Abstract
We report the first case of invasive disease caused by Fusarium chlamydosporum. The patient had aplastic anemia with prolonged neutropenia and was treated with immunosuppressive therapy. While she was receiving empirical amphotericin B, a dark crusted lesion developed on her nasal turbinate. Histologic analysis revealed invasive hyaline hyphae and some darkly pigmented structures that resembled conidia of dematiaceous molds. Only after the mold was grown in culture were characteristic colonial morphology, phialides, conidia, and chlamydospores evident, thus permitting the identification of F. chlamydosporum. This case illustrates the ever-increasing spectrum of pathogenic Fusarium spp. in immunocompromised patients and emphasizes the potential pitfalls in histologic diagnosis, which may have important treatment implications.
Fusarium species have emerged as a major cause of invasive disease and mortality among neutropenic patients (1–5, 8, 17, 19, 21, 23, 28). Fusarium spp. are common soil saprophytes and plant pathogens which, in humans, are mostly associated with superficial mycoses and keratitis. However, with the widespread use of intensive antineoplastic chemotherapy and bone marrow transplantation, more than 100 cases of invasive and disseminated infection caused by Fusarium spp. have been reported—most within the past 10 years.
Most cases of invasive fusariosis are caused by Fusarium solani, Fusarium oxysporum, and Fusarium moniliforme, but in about one-third the species is not identified (8, 17). Disseminated fusariosis commonly manifests with cutaneous lesions, pulmonary infiltrates, and, less often, sinusitis and involvement of the nasal cavity (17). In about one-half of cases of dissemination, Fusarium spp. are isolated from blood culture (5, 17). To our knowledge, the only reported case of human infection by Fusarium chlamydosporum was catheter-related fungemia in a patient with lymphoma (10).
Here, we report the first case of invasive tissue disease caused by F. chlamydosporum. The patient was severely immunocompromised due to aplastic anemia with prolonged neutropenia and was treated with immunosuppressive therapy. While she was receiving empirical amphotericin B for prolonged neutropenic fever, a lesion excised from her nasal turbinate showed invasive hyaline and darkly pigmented hyphal structures. This finding raised the suspicion of a dematiaceous mold, prompting the addition of itraconazole to the therapy. Subsequent evaluation of the mold grown in culture and correlation of the culture results with the histopathology confirmed the identification of F. chlamydosporum.
Case report.
The patient was a 40-year-old female with aplastic anemia who was treated with combination antithymocyte globulin (ATG) and cyclosporin A (CSA) at the National Heart, Lung, and Blood Institute at the Warren-Grant Magnuson Clinical Center, National Institutes of Health (NIH). Prior to transfer to the NIH, she had had fulminant hepatitis of unknown etiology, as well as prolonged neutropenic fever treated with broad-spectrum antibiotics. Approximately 3 weeks after the ATG-CSA protocol was started and while she was receiving empirical amphotericin B (0.5 mg/kg of body weight/day) for persistent neutropenic fever, a raised dark crusted lesion developed on her left middle nasal turbinate. Surgical excision was performed, and material was sent for histologic analysis. Biopsy material was inadvertently not sent for culture. The procedure was complicated by epistaxis requiring packing of the nasal cavity. The gauze used for packing was subsequently sent for culture. Histologic evaluation of the lesion revealed an invasive mold with hyaline as well as darkly pigmented hyphal structures. The appearance was nonspecific and did not permit diagnosis of the genus. The presence of pigment on routine hematoxylin and eosin preparations and Masson-Fontana silver stained materials raised the possibility of phaeohyphomycosis. The dosage of amphotericin B was increased to 1.0 mg/kg/day, and itraconazole (600 mg/day) was added to the schedule. Because of progressive azotemia, amphotericin B lipid complex (5 mg/kg/day) was substituted for amphotericin B. Approximately 2 weeks elapsed before the definitive diagnosis of F. chlamydosporum was made from culture (see below). Once the diagnosis was made, itraconazole was discontinued because of the known resistance of Fusarium spp. to this triazole.
An isolated sputum culture yielded F. chlamydosporum shortly after excision of the nasal lesion. There was no evidence of pulmonary disease on multiple chest radiographs or on a chest computed tomography scan. This isolate therefore most likely colonized the patient’s airway and was not a pulmonary pathogen. Serial computed tomography scans of the sinuses showed opacification of the maxillary, ethmoid, and sphenoid sinuses. However, no bone involvement or progression of disease was detected over the next several weeks. Otolaryngologic examinations showed no recurrence of disease in the nasal cavity. Multiple routine and fungal blood cultures were negative for Fusarium species. Thus, the fusariosis in this patient was considered to be localized and likely to have been cured by surgical excision.
The bone marrow failure did not improve with ATG-CSA treatment, and the patient underwent transplantation with T-cell-replete stem cells. Despite successful engraftment, the patient died of multiorgan failure 5 weeks after transplantation, with no evidence of residual fusariosis.
Mycological methods and diagnosis.
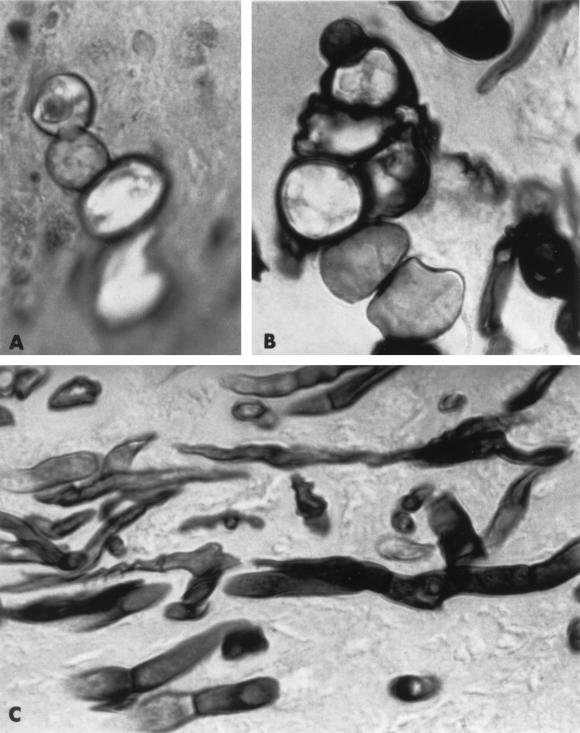
Material from the gauze packing in the nasal cavity was cultured on various mycological agar media. Within 48 h of incubation at 30°C, fluffy colonies of the same fungus grew on all media. The fungus was subcultured on Sabouraud agar and potato dextrose agar. Within 1 week, the isolate produced intensely pinkish red, floccose colonies 6 to 7 cm in diameter. It also grew well at 37°C, reaching a diameter of 4.5 cm in 7 days on potato dextrose agar. On a cornmeal agar slide culture, the isolate produced numerous one- to two-celled, clavate, oblong to fusiform microconidia (2.5 to 4 μm by 8 to 12 μm) directly on short and narrow phialides (denticles) on sympodially proliferating conidiophores (Fig. 1A). Canoe-shaped macroconidia of four or more cells, characteristic of the genus Fusarium, measuring 3 by 30 μm were observed only rarely on the cornmeal agar slide culture (Fig. 1B) and were not observed on the other culture media. Intercalary, smooth or slightly rough, brown-walled chlamydospores (Fig. 1C) were abundantly produced on all cultures in 7 to 10 days. The chlamydospores were mostly globose (6 to 15 μm in diameter) and formed in short chains, but some produced cross septae and became muriform (Fig. 1D). The colony reverse was faintly brown in the beginning but became dark brown as the culture aged due to the increasingly abundant, darkly pigmented chlamydospores.
FIG. 1.
F. chlamydosporum grown on cornmeal agar (magnification, ×1,200). (A) Conidiophores with sympodially proliferating phialides and microconidia; (B) a fusiform macroconidium with four cells and a microconidium with two cells; (C and D) chlamydospores.
Although such chlamydospore morphology was also seen in histopathological sections of the biopsy specimen (Fig. 2A and B), the histologic appearance was not specific enough for the identification of the etiologic agent. The morphology of the invasive hyphal structures in the submucosa was consistent with that of a Fusarium species (Fig. 2C), but the presence of darkly pigmented thick-walled chlamydospores in hematoxylin and eosin-stained materials raised the possibility of a dematiaceous mold. This possibility was strengthened by the results of Masson-Fontana staining. Not only the thick-walled chlamydospores but also portions of many hyphae morphologically consistent with a Fusarium species growing toward the periphery of tissue sections were positive for this staining (data not shown). Only with evaluation of the cultured mold and comparison with the histologic specimens was the diagnosis of invasive fusariosis established.
FIG. 2.
In vivo morphology of F. chlamydosporum (magnification, ×1,200). (A) Dark-walled chlamydospores seen in the hematoxylin and eosin-stained section; (B) muriform chlamydospore (Gomori methenamine-silver stained); (C) septate hyphae (Gomori methenamine-silver stained).
Thus, the isolate was identified as F. chlamydosporum on the basis of intensely pinkish red colonies, sympodially proliferating polyphialidic conidiophores, clavate to fusiform microconidia borne directly on the short and pointed phialides (denticles), numerous intercalary brown-walled chlamydospores, and rarity of macroconidial formation on agar media.
MICs were determined by broth macrodilution methods, as previously described (6). Briefly, a suspension was adjusted with a spectrophotometer to 68 to 71% transmission at a wavelength of 530 nm and diluted 100-fold to yield an inoculum of 1 × 104 to 5 × 104 CFU/ml. Amphotericin B and itraconazole were tested in 0.165 M morpholinepropanesulfonic acid (MOPS)-buffered RPMI 1640 (Bio-Whittaker, Walkersville, Md.) at a pH of 7.0. A 100-μl volume of concentrated antifungal compound was diluted 10-fold with 900 μl of inoculum suspension and incubated at 35°C for 24 and 48 h. The final concentration ranges for amphotericin B (Bristol-Myers Squibb, Princeton, N.J.) and itraconazole (Janssen Pharmaceutica, Piscataway, N.J.) were 0.03 to 16.0 μg/ml. The MIC was graded on a scale of 0 to 4+, with 0 being optically clear, 1+ being slightly hazy, 2+ being a 50% reduction of growth, 3+ being a 25% reduction of growth, and 4+ being equal to MIC for the growth control tube. The MIC was defined as the lowest concentration of antifungal compound which rendered no growth (0) for amphotericin B and a 50% reduction of growth (2+) for itraconazole. The minimum lethal concentration (MLC) was determined by dispensing and streaking 100 μl of broth from the first four tubes containing drug concentrations above the MIC exhibiting no growth onto Sabouraud glucose agar (NIH Media Department, Bethesda, Md.) and incubating it at 35°C. A concentration yielding growth of three or fewer colonies was considered fungicidal. The MLC was defined as the lowest concentration of antifungal compound yielding growth of three or fewer colonies. The MIC and MLC of amphotericin B were 0.5 and 1.0 μg/ml, respectively. The MIC of itraconazole was 1.0 μg/ml.
Discussion.
This is the first reported case of F. chlamydosporum causing invasive tissue disease. More commonly known pathogenic Fusarium species, such as F. solani and F. oxysporum, also produce chlamydospores in vitro, but these structures have not been seen in vivo. The production by F. chlamydosporum of pigmented chlamydospores in tissue in our case is likely an infection site-associated phenomenon rather than a species characteristic. Fungi may produce species-specific structures in tissue exposed to air, such as in the nasal cavity. For example, Aspergillus species produce conidiophores and cleistothecia only in tissue exposed to ambient air (12).
The histologic appearance of Fusarium spp. is variable and can mimic those of several other molds. Therefore, identification of Fusarium spp. requires culture and cannot rely solely on histologic morphology (19). However, as in our case, a significant amount of time may elapse before culture results are available. Thus, therapy under these circumstances must be empirical until a definitive diagnosis is made.
The clinical manifestations and histological appearance of Fusarium spp. may be indistinguishable from those of organisms causing invasive aspergillosis. Both genera infect profoundly immunocompromised patients, and both are associated with vascular invasion, tissue infarction, and hemorrhage. Recently, Liu et al. (14) reviewed biopsy and cytology specimens from culture-confirmed hyalohyphomycosis caused by Fusarium, Paecilomyces, or Acremonium species to identify histologic features that distinguish these molds from Aspergillus species. Sporadic phialide- and phialoconidium-like structures were present in 16 of 19 cases, including 7 of 10 cases of infection by a Fusarium species (14). Phialoconium-like structures seen in tissue were either spherical, oval, curved, or elliptical. These specialized structures may be helpful in alerting the pathologist to the possibility of a non-Aspergillus species but are not readily detected unless inspected with a 100× oil immersion lens (14). The authors point out that the presumptive histologic diagnosis should be confirmed by culture whenever possible (14). In the absence of definitive identification by culturing, the likelihood of infection with a Fusarium species is substantially increased if either widespread cutaneous dissemination or the isolation of the mold from a blood culture occurs (5).
The identification of a Fusarium species in a culture is difficult if macroconidia are not present (8). In these instances confusion with other genera, such as Acremonium, Cylindrocarpon, or Verticillium, may occur (8). Rarely, infection of the nasopharynx and sinuses by a Fusarium species may resemble rhinocerebral zygomycosis (27). Usually the distinction between these two molds can be made histologically because the hyphae of zygomycetes are wider, branch at right angles, and demonstrate a paucity of septations (27).
Typically, Fusarium spp. are not confused with dematiaceous molds. In our case, the presence of pigmented chlamydospores in histologic specimens stained with hematoxylin and eosin raised the suspicion of phaeohyphomycosis. The considerable number of dark-walled hyphae seen after Masson-Fontana staining further supported this presumptive, but incorrect, diagnosis.
Masson-Fontana staining for fungal cells was introduced by Kwon-Chung et al. (13) in an attempt to discern melanin formation by Cryptococcus neoformans in brain tissue. They observed that though Masson-Fontana staining was not specific for melanin, it was useful in differentiating cryptococcal cells from other yeast-like pathogens. The Masson-Fontana reagent stains any phenolic compound, including melanin. Since then, the staining has been frequently used for fungal histopathology to detect melanin-like pigment when the pathogen is suspected to be a dematiaceous mold that fails to produce brown-walled hyphae in tissue (4, 16, 29). It must be emphasized that Masson-Fontana staining is not specific for melanin and that hyphae without melanin can produce positive results as long as phenol compounds are present.
In patients with severe and prolonged neutropenia, early diagnosis of infection by a Fusarium species is important because of the high risk of dissemination. Disseminated fusariosis in this population is associated with a high mortality, and survival is dependent on the rapid recovery of the neutrophil count (17). As in our patient, localized disease may be cured with excision alone; however, we believe that in neutropenic patients, systemic antifungal therapy is warranted because of the high risk of clinically inapparent disseminated disease (20).
In a review of nasopharyngeal and sinus infection caused by Fusarium spp., Lopes et al. (15) reported that of 19 patients, 14 had a hematologic malignancy, 1 had aplastic anemia, 1 had diabetes, and 3 had no risk factors. Disseminated fusariosis occurred in all patients with a hematologic malignancy as well as the patient with aplastic anemia; 10 (67%) of these 15 patients died. In contrast, among the immunocompetent patients, Fusarium disease was localized and surgery, with or without systemic antifungal therapy, was curative (11).
Our patient developed invasive fusariosis while receiving empirical amphotericin B (0.5 mg/kg/day). This is not an unusual occurrence. Infection by Fusarium spp. in neutropenic hosts typically does not respond to conventional dosages of amphotericin B. Even at high dosages (1 to 2 mg/kg/day), the response is generally poor without the rapid recovery from neutropenia (17).
The optimal selection of antifungal therapy for fusariosis is not well defined in the literature. Despite the poor response, high-dose amphotericin B (1 to 2 mg/kg/day) or a lipid formulation of amphotericin is considered standard therapy. Numerous case studies and small-scale studies have investigated various combinations of antifungal regimens (8, 18, 21) and the use of cytokine therapy and granulocyte transfusions (26) in neutropenic patients with infection by Fusarium spp. In a retrospective study of 43 patients with hematologic malignancy and fusariosis treated at the M.D. Anderson Cancer Center, use of transfusions with granulocyte colony-stimulating factor-elicited granulocytes appeared to be associated with a positive response (5). However, the responders in this study also tended to be in remission from the underlying malignancy, to have already recovered their neutrophil counts, and to have localized fusariosis; thus, the independent contribution of the granulocyte transfusions was uncertain (5). No controlled studies of treatment of fusariosis have been published.
Likewise, published data on in vitro susceptibility of Fusarium spp. were generally derived from case studies. Even when the clinical isolates have been susceptible to amphotericin B, the correlation with the clinical response has been poor. Information about the methodology used has not been consistently provided, and there have been significant methodological differences between laboratories in medium composition, pH, inoculum size, incubation time, and temperature (22). With these caveats made, only amphotericin B, natamycin, and miconazole have shown in vitro activity against some Fusarium isolates (22). There is agreement among several published reports that Fusarium spp. are resistant to rifampin, the triazoles, and flucytosine (8, 22, 25).
The number of patients with fusariosis is likely to increase in the future as more patients receive intensive immunosuppressive therapy. Our purposes here are to report the first known case of tissue disease caused by F. chlamydosporum and to alert the clinician and pathologist to the varied histologic appearance of Fusarium spp. Rapid diagnostic methods for mold identification from histologic specimens would increase our diagnostic precision. To this end, immunohistologic techniques which can rapidly identify a number of medically important fungal genera, including Fusarium, have been introduced in a few specialized laboratories (7, 9, 24). Moreover, given the dismal prognosis of fusariosis in neutropenic patients, more effective antifungal therapy and reliable fungal susceptibility testing methods are urgently needed.
REFERENCES
- 1.Anaissie E, Bodey G P, Kantarjian H, Ro J, Vartivarian S E, Hopfer R, Hoy J, Rolston K. New spectrum of fungal infections in patients with cancer. Rev Infect Dis. 1989;11:369–378. doi: 10.1093/clinids/11.3.369. [DOI] [PubMed] [Google Scholar]
- 2.Anaissie E, Kantarjian H, Jones P, Barlogie B, Luna M, Lopez-Berestein G, Bodey G P. Fusarium: a newly recognized fungal pathogen in immunosuppressed patients. Cancer. 1986;57:2141–2145. doi: 10.1002/1097-0142(19860601)57:11<2141::aid-cncr2820571110>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 3.Anaissie E, Kantarjian H, Ro J, Hopfer R, Rolston K, Fainstein V, Bodey G. The emerging role of Fusarium infections in patients with cancer. Medicine. 1988;67:77–83. doi: 10.1097/00005792-198803000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Anaissie E J, Bodey G P, Rinaldi M G. Emerging fungal infections. Eur J Clin Microbiol Infect Dis. 1989;8:323–330. doi: 10.1007/BF01963467. [DOI] [PubMed] [Google Scholar]
- 5.Boutati E I, Anaissie E J. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years’ experience at a cancer center and implications for management. Blood. 1997;90:999–1008. [PubMed] [Google Scholar]
- 6.Espinel-Ingroff A, Dawson K, Pfaller M, Anaissie E, Breslin B, Dixon D, Fothergill A, Paetznick V, Peter J, Rinaldi M, Walsh T. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi: 10.1128/aac.39.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuzawa M, Inaba H, Hayama M, Sakaguchi N, Sano K, Ito M, Hotchi M. Improved detection of medically important fungi by immunoperoxidase staining with polyclonal antibodies. Virchows Arch. 1995;427:407–414. doi: 10.1007/BF00199390. [DOI] [PubMed] [Google Scholar]
- 8.Guarro J, Gené J. Opportunistic fusarial infections in humans. Eur J Clin Microbiol Infect Dis. 1995;14:741–754. doi: 10.1007/BF01690988. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman L, Standard P G, Jalbert M, Kraft D E. Immunohistologic identification of Aspergillus spp. and other hyaline fungi by using polyclonal fluorescent antibodies. J Clin Microbiol. 1997;35:2206–2209. doi: 10.1128/jcm.35.9.2206-2209.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiehn T E, Nelson P E, Bernard E M, Edwards F F, Koziner B, Armstrong D. Catheter-associated fungemia caused by Fusarium chlamydosporum in a patient with lymphocytic lymphoma. J Clin Microbiol. 1985;21:501–504. doi: 10.1128/jcm.21.4.501-504.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurien M, Anandi V, Raman R, Brahmadathan K N. Maxillary sinus fusariosis in immunocompetent hosts. J Laryngol Otol. 1992;106:733–736. doi: 10.1017/s0022215100120729. [DOI] [PubMed] [Google Scholar]
- 12.Kwon-Chung K J, Bennett J E. Medical mycology. Malvern, Pa: Lea & Febiger; 1992. pp. 201–247. [Google Scholar]
- 13.Kwon-Chung K J, Hill W B, Bennett J E. New, special stain for histopathologic diagnosis of cryptococcosis. J Clin Microbiol. 1981;13:383–387. doi: 10.1128/jcm.13.2.383-387.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu K, Howell D N, Perfect J R, Schell W A. Morphologic criteria for the preliminary identification of Fusarium, Paecilomyces, and Acremonium species by histopathology. Am J Clin Pathol. 1998;109:45–54. doi: 10.1093/ajcp/109.1.45. [DOI] [PubMed] [Google Scholar]
- 15.Lopes J O, de Mello E S, Klock C. Mixed intranasal infection caused by Fusarium solani and a zygomycete in a leukaemic patient. Mycoses. 1995;38:281–284. doi: 10.1111/j.1439-0507.1995.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 16.Magnon K C, Jalbert M, Padhye A A. Osteolytic phaeohyphomycosis caused by Phialemonium obovatum. Arch Pathol Lab Med. 1993;117:841–843. [PubMed] [Google Scholar]
- 17.Martino, P., R. Gastaldi, R. Raccah, and C. Girmenia. 1994. Clinical patterns of Fusarium infections in immunocompromised patients. J. Infect. 128(Suppl. 1):7–15. [DOI] [PubMed]
- 18.Merz W G, Karp J E, Hoagland M, Jett-Goheen M, Junknis J M, Hood A F. Diagnosis and successful treatment of fusariosis in the compromised host. J Infect Dis. 1988;158:1046–1055. doi: 10.1093/infdis/158.5.1046. [DOI] [PubMed] [Google Scholar]
- 19.Nelson P E, Dignani M C, Anaissie E J. Taxonomy, biology, and clinical aspects of Fusarium species. Clin Microbiol Rev. 1994;7:479–504. doi: 10.1128/cmr.7.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nucci M, Spector N, Lucena S, Bacha P C, Pulcheri W, Lamosa A, Derossi A, Caiuby M J, Macieira J, Oliveira H O. Three cases of infection with Fusarium species in neutropenic patients. Eur J Clin Microbiol Infect Dis. 1992;11:1160–1162. doi: 10.1007/BF01961136. [DOI] [PubMed] [Google Scholar]
- 21.Rabodonirina M, Piens M A, Monier M F, Guého E, Fière D, Mojon M. Fusarium infections in immunocompromised patients: case reports and literature review. Eur J Clin Microbiol Infect Dis. 1994;13:152–161. doi: 10.1007/BF01982190. [DOI] [PubMed] [Google Scholar]
- 22.Reuben A, Anaissie E, Nelson P E, Hashem R, Legrand C, Ho D H, Bodey G P. Antifungal susceptibility of 44 clinical isolates of Fusarium species determined by using a broth microdilution method. Antimicrob Agents Chemother. 1989;33:1647–1649. doi: 10.1128/aac.33.9.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson S E, Bannatyne R M, Summerbell R C, Milliken J, Gold R, Weitzman S S. Disseminated fusarial infection in the immunocompromised host. Rev Infect Dis. 1988;10:1171–1181. doi: 10.1093/clinids/10.6.1171. [DOI] [PubMed] [Google Scholar]
- 24.Sekhon A S, Kaufman L, Moledina N, Summerbell R C, Padhye A A, Ambroisie E A, Panter T. An exoantigen test for the rapid identification of medically significant Fusarium species. J Med Vet Mycol. 1995;33:287–289. [PubMed] [Google Scholar]
- 25.Sekhon A S, Padhye A A, Garg A K, Ahmad H, Moledina N. In vitro sensitivity of medically significant Fusarium species to various antimycotics. Chemotherapy (Basel) 1994;40:239–244. doi: 10.1159/000239199. [DOI] [PubMed] [Google Scholar]
- 26.Spielberger R T, Falleroni M J, Coene A J, Larson R A. Concomitant amphotericin B therapy, granulocyte transfusions, and GM-CSF administration for disseminated infection with Fusarium in a granulocytopenic patient. Clin Infect Dis. 1993;16:528–530. doi: 10.1093/clind/16.4.528. [DOI] [PubMed] [Google Scholar]
- 27.Valenstein P, Schell W A. Primary intranasal Fusarium infection: potential for confusion with rhinocerebral zygomycosis. Arch Pathol Lab Med. 1986;110:751–754. [PubMed] [Google Scholar]
- 28.Vartivarian, S. E., E. J. Anaissie, and G. P. Bodey. 1993. Emerging fungal pathogens in immunocompromised patients: classification, diagnosis, and management. Clin. Infect. Dis. 17(Suppl. 2):S487–S491. [DOI] [PubMed]
- 29.Zaatari G S, Reed R, Morewessel R. Subcutaneous hyphomycosis caused by Scytalidium hyalinum. Am J Clin Pathol. 1984;82:252–256. doi: 10.1093/ajcp/82.2.252. [DOI] [PubMed] [Google Scholar]