Abstract
From August 1996 to May 1997, six verotoxin-producing Escherichia coli (VTEC) strains were isolated from stool specimens of adults suffering from hemolytic-uremic syndrome (HUS). All the isolates were stx2 positive and belonged to different serotypes: O6:H4, O91:H10, O91:H21, O rough:H16, OX3:H−, and O nontypeable:H−. The enterohemolysin (Ehly)-encoding genes were detected in two isolates, and none of the isolates harbors the intimin (Eae)-encoding gene. These findings suggest that stx2-positive non-O157:H7 VTEC is a major cause of HUS in adults and that several sources of pathogens are responsible for local endemic infections.
Hemolytic-uremic syndrome (HUS) is characterized by acute hemolytic anemia, thrombocytopenia, and acute renal failure. In some cases, these three clinical features are associated with neurological manifestations and fever. The association between HUS and verotoxin-producing Escherichia coli (VTEC) infection is now well established, and usually prodromic gastroenteritis, frequently including bloody diarrhea, is observed (9). Cases of HUS caused by VTEC have been identified in all age groups but most frequently in infants and young children, and they are observed either during the course of outbreaks of VTEC infections or as sporadic cases. Contamination occurs via consumption of contaminated food, and most of the clinical signs observed are due to the absorption from the gastrointestinal tract of Shiga-like toxins (Stx) produced by the bacteria. Two types of Shiga-like toxins (also called verotoxins), Stx1 and Stx2, which presumably cause microangiopathic hemolytic anemia as a result of endothelial-cell injury, have been isolated. Other bacterial virulence factors may play a role in the pathological process, including an outer membrane protein, intimin, the product of the chromosomal gene eae, which is involved in bacterial adhesion to intestinal cells (6), as well as a plasmid-encoded enterohemolysin (Ehly) which has a cytolytic effect (20).
E. coli O157:H7 is the worldwide serotype of VTEC most commonly isolated from HUS patients. Other serogroups have been implicated (O26, O55, O103, O111, and O128) (3, 14, 17, 23), but their occurrence is likely to be underestimated, because isolation of non-O157:H7 VTEC still remains a challenge. Unlike most of the O157:H7 isolates, the majority of non-O157:H7 VTEC strains ferment sorbitol and therefore cannot be isolated by using media such as sorbitol MacConkey agar. Molecular biological and immunological techniques based on the detection of verotoxin genes and toxins, respectively, are so far the most reliable methods for detecting these pathogens in clinical specimens.
Patients and clinical features.
The average number of adults with HUS admitted to the medical intensive-care unit of the Clermont-Ferrand hospital used to be one every 18 months. (This hospital serves a large geographical area with approximately 1.3 million residents.) Between August 1996 and May 1997, this number increased considerably; 14 patients with clinical and biological evidence of HUS were admitted. In six cases, a VTEC strain was identified in the patients’ stools by stx-specific PCR. The patients’ mean age was 64 ± 19 years (range, 39 to 84 years). The male-to-female ratio was 1:5. All the patients developed HUS, defined as a Coombs-negative microangiopathic hemolytic anemia, thrombocytopenia without signs of disseminated intravascular coagulation, and acute renal failure (see Table 1). One of them (patient 1) had previously been admitted to the gastroenterology unit with severe abdominal pain and bloody diarrhea. Eleven days later, development of macroscopic hematuria and acute renal failure prompted her transfer to the intensive-care unit. Coombs-negative microangiopathic hemolytic anemia was defined as a hemoglobin level of <10 g/dl, intravascular hemolysis (serum haptoglobin, ≤0.1 g/liter), negative results of Coombs’ test, and fragmented red cells and schistocytes on blood smear. Acute renal failure occurred in all the patients enrolled; four of them required renal replacement therapy. Fever (body temperature of >38°C) was present in four patients. Prodromal bloody diarrhea was observed in two patients, and nonbloody diarrhea was observed in four. All patients were treated with plasma exchanges, and none of them died. The mean number of plasma exchange treatments was 11 ± 2.
TABLE 1.
General, biological, and clinical data of patients during the acute phase and characteristics of the E. coli strains isolated from patients’ stool specimens
| Characteristic | Patienta
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Sexb | F | M | F | F | F | F |
| Age (yr) | 45 | 84 | 63 | 39 | 76 | 77 |
| Prodromic diarrheac | + (B) | + (NB) | + (NB) | + (NB) | + (B) | + (NB) |
| Body temperature (°C) | 38.5 | 37 | 38.5 | 38 | 39 | 37.2 |
| Biological parameter | ||||||
| Hemoglobin level (g/dl) | 6.6 | 7.4 | 8.6 | 5.9 | 7.1 | 9.8 |
| Haptoglobin level (g/liter) | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Schistocytes | + | + | + | + | + | + |
| Platelets (count/μl) | 94,000 | 25,000 | 22,000 | 22,000 | 32,000 | 25,000 |
| White blood cells (count/μl) | 16,000 | 7,530 | 4,260 | 10,810 | 13,960 | 9,200 |
| Creatinine (μmol/liter) | 647 | 454 | 240 | 1,127 | 370 | 542 |
| E. coli characteristic | ||||||
| Serotyped | O6:H4 | O91:H10 | O91:H21 | O rough:H16 | OX3:H− | Ont:H− |
| stxe | stx2 | stx2 | stx2 | stx2 | stx2 | stx2 |
| ehlye | − | − | + | + | − | − |
| eaef | − | − | − | − | − | − |
+, present; −, not detected.
F, female; M, male.
B, bloody; NB, nonbloody.
Ont, not O serotypeable.
Stx- and Ehly-encoding genes detected by PCR and specific hybridizations.
eae detected by dot blot hybridization.
Isolation of VTEC strains by stx-specific PCR.
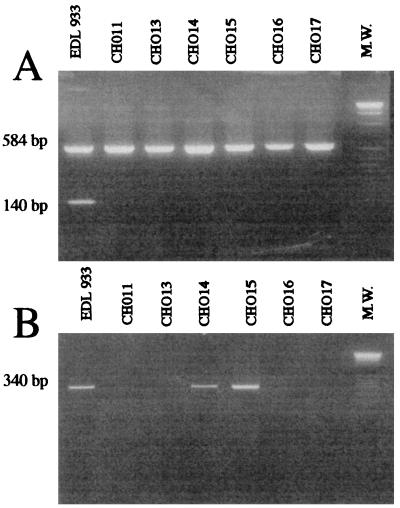
Fecal samples were both cultured in Luria-Bertani (LB) broth (Difco Laboratories, Detroit, Mich.) and streaked out on Drigalski plates (Biomerieux, La Balme les Grottes, France), and they were then incubated at 37°C for 18 h. Bacteria from 1 ml of the LB broth culture or from at least 10 single colonies grown on Drigalski agar and previously suspended in 1 ml of saline were harvested, resuspended in 200 μl of sterile water, and incubated at 100°C for 10 min. Following centrifugation of the lysate, 10 μl of the supernatant was used in PCR. Oligonucleotides specific for amplification were 5′-ACCCTGTAACGAAGTTTGCG-3′ and 5′-ATCTCATGCGACTACTTGAC-3′ for stx1 and 5′-ATCCTATTCCCGGGAGTTTACG-3′ and 5′-GCGTCATCGTATACACAGGAGC-3′ for stx2 (4, 18). The PCR cycle included denaturation for 1 min at 94°C, primer annealing for 1 min at 55°C, and extension for 1 min at 72°C (30 cycles) in a Perkin-Elmer Cetus DNA thermal cycler. Each of the primers was used at 0.125 mM, with 0.2 mM each deoxynucleoside triphosphate (Boehringer Mannheim, Meylan, France), 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1 mM MgCl2, and 1 U of Taq DNA polymerase (Appligène-Oncor, Illkirch, France). The reaction products were then analyzed by electrophoresis on 2% agarose gels after staining with ethidium bromide. DNA from the reference strain E. coli EDL 933 and a reagent blank, which contained all components except the template DNA, were included as positive and negative controls, respectively. The identities of the PCR products were then confirmed by Southern hybridization after transfer to Hybond N+ nylon membranes (Amersham International, Amersham, United Kingdom) and hybridization with a 1.1-kb BamHI stx1-specific or a 0.8-kb PstI stx2-specific DNA probe obtained from the recombinant plasmids pJPN37-19 and pNN111-19, respectively (16). DNA probes were labeled by random priming using the enhanced chemiluminescence system (ECL; Amersham International) according to the manufacturer’s specifications, and hybridized filters were exposed to ECL-Amersham film. As shown in Fig. 1, PCR products of 584 bp were detected with the stx2-specific primers with all stool specimens, but none of them gave a positive reaction with the stx1-specific primers. Similar results were obtained by colony hybridation using Stx1- and Stx2-specific DNA probes (data not shown).
FIG. 1.
Agarose gel electrophoresis of DNA fragments obtained by multiplex PCR with primers specific for stx1 (140 bp) and stx2 (584 bp) (A) and with primers specific for ehly (340 bp) (B) performed with genomic extracts from different E. coli strains: EDL 933, stx1-, stx2-, and ehly-positive O157:H7 reference strain; CH011, CH013, CH014, CH015, CH016, and CH017, isolates from patients 1 through 6, respectively. M.W., 1-kb ladder of molecular size markers (Boehringer Mannheim).
Bacterial identification and characterization.
stx-positive isolates were identified biochemically by using an API 20E test (Biomerieux). All the isolates fermented sorbitol. Determination of their serotypes performed by the International E. coli and Klebsiella Reference Center in Copenhagen, Denmark, revealed that they belonged to different serotypes: O6:H4 (patient 1), O91:H10 (patient 2), O91:H21 (patient 3), O rough:H16 (patient 4), and OX3:H− (patient 5). The O-antigenic nature of the VTEC isolate from patient 6 could not be determined (O+:H−). Ehly-specific genes were detected by PCR using the primers 5′-CACACGGAGCTTATAATATTCTGTCA-3′ and 5′-AATGTTATCCCATTGACATCATTTGACT-3′. Conditions similar to those used for detection of stx genes were used, and the PCR products were identified by hybridization with a 3.4-kb HindIII fragment from pEO40 (20). Two strains, those isolated from patients 3 and 4, harbored Ehly-specific sequences as determined by PCR (Fig. 1) and hybridization; the same two isolates produced detectable hemolysis after 18 h of growth at 37°C on 5% washed sheep blood agar plates. The presence of eae was detected by dot blot hybridization; bacteria were grown in LB broth at 37°C overnight, and DNA was extracted by successive action of lysozyme, proteinase K, and Sarkosyl, followed by a purification step in a cesium chloride gradient. Hybridization was performed as described above by using a DNA probe specific for eae, i.e., a 1.4-kb fragment from an O157:H7 clinical isolate covering the entire eae open reading frame. DNAs from the reference strains E. coli EDL 933 and DH5α were included as positive and negative controls, respectively. None of the VTEC isolates hybridized with this DNA probe when they were tested under high-stringency conditions.
All the VTEC strains isolated in this study harbored Stx2-encoding genes. A higher prevalence of infection with VTEC producing only Stx2 among HUS patients has been reported in several investigations (10, 22). This may reflect the higher pathogenicity previously observed with Stx2- versus Stx1-producing strains both in in vitro assays with endothelial cells (13) and in murine models (24). All the bacterial strains were sorbitol fermenting, and none of them belonged to the O157:H7 serotype. However, although it is unlikely that we would have missed an O157:H7 isolate in the patients’ stools, we cannot exclude the possibility of the occurrence of mixed infections with both a non-O157:H7 and an O157:H7 E. coli strain. Previous studies have described a few cases of mixed infections by detecting anti-O157 antibodies in patients’ sera (2, 5). Unfortunately, we were not able to test patients’ sera for anti-O157 antibody detection in this study. But if we had used routinely performed laboratory procedures with stool specimens, i.e., use of media such as sorbitol MacConkey agar or immunomagnetic separation techniques using anti-O157 antibody-coated beads, none of the present non-O157 isolates would have been detected. Analysis of their ribotype patterns (data not shown) did not reveal any homology, and they all belonged to different serotypes, indicating the sporadic nature of the cases. Three of them belonged to serogroups which have previously been associated with VTEC infections in humans (O91 and O6) (10, 12, 25) and isolated from meat and fecal samples of bovines in both the United States and Europe (15, 19). The O group OX3 is a provisional designation for a new O antigen, but a few isolates from this serogroup, differing from our isolate by the H antigen, have already been isolated from patients suffering from HUS in Europe. In Finland, an Stx2-positive E. coli OX3:H21 was detected in the stools of a 66-year-old woman, and in Denmark, E. coli OX3:H2 was detected in the urine of a patient (8, 11). Since strains belonging to this serogroup are detected in meat samples (19) and in domestic animals (1), they might represent another group of potentially life-threatening VTEC strains causing food infections.
Virulence factors other than toxins are likely to be required during the pathological process, including adherence factors and/or cytolysins.
Among the six VTEC strains isolated in this study, none harbored the intimin-encoding gene (eae), which is involved in the attachment and effacing process, and Ehly sequences were detected in only two isolates. The presence of eae has mostly been described in O157:H7 isolates, but eae-negative non-O157 VTEC strains are also capable of causing disease indistinguishable from that caused by eae-positive O157:H7 (7, 11). It is likely that eae-negative VTEC strains pathogenic for humans may possess adherence factors other than Eae; investigations are currently being performed with isolates from this study in order to identify their adherence factors.
The role of the plasmid-encoded Ehly in the pathologic process of VTEC strains is not yet known. Ehly’s produced by VTEC strains belong to the RTX (defined as repeats in toxin) toxin family and are closely related to the E. coli α hemolysin. They might act by lysing eucaryotic cells or by modulating the immune response, thus enhancing the virulence of VTEC. Previous studies demonstrated that patients infected with Ehly-positive VTEC were at a higher risk for developing HUS than patients infected with Ehly-negative strains (21). Only two bacterial isolates from this study harbored Ehly-encoding genes, indicating that synthesis of Ehly is not an absolute prerequisite for HUS development, although it might contribute.
From this study, we conclude that Shiga toxin-producing bacteria of serotypes other than O157:H7 can cause serious disease, as has been observed in several other instances. Cases of HUS due to non-O157:H7 E. coli are usually sporadic, unlike most of the infections due to serotype O157:H7. The reasons for this difference have not yet been addressed; it might be due to variations in the strains’ virulence, but difficulties in identification of non-O157:H7 E. coli strains might also contribute to underestimation of their virulence potential. Although the cases of HUS observed in this study occurred in the same geographical area in a relatively short period (10 months), characterization of the VTEC isolates demonstrated that they were not related to each other. This might reflect an endemic situation, and since HUS represents the tip of an iceberg of clinical complications, it is likely that the number of mild infections is greatly underestimated. Development of diagnostic tools allowing detection of VTEC regardless of serotype is therefore urgently needed. Rapid and efficient detection of VTEC should be performed not only with patients suffering from HUS, but with anyone suffering from bloody diarrhea, in order to prevent both severe development of the disease and further spread of the pathogens.
Acknowledgments
We thank Kristin Swihart for critical review of this paper.
REFERENCES
- 1.Beutin L, Geier D, Steinruck H, Zimmermann S, Scheutz F. Prevalence and some properties of verotoxin (Shiga-like toxin)-producing Escherichia coli in seven different species of healthy domestic animals. J Clin Microbiol. 1993;31:2483–2488. doi: 10.1128/jcm.31.9.2483-2488.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitzan M, Ludwig K, Klemt M, Konig H, Buren J, Muller-Wiefel D E. The role of Escherichia coli O157 infections in the classical (enteropathic) haemolytic uraemic syndrome: results of a central European, multicentre study. Epidemiol Infect. 1993;110:183–196. doi: 10.1017/s0950268800068102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caprioli A, Luzzi I, Rosmini F, Resti C, Edefonti A, Perfumo F, Farina C, Goglio A, Gianviti A, Rizzoni G. Communitywide outbreak of hemolytic-uremic syndrome associated with non-O157 verocytotoxin-producing Escherichia coli. J Infect Dis. 1994;169:208–211. doi: 10.1093/infdis/169.1.208. [DOI] [PubMed] [Google Scholar]
- 4.Cebula T A, Payne W L, Feng P. Simultaneous identification of strains of Escherichia coli serotype O157:H7 and their Shiga-like toxin type by mismatch amplification mutation assay-multiplex PCR. J Clin Microbiol. 1995;33:248–250. doi: 10.1128/jcm.33.1.248-250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chart H, Smith H, Scotland S M, Rowe B, Milford D V, Taylor C M. Serological identification of Escherichia coli O157:H7 infection in haemolytic uraemic syndrome. Lancet. 1991;337:138–140. doi: 10.1016/0140-6736(91)90801-u. [DOI] [PubMed] [Google Scholar]
- 6.Donnenberg M S, Tzipori S, McKee M L, O’Brien A D, Alroy J, Kaper J B. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J Clin Invest. 1993;92:1418–1424. doi: 10.1172/JCI116718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dytoc M T, Ismaili A, Philpott D J, Soni R, Brunton J L, Sherman P M. Distinct binding properties of eaeA-negative verocytotoxin-producing Escherichia coli of serotype O113:H21. Infect Immun. 1994;62:3494–3505. doi: 10.1128/iai.62.8.3494-3505.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giammanco A, Maggio M, Giammanco G, Morelli R, Minelli F, Scheutz F, Caprioli A. Characteristics of Escherichia coli strains belonging to enteropathogenic E. coli serogroups isolated in Italy from children with diarrhea. J Clin Microbiol. 1996;34:689–694. doi: 10.1128/jcm.34.3.689-694.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin P M, Tauxe R V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–97. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 10.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keskimäki M, Ikäheimo R, Kärkkäinen P, Scheutz F, Ratiner Y, Puohiniemi R, Siitonen A. Shiga toxin-producing Escherichia coli serotype OX3:H21 as a cause of hemolytic-uremic syndrome. Clin Infect Dis. 1997;24:1278–1279. doi: 10.1086/513668. [DOI] [PubMed] [Google Scholar]
- 12.Lindgren S L, Melton A R, O’Brien A D. Virulence of enterohemorrhagic Escherichia coli O91:H21 clinical isolates in an orally infected mouse model. Infect Immun. 1993;61:3832–3842. doi: 10.1128/iai.61.9.3832-3842.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louise C B, Obrig T G. Specific interaction of Escherichia coli O157:H7-derived Shiga-like toxin II with human renal endothelial cells. J Infect Dis. 1995;172:1397–1401. doi: 10.1093/infdis/172.5.1397. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig K, Bitzan M, Zimmermann S, Markus K, Ruder H, Muller-Wiefel D E. Immune response to non-O157 Vero toxin-producing Escherichia coli in patients with hemolytic-uremic syndrome. J Infect Dis. 1996;174:1028–1039. doi: 10.1093/infdis/174.5.1028. [DOI] [PubMed] [Google Scholar]
- 15.Montenegro M A, Bülte M, Trumpf T, Aleksic S, Reuter G, Bulling E, Helmuth R. Detection and characterization of fecal verotoxin-producing Escherichia coli from healthy cattle. J Clin Microbiol. 1990;28:1417–1421. doi: 10.1128/jcm.28.6.1417-1421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newland J W, Neill R J. DNA probes for Shiga-like toxins I and II and for toxin-converting bacteriophages. J Clin Microbiol. 1988;26:1292–1297. doi: 10.1128/jcm.26.7.1292-1297.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paton A W, Ratcliff R M, Doyle R M, Seymour-Murray J, Davos D, Lanser J A, Paton J C. Molecular microbiological investigation of an outbreak of hemolytic-uremic syndrome caused by dry fermented sausage contaminated with Shiga-like toxin-producing Escherichia coli. J Clin Microbiol. 1996;34:1622–1627. doi: 10.1128/jcm.34.7.1622-1627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollard D R, Johnson W M, Lior H, Tyler S D, Rozee K R. Rapid and specific detection of verotoxin genes in Escherichia coli by the polymerase chain reaction. J Clin Microbiol. 1990;28:540–545. doi: 10.1128/jcm.28.3.540-545.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samadpour M, Ongerth J E, Liston J, Tran N, Nguyen D, Whittam T, Wilson R A, Tarr P I. Occurence of Shiga-like toxin-producing Escherichia coli in retail fresh seafood, beef, lamb, pork, and poultry from grocery stores in Seattle, Washington. Appl Environ Microbiol. 1994;60:1038–1040. doi: 10.1128/aem.60.3.1038-1040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt H, Beutin L, Karch H. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect Immun. 1995;63:1055–1061. doi: 10.1128/iai.63.3.1055-1061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt H, Karch H. Enterohemolytic phenotypes and genotypes of Shiga toxin-producing Escherichia coli O111 strains from patients with diarrhea and hemolytic-uremic syndrome. J Clin Microbiol. 1996;34:2364–2367. doi: 10.1128/jcm.34.10.2364-2367.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scotland S M, Willshaw G A, Schmidt H R, Rowe B. Properties of strains of Escherichia coli belonging to serogroup O157 with special reference to production of vero cytotoxins VT1 and VT2. Epidemiol Infect. 1987;99:613–624. doi: 10.1017/s0950268800066462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarr P I, Neill M A. Perspective; the problem of non-O157:H7 Shiga-toxin (verocytotoxin)-producing Escherichia coli. J Infect Dis. 1996;174:1136–1139. doi: 10.1093/infdis/174.5.1136. [DOI] [PubMed] [Google Scholar]
- 24.Wadolkowski E A, Sung L M, Burris J A, Samuel J E, O’Brien A D. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect Immun. 1990;58:3959–3965. doi: 10.1128/iai.58.12.3959-3965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willshaw G A, Scotland S M, Smith H R, Rowe B. Properties of vero cytotoxin-producing Escherichia coli of human origin of O serogroups other than O157. J Infect Dis. 1992;166:797–802. doi: 10.1093/infdis/166.4.797. [DOI] [PubMed] [Google Scholar]