Abstract
CPVT is a rare inherited arrhythmogenic disorder characterized by bidirectional, polymorphic ventricular arrhythmias triggered by catecholamines released during exercise, stress, or sudden emotion in individuals with a normal resting electrocardiogram and structurally normal heart. Mutations in the ryanodine receptor 2 gene are the most common known etiology of this disorder. The c.1195A > G(p.Met399Val) variant in Exon 14 of RyR2 is currently classified as a Variant of Uncertain Significance. We present a case of CPVT caused by this novel disease-causing RyR2 variant and discuss its pathophysiology. The role of SSRIs in treating patients with CPVT unresponsive to mainstream therapies is also highlighted.
Keywords: Catecholaminergic polymorphic ventricular tachycardia, Selective serotonin reuptake inhibitors, Ryanodine receptor 2
1. Introduction
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a rare inherited arrhythmogenic disorder characterized by bidirectional and polymorphic ventricular arrhythmias that are triggered by catecholamines released during exercise, stress, or sudden emotion in individuals with a normal resting electrocardiogram and structurally normal heart. Mutations in the ryanodine receptor 2 gene (RyR2) are the most common known etiology of this disorder. The c.1195A > G (p.Met399Val) variant in Exon 14 of RyR2 is currently classified as a Variant of Uncertain Significance (VUS). To date, this variant has not been reported in the literature in individuals with RyR2-related conditions. Advanced in-silico modeling of protein sequence and biophysical properties indicates that this missense variant is expected to disrupt RyR2 protein function. We present a case of CPVT caused by this novel disease-causing RyR2 variant.
2. Case report
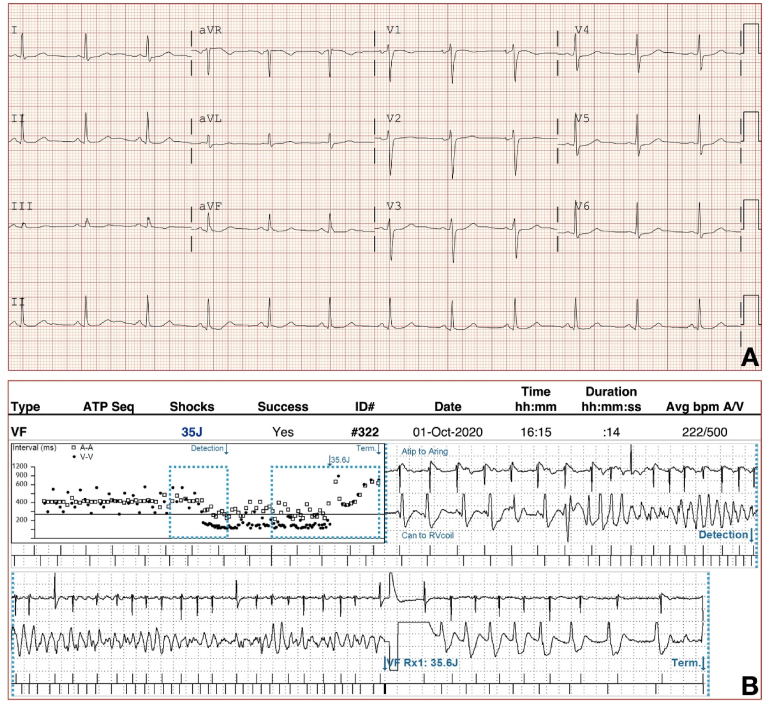
The patient is a 31-year-old woman who had a witnessed out-of-hospital cardiac arrest during physical activity at age 15 years for which she received multiple external defibrillator shocks by EMS. Family history includes a maternal niece who drowned and died in a public swimming pool at age 10 years. The patient underwent dual chamber ICD implantation for secondary prevention. She did not have QT prolongation on ECG but was provisionally diagnosed with “long QT syndrome” based on the occurrence of torsades de pointes. She had received ICD shocks for approximately 20 emotion and exertion induced recurrent polymorphic VT/VF events over the past 16 years. She did not tolerate multiple beta blockers including nadolol, propranolol, metoprolol and bisoprolol due to sedative effects, depression, and fatigue. She then underwent bilateral stellate sympathetic ganglionectomy at age 20 years for refractory VT/VF with a resultant decrease in frequency of arrhythmic events. At age 29 years when she became pregnant, she was referred to our center for high-risk pregnancy management. She had a successful term pregnancy and scheduled C-section delivery at term. Subsequently, as a new mother, she had three emotional stress-provoked polymorphic VT/VF events terminated by appropriate ICD shocks (Fig. 1).
Fig. 1.
(A) 12-lead ECG, (B) A representative episode of ICD treated polymorphic ventricular tachycardia.
Multiple ECGs reviewed showed normal sinus rhythm with normal QT intervals (Fig. 1). Echocardiogram was unremarkable with normal left and right ventricular size and function and absence of any valvular abnormalities. With a presumptive diagnosis of idiopathic ventricular fibrillation, she was started on mexiletine, but this was discontinued after a few months because of subjective adverse effects. Her arrhythmia events were noted to be associated with situations of high adrenergic drive or emotional excitation and in the absence of QT prolongation she was suspected to have CPVT. After genetic counselling, targeted genetic testing comprised of arrhythmia sequencing and deletion/duplication panel of 150 genes revealed a heterozygous VUS with replacement of highly conserved methionine with valine at codon 399 (c.1195A > G) of the RyR2 gene. Even though she did not undergo exercise treadmill testing or Holter monitoring, she still had a high clinical probability of CPVT associated with RYR2 rare variants with a CPVT score of 3.5 based upon her symptoms and relatively short QT interval [1]. Even though a diagnostic CPVT mutation was not identified, after further genetic counselling, her only daughter was tested negative for the identified VUS. No other family members have been tested for or diagnosed with CPVT. Approximately 2 years ago, she was started on paroxetine to manage her anxiety and has had no recurrence of VT/VF since, confirmed on ICD interrogation. We have discussed flecainide as the antiarrhythmic drug of choice with her though this has not yet been initiated due to patient preference as she continues to remain arrhythmia free on paroxetine.
3. Discussion
CPVT is a rare disorder associated with malignant ventricular arrhythmias triggered by adrenergic drive in the setting of stress or exertion. These patients have structurally normal hearts and a normal resting ECG without QT prolongation. This condition can sometimes be difficult to distinguish from long QT syndrome esp. LQT1. Exertion-induced arrhythmias are a common feature of both disorders. Swimming is reported to be a specific trigger for LQT1 but also associated with CPVT. The QT interval on resting ECG in patients with LQT1 syndrome is only mildly prolonged and can even be normal. Moreover, patients with CPVT may have transient QT prolongation after resuscitation from a cardiopulmonary arrest. Holter monitoring and exercise treadmill testing is recommended as part of diagnostic evaluation of CPVT. These can provide both diagnostic and prognostic information and can also facilitate titration of medical therapy. Modified Schwartz score has been developed to aid in the differentiation of these two disorders [2].
The pathophysiology of CPVT is incompletely understood at this time. CPVT occurs because of mutations in either the cardiac ryanodine receptor-Ca2+ release channel (RyR2) gene (an autosomal dominant form) or the sarcoplasmic reticulum protein calsequestrin 2 (CASQ2) gene (a rare recessive form). Both genes encode sarcoplasmic reticulum proteins that play a crucial role in maintaining intracellular calcium homeostasis during the process of excitation–contraction coupling in cardiomyocytes. This process is based on the mechanism of calcium-induced calcium release. Depolarization and activation of the L-type calcium channels on the cardiomyocyte cell membrane causes influx of small amounts of calcium into the cell. This stimulates the opening of the RYR2 channel allowing larger quantities of calcium to be released into the cytoplasm from the sarcoplasm and subsequently leading to myocardial contraction. CASQ2 acts as the major buffering protein for calcium as well as regulates SR calcium release as a component of the RyR2 channel complex.
The RYR2 gene is a large gene containing 105 exons and encodes the 4967-amino acid polypeptide. The RyR2 receptor is a homotetramer of the polypeptide. The channel is formed by the tetramer and 4 FK506-binding proteins. The channel function is modulated by the specific isomer of FK506 interacting with the channel. Mutations in the cardiac RyR2 and its interactive proteins that affect calcium homeostasis are the known leading cause of CPVT. Studies have reported variable penetrance and phenotypic variability with a significant proportion of carriers of pathogenic mutations remaining asymptomatic based on long term monitoring and repeated stress testing [3,4]. RyR2 mutations can also manifest as arrhythmogenic right ventricular cardiomyopathy (ARVC) that presents as monomorphic ventricular tachycardia occurring in the setting of replacement of myocardium, especially in the epicardial right ventricular free wall, by fibrofatty tissue. However, the role of these mutations in the pathogenesis of ARVC remains unclear.
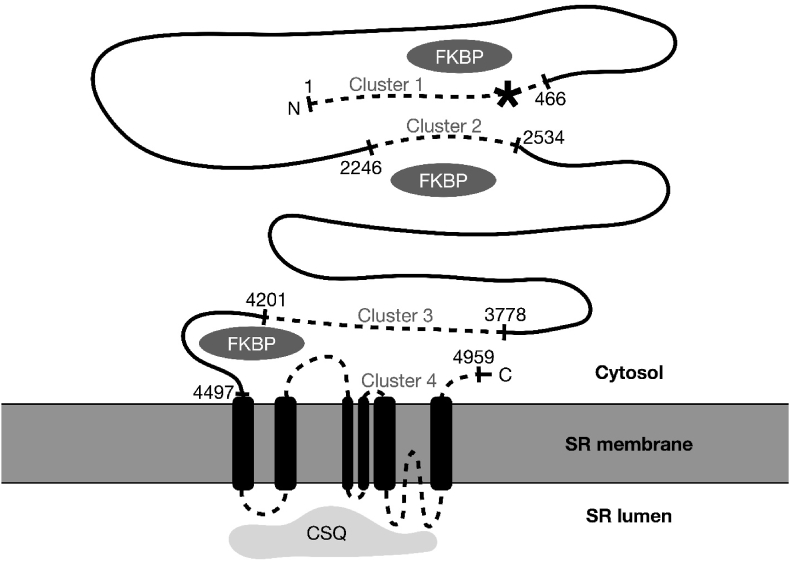
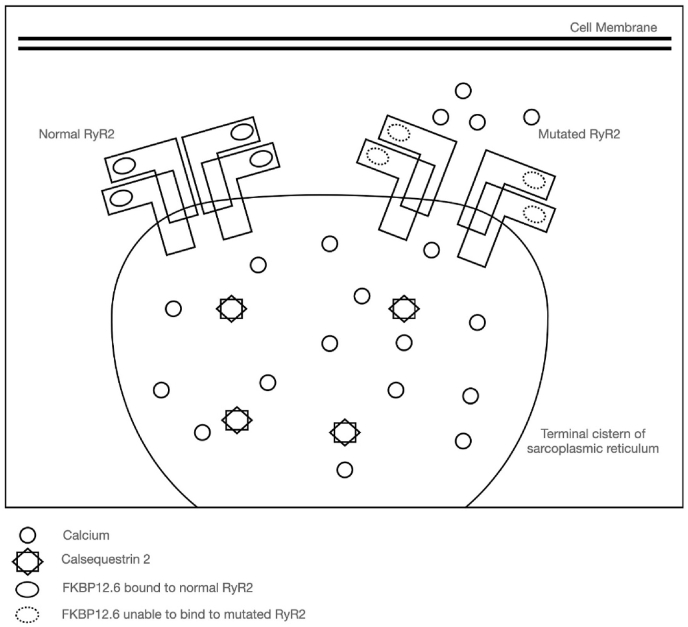
Known CPVT mutation clusters occur in the RyR2 gene corresponding to amino acid residues 1–466, 2246–2534, 3778–4201, 4497–4959, the latter C-terminal fragment encompassing the 6 transmembrane and channel-forming components (Fig. 2). However, in-vitro studies have proposed that a large RyR2 N-terminal fragment (amino acid residues 1–2064 [5] or 305–1937 [6]) is a location of modulatory binding site for a 12.6-kDa FK506-binding protein (calstabin-2 or FKBP12.6), an important regulator of the cardiac RyR2 function. FKBPs are thought to stabilize the closed conformation of RyR2 and prevent diastolic release of calcium from the sarcoplasmic reticulum. It is hypothesized that mutations in this region of RyR2 can affect the ability of FKBP 12.6 to bind to and inhibit the opening of RyR2, especially in response to phosphorylation of RyR2 at multiple sites from adrenergic β-receptor stimulation [7]. This results in the opening of RyR2 during diastole. Aberrant spontaneous leak of calcium from the SR during diastole due to dysfunctional RyR2 contributes to calcium overload in cardiac myocytes (Fig. 3). This can result in fatal arrhythmias initiated by delayed after-depolarizations and triggered activity via the Na–Ca exchanger.
Fig. 2.
Schematic illustration of RyR2, a large 4967-amino acid sarcoplasmic reticulum protein.
∗: Highlighted is the c.1195A > G (p.Met399Val) variant of RyR2, a novel disease-causing RyR2 variant through its role as a binding site for FKBP12.6/calstabin-2. Known CPVT mutation clusters are shown as dotted lines, with amino acid numbers indicating their boundaries (residues 1–466, 2246–2534, 3778–4201, 4497–4959).
Figure adapted from: Blayney LM, Lai FA. Ryanodine receptor-mediated arrhythmias and sudden cardiac death. Pharmacol Ther. 2009; 123(2):151–177. https://doi.org/10.1016/j.pharmthera.2009.03.006.
Fig. 3.
Pathophysiology of RyR2 mutation at binding site of FKBP12.6 leading to aberrant opening of the channel in diastole.
Figure adapted from:
• Wleklinski MJ, Kannankeril PJ, Knollmann BC. Molecular and tissue mechanisms of catecholaminergic polymorphic ventricular tachycardia. J Physiol. 2020; 598(14):2817–2834. https://doi.org/10.1113/JP276757
• Tong M, Jiang Y. FK506-Binding Proteins and Their Diverse Functions. Curr Mol Pharmacol. 2015; 9(1):48–65. https://doi.org/10.2174/1874467208666150519113541.
This patient had c.1195A > G (p.Met399Val) variant in Exon 14 of RyR2 in the region of the FKBP binding site and is classified as a Variant of Uncertain Significance (VUS). Computer modelling of the protein sequence and its biophysical properties (including impact on protein structure and function, spatial conformation, and thermodynamic stability) performed at Invitae Diagnostics, San Francisco, California, USA, in addition to evolutionary genetic conservation suggests that this VUS would disrupt RYR2 protein function. According to the European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) Expert Consensus Statement on the state of genetic testing for cardiac diseases, a CPVT score of ≥3.5 suggests a likelihood of RyR2 mediated CPVT ≥60% and the result of a VUS on genetic testing may be upgraded to at least a ‘likely pathogenic variant’ with very high confidence [8].
The first line treatment of CPVT are beta blockers without intrinsic sympathomimetic activity. In patients who do not tolerate or respond to beta blockers, sodium channel blockers esp. flecainide have demonstrated additional benefit in suppressing ventricular arrhythmias. It has a dual mechanism of anti-arrhythmic action in CPVT by inhibition of arrhythmogenic Ca release via RyR2 receptor channels in addition to reducing triggered activity by reducing availability of sodium channels [9]. Cardiac sympathetic denervation has been shown to be effective and is now recommended in patients with drug refractory ventricular arrhythmias [10]. Implantation of a cardioverter defibrillator may become necessary in patients with recurrent episodes. However, the use of ICDs in this population is associated with significant shock burden and complications. There have been reports of inappropriate ICD shocks leading to fear and anxiety in turn triggering an electrical storm and even death [11].
The effect of psychiatric disorders and antidepressant medications on cardiac sympathetic control has been an area of research interest given the strong association between psychiatric and cardiac disorders. A large-scale cohort study evaluated the effect of tricyclic antidepressants (TCAs), serotonin-norepinephrine reuptake inhibitors (SNRIs) and selective serotonin reuptake inhibitors (SSRIs) on the pre-ejection period as a measure of cardiac sympathetic control. They reported a significantly longer pre-ejection period in SSRI users, as opposed to TCAs and SNRIs, indicating decreased sympathetic control on the heart [12]. This is in line with the findings of studies regarding the effect of SSRI therapy on sympathetic activity determined by a reduction in whole body and cardiac noradrenaline spillover to plasma [13,14]. Cardiac norepinephrine spillover is estimated by the difference in arterial and coronary sinus norepinephrine using a radioactive isotope. Tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors exert their effect through blocking the reuptake of norepinephrine in synaptic clefts increasing its levels and ultimately causing it to spill over into the bloodstream. In contrast, SSRIs appear to reduce overall sympathetic activity by central inhibition of its regulatory circuitry present in the brainstem [12]. SSRIs reduce anxiety and overall sympathetic activity, indirectly decreasing cardiac sympathetic activity. Beyond these hypotheses there is paucity of mechanistic data on impact of SSRI in CPVT.
Another consideration in choosing an antidepressant for this use is their risk for QT prolongation. Among SSRIs, paroxetine is thought to have the lowest risk for prolongation of the QT interval whereas citalopram and escitalopram have the highest risk [15]. There has been a prior report on the utility of SSRIs in preventing recurrence of ventricular arrhythmias in patients with CPVT [16]. Our case represents a similar experience where the patient had arrhythmias refractory to most currently recommended treatment modalities but have been suppressed with SSRI therapy.
4. Conclusions
The variant c.1195A > G (p.Met399Val) in RyR2 gene is a novel autosomal dominant cause for CPVT. Replacement of the highly conserved methionine at codon 399 of this 4967-amino acid sarcoplasmic reticulum calcium channel subunit may disrupt the binding of FKBP12.6/calstabin-2 resulting in diastolic cytosolic calcium release resulting in polymorphic VT. However, beyond β-blockers and cardiac sympathetic denervation, SSRIs may play a role in management of CPVT.
Disclosures
The authors have no conflicts to disclose.
Funding sources
None.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Giudicessi J.R., Lieve K.V.V., Rohatgi R.K., et al. Assessment and validation of a phenotype-enhanced variant classification framework to promote or demote RYR2 missense variants of uncertain significance. Circ Genom Precis Med. May 2019;12(5) doi: 10.1161/circgen.119.002510. [DOI] [PubMed] [Google Scholar]
- 2.Ozawa J., Ohno S., Fujii Y., et al. Differential diagnosis between catecholaminergic polymorphic ventricular tachycardia and long QT syndrome type 1 - modified schwartz score. Circ J. Aug 24 2018;82(9):2269–2276. doi: 10.1253/circj.CJ-17-1032. [DOI] [PubMed] [Google Scholar]
- 3.Postma A.V., Denjoy I., Kamblock J., et al. Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J Med Genet. 2005;42(11):863–870. doi: 10.1136/jmg.2004.028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauce B., Rampazzo A., Basso C., et al. Screening for ryanodine receptor type 2 mutations in families with effort-induced polymorphic ventricular arrhythmias and sudden death. J Am Coll Cardiol. 2002;40(2):341–349. doi: 10.1016/S0735-1097(02)01946-0. [DOI] [PubMed] [Google Scholar]
- 5.Zissimopoulos S., Lai F.A. Interaction of FKBP12.6 with the cardiac ryanodine receptor C-terminal domain. J Biol Chem. Feb 18 2005;280(7):5475–5485. doi: 10.1074/jbc.M412954200. [DOI] [PubMed] [Google Scholar]
- 6.Masumiya H., Wang R., Zhang J., Xiao B., Chen S.R. Localization of the 12.6-kDa FK506-binding protein (FKBP12.6) binding site to the NH2-terminal domain of the cardiac Ca2+ release channel (ryanodine receptor) J Biol Chem. Feb 7 2003;278(6):3786–3792. doi: 10.1074/jbc.M210962200. [DOI] [PubMed] [Google Scholar]
- 7.Sumitomo N. Current topics in catecholaminergic polymorphic ventricular tachycardia. J Arrhythm. Oct 2016;32(5):344–351. doi: 10.1016/j.joa.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilde A.A.M., Semsarian C., Márquez M.F., et al. European heart rhythm association (EHRA)/Heart rhythm society (HRS)/Asia pacific heart rhythm society (APHRS)/Latin American heart rhythm society (LAHRS) Expert Consensus statement on the state of genetic testing for cardiac diseases. Europace. Sep 1 2022;24(8):1307–1367. doi: 10.1093/europace/euac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kryshtal D.O., Blackwell D.J., Egly C.L., et al. RYR2 channel inhibition is the principal mechanism of flecainide action in CPVT. Circ Res. Feb 5 2021;128(3):321–331. doi: 10.1161/circresaha.120.316819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Khatib S.M., Stevenson W.G., Ackerman M.J., et al. AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2017;72(14):e91–e220. doi: 10.1016/j.jacc.2017.10.054. Oct 2 2018. [DOI] [PubMed] [Google Scholar]
- 11.Roston T.M., Jones K., Hawkins N.M., et al. Implantable cardioverter-defibrillator use in catecholaminergic polymorphic ventricular tachycardia: a systematic review. Heart Rhythm. Dec 2018;15(12):1791–1799. doi: 10.1016/j.hrthm.2018.06.046. [DOI] [PubMed] [Google Scholar]
- 12.Licht C.M.M., Penninx B.W.J.H., de Geus E.J.C. Effects of antidepressants, but not psychopathology, on cardiac sympathetic control: a longitudinal study. Neuropsychopharmacology. 2012/10/01 2012;37(11):2487–2495. doi: 10.1038/npp.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barton D.A., Dawood T., Lambert E.A., et al. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J Hypertens. Oct 2007;25(10):2117–2124. doi: 10.1097/HJH.0b013e32829baae7. [DOI] [PubMed] [Google Scholar]
- 14.Shores M.M., Pascualy M., Lewis N.L., Flatness D., Veith R.C. Short-term sertraline treatment suppresses sympathetic nervous system activity in healthy human subjects. Psychoneuroendocrinology. May 2001;26(4):433–439. doi: 10.1016/s0306-4530(01)00002-6. [DOI] [PubMed] [Google Scholar]
- 15.Funk K.A., Bostwick J.R. A comparison of the risk of QT prolongation among SSRIs. Ann Pharmacother. Oct 2013;47(10):1330–1341. doi: 10.1177/1060028013501994. [DOI] [PubMed] [Google Scholar]
- 16.Chen S., Duan Q., Tang K., Zhao D., Xu Y. Serotonin and catecholaminergic polymorphic ventricular tachycardia: a possible therapeutic role for SSRIs? Cardiovasc J Afr. Jul-Aug 2010;21(4):225–228. doi: 10.5830/cvja-2010-023. [DOI] [PMC free article] [PubMed] [Google Scholar]