Graphical abstract
Keywords: Prostate cancer, Biofunctionalization, Prostate-specific diagnosis, Combinational therapy
Highlights
-
•
Nanomaterials for prostate cancer therapy and their current clinical trial status.
-
•
Strategies for conjugating different biomolecules with nanomaterials.
-
•
Biofunctionalized nanosystems used for the detection of different prostate-specific biomarkers and direct visualization of tumor cells.
-
•
Biofunctionalized nanosystems used for the targeted delivery of drugs and biomacromolecules.
-
•
Design and synthesis of biomimetic nanomaterials especially exosomes and viral particle functionalized nanomaterials against prostate cancer.
Abstract
Background
Even with the advancement in the areas of cancer nanotechnology, prostate cancer still poses a major threat to men’s health. Nanomaterials and nanomaterial-derived theranostic systems have been explored for diagnosis, imaging, and therapy for different types of cancer still, for prostate cancer they have not delivered at full potential because of the limitations like in vivo biocompatibility, immune responses, precise targetability, and therapeutic outcome associated with the nanostructured system.
Aim of review
Functionalizing nanomaterials with different biomolecules and bioactive agents provides advantages like specificity towards cancerous tumors, improved circulation time, and modulation of the immune response leading to early diagnosis and targeted delivery of cargo at the site of action.
Key scientific concepts of review
In this review, we have emphasized the classification and comparison of various nanomaterials based on biofunctionalization strategy and source of biomolecules such that it can be used for possible translation in clinical settings and future developments. This review highlighted the opportunities for embedding highly specific biological targeting moieties (antibody, aptamer, oligonucleotides, biopolymer, peptides, etc.) on nanoparticles which can improve the detection of prostate cancer-associated biomarkers at a very low limit of detection, direct visualization of prostate tumors and lastly for its therapy. Lastly, special emphasis was given to biomimetic nanomaterials which include functionalization with extracellular vesicles, exosomes and viral particles and their application for prostate cancer early detection and drug delivery. The present review paves a new pathway for next-generation biofunctionalized nanomaterials for prostate cancer theranostic application and their possibility in clinical translation.
Introduction
Cancer is a major public health concern worldwide. Prostate cancer (PC) is the most diagnosed cancer among men in the United States (USA). The American cancer society predicted that in 2022 around ∼ 268,490 new cases and around ∼ 34,500 new deaths because of PC in the USA [1]. PC comes under adenocarcinoma and is contemplated as non-cutaneous neoplasia [2]. The PC occurs by the mutation in prostate gland cells causing their uncontrolled multiplication which leads finally to metastasis [3]. PC is treatable if detected early however, mostly it has been diagnosed when cancer has been spread to the bone or lymph causing a poor prognosis [4]. PC therapies are primarily divided into surgery, chemo, hormones, and radiotherapy. Moreover, the treatment significantly depends upon cancer stages and once it becomes invasive it’s nearly impossible to cure, thus making early detection of PC a necessity for their prognosis [5]. At the initial stage of PC, patients do not show any symptoms thus making them very hard to treat and causing a very high mortality rate. The early diagnosis of PC is very critical so that it can be treated. Rectal examination and tissue biopsy/identification of prostate-specific antigen (PSA) from standard biochemical assays were used conventionally for diagnosing PC [6], [7], [8], [9], [10], [11], [12]. In most of these methods, PSA was measured which was approved by the United States Food and Drug Administration (FDA) in the year 1984. PSA has been chosen as the gold standard for PC and its concentration > 4 ng/mL in serum indicates PC risk [13], [14]. An overview of current clinically approved PC detection methods as well as a treatment strategy has been summarized in Fig. 1. The conventional biochemical technique is used for attaining initial screening. Afterwards a digital rectal examination (DRE) is carried out for inspecting the prostate gland condition, especially the size and texture [15]. Biopsies also fall under the conventional diagnosis strategy and take place after DRE [16]. Although all three are being used routinely for the detection of PC have several limitations associated with them. The PSA has very poor sensitivity and specificity [17], [18], [19], [20]. Similarly, irrespective of the direct visualization of the tumor gland condition, DRE cannot provide early detection of PC [21]. Furthermore, because of the direct contact with the patient’s prostate gland, the whole detection process is very uncomfortable and unpleasant. The prominent drawback associated with biopsies involves potential infection from the microbe causing inflammation in the diseased gland [1]. Moreover, the treatment of PC is significantly dependent on its stage and all these diagnosis methods cannot detect PC at an early stage [22]. Different clinicopathological factors such as concentration of PSA, tumor stage and histological grade values per Gleason score set the treatment intensity [23]. The treatment strategies as shown in Fig. 1 involve single-modality therapy which includes radiotherapy, surgery, prostatectomy, radiation therapy, or multimodality therapy [24]. In addition to those different chemotherapeutic drugs such as mitoxantrone, docetaxel, cabazitaxel, and some platinum-based anti-cancer drug along with their various combinations were explored for the treatment of PC [25], [26], [27], [28], [29]. Similarly, all the current PC treatments have limitations such as resistance to therapy, local relapse, limited drug bioavailability, and side effects on local tissues [30], [31].
Fig. 1.
Overview of current PC diagnosis and treatment approaches.
Considering the limitations associated with current treatment strategies related to PC, the development of an affordable and safe theranostic system has become very important [32]. The last decade has seen a gradual change from traditional to more targeted and also personalized therapies [33], [34]. Among several innovative technologies available for cancer therapy nanotechnology has been seen as the most promising because of its cost-effective, safe, and versatile nature [35], [36], [37].
Nanomaterials against PC therapy: current clinical trials and limitations
Nanostructured systems have established innovative concepts in smart healthcare. These materials and devices are exclusively developed for cancer theranostics. The electrical, optical, and magnetic properties of nanomaterials provide new capabilities [38] in addition to conventional delivery aspects. Presently, several nanomaterials-based drug-carrier formulations are in the clinical trial and the process to go for the clinical trial or in the clinic [39]. Abraxane (albumin-coated paclitaxel drug) and DOX-loaded liposome are in clinical trials for metastatic breast cancer and ovarian cancer respectively [39]. The nanostructured systems can lead to highly accurate diagnosis with improved sensitivity and specificity in PC tumors. Because of their small size and high surface area, these nanomaterials can easily be loaded with different anti-cancer drug(s)/imaging agent(s) and functionalized with biological molecules for attaining excellent target specificity [40]. Additionally, nanomaterials can utilize tumor leaky vasculature to reach tumors and can stay longer in the tumor due to the enhanced permeability and retention (EPR) effect [41]. Integral or surface modifications of nanomaterials can facilitate controlled or tuned release of loaded theranostics based on tumor disease conditions and/or external stimuli [42]. Thus, drawbacks associated with the current technologies both in the detection and treatment of PCs can easily be overcome by employing nanomaterials.
Table 1 discusses the status of different nanoformulations which are in the clinical trial against PC. BIND-014 nanoformulation having a combination of docetaxel-encapsulating nanoparticle and polyethylene glycol (PEG) and decorated with Prostate-specific membrane antigen (PSMA)-targeted small molecules showed specific delivery of docetaxel in PC tumor [43]. In the phase 1 clinical trial of BIND-014 (dose level of 3.5 to 75 mg/m2 and 15 to 45 mg/m2) was given for every-three weeks (n = 28) or weekly (n = 27) to two mCRPC patients. The phase 1 clinical trial results of BIND-014 were consistent with the preclinical data [43]. Moreover, BIND-014 concentration 60 mg/m2 (for 3 weeks) or 40 mg/m2 (weekly) were recommended for phase 2 study. In the phase 2 study, 42 patients having diverse demography with a median age of 66 and a median dose of 6 were chosen. After delivering BIND-014 the PSMA- positive circulating tumor cells (CTCs) were reduced in these patients. Also, patients showed treatment mediated side effects like fatigue, nausea, neuropathy, and neutropenic fever. The phase 2 clinical finding suggests BIND-014 is active and tolerated in patients and the median radiographic progression-free survival was 9.9 months. The anti-tumor activity was determined through PSMA-positive CTCs, which gets decreased with treatment suggesting PSMA expression can be used for selecting patients and for early response measurement [44]. The major limitation of this phase 2 trial is the lack of comparison in the efficacy between BIND-014 and docetaxel in the patients [45]. In another work, gold nano-shell based localized photothermal therapy was tested on a pilot scale (i.e., 16 patients). The laser power (range- 4.5 W up to 6.5 W) was used in the trial and treatment was given over a 3-to-12-month period. Out of 16 patients, 15 successfully completed their treatment. The median prostate volume decreased from 49 cm3 to 42 cm3 after 3 months and no serious adverse side effects were observed. All patients showed tumor necrosis post-ablation and repeated biopsy [46]. This pilot work also met the primary safety endpoint, and the results were highly promising. The major limitation of this trial was the lack of discussion on the efficacy and cost of treatment. The very little success achieved with nanomaterials for PC theranostics in clinical settings suggests the limitations as well as ample opportunities ahead in this area. Critical factors like side-effect activation, heterogeneity in the target, and detailed immunological and biological clearance mechanism are major limitations needed to be minimized for future advanced theranostics system applications.
Table 1.
Nanosystems for PC treatment are considered or are in a clinical trial.
| Project title | Nanosystem | NCT number | Phase | Sponsoring agency |
|---|---|---|---|---|
| Accuracy of Lymph Node Imaging in Prostate Cancer: PSMA PET-CT and Nano-MRI (MAGNIFY) | Ferumoxtran-10 enhanced MRI 68 Ga PSMA PET-CT |
NCT03223064 | Not Applicable | Radboud University Medical Center |
| Examination of Focal Therapies- MRI-Fusion, HIFU, NanoKnife and Cryotherapy | Focal-driven therapies for diagnosis and treatment of prostate cancer using nano-knife etc. | NCT03982706 | – | Rabin Medical Center |
| The Use of Nanoparticles to Guide the Surgical Treatment of Prostate Cancer | 64Cu-NOTA-PSMA-PEG-Cy5.5-C' dot tracer | NCT04167969 | Phase 1 | Memorial Sloan Kettering Cancer Center |
| Magnetic Nanoparticle Thermoablation-Retention and Maintenance in the Prostate:A Phase 0 Study in Men (MAGNABLATE I) | Magnetic iron nanoparticles | NCT02033447 | Phase 1 | University College London Hospitals |
| Prostate Cancer Circulating Tumor Cells Based on Epithelial-Mesenchymal Transition Biology | Near infrared (NIR) emissive nanotechnology | NCT02022904 | – | Duke University |
Biofunctionalization of nanomaterials: Conjugation strategies
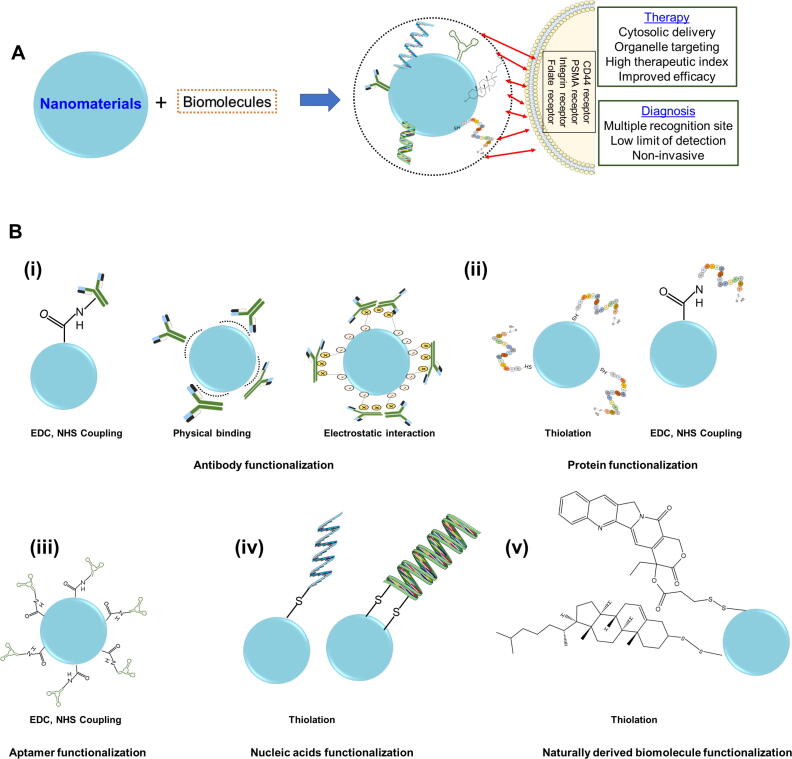
Embellishing nanomaterials with biomolecules has been shown to improve the recognition and targeting ability. Often, biofunctionalization using an antibody, aptamers, nucleic acid, proteins and other naturally derived biomolecules on nanomaterials helps improve the biological performance of the nanomaterials [47]. Fig. 2 presents a schematic overview of biofunctionalized nanomaterial advantages in cancer theranostics. The biofunctionalization of nanomaterials can be done either through non-covalent interaction which includes physical adsorption, biomolecule entrapment, ionic interaction, or covalent interaction on nanomaterial surface [48], [49], [50], [51], [52]. The non-covalent functionalization results in maintaining the conjugated skeleton as well as the inherent electronic structure whereas covalent functionalization provides more stability and reproducibility for biofunctionalized nanomaterials [53]. The last decade has seen very diverse strategies for the biofunctionalization of nanomaterials. In the following subsections, detailed insight related to biofunctionalization is discussed.
Fig. 2.
A. Schematic representation of biofunctionalized nanomaterials advantages for a smart PC theranostic system and B. Schematic depiction of biofunctionalization of (i) antibody (ii) protein, (iii) aptamer, (iv) nucleic acids, and (v) naturally derived biomolecules on nanomaterials.
Aptamers consist of single-stranded oligonucleotides and are usually obtained using the systematic evolution of ligands by the exponential enrichment (SELEX) method [54]. Aptamer provides better selectivity, specificity, stability, and affinity toward a target [55]. In general, functionalization of aptamers with different nanomaterials mostly takes place either with non-covalent (which involves hydrogen bonding, π-π stacking, hydrophobic interactions, etc.) [56], [57] or covalent functionalization [58]. The covalent functionalization of nanomaterials with aptamers gives a stable biofunctionalized system. In the covalent functionalization strategy, a covalent bond takes place through the terminal functional group of nanomaterials and aptamers. As we know, mostly all types of nanomaterials contain amine (-NH2), hydroxyl (–OH) and, carboxyl (–COOH) functional groups which through -Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC)/ N-hydroxysuccinimide (NHS) chemistry gets modified and reacts with NH2-terminated aptamer thus creating a stable biofunctionalized system that can bind specifically with cellular components [59], [60], [61].
Like aptamer functionalization, protein functionalization also can be done through both non-covalent as well as covalent strategies. However, prominently covalent and thiolation reactions produce stable, versatile, and efficient targeted nanomaterials. In general, the covalent functionalization method uses “metal-thiol” bonds between nanomaterials and biomolecules. Nanomaterials functionalized with EDC and NHS react covalently with the protein’s primary amine of lysine [62]. Direct functionalization can also take place between nanomaterials and proteins without using EDC/NHS. In another example, metal thiol chemistry cysteine present in protein gets linked with nanomaterial surface through “S—H or S-metal (e.g., Au) bonding” leading to a direct link between the protein side chain and nanomaterial surface [62], [63], [64].
Antibody functionalized nanomaterials hold significant promise in achieving enhanced detection and therapeutic efficacy while circumventing systemic side effects. The nanomaterials can be functionalized with antibodies through adsorption, covalent functionalization, and ionic interactions [65], [66]. Adsorption of antibodies and ionic interactions comes under the noncovalent functionalization strategy. Commonly, hydrogen bonding, hydrophobic interactions, and van der Waals forces use antibodies to get adsorbed onto nanomaterial surfaces [67]. The ionic interaction occurs between oppositely charged antibodies and nanomaterials surface [68]. The covalent functionalization strategy is based on coupling chemistry. Some of the most prominent covalent functionalization strategies are based on click chemistry, carbodiimide chemistry and maleimide chemistry [69], [70]. As compared with adsorption and ionic functionalization covalent functionalization gives more stable and better reproducibility and antibodies functionalized in this method are present in “end on” orientation [68], [71].
The functionalization of nucleic acid with nanomaterials mostly takes place through thiol or amine-modified bonds. The functionalization reaction involves mostly carbodiimide-assisted amidation reaction [72]. Apart from the above-mentioned biomolecules, there are several other naturally sourced biomolecules that functionalize nanomaterials through covalent, non-covalent, ionic, hydrophobic, etc. interactions. With each biomolecule, the functionalization strategy is different thus leading to different effects on biological systems [73], [74]. The biofunctionalized nanomaterials have led to very good biocompatibility, efficient cellular uptake and selective binding with biomarkers thus covering the entire platform for an advanced theranostic system. This review presents the status of biofunctionalized nanomaterials used for the detection and treatment of PC cells and tumors. This review covers the diagnosis, imaging as well as therapy aspects of biomolecule functionalized nanomaterials against PC. Moreover, in this review special emphasis was made on circulating tumor detection, detection of different components of PSA, imaging of PC tumors, and combinational therapies by discussing all kinds of biofunctionalized nanomaterials to fill the gap of earlier published articles [75], [76], [77], [78], [79], [80]. Lastly, an attempt was made to discuss the possibilities of using extracellular vesicles for biofunctionalization against PC which can be extended to other diseases too.
Biofunctionalized nanomaterials for PC detection, diagnosis, and imaging
The last decade was enriched with the emergence of several active targets present in PC tumors such as STEAP1, B7-H3, and TROP2 apart from CD46 and PSMA [81]. Researchers have focused on targeting these active PC targets through a biofunctionalization approach for detection and treatment. The following sections have discussed different biofunctionalized nanosystems used for the detection of PC.
Antibody functionalized nanomaterials
An attempt was made [82] to detect PSA levels utilizing an electrochemical method by mesoporous silica nanoparticles (Ag@MSNs) conjugated with the prostate-specific antibody. The MSNs provided enhanced fixation capacity of the antibody whereas Ag NPs helped in modulating the electron transfer rate. This sensing platform had a limit of detection (LOD) value of 15 pg ml−1 and linearity in the range of 0.05 to 50.0 ng ml−1. Moreover, the prepared sensing platform was also tested for human serum patient samples achieving very good recovery and low error [82]. A localized surface plasmon resonance (LSPR) method was utilized to detect PSA levels using gold nanoparticles (AuNPs) conjugated with an anti-PSA antibody. [83]. Combining AuNPs with graphene oxide (GO) has become an exciting prospect for immunosensors. An immune sensing platform composed of AuNPs over GO surface and conjugated with an anti-PSA antibody was fabricated for efficient detection of PSA[84]. Similarly, other groups also fabricated bioconjugated nanomaterials especially designing composites of metal nanoparticles with MoS2 and Ti3C2 MXene nanosheets for the detection of PSA using electrochemical methods with very low LOD values and broader linear ranges [85], [86], [87], [88].
Accurate evaluation of different components of PSA i.e., total PSA (t-PSA) and free PSA (f-PSA) can give real time information about PSA in blood serum. Li et al. designed a multiplex electrochemical biosensor by integrating MoS2 with platinum@Au nano prisms and polydopamine film for f-PSA and t-PSA detection in the concentration range of 0.0001 ng/mL-100 ng/mL in serum with LOD values of 0.1 pg/mL and 1.1 fg/mL, respectively. The designed biosensing platform exhibited good performance in real clinical samples with improved specificity in the grey zone [89]. Surface Enhanced Raman scattering (SERS) based immunoassay is also very helpful in determining f-PSA and c-PSA (complexed PSA) in blood serum. A sensing platform was created using two different antibody-conjugated SERS nanotags (f-PSA and c-PSA) and t-PSA conjugated magnetic beads (conjugated via EDC/NHS). A clinical serum-containing both f-PSA and c-PSA antigen, when mixed with t-PSA conjugated magnetic beads, then both types of antigens, were captured on the magnetic bead surface via antibody-antigen reaction. Afterward two different AuNPs having f-PSA antibody with MGITC labelled and c-PSA antibody with XRITC labelled were added to make an immunocomplex sandwich system. These immunocomplexes were separated using the magnetic bead and with the generation of Raman signals from both the nanotags different PSA, components were quantified and detected as shown in Fig. 3 [90]. In another work, a fully automated microfluidic platform was developed to determine f-PSA and t-PSA using the SERS technique. With this approach, a good linear relation (in the range of 0.05 ng mL−1 to 100 ng mL−1) and a low LOD value of 0.1 ng mL−1 were determined for both f-PSA and t-PSA respectively [91]. Kang et al., also performed the SERS based immunoassay using bioconjugate multivalent antibody “(Fab − FcBP” to IgG’s Fc region) gold nanoplates for PSA detection [92].
Fig. 3.
Sequential SERS-based assay process for the simultaneous detection of f-PSA and c-PSA. (i) Mixing of f-PSA, c-PSA, and t-PSA antibody-conjugated magnetic beads. (ii) Addition of SERS nano tags to form sandwich immunocomplexes. (iii) Separation of magnetic immunocomplexes using a magnetic bar. Simultaneous detection of (iv) f-PSA and (v) c-PSA. Adapted with permission from ref [90].
Apart from measuring PSA, reports were presented on direct visualization and imaging of PC tumors using different antibodies functionalized nanomaterials. The direct visualization gives information about localization, risk positioning and PC stages which later can be used for different focal treatments. A commercially available superparamagnetic nanoparticles (SPION) formulation conjugated with Molday ION Rhodamine-B Carboxyl (MIRB) and monoclonal antibody “muJ591” were used for non-invasive and specific detection of PSMA positive PC (LNCaP) cells using 3 T clinical MRI [93]. In another report, MoS2 based quantum dots bioconjugated with an anti-PSMA antibody were used for the imaging of LnCaP cells using a 1064 nm laser [94]. Apart from bioimaging, chemiluminescence (CL) imaging was also explored for PSA detection. The AuNPs were bioconjugated horseradish peroxidase (HRP) and the secondary antibody of PSA (Ab2) to construct HRP-Au NPs-Ab2. The PSA detection was based on multiple signal amplification strategies. The designed sensing platform detects PSA through a photomultiplier tube (PMT) and is visually analyzed by a charge-coupled device (CCD) with LOD values of 0.05 pg mL−1 and 0.1 pg mL−1 with PMT and CCD. PSA levels were also detected in human serum samples with excellent recoveries (in the range of 82.5 % −117 %) [95]. AuNPs functionalized with a prostate-specific antigen (PSMA) were also used to image prostate cancer through X-ray fluorescence imaging [96].
Analysis of circulating tumor cells (CTCs) is also very important to gain insight into cancerous tumor progression. Herein, a highly sensitive profiling approach was designed to monitor CTCs in metastatic castrate-resistant prostate cancer (mCRPC). The cell capture and profiling were done using antibody functionalized magnetic nanoparticles. The mentioned approach was better than the CellSearch method (An FDA cleared method) especially in achieving higher capturing efficiency (i.e., these magnetic nanoparticle-based approaches measured CTCs from 86 % of patients whereas the CellSearch method detected only from 60 % of patients) [97]. In a nutshell, antibody functionalized nanosystems have shown ample potential in PSA detection as well as direct visualization.
Aptamer functionalized nanomaterials
Aptamer provides very high specificity and affinity just like antibodies with long-term stability and lack of immunogenicity [78], [98], [99]. Lots of work were also done on using electrochemical method-based detection with aptamer functionalized nanomaterials. An impedimetric aptasensor was designed by attaching AuNPs to the gold planar surface and co-immobilizing it by DNA aptamer/capto-1-hexanol (MCH) probe layer to detect PSA. A detection value as low as 10 pg/mL was achieved with that sensing platform [100]. Similarly, carboxylic acid CNTs immobilized with a PSA aptasensor were used to detect PSA [101]. Heli et al. designed a PSA aptamer immobilized onto Au nanospheres to electrochemically detect PSA [102]. Some other groups have also explored aptamer functionalized nanomaterials to detect prostate-specific biomarkers, especially the PSA using the electrochemical method [103], [104], [105], [106], [107], [108].
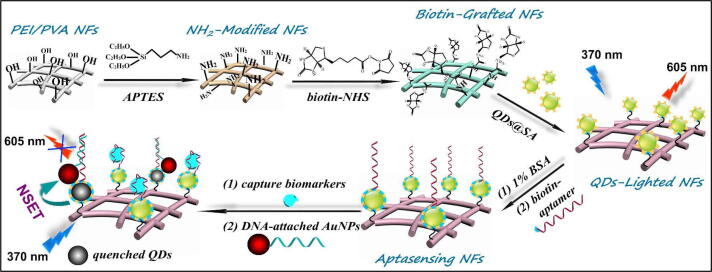
Just like the electrochemical method fluorescence-based detection was also explored for PSA detection. In a very recent report, MoS2 nanoflowers combined with spherical SiO2 nanoparticles were conjugated with a DNA aptamer to detect PSA and sarcosine (SAR) biomarkers for PC detection by measuring variation in fluorescence intensity [109]. Similarly, Su et al., developed daunorubicin (DNR)-loaded MUC1 aptamer-near infrared (NIR) CuInS2 quantum dot (DNR–MUC1–QDs) for PSA detection with the LOD value of 19 nmol L−1 [110]. A prominent work by combining biofunctionalized quantum dot (QDs) nanocomposites with Au nanorod conjugated with PSA-aptamer was reported to detect PSA by measuring the variation in fluorescence intensity [111]. A membrane-based fluorescence sensing platform was designed by combining QDs in the polymeric nanofiber. The QDs were coated with streptavidin to create QD@SA. These QD@SA were bio grafted onto the PEI/PVA based biotin engineered nanofibers (QD/NFs). During the sensing process, the aptamer integrated selectively captures the PSA. Afterward with Au NPs a partial complementary DNA was attached to quench QDs fluorescence because of the NEST mechanism as illustrated in Fig. 4. The amount of PSA was determined from the quenching value. With this method, PSA was detected with a LOD value of 0.46 pg/mL [112]. Combining the surface grafted technique with advanced hierarchical structures can easily be used for other biomarkers too. A fluorescence covalent energy transfer strategy between g-C3N4 quantum dots (g-CNQDs), palladium triangular plates (Pd TPs), and PSA specific aptasensor were used to measure PSA with the LOD value of 4.2 pg mL−1 and in the linear range 10 pg mL−1-50 ng mL−1 [113].
Fig. 4.
Schematic illustration of QDs-lighted electrospun nanofibrous fluorescence aptasensing platform for biomarker assay. Adapted with permission from ref [112].
SERS based many immunosensors were also developed for detecting disease-specific biomarkers against PC functionalized with aptamer. A core–shell nanostructured material was prepared with Au and Ag as “Au@4MBN@Ag”. Here 4MBN acts like a Raman reporter molecule and were anchored on the surface of Au NPs. After the preparation of “Au@4MBN@Ag”, an aptamer was immobilized onto its surface to make a core-satellite like structure for specific recognition and capture of the PSA molecule. In this work, the aptamer was modified with a thiolate group and gets paired and stably fixed between core and outer satellite nanoparticles. Because of the presence of abundant SERS hot spots with this core-satellite like structure enhanced Raman signals were generated. The author detected PSA with a LOD value of 0.38 ag mL−1 in the detection range of 10-2 to 10-15 mg mL−1. Moreover, the author also authenticated PSA detection with patients’ blood having PC and found comparable accuracy with the commonly used ELISA assay. This type of detection method provides an exciting possibility to be explored as a detection tool for PC screening [114]. The aptamer functionalized nanomaterials are prominently used for PSA based detection only however excellent LOD values and broader liner ranges were observed.
Proteins, nucleic acid, and naturally derived biomolecule functionalized nanomaterials
Apart from antibodies and aptamers, several other biomolecules were functionalized with different nanomaterials for diagnosing PC. Huang et al. developed the Förster resonance energy transfer (FRET) method using CDs labelled with hairpin DNA and GO to detect PSA with a LOD value of 0.22 ng mL−1 [115]. In another report, GO-ssDNA based biosensor was developed for electrochemically PSA detection [116]. Biotinylated peptides were used as a functionalizing agent with Cu2+ nanoparticles (Cu-P NPs) for enzyme-free colorimetric detection of PSA [117]. MRI based imaging can improve the early detection needed for PC cells. In the clinical world after MRI, mostly a biopsy procedure takes place in which an ultrasound image (US) gets merged with an MR image to generate a 3D image of the prostate gland. In recent years MRI-US merged strategy has upgraded the cancer biopsy but has certain limitations causing overdiagnosis and over-treatment of patients. Because of this, a recent investigation used PLGA based polymeric nanoparticles functionalized with Clostridium perfringens enterotoxin amino acid sequence (C-CPE) and loaded with SPION for MR imaging and rhodamine dye for FL imaging used to target claudin-3 (Cldn-3) and claudin-4 (Cldn-4). These Cldn-3 and Cldn-4 have a high level and largely expressed PC cells. In two different sized functionalized nanoparticles i.e., CRS1 (average diameter- 152.9 ± 15.7 nm) and CRS4 (average diameter- 421.2 ± 33.8 nm), CRS1 showed greater tumor specificity and upregulated tumour-to-liver signal intensities. Although size-dependent tumor uptake was observed the functionalization improved the tumor-targeting capability. The current study has a strong clinical prospect and in future, by conjugating other antibodies/aptamers, etc. broader PC targets can be explored [118].
As discussed before detecting PSA in the gray zone is also very essential and detecting PSA within CTCs is very novel. For this, facile evaporation induced self-assembly method was developed to prepare CTC chips to achieve PSA detection in its grey zone. Combining CTC detection with PSA-based tests through machine learning, PSA in the grey zone was detected as shown in Fig. 5. This method results in improved diagnostic sensitivity from 58.3 % to 91.7 %. This type of work can accelerate liquid biopsies’ clinical significance and can also be used for other fatal diseases [119].
Fig. 5.
Overview of the efficiency of the CTC chip towards the highly sensitive and non-invasive diagnosis of PC in the PSA gray zone. According to the results of tissue biopsy, PSA exhibits low sensitivity (58.3%) for the diagnosis of PC in the PSA gray zone. In contrast, the combination of CTC detection and PSA-based tests via machine-learning analysis can further upgrade the diagnostic sensitivity to 91.7%, thereby providing a promising non-invasive alternative for the diagnosis of PC in the PSA gray zone. Adapted with permission from ref [119].
Apart from proteins and nucleic acids several naturally derived biopolymers were explored for PC theranostics. Among different biopolymers, hyaluronic acids (HA) are one of the prominent biopolymers used for PC imaging. HA is the main constituent of the extra cellular matrix and plays a major role in the growth of cells and maintaining tissue structural stability [120], [121], [122]. Zhao et al., conjugated HA with zeolitic imidazolate framework-8 (ZIF-8) for MRI imaging of PC-3 cells [123]. Table 2 summarizes the sensing characteristics of biofunctionalized nanomaterials used in recent times for diagnosing PC. Mostly the published work involved the detection of PSA, J1 biomarkers, CTCs, and direct imaging of PC cells. The linearity and LOD values varied based on the source of nanomaterials, biofunctionalization strategy, and detection method. Importantly, in recent times some notable works were done with SERS based diagnosing platforms along with imaging and SPR. However, most of the work for PC diagnosing was still based on an electrochemical approach. Aptamer provides equivalent affinity and specificity to antibodies along with easy modification, immobilization, excellent stability, and very higher reproducibility whereas antibody functionalization provides very specific “antigen–antibody” interactions. Also, with antibody functionalization, we can explore other biomarkers apart from PSA which is specific to PC. Apart from antibodies and aptamers, other biomolecules like harpin DNA functionalized nanomaterial, peptides, etc. provide us with a new possibility to move from an electrochemical to a more calorimetric diagnosis approach, which is simpler, robust, and can be used for high throughput screening. The limitations associated with PSA biomarkers both in clinical management and patients have recently motivated researchers to explore different alternate PC biomarkers [124]. Some of the major biomarkers that have recently been explored include α-Methylacyl coenzyme A racemase (AMACR) and PTEN gene deletions [125], [126], [127], [128]. However, to the best of our knowledge, biofunctionalized nanosystems were not used so far in detecting these two biomarkers for PC detection. Apart from these biomarkers, Tkac et al., reported sarcosine, which is a potential PC biomarker, detection using an amperometric method with Ti3C2TX MXene/Chitosan nanocomposite. The proposed sensing method had an excellent sensing response time i.e. 2 sec and a high recovery index in urine samples [129]. Some other groups also detected sarcosine with different nanomaterials such as SPIONs, platinum/carbon nitride (Pt/g-C3N4), etc. using different analytical techniques like electrochemical, colorimetric, and FRET detection [130], [131], [132]. Exploring the next generation of PC biomarkers provides an exciting opportunity for concrete and decisive guidance for risk assessment and treatment decisions. However clinical implementation limitations are needed to be taken care of for utilizing these non-PSA biomarkers.
Table 2.
Biomolecule functionalized nanomaterials for PC detection.
| Biomolecule | Sensing method | Biomarker detected |
Linear Range | Limit of Detection | Ref. |
|---|---|---|---|---|---|
| Antibody and BSA | Electrochemical | PSA | 1–10,000 pg/mL | 0.48 pg/mL | [86] |
| Aptamer | Electrochemical | PSA | 0.5–350 pg/ mL | 0.14 pg/ mL | [133] |
| PSA antibody | Electrochemical | PSA | 0.001 fg/mL to 0.02 μg/ mL | 5.4 fg/ mL | [84] |
| Hairpin DNA | Fluorescence | PSA | 1–100 ng/ mL | 0.22 ng/ mL | [115] |
| Antibodies and Horseradish peroxidase | Chemiluminescence Imaging platform |
PSA | 0.1–100.0 ng/ mL (PMT) 0.5 – 100.0 ng/ mL (CCD) |
0.05 pg/mL (PMT) 0.1 pg/mL (CCD) |
[95] |
| ssDNA | Electrochemical | PSA VEGF |
1–100 ng/mL 0.05–100 ng/mL |
1 ng/mL 50 pg/mL |
[116] |
| Free-PSA and Total-PSA antibody | SERS | f-PSA t-PSA |
0.05–100 ng/mL | 0.1 ng/ mL | [91] |
| Antibody | ELISA | PSA | 0.0005–0.01 ng/mL | 0.36 pg/mL | [134] |
| Multivalent antibody | SERS | J1 | – | 10 pM | [92] |
| Free-PSA Total-PSA Complex-PSA antibody |
SERS | f-PSA c-PSA |
5.0 pg/mL − 50 ng/mL 45.0 pg/mL − 450 ng/mL |
0.012 ng/mL 0.15 ng/mL |
[90] |
| Antibody | CTC detection + PSA-based hematological | Capturing of Circulating tumor cells | – | – | [119] |
| Antibody | LSPR | PSA | – | – | [83] |
| Monoclonal antibody (MUJ591) | MRI Imaging | PSMA-positive (LNCaP) cells | – | – | [93] |
| Aptamer | SERS | PSA | 10–2 to 10–15 mg/mL | 0.38 mg/mL | [114] |
| Antibody, BSA | Electrochemical | PSA | 0.05 to 50.0 ng/ mL | 15 pg/mL | [82] |
| Antibody | Electrochemical | PSA | 10−3 to200 ng/mL | 10−3 ng/mL | [87] |
| Monoclonal antibody (Ab1) | Electrochemical | PSA | 1–18 ng/mL | 0.001 ng/mL | [85] |
| Heprin, Antibody | Electrochemical | PSA | 0.1 to 50 ng/mL | 0.08 ng/mL | [135] |
| Antibody | Lateral flow immunoassay | PSA | 0.138 ng/mL | [136] | |
| Polydopamine, Free-PSA Total-PSA antibody |
Electrochemical | f-PSA t-PSA |
|
[89] | |
| Aptamer | Electrochemical | PSA Sarcosine |
2.5 fg/mL 14.4 fg/mL |
[109] | |
| Primary antibody, Streptavidin-labeled secondary antibody | Colorimetric | PSA | – | 0.29 ng/mL | [137] |
| Primary antibody, Streptavidin-labeled secondary antibody | Fluorescence | PSA | – | 0.081 ng/mL | [137] |
| Aptamer | Electrochemical | PSA | 0.01 to 10 ng/ml | 0.0079 ng/ml | [138] |
| Aptamer | Colorimetric | PSA | 0.01–15 ng/ml | 0.01 ng/ml | [138] |
| Antibody | Photoelectrochemical immunoassay | PSA | 1.0 to 10000 pg/mL | 0.22 pg/mL | [139] |
| Aptamer | FRET & Inner Filter effect | PSA | 0.9 fg/mL | [140] | |
| DNA | Photoelectrochemical immunoassay | PSA | 1.0 × 10−11 g/ mL to 1.0 × 10−7 g /mL |
0.3 × 10-13 g/mL | [141] |
| Peptide | Colorimetric | PSA | 0.001–1 ng/mL | 1 pg/mL | [117] |
Biofunctionalized nanomaterials for PC therapy and theranostics
Just like detection biofunctionalization with nanomaterials was extensively used for therapy purposes too. The following sections have discussed different biofunctionalized nanosystems used for PC therapy.
Antibody functionalized nanoformulations
Our group evaluated the possibility of bio functionalizing SPION-loaded with docetaxel with antibody J591 (a monoclonal anti PSMA antibody) represented as J591-SPION-Dtxl. The therapeutic superiority of our biofunctionalized nanoformulation was studied with in vitro and ex vivo models with PSMA (+) PC cells i.e., C4-2 and mouse tumors. Our result showed profound selectivity of prepared nanoformulation to target C4-2 cells in both in vitro and ex vivo systems. It must mention here that J591 provides the specific targeting capability against PSMA (+) PC tumors. Taken together, our work provides significant confidence in exploring antibody functionalized nanomaterials for anti-cancerous activities such as overcoming the limitations associated with conventional therapies [142]. In another work, monoclonal anti-PSMA antibodies were conjugated to apoferritin (APO)-encapsulated doxorubicin (DOX) modified gold, represented as APODOX-anti-PSMA. The antibody-conjugated nanocarrier had specific toxicity to PC cells only, which was occurring because of DOX delivery in PC. Around 90 % of LNCaP cells (PC) died with a DOX concentration of 0.25 μM after 72 h. Moreover, the synthesized nanocarrier showed excellent hemocompatible behavior thus showing the possibility for its in vivo use directly in the bloodstream [143]. A similar concept of combining an antibody (for PSMA) with a peptide to achieve site direct conjugation for in vivo delivery of DOX was performed by Adam et al., just after 3 weeks of treatment 55 % decrease in tumor volume was observed. In addition to that, the author also confirmed the targeting capability with different tissues such as the liver, kidney, and heart. This in vivo work further supports the clinical relevance of antibody conjugated nanocarrier for cancer therapeutics [144]. Some other notable works involving antibodies such as D2B, PSMAab, CD44+, AntiSar, and anti-FOLH1 monoclonal antibodies were also conjugated with various nanomaterials such as carbon nanohorns, BSA nanoparticles, lipid-PLGA nanoparticles and chitosan nanoparticles for targeted delivery of various anti-cancerous drugs into PC cells [145], [146], [147], [148], [149]. Apart from delivering a single chemotherapeutic drug, a recent report was published on the co-delivery of DOX and tanshinones (a Chinese traditional medicine) with PSMA conjugated lipid nanoparticles [150].
Antibody conjugated nanosystems were also used for different radiation therapies against PC to minimize the limitation associated with unconjugated radiation therapies. A few notable works also explored α-particles (therapeutic radionuclide) conjugated with PSMA antibody (mAB) to target PSMA for fast binding and intracellular localization of the radioactive particle in the tumor region for achieving higher killing efficiency [151], [152].
The exciting possibility of chemo and radiation therapy with antibody conjugated nanocarrier can lead to very fewer chances of off-target and side effects. Thus, combining both these therapies and conjugating antibodies can generate more synergetic effects in terms of the higher killing of cancerous cells and thus better results. For this kind of work, a theranostic agent- AuNP-5kPEG-PSMA-1-Pc4 was reported to have the combination of AuNPs loaded with a photodynamic drug i.e., Pc4 and conjugated with PSMA-1. The in vitro results showed selective higher uptake of as prepared theranostic agent in PSMA + PC3pip cells. Upon light exposure, more cell ablation was observed in PC3pip cells. Likewise, from the in vivo experiments greater Pc4 accumulation was observed specifically with PSMA-expressing tumors and maximum accumulation was achieved just after 3 h of post-injection. The author also validated their theranostic agent’s photodynamic therapy efficacy in vivo with exciting results [153]. Although the above-mentioned work provided a new avenue for combinational therapies, the drug used there were specific for photodynamic therapy only. To move further, a recent report on combining the chemotherapy drug “docetaxel” with gold nanostars for NIR photo and chemotherapy was reported. The designed multifunctional platform was conjugated with CD133 antibody and represented as GNS@IR820/DTX-CD133. The advantage of this multifunctional platform includes enhanced therapeutic efficacy, higher aqueous stability, elevated drug loading efficiency, and increased tumor-targeting capability. The transmission electron microscope (TEM) revealed the distribution of GNS@IR820/DTX-CD133 into the organelles and cytoplasm of PC3 cells. The in vitro assay provided dose-dependent cytotoxicity and anti-tumor activity whereas the in vivo studies supported satisfactory tumour-targeting capability and anti-tumor capability as confirmed with different biological assays like H&E staining, immunohistochemical staining, and TUNEL experiment as shown in Fig. 6 [154]. Considering the exciting possibility of antibody functionalized nanomaterials for PC therapies it will be interesting to see its clinical transition.
Fig. 6.
Combined therapy in vivo. (A) The representative infrared photothermal images of the tumors treated with PBS, GNS, GNS@IR820/DTX and GNS@IR820/DTX-CD133 after the exposure to NIR-light. (B) The temperature variations in various groups with NIR-light irradiation. (C) The tumor volume in various groups with NIR-light irradiation. Data represent mean ± S.D. (n = 6, ***P < 0.001, Student's t test). (D) Survival curves of tumor-bearing nude mice in various groups after NIR-light irradiation treatment. (E) The weight change curve of tumor-bearing nude mice in various groups after NIR-light irradiation treatment. (F) Representative H&E sections of the tumors were treated with combined therapy for 21 days. Scale bar, 100 μm. (G) Representative immunohistochemical sections of Ki 67 in the tumors were treated with combined therapy for 21 days. Scale bar, 50 μm. (H) Representative TUNEL images of the tumors treated with the combined therapy for 21 days. Scale bar, 50 μm. Adapted with permission from ref [154].
Aptamer functionalized nanoformulations
Langer and their group were one of the pioneers in the area of exploring RNA aptamer functionalized nanomaterials for PC therapies. Therapeutic drugs like dextran, docetaxel, and cisplatin were explored for targeted delivery with polymeric nanoparticles functionalized with nucleic acid aptamers [155], [156], [157]. Farokhzad et al. also documented docetaxel delivery with polymeric nanoparticles functionalized with RNA aptamers. The aptamers chosen here are directly isolated against live cancer cells. This type of strategy can target a range of antigens present in cancerous cells. In addition, the aptamer selection strategy was based on aptamer enriched internalization rather than the highest affinity [158]. Apart from exploring RNA based aptamer for biofunctionalization a single-strand DNA-A9 PSMA RNA hybrid aptamer was tried by conjugating it with dendrimer for the delivery of DOX. As evident DOX uptake was very high in PSMA (+) cells, and the prepared DOX loaded nanocarrier showed greater cytotoxicity with PSMA (+) cells as compared with PSMA (-) cells. The in vivo model further supported the anti-cancer efficacy as around a 78 % decrease in tumor volume was found [159]. The initial work related to aptamer functionalized nanomaterials mostly involved polymeric nanoparticles and delivery of the anti-cancerous drug.
Targeted gene delivery at PC tumor sites is also very beneficial especially in tuning the gene expression (both up-regulation and down-regulation). An RNA aptamer (APT) was functionalized with ATE-based miRNA nanoparticles represented as miRNA/ATE–APT and had a particle size of 221 ± 6.9 nm for its targeted delivery of in vitro and in vivo PC cells. The anti-cancer potency was evaluated using CCK8 assay and IC50 values. Dose-dependent cell growth inhibition was found with miRNA/ATE–APT [160]. miRNA systemic delivery into PC cells was also done using aptamer A10.3.2 functionalized with PEG-PAMAM nanoparticles [161], [162]. Gene therapy has become one of the most influential therapies, especially against castration-resistant PC. Most of the gene delivery studies involved short non-coding RNA delivery, however, some recent reports revealed long non-coding RNAs (lncRNAs) are closely associated with tumor development [163]. IncRNA MEG3 has shown significant promise against castration-resistant PC and so far, its delivery is very rare despite having ample potential. A novel delivery platform was designed using a PAMAM dendrimer conjugated with EpDT3 aptamer to deliver lncRNA MEG3 (pMEG3) in castration-resistant PC cells. The pMEG3 loaded nanocarrier was easily uptake by the cells through endocytosis. The in vitro and in vivo studies further supported the anti-cancer possibility of pMEG3 loaded nanocarrier [164]. In the library of different gene-editing approaches, the most recent addition of CRISPR-Cas9 has generated vast excitement among the scientific community because of its cheaper, faster, and more accurate editing process [165]. The maximized efficacy of CRISPR-Cas9 against PC prominently depends on the delivery route. In a recent report, safe and efficient delivery of CRISPR-Cas9 for PC therapy was developed by loading them in liposome nanoparticles and conjugating them with an RNA aptamer specific for PSMA ligand represented as A10-liposome-CRISPR/Cas9. PLK1 mRNA levels were determined by RT-PCR to evaluate the biological activity of A10-liposome-CRISPR/Cas9. The obtained result demonstrated significant improvement in transfection efficiency due to targeting capability coming from the conjugated aptamer. The cellular uptake assay showed higher uptake of A10-liposome-CRISPR/Cas9 in cell cytoplasm as compared with other control conditions. Cell viability study using MTT assay further confirms the anti-cancer nature and obtained results were in line with other results. From the in vivo results, a substantial reduction in tumor volume was found for A10-liposome-CRISPR/Cas9. A detailed biological assay study with A10-liposome-CRISPR/Cas9 nanocarrier must have delivered a profound impact on smart PC therapies [166].
When we see literatures for aptamer functionalized nanomaterials against PC therapy most of the work was based on the delivery of a single therapeutic agent (both chemotherapeutic drugs and gene therapy materials). Combined chemotherapy has shown itself to be very effective for PC therapies [167]. A lipid hybrid nanoparticle (LPN) conjugated with aptamer and loaded with two anti-cancer drugs curcumin (CUR) and cabazitaxel (CTX) represented as “APT-CUR/CTXLPNs” were explored against PC. From LPN both CUR and CTX had sustained release and aptamer functionalization provided good cell uptake, higher tumor accumulation, and incredible tumor inhibition efficiency both in vitro and in vivo [168]. Apart from combined chemotherapy, combining the drug with some functional material such as SPION has shown interest in PCs therapies. A polymeric nanomaterial loaded with Dtxl (anti-cancer drug) and SPION (MRI agent) was functionalized with aptamer Wy5a for image contrast MRI capability and controlled release. Confocal imaging revealed localization of the nanocarrier in cell cytoplasm whereas concentration-dependent MRI signalling was also observed. The confocal and MRI images confirmed the targeted uptake because of the functionalized Wy5a aptamers. The Wy5a-SPIO-NPs showed tumor regression property assessed using xenograft models of PCA after 5 weeks. Moreover, in vivo toxicity was also assessed from the white blood cell count and the results were in the normal range [169]. A similar approach explored AuNPs dual conjugated with A10 and DUP-1 aptamer and Fe3O4 loaded with DOX and conjugated with PSMA aptamer were explored for photothermal therapy as well as image-guided therapy against PC cells [170], [171]. Designing aptamer functionalization with nanocarrier provides preferential delivery of therapeutic agents at the intended site leading to effective tumor suppression. Just like antibody functionalized nanomaterial for photo and chemotherapy against PC cells, a report by Zheng et al., was on AS1411 aptamer, functionalized with mesoporous polydopamine (MPDA) and loaded with docetaxel for the synergistic killing of PC cells both in vitro and in vivo. It must be mentioned here that aptamer used in this study specifically binds to nucleolin (which can act as a biomarker for PC) thus providing selective uptake with PC-3 cells only and cell death occurred because of mitochondria-mediated apoptosis [172]. These aptamers functionalized nanosystems can be explored for other biomarkers apart from PSMA or PSA based ones so that more precise targeting ability as well as upgraded therapeutic efficacy can be achieved. All these reports showed the importance and promise of aptamer functionalized nanomaterials, especially for the delivery of different chemotherapeutic drugs.
Proteins, nucleic acid, and naturally derived biomolecule functionalized nanoformulations
Apart from exploring antibodies and aptamers based bioconjugation strategies for PC treatment, other biomolecules like proteins, peptides, nucleic acid, etc. were explored for functionalization. To start with, An AuNPs having varied surface ligand density and size were conjugated with PSMA-1 for targeted X-ray radiotherapy as shown in Fig. 7. Regardless of particle size PSMA-1 conjugated AuNPs exhibited specific uptake by PSMA-expressing PC3pip cells. However, the obtained result showed contrasting results with cell sensitization and particle size, i.e., larger-sized (∼19 nm) AuNPs had greater uptake into the cells but smaller sized i.e., (∼2 nm, ∼5 nm) showed better radiotherapy. Interestingly for in vivo, larger-sized AuNPs uptake was lower too which results in a better radiosensitization effect with small AuNPs. The author hypothesized smaller AuNPs ability to extravasate from the vasculature to bind and enter tumor cells thus providing more effective radiotherapy. The author also investigated the elimination and metabolism pathways. Irrespective of size variation, AuNPs showed increased liver retention however with small-sized AuNPs the tendency to be cleared from the urinary system was more. The obtained result gives a better insight into size-dependent off-target toxicity but long-term metabolism is still needed to be investigated in these kinds of studies [173].
Fig. 7.
Schematic illustration of targeted radiotherapy of PC using PSMA-targeted AuNPs of different sizes. Adapted with permission from ref [173].
In another work glutathione-stabilized (Au@GSH) gold nanoparticles were constructed for the delivery of platinum-based anticancer drugs in PC cells [174]. Bombesin (a tetradecapeptide isolated from the skin of the European fire-bellied toad) was functionalized with AuNPs to specifically target GRP-Rs cell surface which is expressed in a larger number in PC cells [175]. Just like Bombesin, gelatin (a biopolymer and responsive towards matrix metalloproteinases) was conjugated with DOX and was coated on AuNPs along with epigallocatechin gallate (EGCG) for inhibiting the growth and proliferation of PC-3 cells and enzyme controlled intracellular DOX release [176]. Considering the small size and high dangling bonds associated with AuNPs, its surface can easily be modified and has shown ample opportunity for cargo delivery. Rahme et al. reported two functionalized AuNPs represented like AuNPs-PEG-Tf (with transferring targeting ligand) and AuNPs-PEI- FA (with folate receptor targeting ligand). The AuNPs-PEG-Tf had good uptake in PC-3 cells through the receptor-mediated uptake process whereas AuNPs-PEI- FA delivered siRNA into LNCaP cells. This work gives a new possibility for non-viral gene delivery against PC cells [177]. Almowalad et. al. also showed enhanced gene transfection ability of Lactoferrin loaded with gold nanocages and conjugated with complexed DNA [178]. Recent advancement of the redox-responsive polyamine cationic dendrimersomes (dendrimer-based vesicles) was successfully validated against the PC treatment leading to possible drug-gene combination therapy, where such polyamine dendrimer (third generation) was modified with either bioactive molecule (camptothecin) or lipid (cholesterol or octadecyl chain) groups [179], [180], [181], [182]. Another biopolymer chitosan nanoparticles were synthesized to deliver a fungal metabolite (TM2) from T. atroviride for anti-cancer activity against PC cells by activating caspase 3 and inhibiting BCL-2 expression in PC cells [183]. In another report, HA based nanoparticles were prepared with polyethylene glycol-gelatin (PEG-gelatin) to deliver epigallocatechin-3-gallate (EGCG) causing inhibition of PC cell growth [184]. Some notable work involved biomolecules and natural products like bacterial genotoxin (CdtB), melanin, polydopamine, curcumin, and folic acid conjugated with different nanomaterials for PCs therapies like radiotherapy, phototherapy, chemotherapy, and gene therapy also showed excellent cell specificity and anti-cancerous effect [185], [186], [187], [188], [189], [190].
Exploring membrane bound PSMA as a target site for anti-cancer drug delivery in PC cells has shown lots of promise both in the lab and in clinical settings. Similarly, PSMA folate hydrolase gives tumor cells advantages for their growth. Thus, exploring PSMA as a target and incorporating a folate-based delivery channel can become advantageous. Taking this, a novel nanoformulation was explored from folate-grafted liposomes to deliver MLP pro drug and DOX. The nanoformulation showed its applicability for delivering drugs both in PSMA-expressing tumors and folate receptor-expressing tumors [191]. A multi functionalized GO nanosheet showed a T1 contrast image of specifically targeted PSCA over-expressed PC cells and delivered DOX both in vitro and in vivo thus showing its potential for dual applications like advanced PC imaging and efficient tumor growth inhibition [192]. A green therapeutic approach was developed with gum acacia-PEG grafted with magnetic iron oxide as “GA-PEG-IONC” against PCs cells by measuring HOXB13 gene expression levels in them. In the presence of IONC, the HOXB 13 gene expression was inhibited causing a cytotoxic effect and PC cell death [193].
In the past few years, innovative biomimetic platforms were designed to inhibit PCs tumors through high-temperature ablation directly or with other therapies. A genetically modified T7 bacteriophage was used as a template such that AuNPs were self-assembled on it forming a hollow nanocluster represented as GP-phage@AuNPs for PC photothermal therapy. LDH and CC8-assay confirmed the biocompatible nature of GP-phage@AuNPs confirming that phase does not cause any side effects to the cells which also supported their future in vivo applicability. ICP-MS confirmed the specificity and direct field microscopy confirmed the targeting ability of GP-phage@AuNPs with PCs cells. With the application of the NIR laser, PC cells died because of the photothermal effect. This type of result opens a new avenue for a genetically engineered system for designing more specific cancer therapies [194]. The emergence of combinational therapy by combining phototherapy and chemotherapy has made castration-resistant prostate cancer (CRPC) treatment more feasible. A new approach based on the activation of nanoformulation “PGP/CaCO3@IR820/DTX-HA” having CaCO3 (for docetaxel delivery and entrapment of IR820 photosensitizer) grown on the pentagonal gold prism and modified with hyaluronic acid was used for tumor-targeted PC therapy as shown in Fig. 8. With 808 NIR laser irradiation, PGP/CaCO3@IR820/DTX-HA suspension reached a temperature of 68.5 °C within 6 min showing its excellent PTT conversion efficiency similarly PGP/CaCO3@IR820/DTX-HA also showed enhanced singlet oxygen generation confirming its suitability for PDT. The TEM images revealed the distribution of PGP/CaCO3@IR820/DTX-HA in PC cell cytoplasm which takes place because of micropinocytosis. The confocal laser scanning microscopy (CLSM) image confirmed the delivery of docetaxel and IR820 into PC cells. The CC8-assay showed dose-dependent anti-cancer behavior upon laser irradiation which was supported by a flow cytometry assay. The in vivo studies also demonstrated the accumulation of PGP/CaCO3@IR820/DTX-HA in the tumor region. This targeted accumulation takes place because of the EPR effect and the presence of HA onto the nanoplatform surface. The obtained result provides an excellent promise in the area of integrated smart nanoplatforms for CRPC theranostics [195]. A report based on polymeric epigallocatechin 3-gallate (EGCG)-loaded nanoparticles (NPs) conjugated with folic acid showed very good anti-cancer behavior towards PSMA (+) 22Rv1 PC cells [196].
Fig. 8.
Schematic representation of PGP/CaCO3@IR820/DTX-HA nanoprobes for combined PTT/PDT/CT and multimodal imaging. Adapted with permission from ref [195].
Autophagy has been considered the most prolific and smart rescuer and reinforced mechanism for cancerous cells against photothermal therapy. Moreover, because of autophagy the recurrence and metastasis of the treated tumor may occur which can worsen the disease [197], [198]. Herein, a biomimetic nanoplatform represented as “mPDA@CMs NPs-CQ” was designed using mesoporous polydopamine nanoparticles (mPDA NPs) coated with the PCs cell membrane (CMs) and encapsulated with chloroquine (CQ), an autophagy inhibitor for achieving synergistic photothermal effect as shown in Fig. 9. The designed nanoplatform escaped the macrophage phagocytosis and overcame the vascular barrier so that it can target homologously the PC cells. The mPDA@CMs NPs-CQ nanoplatform exhibited decent photothermal conversion efficiency of 39.36 % so that cell-based autophagy can be activated and the delivery of CQ inhibited the autophagy thus providing synergistic photothermal killing. Major cell-based events like induction of autophagosome accumulation, reactive oxygen species generation, generation of endoplasmic stress, damage to mitochondrial membrane, and generation of apoptotic signals occurred with the application of NIR light causing very powerful suppression of PC cells and prostate tumor xenografts. Moreover, no histopathological damage against major organs like the liver/ kidney was found when mPDA@CMs NPs-CQ were subjected to NIR light confirming the good biosafety and in vivo biocompatibility associated with mPDA@CMs NPs-CQ [199]. However, the mentioned work was done with subcutaneous prostate tumor thus further studies with tumor microenvironments are needed to be performed.
Fig. 9.
Schematics of the fabrication processes of mPDA@CMs NPs-CQ and the subsequent synergistic antitumor strategy combining PTT killing and autophagy blocking. Adapted with permission from ref [199].
Table 3 summarizes the biofunctionalized nanomaterials used in recent times for destroying PC cells and tumors. Mostly covalent functionalization was used for conjugating the biomolecules onto nanomaterial surfaces. Biofunctionalization provides enhanced therapeutic efficacy as well as PC cell selectivity. Antibody functionalized nanomaterials have stronger and specific interaction possibilities with PC cells/tumors but having a larger size may create difficulty in targeting. Also, all the antibody functionalization strategies are based on the type of target and with PC cells mostly the antibodies were based on PSMAs. However, the efficacy achieved with antibody functionalized is still remarkable especially for combinational therapy as done by Cui et al., for delivering docetaxel as well as phototherapy [154]. With the innovation in the areas of SELEX technology, aptamer functionalized nanomaterials have explored wider PC targets and have delivered a wide range of anti-cancer therapeutics. The aptamer functionalized nanomaterials were explored for the delivery of anti-cancer drugs as well as in immunotherapy by loading and delivering a wide variety of genes (mRNA, siRNA, CRISPR-Cas9, and lncRNAs). However, literature related to aptamer functionalized nanomaterials for radiotherapy as well as hyperthermia applications are very rare thus it needed to be explored so that higher efficacy in killing PC tumors can be achieved. Apart from antibodies and aptamers, several other biomolecules functionalized nanosystems were explored for PC therapies which include polydopamine, gelatin, peptides, and amino acid sequences. The driving force behind targeting these biomolecules with PC cells is system-specific and depends upon the choice of biomolecules. Considering the wide range of peptides, proteins, amino acid, and naturally extracted biomolecules present, attaining a uniform interaction mechanism with PC cells is nearly impossible. However, the encouraging results in terms of efficacy with these systems can help create future delivery systems for PC therapies in clinical settings.
Table 3.
Biomolecule functionalized nanomaterials for PC treatment.
| Biomolecules | Nanomaterials | Payload | Conjugation | Ref. |
|---|---|---|---|---|
| mAbPSCA | Graphene Oxide | DOX | Covalent | [192] |
| Gum acacia | PEG Iron Oxide | – | – | [193] |
| DNA-A9 PSMA | Dendrimer | DOX | Covalent | [159] |
| Transferrin and Folic Acid |
AuNPs | siRNA | Complexation Electrostatic interaction |
[177] |
| A10-3.2 aptamer | Lipid-polymer hybrid nanoparticles | Curcumin and Cabazitaxel | Covalent | [168] |
| J591 | SPION | Docetaxel | Thiolated | [142] |
| Goserelin | AuNPs | – | Covalent to PEG | [200] |
| CD133 | Gold nanostars | Docetaxel | Covalent to PEG | [154] |
| Clostridium perfringens enterotoxin | SPION | – | – | [118] |
| A10 2′-fluoropyrimidine RNA aptamers |
PLA-PEG | Docetaxel | Covalent | [156] |
| A10 PSMA | PLA-PEG | Dextran | Covalent | [201] |
| EpDT3 | PAMAM | lncRNA MEG3 | Thiolated | [164] |
| Wy5a | PLGA-PEG | Docetaxel | Covalent | [169] |
| T7 phages | AuNPs | – | – | [194] |
| Anti-PSMA antibodies | AuNPs | DOX | Conjugated with HWR peptide | [143] |
| Anti-FOLH1 | Mesoporous silica nanoparticles | Docetaxel | Covalent | [149] |
| D2B | Carbon nanohorns | Cisplatin | Covalent | [145] |
| PSMA-1 | AuNPs | – | Covalent to PEG | [173] |
| AS1411 | Mesoporous polydopamine | Docetaxel | Covalent | [172] |
| A10-3.2 | miRNA/ATE | siRNA | Covalent | [160] |
| A10-3-J1 | SPION | DOX | Peptide bond | [202] |
| anti-PSMA APT | PEG-PAMAM | miRNA | Covalent to PEG | [162] |
| Bombesin | AuNPs | – | I-SPAAC reaction | [175] |
| A10, DUP-1 | AuNS | – | Thiol modified | [170] |
| PSMAab | BSA-PEILBLNPs | Docetaxel/siRNA | Amine bond | [146] |
| AntiSar | Chitosan nanoparticles | DOX | Electrostatic interaction | [148] |
| XEO2 mini | Lipid-polymer nanoparticle | Docetaxel | Maleimide-thiol chemistry | [158] |
| Glutathione | AuNPs | Platinum (IV) drug | Direct as reducing agent | [174] |
| CD44 antibodies | Lipid-poly (lactic-co-glycolic acid) nanoparticles | Salinomycin | Thiolated | [147] |
| Gelatin | AuNPS | DOX and EGCG | – | [176] |
| Hyaluronic acid | Pentagonal gold prisms | Docetaxel | – | [195] |
| Folic acid | Polymeric epigallocatechin 3-gallate | – | – | [196] |
| A11 mini body | Gold nanoshells | Dative bond | [203] | |
| Anti-FOLH1 monoclonal antibody (clone C803N) and 11-aminoundecanoic acid | Mesoporous silica nanoparticles | Docetaxel | – | [149] |
Exosomes and cloaked nanomaterials against PC
In the past few years, there have been considerable efforts in the areas of biomimetic nanotechnology for achieving an effective in vivo model [204], [205], [206]. These biomimetic nanosystems have favorable interactions with proteins, cells, and extracellular matrices. The biomimetic designing of nanosystems has been done by functionalizing nanomaterials with different extracellular vesicles (EVs) or exogenous substances like viruses. These biomimetic nanostructured materials provide camouflage for direct and indirect delivery. The functionalization strategy includes three critical steps. The first step is separation of biological vectors using cell lysis, ultracentrifugation, and purification [207], [208]. The next step include extraction of functional agents from separated biological vectors [209] and the final step includes functionalization of bioagents onto nanomaterials using bioconjugation, polymerization, electroporation etc [210], [211]. To preserve the bioactivity whole functionalization needed to be performed very gently [212]. Major bioactive agents contains RNA, lipids and proteins and are either microparticles or nanosized exosomes [213]. EVs have shown ample potential for different therapeutic applications and have been explored in cancer therapies [214], [215]. Venofer (an iron oxide carbohydrate nanoparticle) labelled with human mesenchymal stem cells (MSCs) releases exosomes containing iron oxide. These exosomes were engulfed efficiently by tumor cells. Moreover, the exosomes effectively killed cancerous tumors through hyperthermia or the release of the anti-cancerous prodrug 5-FC [216]. To further extend this concept a multifunctional theranostic extracellular vesicle (EVs) having the presence of iron oxide and mTHPC photosensitizer were explored for photo and magnetic therapy along with MRI and magnetic manipulation against PC3 cells. It must be mentioned here that EVs were produced from human umbilical vein endothelial cells [217]. In the majority of EVs based work, EVs have been extracted from cells however very tedious purification and achieving very less content of highly pure EVs limits their applicability. A nano vector “exo-PMA/Fe-HSA@DOX” was developed from EVs from exosomes extracted from urine samples. These urinary exosome-based nanovectors contain similar cancer cell membrane antigen and were taken from prostate patients. These nanovectors get deeply penetrated PC DU145 3D MCTS and showed a synergistic effect (i.e., low dose high killing) [218]. Utilizing exosomes from a natural source provides minimization with contamination thus leading to very pure exosomes thus no generation of undesirable cell inflammation and proliferation. These pioneering studies in EVs cloaked nanomaterials give a new approach to exploiting exosomes from PC patients which can be used for targeted tumor therapy. Also, EVs generated from urine can be explored for PC specific biomarkers for early detection. Apart from EVs some other biomimetic nanosystems were constructed using cancerous cells and viruses. Liu and group functionalized LNCaP PC cells with CaCO3 capped mesoporous silica nanoparticles for inhibiting prostate tumor growth [219]. In another approach a recombinant bacteriophage MS2 virus-like particles (VLPs) were prepared for immunotherapy against PC [220]. A tobacco mosaic virus (TMV) nanoparticle complexed with fluorescence (NIRF) dye Cy7.5 were also used for Near-Infrared Fluorescence Imaging (NIRF) and T2-MRI imaging of PC cells and tumors. Moreover, author performed imaging both in vitro and in vivo [221].
The biomimetic nanomaterials inherit all the main factors needed for immune escape and tumor tropism. These nanosystems can minimize the current limitations associated with diagnosis as well as medications however the major problem associated with these nanosystems is their industrial translations. The three major challenges associated with biomimetic nanosystems are stability, large-scale production, and effectiveness. Thus, more detailed research and innovations are needed to be explored in this area which can transform the cancer theranostics and can be extended to other major diseases [222].
Conclusion and Future Perspectives
So far significant efforts have been made to amalgamate biomolecules with nanomaterials to make a versatile smart bio-nano conjugated system against PC cells and tumors. In this review, we divided the biofunctionalized nanomaterials broadly into antibodies, aptamers, and all other biomolecules (including proteins, peptides, nucleic acids, bioactive molecules, and lipids) for PC diagnosis and treatment. It is evident that functionalized nanosystems provided specificity and prostate-specific targeting ability both for diagnosis and therapy. All these biofunctionalized systems have their advantages and limitations but can be seen as the most impactful prospect for future research. The early diagnosis of PC is still very much conundrum and most of the diagnosis-related studies were based on PSAs. Although very limited work was done in exploring other domains of research related to CTCs, real-time visualization of PC cells and more detailed analysis related to PSA which includes t-PSA, f-PSA, c-PSA, and detection of PSA in the grey zone. Some of the published work has shown very innovative sensing design but the tedious and complex design process may tend to increase the cost of sensor fabrication as well as analysis time. Furthermore, one of the major limitations associated with these diagnosis systems includes a lack of information related to high throughput studies. Also, the need of the hour is to merge different diagnostic techniques such as calorimetric studies can be merged with SERS based techniques so that more qualitative, as well as quantitative information, can be extracted from the same set of samples. Designing biofunctionalized nanomaterials for identifying new biomarkers can be explored such that we can move from conventional yet very uncomfortable biopsies or DRE. Similarly, more in vivo work from biofunctionalized nanomaterials therapy is needed, to validate these systems for human trials. A detailed study related to protein-corona formation; long-term toxicity is very critical. In addition, a more robust functionalization process needs to be explored so that it cannot suppress the original properties of nanomaterials. Lastly, the emergence of artificial intelligence and machine learning in the last three years can help achieve more optimized applications of these biofunctionalized nanomaterials against PC therapies. By integrating machine learning and deep learning with an imaging system, a simpler 3D image of the prostate gland can be visualized. Integrating automated systems with SERS/calorimetric or electrochemical approaches can help diagnose multiple samples. Similarly, artificial intelligence can be combined with radiotherapy, phototherapy, and hyperthermia so that patient’s real-time condition can be monitored. Apart from functionalizing the nanomaterials with different classes of biomolecules exploring cell membrane cloaked nanomaterials against PC is needed to be explored in the future for both diagnosis and therapy. The cell membrane cloaked nanomaterials can provide an effective bio interface environment which can improve the targeting capability. Some work has been done using this aspect and still needs detailed study for the diagnosis, especially in CTCs, and combinational therapies. Exploring hybrid cell membranes for coating and incorporating diverse biological moieties (like antibodies, peptides, and proteins) into the nanosystems may lead to synergistic in vivo performances.
Lastly “there is still plenty of room at the bottom” in the areas of cancer nanomedicine, and together by combining a range of biomarkers, exploration of non-invasive techniques and artificial intelligence a smart biofunctionalized PC theranostic system can transform the whole treatment process and can also be used for all other cancer therapies.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Ethics approval and consent to participate
We have not used cell lines, animals, and human tissues in this work. This study does not involve animal experiments and clinical patient recruitment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the start-up research support from the Department of Immunology and Microbiology, School of Medicine, University of Texas Rio Grande Valley given to MJ, MMY, and SCC. This work was supported by the National Institute of Health/National Cancer Institute’s funding SC1GM139727, R01 CA210192, and R01 CA206069.
Biographies

Dr. Pranav obtained his PhD degree from Indian Institute of Technology Indore, India in 2021. His PhD research was focused on sustainable carbon nanomaterials for healthcare and environmental applications. He then worked as a research associate (February 2021 to August 2021) in the department of chemistry at Indian Institute of Technology Indore where he initiated and successfully developed innovative strategies using kitchen blender for degradation of antibiotics and dyes for minimizing environmental pollutant. Currently, he is a post-doctoral research fellow at the Department of Immunology and Microbiology, School of Medicine, University of Texas Rio Grande Valley, USA, where his main interest is the development chiral nanomaterials for sensing of amino acid enantiomers and against prostate, breast, colon, liver, and pancreatic cancer.

Dr. Partha Laskar obtained his PhD degree from the Indian Institute of Technology Kharagpur, India in 2016. His research was focused on the synthesis of stimuli-responsive amphiphilic and hydrophilic random copolymers towards cancer therapy. He then worked as a post-doctoral research associate (December 2016 to February 2020) at the Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, United Kingdom, where he initiated and successfully developed smart dendrimersomes towards combination therapy against prostate cancer. Currently, he is a post-doctoral research fellow at the Department of Immunology and Microbiology, School of Medicine, University of Texas Rio Grande Valley, USA, where his main interest is the development of targeted combination therapy against pancreatic cancer.

Dr. Meena Jaggi is tenured Professor of the Immunology and Microbiology Department and Member of South Texas Center of Excellence in Cancer Research (ST-CECR) at the School of Medicine, University of Texas Rio Grande Valley (UTRGV), McAllen, Texas, USA. The primary focus of my research is to identify and evaluate the functional significance of cell-cell adhesion molecules known as cadherins and catenins in cancer progression and to understand the regulation of cadherin/catenin complex activity by Protein Kinase D signaling. In-depth knowledge of molecular mechanisms involved in signal transduction of human cancers is critical for the development of biomarker for early detection of cancer and rationalized structure-based drug designing.

Dr. Subhash Chauhan is a tenured Associate Professor of the Immunology and Microbiology Department and Member of South Texas Center of Excellence in Cancer Research (ST-CECR) at the School of Medicine, University of Texas Rio Grande Valley (UTRGV), McAllen, Texas, USA. Primary research interest of Dr. Chauhan’s lab is to identify and characterize the diagnostic and therapeutic targets for cancer. This research is aimed for the identification and characterization of biomarkers that aberrantly express or localize in cancer cells to develop newer tools for early disease diagnosis. We are utilizing genomics and proteomics approach for identification of novel early diagnostic markers. Recently we have identified a novel trans-membrane mucin MUC13 which is highly over-expressed ovarian and pancreatic and colon cancers. This may be potential biomarker for early cancer diagnosis as well as a good target for antibody guided targeted therapy. Nonspecific distribution and suboptimal delivery of the anti-cancer drug(s) to the tumor cells are the major hindrances in the successful use of traditional chemotherapy. Dr. Chauhan’s lab is developing novel targeted therapeutic modalities for the treatment and diagnosis of cancers. Cancer tissues overexpress certain cancer associated antigens, and antibodies against these antigens will potentially recognize cancer cells. These antibodies can be used to deliver the radionuclides and nanoparticles-encapsulated drugs specifically to the tumors. Research work from our lab has been presented at National and International symposiums/conferences. I have been actively involved in the peer review process of manuscripts for numerous journals, NIH study sections, multiple external funding agencies, training of junior faculties and graduate students.

Dr. Murali Yallapu is a tenured Associate Professor of the Immunology and Microbiology Department and Member of South Texas Center of Excellence in Cancer Research (ST-CECR) at the School of Medicine, University of Texas Rio Grande Valley (UTRGV), McAllen, Texas, USA. Dr. Yallapu is a recipient of the “Prof. A. Kameswara Rao's Gold Medal‐1999”. Dr. Yallapu has received his PhD degree in Polymer Science & Technology and completed postdoctoral training in materials science, drug delivery, nanomedicine, and cancer biology from Cleveland Clinic, University of Nebraska Medical Center, Sanford Research, and Gwangju Institute of Science & Technology. Before Joining the UTRGV, Dr. Yallapu served as Assistant Professor at the Department of Pharmaceutical Sciences, The University of Tennessee Health Science Center. Dr. Yallapu has been serving as Editorial Board Member for various drug delivery, nanotechnology, and cancer related journals. He has been an ad‐hoc reviewer for NIH and various other funding agency study sections. He has published over 175 peer‐reviewed articles and reviews in journals, book chapters, and over 125 conference abstracts. His work was cited over 13717 times, with an H‐index of 57 and an i10‐index of 138. His current research primarily focuses on the development of nanomaterials for improved therapeutic potential of clinical drug(s) and developing new constructs for biomedical applications. Additionally, through cancer immunology institute we will generate safe and effective nanoformulations for cancer immunotherapy.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Nader R., El Amm J., Aragon-Ching J.B. Role of chemotherapy in prostate cancer. Asian J Androl. 2018;20(3):221–229. doi: 10.4103/aja.aja_40_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costello L.C., Franklin R.B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch Biochem Biophys. 2016;611:100–112. doi: 10.1016/j.abb.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 5.Miller K.D., Fidler-Benaoudia M., Keegan T.H., Hipp H.S., Jemal A., Siegel R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020;70(6):443–459. doi: 10.3322/caac.21637. [DOI] [PubMed] [Google Scholar]
- 6.Zelefsky M., Leibel S., Gaudin P., Kutcher G., Fleshner N., Venkatramen E.S., et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol *Biology* Phys. 1998;41(3):491–500. doi: 10.1016/s0360-3016(98)00091-1. [DOI] [PubMed] [Google Scholar]
- 7.Perdonà S., Cavadas V., Di Lorenzo G., Damiano R., Chiappetta G., Del Prete P., et al. Prostate cancer detection in the “grey area” of prostate-specific antigen below 10 ng/ml: head-to-head comparison of the updated PCPT calculator and Chun's nomogram, two risk estimators incorporating prostate cancer antigen 3. Eur Urol. 2011;59(1):81–87. doi: 10.1016/j.eururo.2010.09.036. [DOI] [PubMed] [Google Scholar]
- 8.Melichar B. PSA, PCA3 and the phi losophy of prostate cancer management. Clin Chem Laborat Med (CCLM) 2013;51(4):707–712. doi: 10.1515/cclm-2013-0156. [DOI] [PubMed] [Google Scholar]
- 9.Campodonico F., Casarico A., Gavazzi L., Calcagno T., Capponi G., Canepa G., et al. Cancer detection with TRUS-guided 10-core biopsy of the prostate. An institutional assessment at the first, repeated and surgical specimen biopsy. Arch Ital Urol Androl. 2006;78(2):39–43. [PubMed] [Google Scholar]
- 10.Shinohara K. Improving cancer detection by prostate biopsy: the role of core number and site. Nat Clin Pract Urol. 2006;3(10):526–527. doi: 10.1038/ncpuro0602. [DOI] [PubMed] [Google Scholar]
- 11.Naji L., Randhawa H., Sohani Z., Dennis B., Lautenbach D., Kavanagh O., et al. Digital rectal examination for prostate cancer screening in primary care: a systematic review and meta-analysis. Ann Fam Med. 2018;16(2):149–154. doi: 10.1370/afm.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barry M.J., Simmons L.H. Prevention of prostate cancer morbidity and mortality: primary prevention and early detection. Med Clin North Am. 2017;101(4):787–806. doi: 10.1016/j.mcna.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Pepe P., Aragona F. PCA3 score vs PSA free/total accuracy in prostate cancer diagnosis at repeat saturation biopsy. Anticancer Res. 2011;31(12):4445–4449. [PubMed] [Google Scholar]
- 14.Karunasinghe N., Minas T.Z., Bao B.-Y., Lee A., Wang A., Zhu S., et al. Assessment of factors associated with PSA level in prostate cancer cases and controls from three geographical regions. Sci Rep. 2022;12(1):55. doi: 10.1038/s41598-021-04116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okotie O.T., Roehl K.A., Han M., Loeb S., Gashti S.N., Catalona W.J. Characteristics of prostate cancer detected by digital rectal examination only. Urology. 2007;70(6):1117–1120. doi: 10.1016/j.urology.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Scattoni V., Zlotta A., Montironi R., Schulman C., Rigatti P., Montorsi F. Extended and saturation prostatic biopsy in the diagnosis and characterisation of prostate cancer: a critical analysis of the literature. Eur Urol. 2007;52(5):1309–1322. doi: 10.1016/j.eururo.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Catalona W.J., Smith D.S., Ratliff T.L., Dodds K.M., Coplen D.E., Yuan J.J., et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324(17):1156–1161. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 18.Thompson I.M., Pauler D.K., Goodman P.J., Tangen C.M., Lucia M.S., Parnes H.L., et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 19.Schröder F.H., van der Cruijsen-Koeter I., de Koning H.J., Vis A.N., Hoedemaeker R.F., Kranse R. Prostate cancer detection at low prostate specific antigen. J Urol. 2000;163(3):806–812. [PubMed] [Google Scholar]
- 20.Catalona W.J., Richie J.P., Ahmann F.R., Hudson M.A., Scardino P.T., Flanigan R.C., et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6630 men. J Urol. 2017;197(2s):S200–S207. doi: 10.1016/j.juro.2016.10.073. [DOI] [PubMed] [Google Scholar]
- 21.Kang B.J., Jeun M., Jang G.H., Song S.H., Jeong I.G., Kim C.S., et al. Diagnosis of prostate cancer via nanotechnological approach. Int J Nanomed. 2015;10:6555–6569. doi: 10.2147/IJN.S91908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandhu G.S., Andriole G.L. Overdiagnosis of prostate cancer. JNCI Monographs. 2012;2012(45):146–151. doi: 10.1093/jncimonographs/lgs031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epstein J.I., Zelefsky M.J., Sjoberg D.D., Nelson J.B., Egevad L., Magi-Galluzzi C., et al. A contemporary prostate cancer grading system: a validated alternative to the gleason score. Eur Urol. 2016;69(3):428–435. doi: 10.1016/j.eururo.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandhu S., Moore C.M., Chiong E., Beltran H., Bristow R.G., Williams S.G. Prostate cancer. The Lancet. 2021;398(10305):1075–1090. doi: 10.1016/S0140-6736(21)00950-8. [DOI] [PubMed] [Google Scholar]
- 25.Tannock I.F., Osoba D., Stockler M.R., Ernst D.S., Neville A.J., Moore M.J., et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14(6):1756–1764. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 26.Petrylak D.P., Tangen C.M., Hussain M.H., Lara P.N., Jr., Jones J.A., Taplin M.E., et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 27.Tannock I.F., de Wit R., Berry W.R., Horti J., Pluzanska A., Chi K.N., et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 28.de Bono J.S., Oudard S., Ozguroglu M., Hansen S., Machiels J.P., Kocak I., et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 29.Sternberg C.N., Petrylak D.P., Sartor O., Witjes J.A., Demkow T., Ferrero J.M., et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial. J Clin Oncol. 2009;27(32):5431–5438. doi: 10.1200/JCO.2008.20.1228. [DOI] [PubMed] [Google Scholar]
- 30.Sun B., Straubinger R.M., Lovell J.F. Current taxane formulations and emerging cabazitaxel delivery systems. Nano Res. 2018;11(10):5193–5218. [Google Scholar]
- 31.Fujita M., Murao T., Osawa M., Hirai S., Fukushima S., Yo S., et al. Colonic endoscopic mucosal resection in patients taking anticoagulants: is heparin bridging therapy necessary? J Dig Dis. 2018;19(5):288–294. doi: 10.1111/1751-2980.12598. [DOI] [PubMed] [Google Scholar]
- 32.Brigger I., Dubernet C., Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54(5):631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 33.Guidolin K., Zheng G. Nanomedicines lost in translation. ACS Nano. 2019;13(12):13620–13626. doi: 10.1021/acsnano.9b08659. [DOI] [PubMed] [Google Scholar]
- 34.Haseeb Q., Hamdani S.D.A., Akram A., Khan D.A., Rajput T.A., Babar M.M. Nanobiotechnology: paving the way to personalized medicine. Springer Singapore. 2020:17–32. [Google Scholar]
- 35.Faintuch B.L., Faintuch S. In: Precision Medicine for Investigators. Faintuch J., Faintuch S., editors. Academic Press; Practitioners and Providers: 2020. Chapter 44 - Nanotheranostics in oncology and drug development for imaging and therapy; pp. 453–458. [Google Scholar]
- 36.Prasad R., Jain N.K., Conde J., Srivastava R. Localized nanotheranostics: recent developments in cancer nanomedicine. Mater Today Adv. 2020;8 [Google Scholar]
- 37.Tiwari P., Agarwal S., Srivastava S., Jain S. The combined effect of thermal and chemotherapy on HeLa cells using magnetically actuated smart textured fibrous system. J Biomed Mater Res B Appl Biomater. 2018;106(1):40–51. doi: 10.1002/jbm.b.33812. [DOI] [PubMed] [Google Scholar]
- 38.Gomez-Marquez J., Hamad-Schifferli K. Local development of nanotechnology-based diagnostics. Nat Nanotechnol. 2021;16(5):484–486. doi: 10.1038/s41565-021-00907-2. [DOI] [PubMed] [Google Scholar]
- 39.Hua S., de Matos M.B.C., Metselaar J.M., Storm G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization. Front Pharmacol. 2018;9:790. doi: 10.3389/fphar.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M.D.P., Acosta-Torres L.S., et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura Y., Mochida A., Choyke P.L., Kobayashi H. Nanodrug delivery: is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug Chem. 2016;27(10):2225–2238. doi: 10.1021/acs.bioconjchem.6b00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navya P.N., Kaphle A., Srinivas S.P., Bhargava S.K., Rotello V.M., Daima H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019;6(1):23. doi: 10.1186/s40580-019-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Von Hoff D.D., Mita M.M., Ramanathan R.K., Weiss G.J., Mita A.C., LoRusso P.M., et al. Phase I study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin Can Res. 2016;22(13):3157–3163. doi: 10.1158/1078-0432.CCR-15-2548. [DOI] [PubMed] [Google Scholar]
- 44.Autio K.A., Dreicer R., Anderson J., Garcia J.A., Alva A., Hart L.L., et al. Safety and efficacy of BIND-014, a docetaxel nanoparticle targeting prostate-specific membrane antigen for patients with metastatic castration-resistant prostate cancer: a phase 2 clinical trial. JAMA Oncology. 2018;4(10):1344–1351. doi: 10.1001/jamaoncol.2018.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morales-Barrera R., Suárez C., Mateo J., González M., Carles J. Nanoparticles as theranostic vehicles in prostate cancer. Ann Translat Med. 2019:29. doi: 10.21037/atm.2019.01.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rastinehad AR, Anastos H, Wajswol E, Winoker JS, Sfakianos JP, Doppalapudi SK et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc Natl Acad Sci 2019;116(37):18590-18596. [DOI] [PMC free article] [PubMed]
- 47.Wang J., Wang T.T., Gao P.F., Huang C.Z. Biomolecules-conjugated nanomaterials for targeted cancer therapy. J Mater Chem B. 2014;2(48):8452–8465. doi: 10.1039/c4tb01263a. [DOI] [PubMed] [Google Scholar]
- 48.Oliveira S.F., Bisker G., Bakh N.A., Gibbs S.L., Landry M.P., Strano M.S. Protein functionalized carbon nanomaterials for biomedical applications. Carbon. 2015;95:767–779. [Google Scholar]
- 49.Murray R.W. Nanoelectrochemistry: metal nanoparticles, nanoelectrodes, and nanopores. Chem Rev. 2008;108(7):2688–2720. doi: 10.1021/cr068077e. [DOI] [PubMed] [Google Scholar]
- 50.Sanità G., Carrese B., Lamberti A. Nanoparticle surface functionalization: how to improve biocompatibility and cellular internalization. Front Mol Biosci. 2020;7 doi: 10.3389/fmolb.2020.587012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sperling R.A., Parak W.J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos Trans Royal Soc A: Math, Phys Eng Sci. 2010;368(1915):1333–1383. doi: 10.1098/rsta.2009.0273. [DOI] [PubMed] [Google Scholar]
- 52.Cheng X., Jiang J., Liang G. Covalently conjugated hydrogelators for imaging and therapeutic applications. Bioconjug Chem. 2020;31(3):448–461. doi: 10.1021/acs.bioconjchem.9b00867. [DOI] [PubMed] [Google Scholar]
- 53.Ju H., Zhang X., Wang J. Springer, New York, New York, NY; Development and Application: 2011. Biofunctionalization of Nanomaterials, NanoBiosensing: Principles; pp. 1–38. [Google Scholar]
- 54.Catuogno S., Esposito C.L. Aptamer cell-based selection: overview and advances. Biomedicines. 2017;5(3) doi: 10.3390/biomedicines5030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meng H.M., Liu H., Kuai H., Peng R., Mo L., Zhang X.B. Aptamer-integrated DNA nanostructures for biosensing, bioimaging and cancer therapy. Chem Soc Rev. 2016;45(9):2583–2602. doi: 10.1039/c5cs00645g. [DOI] [PubMed] [Google Scholar]
- 56.Ding S., Lyu Z., Niu X., Zhou Y., Liu D., Falahati M., et al. Integrating ionic liquids with molecular imprinting technology for biorecognition and biosensing: a review. Biosens Bioelectron. 2020;149 doi: 10.1016/j.bios.2019.111830. [DOI] [PubMed] [Google Scholar]
- 57.Du C, Zhang N, Ding S, Gao X, Guan P, Hu X. Preparation of highly cross-linked raspberry-like nano/microspheres and surface tailoring for controlled immunostimulating peptide adsorption11Electronic supplementary information (ESI) available: Adsorption isotherms and binding kinetics study of IL-functionalized microspheres. See DOI: 10.1039/c6py00747c, Polymer Chemistry 7(27) (2016) 4531-4541.
- 58.Ding S., Khan A.I., Cai X., Song Y., Lyu Z., Du D., et al. Overcoming blood-brain barrier transport: Advances in nanoparticle-based drug delivery strategies. Mater Today (Kidlington) 2020;37:112–125. doi: 10.1016/j.mattod.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahmoudpour M, Ding S, Lyu Z, Ebrahimi G, Du D, Ezzati Nazhad Dolatabadi J et al. Aptamer functionalized nanomaterials for biomedical applications: recent advances and new horizons. Nano Today 39 (2021) 101177.
- 60.Odeh F, Nsairat H, Alshaer W, Ismail MA, Esawi E, Qaqish B. Aptamers chemistry: chemical modifications and conjugation strategies. Molecules (Basel, Switzerland) 2019;25(1):3. [DOI] [PMC free article] [PubMed]
- 61.Colak B., Da Silva J.C.S., Soares T.A., Gautrot J.E. Impact of the molecular environment on thiol-ene coupling for biofunctionalization and conjugation. Bioconjug Chem. 2016;27(9):2111–2123. doi: 10.1021/acs.bioconjchem.6b00349. [DOI] [PubMed] [Google Scholar]
- 62.Aubin-Tam M.E., Hamad-Schifferli K. Structure and function of nanoparticle-protein conjugates. Biomed Mater. 2008;3(3) doi: 10.1088/1748-6041/3/3/034001. [DOI] [PubMed] [Google Scholar]
- 63.Zheng M., Li Z., Huang X. Ethylene glycol monolayer protected nanoparticles: synthesis, characterization, and interactions with biological molecules. Langmuir. 2004;20(10):4226–4235. doi: 10.1021/la035981i. [DOI] [PubMed] [Google Scholar]
- 64.Saha K., Agasti S.S., Kim C., Li X., Rotello V.M. Gold nanoparticles in chemical and biological sensing. Chem Rev. 2012;112(5):2739–2779. doi: 10.1021/cr2001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marques A.C., Costa P.J., Velho S., Amaral M.H. Functionalizing nanoparticles with cancer-targeting antibodies: a comparison of strategies. J Control Release. 2020;320:180–200. doi: 10.1016/j.jconrel.2020.01.035. [DOI] [PubMed] [Google Scholar]
- 66.Maddahfar M., Wen S., Hosseinpour Mashkani S.M., Zhang L., Shimoni O., Stenzel M., et al. Stable and highly efficient antibody-nanoparticles conjugation. Bioconjug Chem. 2021;32(6):1146–1155. doi: 10.1021/acs.bioconjchem.1c00192. [DOI] [PubMed] [Google Scholar]
- 67.Tallawi M., Rosellini E., Barbani N., Cascone M.G., Rai R., Saint-Pierre G., et al. Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: a review. J R Soc Interface. 2015;12(108):20150254. doi: 10.1098/rsif.2015.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goossens J., Sein H., Lu S., Radwanska M., Muyldermans S., Sterckx Y.-G.-J., et al. Functionalization of gold nanoparticles with nanobodies through physical adsorption. Anal Methods. 2017;9(23):3430–3440. [Google Scholar]
- 69.Ou X., Jiang L., Chen P., Zhu M., Hu W., Liu M., et al. Highly stable graphene-based multilayer films immobilized via covalent bonds and their applications in organic field-effect transistors. Adv Funct Mater. 2013;23(19):2422–2435. [Google Scholar]
- 70.Okyem S., Awotunde O., Ogunlusi T., Riley M.B., Driskell J.D. High-affinity points of interaction on antibody allow synthesis of stable and highly functional antibody-gold nanoparticle conjugates. Bioconjug Chem. 2021;32(8):1753–1762. doi: 10.1021/acs.bioconjchem.1c00261. [DOI] [PubMed] [Google Scholar]
- 71.Song H.Y., Zhou X., Hobley J., Su X. Comparative study of random and oriented antibody immobilization as measured by dual polarization interferometry and surface Plasmon resonance spectroscopy. Langmuir. 2012;28(1):997–1004. doi: 10.1021/la202734f. [DOI] [PubMed] [Google Scholar]
- 72.Hazani M., Naaman R., Hennrich F., Kappes M.M. Confocal fluorescence imaging of DNA-functionalized carbon nanotubes. Nano Lett. 2003;3(2):153–155. [Google Scholar]
- 73.Veerapandian M., Yun K. Functionalization of biomolecules on nanoparticles: specialized for antibacterial applications. Appl Microbiol Biotechnol. 2011;90(5):1655–1667. doi: 10.1007/s00253-011-3291-6. [DOI] [PubMed] [Google Scholar]
- 74.Walsh T.R., Knecht M.R. Biomolecular material recognition in two dimensions: peptide binding to graphene, h-BN, and MoS2 nanosheets as unique bioconjugates. Bioconjug Chem. 2019;30(11):2727–2750. doi: 10.1021/acs.bioconjchem.9b00593. [DOI] [PubMed] [Google Scholar]
- 75.de Araújo J.T.C., Duarte J.L., Di Filippo L.D., Araújo V.H.S., Carvalho G.C., Chorilli M. Nanosystem functionalization strategies for prostate cancer treatment: a review. J Drug Target. 2021;29(8):808–821. doi: 10.1080/1061186X.2021.1892121. [DOI] [PubMed] [Google Scholar]
- 76.Omabe K., Paris C., Lannes F., Taieb D., Rocchi P. Nanovectorization of prostate cancer treatment strategies: a new approach to improved outcomes. Pharmaceutics. 2021;13(5) doi: 10.3390/pharmaceutics13050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cohen L., Livney Y.D., Assaraf Y.G. Targeted nanomedicine modalities for prostate cancer treatment. Drug Resist Updat. 2021;56 doi: 10.1016/j.drup.2021.100762. [DOI] [PubMed] [Google Scholar]
- 78.Farshchi F., Hasanzadeh M. Nanomaterial based aptasensing of prostate specific antigen (PSA): Recent progress and challenges in efficient diagnosis of prostate cancer using biomedicine. Biomed Pharmacother. 2020;132 doi: 10.1016/j.biopha.2020.110878. [DOI] [PubMed] [Google Scholar]
- 79.Zhang J., Wang L., You X., Xian T., Wu J., Pang J. Nanoparticle therapy for prostate cancer: overview and perspectives. Curr Top Med Chem. 2019;19(1):57–73. doi: 10.2174/1568026619666190125145836. [DOI] [PubMed] [Google Scholar]
- 80.Yari H., Gali H., Awasthi V. Nanoparticles for targeting of prostate cancer. Curr Pharm Des. 2020;26(42):5393–5413. doi: 10.2174/1381612826666200721001500. [DOI] [PubMed] [Google Scholar]
- 81.Rosellini M., Santoni M., Mollica V., Rizzo A., Cimadamore A., Scarpelli M., et al. Treating prostate cancer by antibody-drug conjugates. Int J Mol Sci. 2021;22(4) doi: 10.3390/ijms22041551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang H., Zhang Y., Yu H., Wu D., Ma H., Li H., et al. Label-free electrochemical immunosensor for prostate-specific antigen based on silver hybridized mesoporous silica nanoparticles. Anal Biochem. 2013;434(1):123–127. doi: 10.1016/j.ab.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 83.Jazayeri M.H., Amani H., Pourfatollah A.A., Avan A., Ferns G.A., Pazoki-Toroudi H. Enhanced detection sensitivity of prostate-specific antigen via PSA-conjugated gold nanoparticles based on localized surface plasmon resonance: GNP-coated anti-PSA/LSPR as a novel approach for the identification of prostate anomalies. Cancer Gene Ther. 2016;23(10):365–369. doi: 10.1038/cgt.2016.42. [DOI] [PubMed] [Google Scholar]
- 84.Pal M., Khan R. Graphene oxide layer decorated gold nanoparticles based immunosensor for the detection of prostate cancer risk factor. Anal Biochem. 2017;536:51–58. doi: 10.1016/j.ab.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 85.Suresh L, Brahman PK, Reddy KR, J SB. Development of an electrochemical immunosensor based on gold nanoparticles incorporated chitosan biopolymer nanocomposite film for the detection of prostate cancer using PSA as biomarker. Enzyme Microb Technol 2018;112:43–51. [DOI] [PubMed]
- 86.Ghanavati M., Tadayon F., Bagheri H. A novel label-free impedimetric immunosensor for sensitive detection of prostate specific antigen using Au nanoparticles/MWCNTs- graphene quantum dots nanocomposite. Microchem J. 2020;159 [Google Scholar]
- 87.Kukkar M., Tuteja S.K., Kumar P., Kim K.H., Bhadwal A.S., Deep A. A novel approach for amine derivatization of MoS2 nanosheets and their application toward label-free immunosensor. Anal Biochem. 2018;555:1–8. doi: 10.1016/j.ab.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 88.Chen J., Tong P., Huang L., Yu Z., Tang D. Ti3C2 MXene nanosheet-based capacitance immunoassay with tyramine-enzyme repeats to detect prostate-specific antigen on interdigitated micro-comb electrode. Electrochim Acta. 2019;319:375–381. [Google Scholar]
- 89.Li S., Zhang J., Tan C.S., Chen C., Hu C., Bai Y., et al. Electrochemical immunosensor based on hybrid MoS2/Pt@Au-nanoprism/PDA for simultaneous detection of free and total prostate specific antigen in serum. Sens Actuat B. 2022;357 [Google Scholar]
- 90.Cheng Z., Choi N., Wang R., Lee S., Moon K.C., Yoon S.Y., et al. Simultaneous detection of dual prostate specific antigens using surface-enhanced Raman scattering-based immunoassay for accurate diagnosis of prostate cancer. ACS Nano. 2017;11(5):4926–4933. doi: 10.1021/acsnano.7b01536. [DOI] [PubMed] [Google Scholar]
- 91.Gao R., Cheng Z., Wang X., Yu L., Guo Z., Zhao G., et al. Simultaneous immunoassays of dual prostate cancer markers using a SERS-based microdroplet channel. Biosens Bioelectron. 2018;119:126–133. doi: 10.1016/j.bios.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 92.Lee M., Kim H., Kim E., Yi S.Y., Hwang S.G., Yang S., et al. Multivalent antibody-nanoparticle conjugates to enhance the sensitivity of surface-enhanced Raman scattering-based immunoassays. ACS Appl Mater Interfaces. 2018;10(44):37829–37834. doi: 10.1021/acsami.8b13180. [DOI] [PubMed] [Google Scholar]
- 93.Bates D., Abraham S., Campbell M., Zehbe I., Curiel L. Development and characterization of an antibody-labeled super-paramagnetic iron oxide contrast agent targeting prostate cancer cells for magnetic resonance imaging. PLoS ONE. 2014;9(5):e97220. doi: 10.1371/journal.pone.0097220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sweet C., Pramanik A., Jones S., Ray P.C. Two-photon fluorescent molybdenum disulfide dots for targeted prostate cancer imaging in the biological II window. ACS Omega. 2017;2(5):1826–1835. doi: 10.1021/acsomega.7b00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao L.Z., Fu Y.Z., Ren S.W., Cao J.T., Liu Y.M. A novel chemiluminescence imaging immunosensor for prostate specific antigen detection based on a multiple signal amplification strategy. Biosens Bioelectron. 2021;171 doi: 10.1016/j.bios.2020.112729. [DOI] [PubMed] [Google Scholar]
- 96.Hara D., Tao W., Totiger T.M., Pourmand A., Dogan N., Ford J.C., et al. Prostate cancer targeted X-ray fluorescence imaging via gold nanoparticles functionalized with prostate-specific membrane antigen (PSMA) Int J Radiat Oncol Biol Phys. 2021;111(1):220–232. doi: 10.1016/j.ijrobp.2021.04.032. [DOI] [PubMed] [Google Scholar]
- 97.Green B.J., Nguyen V., Atenafu E., Weeber P., Duong B.T.V., Thiagalingam P., et al. Phenotypic profiling of circulating tumor cells in metastatic prostate cancer patients using nanoparticle-mediated ranking. Anal Chem. 2019;91(15):9348–9355. doi: 10.1021/acs.analchem.9b01697. [DOI] [PubMed] [Google Scholar]
- 98.Tan W., Donovan M.J., Jiang J. Aptamers from cell-based selection for bioanalytical applications. Chem Rev. 2013;113(4):2842–2862. doi: 10.1021/cr300468w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yi K., Rong Y., Huang L., Tang X., Zhang Q., Wang W., et al. Aptamer-exosomes for tumor theranostics. ACS Sens. 2021;6(4):1418–1429. doi: 10.1021/acssensors.0c02237. [DOI] [PubMed] [Google Scholar]
- 100.Jolly P., Zhurauski P., Hammond J.L., Miodek A., Liébana S., Bertok T., et al. Self-assembled gold nanoparticles for impedimetric and amperometric detection of a prostate cancer biomarker. Sens Actuat B. 2017;251:637–643. [Google Scholar]
- 101.Tahmasebi F., Noorbakhsh A. Sensitive electrochemical prostate specific antigen aptasensor: effect of carboxylic acid functionalized carbon nanotube and glutaraldehyde linker. Electroanalysis. 2016;28(5):1134–1145. [Google Scholar]
- 102.Rahi A., Sattarahmady N., Heli H. Label-free electrochemical aptasensing of the human prostate-specific antigen using gold nanospears. Talanta. 2016;156–157:218–224. doi: 10.1016/j.talanta.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 103.Kavosi B., Salimi A., Hallaj R., Moradi F. Ultrasensitive electrochemical immunosensor for PSA biomarker detection in prostate cancer cells using gold nanoparticles/PAMAM dendrimer loaded with enzyme linked aptamer as integrated triple signal amplification strategy. Biosens Bioelectron. 2015;74:915–923. doi: 10.1016/j.bios.2015.07.064. [DOI] [PubMed] [Google Scholar]
- 104.Yu Q., Wu Y., Liu Z., Lei S., Li G., Ye B. Novel electrochemical biosensor based on cationic peptide modified hemin/G-quadruples enhanced peroxidase-like activity. Biosens Bioelectron. 2018;107:178–183. doi: 10.1016/j.bios.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 105.Zhao Y., Liu H., Shi L., Zheng W., Jing X. Electroactive Cu2O nanoparticles and Ag nanoparticles driven ratiometric electrochemical aptasensor for prostate specific antigen detection. Sens Actuat B. 2020;315 [Google Scholar]
- 106.Duan F., Zhang S., Yang L., Zhang Z., He L., Wang M. Bifunctional aptasensor based on novel two-dimensional nanocomposite of MoS(2) quantum dots and g-C(3)N(4) nanosheets decorated with chitosan-stabilized Au nanoparticles for selectively detecting prostate specific antigen. Anal Chim Acta. 2018;1036:121–132. doi: 10.1016/j.aca.2018.06.070. [DOI] [PubMed] [Google Scholar]
- 107.Tian L., Liu L., Li Y., Wei Q., Cao W. 3D sandwich-type prostate specific antigen (PSA) immunosensor based on rGO–MWCNT–Pd nanocomposite. New J Chem. 2015;39(7):5522–5528. [Google Scholar]
- 108.Karimipour M., Heydari-Bafrooei E., Sanjari M., Johansson M.B., Molaei M. A glassy carbon electrode modified with TiO(2)(200)-rGO hybrid nanosheets for aptamer based impedimetric determination of the prostate specific antigen. Mikrochim Acta. 2018;186(1):33. doi: 10.1007/s00604-018-3141-7. [DOI] [PubMed] [Google Scholar]
- 109.Yan R., Lu N., Han S., Lu Z., Xiao Y., Zhao Z., et al. Simultaneous detection of dual biomarkers using hierarchical MoS2 nanostructuring and nano-signal amplification-based electrochemical aptasensor toward accurate diagnosis of prostate cancer. Biosens Bioelectron. 2022;197 doi: 10.1016/j.bios.2021.113797. [DOI] [PubMed] [Google Scholar]
- 110.Lin Z., Ma Q., Fei X., Zhang H., Su X. A novel aptamer functionalized CuInS2 quantum dots probe for daunorubicin sensing and near infrared imaging of prostate cancer cells. Anal Chim Acta. 2014;818:54–60. doi: 10.1016/j.aca.2014.01.057. [DOI] [PubMed] [Google Scholar]
- 111.Wu X., Gao F., Xu L., Kuang H., Wang L., Xu C. A fluorescence active gold nanorod–quantum dot core–satellite nanostructure for sub-attomolar tumor marker biosensing. RSC Adv. 2015;5(118):97898–97902. [Google Scholar]
- 112.Yang T., Hou P., Zheng L.L., Zhan L., Gao P.F., Li Y.F., et al. Surface-engineered quantum dots/electrospun nanofibers as a networked fluorescence aptasensing platform toward biomarkers. Nanoscale. 2017;9(43):17020–17028. doi: 10.1039/c7nr04817c. [DOI] [PubMed] [Google Scholar]
- 113.Wang H.M., Huang X.Q., Wang A.J., Luo X., Liu W.D., Yuan P.X., et al. Construction of efficient “on-off-on” fluorescence aptasensor for ultrasensitive detection of prostate specific antigen via covalent energy transfer between g-C(3)N(4) quantum dots and palladium triangular plates. Anal Chim Acta. 2020;1104:53–59. doi: 10.1016/j.aca.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 114.Wu Q., Chen G., Qiu S., Feng S., Lin D. A target-triggered and self-calibration aptasensor based on SERS for precise detection of a prostate cancer biomarker in human blood. Nanoscale. 2021;13(16):7574–7582. doi: 10.1039/d1nr00480h. [DOI] [PubMed] [Google Scholar]
- 115.He J.H., Cheng Y.Y., Zhang Q.Q., Liu H., Huang C.Z. Carbon dots-based fluorescence resonance energy transfer for the prostate specific antigen (PSA) with high sensitivity. Talanta. 2020;219 doi: 10.1016/j.talanta.2020.121276. [DOI] [PubMed] [Google Scholar]
- 116.Pan L.H., Kuo S.H., Lin T.Y., Lin C.W., Fang P.Y., Yang H.W. An electrochemical biosensor to simultaneously detect VEGF and PSA for early prostate cancer diagnosis based on graphene oxide/ssDNA/PLLA nanoparticles. Biosens Bioelectron. 2017;89(Pt 1):598–605. doi: 10.1016/j.bios.2016.01.077. [DOI] [PubMed] [Google Scholar]
- 117.Sun T., Xia N., Yuan F., Liu X., Chang Y., Liu S., et al. A colorimetric method for determination of the prostate specific antigen based on enzyme-free cascaded signal amplification via peptide-copper(II) nanoparticles. Mikrochim Acta. 2020;187(2):116. doi: 10.1007/s00604-019-4074-5. [DOI] [PubMed] [Google Scholar]
- 118.Martin D.T., Lee J.S., Liu Q., Galiana G., Sprenkle P.C., Humphrey P.A., et al. Targeting prostate cancer with Clostridium perfringens enterotoxin functionalized nanoparticles co-encapsulating imaging cargo enhances magnetic resonance imaging specificity. Nanomedicine. 2021;40 doi: 10.1016/j.nano.2021.102477. [DOI] [PubMed] [Google Scholar]
- 119.Wang B., Zhang S., Meng J., Min L., Luo J., Zhu Z., et al. Evaporation-induced rGO coatings for highly sensitive and non-invasive diagnosis of prostate cancer in the PSA gray zone. Adv Mater. 2021;33(40):e2103999. doi: 10.1002/adma.202103999. [DOI] [PubMed] [Google Scholar]
- 120.Robert L. Hyaluronan, a truly “youthful” polysaccharide. Its medical applications. Pathol Biol (Paris) 2015;63(1):32–34. doi: 10.1016/j.patbio.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 121.Reed R.K., Lilja K., Laurent T.C. Hyaluronan in the rat with special reference to the skin. Acta Physiol Scand. 1988;134(3):405–411. doi: 10.1111/j.1748-1716.1988.tb08508.x. [DOI] [PubMed] [Google Scholar]
- 122.Schaefer L., Schaefer R.M. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res. 2009;339(1):237. doi: 10.1007/s00441-009-0821-y. [DOI] [PubMed] [Google Scholar]
- 123.Shu F., Lv D., Song X.-L., Huang B., Wang C., Yu Y., et al. Fabrication of a hyaluronic acid conjugated metal organic framework for targeted drug delivery and magnetic resonance imaging. RSC Adv. 2018;8(12):6581–6589. doi: 10.1039/c7ra12969f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saini S. PSA and beyond: alternative prostate cancer biomarkers. Cell Oncol (Dordr) 2016;39(2):97–106. doi: 10.1007/s13402-016-0268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rubin M.A., Zhou M., Dhanasekaran S.M., Varambally S., Barrette T.R., Sanda M.G., et al. alpha-Methylacyl coenzyme A racemase as a tissue biomarker for prostate cancer. JAMA. 2002;287(13):1662–1670. doi: 10.1001/jama.287.13.1662. [DOI] [PubMed] [Google Scholar]
- 126.Rubin M.A., Bismar T.A., Andrén O., Mucci L., Kim R., Shen R., et al. Decreased alpha-methylacyl CoA racemase expression in localized prostate cancer is associated with an increased rate of biochemical recurrence and cancer-specific death. Can Epidemiol Biomarkers Prev. 2005;14(6):1424–1432. doi: 10.1158/1055-9965.EPI-04-0801. [DOI] [PubMed] [Google Scholar]
- 127.Carver B.S., Chapinski C., Wongvipat J., Hieronymus H., Chen Y., Chandarlapaty S., et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19(5):575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Boström P.J., Bjartell A.S., Catto J.W.F., Eggener S.E., Lilja H., Loeb S., et al. Genomic predictors of outcome in prostate cancer. Eur Urol. 2015;68(6):1033–1044. doi: 10.1016/j.eururo.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 129.Hroncekova S., Bertok T., Hires M., Jane E., Lorencova L., Vikartovska A., et al. Ultrasensitive Ti3C2TX MXene/chitosan nanocomposite-based amperometric biosensor for detection of potential prostate cancer marker in urine samples. Processes. 2020;8(5):580. doi: 10.3390/pr8050580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Uhlirova D., Stankova M., Docekalova M., Hosnedlova B., Kepinska M., Ruttkay-Nedecky B., et al. A rapid method for the detection of sarcosine using SPIONs/Au/CS/SOX/NPs for prostate cancer sensing. Int J Mol Sci. 2018;19(12) doi: 10.3390/ijms19123722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Feng J., Chen X., Shi X., Zheng W., Zhang X., Yang H. Sarcosine biosensor based on Pt/g-C3N4 nanocomposites with high electrocatalytic activity. ECS J Solid State Sci Technol. 2022;11(4) [Google Scholar]
- 132.Heger Z., Cernei N., Krizkova S., Masarik M., Kopel P., Hodek P., et al. Paramagnetic nanoparticles as a platform for FRET-based sarcosine picomolar detection. Sci Rep. 2015;5(1):8868. doi: 10.1038/srep08868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Soleimani S., Arkan E., Jalalvand A.R., Goicoechea H.C. Fabrication of a novel electrochemical aptasensor assisted by a novel computerized monitoring system for real-time determination of the prostate specific antigen: a computerized experimental method brought elegancy. Microchem J. 2020;157 [Google Scholar]
- 134.Shao F., Zhang L., Jiao L., Wang X., Miao L., Li H., et al. Enzyme-free immunosorbent assay of prostate specific antigen amplified by releasing pH indicator molecules entrapped in mesoporous silica nanoparticles. Anal Chem. 2018;90(14):8673–8679. doi: 10.1021/acs.analchem.8b02019. [DOI] [PubMed] [Google Scholar]
- 135.Talamini L., Zanato N., Zapp E., Brondani D., Westphal E., Gallardo H., et al. Heparin-gold nanoparticles and liquid crystal applied in label-free electrochemical immunosensor for prostate-specific antigen. Electroanalysis. 2018;30(2):353–360. [Google Scholar]
- 136.Bock S., Kim H.-M., Kim J., An J., Choi Y.-S., Pham X.-H., et al. Lateral flow immunoassay with quantum-dot-embedded silica nanoparticles for prostate-specific antigen detection. Nanomaterials. 2021;12(1):33. doi: 10.3390/nano12010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li M., Yang W., Hu H., Feng Y., Zhu S., Li C., et al. A dual-readout sandwich immunoassay based on biocatalytic perovskite nanocrystals for detection of prostate specific antigen. Biosens Bioelectron. 2022;113979 doi: 10.1016/j.bios.2022.113979. [DOI] [PubMed] [Google Scholar]
- 138.Cao X., Liu M., Zhao M., Li J., Xia J., Zou T., et al. Synergetic PtNP@Co3O4 hollow nanopolyhedrals as peroxidase-like nanozymes for the dual-channel homogeneous biosensing of prostate-specific antigen. Anal Bioanal Chem. 2022;414(5):1921–1932. doi: 10.1007/s00216-021-03827-1. [DOI] [PubMed] [Google Scholar]
- 139.Li B., Guo L., Chen M., Guo Y., Ge L., Kwok H.F. Single-atom Pt-anchored Zn0.5Cd0.5S boosted photoelectrochemical immunoassay of prostate-specific antigen. Biosens Bioelectron. 2022;202 doi: 10.1016/j.bios.2022.114006. [DOI] [PubMed] [Google Scholar]
- 140.Sun Y., Fan J., Cui L., Ke W., Zheng F., Zhao Y. Fluorometric nanoprobes for simultaneous aptamer-based detection of carcinoembryonic antigen and prostate specific antigen. Mikrochim Acta. 2019;186(3):152. doi: 10.1007/s00604-019-3281-4. [DOI] [PubMed] [Google Scholar]
- 141.Zhu Y.-C., Xu F., Zhang N., Zhao W.-W., Xu J.-J., Chen H.-Y. DNA sequence functionalized with heterogeneous core–satellite nanoassembly for novel energy-transfer-based photoelectrochemical bioanalysis. Biosens Bioelectron. 2017;91:293–298. doi: 10.1016/j.bios.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 142.Nagesh P.K.B., Johnson N.R., Boya V.K.N., Chowdhury P., Othman S.F., Khalilzad-Sharghi V., et al. PSMA targeted docetaxel-loaded superparamagnetic iron oxide nanoparticles for prostate cancer. Colloids Surf B Biointerfaces. 2016;144:8–20. doi: 10.1016/j.colsurfb.2016.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dostalova S., Cerna T., Hynek D., Koudelkova Z., Vaculovic T., Kopel P., et al. Site-directed conjugation of antibodies to apoferritin nanocarrier for targeted drug delivery to prostate cancer cells. ACS Appl Mater Interfaces. 2016;8(23):14430–14441. doi: 10.1021/acsami.6b04286. [DOI] [PubMed] [Google Scholar]
- 144.Dostalova S., Polanska H., Svobodova M., Balvan J., Krystofova O., Haddad Y., et al. Prostate-specific membrane antigen-targeted site-directed antibody-conjugated apoferritin nanovehicle favorably influences in vivo side effects of doxorubicin. Sci Rep. 2018;8(1):8867. doi: 10.1038/s41598-018-26772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lucío M.I., Opri R., Pinto M., Scarsi A., Fierro J.L.G., Meneghetti M., et al. Targeted killing of prostate cancer cells using antibody–drug conjugated carbon nanohorns. J Mater Chem B. 2017;5(44):8821–8832. doi: 10.1039/c7tb02464a. [DOI] [PubMed] [Google Scholar]
- 146.Pang S.T., Lin F.W., Chuang C.K., Yang H.W. Co-delivery of docetaxel and p44/42 MAPK siRNA using PSMA antibody-conjugated BSA-PEI layer-by-layer nanoparticles for prostate cancer target therapy. Macromol Biosci. 2017;17(5) doi: 10.1002/mabi.201600421. [DOI] [PubMed] [Google Scholar]
- 147.Wei J., Sun J., Liu Y. Enhanced targeting of prostate cancer-initiating cells by salinomycin-encapsulated lipid-PLGA nanoparticles linked with CD44 antibodies. Oncol Lett. 2019;17(4):4024–4033. doi: 10.3892/ol.2019.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Skalickova S, Loffelmann M, Gargulak M, Kepinska M, Docekalova M, Uhlirova D et al. Zinc-Modified Nanotransporter of Doxorubicin for Targeted Prostate Cancer Delivery, Nanomaterials (Basel) 7(12) (2017). [DOI] [PMC free article] [PubMed]
- 149.Rivero-Buceta E., Vidaurre-Agut C., Vera-Donoso C.D., Benlloch J.M., Moreno-Manzano V., Botella P. PSMA-targeted mesoporous silica nanoparticles for selective intracellular delivery of docetaxel in prostate cancer cells. ACS Omega. 2019;4(1):1281–1291. [Google Scholar]
- 150.Sun G., Sun K., Sun J. Combination prostate cancer therapy: Prostate-specific membranes antigen targeted, pH-sensitive nanoparticles loaded with doxorubicin and tanshinone. Drug Deliv. 2021;28(1):1132–1140. doi: 10.1080/10717544.2021.1931559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhu C., Bandekar A., Sempkowski M., Banerjee S.R., Pomper M.G., Bruchertseifer F., et al. Nanoconjugation of PSMA-targeting ligands enhances perinuclear localization and improves efficacy of delivered alpha-particle emitters against tumor endothelial analogues. Mol Cancer Ther. 2016;15(1):106–113. doi: 10.1158/1535-7163.MCT-15-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bandekar A., Zhu C., Jindal R., Bruchertseifer F., Morgenstern A., Sofou S. Anti-prostate-specific membrane antigen liposomes loaded with 225Ac for potential targeted antivascular α-particle therapy of cancer. J Nucl Med. 2014;55(1):107–114. doi: 10.2967/jnumed.113.125476. [DOI] [PubMed] [Google Scholar]
- 153.Mangadlao J.D., Wang X., McCleese C., Escamilla M., Ramamurthy G., Wang Z., et al. Prostate-specific membrane antigen targeted gold nanoparticles for theranostics of prostate cancer. ACS Nano. 2018;12(4):3714–3725. doi: 10.1021/acsnano.8b00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tan H., Hou N., Liu Y., Liu B., Cao W., Zheng D., et al. CD133 antibody targeted delivery of gold nanostars loading IR820 and docetaxel for multimodal imaging and near-infrared photodynamic/photothermal/chemotherapy against castration resistant prostate cancer. Nanomedicine. 2020;27 doi: 10.1016/j.nano.2020.102192. [DOI] [PubMed] [Google Scholar]
- 155.Farokhzad O.C., Jon S., Khademhosseini A., Tran T.-N.-T., Lavan D.A., Langer R. Nanoparticle-aptamer bioconjugates. Can Res. 2004;64(21):7668–7672. doi: 10.1158/0008-5472.CAN-04-2550. [DOI] [PubMed] [Google Scholar]
- 156.Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. In Proceedings of the national academy of sciences 2006;103(16):6315–6320. [DOI] [PMC free article] [PubMed]
- 157.Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA–PEG nanoparticles. In: Proceedings of the National Academy of Sciences 105(45) (2008) 17356-17361. [DOI] [PMC free article] [PubMed]
- 158.Xiao Z., Levy-Nissenbaum E., Alexis F., Lupták A., Teply B.A., Chan J.M., et al. Engineering of targeted nanoparticles for cancer therapy using internalizing aptamers isolated by cell-uptake selection. ACS Nano. 2012;6(1):696–704. doi: 10.1021/nn204165v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee I.H., An S., Yu M.K., Kwon H.K., Im S.H., Jon S. Targeted chemoimmunotherapy using drug-loaded aptamer-dendrimer bioconjugates. J Control Release. 2011;155(3):435–441. doi: 10.1016/j.jconrel.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 160.Hao Z., Fan W., Hao J., Wu X., Zeng G.Q., Zhang L.J., et al. Efficient delivery of micro RNA to bone-metastatic prostate tumors by using aptamer-conjugated atelocollagen in vitro and in vivo. Drug Deliv. 2016;23(3):874–881. doi: 10.3109/10717544.2014.920059. [DOI] [PubMed] [Google Scholar]
- 161.Wu X., Ding B., Gao J., Wang H., Fan W., Wang X., et al. Second-generation aptamer-conjugated PSMA-targeted delivery system for prostate cancer therapy. Int J Nanomed. 2011;6:1747–1756. doi: 10.2147/IJN.S23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu X., Tai Z., Zhu Q., Fan W., Ding B., Zhang W., et al. Study on the prostate cancer-targeting mechanism of aptamer-modified nanoparticles and their potential anticancer effect in vivo. Int J Nanomed. 2014;9:5431–5440. doi: 10.2147/IJN.S71101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yao R.-W., Wang Y., Chen L.-L. Cellular functions of long noncoding RNAs. Nat Cell Biol. 2019;21(5):542–551. doi: 10.1038/s41556-019-0311-8. [DOI] [PubMed] [Google Scholar]
- 164.Tai Z., Ma J., Ding J., Pan H., Chai R., Zhu C., et al. Aptamer-functionalized dendrimer delivery of plasmid-encoding lncRNA MEG3 enhances gene therapy in castration-resistant prostate cancer. Int J Nanomed. 2020;15:10305–10320. doi: 10.2147/IJN.S282107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhen S., Takahashi Y., Narita S., Yang Y.C., Li X. Targeted delivery of CRISPR/Cas9 to prostate cancer by modified gRNA using a flexible aptamer-cationic liposome. Oncotarget. 2017;8(6):9375–9387. doi: 10.18632/oncotarget.14072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li K, Zhan W, Chen Y, Jha RK, Chen X. Docetaxel and doxorubicin codelivery by nanocarriers for synergistic treatment of prostate cancer. Front Pharmacol 2019;10:1436-1436. [DOI] [PMC free article] [PubMed]
- 168.Chen Y., Deng Y., Zhu C., Xiang C. Anti prostate cancer therapy: Aptamer-functionalized, curcumin and cabazitaxel co-delivered, tumor targeted lipid-polymer hybrid nanoparticles. Biomed Pharmacother. 2020;127 doi: 10.1016/j.biopha.2020.110181. [DOI] [PubMed] [Google Scholar]
- 169.Fang Y., Lin S., Yang F., Situ J., Lin S., Luo Y. Aptamer-Conjugated multifunctional polymeric nanoparticles as cancer-targeted, MRI-ultrasensitive drug delivery systems for treatment of castration-resistant prostate cancer. Biomed Res Int. 2020;2020:9186583. doi: 10.1155/2020/9186583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jo H., Youn H., Lee S., Ban C. Ultra-effective photothermal therapy for prostate cancer cells using dual aptamer-modified gold nanostars. J Mater Chem B. 2014;2(30):4862–4867. doi: 10.1039/c4tb00643g. [DOI] [PubMed] [Google Scholar]
- 171.Yu M.K., Kim D., Lee I.H., So J.S., Jeong Y.Y., Jon S. Image-guided prostate cancer therapy using aptamer-functionalized thermally cross-linked superparamagnetic iron oxide nanoparticles. Small. 2011;7(15):2241–2249. doi: 10.1002/smll.201100472. [DOI] [PubMed] [Google Scholar]
- 172.Dai L., Wei D., Zhang J., Shen T., Zhao Y., Liang J., et al. Aptamer-conjugated mesoporous polydopamine for docetaxel targeted delivery and synergistic photothermal therapy of prostate cancer. Cell Prolif. 2021;54(11):e13130. doi: 10.1111/cpr.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Luo D., Wang X., Zeng S., Ramamurthy G., Burda C., Basilion J.P. Prostate-specific membrane antigen targeted gold nanoparticles for prostate cancer radiotherapy: does size matter for targeted particles? Chem Sci. 2019;10(35):8119–8128. doi: 10.1039/c9sc02290b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kumar A., Huo S., Zhang X., Liu J., Tan A., Li S., et al. Neuropilin-1-targeted gold nanoparticles enhance therapeutic efficacy of platinum(IV) drug for prostate cancer treatment. ACS Nano. 2014;8(5):4205–4220. doi: 10.1021/nn500152u. [DOI] [PubMed] [Google Scholar]
- 175.Simpson E.J., Gobbo P., Bononi F.C., Murrell E., Workentin M.S., Luyt L.G. Bombesin-functionalized water-soluble gold nanoparticles for targeting prostate cancer. J Interdiscip Nanomed. 2017;2(4):174–187. [Google Scholar]
- 176.Tsai L.C., Hsieh H.Y., Lu K.Y., Wang S.Y., Mi F.L. EGCG/gelatin-doxorubicin gold nanoparticles enhance therapeutic efficacy of doxorubicin for prostate cancer treatment. Nanomedicine (Lond) 2016;11(1):9–30. doi: 10.2217/nnm.15.183. [DOI] [PubMed] [Google Scholar]
- 177.Guo J., O'Driscoll C.M., Holmes J.D., Rahme K. Bioconjugated gold nanoparticles enhance cellular uptake: a proof of concept study for siRNA delivery in prostate cancer cells. Int J Pharm. 2016;509(1–2):16–27. doi: 10.1016/j.ijpharm.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 178.Almowalad J., Somani S., Laskar P., Meewan J., Tate R.J., Mullin M., et al. Lactoferrin-bearing gold nanocages for gene delivery in prostate cancer cells in vitro. Int J Nanomed. 2021;16:4391–4407. doi: 10.2147/IJN.S316830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Laskar P., Somani S., Campbell S.J., Mullin M., Keating P., Tate R.J., et al. Camptothecin-based dendrimersomes for gene delivery and redox-responsive drug delivery to cancer cells. Nanoscale. 2019;11(42):20058–20071. doi: 10.1039/c9nr07254c. [DOI] [PubMed] [Google Scholar]
- 180.Laskar P., Somani S., Altwaijry N., Mullin M., Bowering D., Warzecha M., et al. Redox-sensitive, cholesterol-bearing PEGylated poly(propylene imine)-based dendrimersomes for drug and gene delivery to cancer cells. Nanoscale. 2018;10(48):22830–22847. doi: 10.1039/c8nr08141g. [DOI] [PubMed] [Google Scholar]
- 181.Laskar P., Somani S., Mullin M., Tate R.J., Warzecha M., Bowering D., et al. Octadecyl chain-bearing PEGylated poly(propyleneimine)-based dendrimersomes: physicochemical studies, redox-responsiveness, DNA condensation, cytotoxicity and gene delivery to cancer cells. Biomater Sci. 2021;9(4):1431–1448. doi: 10.1039/d0bm01441a. [DOI] [PubMed] [Google Scholar]
- 182.Almowalad J., Laskar P., Somani S., Meewan J., Tate R.J., Dufès C. Lactoferrin- and dendrimer-bearing gold nanocages for stimulus-free DNA delivery to prostate cancer cells. Int J Nanomed. 2022;17:1409–1421. doi: 10.2147/IJN.S347574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Saravanakumar K., Mariadoss A.V.A., Sathiyaseelan A., Venkatachalam K., Hu X., Wang M.-H. pH-sensitive release of fungal metabolites from chitosan nanoparticles for effective cytotoxicity in prostate cancer (PC3) cells. Process Biochem. 2021;102:165–172. [Google Scholar]
- 184.Huang W.-Y., Lin J.-N., Hsieh J.-T., Chou S.-C., Lai C.-H., Yun E.-J., et al. Nanoparticle targeting CD44-positive cancer cells for site-specific drug delivery in prostate cancer therapy. ACS Appl Mater Interfaces. 2016;8(45):30722–30734. doi: 10.1021/acsami.6b10029. [DOI] [PubMed] [Google Scholar]
- 185.Dai L., Shen G., Wang Y., Yang P., Wang H., Liu Z. PSMA-targeted melanin-like nanoparticles as a multifunctional nanoplatform for prostate cancer theranostics. J Mater Chem B. 2021;9(4):1151–1161. doi: 10.1039/d0tb02576c. [DOI] [PubMed] [Google Scholar]
- 186.Mahmoud N.N., Aqabani H., Hikmat S., Abu-Dahab R. Colloidal stability and cytotoxicity of polydopamine-conjugated gold nanorods against prostate cancer cell lines. Molecules. 2021;26(5) doi: 10.3390/molecules26051299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nambiar S., Osei E., Fleck A., Darko J., Mutsaers A.J., Wettig S. Synthesis of curcumin-functionalized gold nanoparticles and cytotoxicity studies in human prostate cancer cell line. Appl Nanosci. 2018;8(3):347–357. [Google Scholar]
- 188.Singh N., Sallem F., Mirjolet C., Nury T., Sahoo S.K., Millot N., et al. Polydopamine modified superparamagnetic iron oxide nanoparticles as multifunctional nanocarrier for targeted prostate cancer treatment. Nanomaterials (Basel) 2019;9(2) doi: 10.3390/nano9020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Thambiraj S., Vijayalakshmi R., Ravi Shankaran D. An effective strategy for development of docetaxel encapsulated gold nanoformulations for treatment of prostate cancer. Sci Rep. 2021;11(1):2808. doi: 10.1038/s41598-020-80529-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chen Y.A., Lai Y.R., Wu H.Y., Lo Y.J., Chang Y.F., Hung C.L., et al. Bacterial genotoxin-coated nanoparticles for radiotherapy sensitization in prostate cancer. Biomedicines. 2021;9(2) doi: 10.3390/biomedicines9020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Patil Y., Shmeeda H., Amitay Y., Ohana P., Kumar S., Gabizon A. Targeting of folate-conjugated liposomes with co-entrapped drugs to prostate cancer cells via prostate-specific membrane antigen (PSMA) Nanomedicine. 2018;14(4):1407–1416. doi: 10.1016/j.nano.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 192.Guo L., Shi H., Wu H., Zhang Y., Wang X., Wu D., et al. Prostate cancer targeted multifunctionalized graphene oxide for magnetic resonance imaging and drug delivery. Carbon. 2016;107:87–99. [Google Scholar]
- 193.Namasivayam S.K.R., Rabel A.M., Prasana R., Arvind Bharani R.S., Nachiyar C.V. Gum acacia PEG iron oxide nanocomposite (GA-PEG-IONC) induced pharmacotherapeutic activity on the Las R gene expression of Pseudomonas aeruginosa and HOXB13 expression of prostate cancer (Pc 3) cell line. A green therapeutic approach of molecular mechanism inhibition. Int J Biol Macromol. 2021;190:940–959. doi: 10.1016/j.ijbiomac.2021.08.162. [DOI] [PubMed] [Google Scholar]
- 194.Oh M.H., Yu J.H., Kim I., Nam Y.S. Genetically programmed clusters of gold nanoparticles for cancer cell-targeted photothermal therapy. ACS Appl Mater Interfaces. 2015;7(40):22578–22586. doi: 10.1021/acsami.5b07029. [DOI] [PubMed] [Google Scholar]
- 195.Tan H., Liu Y., Hou N., Cui S., Liu B., Fan S., et al. Tumor microenvironment pH-responsive pentagonal gold prism-based nanoplatform for multimodal imaging and combined therapy of castration-resistant prostate cancer. Acta Biomater. 2022 doi: 10.1016/j.actbio.2022.01.012. [DOI] [PubMed] [Google Scholar]
- 196.Alserihi R.F., Mohammed M.R.S., Kaleem M., Khan M.I., Sechi M., Sanna V., et al. Development of (−)-epigallocatechin-3-gallate-loaded folate receptor-targeted nanoparticles for prostate cancer treatment. Nanotechnol Rev. 2022;11(1):298–311. [Google Scholar]
- 197.Zhang X., Du J., Guo Z., Yu J., Gao Q., Yin W., et al. Efficient near infrared light triggered nitric oxide release nanocomposites for sensitizing mild photothermal therapy. Adv Sci (Weinh) 2019;6(3):1801122. doi: 10.1002/advs.201801122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vera-Ramirez L., Vodnala S.K., Nini R., Hunter K.W., Green J.E. Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence. Nat Commun. 2018;9(1):1944. doi: 10.1038/s41467-018-04070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huang X., Chen L., Lin Y., Tou K.I., Cai H., Jin H., et al. Tumor targeting and penetrating biomimetic mesoporous polydopamine nanoparticles facilitate photothermal killing and autophagy blocking for synergistic tumor ablation. Acta Biomater. 2021;136:456–472. doi: 10.1016/j.actbio.2021.09.030. [DOI] [PubMed] [Google Scholar]
- 200.Wolfe T., Chatterjee D., Lee J., Grant J.D., Bhattarai S., Tailor R., et al. Targeted gold nanoparticles enhance sensitization of prostate tumors to megavoltage radiation therapy in vivo. Nanomedicine. 2015;11(5):1277–1283. doi: 10.1016/j.nano.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Farokhzad O.C., Jon S., Khademhosseini A., Tran T.N., Lavan D.A., Langer R. Nanoparticle-aptamer bioconjugates: a new approach for targeting prostate cancer cells. Can Res. 2004;64(21):7668–7672. doi: 10.1158/0008-5472.CAN-04-2550. [DOI] [PubMed] [Google Scholar]
- 202.Leach J.C., Wang A., Ye K., Jin S. A RNA-DNA hybrid aptamer for nanoparticle-based prostate tumor targeted drug delivery. Int J Mol Sci. 2016;17(3):380. doi: 10.3390/ijms17030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mayle K.M., Dern K.R., Wong V.K., Chen K.Y., Sung S., Ding K., et al. Engineering A11 minibody-conjugated, polypeptide-based gold nanoshells for prostate stem cell antigen (PSCA)-targeted photothermal therapy. SLAS Technol. 2017;22(1):26–35. doi: 10.1177/2211068216669710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kozlovskaya V., Alexander J.F., Wang Y., Kuncewicz T., Liu X., Godin B., et al. Internalization of red blood cell-mimicking hydrogel capsules with pH-triggered shape responses. ACS Nano. 2014;8(6):5725–5737. doi: 10.1021/nn500512x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Li R., He Y., Zhang S., Qin J., Wang J. Cell membrane-based nanoparticles: a new biomimetic platform for tumor diagnosis and treatment. Acta Pharm Sinica B. 2018;8(1):14–22. doi: 10.1016/j.apsb.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dehaini D., Fang R.H., Zhang L. Biomimetic strategies for targeted nanoparticle delivery. Bioeng Transl Med. 2016;1(1):30–46. doi: 10.1002/btm2.10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhai Y., Su J., Ran W., Zhang P., Yin Q., Zhang Z., et al. Preparation and application of cell membrane-camouflaged nanoparticles for cancer therapy. Theranostics. 2017;7(10):2575–2592. doi: 10.7150/thno.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tarasov V.V., Svistunov A.A., Chubarev V.N., Dostdar S.A., Sokolov A.V., Brzecka A., et al. Extracellular vesicles in cancer nanomedicine. Semin Can Biol. 2021;69:212–225. doi: 10.1016/j.semcancer.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 209.Chung Y.H., Cai H., Steinmetz N.F. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv Drug Deliv Rev. 2020;156:214–235. doi: 10.1016/j.addr.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Choi B., Park W., Park S.B., Rhim W.K., Han D.K. Recent trends in cell membrane-cloaked nanoparticles for therapeutic applications. Methods. 2020;177:2–14. doi: 10.1016/j.ymeth.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 211.Chen H.Y., Deng J., Wang Y., Wu C.Q., Li X., Dai H.W. Hybrid cell membrane-coated nanoparticles: a multifunctional biomimetic platform for cancer diagnosis and therapy. Acta Biomater. 2020;112:1–13. doi: 10.1016/j.actbio.2020.05.028. [DOI] [PubMed] [Google Scholar]
- 212.Xu C.H., Ye P.J., Zhou Y.C., He D.X., Wei H., Yu C.Y. Cell membrane-camouflaged nanoparticles as drug carriers for cancer therapy. Acta Biomater. 2020;105:1–14. doi: 10.1016/j.actbio.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 213.Thakur A., Parra D.C., Motallebnejad P., Brocchi M., Chen H.J. Exosomes: small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact Mater. 2022;10:281–294. doi: 10.1016/j.bioactmat.2021.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kao C.-Y., Papoutsakis E.T. Extracellular vesicles: exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr Opin Biotechnol. 2019;60:89–98. doi: 10.1016/j.copbio.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 215.Adriano B., Cotto N.M., Chauhan N., Jaggi M., Chauhan S.C., Yallapu M.M. Milk exosomes: nature's abundant nanoplatform for theranostic applications. Bioact Mater. 2021;6(8):2479–2490. doi: 10.1016/j.bioactmat.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Altanerova U., Babincova M., Babinec P., Benejova K., Jakubechova J., Altanerova V., et al. Human mesenchymal stem cell-derived iron oxide exosomes allow targeted ablation of tumor cells via magnetic hyperthermia. Int J Nanomed. 2017;12:7923–7936. doi: 10.2147/IJN.S145096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Piffoux M., Silva A.K.A., Lugagne J.-B., Hersen P., Wilhelm C., Gazeau F. Extracellular vesicle production loaded with nanoparticles and drugs in a trade-off between loading, yield and purity: towards a personalized drug delivery system. Adv Biosyst. 2017;1(5):1700044. doi: 10.1002/adbi.201700044. [DOI] [PubMed] [Google Scholar]
- 218.Pan S., Zhang Y., Huang M., Deng Z., Zhang A., Pei L., et al. Urinary exosomes-based engineered nanovectors for homologously targeted chemo-chemodynamic prostate cancer therapy via abrogating EGFR/AKT/NF-kB/IkB signaling. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120946. [DOI] [PubMed] [Google Scholar]
- 219.Liu C.-M., Chen G.-B., Chen H.-H., Zhang J.-B., Li H.-Z., Sheng M.-X., et al. Cancer cell membrane-cloaked mesoporous silica nanoparticles with a pH-sensitive gatekeeper for cancer treatment. Colloids Surf, B. 2019;175:477–486. doi: 10.1016/j.colsurfb.2018.12.038. [DOI] [PubMed] [Google Scholar]
- 220.Li J., Sun Y., Jia T., Zhang R., Zhang K., Wang L. Messenger RNA vaccine based on recombinant MS2 virus-like particles against prostate cancer. Int J Can. 2014;134(7):1683–1694. doi: 10.1002/ijc.28482. [DOI] [PubMed] [Google Scholar]
- 221.Hu H., Zhang Y., Shukla S., Gu Y., Yu X., Steinmetz N.F. Dysprosium-modified tobacco mosaic virus nanoparticles for ultra-high-field magnetic resonance and near-infrared fluorescence imaging of prostate cancer. ACS Nano. 2017;11(9):9249–9258. doi: 10.1021/acsnano.7b04472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chen L., Hong W., Ren W., Xu T., Qian Z., He Z. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduction Targeted Therapy. 2021;6(1):225. doi: 10.1038/s41392-021-00631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]