Dear Editor,
Inner ear drug delivery has traditionally involved lateral approaches to the cochlea, including transtympanic injection, round window injection, oval window injection, cochleostomy, and semicircular canal (SSC) fenestration. The connection between cochlear fluid spaces and the cerebrospinal fluid (CSF) space, on the other hand, remains poorly understood. This connection has important implications for routes of therapeutic administration.
Cisternograms are employed clinically to diagnose and localize CSF leaks at the skull base. Spontaneous CSF leaks of the temporal bone occur without an inciting event and are associated with calvarial thinning and bony defects of the skull base through which CSF leaks into the middle ear or mastoid.1 In challenging diagnostic cases, our team utilizes MRI cisternograms, which involve fluoroscopic lumbar puncture with injection of 500 μL of gadolinium contrast chelate (Gd-BOPTA, MultiHance, Bracco, Milan, Italy) into the intrathecal (CSF) space followed by same-day serial MR imaging performed at 3T field strength.2 Unexpectedly, we observed rapid and progressive diffusion of gadolinium contrast into the human cochleae and vestibule (Figure 1).
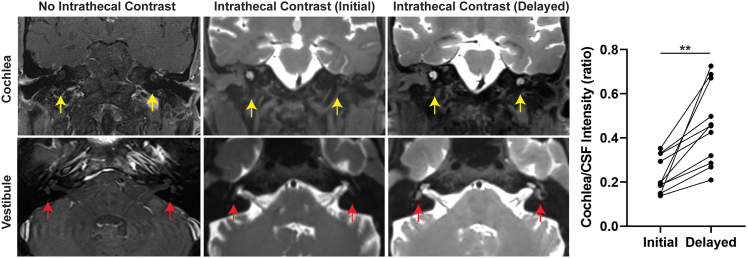
Figure 1.
Gadolinium contrast transduction of the human cochlea and vestibule via CSF
Comparison of enhancement of cochlea in T1-weighted SPACE fat-saturated (FS) MRI without intrathecal contrast (patient received intravenous contrast, left column), at time of initial imaging approximately 1 h after administration of intrathecal contrast (middle column) and at time of delayed imaging (right column). The graph to the right demonstrates the change in cochlea-to-CSF contrast intensity ratio between initial and delayed images for each qualifying patient. ∗∗p < 0.005.
We assessed MRI cisternograms from 15 consecutive patients suspected of having a spontaneous CSF leak from 2019 to 2021 (Tables S1 and S2). Eight patients (53%) were found to have a CSF leak on initial or delayed imaging, while seven patients did not. Among the eight patients with a confirmed CSF leak, five were spontaneous, two were traumatic, and one occurred postoperatively following a translabyrinthine resection of a vestibular schwannoma. Gadolinium transduction of the cochlea was observed in 100% of the patients on initial imaging (Figure 1). Vestibular uptake, upon either initial or delayed scan, was observed in 12 patients (80%). The average time to initial scan after lumbar puncture and intrathecal administration of gadolinium contrast was 74 (±51) min, while the average time to delayed scan was 341 (±83) min. The average peak contrast signal intensity of the cochlea was 53% (±23%) of the peak intensity of CSF on initial MRI. This ratio increased to 65.5% (±21.5%) for delayed imaging. When both cochlear and CSF intensity were referenced to the medulla for cross-image standardization purposes (supplemental information and Figure S1), cochlea-to-CSF ratio demonstrated a significant 215% increase from 22.7% to 45.5% (p = 0.002) (Figure 1). Spontaneous CSF leaks have been associated with elevated intracranial pressure, which may impact the rate of cochlear diffusion of CSF contrast. However, no difference in cochlear diffusion (standardized to medulla intensity) was observed in patients with CSF leaks (3.44 ± 0.51) compared to those without CSF leaks (3.51 ± 0.32, p = 0.77). Patients tolerated the procedure well with minimal side effects such as headache and nausea, which are well-known side effects of lumbar punctures and cisternograms. None of the patients complained of worsening tinnitus, subjective hearing loss, or dizziness.
Hearing loss is the most prevalent sensory disorder globally, significantly impacting individuals of all ages.3 Most adult-onset hearing loss is due to environmental exposure and presbycusis, but genetic factors account for up to 80% of congenital and 25% of adult-onset cases.3,4 As a result, gene therapy vectors, particularly adeno-associated viruses (AAVs) or similar helper-dependent adenoviral vectors, have gained interest as a potential treatment or cure for those suffering from genetic forms of hearing loss. Several studies have shown promising hearing outcomes after AAV gene therapy administration in animal models, as reviewed by Delmaghani et al. and Omichi et al.5,6,7,8,9,10,11,12,13,14
A significant challenge in administration of therapeutics such as viral mediated gene transfer is the mode of delivery, which is especially true for the inner ear. Many previous studies have attempted AAV administration to the inner ear through lateral approaches.15,16 Some of these approaches have inherent risks of permanent hearing loss and/or vestibular dysfunction in addition to other standard risks of mastoidectomy, such as facial nerve injury. Lateral approaches to AAV administration have also been demonstrated to result in contralateral cochlea transduction, implicating communication between the cochlea and CSF space.6 Consequently, intrathecal administration of AAV or alternative gene therapeutics may offer an effective and potentially safe route of administration.
A recent “letter to the editor” in Molecular Therapy discussed intracerebroventricular administration of AAV9.EGFP into non-human primates (NHPs), Macaca mulatta monkeys, demonstrated bilateral cochlear transduction.7 Our data support this NHP study, demonstrating that communication between the human cochleae and the CSF space can be highly efficient. Considering particle size, AAV is estimated to be approximately 25 nm, while gadolinium contrast chelate (Gd-BOPTA, Multihance) is approximately 50–100 nm.17,18 If diffusion is the mechanism of transduction into the human cochlea, similar efficiencies would be expected with gadolinium and AAV or similarly sized gene therapy vectors. While entry into the cochlea is just one step, therapeutic uptake within cells of interest (cochlear hair cells, stria vascularis, spiral ganglion neurons) could vary based upon specific AAV serotypes, receptors, and/or channels.
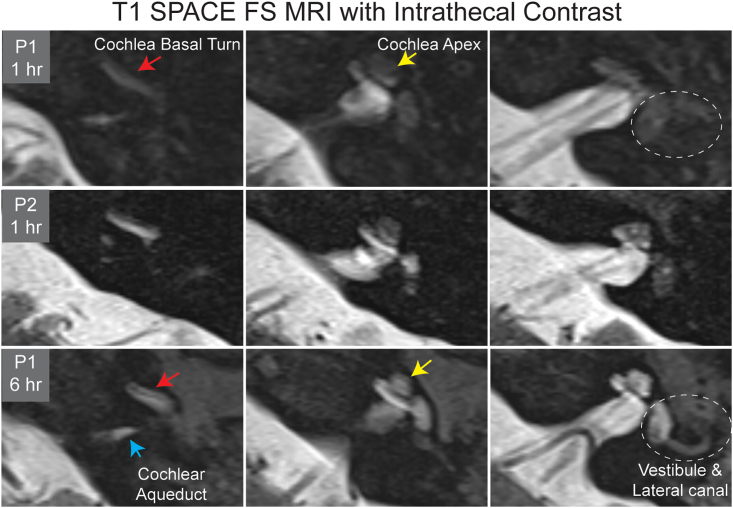
The cochlear aqueduct contains CSF and is theorized to insert into the basal turn of the cochlea in humans.19 We initially observed contrast diffusion into the basal turn of the cochlea followed by diffusion into the apex of the cochlea (Figure 2). Subsequently, contrast diffused into the vestibule and ultimately the semicircular canals. While we hypothesize that cochlea transduction occurs via the aqueduct, the CSF fluid signal within the cochlear aqueduct becomes undetectable on MRI as it approaches the basal turn of the cochlea, making this hypothesis challenging to prove with 3T MRI (Figure 2). Ranum et al. also observed variable AAV uptake throughout the cochlea from apex to base,7 while Nagawana et al. argue for diffusion via the modiolus.20 Regardless of the route of entry, we ultimately observe contrast diffusion throughout the scala of the cochlea (Figure 2).
Figure 2.
Progressive contrast diffusion within the cochlea and vestibule
Two patients (P1 and P2) demonstrated variable initial contrast uptake into the cochlea at 1 h on T1-weighted SPACE fat-saturated (FS) MRI after intrathecal gadolinium contrast administration. Contrast is initially observed in the basal turn (red arrow) of the cochlea (P1) and not the cochlear apex (yellow arrow) nor vestibule (dashed circle). P2 exhibited more intense contrast uptake through the cochlea and initial vestibule uptake. On delayed imaging, contrast is seen throughout the cochlea and vestibule and semicircular canals. The blue arrow indicates contrast within the cochlear aqueduct, where contrast does not macroscopically connect to the basal turn of the cochlea.
The increase in contrast intensity from initial to delayed scan suggests continued diffusion of contrast material into the cochlea, although the much higher CSF contrast levels decrease slightly over time due to washout. All delayed MRIs were performed 2–8 h after lumbar puncture. Contrast initially progresses from CSF to the cochlea, followed by the vestibule. Vestibular uptake was observed in 40% of patients during initial imaging, and vestibular uptake increased to 80% of patients who had delayed imaging. It is reasonable to presume that contrast would be observed in the vestibule and labyrinths in all patients if given necessary time for diffusion.
To evaluate if CSF gadolinium diffused into the systemic circulation and was ultimately concentrated into the cochlea from the blood, we identified three patients who underwent sequential MR imaging after receiving intravenous gadolinium (Table S3). Two patients had 15–17 mL of intravenous gadolinium (Gd-BOPTA, MultiHance, Bracco, Milan, Italy) and brain MR imaging followed by delayed imaging with an additional 15–17 mL of intravenous gadolinium approximately 24 h later. The remaining patient received 3.2 mL of intravenous gadolinium (DOTA-Gd, Dotarem, Guerbet, Villepinte, France) on initial imaging and an additional 3.2 mL of intravenous contrast approximately 24 h later. We did not observe gadolinium contrast in cochleae of any of the three patients with direct intravenous injections (Figure S2), suggesting against the possibility that only 0.5 mL of intrathecal gadolinium contrast (MultiHance) enters the cochlea via the systemic circulation. Rather, these data implicate CSF gadolinium diffusion into the cochlea directly from the CSF.
Lateral approaches to gene therapy administration present significant risk to hearing and balance, particularly with SSC fenestration or round window injections. Intrathecal contrast administration, however, does not pose any structural or surgical risk to the inner ear itself. While risk to the central nervous system (CNS) may vary by vector or substance, this would need to be determined for any therapeutic before widespread adoption of new mode of administration. When administered at low dose, intrathecal gadolinium contrast does not appear to pose a significant risk to the CNS, although gadolinium deposition in brain tissues is a reported phenomenon.21 In particular, intrathecal gadolinium is considered off-label administration, necessitating informed consent of the patient prior to administration. The observed safety profile of low-dose intrathecal gadolinium administration suggests that future gene therapy vectors targeting the inner ear may be safely administered through this route. More work is needed to determine and demonstrate the safety and efficacy of intrathecal administration for future inner ear therapies. For example, the dose and volume required to reach the inner ear may be higher than direct inner ear injections. Ranum et al. injected 4 mL of virus (3E13 vector genomes) into the CSF and demonstrated transduction into the cochlea.7 Studies using the traditional lateral approach for delivery into the NHP inner ear, however, have observed robust transgene expression with as little as 10–20 μL of virus (vector genomes ranging from 5.8E10–3E11).22,23,24 It is difficult to compare the transduction efficiency of the inner ear between these studies as different NHP species were utilized and different capsid variants with different tropisms were used. More work is needed to identify the minimum dosage needed to robustly deliver AAV to the inner ear via the CSF. Additionally, the clearance of the virus from the CSF would be through systemic circulation, and thus, it will be important for future studies to design vectors or small molecules that are highly specific in their targeting of the cochlea tissue. Finally, lumbar puncture is a frequently performed procedure in the clinical setting. However, potential risks of headache, CSF leak, and pain need to be considered.
Future clinical trials for inner ear therapeutics will require measuring human cochleae diffusion efficiency combined with functional outcomes. Given the variability we observe with CSF contrast diffusion into the cochlea, clinical trials should combine inner ear therapeutic agents with gadolinium contrast to correlate clinical hearing outcomes with gadolinium diffusion efficiency. To our knowledge, only one study has combined intrathecal administration of MRI contrast (gadoteridol) with AAV9 in NHPs and demonstrated different patterns of gadoteridol distribution and AAV9 clearance from the CSF depending on the route of delivery.25 Assessment of cochleae cellular uptake of therapeutics (e.g., AAV) with traditional histologic techniques would not be possible in human patients since biopsy of the inner ear would result in deafness. Our current study demonstrates the favorable safety profile of intrathecal administration of gadolinium. Future studies could determine the minimum volume necessary to observe cochlear diffusion.
Acknowledgments
This work was funded by the NIH (K08-DC016034 and R01 DC020574 to R.F.N.) and the Triological Society and American College of Surgeons Clinician Scientist Development Award (to R.F.N.).
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.08.001.
Supplemental information
References
- 1.Nelson R.F., Hansen K.R., Gantz B.J., Hansen M.R. Calvarium thinning in patients with spontaneous cerebrospinal fluid leak. Otol. Neurotol. 2015;36:481–485. doi: 10.1097/MAO.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 2.Alwani M.M., Saltagi M.Z., MacPhail M.E., Nelson R.F. Middle Cranial Fossa Repair of Temporal Bone Spontaneous CSF Leaks With Hydroxyapatite Bone Cement. Laryngoscope. 2021;131:624–632. doi: 10.1002/lary.28761. [DOI] [PubMed] [Google Scholar]
- 3.Lahlou G., Calvet C., Giorgi M., Lecomte M.J., Safieddine S. Towards the Clinical Application of Gene Therapy for Genetic Inner Ear Diseases. J. Clin. Med. 2023;12:1046. doi: 10.3390/jcm12031046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith R.J.H., Bale J.F., Jr., White K.R. Sensorineural hearing loss in children. Lancet. 2005;365:879–890. doi: 10.1016/S0140-6736(05)71047-3. [DOI] [PubMed] [Google Scholar]
- 5.Naso M.F., Tomkowicz B., Perry W.L., 3rd, Strohl W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs. 2017;31:317–334. doi: 10.1007/s40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landegger L.D., Pan B., Askew C., Wassmer S.J., Gluck S.D., Galvin A., Taylor R., Forge A., Stankovic K.M., Holt J.R., Vandenberghe L.H. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat. Biotechnol. 2017;35:280–284. doi: 10.1038/nbt.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranum P.T., Tecedor L., Keiser M.S., Chen Y.H., Leib D.E., Liu X., Davidson B.L. Cochlear transduction via cerebrospinal fluid delivery of AAV in non-human primates. Mol. Ther. 2023;31:609–612. doi: 10.1016/j.ymthe.2022.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delmaghani S., El-Amraoui A. Inner Ear Gene Therapies Take Off: Current Promises and Future Challenges. J. Clin. Med. 2020;9:2309. doi: 10.3390/jcm9072309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omichi R., Shibata S.B., Morton C.C., Smith R.J.H. Gene therapy for hearing loss. Hum. Mol. Genet. 2019;28:R65–R79. doi: 10.1093/hmg/ddz129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarabichi O., Correa T., Kul E., Phillips S., Darkazanly B., Young S.M., Jr., Hansen M.R. Development and evaluation of helper dependent adenoviral vectors for inner ear gene delivery. Hear. Res. 2023;435:108819. doi: 10.1016/j.heares.2023.108819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters C.W., Hanlon K.S., Ivanchenko M.V., Zinn E., Linarte E.F., Li Y., Levy J.M., Liu D.R., Kleinstiver B.P., Indzhykulian A.A., Corey D.P. Rescue of hearing by adenine base editing in a humanized mouse model of Usher syndrome type 1F. Mol. Ther. 2023;31:2439–2453. doi: 10.1016/j.ymthe.2023.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishibashi Y., Sung C.Y.W., Grati M., Chien W. Immune responses in the mammalian inner ear and their implications for AAV-mediated inner ear gene therapy. Hear. Res. 2023;432:108735. doi: 10.1016/j.heares.2023.108735. [DOI] [PubMed] [Google Scholar]
- 13.Ivanchenko M.V., Hathaway D.M., Klein A.J., Pan B., Strelkova O., De-la-Torre P., Wu X., Peters C.W., Mulhall E.M., Booth K.T., et al. Mini-PCDH15 gene therapy rescues hearing in a mouse model of Usher syndrome type 1F. Nat. Commun. 2023;14:2400. doi: 10.1038/s41467-023-38038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J., Li M., Liu Q., Xu X., Lu X., Ma G., Ma C., Zhu X., Wu X., Ren J. Single and Dual Vector Gene Therapy with AAV9-PHP.B Rescues Hearing in Tmc1 Mutant Mice. Mol. Ther. 2021;22:973–982. doi: 10.1016/j.ymthe.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki J., Hashimoto K., Xiao R., Vandenberghe L.H., Liberman M.C. Cochlear gene therapy with ancestral AAV in adult mice: complete transduction of inner hair cells without cochlear dysfunction. Sci. Rep. 2017;7:45524. doi: 10.1038/srep45524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshimura H., Shibata S.B., Ranum P.T., Smith R.J.H. Enhanced viral-mediated cochlear gene delivery in adult mice by combining canal fenestration with round window membrane inoculation. Sci. Rep. 2018;8:2980. doi: 10.1038/s41598-018-21233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horowitz E.D., Rahman K.S., Bower B.D., Dismuke D.J., Falvo M.R., Griffith J.D., Harvey S.C., Asokan A. Biophysical and ultrastructural characterization of adeno-associated virus capsid uncoating and genome release. J. Virol. 2013;87:2994–3002. doi: 10.1128/JVI.03017-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aime S., Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J. Magn. Reson. Imaging. 2009;30:1259–1267. doi: 10.1002/jmri.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thalen E.O., Wit H.P., Segenhout J.M., Albers F.W. Dynamics of inner ear pressure change caused by intracranial pressure manipulation in the guinea pig. Acta Otolaryngol. 2001;121:470–476. [PubMed] [Google Scholar]
- 20.Naganawa S., Satake H., Iwano S., Sone M., Nakashima T. Communication between cochlear perilymph and cerebrospinal fluid through the cochlear modiolus visualized after intratympanic administration of Gd-DTPA. Radiat. Med. 2008;26:597–602. doi: 10.1007/s11604-008-0286-z. [DOI] [PubMed] [Google Scholar]
- 21.Mallio C.A., Quattrocchi C.C., Rovira À., Parizel P.M. Gadolinium Deposition Safety: Seeking the Patient's Perspective. AJNR. Am. J. Neuroradiol. 2020;41:944–946. doi: 10.3174/ajnr.A6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.György B., Meijer E.J., Ivanchenko M.V., Tenneson K., Emond F., Hanlon K.S., Indzhykulian A.A., Volak A., Karavitaki K.D., Tamvakologos P.I., et al. Gene Transfer with AAV9-PHP.B Rescues Hearing in a Mouse Model of Usher Syndrome 3A and Transduces Hair Cells in a Non-human Primate. Mol. Ther. Methods Clin. Dev. 2019;13:1–13. doi: 10.1016/j.omtm.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivanchenko M.V., Hanlon K.S., Hathaway D.M., Klein A.J., Peters C.W., Li Y., Tamvakologos P.I., Nammour J., Maguire C.A., Corey D.P. AAV-S: A versatile capsid variant for transduction of mouse and primate inner ear. Mol. Ther. Methods Clin. Dev. 2021;21:382–398. doi: 10.1016/j.omtm.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivanchenko M.V., Hanlon K.S., Devine M.K., Tenneson K., Emond F., Lafond J.F., Kenna M.A., Corey D.P., Maguire C.A. Preclinical testing of AAV9-PHP.B for transgene expression in the non-human primate cochlea. Hear. Res. 2020;394:107930. doi: 10.1016/j.heares.2020.107930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohno K., Samaranch L., Hadaczek P., Bringas J.R., Allen P.C., Sudhakar V., Stockinger D.E., Snieckus C., Campagna M.V., San Sebastian W., et al. Kinetics and MR-Based Monitoring of AAV9 Vector Delivery into Cerebrospinal Fluid of Nonhuman Primates. Mol. Ther. Methods Clin. Dev. 2019;13:47–54. doi: 10.1016/j.omtm.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.