SOX2 was identified as having pro-metastatic activity from primary tumor-derived organoids matched with liver metastases of CRC patients. Thus, SOX2 is thought to be associated with CRC invasion and proliferation as well as liver metastasis.54 In addition, organoids were generated from primary and metastatic breast cancer tissues and accurately replicated histopathology, hormone receptor status, HER2 status, and DNA copy number changes in a previous study conducted by Sachs N et al.14 This suggests that organoids can accurately mimic native as well as metastatic tumors and provide a better understanding of cancer biology. In short, cancer organoids provide an effective tumor model to investigate the mechanisms of promotion and inhibition of tumor invasion.
Drug assays
Most cytotoxic agents are more effective in tumor cell lines than in patients, in whom tumor responsiveness varies greatly according to tumor type.129 Voskoglou-Nomikos et al established that cell line in vitro models predict cytotoxic responses of non-small cell lung cancer but not of colon cancer under a disease-oriented approach.130 Organoids are closer to physiological structures and more realistically reproduce drug responses filling a gap between drug screening and clinical trials. Several tumor organoid biobanks (Table 2) have been established for the identification and testing of new drugs and toxicology assessments.14,16,18,30,32
Table 2.
Cancer organoid biobanks.
| Tumor type | Source |
Success rate (%) | Achievement | Rf. | |
|---|---|---|---|---|---|
| Type | Quantity | ||||
| Breast cancer | Ductal adenocarcinoma, lobular adenocarcinoma | 100 patients | >80 | Used for cancer research, drug development, and to assess personalized in vitro drug responses. | 14 |
| invasive ductal carcinoma, invasive lobular carcinoma | 33 patients | 87.5 | New protocol for obtaining patient-derived oganoids from breast cancer | 197 | |
| Bladder Cancer | Urothelial carcinoma Squamous-cell carcinoma | 16 patients | 70 | In vitro model of tumor evolution and therapeutic response to precision cancer medicine. | 198 |
| Primary tumor | 50 patients | 50 | Bladder organoids biobank for drug testing in the future. | 16 | |
| Colorectal cancer | Primary tumor | 20 patients | 90 | Tumor organoids may fill the gap between cancer genetics and patient trials for drug research as well as personalized therapy. | 17 |
| Primary tumor | 43 patients | 100 | Functional links between genetic alterations, niche requirements and biological phenotypes of tumors, providing a multifunctional platform for biomedical research. | 18 | |
| Metastases | 14 patients | 71 | Personalized screening tool using patient-derived tumor organoids. | 37 | |
| Esophageal cancer | Oesophageal squamous- cell carcinoma, Oropharyngeal squamous- cell carcinoma |
21 patients | 71.4 | Platform to analyze cancer cell heterogeneity, assess personalized drug treatment response and treatment resistance. | 110 |
| Gastric cancer | Normal, dysplastic, cancer, and lymph node metastases | 34 patients | >50 (tumor)/>90 (normal) | Identified potential targeted drugs to guide patient drug selection. | 199 |
| Glioblastoma | IDH1 mutant tumors, recurrent tumors | 53 patients | 91.4 | The establishment a large cohort of unique organoids and patient-derived orthotopic xenografts of various glioma subtypes. | 24 |
| IDH1 mutant IV glioblastoma (GBM), IDH1 mutant II-III gliomas | 173 patients | 79/68 | The establishment a large cohort of unique organoids and patient-derived orthotopic xenografts of various glioma subtypes | 117 | |
| Head and neck squamous cell carcinoma | Primary tumor | 40 patients | 65 | Comparison of organoids with normal epithelium applied to in vitro drug screening. | 25 |
| Kidney cancer | Wilms tumors, malignant rhabdoid tumors (MRTK), renal cell carcinomas (RCC), and congenital mesoblastic nephromas | 50 children | 100(normal)/75 (Wilms tumors)/100(MRTK)/75(RCC) | Captures heterogeneity of pediatric renal tumors; well-characterized model for basic cancer research, drug screening and personalized medicine. | 27 |
| Lung cancer | Non-small cell lung cancer | 14 patients | 71.43 | A living biobank of patient-derived organoids from non-small cell lung cancer patients was established. | 200 |
| adenocarcinoma, squamous cell carcinoma, adenosquamous carcinoma, large cell carcinoma, and small cell carcinoma | 36 patients | 87 | Successfully construct biobank of lung cancer organoids | 30 | |
| Liver cancer | Hepatocellular carcinoma | 10 patients | 26 | Hepatocellular carcinoma (HCC) organoids generated from needle biopsies of patients with liver cancer; response to sorafenib treatment. | 201 |
| ovarian cancer | Borderline tumors, Clear-cell carcinoma, Endometrioid carcinoma, Mucinous carcinoma, Serous carcinoma |
32 patients | 65 | Ovarian cancer organoids can be used for drug-screening assays and different tumor subtype responses to platinum-based chemotherapy. | 32 |
| Pancreatic cancer | Ductal adenocarcinoma (primary tumor and Metastases) | 138 patients | 75 | Predict drug responses in pancreatic cancer patients and provide a rational for prioritizing therapeutic regimens. | 202 |
| pancreatic ductal adenocarcinoma (PDAC) | 39 patients | / | Revealed functional heterogeneity in Wnt niche independence in PDAC. | 203 | |
| Intraductal papillary mucinous neoplasms (IPMNs) | 8 patients | / | Potential drivers of IPMN tumor development were identified. | 104 | |
| Intraductal papillary mucinous neoplasms (IPMNs) | 15 patients | 81(tumor)/87(normal) | The mechanism of IPMN invasion was revealed. | 105 | |
| Prostate Cancer | Adenocarcinoma metastases Circulating tumor cells |
7 patients | 15–20 | Recapitulated the molecular diversity of prostate cancer subtypes, providing an in vitro model for understanding disease pathogenesis and response to therapy. | 13 |
| Neuroendocrine tumors | Gastroenteropancreatic (GEP) neuroendocrine neoplasm (NEN) | 39 patients | / | Understanding of GEP-NEN and its genetic and biological phenotypes. | 204 |
Drug efficacy testing
As an in vitro individualized preclinical model, organoids have great potential in susceptibility testing of individualized drugs. Organoids from metastatic gastrointestinal cancer (colorectal and gastroesophageal) have been tested for their ability to predict a patient's treatment response. It accurately reproduced the patient treatment response of gastrointestinal cancer with high sensitivity (100%), specificity (93%), positive predictive value (88%), and negative predictive value (100%).131 Li et al used signet ring cell carcinoma (SRCC) and non-SRCC organoids to observe treatment responses to 5-fluorouracil, oxaliplatin, docetaxel, and irinotecan. The SRCC organoid system had a lower IC50 value for docetaxel than the non-SRCC. No further differences were found between the two types of organoids for other drugs.76 The sensitivity of 40 drugs and gene expression profiles of 38 CRC liver metastases from 22 patients were tested using PDOs. Sensitivity to several anticancer drugs and antimetabolites that have not previously been used to treat CRC was revealed while some drugs without clear genomic markers (alisetidine b and navitoclax) showed heterogeneity in anticancer activity.59 In addition, cancer organoids are also effective tools to interrogate gene–drug associations. A study showed that TP53 mutant organoids were insensitive to nutlin-3a in the biliary tract and CRC organoids.132 This result is consistent with clinical findings in cancer patients with TP53 mutations. Cancer organoids have also facilitated research into mechanisms of therapeutic resistance.30,70,133,134 Among oxaliplatin-resistant GCOs, the presence of myoferlin was shown to be strongly associated with the acquisition of oxaliplatin resistance.70 In another study, expression of atypical cyclin P was found to promote the stem-like phenotype of intestinal cancer organoids, which often leads to tumor recurrence, metastasis, and treatment resistance.134
Personalized treatment options have been explored via organoid predictions. Combination therapy with RAS pathway inhibitors and drugs (HER2 inhibitor selafatinib, MEK inhibitor selumetinib, and ERK inhibitor SCH772984) in CRC organoids with wild-type or mutant RAS were evaluated and further oncogenic mutations were introduced by CRISPR. The combination of afatinib with selumetinib and SCH772984 with selumetinib both inhibited mutant organoid growths, demonstrating the potential of patient-derived CRC organoid repertoires for preclinical assessment of inhibitors and drug combinations.135 A blinded study cultured 77 samples from 57 patients with stage IV colorectal cancer to generate organoids and evaluate the predictive accuracy of chemotherapy responses. Organoids and metastatic tissues were very consistent with the original cancer tissues, preserving histological features and marker expression. The sensitivity, specificity, and precision of patient-derived tumor organoid models for chemotherapy response prediction were 63.33%, 94.12%, and 79.69%, respectively.136 These studies have shown that for patients, organoid sensitivity testing technology can quickly detect the most appropriate drugs, develop the best and most effective drug treatment regimen, and reduce the probability of drug side effects, drug resistance, and tumor recurrence, so as to obtain the best treatment.
Cancer organoid biobanks can serve as critical models for new drug discovery. This may shorten the preclinical trial cycle, reduce development costs and risks, improve the success rate, and facilitate drug discovery. Plocabulin is a novel microtubule-disrupting antitumor agent of marine origin currently undergoing phase II clinical trials. Costales-Carrera et al used 3D tumor organoids from 3 CRC patients to show that plocabulin is strongly cytotoxic to CRC.137 Organoids represent a reproducible platform for personalized drug screening which addresses inter- and intra-tumoral heterogeneity despite the small sample sizes of most studies to date although there is more work to be done before they are a predictive tool for clinical decision-making.
Drug toxicity testing
Tissue-derived organoids allow the selection of drugs that specifically target cancer cells but do not damage healthy cells. At present, general cell screening and animal model screening often fail to accurately predict adverse reactions in humans. The organoid model plays a vital role in drug toxicity screening. Indeed, hepatotoxicity, cardiotoxicity, and nephrotoxicity are the main causes of drug clinical trial failure. Liver organoids have been used to assess hepatotoxicity.138, 139, 140 For example, Mekky et al used human liver organoids to test the toxicity of aspartic acid-coated magnesium oxide nanoparticles and valproate showing that these drugs decrease cell viability, decrease ATP, and increase reactive oxygen species in hepatocytes.139 Similarly, cardiac organoids were used to demonstrate that hypoxic cardiac injury aggravates the cardiotoxicity of doxorubicin.141 Cardiac organoids from mouse embryonic stem cell-derived embryoid bodies142 and human iPSCs have also been used for drug testing,143 as have iPSC-derived kidney organoids for nephrotoxicity screening.144 These organoids provide patient-specific models for investigating the toxicity of anticancer drugs in personalized medicine.
Immunological studies
Immunotherapy involves tumor recognition by the immune system and initiation or enhancement of immune responses. Non-specific immunotherapy introduces adjuvants, such as cytokines or other cell signaling molecules, to enhance overall immune activity while specific immunotherapy induces antigen-specific immune responses to one or more tumor targets. Anti-cancer vaccines, dendritic cell therapy, or oncolytic viruses may be used. T-cell and antibody-based therapies administer immune system components to act directly on the tumor or enhance existing immunity.145, 146, 147 However, most patients are insensitive to immunotherapy due to poor tumor immunogenicity and immune escape mediated by the tumor microenvironment.148 Immunotherapy cannot simulate the complex tumor microenvironment of pericytes and fibroblasts, resident or infiltrating vascular structures (endothelial cells), and immune cells because cancer cell lines do not reproduce the heterogeneity of tumor epithelial cells and the mouse immune system is too different from the human. However, cancer organoids preserve the characteristics of the human immune system and are gradually becoming a powerful tool for studying cancer immunotherapy.
Two conceptually different approaches have been described to construct the tumor microenvironment. (i) In reconstitution models, cancer organoids are cultured in an extracellular matrix, such as Matrigel or BME-2, and immersed in a tissue culture medium; exogenous immune cells, from autologous peripheral blood or tumors, are co-cultured with the organoids.50,61,72,78,97,149 (ii) In the overall native organoid model, the innate immune microenvironment (endogenous immune cells and other non-epithelial cell types) of tumor specimens is preserved without reconstitution. Organotypic tumor spheroids (40–100 μm) have been cultured in a microfluidic device containing collagen for 5–9 days, preserving tumor cells and endogenous immune cells, such as lymphocytes and medullary cell populations.150 Alternatively, primary tissue fragments containing tumor cells and immune components were embedded in collagen gels within Transwell dishes for air-liquid interface (ALI) culture. The top of the collagen gel is exposed to air, allowing the cells to obtain an adequate supply of oxygen. Many different primary tumors, including colon, lung, pancreas, and kidney have been cultured for up to 30 days. The complex histological tumor microenvironment is preserved with tumor parenchyma and stroma, including functional and tumor-infiltrating lymphocytes (TILs).151,152
The lack of functional blood vessels may lead to immature organoids, necrotic kernels, and premature differentiation, and vascularization would support the growth of more viable organoids. Organoid angiogenesis may be achieved by implanting organoids into highly vascular animal tissues, such as chicken chorion allantois membrane, where host blood vessels penetrate the organoid.153 Mesodermal precursor cells have been incorporated into human tumors and neural organoids, thereby forming vascularized organoids in vitro.153 Vessels may add layer-by-layer deposition to in vitro co-cultures or form tubular voids by selective removal of material that is seeded with endothelial cells and connected to the perfusion network.154 Alternatively, vascular endothelial growth factor (VEGF) and hypoxia gradients within a compartmentalized microfluidic chip have been used to induce vascularization in organoid-endothelial cell co-culture.155
Tumor immunobiology
Bone marrow and thymus are the sites of development, differentiation, and maturation of immune cells and aid the development of peripheral immune organs. Stromal cells form a scaffold for thymic tissue that promotes differentiation and proliferation of thymic epithelial cells (TECs) to support thymic function and T lymphocyte maturation.156,157 3D thymic organoids may be generated by thymocytes isolated from NOD SCID mice or postpartum tissue which are dissociated with deoxyguanosine and cultured on gel foam sponges during the process of reaggregation fetal thymic organ culture (FTOC). Thymocyte aggregates are cultured with hematopoietic stem cells (HSCs) to support the development of T cells after transplantation into athymic mice.158, 159, 160 However, to avoid T cell dysplasia due to different sizes of tissue samples, isolated tissues have been cultured in a single suspension and on gelatin foam at the air-liquid interface, in the process of reaggregation thymic organ culture (RTOC) which maintains the 3D conformation necessary for T cell function.161 An artificial thymic organoid system (ATO) has also been constructed by adding aggregates co-cultured from HSCs and MS5-hDLL1 cells (genetically modified allantois cell line expressing human DLL1 or DLL4) to cell culture inserts at the air-liquid interface in serum-free medium.162 The ATO system has been used to assist ESC and iPSC in the production of conventional T cells, providing a controllable, efficient, and stable tool for studying human thymus function.163,164
The lymph node peripheral cortex is characterized by a spatially organized extracellular matrix (ECM) with lymphoid follicles, rich in B cells and follicular dendritic cells (FDCs).165 Suematsu et al implanted thymus-derived stromal cell lines together with dendritic cells into biocompatible scaffolds, after which they were transplanted into the subcapsular space of mouse kidneys and generated a lymphoid tissue organoid which retains a secondary lymphoid organ structure and contains a network of compartmentalized B and T cell clusters, high endothelial venule-like vessels, germinal centers, and follicular dendritic cells. This simplified lymphoid tissue organoid assists with studies of secondary lymphoid organ development and induction of adaptive immune responses and may be used to interrogate tumor immune escape mechanisms.166 Votanopoulos et al generated melanoma/lymph node organoids by introducing patient tissue into an ECM-based hydrogel system to create three-dimensional (3D) mixed immune-enhanced patient-derived tumor organoid (iPDO) after washing them with saline, antibiotics, and erythrolysis buffer. This mixed organoid preserves both tumor heterogeneity and stromal and immune cell components. The iPDO reflects immunotherapy responses in 85% of patients and activates patient-matched peripheral blood T cells for killing tumor cells in the naïve PDO.167 Immune organoids allow the immune system and tumor cells of individual patients to remain viable and permit the mechanistic study of hematological malignancies. Using these organoids, mutations in mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1) protease and caspase recruitment domain-containing protein 11 (CARD11) mutations were found to be involved in activated B-cell like diffuse large B-cell lymphoma, the molecular subtypes of which are activated through different genetic pathways.168,169
Tumor immunotherapy
Immune checkpoint inhibitors (ICIs)
Targeting of PD1/PD-L1 and CTLA-4 by ICIs produces a clinical response among only some patients with melanoma,170 skin squamous cell carcinoma,171 non-small cell lung cancer,172 renal cell carcinoma,173 head and neck cancer,174 and tumors with mismatch repair defects.175 In vitro systems that preserve the tumor microenvironment (TME) may facilitate the development of more precise immuno-oncology. Kong et al co-cultured CRC organoids with autologous TILs to demonstrate the migratory and tumor cytotoxic activities of TILs, indicating the maintenance of the immune checkpoint, the PD-1/PD-L1 pathway.176 Courau and colleagues demonstrated that CRC organoids respond to immune infiltration by up-regulating HLA-E, a ligand for the CD8 and NK cell receptor, NKG2A, and that anti-MICA/B increases NKG2A while stimulating anti-tumor responses. Synergistic actions of anti-MICA/B and anti-NKG2A may be a potential immunomodulator in CRC patients.177 Chakrabarti et al investigated the potential link between the human epidermal growth factor receptor 2 (HER2) and PD-1/PD-L1 in co-cultures of human gastric cancer organoids, cytotoxic T lymphocytes (CTLs), and myeloid-derived suppressor cells (MDSCs). Knockdown of HER2 decreased PD-L1 expression and increased CTL infiltration and an antitumor effect. Co-expression of HER2 and PD-L1 may contribute to tumor cell immune escape, indicating a potential treatment for patients with anti-PD-1/anti-PD-L1 resistance.72 However, it must be acknowledged that the addition of a single immune cell type may not completely reproduce the complex interactions between different immune cell populations.
Holistic culture systems, including microfluidic and ALI methods, can be used for the functional modeling of ICIs. 3D microfluidic culture can reproduce in vivo therapeutic sensitivity when organoids were generated from MC38 and GL261 tumors (sensitive to PD1), CT26 (moderately sensitive to PD1) or B16F10, human melanoma and Merkel cell carcinoma (resistant to PD-1). Moreover, a combination of anti-PD-1 and TBK1/IKKε inhibitors helped to overcome immunosuppressive TME.150 Deng et al identified the CDK4/6 inhibitor which increased T cell infiltration and had a synergistic action with that of anti-PD-1, thereby improving anti-tumor activity.178 ALI culture of organoids with a blockade by anti-PD-1 or/and anti-PD-L1 resulted in expansion and activation of tumor antigen-specific T cells.179 These examples show the potential of immune-tumor organoids in detecting antibody-mediated therapy, identifying novel therapeutic targets, and visualizing and enhancing immune cell migration and tumor infiltration.
Adoptive cell transfer-therapy
Adoptive cell transfer therapy (ACT) includes TILs, chimeric antigen receptor T cell therapy (CAR-T), and T cell receptor therapy (TCR).180 CAR-T and TCR cell therapies involve extracting T cells from the patient's peripheral blood and genetically engineering them to express tumor-specific ligands. Cancer organoid models, particularly those containing TME components, are capable of monitoring and enhancing CAR cell recruitment and penetration in tumor tissue and subsequent killing. CAR NK-92 cells expressing EGFRvIII181 and CAR-T cells targeting bladder cancer-specific antigens52 have been analyzed using organoids. Glioblastoma organoids co-cultured with CAR-T cells have been a tool in immunotherapy research.115 These organoid immune cell co-culture systems can be used to assess car-mediated tumor-specific cytotoxicity in normal and tumor organoids. In addition, PDOs can be used as a source of tumor-reactive T cells, a culture platform to enrich them, and can induce and assess the killing efficiency of tumor-specific T cells. CAR-T cells expressing ligands for CD39 hepatitis B virus (HBV) surface protein and personalized tumor-reactive CD8 T had activity against hepatocellular carcinoma organoids.182 Although this study is currently limited to low patient numbers it proves the concept of organoid models being suitable for immunotherapy development.
Oncolytic virus-therapy
Oncolytic viruses are replication-competent and tumor-targeted to infect and kill tumor cells while stimulating the host to produce an anti-tumor immune response.183 Organoid models of pancreatic cancer,102 bladder cancer,184 glioblastomas,184 and breast cancer48 have been used as a screening platform for oncolytic viral therapy. A renal cell carcinoma PDO (RCC PDO) was tested with an oncolytic adenovirus into which a cross-hybridized Fc-fusion peptide, consisting of Fc with IgA1 and IgG1 constant domains, attached to the PD-1 outer domain had been cloned. The fusion peptide bound to PD-L1 and activated IgA1 neutrophils and IgG1 natural killer and complement activation functions enhancing tumor killing relative to anti-PD-L1 (atezolizumab), IgG1-PDL1, and IgA-PDL1.185 Although these studies suggest promise for PDOs in studying oncolytic virus infectivity and cytotoxicity, to our knowledge, immune responses triggered by oncolytic viruses have not been investigated in complex immune-organoids.
Immunotherapy research has driven the development of novel cancer therapies, either alone or in combination with other therapies, to target immunomodulatory pathways and advance the control of cancer cells. PDOs combine TME characteristics and mimic immunotherapy responses, while co-culture with immune cells mimics the mechanism of immunotherapy efficiency and drug resistance. Overall, cancer organoids can not only elucidate potential resistance pathways but may also contribute to therapeutic efforts, such as in vitro screening and optimization of drugs and cellular immunotherapies; if short-term responses in culture do accurately reflect clinical responses and long-term outcomes, they can also determine patient sensitivity to single or combination therapies in real time. In the future, organoid approaches will greatly facilitate the basic science and translation of immuno-oncology and accelerate the development of personalized immunotherapy for human tumors.
Current limitations and future opportunities
Many features of human cancer development and progression are reproduced by cancer organoids but the pathophysiology and clinical relevance could be improved. Firstly, although three-dimensional organoids constructed in vitro already have structures of some organs compared with intact organs in vivo, the structures are still relatively simple and can only partially reflect tissue characteristics. Additional supplements, such as IL-2, anti-CD3, and anti-CD28 antibodies, are often required to maintain immune cells in co-culture with organoids. The animal origins of the 3-D collagen-rich lamin, Matrigel, which is essential for organoid culture may also influence treatment outcomes. There is also variation in PDO generation due to a lack of standardized methodology plus ethical issues around the transplantation of organoids into humans, all of which must be addressed. Secondly, organoid culture varies according to the tumor type and starting material with treatment-directed tumor shrinkage affecting the success of the culture. Similarly, metastasis-derived organoids are difficult to generate, especially with inter-organ spread, and must be validated using mouse xenograft models. Thirdly, tumor-derived organoids usually grow more slowly than healthy tissue-derived organoids which leads to the contamination of tumor organoids with healthy tissue.85,186 Non-solid tumors cannot be reproduced as organoids and more complex brain organoids are still a major challenge. Fourthly, PDOs establishment takes 4–6 weeks plus approximately 2–4 weeks to screen for anticancer drugs, reducing the chances of coinciding with the optimal therapeutic window for patients. Moreover, the production of PDOs is currently very expensive, and the technology is immature and difficult to incorporate into existing healthcare systems. The ethical significance of tumor organoid biobanking also requires further consideration. Despite these limitations, organoid cultures are a physiologically relevant model for translational applications and personalized cancer drug development.
Yin et al187 established an in vitro tumor model of a patient-derived tumor cell cluster by simultaneously optimizing the medium in Matrigel-free conditions. This model allows short-term cell expansions from primary tumors, including immune and fibroblast populations, allowing the investigation of hundreds of therapeutic options per patient in a manner that correlated with clinical performance. This model excludes the influence of extrinsic factors such as Matrigel on subsequent studies while allowing drug prediction within the patient's optimal therapeutic time window. However, the chronic culture of patient-derived tumor cell clusters leads to the loss of stromal cells and their drug response patterns may be altered.
At present, the “Organ-on-a-chip” technology formed by the integration of organ chip and organoid can address some problems very well. A typical example is the application of microfluidic devices in an in vitro tumor organoid model. The interaction between tumor and stroma was investigated mechanically by simulating the spatial organization of the tumor microenvironment on a chip. It has been observed that tumor-associated fibroblasts (CAF) enhance the expression of glycoprotein non-metastatic B in breast cancer cells, thereby promoting invasion.188 The organoid-on-a-chip allows simulation of the microenvironment and establishment of interrelationships between tissues and multiple organs.189 Lung cancer organoids combined with microfluidic chip technology have produced drug responses within a week.190 In addition, Shirure et al191 used this technique and organoid integration to mimic perfused vessels for investigating the progression and response of cell lines and PDOs to chemotherapy and anti-angiogenic therapy. Many aspects remain to be improved in order to obtain more accurate and reliable results. Skilled and experienced researchers are required for the fabrication of microfluidic devices and sophisticated tissue engineering techniques. Another potential limitation is that biomaterials used to fabricate many organ chips cannot be effectively used for drug research due to drug absorption.
Optical metabolic imaging (OMI) measures drug-induced changes in cellular metabolism and may shorten drug turnover times and be used to test drug responses in individualized cancer treatment.192,193 The susceptibility of gastroenteropancreatic neuroendocrine tumor (GEP-NET) organoids to navitoclaxa (Bcl-2 inhibitor) and everolimus (mTOR inhibitor GEP-NET treatment) has been evaluated by OMI.194 3D bioprinting allows precise control of spatial heterogeneity in the tumor microenvironment through spatially deterministic deposition of predefined biobanks that may contain multiple cell types, biochemical factors, and ECM. Reid et al generated chimeric organoids by co-printing cancer cells and normal breast epithelial cells and showed that cancer cells within the chimeric construct had significantly increased levels of 5-hydroxymethylcytosine compared to bio-printed tumor-like cells.195 In addition, organoid technology, microfluidic technology, and 3D printing technology have been combined into an automated organoid platform for one-week high-throughput drug screening and personalized medicines.196 When limitations are eventually overcome, organoids may give new hope to cancer patients and greatly promote human health.
Conclusions
Patient-derived tumor organoids are physiologically and clinically more advanced and maintain patient-specific tumor heterogeneity, constituting a good platform for different tumors. This model allows the simulation of tumor development to provide new targets for cancer therapy and facilitate appropriate treatment selection. Some complex cancer organoids may become tools for personalized immunotherapy validation, response and toxicity testing, and therapeutic development in early clinical trials. Therefore, tumor-derived organoids are an important tool through which we can improve the understanding of cancer and promote the treatment of cancer patients in the future.
Author contributions
XM and QW collected the related paper and drafted the manuscript. GL and HL revised the manuscript and prepared the figures. DP and SX participated in the design of the review and helped draft and modify the manuscript. All authors read and approved the final manuscript.
Conflict of interests
The authors declared that there is no conflict of interests.
Funding
This work was supported by grants from the Project Nn10 of Harbin Medical University Cancer Hospital, Heilongjiang, China (No. Nn102017-02), the National Natural Science Foundation of China (No. 81972706, 81872149, 82173235, 82072904, 82103325), Outstanding Youth Project of Heilongjiang Provincial Natural Science Foundation, Heilongjiang, China (No. YQ2019H027), Distinguished Young Scholars of Harbin Medical University Cancer Hospital, Heilongjiang, China (No. JCQN2018-03), Yong Elite Training Foundation Grant of Harbin Medical University Cancer Hospital, Heilongjiang, China (No. JY2016-02), and Haiyan Fund Project of Harbin Medical University Cancer Hospital, Heilongjiang, China (No. JJMS 2022–17).
Acknowledgements
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Shouping Xu, Email: Shoupingxu@hrbmu.edu.cn.
Da Pang, Email: pangda@ems.hrbmu.edu.cn.
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Kapałczyńska M., Kolenda T., Przybyła W., et al. 2D and 3D cell cultures – a comparison of different types of cancer cell cultures. Arch Med Sci. 2018;14(4):910–919. doi: 10.5114/aoms.2016.63743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato T., Vries R.G., Snippert H.J., et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 4.Lancaster M.A., Knoblich J.A. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194):1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 5.Tuveson D., Clevers H. Cancer modeling meets human organoid technology. Science. 2019;364(6444):952–955. doi: 10.1126/science.aaw6985. [DOI] [PubMed] [Google Scholar]
- 6.Qu J., Kalyani F.S., Liu L., et al. Tumor organoids: synergistic applications, current challenges, and future prospects in cancer therapy. Cancer Commun. 2021;41(12):1331–1353. doi: 10.1002/cac2.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H., Wang Y., Wang P., et al. Tumor organoids for cancer research and personalized medicine. Cancer Biol Med. 2021;19(3):319–332. doi: 10.20892/j.issn.2095-3941.2021.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato T., Stange D.E., Ferrante M., et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141(5):1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 9.Pringle S., Maimets M., van der Zwaag M., et al. Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cell. 2016;34(3):640–652. doi: 10.1002/stem.2278. [DOI] [PubMed] [Google Scholar]
- 10.Li X., Nadauld L., Ootani A., et al. Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat Med. 2014;20(7):769–777. doi: 10.1038/nm.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan H., Demirci U., Chen P. Emerging organoid models: leaping forward in cancer research. J Hematol Oncol. 2019;12:142. doi: 10.1186/s13045-019-0832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Driehuis E., Kretzschmar K., Clevers H. Establishment of patient-derived cancer organoids for drug-screening applications. Nat Protoc. 2020;15(10):3380–3409. doi: 10.1038/s41596-020-0379-4. [DOI] [PubMed] [Google Scholar]
- 13.Gao D., Vela I., Sboner A., et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159(1):176–187. doi: 10.1016/j.cell.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sachs N., de Ligt J., Kopper O., et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172(1–2):373–386. doi: 10.1016/j.cell.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Dekkers J.F., van Vliet E.J., Sachs N., et al. Long-term culture, genetic manipulation and xenotransplantation of human normal and breast cancer organoids. Nat Protoc. 2021;16(4):1936–1965. doi: 10.1038/s41596-020-00474-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullenders J., de Jongh E., Brousali A., et al. Mouse and human urothelial cancer organoids: a tool for bladder cancer research. Proc Natl Acad Sci U S A. 2019;116(10):4567–4574. doi: 10.1073/pnas.1803595116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van de Wetering M., Francies H., Francis J., et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161(4):933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujii M., Shimokawa M., Date S., et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell. 2016;18(6):827–838. doi: 10.1016/j.stem.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Francies H.E., Barthorpe A., McLaren-Douglas A., et al. Drug sensitivity assays of human cancer organoid cultures. Methods Mol Biol. 2019;1576:339–351. doi: 10.1007/7651_2016_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartfeld S., Bayram T., van de Wetering M., et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 2015;148(1):126–136. doi: 10.1053/j.gastro.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z., Guo Y., Jin Y., et al. Establishment and drug screening of patient-derived extrahepatic biliary tract carcinoma organoids. Cancer Cell Int. 2021;21:519. doi: 10.1186/s12935-021-02219-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan B., Zhao X., Wang X., et al. Patient-derived organoids for personalized gallbladder cancer modelling and drug screening. Clin Transl Med. 2022;12(1):e678. doi: 10.1002/ctm2.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubert C.G., Rivera M., Spangler L.C., et al. A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Cancer Res. 2016;76(8):2465–2477. doi: 10.1158/0008-5472.CAN-15-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacob F., Salinas R.D., Zhang D.Y., et al. A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell. 2020;180(1):188–204. doi: 10.1016/j.cell.2019.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Driehuis E., Kolders S., Spelier S., et al. Oral mucosal organoids as a potential platform for personalized cancer therapy. Cancer Discov. 2019;9(7):852–871. doi: 10.1158/2159-8290.CD-18-1522. [DOI] [PubMed] [Google Scholar]
- 26.Schutgens F., Rookmaaker M.B., Margaritis T., et al. Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat Biotechnol. 2019;37(3):303–313. doi: 10.1038/s41587-019-0048-8. [DOI] [PubMed] [Google Scholar]
- 27.Calandrini C., Schutgens F., Oka R., et al. An organoid biobank for childhood kidney cancers that captures disease and tissue heterogeneity. Nat Commun. 2020;11:1310. doi: 10.1038/s41467-020-15155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huch M., Gehart H., van Boxtel R., et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160(1–2):299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broutier L., Mastrogiovanni G., Verstegen M.M., et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23(12):1424–1435. doi: 10.1038/nm.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim M., Mun H., Sung C.O., et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun. 2019;10:3991. doi: 10.1038/s41467-019-11867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi R., Radulovich N., Ng C., et al. Organoid cultures as preclinical models of non-small cell lung cancer. Clin Cancer Res. 2020;26(5):1162–1174. doi: 10.1158/1078-0432.CCR-19-1376. [DOI] [PubMed] [Google Scholar]
- 32.Kopper O., de Witte C.J., Lõhmussaar K., et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat Med. 2019;25(5):838–849. doi: 10.1038/s41591-019-0422-6. [DOI] [PubMed] [Google Scholar]
- 33.Hill S.J., Decker B., Roberts E.A., et al. Prediction of DNA repair inhibitor response in short-term patient-derived ovarian cancer organoids. Cancer Discov. 2018;8(11):1404–1421. doi: 10.1158/2159-8290.CD-18-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boj S.F., Hwang C.I., Baker L.A., et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160(1–2):324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang L., Holtzinger A., Jagan I., et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med. 2015;21(11):1364–1371. doi: 10.1038/nm.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karkampouna S., La Manna F., Benjak A., et al. Patient-derived xenografts and organoids model therapy response in prostate cancer. Nat Commun. 2021;12:1117. doi: 10.1038/s41467-021-21300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weeber F., van de Wetering M., Hoogstraat M., et al. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc Natl Acad Sci U S A. 2015;112(43):13308–13311. doi: 10.1073/pnas.1516689112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sachs N., Clevers H. Organoid cultures for the analysis of cancer phenotypes. Curr Opin Genet Dev. 2014;24:68–73. doi: 10.1016/j.gde.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Bhatia S., Kramer M., Russo S., et al. Patient-derived triple-negative breast cancer organoids provide robust model systems that recapitulate tumor intrinsic characteristics. Cancer Res. 2022;82(7):1174–1192. doi: 10.1158/0008-5472.CAN-21-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo X., She J., Xu T., et al. Establishment and characterization of organoids from a patient with adenomyoepithelioma of the breast. Bioengineered. 2021;12(2):11578–11585. doi: 10.1080/21655979.2021.1974809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X., Pan B., Song X., et al. Breast cancer organoids from a patient with giant papillary carcinoma as a high-fidelity model. Cancer Cell Int. 2020;20:86. doi: 10.1186/s12935-020-01171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan B., Zhao D., Liu Y., et al. Establishment and characterization of breast cancer organoids from a patient with mammary Paget's disease. Cancer Cell Int. 2020;20:365. doi: 10.1186/s12935-020-01459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan B., Zhao D., Liu Y., et al. Breast cancer organoids from malignant pleural effusion-derived tumor cells as an individualized medicine platform. In Vitro Cell Dev Biol Anim. 2021;57(5):510–518. doi: 10.1007/s11626-021-00563-9. [DOI] [PubMed] [Google Scholar]
- 44.Dekkers J.F., Whittle J.R., Vaillant F., et al. Modeling breast cancer using CRISPR-Cas9-mediated engineering of human breast organoids. J Natl Cancer Inst. 2020;112(5):540–544. doi: 10.1093/jnci/djz196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen P., Zhang X., Ding R., et al. Patient-derived organoids can guide personalized-therapies for patients with advanced breast cancer. Adv Sci. 2021;8(22) doi: 10.1002/advs.202101176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan B., Li X., Zhao D., et al. Optimizing individualized treatment strategy based on breast cancer organoid model. Clin Transl Med. 2021;11(4) doi: 10.1002/ctm2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao N., Powell R.T., Yuan X., et al. Morphological screening of mesenchymal mammary tumor organoids to identify drugs that reverse epithelial-mesenchymal transition. Nat Commun. 2021;12:4262. doi: 10.1038/s41467-021-24545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carter M.E., Hartkopf A.D., Wagner A., et al. A three-dimensional organoid model of primary breast cancer to investigate the effects of oncolytic virotherapy. Front Mol Biosci. 2022;9:826302. doi: 10.3389/fmolb.2022.826302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai E.Y., Garcia J., Liu Y., et al. A bladdercancerpatient-derived xenograft displays aggressive growth dynamics in vivo and in organoid culture. Sci Rep. 2021;11:4609. doi: 10.1038/s41598-021-83662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gong Z., Huang L., Tang X., et al. Acoustic droplet printing tumor organoids for modeling bladder tumor immune microenvironment within a week. Adv Healthc Mater. 2021;10(22) doi: 10.1002/adhm.202101312. [DOI] [PubMed] [Google Scholar]
- 51.Kong J., Lee H., Kim D., et al. Network-based machine learning in colorectal and bladder organoid models predicts anti-cancer drug efficacy in patients. Nat Commun. 2020;11:5485. doi: 10.1038/s41467-020-19313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu L., Li Z., Mei H., et al. Patient-derived organoids of bladder cancer recapitulate antigen expression profiles and serve as a personal evaluation model for CAR-T cells in vitro. Clin Transl Immunology. 2021;10(2):e1248. doi: 10.1002/cti2.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan H.H.N., Siu H.C., Ho S.L., et al. Organoid cultures of early-onset colorectal cancers reveal distinct and rare genetic profiles. Gut. 2020;69(12):2165–2179. doi: 10.1136/gutjnl-2019-320019. [DOI] [PubMed] [Google Scholar]
- 54.Li H., Dai W., Xia X., et al. Modeling tumor development and metastasis using paired organoids derived from patients with colorectal cancer liver metastases. J Hematol Oncol. 2020;13:119. doi: 10.1186/s13045-020-00957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao H., Yan C., Hu Y., et al. Differentiated cancer cell-originated lactate promotes the self-renewal of cancer stem cells in patient-derived colorectal cancer organoids. Cancer Lett. 2020;493:236–244. doi: 10.1016/j.canlet.2020.08.044. [DOI] [PubMed] [Google Scholar]
- 56.Costales-Carrera A., Fernández-Barral A., Bustamante-Madrid P., et al. Comparative study of organoids from patient-derived normal and tumor colon and rectal tissue. Cancers. 2020;12(8):2302. doi: 10.3390/cancers12082302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu X., Zhang L., Li Y., et al. Organoid modelling identifies that DACH1 functions as a tumour promoter in colorectal cancer by modulating BMP signalling. EBioMedicine. 2020;56:102800. doi: 10.1016/j.ebiom.2020.102800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Post J.B., Roodhart J.M.L., Snippert H.J.G. Colorectal cancer modeling with organoids: discriminating between oncogenic RAS and BRAF variants. Trends Cancer. 2020;6(2):111–129. doi: 10.1016/j.trecan.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 59.Bruun J., Kryeziu K., Eide P.W., et al. Patient-derived organoids from multiple colorectal cancer liver metastases reveal moderate intra-patient pharmacotranscriptomic heterogeneity. Clin Cancer Res. 2020;26(15):4107–4119. doi: 10.1158/1078-0432.CCR-19-3637. [DOI] [PubMed] [Google Scholar]
- 60.Nagai H., Kuroha M., Handa T., et al. Comprehensive analysis of microRNA profiles in organoids derived from human colorectal adenoma and cancer. Digestion. 2021;102(6):860–869. doi: 10.1159/000513882. [DOI] [PubMed] [Google Scholar]
- 61.Luo X., Fong E.L.S., Zhu C., et al. Hydrogel-based colorectal cancer organoid co-culture models. Acta Biomater. 2021;132:461–472. doi: 10.1016/j.actbio.2020.12.037. [DOI] [PubMed] [Google Scholar]
- 62.Cho Y.W., Min D.W., Kim H.P., et al. Patient-derived organoids as a preclinical platform for precision medicine in colorectal cancer. Mol Oncol. 2022;16(12):2396–2412. doi: 10.1002/1878-0261.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Q., Sun H., Luo D., et al. Lnc-RP11-536 K7.3/SOX2/HIF-1α signaling axis regulates oxaliplatin resistance in patient-derived colorectal cancer organoids. J Exp Clin Cancer Res. 2021;40:348. doi: 10.1186/s13046-021-02143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yao Y., Xu X., Yang L., et al. Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell. 2020;26(1):17–26. doi: 10.1016/j.stem.2019.10.010. e6. [DOI] [PubMed] [Google Scholar]
- 65.Engel R.M., Chan W.H., Nickless D., et al. Patient-derived colorectal cancer organoids upregulate revival stem cell marker genes following chemotherapeutic treatment. J Clin Med. 2020;9(1):128. doi: 10.3390/jcm9010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ooft S.N., Weeber F., Schipper L., et al. Prospective experimental treatment of colorectal cancer patients based on organoid drug responses. ESMO Open. 2021;6(3):100103. doi: 10.1016/j.esmoop.2021.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jung Y.H., Choi D.H., Park K., et al. Drug screening by uniform patient derived colorectal cancer hydro-organoids. Biomaterials. 2021;276 doi: 10.1016/j.biomaterials.2021.121004. [DOI] [PubMed] [Google Scholar]
- 68.Norkin M., Ordóñez-Morán P., Huelsken J. High-content, targeted RNA-seq screening in organoids for drug discovery in colorectal cancer. Cell Rep. 2021;35(3):109026. doi: 10.1016/j.celrep.2021.109026. [DOI] [PubMed] [Google Scholar]
- 69.Ukai S., Honma R., Sakamoto N., et al. Molecular biological analysis of 5-FU-resistant gastric cancer organoids; KHDRBS3 contributes to the attainment of features of cancer stem cell. Oncogene. 2020;39(50):7265–7278. doi: 10.1038/s41388-020-01492-9. [DOI] [PubMed] [Google Scholar]
- 70.Harada K., Sakamoto N., Ukai S., et al. Establishment of oxaliplatin-resistant gastric cancer organoids: importance of myoferlin in the acquisition of oxaliplatin resistance. Gastric Cancer. 2021;24(6):1264–1277. doi: 10.1007/s10120-021-01206-4. [DOI] [PubMed] [Google Scholar]
- 71.Koh V., Chakrabarti J., Torvund M., et al. Hedgehog transcriptional effector GLI mediates mTOR-Induced PD-L1 expression in gastric cancer organoids. Cancer Lett. 2021;518:59–71. doi: 10.1016/j.canlet.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chakrabarti J., Koh V., Steele N., et al. Disruption of Her2-induced PD-L1 inhibits tumor cell immune evasion in patient-derived gastric cancer organoids. Cancers. 2021;13(24):6158. doi: 10.3390/cancers13246158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lo Y.H., Kolahi K.S., Du Y., et al. A CRISPR/Cas9-engineered ARID1A-deficient human gastric cancer organoid model reveals essential and nonessential modes of oncogenic transformation. Cancer Discov. 2021;11(6):1562–1581. doi: 10.1158/2159-8290.CD-20-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li H., Wang C., Lan L., et al. PARP1 inhibitor combined with oxaliplatin efficiently suppresses oxaliplatin resistance in gastric cancer-derived organoids via homologous recombination and the base excision repair pathway. Front Cell Dev Biol. 2021;9:719192. doi: 10.3389/fcell.2021.719192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ku C.C., Wuputra K., Pan J.B., et al. Generation of human stomach cancer iPSC-derived organoids induced by Helicobacter pylori infection and their application to gastric cancer research. Cells. 2022;11(2):184. doi: 10.3390/cells11020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li G., Ma S., Wu Q., et al. Establishment of gastric signet ring cell carcinoma organoid for the therapeutic drug testing. Cell Death Dis. 2022;8:6. doi: 10.1038/s41420-021-00803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang S., Wang Y., Xun X., et al. Hedgehog signaling promotes sorafenib resistance in hepatocellular carcinoma patient-derived organoids. J Exp Clin Cancer Res. 2020;39:22. doi: 10.1186/s13046-020-1523-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu J., Li P., Wang L., et al. Cancer-associated fibroblasts provide a stromal niche for liver cancer organoids that confers trophic effects and therapy resistance. Cell Mol Gastroenterol Hepatol. 2021;11(2):407–431. doi: 10.1016/j.jcmgh.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dost A.F.M., Moye A.L., Vedaie M., et al. Organoids model transcriptional hallmarks of oncogenic KRAS activation in lung epithelial progenitor cells. Cell Stem Cell. 2020;27(4):663–678. doi: 10.1016/j.stem.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim S.Y., Kim S.M., Lim S., et al. Modeling clinical responses to targeted therapies by patient-derived organoids of advanced lung adenocarcinoma. Clin Cancer Res. 2021;27(15):4397–4409. doi: 10.1158/1078-0432.CCR-20-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma X., Yang S., Jiang H., et al. Transcriptomic analysis of tumor tissues and organoids reveals the crucial genes regulating the proliferation of lung adenocarcinoma. J Transl Med. 2021;19:368. doi: 10.1186/s12967-021-03043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miura A., Yamada D., Nakamura M., et al. Oncogenic potential of human pluripotent stem cell-derived lung organoids with HER2 overexpression. Int J Cancer. 2021;149(8):1593–1604. doi: 10.1002/ijc.33713. [DOI] [PubMed] [Google Scholar]
- 83.Choi S.Y., Cho Y.H., Kim D.S., et al. Establishment and long-term expansion of small cell lung cancer patient-derived tumor organoids. Int J Mol Sci. 2021;22(3):1349. doi: 10.3390/ijms22031349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lu T., Cao Y., Zhao P., et al. Organoid: a powerful tool to study lung regeneration and disease. Cell Regen. 2021;10:21. doi: 10.1186/s13619-021-00082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dijkstra K.K., Monkhorst K., Schipper L.J., et al. Challenges in establishing pure lung cancer organoids limit their utility for personalized medicine. Cell Rep. 2020;31(5):107588. doi: 10.1016/j.celrep.2020.107588. [DOI] [PubMed] [Google Scholar]
- 86.Gmeiner W.H., Miller L.D., Chou J.W., et al. Dysregulated pyrimidine biosynthesis contributes to 5-FU resistance in SCLC patient-derived organoids but response to a novel polymeric fluoropyrimidine, CF10. Cancers. 2020;12(4):788. doi: 10.3390/cancers12040788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen J.H., Chu X.P., Zhang J.T., et al. Genomic characteristics and drug screening among organoids derived from non-small cell lung cancer patients. Thorac Cancer. 2020;11(8):2279–2290. doi: 10.1111/1759-7714.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoffmann K., Berger H., Kulbe H., et al. Stable expansion of high-grade serous ovarian cancer organoids requires a low-Wnt environment. EMBO J. 2020;39(6) doi: 10.15252/embj.2019104013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen H., Gotimer K., De Souza C., et al. Short-term organoid culture for drug sensitivity testing of high-grade serous carcinoma. Gynecol Oncol. 2020;157(3):783–792. doi: 10.1016/j.ygyno.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nanki Y., Chiyoda T., Hirasawa A., et al. Patient-derived ovarian cancer organoids capture the genomic profiles of primary tumours applicable for drug sensitivity and resistance testing. Sci Rep. 2020;10:12581. doi: 10.1038/s41598-020-69488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Witte C.J., Espejo Valle-Inclan J., Hami N., et al. Patient-derived ovarian cancer organoids mimic clinical response and exhibit heterogeneous inter- and intrapatient drug responses. Cell Rep. 2020;31(11):107762. doi: 10.1016/j.celrep.2020.107762. [DOI] [PubMed] [Google Scholar]
- 92.Bi J., Newtson A.M., Zhang Y., et al. Successful patient-derived organoid culture of gynecologic cancers for disease modeling and drug sensitivity testing. Cancers. 2021;13(12):2901. doi: 10.3390/cancers13122901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang S., Iyer S., Ran H., et al. Genetically defined, syngeneic organoid platform for developing combination therapies for ovarian cancer. Cancer Discov. 2021;11(2):362–383. doi: 10.1158/2159-8290.CD-20-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gorski J.W., Zhang Z., McCorkle J.R., et al. Utilizing patient-derived epithelial ovarian cancer tumor organoids to predict carboplatin resistance. Biomedicines. 2021;9(8):1021. doi: 10.3390/biomedicines9081021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nelson S.R., Zhang C., Roche S., et al. Modelling of pancreatic cancer biology: transcriptomic signature for 3D PDX-derived organoids and primary cell line organoid development. Sci Rep. 2020;10:2778. doi: 10.1038/s41598-020-59368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang W., Navarro-Serer B., Jeong Y.J., et al. Pattern of invasion in human pancreatic cancer organoids is associated with loss of SMAD4 and clinical outcome. Cancer Res. 2020;80(13):2804–2817. doi: 10.1158/0008-5472.CAN-19-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Holokai L., Chakrabarti J., Lundy J., et al. Murine- and human-derived autologous organoid/immune cell Co-cultures as pre-clinical models of pancreatic ductal adenocarcinoma. Cancers. 2020;12(12):3816. doi: 10.3390/cancers12123816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matsuura T., Maru Y., Izumiya M., et al. Organoid-based ex vivo reconstitution of Kras-driven pancreatic ductal carcinogenesis. Carcinogenesis. 2020;41(4):490–501. doi: 10.1093/carcin/bgz122. [DOI] [PubMed] [Google Scholar]
- 99.Braun L.M., Lagies S., Klar R.F.U., et al. Metabolic profiling of early and late recurrent pancreatic ductal adenocarcinoma using patient-derived organoid cultures. Cancers. 2020;12(6):1440. doi: 10.3390/cancers12061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Choi J.I., Jang S.I., Hong J., et al. Cancer-initiating cells in human pancreatic cancer organoids are maintained by interactions with endothelial cells. Cancer Lett. 2021;498:42–53. doi: 10.1016/j.canlet.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 101.Lacomb J.F., Plenker D., Tiriac H., et al. Single-pass vs 2-pass endoscopic ultrasound-guided fine-needle biopsy sample collection for creation of pancreatic adenocarcinoma organoids. Clin Gastroenterol Hepatol. 2021;19(4):845–847. doi: 10.1016/j.cgh.2020.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raimondi G., Mato-Berciano A., Pascual-Sabater S., et al. Patient-derived pancreatic tumour organoids identify therapeutic responses to oncolytic adenoviruses. EBioMedicine. 2020;56:102786. doi: 10.1016/j.ebiom.2020.102786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Palzer J., Mues B., Goerg R., et al. Magnetic fluid hyperthermia as treatment option for pancreatic cancer cells and pancreatic cancer organoids. Int J Nanomed. 2021;16:2965–2981. doi: 10.2147/IJN.S288379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang B., Trujillo M.A., Fujikura K., et al. Molecular characterization of organoids derived from pancreatic intraductal papillary mucinous neoplasms. J Pathol. 2020;252(3):252–262. doi: 10.1002/path.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beato F., Reverón D., Dezsi K.B., et al. Establishing a living biobank of patient-derived organoids of intraductal papillary mucinous neoplasms of the pancreas. Lab Invest. 2021;101(2):204–217. doi: 10.1038/s41374-020-00494-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dantes Z., Yen H.Y., Pfarr N., et al. Implementing cell-free DNA of pancreatic cancer patient-derived organoids for personalized oncology. JCI Insight. 2020;5(15) doi: 10.1172/jci.insight.137809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Servant R., Garioni M., Vlajnic T., et al. Prostate cancer patient-derived organoids: detailed outcome from a prospective cohort of 81 clinical specimens. J Pathol. 2021;254(5):543–555. doi: 10.1002/path.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heninger E., Kosoff D., Rodems T.S., et al. Live cell molecular analysis of primary prostate cancer organoids identifies persistent androgen receptor signaling. Med Oncol. 2021;38(11):135. doi: 10.1007/s12032-021-01582-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Choo N., Ramm S., Luu J., et al. High-throughput imaging assay for drug screening of 3D prostate cancer organoids. SLAS Discov. 2021;26(9):1107–1124. doi: 10.1177/24725552211020668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kijima T., Nakagawa H., Shimonosono M., et al. Three-dimensional organoids reveal therapy resistance of esophageal and oropharyngeal squamous cell carcinoma cells. Cell Mol Gastroenterol Hepatol. 2019;7(1):73–91. doi: 10.1016/j.jcmgh.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Derouet M.F., Allen J., Wilson G.W., et al. Towards personalized induction therapy for esophageal adenocarcinoma: organoids derived from endoscopic biopsy recapitulate the pre-treatment tumor. Sci Rep. 2020;10:14514. doi: 10.1038/s41598-020-71589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karakasheva T.A., Gabre J.T., Sachdeva U.M., et al. Patient-derived organoids as a platform for modeling a patient's response to chemoradiotherapy in esophageal cancer. Sci Rep. 2021;11:21304. doi: 10.1038/s41598-021-00706-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Driehuis E., Spelier S., Beltrán Hernández I., et al. Patient-derived head and neck cancer organoids recapitulate EGFR expression levels of respective tissues and are responsive to EGFR-targeted photodynamic therapy. J Clin Med. 2019;8(11):1880. doi: 10.3390/jcm8111880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Krieger T.G., Tirier S.M., Park J., et al. Modeling glioblastoma invasion using human brain organoids and single-cell transcriptomics. Neuro Oncol. 2020;22(8):1138–1149. doi: 10.1093/neuonc/noaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jacob F., Ming G.L., Song H. Generation and biobanking of patient-derived glioblastoma organoids and their application in CAR T cell testing. Nat Protoc. 2020;15(12):4000–4033. doi: 10.1038/s41596-020-0402-9. [DOI] [PubMed] [Google Scholar]
- 116.Darrigues E., Zhao E.H., De Loose A., et al. Biobanked glioblastoma patient-derived organoids as a precision medicine model to study inhibition of invasion. Int J Mol Sci. 2021;22(19):10720. doi: 10.3390/ijms221910720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Golebiewska A., Hau A.C., Oudin A., et al. Patient-derived organoids and orthotopic xenografts of primary and recurrent gliomas represent relevant patient avatars for precision oncology. Acta Neuropathol. 2020;140(6):919–949. doi: 10.1007/s00401-020-02226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pon J.R., Marra M.A. Driver and passenger mutations in cancer. Annu Rev Pathol. 2015;10:25–50. doi: 10.1146/annurev-pathol-012414-040312. [DOI] [PubMed] [Google Scholar]
- 119.Helleday T., Eshtad S., Nik-Zainal S. Mechanisms underlying mutational signatures in human cancers. Nat Rev Genet. 2014;15(9):585–598. doi: 10.1038/nrg3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guernet A., Grumolato L. CRISPR/Cas9 editing of the genome for cancer modeling. Methods. 2017;121–122:130–137. doi: 10.1016/j.ymeth.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 121.Drost J., van Jaarsveld R.H., Ponsioen B., et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature. 2015;521(7550):43–47. doi: 10.1038/nature14415. [DOI] [PubMed] [Google Scholar]
- 122.Matano M., Date S., Shimokawa M., et al. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med. 2015;21(3):256–262. doi: 10.1038/nm.3802. [DOI] [PubMed] [Google Scholar]
- 123.Naruse M., Masui R., Ochiai M., et al. An organoid-based carcinogenesis model induced by in vitro chemical treatment. Carcinogenesis. 2020;41(10):1444–1453. doi: 10.1093/carcin/bgaa011. [DOI] [PubMed] [Google Scholar]
- 124.Han H., Davidson L.A., Fan Y.Y., et al. Loss of aryl hydrocarbon receptor suppresses the response of colonic epithelial cells to IL22 signaling by upregulating SOCS3. Am J Physiol Gastrointest Liver Physiol. 2022;322(1):G93–G106. doi: 10.1152/ajpgi.00074.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Artegiani B., Hendriks D., Beumer J., et al. Fast and efficient generation of knock-in human organoids using homology-independent CRISPR-Cas9 precision genome editing. Nat Cell Biol. 2020;22(3):321–331. doi: 10.1038/s41556-020-0472-5. [DOI] [PubMed] [Google Scholar]
- 126.Hendriks D., Artegiani B., Hu H., et al. Establishment of human fetal hepatocyte organoids and CRISPR-Cas9-based gene knockin and knockout in organoid cultures from human liver. Nat Protoc. 2021;16(1):182–217. doi: 10.1038/s41596-020-00411-2. [DOI] [PubMed] [Google Scholar]
- 127.Steeg P.S. Targeting metastasis. Nat Rev Cancer. 2016;16(4):201–218. doi: 10.1038/nrc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roukens M.G., Frederiks C.L., Seinstra D., et al. Regulation of a progenitor gene program by SOX4 is essential for mammary tumor proliferation. Oncogene. 2021;40(45):6343–6353. doi: 10.1038/s41388-021-02004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hay M., Thomas D.W., Craighead J.L., et al. Clinical development success rates for investigational drugs. Nat Biotechnol. 2014;32(1):40–51. doi: 10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]
- 130.Voskoglou-Nomikos T., Pater J.L., Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9(11):4227–4239. [PubMed] [Google Scholar]
- 131.Vlachogiannis G., Hedayat S., Vatsiou A., et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359(6378):920–926. doi: 10.1126/science.aao2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Saito Y., Muramatsu T., Kanai Y., et al. Establishment of patient-derived organoids and drug screening for biliary tract carcinoma. Cell Rep. 2019;27(4):1265–1276. doi: 10.1016/j.celrep.2019.03.088. [DOI] [PubMed] [Google Scholar]
- 133.Boos S.L., Loevenich L.P., Vosberg S., et al. Disease modeling on tumor organoids implicates AURKA as a therapeutic target in liver metastatic colorectal cancer. Cell Mol Gastroenterol Hepatol. 2022;13(2):517–540. doi: 10.1016/j.jcmgh.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sánchez-Botet A., Quandt E., Masip N., et al. Atypical cyclin P regulates cancer cell stemness through activation of the WNT pathway. Cell Oncol. 2021;44(6):1273–1286. doi: 10.1007/s13402-021-00636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Verissimo C.S., Overmeer R.M., Ponsioen B., et al. Targeting mutant RAS in patient-derived colorectal cancer organoids by combinatorial drug screening. Elife. 2016;5 doi: 10.7554/eLife.18489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang T., Pan W., Zheng H., et al. Accuracy of using a patient-derived tumor organoid culture model to predict the response to chemotherapy regimens in stage IV colorectal cancer: a blinded study. Dis Colon Rectum. 2021;64(7):833–850. doi: 10.1097/DCR.0000000000001971. [DOI] [PubMed] [Google Scholar]
- 137.Costales-Carrera A., Fernández-Barral A., Bustamante-Madrid P., et al. Plocabulin displays strong cytotoxic activity in a personalized colon cancer patient-derived 3D organoid assay. Mar Drugs. 2019;17(11):648. doi: 10.3390/md17110648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Katsuda T., Kawamata M., Hagiwara K., et al. Conversion of terminally committed hepatocytes to culturable bipotent progenitor cells with regenerative capacity. Cell Stem Cell. 2017;20(1):41–55. doi: 10.1016/j.stem.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 139.Mekky G., Seeds M., Diab A.E.A., et al. The potential toxic effects of magnesium oxide nanoparticles and valproate on liver tissue. J Biochem Mol Toxicol. 2021;35(3) doi: 10.1002/jbt.22676. [DOI] [PubMed] [Google Scholar]
- 140.Shinozawa T., Kimura M., Cai Y., et al. High-fidelity drug-induced liver injury screen using human pluripotent stem cell–derived organoids. Gastroenterology. 2021;160(3):831–846. doi: 10.1053/j.gastro.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Richards D.J., Li Y., Kerr C.M., et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat Biomed Eng. 2020;4(4):446–462. doi: 10.1038/s41551-020-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee J., Sutani A., Kaneko R., et al. In vitro generation of functional murine heart organoids via FGF4 and extracellular matrix. Nat Commun. 2020;11:4283. doi: 10.1038/s41467-020-18031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee S.J., Kim H.A., Kim S.J., et al. Improving generation of cardiac organoids from human pluripotent stem cells using the aurora kinase inhibitor ZM447439. Biomedicines. 2021;9(12):1952. doi: 10.3390/biomedicines9121952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Freedman B.S., Brooks C.R., Lam A.Q., et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun. 2015;6:8715. doi: 10.1038/ncomms9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Galluzzi L., Vacchelli E., Pedro J.M.B.S., et al. Classification of current anticancer immunotherapies. Oncotarget. 2014;5(24):12472–12508. doi: 10.18632/oncotarget.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Waldman A.D., Fritz J.M., Lenardo M.J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Palucka A., Coussens L. The basis of oncoimmunology. Cell. 2016;164(6):1233–1247. doi: 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Christofi T., Baritaki S., Falzone L., et al. Current perspectives in cancer immunotherapy. Cancers. 2019;11(10):1472. doi: 10.3390/cancers11101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dijkstra K.K., Cattaneo C.M., Weeber F., et al. Generation of tumor-reactive T cells by Co-culture of peripheral blood lymphocytes and tumor organoids. Cell. 2018;174(6):1586–1598. doi: 10.1016/j.cell.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jenkins R.W., Aref A.R., Lizotte P.H., et al. Ex vivo profiling of PD-1 blockade using organotypic tumor spheroids. Cancer Discov. 2018;8(2):196–215. doi: 10.1158/2159-8290.CD-17-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Neal J.T., Li X., Zhu J., et al. Organoid modeling of the tumor immune microenvironment. Cell. 2018;175(7):1972–1988. doi: 10.1016/j.cell.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen J., Sun H.W., Yang Y.Y., et al. Reprogramming immunosuppressive myeloid cells by activated T cells promotes the response to anti-PD-1 therapy in colorectal cancer. Signal Transduct Target Ther. 2021;6(1):4. doi: 10.1038/s41392-020-00377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wörsdörfer P., Dalda N., Kern A., et al. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci Rep. 2019;9(1):15663. doi: 10.1038/s41598-019-52204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Grebenyuk S., Ranga A. Engineering organoid vascularization. Front Bioeng Biotechnol. 2019;7:39. doi: 10.3389/fbioe.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nashimoto Y., Hayashi T., Kunita I., et al. Integrating perfusable vascular networks with a three-dimensional tissue in a microfluidic device. Integr Biol. 2017;9(6):506–518. doi: 10.1039/c7ib00024c. [DOI] [PubMed] [Google Scholar]
- 156.Rodewald H.R. Thymus organogenesis. Annu Rev Immunol. 2008;26:355–388. doi: 10.1146/annurev.immunol.26.021607.090408. [DOI] [PubMed] [Google Scholar]
- 157.Farley A.M., Morris L.X., Vroegindeweij E., et al. Dynamics of thymus organogenesis and colonization in early human development. Development. 2013;140(9):2015–2026. doi: 10.1242/dev.087320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Robin C., Bennaceur-Griscelli A., Louache F., et al. Identification of human T-lymphoid progenitor cells in CD34+ CD38low and CD34+ CD38+ subsets of human cord blood and bone marrow cells using NOD-SCID fetal thymus organ cultures. Br J Haematol. 1999;104(4):809–819. doi: 10.1046/j.1365-2141.1999.01266.x. [DOI] [PubMed] [Google Scholar]
- 159.Deng Z., Liu H., Rui J., et al. Reconstituted Thymus organ culture. Methods Mol Biol. 2016;1323:151–158. doi: 10.1007/978-1-4939-2809-5_13. [DOI] [PubMed] [Google Scholar]
- 160.Selvaratnam J.S., T S.H., Anderson M.K. Fetal thymic organ culture (FTOC) optimized for gamma-delta T cell studies. Methods Mol Biol. 2022;2421:243–265. doi: 10.1007/978-1-0716-1944-5_17. [DOI] [PubMed] [Google Scholar]
- 161.Sharma H., Moroni L. Recent advancements in regenerative approaches for Thymus rejuvenation. Adv Sci. 2021;8(14):2100543. doi: 10.1002/advs.202100543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Seet C.S., He C., Bethune M.T., et al. Generation of mature T cells from human hematopoietic stem and progenitor cells in artificial thymic organoids. Nat Methods. 2017;14(5):521–530. doi: 10.1038/nmeth.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gardner C.L., Pavel-Dinu M., Dobbs K., et al. Gene editing rescues in vitro T cell development of RAG2-deficient induced pluripotent stem cells in an artificial thymic organoid system. J Clin Immunol. 2021;41(5):852–862. doi: 10.1007/s10875-021-00989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bosticardo M., Pala F., Calzoni E., et al. Artificial thymic organoids represent a reliable tool to study T-cell differentiation in patients with severe T-cell lymphopenia. Blood Adv. 2020;4(12):2611–2616. doi: 10.1182/bloodadvances.2020001730. Blood Adv. 2020;4(15):3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gasteiger G., Ataide M., Kastenmüller W. Lymph node - an organ for T-cell activation and pathogen defense. Immunol Rev. 2016;271(1):200–220. doi: 10.1111/imr.12399. [DOI] [PubMed] [Google Scholar]
- 166.Suematsu S., Watanabe T. Generation of a synthetic lymphoid tissue-like organoid in mice. Nat Biotechnol. 2004;22(12):1539–1545. doi: 10.1038/nbt1039. [DOI] [PubMed] [Google Scholar]
- 167.Votanopoulos K.I., Forsythe S., Sivakumar H., et al. Model of patient-specific immune-enhanced organoids for immunotherapy screening: feasibility study. Ann Surg Oncol. 2020;27(6):1956–1967. doi: 10.1245/s10434-019-08143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xia X., Zhou W., Guo C., et al. Glutaminolysis mediated by MALT1 protease activity facilitates PD-L1 expression on ABC-DLBCL cells and contributes to their immune evasion. Front Oncol. 2018;8:632. doi: 10.3389/fonc.2018.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lenz G., Davis R.E., Ngo V.N., et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319(5870):1676–1679. doi: 10.1126/science.1153629. [DOI] [PubMed] [Google Scholar]
- 170.Larkin J., Chiarion-Sileni V., Gonzalez R., et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 171.Migden M.R., Rischin D., Schmults C.D., et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 172.Socinski M.A., Jotte R.M., Cappuzzo F., et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 173.Motzer R.J., Escudier B., McDermott D.F., et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bins S., van Meerten E., Mathijssen R.H. Nivolumab for squamous-cell cancer of head and neck. N Engl J Med. 2017;376(6):595–596. doi: 10.1056/NEJMc1615565. [DOI] [PubMed] [Google Scholar]
- 175.Syn N.L., Teng M.W.L., Mok T.S.K., et al. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18(12):e731–e741. doi: 10.1016/S1470-2045(17)30607-1. [DOI] [PubMed] [Google Scholar]
- 176.Kong J.C.H., Guerra G.R., Millen R.M., et al. Tumor-infiltrating lymphocyte function predicts response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. JCO Precis Oncol. 2018;2:1–15. doi: 10.1200/PO.18.00075. [DOI] [PubMed] [Google Scholar]
- 177.Courau T., Bonnereau J., Chicoteau J., et al. Cocultures of human colorectal tumor spheroids with immune cells reveal the therapeutic potential of MICA/B and NKG2A targeting for cancer treatment. J Immunother Cancer. 2019;7:74. doi: 10.1186/s40425-019-0553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Deng J., Wang E.S., Jenkins R.W., et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 2018;8(2):216–233. doi: 10.1158/2159-8290.CD-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Esser L.K., Branchi V., Leonardelli S., et al. Cultivation of clear cell renal cell carcinoma patient-derived organoids in an air-liquid interface system as a tool for studying individualized therapy. Front Oncol. 2020;10:1775. doi: 10.3389/fonc.2020.01775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.June C.H., O'Connor R.S., Kawalekar O.U., et al. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 181.Schnalzger T.E., de Groot M.H., Zhang C., et al. 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J. 2019;38(12) doi: 10.15252/embj.2018100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zou F., Tan J., Liu T., et al. The CD39+ HBV surface protein-targeted CAR-T and personalized tumor-reactive CD8+ T cells exhibit potent anti-HCC activity. Mol Ther. 2021;29(5):1794–1807. doi: 10.1016/j.ymthe.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kaufman H.L., Kohlhapp F.J., Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2016;15(9):660. doi: 10.1038/nrd.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhu Z., Gorman M.J., McKenzie L.D., et al. Zika virus has oncolytic activity against glioblastoma stem cells. J Exp Med. 2017;214(10):2843–2857. doi: 10.1084/jem.20171093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hamdan F., Ylösmäki E., Chiaro J., et al. Novel oncolytic adenovirus expressing enhanced cross-hybrid IgGA Fc PD-L1 inhibitor activates multiple immune effector populations leading to enhanced tumor killing in vitro, in vivo and with patient-derived tumor organoids. J Immunother Cancer. 2021;9(8) doi: 10.1136/jitc-2021-003000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Karthaus W.R., Iaquinta P.J., Drost J., et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell. 2014;159(1):163–175. doi: 10.1016/j.cell.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yin S., Xi R., Wu A., et al. Patient-derived tumor-like cell clusters for drug testing in cancer therapy. Sci Transl Med. 2020;12(549):eaaz1723. doi: 10.1126/scitranslmed.aaz1723. [DOI] [PubMed] [Google Scholar]
- 188.Truong D.D., Kratz A., Park J.G., et al. A human organotypic microfluidic tumor model permits investigation of the interplay between patient-derived fibroblasts and breast cancer cells. Cancer Res. 2019;79(12):3139–3151. doi: 10.1158/0008-5472.CAN-18-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Park S.E., Georgescu A., Huh D. Organoids-on-a-chip. Science. 2019;364(6444):960–965. doi: 10.1126/science.aaw7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hu Y., Sui X., Song F., et al. Lung cancer organoids analyzed on microwell arrays predict drug responses of patients within a week. Nat Commun. 2021;12:2581. doi: 10.1038/s41467-021-22676-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shirure V.S., Bi Y., Curtis M.B., et al. Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids. Lab Chip. 2018;18(23):3687–3702. doi: 10.1039/c8lc00596f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Walsh A.J., Castellanos J.A., Nagathihalli N.S., et al. Optical imaging of drug-induced metabolism changes in murine and human pancreatic cancer organoids reveals heterogeneous drug response. Pancreas. 2016;45(6):863–869. doi: 10.1097/MPA.0000000000000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Walsh A.J., Cook R.S., Skala M.C. Functional optical imaging of primary human tumor organoids: development of a personalized drug screen. J Nucl Med. 2017;58(9):1367–1372. doi: 10.2967/jnumed.117.192534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gillette A.A., Babiarz C.P., VanDommelen A.R., et al. Autofluorescence imaging of treatment response in neuroendocrine tumor organoids. Cancers. 2021;13(8):1873. doi: 10.3390/cancers13081873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Reid J.A., Palmer X.L., Mollica P.A., et al. A 3D bioprinter platform for mechanistic analysis of tumoroids and chimeric mammary organoids. Sci Rep. 2019;9:7466. doi: 10.1038/s41598-019-43922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jiang S., Zhao H., Zhang W., et al. An automated organoid platform with inter-organoid homogeneity and inter-patient heterogeneity. Cell Rep Med. 2020;1(9):100161. doi: 10.1016/j.xcrm.2020.100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mazzucchelli S, Piccotti F, Allevi R, et al. Establishment and morphological characterization of patient-derived organoids from breast cancer. Biol Proced Online. 2019;21:12. doi: 10.1186/s12575-019-0099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lee SH, Hu W, Matulay JT, et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell. 2018;173(2):515–528.e17. doi: 10.1016/j.cell.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yan HHN, Siu HC, Law S, et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. 2018;23(6):882–897.e11. doi: 10.1016/j.stem.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 200.Li Y, Gao Y, Liang B, et al. Patient-derived organoids of non-small cells lung cancer and their application for drug screening. Neoplasma. 2020;67(2):430–437. doi: 10.4149/neo_2020_190417N346. [DOI] [PubMed] [Google Scholar]
- 201.Nuciforo S, Fofana I, Matter MS, et al. Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep. 2018;24(5):1363–1376. doi: 10.1016/j.celrep.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tiriac H, Belleau P, Engle DD, et al. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov. 2018;8(9):1112–1129. doi: 10.1158/2159-8290.CD-18-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Seino T, Kawasaki S, Shimokawa M, et al. Human pancreatic tumor organoids reveal loss of stem cell niche factor dependence during disease progression. Cell Stem Cell. 2018;22(3):454–467.e6. doi: 10.1016/j.stem.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 204.Kawasaki K, Toshimitsu K, Matano M, et al. An Organoid Biobank of Neuroendocrine Neoplasms Enables Genotype-Phenotype Mapping. Cell. 2020;183(5):1420–1435.e21. doi: 10.1016/j.cell.2020.10.023. [DOI] [PubMed] [Google Scholar]