Main text
In recent years, adoptive cell therapy has made significant strides in treating various diseases. Yet, the full potential of this promising field remains hindered by complex challenges. Intricate procedures for cell manipulation, high manufacturing costs, extended production timelines, and the risk of adverse effects like off-target toxicities constrain its broader application.1,2 To overcome these obstacles, cutting-edge in vivo gene editing approaches are being developed that necessitate high precision in targeted editing and the use of safe, non-toxic delivery systems,3,4,5,6 both of which are paramount in ensuring patient safety (Table 1).
Table 1.
Comparison between main ex vivo and in vivo editing features
| Criteria | Ex vivo engineering | In vivo engineering |
|---|---|---|
| Patient convenience | may require cell collection from patient, waiting times | no need for cell collection, timely availability |
| Preconditioning | preconditioning regimen is recommended | may bypass the need for preconditioning |
| Precision & control | high precision in manufacturing, quality checks | high percision in delivery vector design |
| Validation & efficiency | clinically validated, high editing efficiency | still under validation, varying efficiency |
| Manufacturing & logistics | complex and lengthy process, logistical burden | simplified process, minimal logistics |
| Cost & infrastructure | high cost, complex infrastructure required | generally reduced cost and infrastructure needs |
| Cell fitness considerations | possibility of reduced cell fitness due to ex vivo culturing | possibility of preserved cell fitness due to direct in vivo editing |
| Toxicity risks | controlled ex vivo editing, potential risks of toxicity and off-target effects | uncontrolled in vivo editing, increased risk of toxicity and off-target effects |
| Immunogenicity | potential risks of immunogenic responses, method-dependent | higher risk of immunogenicity |
The selection of an appropriate delivery system is crucial for in vivo gene editing. An ideal system would streamline the manufacturing process to cut costs and broaden access, handle large genetic payloads to accommodate next-generation editing technologies, and ensure receptor-specific targeting to mitigate off-target effects. Adenoviral vectors (AdVs) are emerging as a promising tool in this context. Their cargo capacity of 30–40 kb allows for the transportation of large genetic materials, while their episomal DNA delivery method reduces the risk of insertional mutagenesis. The structural flexibility of AdVs further facilitates targeted delivery, contributing to the development of safe and efficient clinical delivery systems.7
In a study published in Molecular Therapy, Rice-Boucher and colleagues investigated the use of AdVs for targeted in vivo B cell editing.8 They employed a triple AdV editing strategy that involved structural protein engineering, the utilization of tissue-specific promoters, and virus modification to lower liver tropism, thus increasing the availability of the vector for B cell targeting. They screened three in-house generated AdV variants—AdRGD, AdPK4, and SAd36—each utilizing distinct infection pathways through coxsackie and adenovirus receptor, integrin, or glycan proteins. Specific promoters (SFFV, EEK, MH, and EBVW) were further evaluated for their ability to drive reporter gene expression within B cells, with the results compared to the standard AdV5.CMV virus. The AdRGD.EEK combination demonstrated the highest reporter gene expression in B cells in vivo while also minimizing off-target toxicity. Contrary to expectations, reducing liver tropism did not lead to an increase in gene transfer to B cells in other organs such as the spleen, a key site for B cell maturation and activation.
Furthermore, the authors discovered that AdVs preferentially infect B cells in vivo through the penton base, highlighting the potential for engineering this component to enhance B cell editing efficiency. They also revealed a significant correlation between B cell phenotype and editing efficiency, with activated B cells enriched with CD95 and GL7, and rapidly dividing subtypes such as plasmablasts, germinal center, and marginal zone B cells, exhibiting the highest editing rates. The results from this study contribute valuable insights for targeted in vivo gene delivery, including methods for minimizing off-target toxicity. While the findings are noteworthy, certain limitations in the study—such as the use of a surrogate reporter gene, the low number of edited cells, and the absence of immunogenicity assessments—suggest that further research is warranted to continue to explore these mechanisms and tounderstand the long-term effects of AdV-mediated gene transfer to B cells.
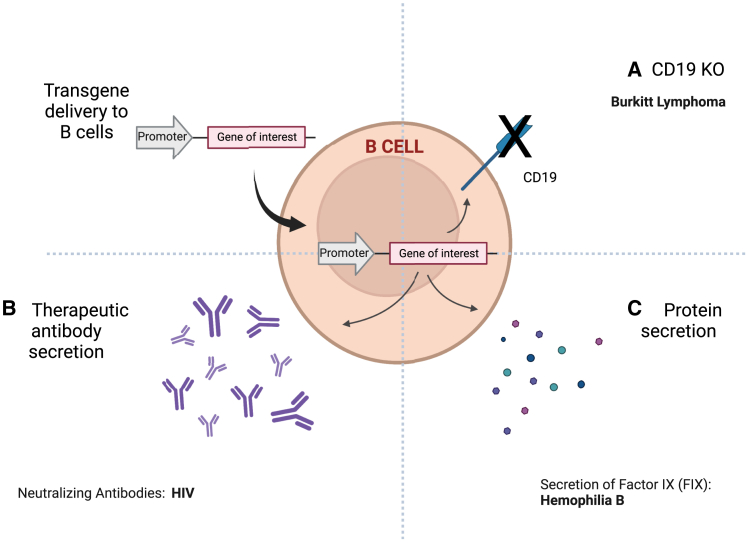
B cell editing has emerged as a promising alternative to T cell engineering for therapeutic applications (Figure 1).9 B cells offer unique advantages, such as transformation into long-lasting plasma cells, high protein production, and a rapid response to antigens, making them attractive targets for the treatment of conditions such as genetic disorders, infections, cancer, and autoimmune diseases. While challenges such as low editing efficiency and potential toxicity must be managed, recent advances in ex vivo B cell engineering signal promise for translating these approaches into in vivo applications. For instance, targeted manipulation of B cells may be applied in vivo to treat disorders like Burkitt Lymphoma,10 provided that precise targets can be identified within the aberrant cells. Similarly, in vivo engineering of B cells may offer alternatives to conventional therapies for diseases like hemophilia B11 or be used to express therapeutic monoclonal antibodies12 and deliver immune checkpoint inhibitors.13 Initial in vivo studies employing B cells to secrete neutralizing antibodies against HIV-1 have already demonstrated the high potential of this approach.14
Figure 1.
Potential clinical applications of in vivo B cell editing
(A) CD19 knockout using CRISPR-Cas9 for Burkitt lymphoma treatment. (B) Engineering B cells to secrete specific therapeutic antibodies, e.g., for HIV treatment. (C) B cell manipulation for protein secretion, such as factor IX, for hemophilia B therapy. Created with BioRender.com.
In conclusion, the development of in vivo gene editing has emerged as a promising avenue in the treatment of various medical conditions, necessitating the creation of safe and specific delivery systems. Research like that conducted by Rice-Boucher and colleagues plays a pivotal role in this advancement, particularly in the area of targeted B cell editing. An immediate priority in this field appears to be the development of adaptable delivery systems tailored to different cells and tissues, complemented by predictive models to assess toxicity and off-target effects before human trials. This ongoing progress in in vivo gene editing represents a significant milestone, consistently paving the way for more timely, effective, and targeted therapeutic interventions.
References
- 1.Abou-el-Enein M., Elsallab M., Feldman S.A., Fesnak A.D., Heslop H.E., Marks P., Till B.G., Bauer G., Savoldo B. Scalable Manufacturing of CAR T Cells for Cancer Immunotherapy. Blood Cancer Discov. 2021;2:408–422. doi: 10.1158/2643-3230.BCD-21-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flugel C.L., Majzner R.G., Krenciute G., Dotti G., Riddell S.R., Wagner D.L., Abou-El-Enein M. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat. Rev. Clin. Oncol. 2023;20:49–62. doi: 10.1038/s41571-022-00704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfeiffer A., Thalheimer F.B., Hartmann S., Frank A.M., Bender R.R., Danisch S., Costa C., Wels W.S., Modlich U., Stripecke R., et al. In vivo generation of human CD 19- CAR T cells results in B-cell depletion and signs of cytokine release syndrome. EMBO Mol. Med. 2018;10 doi: 10.15252/emmm.201809158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal S., Hanauer J.D.S., Frank A.M., Riechert V., Thalheimer F.B., Buchholz C.J. In Vivo Generation of CAR T Cells Selectively in Human CD4+ Lymphocytes. Mol. Ther. 2020;28:1783–1794. doi: 10.1016/j.ymthe.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith T.T., Stephan S.B., Moffett H.F., McKnight L.E., Ji W., Reiman D., Bonagofski E., Wohlfahrt M.E., Pillai S.P.S., Stephan M.T. In situ programming of leukaemia-specific t cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 2017;12:813–820. doi: 10.1038/nnano.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rurik J.G., Tombácz I., Yadegari A., Méndez Fernández P.O., Shewale S.V., Li L., Kimura T., Soliman O.Y., Papp T.E., Tam Y.K., et al. CAR T cells produced in vivo to treat cardiac injury. Science. 2022;375:91–96. doi: 10.1126/science.abm0594. https://www.science.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulcha J.T., Wang Y., Ma H., Tai P.W.L., Gao G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021;6:53. doi: 10.1038/s41392-021-00487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rice-Boucher P.J., Mendonça S.A., Alvarez A.B., Sturtz A.J., Lorincz R., Dmitriev I.P., Kashentseva E.A., Lu Z.H., Romano R., Selby M., et al. Adenoviral vectors infect B lymphocytes. Mol. Ther. 2023 doi: 10.1016/j.ymthe.2023.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers G.L., Cannon P.M. Genome edited B cells: a new frontier in immune cell therapies. Mol. Ther. 2021;29:3192–3204. doi: 10.1016/j.ymthe.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson M.J., Laoharawee K., Lahr W.S., Webber B.R., Moriarity B.S. Engineering of Primary Human B cells with CRISPR/Cas9 Targeted Nuclease. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-30358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung K.L., Meitlis I., Hale M., Chen C.Y., Singh S., Jackson S.W., Miao C.H., Khan I.F., Rawlings D.J., James R.G. Engineering Protein-Secreting Plasma Cells by Homology-Directed Repair in Primary Human B Cells. Mol. Ther. 2018;26:456–467. doi: 10.1016/j.ymthe.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greiner V., Bou Puerto R., Liu S., Herbel C., Carmona E.M., Goldberg M.S. CRISPR-Mediated Editing of the B Cell Receptor in Primary Human B Cells. iScience. 2019;12:369–378. doi: 10.1016/j.isci.2019.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo B., Zhan Y., Luo M., Dong H., Liu J., Lin Y., Zhang J., Wang G., Verhoeyen E., Zhang Y., Zhang H. Engineering of α-PD-1 antibody-expressing long-lived plasma cells by CRISPR/Cas9-mediated targeted gene integration. Cell Death Dis. 2020;11:973. doi: 10.1038/s41419-020-03187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nahmad A.D., Lazzarotto C.R., Zelikson N., Kustin T., Tenuta M., Huang D., Reuveni I., Nataf D., Raviv Y., Horovitz-Fried M., et al. In vivo engineered B cells secrete high titers of broadly neutralizing anti-HIV antibodies in mice. Nat. Biotechnol. 2022;40:1241–1249. doi: 10.1038/s41587-022-01328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]