Abstract
Objective
Disparities exist in health care access, diagnosis, and treatment of chronic pain in Latino populations and other minority populations. Cognitive behavioral–based physical therapy (CBPT) interventions have been shown to be effective in predominantly non-Hispanic white populations with chronic spine pain. However, there is a need for culturally adapted CBPT interventions that focus on the conservative management of chronic spine pain. The primary purpose of the study described in this protocol is to test the efficacy of an adapted cognitive behavioral–based hybrid telerehabilitation intervention for Latino patients with chronic spine pain.
Methods
A single-blind, 2-arm parallel group, superiority randomized clinical trial is planned to compare an adapted CBPT intervention to Usual Care physical therapy. Goal Oriented Activity for Latinos with chronic Spine pain (GOALS/Metas) is an 8-week hybrid telerehabilitation intervention that integrates guideline-based physical therapy and pain management interventions using cognitive behavioral approaches and has been adapted for Latino patients with chronic spine pain. Usual Care physical therapy will be administered based on institutional standards at the referring health center. Outcome measures will be evaluated preintervention and at 1-week, 3-months, and 6-months postintervention. The primary outcome is pain-related disability 1-week postintervention using the Brief Pain Inventory Pain Interference subscale. Secondary outcome measures include behavioral measures of functional activity, social participation, physical activity, and sleep. Determinants of treatment effect, including pain-related psychological measures, posture and movement, self-efficacy, treatment expectancy, and therapeutic alliance, will be included in the secondary moderation and mediation analyses.
Impact
This clinical trial will provide information on the extent to which an adapted CBPT hybrid telerehabilitation intervention is effective in reducing pain-related disability for Latino patients with chronic spine pain. This information will be useful for clinicians to integrate in their practice, given the growing population of Latino patients who experience disparities in health care management of chronic pain.
Keywords: Adapted, Chronic, Cognitive Behavioral, Hispanic, Latino, Low Back, Neck, Pain, Physical Therapy, Spine
Introduction
Chronic spine pain is a highly prevalent health condition, which has a profound impact on the lives of individuals affected and society. Up to 80% of the population experiences low back pain (LBP) at some point in their lives,1 with an average of 62% reporting chronic LBP after an initial episode.2 Neck pain affects 30% to 50% of the general population each year,3 with 14% to 17% of people reporting chronic neck pain.4,5 Further, LBP ranked highest and neck pain ranked fourth as most disabling among 291 diseases globally.6,7 The impact of chronic spine pain on affected individuals and their families is substantial, including poor mood, persistent disability, reduced participation in leisure time and social activities, difficulty engaging in work activities, and inability to care for children.8–11 The resulting socioeconomic impacts of chronic spine pain in the United States are profound; the estimated annual cost of LBP alone is approximately $100 billion due to reduced productivity and lost wages.12
Chronic spine pain is a problem for Hispanic and Latino-Americans in particular, with studies reporting chronic LBP in 24% to 55% of Hispanic and Latino adults.13,14 This is likely an underestimate of prevalence, since Hispanic and Latino adults are less likely than non-Hispanic white adults to report chronic pain.15 Further, compared to other racial and ethnic groups, Hispanic and Latino adults report higher pain-related anxiety,16 which is a known risk factor for the development and persistence of spine pain.17 Disparities in access to culturally competent care also have been reported for Hispanic and Latino adults with chronic pain.15 Specifically, there are no existing culturally and linguistically adapted physical therapist interventions that focus on the conservative management of chronic spine pain in Hispanic and Latino adults.
Evidence-based physical therapist clinical practice guidelines have been established for chronic spine pain, with demonstrated effectiveness for reducing pain and improving function.18,19 In addition, psychologically informed physical therapist interventions that incorporate cognitive behavioral approaches for pain management have been developed and tested to address the psychological risk factors associated with chronic spine pain.17,20–26 Cognitive behavioral–based physical therapy (CBPT) interventions, in particular, have demonstrated efficacy for spine pain27–32; however, these interventions have yet to be culturally and linguistically adapted for Hispanic and Latino adults.
Difficulty with access to health care poses an additional barrier for Hispanic and Latino adults with chronic spine pain.15 Telerehabilitation has been increasingly used to help improve access to medical care for populations that historically have had difficulty with access.33 Further, the COVID-19 pandemic forced a shift to telerehabilitation in many physical therapy settings, including outpatient management of musculoskeletal conditions like chronic spine pain.34 Preliminary evidence on telehealth for spine pain indicates that a hybrid approach is more effective for reducing disability.35 Further, cognitive behavioral–based interventions can be readily delivered via telerehabilitation with outcomes that are comparable to traditional face-to-face delivery methods.36
An existing multimodal CBPT telerehabilitation intervention, with demonstrated efficacy for reducing pain and improving function in patients after spine surgery, was culturally and linguistically adapted for Latino adults with chronic spine pain.28,37 The primary purpose of the study described in this protocol is to test the efficacy of the adapted cognitive behavioral–based hybrid telerehabilitation intervention for Latino adults with chronic spine pain, entitled Goal Oriented Activity for Latinos with chronic Spine pain (GOALS/Metas). We hypothesize that participants in the GOALS/Metas intervention will experience a greater improvement in pain-related disability than participants in a Usual Care group. A secondary purpose of this study is to examine the psychological and biological moderators and mediators of treatment response.
Methods
Overview of Study and Setting
The GOALS/Metas intervention will be compared to Usual Care physical therapy by conducting a single-blind, 2-arm parallel group, superiority randomized clinical trial (Fig. 1). This study is being conducted in partnership with a Federally Qualified Health Center (FQHC), serving uninsured, low-income, and medically underserved persons in San Diego County. Enrolled participants will be randomized into either the GOALS/Metas intervention arm or the Usual Care control arm. GOALS/Metas is an 8-week CBPT intervention for Latino adults with chronic spine pain, which is delivered in a hybrid format with 2 in-person sessions and 6 telerehabilitation sessions (Fig. 2). The Usual Care comparison intervention is 8 weeks of standard physical therapy at the FQHC. Outcome measures include questionnaires and objective measures of spine posture and movement, physical activity, and sleep behaviors. Study assessments will be conducted by the research staff 1 week before (baseline) and at 1 week (1-wk Post), 3 months (3-mo Post), and 6 months (6-mo Post) after completing the assigned intervention (Fig. 1). The primary outcome is pain-related disability as measured by the Brief Pain Inventory (BPI) Pain Interference subscale.38
Figure 1.

Study design for parallel group randomized clinical trial comparing the Goal Oriented Activity for Latinos with chronic Spine pain (GOALS/Metas) intervention to Usual Care physical therapy. EHR = Electronic Health Records.
Figure 2.
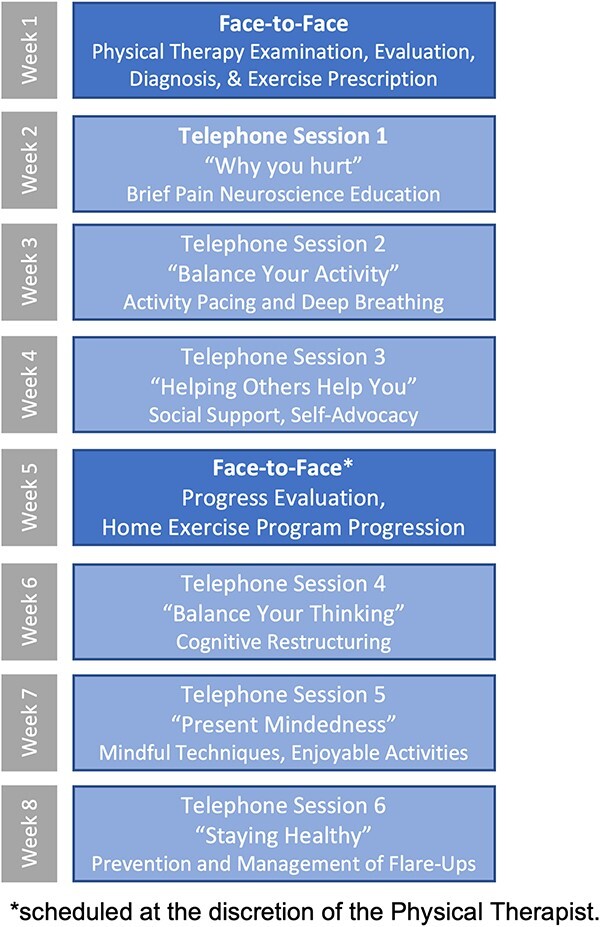
GOALS/Metas hybrid telerehabilitation intervention, including content of face-to-face sessions and cognitive behavioral pain coping skills for each telephone session (Note: Each telephone session also includes goal setting for graded aerobic activity, impairment-based therapeutic exercise, and graded functional activity).
Research staff conducting assessments and biostatisticians conducting analyses will be blinded to the treatment group. All research staff conducting recruitment and assessments and physical therapists implementing the GOALS/Metas intervention will be bilingual (English/Spanish) and have demonstrated fluency in oral and written communication in both languages. Study materials were professionally translated, and these are available in English and Spanish. All translated materials were pilot tested and adapted as appropriate during a 2-stage adaptation process. Assessments and GOALS/Metas intervention visits will be conducted in the participant’s preferred language.
Participant Eligibility and Recruitment
One hundred thirty-eight Hispanic patients between the ages of 18 and 66, who are referred by their primary care physician to physical therapy for neck or LBP, will be enrolled. Consecutive patients from the FQHC’s physical therapy referral database, who meet prescreening eligibility criteria for Hispanic ethnicity, age, and medical condition (neck pain or LBP), will be contacted by bilingual research staff to determine eligibility using a phone screening questionnaire. Additional symptom-related eligibility criteria assessed during the phone screening are included in Supplementary Appendix 1. Participants who are deemed eligible based on the phone screening will be enrolled in the study and will be scheduled for the first study assessment, where they will provide written informed consent prior to participation.
Randomization and Allocation of Interventions
Enrolled participants will be stratified based on the pain region (cervical and lumbar) and sex (female and male) and will be randomized into the GOALS/Metas or Usual Care intervention arm. The stratified randomization scheme was developed based on the prevalence of referrals for cervical (25%) and lumbar spine (75%) pain and for female (60%) and male (40%) patients extracted from the FQHC electronic health records. The randomization scheme will be generated in Statistical Analysis System (SAS) analytics software (Cary, NC, USA) and implemented using the custom randomization schedule function on the REDCap Cloud platform (Encinitas, CA, USA), with random assignments completed after eligible participants complete the baseline assessment. The allocation sequence and group assignment will be concealed from the blinded research staff conducting assessments by including this information in an event on REDCap Cloud, which is only accessible to unblinded research staff using permissions’ restrictions. An unblinded recruitment coordinator will schedule participants with providers for their assigned group.
Interventions
GOALS/Metas Intervention
The GOALS/Metas intervention was systematically adapted for Latino patients with chronic spine pain from an existing evidence-based CBPT intervention.28 GOALS/Metas combines clinical practice guideline-based physical therapy18,19 with cognitive-behavioral skill training to address the physical, psychosocial, and behavioral factors known to influence the experience of chronic spine pain. Systematic adaptation of the intervention was conducted by a panel, including a team of physical therapists, a clinical psychologist, clinical scientists, and implementation scientists, and included input from Latino patients with chronic spine pain. The adaptation panel used Intervention Mapping39 and considered the cultural and linguistic factors specifically relevant to Latino patients with chronic spine pain. Adaptation of the GOALS/Metas intervention has been presented in abstract form40 and will be detailed in future reports.
Briefly, GOALS/Metas is an 8-week, hybrid telerehabilitation intervention implemented by a bilingual research physical therapist with formal training in the manualized intervention (see Supplemental Materials). The hybrid intervention begins with an initial face-to-face physical therapy evaluation, followed by 6 remote treatment sessions conducted once per week by telephone. A second face-to-face session is scheduled near the midpoint of the intervention to assess the progress and advance the participant’s home exercise program (Fig. 2). Weekly remote treatment sessions are comprised of a graded activity program focused on goal setting in 4 treatment domains: pain coping skills, therapeutic exercise, aerobic activity, and functional activity. Pain coping skills adapted from cognitive behavioral–based interventions described by Archer et al28 and Thorn et al41 include instruction in pain neuroscience,42 under/overactive activity types and activity pacing,43,44 deep breathing/relaxation,45,46 soliciting helpful social support,47 cognitive restructuring,48,49 present mindedness,46,48,50 and creating a plan for symptom self-management.51 Therapeutic exercise, aerobic activity, and functional activities are individually tailored and progressed at the physical therapists’ discretion based on impairments from the examination and to meet patient-identified goals using a bank of evidence-based exercises for neck pain and LBP18,19 along with standard guidelines for progression of exercise time, intensity, and complexity.52 To reduce fear of movement and promote increases in functional activity, the physical therapist also provides education on behavioral strategies to reduce pain-provoking spine postures and movements during daily activities.53 Finally, principles of motivational interviewing are used throughout the intervention to facilitate adherence to patient-identified goals.54 Participants receive an informational binder with worksheets to follow along with the therapist during treatment sessions. Each session includes an action plan and weekly assignments that are individually tailored to the participant’s goals.
Usual Care Intervention
The Usual Care control intervention is delivered by physical therapists employed by the FQHC according to the institutional standards of care. Participants in the Usual Care group are scheduled for an initial physical therapy evaluation at 1 of 4 FQHC Rehabilitation clinics. The plan of care is then determined by the treating physical therapist. The number of physical therapy sessions and types of interventions provided during Usual Care will be characterized by extracting Current Procedural Terminology billing dates and codes from the participants’ electronic health record over the 8-week study period. Physical therapists who provide the Usual Care intervention will be naive to the purpose of the study and details of the GOALS/Metas intervention. Participants in the Usual Care group will be instructed not to discuss their study participation with the treating physical therapist.
Intervention Fidelity, Adherence, and Adverse Event Monitoring
Delivery of the GOALS/Metas intervention will adhere to a manualized protocol. Physical therapists will self-monitor intervention fidelity for each session of GOALS/Metas by completing a checklist of required components and noting the reasons for any protocol deviations. Ten percent of audio-recorded treatment sessions will be reviewed by a principal investigator (K.S.M.) using the same fidelity checklist and discussed in biweekly meetings with GOALS/Metas physical therapists. Fidelity check meetings will occur throughout the duration of the trial to address protocol deviations and to review the cognitive behavioral and motivational interviewing skills as needed to minimize intervention drift.
Participants' adherence to the intervention will be monitored by assessing the percentage of scheduled physical therapy sessions attended by each participant. Adherence to behavioral goals for each GOALS/Metas treatment session will be monitored by the physical therapist using a Goal Attainment Scale.55 Adverse events will be directly assessed and recorded by the research physical therapists. Adverse events will be reviewed weekly by the investigative team and biannually by a Data Safety and Monitoring Board and will be reported to the Institutional Review Board.
Outcome Assessments
Assessments will be conducted by blinded, bilingual research staff at baseline and at 1-week, 3-months, and 6-months postintervention (see Supplemental Materials). Assessments will include questionnaires administered electronically on REDCap Cloud, sensor measures of lumbar spine posture and movement (DorsaVi Inc, New York, NY, USA) and physical activity and sleep (Actigraph Inc, Pensacola, FL, USA). For questionnaires, assessors will read questions aloud in the participant’s preferred language and will record the participant’s responses in REDCap Cloud. To facilitate access and retention, participants will be offered the opportunity to complete assessments remotely via video or voice call if unable to attend in-person assessment visits. For remote assessments, participants will have a visual reference (paper copy) for all questionnaires, and sensors will be delivered directly to participants for remote monitoring. Assessment questionnaires that were available and validated in Mexican-American Spanish were used.56–65 When validated Mexican-American translations were not available, 2-panel consensus translation was conducted to translate questionnaires.66 All measures, constructs, time points at which they are collected, and references for validated English and Spanish measures are listed in Table 1.
Table 1.
GOALS/Metas timeline, measures and associated constructs for outcomes, behaviors, determinants, and cultural and psychosocial contexta
| Measure | Construct | Baseline | Postintervention | ||
|---|---|---|---|---|---|
| 1-week | 3-months | 6-months | |||
| Primary Outcome | |||||
| Brief Pain Inventory (BPI) – Pain Interference Subscale38,59,67,68,70 | Pain-Related Disability | X | X | X | X |
| Secondary Clinical and Behavioral Outcomes | |||||
| Brief Pain Inventory (BPI) – Pain Severity Subscale38,59,67,68,70 | Pain Intensity | X | X | X | X |
| Pain, Enjoyment of Life, and General Activity Scale (PEG-3)67,71 | Pain Intensity and Interference | X | X | X | X |
| Modified Oswestry Disability Index (ODI)b,57,58,72,84 | Pain-Related Disability (Low Back) | X | X | X | X |
| Neck Disability Index (NDI)57,62,74,76,78 | Pain-Related Disability (Neck) | X | X | X | X |
| Patient Specific Functional Scale (PSFS)79,80 | Functional Activities (Behavior) | X | X | X | X |
| PROMIS Short-Form Questionnaires (Physical Function, Participation)85 | Physical and Social Participation | X | X | X | NT |
| Physical Activity (Actigraph)86,87 | Physical Activity (Behavior) | X | X | NT | NT |
| Rapid Assessment of Physical Activity (RAPA)65,88 | Physical Activity (Behavior) | X | X | X | NT |
| Sleep (Actigraph)89,90 | Sleep (Behavior) | X | X | NT | NT |
| PROMIS Short-Form Sleep Disturbance Questionnaire85,91 | Sleep (Behavior) | X | X | X | NT |
| Global Impression of Change (GIC)83 | Perceived Change | NT | X | X | X |
| Global Rating of Satisfaction (GRS)83 | Satisfaction | NT | X | X | X |
| Determinants | |||||
| Demographic & Health History Questionnaire83,92,93 | Sociodemographic & Clinical Characteristics | X | NT | NT | NT |
| Treatment Expectancy Measure (TEM)41,94 | Treatment Expectations | X | NT | NT | NT |
| Fear Avoidance Beliefs Questionnaire (FABQ)61,95,96 | Fear of Movement | X | X | X | NT |
| Pain Catastrophizing Scale (PCS)b,97,98 | Pain Catastrophizing | X | X | X | NT |
| Pain Self-Efficacy Questionnaire (PSEQ)b,99,100 | Pain Self-Efficacy | X | X | X | NT |
| New General Self-Efficacy Scale (GSES)b,101 | General Self-Efficacy | X | X | X | NT |
| Self-Efficacy & Exercise Habits Survey (SEHS)a,102 | Exercise Self-Efficacy | X | X | X | NT |
| Clinical & Functional Movement Testing (DorsaVi)103–105 | Spine Posture and Movement | X | X | NT | NT |
| Kim Alliance Scale (KAS)b,106–108 | Therapeutic Alliance | NT | X | NT | NT |
| Cultural & Psychosocial Context | |||||
| Bidimensional Acculturation Scale for Hispanics (BAS)56,109–111 | Acculturation | X | NT | NT | NT |
| Connor-Davidson Resilience Scale60,112–114 | Resilience | X | NT | NT | NT |
| Medical Outcomes Study – Social Support Measure (MOS-SS)63,64,115 | Social Support | X | X | NT | NT |
| PROMIS Short-Form Questionnaires (Anxiety, Depression)85 | Psychological, Anxiety and Depression | X | X | X | NT |
NT = not tested; PROMIS = Patient-Reported Outcomes Measurement Information System.
Translated into Spanish using the 2-panel consensus method.66
Measures were selected to capture the demographic and clinical characteristics of the study sample as well as the outcomes, behaviors, determinants, and the cultural and psychosocial contextual factors hypothesized to influence treatment response in Latino patients with chronic spine pain. The primary outcome is the change in pain-related disability from baseline to 1-week postintervention based on the BPI Pain Interference subscale.38,59,67–70 This primary outcome was selected because it reflects the extent to which spine pain interferes with various aspects of daily life, is a clinically meaningful outcome, and significant improvements in pain interference were observed in the previous CBPT intervention for postsurgical spine pain.28 Secondary outcome measures include change in the following measures from baseline: BPI Pain Interference subscale at 3-month and 6-month time points; the BPI Pain Severity subscale38,59,67–70; the Pain, Enjoyment of Life, and General Activity Scale (PEG-3)67,71; and region-specific measures of pain-related disability, including the modified Oswestry Disability Index for LBP57,58,72,73; the Neck Disability Index for neck pain57,62,74–78; and the Patient-Specific Functional Scale79,80 at all follow-up time points, and performance on functional tasks, including a self-paced and fast-paced 6-m walk (speed), overhead lift for neck pain (speed and repetitions), squat lift for LBP (speed and repetitions), and 30-s sit-to-stand for LBP (repetitions) at 1-week postintervention. More information on additional outcome measures and measures of behaviors, determinants, and the cultural and psychosocial contextual factors are described in Supplementary Appendix 2.
Data Analysis
Sample Size Calculation
Sample size calculations were derived based on the effect sizes for change in the BPI Pain Interference subscale between intervention and control groups 3-months postintervention as reported in the CBPT study that provided the basis for the GOALS/Metas intervention.28 A total sample size of 138 participants (N = 69 per group) is needed to detect a group difference in change in BPI Pain Interference of 1.5 (effect size = 0.57) with 80% power and an alpha of 0.0125 to govern the family-wise error rate to account for the secondary moderation and mediation analyses. This sample size assumes a within-subject correlation (rho) of 0.38 and actual drop-out rates (40%) observed during pilot testing of the adapted GOALS/Metas intervention. The high rate of attrition during pilot testing is consistent with the high nonadherence rates for physical therapy referrals at the FQHC based on a historical review of electronic health record data.
Primary Analysis of Treatment Effect
A modified intention-to-treat (mITT) approach will be used to test our primary hypothesis that participants in the GOALS/Metas intervention will experience a greater improvement in pain-related disability than participants in the Usual Care group. The analysis is considered a modified ITT approach because all participants who are randomized may not be included in the analysis due to the inclusion criterion that participants must complete at least 1 physical therapy session, which occurs after randomization. The mITT, which includes only participants who attend the first physical therapy session after randomization, was selected to account for the unusually high rates of nonadherence to physical therapy referral in the FQHC setting. Therefore, the primary mITT analysis will be conducted for all participants who complete the baseline assessment, are randomized, and complete at least 1 physical therapy session of either the GOALS/Metas or Usual Care intervention. Descriptive statistics and tests for data normality will be conducted, and missing data will be handled using a multiple imputation approach. A linear regression model will be used to test for the intervention group by time interaction effects for the primary outcome of BPI Pain Interference at 1-week postintervention. The model will be adjusted for the sociodemographic and clinical characteristics that may affect treatment response and are found to differ between groups.
Secondary Analyses
Secondary outcome measures will be analyzed as described above. Per protocol analyses also will be conducted using a similar approach for primary (1-wk Post) and secondary (3-mo Post and 6-mo Post) endpoints to assess the magnitude of treatment effects that can be expected for participants who complete at least 80% of scheduled physical therapy sessions in each treatment arm. Finally, planned secondary analyses will explore the physiological, psychological, and environmental moderators and mediators of treatment response. Multivariate logistic regression analyses will be conducted to explore candidate moderators (determinants) within each measurement domain. Potential mediators of treatment response will be explored with generalized structural equation modeling using maximum likelihood estimation methods. Treatment response will be characterized using a dichotomous variable distinguished by  30% reduction in BPI Pain Interference, which is considered a clinically meaningful improvement in people with chronic pain based on the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) guidelines.81–83 All data analyses will be conducted using SAS, version 9.4, and Stata, Version 16.1 analytics software.
30% reduction in BPI Pain Interference, which is considered a clinically meaningful improvement in people with chronic pain based on the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) guidelines.81–83 All data analyses will be conducted using SAS, version 9.4, and Stata, Version 16.1 analytics software.
Role of the Funding Source
The funding source had no role in the study's design, conduct, and reporting.
Discussion
This is the first clinical trial to examine the efficacy of a culturally adapted cognitive behavioral–based telerehabilitation intervention for Latino patients with chronic pain. The intervention integrates guideline-based physical therapy and pain management interventions using cognitive behavioral approaches. This study protocol design includes several novel culturally informed components specifically designed for implementation in a population of Latino patients with chronic spine pain from an FQHC, including (1) a culturally and linguistically adapted intervention for Latino patients; (2) study materials available in English and Spanish, delivered in the participant’s preferred language; (3) bilingual (English/Spanish) research staff and physical therapists implementing the GOALS/Metas intervention; and (4) a hybrid telerehabilitation approach to facilitate access to care and participation for Latino patients from an FQHC. One concern that has arisen with implementation of telehealth interventions in low-income and medically underserved populations is limitations in access to technology. A strength of the GOALS/Metas intervention is that it was specifically adapted as a hybrid intervention with telerehabilitation components delivered via telephone to avoid access barriers. If effective, this intervention could be integrated with the existing practice and implemented to provide the first culturally appropriate evidence-based physical therapist intervention for Latino patients with chronic spine pain.
There are several limitations of the proposed study protocol design. First, the GOALS/Metas intervention is complex and includes multiple components that require extensive training. However, this training is critical to implementing cognitive behavioral aspects of the program, given that prior studies have demonstrated the failed implementation of cognitive behavioral approaches which were attributed to lack of provider training.24 Second, the complexity of a behavioral intervention, such as GOALS/Metas, limits the ability to isolate the effects of individual treatment elements. However, secondary responder, mediation, and moderation analyses will allow us to examine the influence of determinants of treatment response. Third, the comparison group is Usual Care physical therapy as determined by the physical therapist at the FQHC, which could result in variability in the intervention components for the comparison intervention. However, a Usual Care comparison group was selected because it is representative of physical therapy treatment that is currently available to the population of interest, and the intervention variability can be measured through precise chart-review. The current study was powered to test efficacy using the primary outcome at 1 postintervention endpoint (1-wk Post), but it is possible that it may not be adequately powered to test additional postassessment time points and/or secondary outcomes. However, this rich dataset will inform mechanistic modeling of treatment effects that can be used to refine future iterations of the intervention for clinical implementation. Last, the mITT analytic approach could introduce bias if engagement in the first physical therapy session is different between intervention groups. However, given the high rates of nonadherence to physical therapy referral at FHCSD, this approach was selected instead of a traditional ITT approach to ensure adequate enrollment of participants who initiate treatment as recommended by their physician. Results from the mITT analysis will not reflect outcomes from those who are unwilling or unable to initiate physical therapy for the management of spine pain.
This clinical trial will provide clinicians with information on the extent to which an adapted CBPT intervention is effective in reducing pain interference for Latino patients with chronic spine pain. This information can be useful for clinicians to integrate in their practice, given the growing population of Latino patients who face disparities in the health care management of chronic pain.
Supplementary Material
Acknowledgments
The authors thank SDSU HealthLINK Center staff: Drs Guadalupe X. Ayala and Elva Arredondo, and Ms Karla Armenta; research assistants Carlos Sanchez-Rojas, Rebecca Pierce, Patricia Dionicio, and Martha Ceballos for their contributions in the development of this RCT protocol; Ms Maria Tolman for professional translation of study materials; Dr Josephine Mitchell for her contributions to illustrations in the GOALS/Metas patient manual. The authors also thank the patients, staff, and administrative leadership at the Family Health Centers of San Diego for their support of the pilot work that has led to this study.
Contributor Information
Sara P Gombatto, Doctor of Physical Therapy Program, Department of Exercise and Nutritional Sciences, San Diego State University, San Diego, California, USA; SDSU HealthLINK Center for Transdisciplinary Health Disparities Research, San Diego, California, USA.
Kristin R Archer, Orthopaedic Surgery and Physical Medicine and Rehabilitation, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Stephen T Wegener, Department of Physical Medicine and Rehabilitation, Johns Hopkins University, Baltimore, Maryland, USA.
Yessenia Hernandez, SDSU HealthLINK Center for Transdisciplinary Health Disparities Research, San Diego, California, USA.
Shih-Fan Lin, SDSU HealthLINK Center for Transdisciplinary Health Disparities Research, San Diego, California, USA.
Job Godino, Laura Rodriguez Research Institute, Family Health Centers of San Diego, San Diego, California, USA.
Jason Van Dyke, Laura Rodriguez Research Institute, Family Health Centers of San Diego, San Diego, California, USA.
Jie Liu, Laura Rodriguez Research Institute, Family Health Centers of San Diego, San Diego, California, USA.
Katrina S Monroe, Doctor of Physical Therapy Program, Department of Exercise and Nutritional Sciences, San Diego State University, San Diego, California, USA; SDSU HealthLINK Center for Transdisciplinary Health Disparities Research, San Diego, California, USA.
Author Contributions
Concept/idea/research design: S.P. Gombatto, K.S. Monroe, K.R. Archer, S.T. Wegener, , S.-F. Lin
Writing: S.P. Gombatto, K.S. Monroe
Data analysis: S.-F. Lin, K.S. Monroe, S.P. Gombatto
Project management: S.P. Gombatto, Y. Hernandez, K.S. Monroe
Fund procurement: S.P. Gombatto, K.S. Monroe
Providing facilities/equipment: S.P. Gombatto, J. Godino, J. Van Dyke, J. Liu, K.S. Monroe
Providing institutional liaisons: S.P. Gombatto, J. Godino, J. Van Dyke, J. Liu, K.S. Monroe
Consultation (including review of manuscript before submitting): S.P. Gombatto, K.R. Archer, S.T. Wegener, J. Godino, J. Van Dyke, J. Liu, K.S. Monroe, Y. Hernandez, S.-F. Lin
Funding
Research reported in this manuscript was supported by a grant from the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number U54MD012397. REDCap Cloud services at SDSU are supported by grants from the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Numbers S21MD010690 (SDSU HealthLINK Endowment) and U54MD012397 (SDSU HealthLINK Center).
Ethics Approval
This study was approved and is being monitored by the San Diego State University Institutional Review Board (HS-2021-0121).
Clinical Trial Registration
GOALS/Metas is registered on clinicaltrials.gov (protocol ID: 228489; NCT05005416). Trial enrollment began in August 2021, and data collection is ongoing.
Data Availability
The data that support the findings of this study will be openly available in Open Science once the trial is completed.
Disclosures
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. [DOI] [PubMed] [Google Scholar]
- 2. Hestbaek L, Leboeuf-Yde C, Manniche C. Low back pain: What is the long-term course? A review of studies of general patient populations. Eur Spine J. 2003;12:149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hogg-Johnson S, Velde G, Carroll LJ, et al. The burden and determinants of neck pain in the general population: results of the bone and joint decade 2000-2010 task force on neck pain and its associated disorders. Spine (Phila Pa 1976). 2008;33:S39–S51. [DOI] [PubMed] [Google Scholar]
- 4. Bovim G, Schrader H, Sand T. Neck pain in the general population. Spine (Phila Pa 1976). 1994;19:1307–1309. [DOI] [PubMed] [Google Scholar]
- 5. Fejer R, Kyvik KO, Hartvigsen J. The prevalence of neck pain in the world population: a systematic critical review of the literature. Eur Spine J. 2006;15:834–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from The Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:968–974. [DOI] [PubMed] [Google Scholar]
- 7. Hoy D, March L, Woolf A, et al. The global burden of neck pain: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1309–1315. [DOI] [PubMed] [Google Scholar]
- 8. Hoy D, Brooks P, Blyth F, Buchbinder R. The epidemiology of low back pain. Best Pract Res Clin Rheumatol. 2010;24:769–781. [DOI] [PubMed] [Google Scholar]
- 9. Hoy DG, Protani M, De R, Buchbinder R. The epidemiology of neck pain. Best Pract Res Clin Rheumatol. 2010;24:783–792. [DOI] [PubMed] [Google Scholar]
- 10. Van Randeraad-van der Zee CH, Beurskens AJ, Swinkels RA, et al. The burden of neck pain: its meaning for persons with neck pain and healthcare providers, explored by concept mapping. Qual Life Res. 2016;25:1219–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290:2443–2454. [DOI] [PubMed] [Google Scholar]
- 12. Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88 Suppl 2:21–24. [DOI] [PubMed] [Google Scholar]
- 13. Bazargan M, Loeza M, Ekwegh T, et al. Multi-dimensional impact of chronic low back pain among underserved African American and Latino older adults. Int J Environ Res Public Health. 2021;18:7246. 10.3390/ijerph18147246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Plesh O, Adams SH, Gansky SA. Racial/ethnic and gender prevalences in reported common pains in a national sample. J Orofac Pain Winter. 2011;25:25–31. [PMC free article] [PubMed] [Google Scholar]
- 15. Hollingshead NA, Ashburn-Nardo L, Stewart JC, Hirsh AT. The pain experience of Hispanic Americans: a critical literature review and conceptual model. J Pain. 2016;17:513–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gagnon CM, Matsuura JT, Smith CC, Stanos SP. Ethnicity and interdisciplinary pain treatment. Pain Pract. 2014;14:532–540. [DOI] [PubMed] [Google Scholar]
- 17. Linton SJ. A review of psychological risk factors in back and neck pain. Spine (Phila Pa 1976). 2000;25:1148–1156. [DOI] [PubMed] [Google Scholar]
- 18. Delitto A, George SZ, Van Dillen L, et al. Low back pain clinical practice guidelines linked to the international classification of functioning, disability, and health from the orthopaedic section of the American Physical Therapy Association. J Orthop Sport Phys. 2012;42:A1–A57. [DOI] [PubMed] [Google Scholar]
- 19. Blanpied PR, Gross AR, Elliott JM, et al. Neck pain: revision 2017. J Orthop Sports Phys Ther. 2017;47:A1–A83. [DOI] [PubMed] [Google Scholar]
- 20. Crombez G, Vlaeyen JW, Heuts PH, Lysens R. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80:329–339. [DOI] [PubMed] [Google Scholar]
- 21. Shmagel A, Foley R, Ibrahim H. Epidemiology of chronic low back pain in US adults: data from the 2009-2010 National Health and Nutrition Examination Survey. Arthritis Care Res (Hoboken). 2016;68:1688–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schiltenwolf M, Akbar M, Neubauer E, et al. The cognitive impact of chronic low back pain: positive effect of multidisciplinary pain therapy. Scand J Pain. 2017;17:273–278. [DOI] [PubMed] [Google Scholar]
- 23. Foster NE, Delitto A. Embedding psychosocial perspectives within clinical management of low back pain: integration of psychosocially informed management principles into physical therapist practice--challenges and opportunities. Phys Ther. 2011;91:790–803. [DOI] [PubMed] [Google Scholar]
- 24. Nicholas MK, George SZ. Psychologically informed interventions for low back pain: an update for physical therapists. Phys Ther. 2011;91:765–776. [DOI] [PubMed] [Google Scholar]
- 25. Coronado RA, Brintz CE, McKernan LC, et al. Psychologically informed physical therapy for musculoskeletal pain: current approaches, implications, and future directions from recent randomized trials. Pain Rep Sep-Oct. 2020;5:e847. 10.1097/pr9.0000000000000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Söderlund A, Elvén M, Sandborgh M, Fritz J. Implementing a behavioral medicine approach in physiotherapy for patients with musculoskeletal pain: a scoping review. Pain Rep Sep-Oct. 2020;5:e844. 10.1097/pr9.0000000000000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vibe Fersum K, O'Sullivan P, Skouen JS, Smith A, Kvåle A. Efficacy of classification-based cognitive functional therapy in patients with non-specific chronic low back pain: a randomized controlled trial. Eur J Pain. 2013;17:916–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Archer KR, Devin CJ, Vanston SW, et al. Cognitive-Behavioral-based physical therapy for patients with chronic pain undergoing lumbar spine surgery: a randomized controlled trial. J Pain. 2016;17:76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hall A, Richmond H, Copsey B, et al. Physiotherapist-delivered cognitive-behavioural interventions are effective for low back pain, but can they be replicated in clinical practice? A systematic review. Disabil Rehabil. 2018;40:1–9. [DOI] [PubMed] [Google Scholar]
- 30. Sterling M, Smeets R, Keijzers G, Warren J, Kenardy J. Physiotherapist-delivered stress inoculation training integrated with exercise versus physiotherapy exercise alone for acute whiplash-associated disorder (StressModex): a randomised controlled trial of a combined psychological/physical intervention. Br J Sports Med. 2019;53:1240–1247. [DOI] [PubMed] [Google Scholar]
- 31. Godfrey E, Wileman V, Galea Holmes M, et al. Physical Therapy Informed by Acceptance and Commitment Therapy (PACT) versus usual care physical therapy for adults with chronic low back pain: a randomized controlled trial. J Pain. 2020;21:71–81. [DOI] [PubMed] [Google Scholar]
- 32. O'Keeffe M, O'Sullivan P, Purtill H, Bargary N, O'Sullivan K. Cognitive functional therapy compared with a group-based exercise and education intervention for chronic low back pain: a multicentre randomised controlled trial (RCT). Br J Sports Med. 2020;54:782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lovo S, Harrison L, O'Connell ME, Trask C, Bath B. Experience of patients and practitioners with a team and technology approach to chronic back disorder management. J Multidiscip Healthc. 2019;12:855–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee AC. COVID-19 and the advancement of digital physical therapist practice and telehealth. Phys Ther. 2020;100:1054–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dario AB, Moreti Cabral A, Almeida L, et al. Effectiveness of telehealth-based interventions in the management of non-specific low back pain: a systematic review with meta-analysis. Spine J. 2017;17:1342–1351. [DOI] [PubMed] [Google Scholar]
- 36. Greenwood H, Krzyzaniak N, Peiris R, et al. Telehealth versus face-to-face psychotherapy for less common mental health conditions: systematic review and meta-analysis of randomized controlled trials. JMIR Ment Health. 2022;9:e31780. 10.2196/31780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Archer KR, Motzny N, Abraham CM, et al. Cognitive-behavioral-based physical therapy to improve surgical spine outcomes: a case series. Phys Ther. 2013;93:1130–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap. 1994;23:129–138. [PubMed] [Google Scholar]
- 39. Bartholomew ELKMCM, Ruiter RAC, Fernández ME, Kok G, Parcel GS. Chapter 10 using intervention mapping to adapt evidence-based interventions. In: Planning Health Promotion Programs: An Intervention Mapping Approach. 4th ed. San Francisco, CA: Jossey-Bass; 2016: 704. [Google Scholar]
- 40. Maluf KSRC, Van Dyke J, Liu J, et al. Goal Oriented Activity for Latinos/Hispanics with Spine Pain (GOALS): Use of Intervention Mapping and FRAME to Adapt a Cognitive-Behavioral based Telerehabilitation Intervention. Presented at: International Association for the Study of Pain World Congress on Pain. Toronto, Canada; 2022. [Google Scholar]
- 41. Thorn BE, Day MA, Burns J, et al. Randomized trial of group cognitive behavioral therapy compared with a pain education control for low-literacy rural people with chronic pain. Pain. 2011;152:2710–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Siddall B, Ram A, Jones MD, Booth J, Perriman D, Summers SJ. Short-term impact of combining pain neuroscience education with exercise for chronic musculoskeletal pain: a systematic review and meta-analysis. Pain. 2022;163:e20–e30. [DOI] [PubMed] [Google Scholar]
- 43. Andrews NE, Strong J, Meredith PJ. Activity pacing, avoidance, endurance, and associations with patient functioning in chronic pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2012;93:2109–2121.e7. [DOI] [PubMed] [Google Scholar]
- 44. Guy L, McKinstry C, Bruce C. Effectiveness of pacing as a learned strategy for people with chronic pain: a systematic review. Am J Occup Ther May/Jun. 2019;73:7303205060p1–7303205060p10. 10.5014/ajot.2019.028555. [DOI] [PubMed] [Google Scholar]
- 45. Vambheim SM, Kyllo TM, Hegland S, Bystad M. Relaxation techniques as an intervention for chronic pain: a systematic review of randomized controlled trials. Heliyon. 2021;7:e07837. 10.1016/j.heliyon.2021.e07837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee C, Crawford C, Hickey A. Mind-body therapies for the self-management of chronic pain symptoms. Pain Med. 2014;15:S21–S39. [DOI] [PubMed] [Google Scholar]
- 47. Che X, Cash R, Ng SK, Fitzgerald P, Fitzgibbon BM. A systematic review of the processes underlying the main and the buffering effect of social support on the experience of pain. Clin J Pain. 2018;34:1061–1076. [DOI] [PubMed] [Google Scholar]
- 48. Day MA, Ward LC, Ehde DM, et al. A pilot randomized controlled trial comparing mindfulness meditation, cognitive therapy, and mindfulness-based cognitive therapy for chronic low back pain. Pain Med. 2019, 2148;20:2134. 10.1093/pm/pny273. [DOI] [PubMed] [Google Scholar]
- 49. Organista KC, Munoz RF. Cognitive behavioral therapy with Latinos. Cogn Behav Pract Win. 1996;3:255–270. [Google Scholar]
- 50. Pardos-Gascón EM, Narambuena L, Leal-Costa C, van-der Hofstadt-Román CJ.. Differential efficacy between cognitive-behavioral therapy and mindfulness-based therapies for chronic pain: systematic review. Int J Clin Health Psychol Jan-Apr. 2021;21:100197. 10.1016/j.ijchp.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rathnayake APS, Sparkes V, Sheeran L. What is the effect of low back pain self-management interventions with exercise components added? A systematic review with meta-analysis. Musculoskelet Sci Pract. 2021;56:102469. 10.1016/j.msksp.2021.102469. [DOI] [PubMed] [Google Scholar]
- 52. U.S. Department of Health and Human Services . Physical Activity Guidelines for Americans. 2018. Accessed August 2, 2023. https://health.gov/our-work/nutrition-physical-activity/physical-activity-guidelines/current-guidelines.
- 53. Laird RA, Kent P, Keating JL. Modifying patterns of movement in people with low back pain -does it help? A systematic review. BMC Musculoskelet Disord. 2012;13:169. 10.1186/1471-2474-13-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alperstein D, Sharpe L. The efficacy of motivational interviewing in adults with chronic pain: a meta-analysis and systematic review. J Pain. 2016;17:393–403. [DOI] [PubMed] [Google Scholar]
- 55. Haladay D, Swisher L, Hardwick D. Goal attainment scaling for patients with low back pain in rehabilitation: a systematic review. Health Sci Rep. 2021;4:e378. 10.1002/hsr2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Marin G, Gamba R. A new measurement of acculturation for Hispanics: the Bidimensional Acculturation Scale for Hispanics. Hisp J Behav Sci. 1996;18:297–316. [Google Scholar]
- 57. Murphy DR, Lopez M. Neck and back pain specific outcome assessment questionnaires in the Spanish language: a systematic literature review. Spine J. 2013;13:1667–1674. [DOI] [PubMed] [Google Scholar]
- 58. Alcántara-Bumbiedro S, Flórez-García MT, Echávarri-Pérez C, García-Pérez F. Escala de incapacidad por dolor lumbar de Oswestry. Rehabilitación. 2006;40:150–158. [Google Scholar]
- 59. Andres AJ, Cruces Prado LM, Canos Verdecho MA, et al. Validation of the short form of the brief pain inventory (BPI-SF) in Spanish patients with non-cancer-related pain. Pain Pract. 2015;15:643–653. [DOI] [PubMed] [Google Scholar]
- 60. Notario B, Martinez Vizcaino V, Trillo-Calvo E, Pérez-Yus MC, Serrano-Parra M, Garcia-Campayo J. Validity and reliability of the Spanish version of the 10-item CD-RISC in patients with fibromyalgia. Health Qual Life Outcomes. 2014;12:14. 10.1186/1477-7525-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kovacs FM, Muriel A, Medina JM, et al. Psychometric characteristics of the Spanish version of the FAB questionnaire. Spine (Phila Pa 1976). 2006;31:104–110. [DOI] [PubMed] [Google Scholar]
- 62. Kovacs FM, Bago J, Royuela A, et al. Psychometric characteristics of the Spanish version of instruments to measure neck pain disability. BMC Musculoskelet Disord. 2008;9:42. 10.1186/1471-2474-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Costa Requena G, Salamero M, Gil F. Validación del cuestionario MOS-SSS de apoyo social en pacientes con cáncer. Med Clin-Barcelona. 2007;128:687–691. [DOI] [PubMed] [Google Scholar]
- 64. Gómez-Campelo P, Pérez-Moreno EM, Burgos-Lunar C, Bragado-Álvarez C, Jiménez-García R, Salinero-Fort M. Psychometric properties of the eight-item modified medical outcomes study social support survey based on Spanish outpatients. Qual Life Res. 2014;23:2073–2078. [DOI] [PubMed] [Google Scholar]
- 65. Vega-Lopez S, Chavez A, Farr KJ, Ainsworth BE. Validity and reliability of two brief physical activity questionnaires among Spanish-speaking individuals of Mexican descent. BMC Res Notes. 2014;7:29. 10.1186/1756-0500-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McKenna SP, Doward LC. The translation and cultural adaptation of patient-reported outcome measures. Value Health Mar-Apr. 2005;8:89–91. [DOI] [PubMed] [Google Scholar]
- 67. Kean J, Monahan PO, Kroenke K, et al. Comparative responsiveness of the PROMIS pain interference short forms, brief pain inventory, PEG, and SF-36 bodily pain subscale. Med Care. 2016;54:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chiarotto A, Maxwell LJ, Ostelo RW, Boers M, Tugwell P, Terwee CB. Measurement properties of visual analogue scale, numeric rating scale and pain severity subscale of the brief pain inventory in patients with low back pain: a systemic review. J Pain. 2019;20:245–263. [DOI] [PubMed] [Google Scholar]
- 69. D'Alonzo KT. Evaluation and revision of questionnaires for use among low-literacy immigrant Latinos. Latino-Am Enfermagem. 2011;19:1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Song C, Lin S, Huang C, Wu HC, Chen CH, Hsieh CL. Validation of the brief pain inventory in patients with low back pain. Spine (Phila Pa 1976). 2016;41:E937–E942. [DOI] [PubMed] [Google Scholar]
- 71. Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24:733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fairbank JC, Pynsent PB. The Oswestry disability index. Spine (Phila Pa 1976). 2000;25:2940–2952. discussion 2952. 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 73. Pomares Avalos AJ, López Fernández R, Zaldívar Pérez DF. Validación de la escala de incapacidad por dolor lumbar de Oswestry, en paciente con dolor crónico de la espalda. Cienfuegos, 2017-2018. Rehabilitación. 2020;54:25–30. [DOI] [PubMed] [Google Scholar]
- 74. MacDermid JC, Walton DM, Avery S, et al. Measurement properties of the neck disability index: a systematic review. J Orthop Sports Phys Ther. 2009;39:400–C12. [DOI] [PubMed] [Google Scholar]
- 75. Andrade Ortega JA, Delgado Martínez AD, Almécija RR. Validation of the Spanish version of the neck disability index. Spine (Phila Pa 1976). 2010;35:E114–E118. [DOI] [PubMed] [Google Scholar]
- 76. Cleland JA, Fritz JM, Whitman JM, Palmer JA. The reliability and construct validity of the neck disability index and patient specific functional scale in patients with cervical radiculopathy. Spine (Phila Pa 1976). 2006;31:598–602. [DOI] [PubMed] [Google Scholar]
- 77. Ortega JAA, Martinez ADD, Ruiz RA. Validation of the Spanish version of the neck disability index. Spine. 2010;35:E114–E118. [DOI] [PubMed] [Google Scholar]
- 78. Vernon H, Mior S. The neck disability index: a study of reliability and validity. J Manip Physiol Ther. 1991;14:409–415. [PubMed] [Google Scholar]
- 79. Hefford C, Abbott JH, Arnold R, Baxter GD. The patient-specific functional scale: validity, reliability, and responsiveness in patients with upper extremity musculoskeletal problems. J Orthop Sports Phys Ther. 2012;42:56–65. [DOI] [PubMed] [Google Scholar]
- 80. Westaway MD, Stratford PW, Binkley JM. The patient-specific functional scale: validation of its use in persons with neck dysfunction. J Orthop Sports Phys Ther. 1998;27:331–338. [DOI] [PubMed] [Google Scholar]
- 81. Dworkin RH, Turk DC, Peirce-Sandner S, et al. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain. 2010;149:177–193. [DOI] [PubMed] [Google Scholar]
- 82. Gewandter JS, Dworkin RH, Turk DC, et al. Research design considerations for chronic pain prevention clinical trials: IMMPACT recommendations. Pain. 2015;156:1184–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- 84. Fritz JM, Irrgang JJ. A comparison of a modified Oswestry low back pain disability questionnaire and the Quebec back pain disability scale. Phys Ther. 2001;81:776–788. [DOI] [PubMed] [Google Scholar]
- 85. Deyo, Ramsey, Buckley, et al. Performance of a patient reported outcomes measurement information system (PROMIS) short form in older adults with chronic musculoskeletal pain. Pain Med. 2016;17:pnv046–pnv324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med. 2017;47:1821–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Arguello D, Andersen K, Morton A, Freedson PS, Intille SS, John D. Validity of proximity sensor-based wear-time detection using the ActiGraph GT9X. J Sports Sci. 2018;36:1502–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. McArthur LH, Holbert D, Pena M. Development and application of rapid assessment diet and physical activity indexes, which suggest high consumption of energy-dense foods and inadequate exercise among adolescents from 6 Latin American cities: a pilot study. Nutr Res. 2008;28:590–599. [DOI] [PubMed] [Google Scholar]
- 89. Souza L, Benedito-Silva AA, Pires ML, Poyares D, Tufik S, Calil HM. Further validation of actigraphy for sleep studies. Sleep. 2003;26:81–85. [DOI] [PubMed] [Google Scholar]
- 90. Monk TH, Reynolds CF, Kupfer DJ, et al. The Pittsburgh sleep diary. J Sleep Res. 1994;3:111–120. [PubMed] [Google Scholar]
- 91. Burgess HJ, Burns JW, Buvanendran A, et al. Associations between sleep disturbance and chronic pain intensity and function: a test of direct and indirect pathways. Clin J Pain. 2019;35:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. [DOI] [PubMed] [Google Scholar]
- 93. Deyo RA, Dworkin SF, Amtmann D, et al. Report of the NIH task force on research standards for chronic low back pain. J Pain. 2014;15:569–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Goossens ME, Vlaeyen JW, Hidding A, Kole-Snijders A, Evers SM. Treatment expectancy affects the outcome of cognitive-behavioral interventions in chronic pain. Clin J Pain Jan-Feb. 2005;21:18–26. discussion 69-72. [DOI] [PubMed] [Google Scholar]
- 95. Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A fear-avoidance beliefs questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52:157–168. [DOI] [PubMed] [Google Scholar]
- 96. George SZ, Valencia C, Beneciuk JM. A psychometric investigation of fear-avoidance model measures in patients with chronic low back pain. J Orthop Sports Phys Ther. 2010;40:197–205. [DOI] [PubMed] [Google Scholar]
- 97. Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7:524–532. 10.1037/1040-3590.7.4.524. [DOI] [Google Scholar]
- 98. Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O'Neill E. Factor structure, reliability, and validity of the pain catastrophizing scale. J Behav Med. 1997;20:589–605. [DOI] [PubMed] [Google Scholar]
- 99. Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain. 2007;11:153–163. [DOI] [PubMed] [Google Scholar]
- 100. Chiarotto A, Vanti C, Cedraschi C, et al. Responsiveness and minimal important change of the pain self-efficacy questionnaire and short forms in patients with chronic low back pain. J Pain. 2016;17:707–718. [DOI] [PubMed] [Google Scholar]
- 101. Chen G, Gully SM, Eden D. Validation of a new general self-efficacy scale. Organ Res Methods. 2001;4:62–83. [Google Scholar]
- 102. Sallis JF, Pinski R, Grossman R, Patterson TL, Nader PR. The development of self-efficacy scales for health-related diet and exercise behaviors. Health Educ Res. 1988;3:283–292. [Google Scholar]
- 103. Kent P, Laird R, Haines T. The effect of changing movement and posture using motion-sensor biofeedback, versus guidelines-based care, on the clinical outcomes of people with sub-acute or chronic low back pain-a multicentre, cluster-randomised, placebo-controlled, pilot trial. BMC Musculoskelet Disord. 2015;16:131. 10.1186/s12891-015-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Laird RA, Kent P, Keating JL. How consistent are lordosis, range of movement and lumbo-pelvic rhythm in people with and without back pain? BMC Musculoskelet Disord. 2016;17:403. 10.1186/s12891-016-1250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mjosund HL, Boyle E, Kjaer P, Mieritz RM, Skallgard T, Kent P. Clinically acceptable agreement between the ViMove wireless motion sensor system and the Vicon motion capture system when measuring lumbar region inclination motion in the sagittal and coronal planes. BMC Musculoskelet Disord. 2017;18:124. 10.1186/s12891-017-1489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kim SC, Boren D, Solem SL. The Kim alliance scale: development and preliminary testing. Clin Nurs Res. 2001;10:314–331. [DOI] [PubMed] [Google Scholar]
- 107. Kim SC, Kim S, Boren D. The quality of therapeutic alliance between patient and provider predicts general satisfaction. Mil Med. 2008;173:85–90. [DOI] [PubMed] [Google Scholar]
- 108. Alvarez K, Wang Y, Alegria M, et al. Psychometrics of shared decision making and communication as patient centered measures for two language groups. Psychol Assess. 2016;28:1074–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Dillon F, Ortiz M, Rice C. Validating the multidimensional measure of cultural identity scales for Latinos among Latina mothers and daughters. Cult Divers Ethn Minor Psychol. 2009;15:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lopez DS, Fernandez ME, Cano MA, et al. Association of acculturation, nativity, and years living in the United States with biobanking among individuals of Mexican descent. Cancer Epidemiol Biomark Prev. 2014;23:402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Marin G, Sabogal F. Development of a short acculturation scale for Hispanics. Hisp J Behav Sci. 1987;9:183–205. [Google Scholar]
- 112. Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson resilience scale (CD-RISC). Depress Anxiety. 2003;18:76–82. [DOI] [PubMed] [Google Scholar]
- 113. Gras M-E, Font-Mayolas S, Baltasar A, Patiño J, Sullman MJM, Planes M. The Connor-Davidson resilience scale (CD-RISC) amongst young Spanish adults. Clínica y Salud. 2019;30:73–79. [Google Scholar]
- 114. Manzano-García G, Ayala Calvo JC. Psychometric properties of Connor-Davidson resilience scale in a Spanish sample of entrepreneurs. Psicothema. 2013;25:245–251. [DOI] [PubMed] [Google Scholar]
- 115. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study will be openly available in Open Science once the trial is completed.


