Abstract
Hearing loss is a common disorder affecting nearly 20% of the world’s population. Recently, studies have shown that inner ear gene therapy can improve auditory function in several mouse models of hereditary hearing loss. In most of these studies, the underlying mutations affect only a small number of cell types of the inner ear (e.g., sensory hair cells). Here, we applied inner ear gene therapy to the Ildr1Gt(D178D03)Wrst (Ildr1w−/−) mouse, a model of human DFNB42, non-syndromic autosomal recessive hereditary hearing loss associated with ILDR1 variants. ILDR1 is an integral protein of the tricellular tight junction complex and is expressed by diverse inner ear cell types in the organ of Corti and the cochlear lateral wall. We simultaneously applied two synthetic adeno-associated viruses (AAVs) with different tropism to deliver Ildr1 cDNA to the Ildr1w−/− mouse inner ear: one targeting the organ of Corti (AAV2.7m8) and the other targeting the cochlear lateral wall (AAV8BP2). We showed that combined AAV2.7m8/AAV8BP2 gene therapy improves cochlear structural integrity and auditory function in Ildr1w−/− mice.
Keywords: gene therapy, hearing loss, tight junction, ILDR1, tricellulin, atomic force microscopy
Graphical abstract
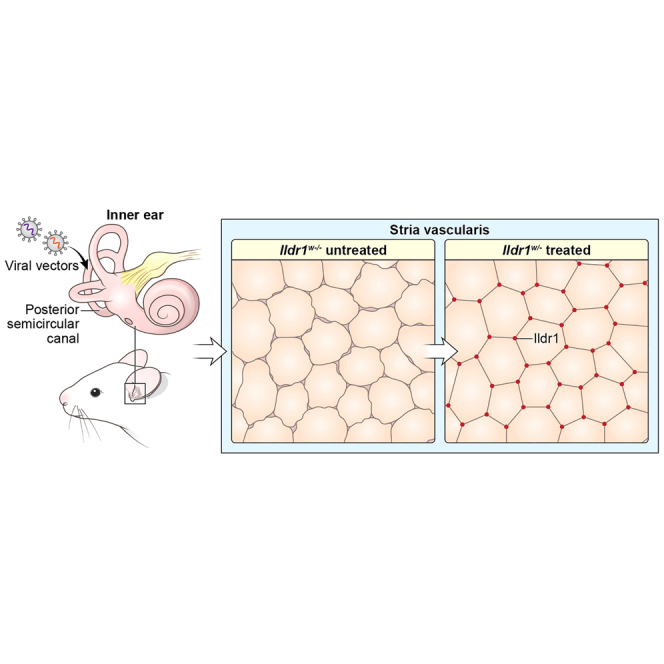
Because most of AAVs transduce selective cell populations, it is challenging to augment deafness genes expressed in diverse cell types of the cochlea. We utilized two AAVs with different tropism to transduce various cell types to improve auditory function in an ILDR1-deficient mouse model of human deafness (DFNB42).
Introduction
Hearing loss is a common disorder affecting approximately 1 in 1,000 newborns every year.1 Currently, over 100 non-syndromic deafness genes have been discovered in humans.2 The majority of these genes play important roles in development and maintenance of normal inner ear function.3 One important structural network that is critical for proper functioning of the inner ear is the tight junction network. Tight junctions (TJs) are intricate structures in the inner ear that are formed between adjacent cells4,5 and regulate transport of solutes through the paracellular pathway.6 Bicellular TJs (bTJs) are formed between two adjacent cells, while tricellular TJs (tTJs) are formed between three adjacent cells. Sheets of epithelial cells in the inner ear create ionic barriers separating the perilymph with high sodium concentration in the scala tympani and scala vestibuli from the endolymph with high potassium concentration in the scala media.7,8 In addition, reticular lamina TJs separate high-potassium endolymph from high-sodium perilymph that surrounds the outer hair cell (OHC) bodies in the tunnel of Corti.9,10 This separation of membranous compartments with unique ionic compositions is critical to maintain the +80-mV endocochlear potential (EP), the driving force for mechanotransduction of sound by the cochlear hair cells.11,12
Pathogenic variants of genes encoding TJ proteins, such as claudin-14 and tricellulin (also known as MARVELD2), have been shown to cause hereditary hearing loss in humans, DFNB29 and DFNB49, respectively.11,13,14 Immunoglobulin-like domain-containing receptor 1 (ILDR1; also known as angulin-2) is a tTJ-associated protein that has been reported previously to be responsible for localization of tricellulin in tTJs in vitro, using heterologous cell lines.15 However, in mice lacking ILDR1 at the tTJs of the organ of Corti, recruitment and initial localization of tricellulin are not affected, but retention of tricellulin at the tTJs is impaired.16 Mutations in human ILDR1 have been shown to cause non-syndromic autosomal recessive hereditary hearing loss (DFNB42).17 Studies in Ildr1 knockout mice have shown progressive cochlear hair cell degeneration from base to apex, starting at the onset of hearing in mice on post-natal day 12 (P12). These mice develop profound hearing loss by P30.15,16,18
Current treatment options for hearing loss are limited. Patients with mild to moderate hearing loss can be fitted with hearing aids, and patients with severe to profound hearing loss may be candidates for cochlear implantation. While these devices are effective and help many patients, they have significant shortcomings, including difficulty deciphering specific sounds and speech in a noisy environment.19,20 Over the last few years, inner ear gene therapy has emerged as a promising therapeutic option for hearing loss.21,22 While many inner ear gene therapy studies have shown successful improvement of auditory function in various mouse models of human hereditary hearing loss, most of these studies have focused on genes that are predominantly expressed in a specific cell type in the mouse inner ear; for example, VGlut3 in inner hair cells.23,24 However, efforts to apply inner ear gene therapy replacing or augmenting genes that are expressed by a variety of different cell types in the inner ear have been much more challenging (e.g., Gjb2).25 It is difficult to find a single adeno-associated virus (AAV) serotype that can efficiently and concomitantly transduce a heterogeneous cell population in the mammalian inner ear.
Ildr1 is expressed in a heterogeneous epithelial cell population across the inner ear, including hair cells and supporting cells of the organ of Corti and marginal cells of the stria vascularis.16 In this study, we applied combined AAV2.7m8/AAV8BP2-Ildr1 gene therapy to a mouse model of DFNB42 (Ildr1w−/−).16 We show that combined AAV2.7m8/AAV8BP2-Ildr1 cDNA delivery (termed “combined AAV-Ildr1 gene therapy”) resulted in restoration in ILDR1 expression in the inner ear of Ildr1w−/− mice. Restoration of ILDR1 expression led to an improvement in TJ organization, tricellulin retention at tTJs, and structural integrity of the Ildr1w−/− cochlea. Furthermore, combined AAV-Ildr1 gene therapy improved auditory function in Ildr1w−/− mice. These results offer a novel strategy for applying inner ear gene therapy to hereditary hearing loss where the causative gene is expressed in diverse cell types in the cochlea.
Results
Combined AAV2.7m8/AAV8BP2-GFP transduces sensory and non-sensory cells throughout the cochlea
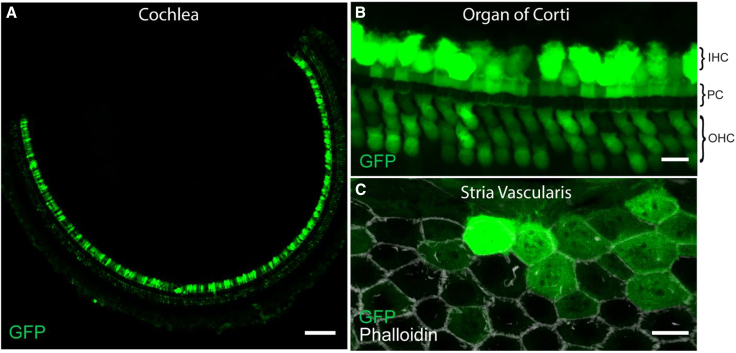
ILDR1 is expressed in sensory and non-sensory cells in the cochlea throughout the organ of Corti and in the marginal cells of the stria vascularis.16 For gene therapy to successfully improve auditory function in a mouse model of DFNB42, we hypothesized that Ildr1 cDNA must be delivered to a variety of cell types in the cochlea. Thus, we used a combination of two AAV vectors with different tropism that distinctly target the cell types in the organ of Corti and the cochlear lateral wall. We have shown previously that AAV2.7m8 is an effective viral vector for targeting inner hair cells (IHCs) and OHCs as well as some supporting cells in the organ of Corti, while AAV8BP2 is capable of transducing different cell types in the cochlear lateral wall.26,27 Here we tested whether we could transduce the diverse cell types in the organ of Corti and cochlear lateral wall by simultaneously injecting AAV2.7m8-GFP (4.88 × 109 genome copies [GCs]) and AAV8BP2-GFP (5.50 × 109 GCs) into neonatal (P0–P5) wild-type (Ildr1w+/+) mouse inner ears using a posterior semicircular canal (PSC) approach. We found that GFP expression was detected in IHCs and OHCs and supporting cells in the organ of Corti (Figures 1A and 1B) as well as in the marginal cell layer of the stria vascularis (Figure 1C). This indicates that combined injection of AAV2.7m8 and AAV8BP2 can successfully transduce different cell types that express ILDR1 across the scala media of the mouse cochlea.
Figure 1.
Combined AAV2.7m8-GFP and AAV8BP2-GFP delivery transduced sensory and non-sensory cells throughout the scala media
(A–C) When AAV2.7m8-GFP and AAV8BP2-GFP were delivered simultaneously into neonatal Ildr1+/+ mice via the PSC, many inner ear cell types were transduced by the AAVs and expressed GFP (A). In the organ of Corti, GFP expression was seen in many IHCs, OHCs, and supporting cells (B). Examination of the marginal cell layer of the stria vascularis (delineated by phalloidin staining in white) revealed GFP expression in many marginal cells (C). Images were taken on P30. Scale bars represent 100 μm in (A) and 10 μm in (B) and (C). IHC, inner hair cell; OHC, outer hair cell; PC, pillar cell.
Combined AAV-Ildr1 gene therapy restores ILDR1 expression and improves TJ organization in Ildr1w−/− mice
The TJ network separates the endolymphatic space from the perilymphatic space in the organ of Corti.8 ILDR1 is expressed at tTJs (Figures 2A and 2D). The Ildr1w−/− mouse model of DFNB42 has a gene trap in intron 2 and does not express ILDR1;16 consequently, the TJ network is disorganized in the organ of Corti (Figure 2B).16 When combined AAV-Ildr1 gene therapy (2.91 × 109 GCs for AAV2.7m8-Cmv-Ildr1-EGFP and 0.58 × 109 GC for AAV8BP2-Cmv-Ildr1-EGFP) was delivered to neonatal (P0–P5) Ildr1w−/− mice using the PSC approach, we found that many cell types in the treated cochlea expressed GFP, indicating viral transduction (Figures 2C and 2F). We quantified the viral transduction efficiency of IHCs and OHCs, pillar cells, and marginal cells in the stria vascularis by counting the cells that express GFP at P14. The transduction rates were 78.2% ± 5.60% for IHCs (n = 5 mice), 71.9% ± 9.08% for OHCs (n = 5 mice), 42.7% ± 4.24% for pillar cells (n = 5 mice), and 77.4% ± 8.26% for marginal cells of the stria vascularis (n = 5 mice) (Figures 2G–2I). Treated Ildr1w−/− mice also showed restoration of ILDR1 in tTJs in the organ of Corti and the cochlear lateral wall (Figures 2C and 2F). In addition, in treated mice, the overall morphology and cellular organization of the organ of Corti showed significant improvement compared with non-treated mutant mouse ears because of the partially restored TJ network in treated mice. (Figure 2C). Furthermore, ILDR1 expression was detected in the organ of Corti and marginal cells of the stria vascularis 2 months post treatment and appears to be long lasting (Figures S1A–S1F). These results suggest that combined AAV-Ildr1 gene therapy was capable of restoring ILDR1 expression in a variety of cell types in the Ildr1w−/− cochlea, leading to significant improvement of TJ network organization.
Figure 2.
Combined AAV-Ildr1 gene therapy restored ILDR1 expression in Ildr1w−/− mice
(A–F) Confocal images showing the organ of Corti and stria vascularis on P14 (∼10–14 days after AAV-mediated gene delivery). Images were taken from the cochlear middle turn. In wild-type mice, ILDR1 (magenta) is expressed in tTJs between hair cells and supporting cells in the organ of Corti (A) and in the marginal cell layer of the stria vascularis (D, white arrows). In Ildr1w−/− mice, ILDR1 expression is absent in the organ of Corti (B) and stria vascularis (E). Ildr1w−/− mice that were treated with combined AAV-Ildr1 gene therapy showed restoration of ILDR1 expression (magenta) at tTJs in the organ of Corti (C, white arrows) and in the marginal cell layer of the stria vascularis (F, white arrows). GFP expression was observed in transduced cells (C and F). (G–I) Quantification of transduction rates are shown for IHCs (G), OHCs (G), PCs (H), and marginal cells (MCs, I) examined on P14. Error bars represents SEM. Scale bars represent 10 μm; n = number of ears examined.
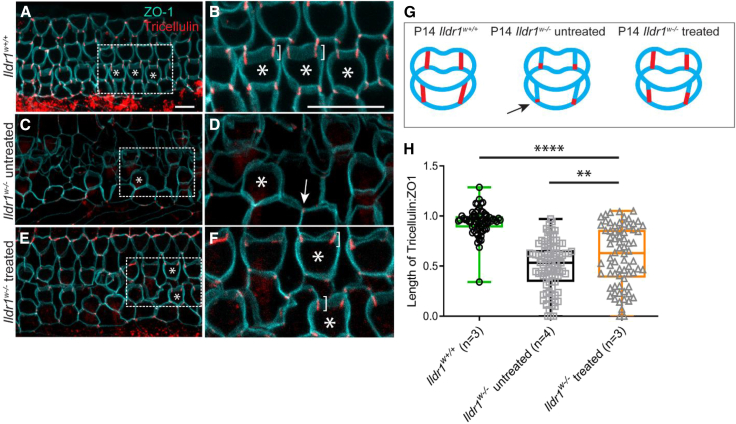
Combined AAV-Ildr1 gene therapy improves tricellulin localization in Ildr1w−/− mice
ILDR1 plays an important role in maintenance and retention of tricellulin in tTJs.16 In wild-type mice, tricellulin expression can be seen throughout the entire length of tTJs in the cochlea (Figures 3A, 3B, and 3G). In Ildr1w−/− mice, mislocalization of tricellulin begins to be observable at P6–P10, and by P11–P14, the tricellulin signal is limited to the basal portion of tTJs (Figures 3C, 3D, and 3G).16 When Ildr1w−/− mice were treated with combined AAV-Ildr1 gene therapy, we found that tricellulin expression can be seen throughout the entire length of tTJs (Figures 3E, 3F, and 3G). We quantified recovery of tricellulin localization by measuring the length of tricellulin expression along tTJs using the 3D rendering software Volocity (PerkinElmer, Waltham, MA, USA). We found that Ildr1w−/− mice that were treated with combined AAV-Ildr1 gene therapy had tricellulin expression along a longer portion of tTJs compared with non-treated Ildr1w−/− mice (0.60 ± 0.04 μm vs. 0.50 ± 0.02 μm, p < 0.01, one-way ANOVA with Holm-Šidák correction; Figure 3H). However, the mean length of tricellulin expression at tTJs in treated Ildr1w−/− mice was still lower than in wild-type mice (0.60 ± 0.04 μm vs. 0.94 ± 0.02 μm, p < 0.0001, one-way ANOVA with Holm-Šidák correction; Figure 3H). These results suggest that combined AAV-Ildr1 gene therapy can improve tricellulin localization because of its improved retention in tTJs.
Figure 3.
Combined AAV-Ildr1 gene therapy improved tricellulin retention and localization at tTJs in P14 Ildr1w−/− mice
(A–G) In wild-type mice (A and B), tricellulin (red) can be detected along the entire length of tTJs (brackets in B), similar to the cartoon (G). In contrast, tricellulin expression in Ildr1w−/− mice is predominantly limited to the basal end of tTJs (C, D, and G; see arrows in D and G). Tricellulin localization in Ildr1w−/− mice is improved after combined AAV-Ildr1 gene therapy treatment (E–G). A closer examination reveals tricellulin expression throughout the entire length of tTJ (brackets in F), comparable with wild-type expression in some cases (B and F). The asterisks in (A)–(F) are included to facilitate OHC identification. (H) Quantification of tricellulin localization at tTJs was performed using the Volocity software. Treated Ildr1w−/− mice showed improved tricellulin localization compared with untreated Ildr1w−/− mice. Statistical analysis was performed using one-way ANOVA with Holm-Šidák correction. ∗∗p < 0.01, ∗∗∗∗p < 0.0001. Confocal images were taken from the apex of the cochlea. Scale bars represent 10 μm.
Combined AAV-Ildr1 gene therapy improves the structural integrity of the cochlear epithelium in Ildr1w−/− mice
Because the combined AAV-Ildr1 gene therapy improved TJ network organization and tricellulin localization, we wanted to see whether the structural integrity of the inner ear would be amended after this gene therapy. We used atomic force microscopy (AFM) to measure the structural integrity of the Ildr1w−/− mouse cochlear lateral wall after gene therapy treatment (Figure 4A). Atomic force scanning probe microscopy has been used successfully to measure the elastic and viscous properties of samples.28 When cochlear lateral wall tissues from Ildr1w−/− mice treated with combined AAV-Ildr1 gene therapy (n = 3) were analyzed using AFM on P14, we found that they had significantly increased epithelial tension compared with the untreated contralateral ear (n = 3) (1.78 ± 0.18 nN μm−1 vs. 0.75 ± 0.12 nN μm−1, p < 0.0001) and untreated Ildr1w−/− mice (n = 3) (1.78 ± 0.18 nN μm−1 vs. 0.99 ± 0.07 nN μm−1, p < 0.01, one-way ANOVA with Holm-Šidák correction; Figure 4B). The epithelial tension measurements of treated Ildr1w−/− mice were similar to those of wild-type mice (n = 3) (1.80 ± 0.19 nN μm−1, p = 0.93, one-way ANOVA with Holm-Šidák correction; Figure 4B). On P14, we did not observe any significant difference in epithelial viscosity in cochlear lateral wall tissues between wild-type and untreated Ildr1w−/− mice (1.21 ± 0.13 nN μm−1 vs. 0.93 ± 0.10 nN μm−1, p = 0.21, one-way ANOVA with Holm-Šidák correction, n = 3 for each group; Figure 4C). This is likely a reflection of the relatively immature state of the cochlea at this age.29,30 Not surprisingly, we also did not observe any significant difference in mean epithelial viscosity in P14 cochlear lateral wall tissues between Ildr1w−/− mice treated with combined AAV-Ildr1 gene therapy and untreated Ildr1w−/− mice (1.31 ± 0.14 nN μm−1 vs. 0.93 ± 0.10 nN μm−1, p = 0.10, one-way ANOVA with Holm-Šidák correction, n = 3 for each group; Figure 4C). However, the mean epithelial viscosity in treated P14 Ildr1w−/− ears was higher than in the contralateral untreated ears of the same mice (1.31 ± 0.14 mPa s−1 vs. 0.61 ± 0.11 mPa s−1, p < 0.001, one-way ANOVA with Holm-Šidák correction, n = 3 for each group; Figure 4C).
Figure 4.
Combined AAV-Ildr1 gene therapy restored the structural integrity in the Ildr1w−/− cochlea
(A) Atomic force microscopy (AFM) was used to measure the integrity of the cochlear lateral wall. (B–E) Measurements of epithelial tension (B and D) and epithelial viscosity (C and E) of microdissected cochlear lateral wall tissues were made. AFM measurements on P14 of Ildr1w−/− mice treated with combined AAV-Ildr1 gene therapy (labeled “Ildr1w−/− treated”) revealed significant improvement in epithelial tension compared with untreated Ildr1w−/− as well as the untreated contralateral ears (labeled “Ildr1w−/− contralateral ear”) (B). Furthermore, no significant tension differences were measured in wild-type (labeled “Ildr1w+/+”) and treated tissue (B). Epithelial viscosity measurements in treated mice showed improvement when compared to the untreated contralateral ears (C). In P30 Ildr1w−/− treated mice, the epithelial tension was significantly greater than in untreated Ildr1w−/− mice and untreated contralateral ears (D). No significant differences were seen between wild-type and treated tissue. A similar pattern was also observed with epithelial viscosity measurements, with Ildr1w−/− mice treated with combined AAV-Ildr1 gene therapy showing significant greater epithelial viscosity compared with non-treated Ildr1w−/− mice (E). However, the epithelial viscosity in treated Ildr1w−/− tissues was lower than in wild-type tissues. Statistical analysis was performed using one-way ANOVA with Holm-Šidák correction. ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
We also used AFM to examine the structural integrity of the cochlear lateral wall at P30. We found that P30 cochlear lateral wall tissues from Ildr1w−/− mice treated with combined AAV-Ildr1 gene therapy (n = 4) had significantly increased epithelial tension and epithelial viscosity compared with cochlear lateral wall tissues from untreated Ildr1w−/− mice (n = 5) (1.96 ± 0.18 nN μm−1 vs. 0.82 ± 0.06 nN μm−1, p < 0.0001 for epithelial tension; 1.32 ± 0.17 mPa s−1 vs. 0.66 ± 0.05 mPa s−1, p < 0.01 for epithelial viscosity; one-way ANOVA with Holm-Šidák correction; Figures 4D and 4E). Similarly, we found that P30 cochlear lateral wall tissues from the Ildr1w−/− mice treated with combined AAV-Ildr1 gene therapy (n = 4) had significantly increased epithelial tension and epithelial viscosity compared with cochlear lateral wall tissues from untreated contralateral ears of the same animals (1.96 ± 0.18 nN μm−1 vs. 0.98 ± 0.11 nN μm−1, p < 0.0001 for epithelial tension; 1.32 ± 0.17 mPa s−1 vs. 0.55 ± 0.07 mPa s−1, p < 0.01, for epithelial viscosity, one-way ANOVA with Holm-Šidák correction; Figures 4D and 4E). Epithelial tension measurements of treated Ildr1w−/− mice were similar to those of wild-type mice (n = 4) (1.72 ± 0.17 nN μm−1, p = 0.35, one-way ANOVA with Holm-Šidák correction; Figure 4D), while a significant difference in epithelial viscosity was still present between treated Ildr1w−/− mice and wild-type mice (2.44 ± 0.30 mPa s−1, p < 0.0001, one-way ANOVA with Holm-Šidák correction; Figure 4E).
Finally, AFM nanoindentation-based mechanical mapping was used to assess the tissue integrity of the organ of Corti by measuring the stiffness of the inner pillar cells. The reason why nanoindentation-based mechanical mapping AFM was utilized for the organ of Corti specimens instead of the noncontact frequency-modulation AFM utilized for the lateral wall specimens is that stereocilium bundles on cochlear hair cells in the organ of Corti can interfere with noncontact frequency-modulation AFM measurements. Ildr1w−/− mice that were treated with combined AAV-Ildr1 gene therapy (n = 3) had a significantly higher elastic Young’s modulus, an indicator of stiffness, compared with untreated Ildr1w−/− mice (n = 3) (84.2 ± 4.78 kPa vs. 35.6 ± 2.81 kPa, p < 0.0001, one-way ANOVA with Holm-Šidák correction; Figure S2). We also observed a significant increase in elastic Young’s modulus in treated Ildr1w−/− mice (n = 3) compared with untreated contralateral ears of the same animals (n = 3) (84.2 ± 4.78 kPa vs. 29.6 ± 2.38 kPa, p < 0.0001, one-way ANOVA with Holm-Šidák correction; Figure S2). Despite this significant increase, treated Ildr1w−/− mice (n = 3) still show a significantly lower elastic Young’s modulus compared with wild-type mice (n = 3) (84.2 ± 4.78 kPa vs. 132.8 ± 5.27 kPa, p < 0.0001, one-way ANOVA with Holm-Šidák correction; Figure S2A). These results suggest that combined AAV-Ildr1 gene therapy can partially restore cochlear epithelium stiffness and structural integrity in Ildr1w−/− mice.
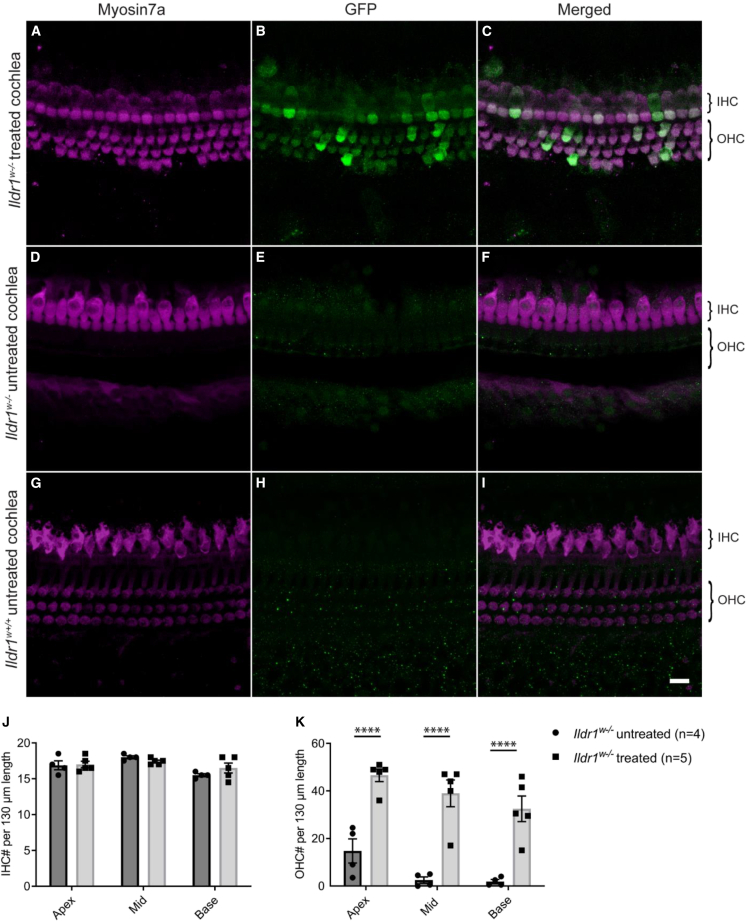
Combined AAV-Ildr1 gene therapy improves OHC survival and auditory function in Ildr1w−/− mice
Ildr1w−/− mice undergo progressive hair cell loss starting at P12, leading to severe deafness by P14.16 We examined IHC and OHC survival in Ildr1w−/− mice on P14 compared with wild-type mice (Figures 5G, 5H, and 5I). Confocal images taken from an untreated P14 Ildr1w−/− mouse showed largely intact IHCs, but significant OHC degeneration had occurred (Figures 5D, 5E, and 5F). In contrast, Ildr1w−/− mice that were treated with combined AAV-Ildr1 gene therapy showed significant improvement in OHC survival on P14 (Figures 5A and 5C). Quantification of IHC survival on P14 showed no significant difference between treated and untreated Ildr1w−/− mice (apex: 17.0 ± 0.47 vs. 16.9 ± 0.63, p = 0.85; middle: 17.4 ± 0.19 vs. 18.0 ± 0.20, p = 0.60; base: 16.5 ± 0.69 vs. 15.5 ± 0.20, p = 0.37; two-way ANOVA with Holm-Šidák correction; Figure 5J). However, quantification of OHC survival showed significant differences between treated (n = 5) and untreated (n = 4) Ildr1w−/− mice at the apical (46.6 ± 2.69 vs. 14.8 ± 5.06, respectively; p < 0.0001), middle (39.0 ± 5.65 vs. 2.50 ± 1.31, respectively; p < 0.0001), and basal (32.5 ± 5.40 vs. 1.88 ± 0.85, respectively; p < 0.0001, two-way ANOVA with Holm-Šidák correction) turns of the cochlea (Figure 5K). Treated Ildr1w−/− mice also showed more organized stereocilium morphology in cochlear hair cells, particularly in OHCs, likely indicating a healthier state of cochlear hair cells in treated Ildr1w−/− mice compared with untreated ones (Figure S3). These results indicate that combined AAV-Ildr1 gene therapy improves OHC survival in Ildr1w−/− mice.
Figure 5.
Combined AAV-Ildr1 gene therapy improved OHC survival
(A–I) Representative confocal images of the P14 organ of Corti from the middle turn of the cochlea. In untreated Ildr1w−/− mice, a majority of OHCs have undergone degeneration by P14 (D–F). IHCs are still intact at this age. In contrast, Ildr1w−/− mice treated with combined AAV-Ildr1 gene therapy showed significantly more OHCs at P14 (A–C). These same OHCs show GFP expression, indicating transduction by combined AAV-Ildr1 (B and C). Confocal images from a wild-type littermate are shown for comparison (G–I). (J and K) Quantification of OHCs showed an increase in survival throughout all three turns of the cochlea in Ildr1w−/− mice treated with combined AAV-Ildr1 gene therapy (K). In both groups, IHCs were largely intact at P14 (J). Dark gray bars represent untreated Ildr1w−/− mice, while light gray bars represent Ildr1w−/− mice treated with combined AAV-Ildr1 gene therapy. Error bars represents SE. Statistical analysis was performed using two-way ANOVA with Holm-Šidák correction. ∗∗∗∗p < 0.0001. The scale bar represents 10 μm.
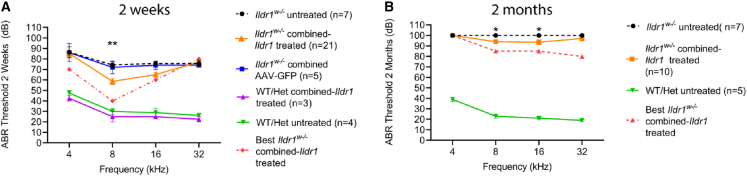
We examined the auditory function of Ildr1w−/− mice on P14 by measuring auditory brain stem responses (ABRs). Ildr1w−/− mice that were treated with combined AAV-Ildr1 gene therapy (n = 21) had significantly improved auditory function compared with non-treated Ildr1w−/− mice (n = 7, p < 0.0001, two-way ANOVA). Post hoc analysis showed a statistically significant difference at 8 kHz between treated and untreated animals (58.6 ± 2.99 dB SPL vs. 74.3 ± 2.97 dB SPL, respectively; p < 0.01, two-way ANOVA with Holm-Šidák correction) and a nearly statistically significant difference at 16 kHz (65.0 ± 2.24 dB SPL vs. 75.7 ± 0.71 dB SPL, respectively; p = 0.055, two-way ANOVA with Holm-Šidák correction; Figure 6A). In the best-performing Ildr1w−/− mouse that received combined AAV-Ildr1 gene therapy treatment, the ABR threshold at 8 kHz was 40 dB SPL, similar to that of untreated wild-type and heterozygous littermates (n = 4). Some wild-type and heterozygous littermates also underwent combined AAV-Ildr1 gene therapy treatment (n = 3), and their ABR thresholds were similar to that of untreated wild-type and heterozygous littermates, indicating that the combined AAV-Ildr1 gene therapy did not cause toxicity to the inner ear (Figure 6A). A control group of Ildr1w−/− mice (n = 5) was injected with combined AAV2.7m8/AAV8BP2-GFP and showed no improvement in ABR thresholds compared with the untreated Ildr1w−/− mice (Figure 6A). In 7 of 21 Ildr1w−/− mice that were treated with combined AAV-Ildr1 gene therapy, measurable DPOAEs above the noise floor were detected, whereas none of the non-treated Ildr1w−/− mice showed DPOAEs above the noise floor (n = 6; Figure S4). The fact that not all treated Ildr1w−/− mice had measurable DPOAEs is likely due to the partial improvement in auditory function seen in these animals. Therefore, the ABR thresholds in many of these treated Ildr1w−/− mice may still be too high for consistent DPOAE measurements.
Figure 6.
Combined AAV-Ildr1 gene therapy improved auditory function in Ildr1w−/− mice
(A and B) Auditory brainstem response (ABR) was used to assess the auditory function on P14 (A) and P60 (B). Untreated Ildr1w−/− mice have significantly elevated ABR thresholds on P14 (dotted black line). A control group of Ildr1w−/− mice injected with combined AAV-GFP showed similar ABR thresholds as untreated Ildr1w−/− mice (blue line). Ildr1w−/− mice that were treated with combined AAV-Ildr1 gene therapy (orange line) displayed significant improvement in ABR thresholds at 8 kHz. Statistical analysis was performed using two-way ANOVA with Holm-Šidák correction for multiple comparisons (A). The ABR thresholds of the best performer are shown as a red dotted line. The ABR thresholds of treated and untreated wild-type (WT) and heterozygous (Het) littermates are shown for comparison (purple and green lines, respectively). On P60, 5 of 10 Ildr1w−/− mice treated with combined AAV-Ildr1 gene therapy (orange line) exhibited recordable ABR thresholds (B). Untreated Ildr1w−/− mice showed no recordable ABR thresholds (dotted black line). A two-way ANOVA with Holm-Šidák correction showed significant improvements in hearing at 8 kHz and 16 kHz when comparing mice treated with combined AAV-Ildr1 gene therapy with untreated Ildr1w−/− mice. The ABR thresholds of the best performer are shown as a red dotted line. Error bars represents SE. ∗p < 0.05, ∗∗p < 0.01.
We assessed the longevity of the improvement in auditory function in treated Ildr1w−/− mice by performing ABR testing in some of these animals on P60. We found that the ABR thresholds were elevated in treated Ildr1w−/− mice (n = 10) on P60 compared with P14 (Figure 6B). This may be due to progressive HC degeneration in treated Ildr1w−/− mice on P60 (Figure S5). However, 5 of the 10 Ildr1w−/− mice treated with the combined AAV-Ildr1 gene therapy still showed recordable ABR waveforms, with the best performer having an ABR threshold of 80 dB SPL at 32 kHz. In contrast, none of the untreated Ildr1w−/− mice (n = 7) had measurable ABR waveforms (p < 0.01, two-way ANOVA with Holm-Šidák correction). Post hoc analysis showed statistically significant differences between the treated and untreated animals at 8 kHz (94.0 ± 2.08 dB SPL vs. 100 ± 0.00 dB SPL, respectively; p = 0.03, two-way ANOVA with Holm-Šidák correction) and at 16 kHz (93.5 ± 2.24 dB SPL vs. 100 ± 0.00 dB SPL, respectively; p = 0.02, two-way ANOVA with Holm-Šidák correction).
We also tested whether treatment with AAV2.7m8-Ildr1 or AAV8BP2-Ildr1 alone would result in similar hearing improvement in Ildr1w−/− mice. Neonatal (P0–P5) Ildr1w−/− mice were injected with either 2.91 × 109 GCs of AAV2.7m8-Cmv-Ildr1-EGFP (AAV2.7m8-Ildr1) or 0.58 × 109 GCs of AAV8BP2-Cmv-Ildr1-EGFP (AAV8BP2-Ildr1) through the PSC approach. In neonatal Ildr1w−/− mice that were treated with AAV2.7m8-Ildr1 alone, ILDR1 and GFP expression was seen only in hair cells and supporting cells in the organ of Corti (Figures S6A–S6C). ILDR1 and GFP expression was not observed in stria vascularis marginal cells (Figures S6D–S6F). This is consistent with the tropism of AAV2.7m8 in the mouse cochlea.26,27 In neonatal Ildr1w−/− mice that were treated with AAV8BP2-Ildr1 alone, ILDR1 and GFP expression was seen only in hair cells in the organ of Corti but not supporting cells (Figures S6G–S6I). ILDR1 and GFP expression was also seen in marginal cells of the stria vascularis in these mice (Figures S6J–S6L). This is consistent with the tropism of AAV8BP2 in the mouse cochlea.26,27 The auditory function of neonatal Ildr1w−/− mice treated with either AAV2.7m8-Ildr1 or AAV8BP2-Ildr1 alone was tested using ABR. We found that, on P14, Ildr1w−/− mice that were treated with either AAV2.7m8-Ildr1 alone or AAV8BP2-Ildr1 alone showed similar improvement in ABR thresholds compared with Ildr1w−/− mice that were treated with combined AAV-Ildr1 gene therapy (Figure S6M). However, none of the Ildr1w−/− mice that were treated with AAV2.7m8-Ildr1 or AAV8BP2-Ildr1 alone had measurable ABR thresholds by P60 (Figure S6N). These results suggest that, while AAV2.7m8-Ildr1 and AAV8BP2-Ildr1 could individually improve auditory function in Ildr1w−/− mice on P14, the improvement in auditory function seen in Ildr1w−/− mice that were treated with combined AAV-Ildr1 gene therapy was much longer lasting than AAV2.7m8-Ildr1 or AAV8BP2-Ildr1 alone.
Discussion
Selection of the appropriate viral vector for gene delivery plays a decisive role in the success of inner ear gene therapy studies.31 The ideal viral vector should be able to transduce only cell types where the target gene is normally expressed. One strategy for overcoming this challenge is to design synthetic AAVs that would potentially target the specific cell types that express the target gene. This strategy will likely be costly and time consuming because new synthetic AAVs will need to be designed and tested for each target gene and cell type(s) of interest. Another strategy to overcome this challenge is to utilize available AAVs with known transduction tropism and to combine them for simultaneous gene delivery to various cell types.
While several AAV serotypes have been shown to transduce selective cell types in the mammalian cochlea, few (e.g., AAV9-PHP.B and AAV-S) are capable of transducing a large number of the diverse cell types simultaneously.32,33 This is likely one of the reasons why most successful inner ear gene therapy studies have focused on genes that are expressed in a small cell population in the cochlea, such as IHCs.21,22 However, some of the most common types of hereditary hearing loss, such as DFNB1A (Gjb2), are caused by mutations in genes that are expressed in diverse cell types in the cochlea.31,34 This presents a significant challenge for effective inner ear gene therapy for these disease processes. The current study examines the strategy of combining two different AAVs with known tropism for simultaneous gene delivery to restore expression of functional ILDR1 in multiple cell types of the Ildr1w−/− mouse model of DFNB42.
Our results demonstrate that simultaneous injections of two synthetic AAVs with different cellular tropism in the inner ear is effective not only to improve hearing function in Ildr1w−/− mice but also to deliver a longer-lasting therapeutic effect of gene therapy. ILDR1 encodes a type I transmembrane protein containing an extracellular immunoglobulin-like domain.17,35,36 ILDR1 was first described in lymphoma cells, and it is a lipoprotein receptor in the small intestines, mediating fat-stimulated cholecystokinin secretion.35,36 In the mammalian cochlea, ILDR1 is responsible for maintenance/retention of tricellulin in tTJs.15,16 Because ILDR1 is expressed in diverse cell types in the cochlea, a method was devised to effectively target this varied population of cells. Instead of searching for a single AAV serotype that might simultaneously target all of these different cell types, we decided to utilize two existing synthetic AAVs that have differing tropism in the mouse inner ear to target the various cell types in the cochlea. Our previous publication reported that AAV2.7m8 can transduce the IHCs and OHCs as well as some supporting cells in the organ of Corti.26 We have also shown recently that AAV8BP2 can successfully transduce various cell types in the cochlear lateral wall, including marginal cells and intermediate cells of the stria vascularis.27 This combined AAV approach for inner ear gene delivery was first tested by injecting AAV2.7m8-GFP and AAV8BP2-GFP simultaneously into Ildr1w+/+ mice using the PSC approach. We observed a large population of GFP-positive cells in the organ of Corti and cochlear lateral wall, indicating successful viral transduction of the different cell types in the mouse cochlea (Figure 1).
We injected AAV2.7m8-Ildr1 and AAV8BP2-Ildr1 simultaneously into neonatal Ildr1w−/− mice using the PSC surgical approach. We found that combined AAV-Ildr1 gene therapy resulted in restoration of ILDR1 expression not only in tTJs of the organ of Corti but also in tTJs of stria vascularis marginal cells of Ildr1w−/− mice. The therapy appears to be durable, with ILDR1 expression still detected 2 months after injection. Restoration of ILDR1 expression resulted in improvement of TJ network organization in Ildr1w−/− mice. The combined AAV-Ildr1 gene therapy improved retention of tricellulin in tTJs, which resulted in nearly wild-type localization of tricellulin. Not only has tricellulin been shown to play an important role in maintaining the organization of tTJs, it is also critical for normal auditory function.37 Structurally, tricellulin appears to be essential for maintaining the integrity of tTJs. Tricellulin may also control the ionic permeability and movement of uncharged solutes in the paracellular space.8,38 Expression of ILDR1 in Ildr1w−/− mice after combined AAV-Ildr1 gene therapy resulted in a significant improvement in tricellulin localization because of better retention at tTJs, which improved the overall TJ network architecture. This improvement in cochlear TJ network organization in treated Ildr1w−/− mice translated into a quantifiable increase in cochlear tissue integrity in these animals. AFM was used to evaluate the integrity of the cochlear lateral wall and organ of Corti in Ildr1w−/− mice. AFM is a sensitive method for measuring tissue integrity.30,39,40 It has been applied successfully to measure stereocilium bundle stiffness and damping in the mouse inner ear.30,39,40 The cochlear lateral wall from Ildr1w−/− mice treated with combined AAV-Ildr1 gene therapy showed significant improvement in epithelial tension and epithelial viscosity compared with non-treated Ildr1w−/− mice. In fact, combined AAV-Ildr1 gene therapy improved epithelial tension in treated Ildr1w−/− mice to levels comparable with those of wild-type mice. In addition, the organ of Corti from Ildr1w−/− mice treated with combined AAV-Ildr1 gene therapy also showed significantly increased stiffness (higher Young’s modulus) compared with non-treated Ildr1w−/− mice. These results suggest that restoration of tTJs in Ildr1w−/− mice that received combined AAV-Ildr1 gene therapy translated into improved cochlear tissue integrity.
Ildr1w−/− mice that were treated with combined AAV-Ildr1 gene therapy also showed significant improvement in OHC survival compared with untreated Ildr1w−/− mice on P14. In addition, the ABR thresholds of Ildr1w−/− mice that received combined AAV-Ildr1 gene therapy treatment were significantly lower at 8 kHz compared with non-treated Ildr1w−/− mice. However, the ABR thresholds in treated Ildr1w−/− mice were still elevated compared with wild-type mice. It is likely that, for the cochlea to function normally, the TJ network needs to be completely intact because even a small amount of leakage of the high-potassium endolymph through the TJ network may be sufficient to cause deleterious effects on cochlear homeostasis and hearing function over time.41 The notion that the leakiness of the TJ network is correlated with the degree and progression of hearing loss is supported by the fact that even though Ildr1w−/− mice that were treated with either AAV2.7m8-Ildr1 or AAV8BP2-Ildr1 alone showed an initial improvement in ABR thresholds on P14, they were completely deaf by P60. Even though we observed a high level of hair cell, supporting cell, and marginal cell transduction in Ildr1w−/− mice that were treated with combined AAV-Ildr1 gene therapy (∼40%–80%), this may not be sufficient to completely restore and sustainably maintain hearing in these animals for a prolonged period of time. In addition, it has been shown that tricellulin plays a critical role in regulating paracellular transport of small uncharged molecules such as ATP, which can act as a signaling molecule.37,42,43 Increasing concentrations of ATP may have leaked into areas where ILDR1 expression was absent, increasingly inducing HC death.16,37,43 Finally, by P60, Ildr1w−/− mice treated with combined AAV-Ildr1 gene therapy still expressed ILDR1 in surviving cells. Measurable ABR thresholds were still present in 5 of the 10 treated mice, whereas no ABR thresholds were observed in all untreated Ildr1w−/− mice. These results showed that combined AAV-Ildr1 gene therapy could delay hearing loss progression in some Ildr1w−/− mice.
In this study, we demonstrate that simultaneous injection of two synthetic AAVs with different tropism in the inner ear can be used as an effective strategy to improve auditory function in Ildr1w−/− mice. This strategy allowed us to efficiently and simultaneously transduce a variety of cell types in the mammalian cochlea. Restoration of ILDR1 expression in a large percentage of targeted cell types of the organ of Corti and cochlear lateral wall resulted in an improvement in TJ network organization, tricellulin localization, OHC survival, and structural integrity of the cochlea. This study serves as a proof of concept for utilization of existing AAVs with different tropism to target various cell populations in the cochlea, which could improve inner ear gene therapy efficacy when functional rescue of heterogeneous cell types is required to restore normal hearing function.
Materials and methods
AAV vector construction
AAV2.7m8-Cmv-Ildr1-EGFP (5.82 × 1012 GCs/mL) and AAV8BP2-Cmv-Ildr1-EGFP (1.16 × 1012 GCs/mL) were produced by the Research Vector Core at the Center for Advanced Retinal and Ocular Therapeutics (University of Pennsylvania). Both synthetic vectors contained the full-length isoform of mouse Ildr1 cDNA (GenBank: NM_001285788). The virus was manufactured after triple transfection of HEK293 cells and was isolated and purified by microfluidization, filtration, cation exchange chromatography, density gradient ultracentrifugation, and diafiltration in PBS.44,45 The purified virus was passed through a 0.22-μm filter using a sterile 60-mL syringe and syringe filtered and stored frozen at −80°C in sterile tubes until use. The AAV2.7m8-CAG-eGFP (9.75 × 1012 GC/mL) and AAV8BP2-CAG-eGFP (1.10 × 1013 GC/mL) vectors were produced by the same facility using the same protocols.
Animal surgery
Animal surgery was approved by the Animal Care and Use Committee at the National Institute on Deafness and Other Communication Disorders (NIDCD ASP1378). Anesthesia in neonatal (P0–P5) pups was induced and maintained through hypothermia. Surgery was performed on the left ear of each animal using the PSC approach.46 The right ear served as a control. A post-auricular incision was created, and the tissue was dissected to expose the PSC. Care was taken to avoid the facial nerve during the procedure. A nanoliter microinjection system (Nanoliter2000; World Precision Instruments, Sarasota, FL, USA) was used in conjunction with a glass micropipette for inner ear injection. For animals that underwent combined AAV-Ildr1 treatment, 0.5 μL of AAV2.7m8-Cmv-Ildr1-EGFP (2.91 × 109 GCs) and 0.5 μL of AAV8BP2-Cmv-Ildr1-EGFP (0.58 × 109 GCs) were injected. For animals that received AAV2.7m8-Ildr1 alone, 0.5 μL of AAV2.7m8-Cmv-Ildr1-EGFP (2.91 × 109 GCs) was injected. For animals that received AAV8BP2-Ildr1 alone, 0.5 μL of AAV8BP2-Cmv-Ildr1-EGFP (0.58 × 109 GC) was injected. The incision was closed with 5-0 Vicryl sutures.
ABR and DPOAE measurements
ABR and DPOAE testing was done using the RZ6 Multi I/O Processor (Tucker-Davis Technologies, Gainesville, FL, USA) and BioSigRx v.5.1) software (Tucker-Davis Technologies). Animals were anesthetized with ketamine (100 mg/kg) and dexmedetomidine (0.5 mg/mL) via intraperitoneal injection and placed on a warming pad in a sound booth (ETS-Lindgren Acoustic Systems, Cedar Park, TX, USA). The animals’ temperature was maintained using a closed feedback loop and monitored using a rectal probe (TC-1000; SWE, Ardmore, PA, USA). DPOAEs were measured using two TDT MF-1 speakers and an ER-10B+ microphone (Etymotic, Elk Grove Village, IL, USA) inserted into the mouse’s ear using a modified pipette tip. DPOAE levels were obtained in response to two primary tones at f1 = 65 dB SPL and f2 = 55 dB SPL, with f2 varied between 4 and 44.8 kHz (5 pts/octave) and f2/f1 = 1.25. The mean noise floor was calculated from three points sampled above and three points sampled below the 2f1-f2 frequency. Output data presented in dB V were converted to dB SPL offline based on the ER-10B+ microphone’s calibration voltage (dB SPL = 20 × log([10(dB V/20)] / 0.05) + 93.9).
For ABR measurements, subdermal needle electrodes were inserted at the vertex (+) and test-ear mastoid (−) with a ground electrode under the contralateral ear. ABR thresholds were measured at 4, 8, 16, and 32 kHz using 3-ms, Blackman-gated tone pips presented at 29.9/s with alternating stimulus polarity. At each stimulus level, 512–1,024 responses were averaged. Thresholds were determined by visual inspection of the waveforms and defined at the lowest stimulus level at which any wave could be reliably detected. A minimum of two waveforms were obtained at the threshold level to ensure repeatability of the response. Physiological results were analyzed for individual frequencies and then averaged for each of these frequencies from 4–32 kHz.
Noncontact frequency-modulation AFM
Wild-type Ildr1w+/+, heterozygous Ildr1w−/+, and homozygous Ildr1w−/− mice and gene therapy-treated Ildr1w−/− mice were used. Animals of each genotype were sacrificed by CO2 asphyxia followed by decapitation according to the National Institutes of Health Guidelines for Care and Use of Laboratory Animals. The bullae were removed from mouse temporal bones and submerged in cold Leibovitz medium (L-15, 21083-027; Life Technologies). The lateral wall epithelium was microdissected from the cochleae, cut into small pieces, and plated on a glass-bottom Petri dish (HBST-5040, Willco Wells) precoated with 10 μL of Cell-Tak (354240, Corning) to immobilize it and immersed in Leibovitz medium. The lateral wall culture was incubated at 37°C and 5% CO2 for 15–30 min to achieve firm attachment of the specimen before AFM measurements. Non-contact acoustic frequency-modulation AFM (FM-AFM) experiments were performed, utilizing the Bioscope Catalyst AFM system (Bruker) mounted on an inverted Axiovert 200M optical microscope (Carl Zeiss) equipped with a confocal laser scanning microscope 510 Meta (Carl Zeiss) and using a 40× objective lens (0.95 NA, Plan-Apochromat, Carl Zeiss).30,39 During experiments, lateral wall explants were maintained at 37°C using a heating sample stage (Bruker). A modified AFM microcantilever with an attached 25-μm polystyrene bead (Novascan) was used for all FM-AFM measurements. Using the thermal tune fluctuation method built into the AFM system, the calibrated spring constant was 0.55 N/m-0.9 N/m. Next, explants were placed on the AFM sample stage and tapping mode was selected. Then, the cantilever tune curve mode was engaged, and the cantilever driving frequency was chosen to be the largest frequency peak near the cantilever natural resonance frequency (predetermined by the thermal tune method).39 After gently engaging the lateral wall tissue apical surface, the cantilever tune was launched, and the cantilever was positioned 8 μm away from the epithelium. A frequency sweep was recorded, and the cantilever phase lag was corrected to be π/2. Next, the acoustically vibrating bead was moved from an 8-μm to a 1-μm gap distance by 500-nm intervals. A frequency sweep was recorded for each interval. Supracellular apical epithelial tension and viscosity calculations were performed using a custom-made MATLAB script (MathWorks). The apical epithelial tension model based on lubrication theory for linearized Stokes flow used here is described in detail in Cartagena-Rivera et al.39
AFM nanoindentation-based mechanical mapping
The same genotypes and conditions and euthanasia and inner ear extraction procedures were used for cochlear explant culture experiments. The organ of Corti sensory epithelium was microdissected from the cochlea, the tectorial membrane was removed using a 27G needle, and the entire spiral of the organ of Corti was plated on a glass-bottom Petri dish (FuoroDish FD35-100, World Precision Instruments) precoated with 10 μL of Cell-Tak (354240, Corning) to immobilize it and immersed in Leibovitz medium. The organ of Corti culture was incubated at 37°C and 5% CO2 for 15–30 min to achieve firm attachment of the specimen before AFM measurements. A similar sample preparation procedure has been described previously.40,47
Quantitative imaging (QI) mode AFM maps were obtained using the JPK NanoWizard 4XP BioScience AFM system (Bruker) mounted on an inverted Axio Observer.7 optical microscope (Carl Zeiss) equipped with a confocal laser scanning microscope with Airyscan 2 and multiplex module (LSM 900, Carl Zeiss) and with a 40× objective (0.95 NA, Plan-Apochromat, Carl Zeiss). During QI-AFM experiments, the organ of Corti sensory epithelium explants were maintained at 37°C using a Bruker Petri dish heating stage. Explants were scanned using a probe appropriate for live-cell imaging with a tip height of 17 μm, controlled tip radius of 65 nm, and opening angle of 15° (PFQNM-LC, Bruker). The cantilever probes used had spring constant values ranging between 0.06 and 0.12 N/m and were pre-calibrated by the AFM system using the thermal tune method. Probes were replaced for each new experiment or more frequently as needed. The applied force was set to be 800 pN to 1.5 nN, yielding indentations between 300 nm and 1 μm. The force curve ramp rate was set to 120 Hz with speeds between 150 and 250 μm/s. The images were collected at a scan resolution of 128 × 128 pixels. Each tissue scan lasted ∼10 min.
The Young’s modulus (E; in Pa) maps of the cochlear explants were analyzed and extracted using the JPK/SPM data processing software (Bruker) using the Sneddon’s contact mechanics model for conical indenters, in which , where F is the applied force, α is the tip half-opening angle, and δ is the sample mean indentation. For quantitative statistical analysis of the extracted stiffness values, regions of interest (ROIs) on inner pillar cells were determined, and the values were extracted and analyzed.
Immunohistochemistry and quantification
After completion of auditory testing, mice were euthanized by CO2 asphyxiation followed by decapitation. Temporal bones were harvested and fixed in Glyoxal (Prefer) for 2 h or overnight in 4% paraformaldehyde followed by decalcification in 120 mM ethylenediaminetetraacetic acid for 4 days. The organ of Corti and portions of the lateral wall were microdissected for immunohistochemistry. Dissected tissue fixed in Glyoxal over 2 h was used to visualize ILDR1 and tricellulin expression. These tissues were blocked and labeled with rabbit anti-ILDR1 (Sigma-Aldrich) or rabbit anti-tricellulin (Life Technologies), mouse anti-ZO-1 (Life Technologies), chicken anti-GFP (Abcam), and Phalloidin-Atto 390 (Sigma-Aldrich).
Hair cell and infection quantification was completed using microdissected tissues fixed with 4% paraformaldehyde. Cochlear tissues were stained with rabbit anti-myosin 7a antibody to label hair cells (Proteus BioSciences), mouse anti-ZO-1, chicken anti-GFP, and Phalloidin-Atto 390. Primary and secondary antibodies were diluted in phosphate-buffered saline. Z stacks of images were obtained using a Zeiss LSM880 laser scanning confocal microscope with plan-Apochromat 10×, 0.45 NA and 63×, 1.4 NA objectives.
Quantification of cochlear hair cell survival was performed using two 63× images taken at the apical, middle, and basal turns of the cochlea. IHCs and OHCs were counted and averaged at each location through positive myosin 7a staining. The overall infection efficiency was determined by averaging GFP-positive cells throughout the cochlea.
Tricellulin localization was analyzed using Volocity 3D image analysis software (PerkinElmer) as described previously.16 Images of the organ of Corti were taken at 1.5× magnification using a 63× objective. Two sets of images were taken at each turn of the cochlea. Twelve tricellular points were randomly selected in each confocal z stack image, and its mislocalization was determined by comparing the ratio of ZO-1 length to tricellulin length.16
Statistics
All data shown include mean ± SEM. One-way and two-way ANOVA with post hoc test was used to compare multiple means. GraphPad Prism 9.3.0 was used to analyze and plot data. A pvalue less than 0.05 was considered significant.
Study approval
All animal procedures were approved by the animal care and use Committee at the National Institute on Deafness and Other Communication Disorders (NIDCD; ASP1378). All animal procedures were performed in compliance with the ethical guidelines and regulations set forth by the Animal Care and Use Committee at the NIDCD.
Acknowledgments
This work was supported by NIDCD Division of Intramural Research grants DC000082, DC000039, and DC000079; NIBIB Division of Intramural Research grant 1ZIAEB000094; and the NIH Distinguished Scholars Program (to A.X.C.-R.). We are grateful for Dr. Jean Bennett and Dr. Shangzhen Zhou at the University of Pennsylvania Perelman School of Medicine for manufacturing the viral vectors used in this study. We are grateful to the mouse auditory testing facility at NIDCD for providing assistance with animal auditory testing (DC000080). We are grateful to the NIDCD animal facility staff for caring for our animals. We are also grateful to Dr. Shangzhen Zhou for assistance with generating the recombinant AAVs.
Author contributions
W.C., T.B.F., I.A.B., and A.X.C.-R. conceived and designed the study. K.I., H.J.W., A.X.C.-R., K.A.F., and W.C. performed and analyzed the experiments. W.C. and I.A.B. supervised the work. M.G., T.B.F., and I.A.B. provided support for the study. K.I. and W.C. wrote the manuscript with participation of all authors.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.07.014.
Supplemental information
Data and code availability
Data will be made available upon request.
References
- 1.Morton C.C., Nance W.E. Newborn hearing screening--a silent revolution. N. Engl. J. Med. 2006;354:2151–2164. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- 2.Azaiez H., Booth K.T., Ephraim S.S., Crone B., Black-Ziegelbein E.A., Marini R.J., Shearer A.E., Sloan-Heggen C.M., Kolbe D., Casavant T., et al. Genomic Landscape and Mutational Signatures of Deafness-Associated Genes. Am. J. Hum. Genet. 2018;103:484–497. doi: 10.1016/j.ajhg.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dror A.A., Avraham K.B. Hearing impairment: a panoply of genes and functions. Neuron. 2010;68:293–308. doi: 10.1016/j.neuron.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Aijaz S., Balda M.S., Matter K. Tight junctions: molecular architecture and function. Int. Rev. Cytol. 2006;248:261–298. doi: 10.1016/s0074-7696(06)48005-0. [DOI] [PubMed] [Google Scholar]
- 5.Zihni C., Mills C., Matter K., Balda M.S. Tight junctions: from simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016;17:564–580. doi: 10.1038/nrm.2016.80. [DOI] [PubMed] [Google Scholar]
- 6.Steed E., Balda M.S., Matter K. Dynamics and functions of tight junctions. Trends Cell Biol. 2010;20:142–149. doi: 10.1016/j.tcb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Ferrary E., Sterkers O. Mechanisms of endolymph secretion. Kidney Int. Suppl. 1998;65:S98–S103. [PubMed] [Google Scholar]
- 8.Kitajiri S.I., Katsuno T. Tricellular Tight Junctions in the Inner Ear. Biomed. Res. Int. 2016;2016 doi: 10.1155/2016/6137541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glueckert R., Johnson Chacko L., Rask-Andersen H., Liu W., Handschuh S., Schrott-Fischer A. Anatomical basis of drug delivery to the inner ear. Hear. Res. 2018;368:10–27. doi: 10.1016/j.heares.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Johnstone B.M., Patuzzi R., Syka J., Syková E. Stimulus-related potassium changes in the organ of Corti of guinea-pig. J. Physiol. 1989;408:77–92. doi: 10.1113/jphysiol.1989.sp017448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitano T., Kitajiri S.-I., Nishio S.-Y., Usami S.-I. Detailed Clinical Features of Deafness Caused by a Claudin-14 Variant. Int. J. Mol. Sci. 2019;20:4579. doi: 10.3390/ijms20184579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudspeth A.J. How the ear's works work. Nature. 1989;341:397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- 13.Riazuddin S., Ahmed Z.M., Fanning A.S., Lagziel A., Kitajiri S.i., Ramzan K., Khan S.N., Chattaraj P., Friedman P.L., Anderson J.M., et al. Tricellulin is a tight-junction protein necessary for hearing. Am. J. Hum. Genet. 2006;79:1040–1051. doi: 10.1086/510022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilcox E.R., Burton Q.L., Naz S., Riazuddin S., Smith T.N., Ploplis B., Belyantseva I., Ben-Yosef T., Liburd N.A., Morell R.J., et al. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell. 2001;104:165–172. doi: 10.1016/s0092-8674(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 15.Higashi T., Tokuda S., Kitajiri S.i., Masuda S., Nakamura H., Oda Y., Furuse M. Analysis of the 'angulin' proteins LSR, ILDR1 and ILDR2--tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. J. Cell Sci. 2013;126:966–977. doi: 10.1242/jcs.116442. [DOI] [PubMed] [Google Scholar]
- 16.Morozko E.L., Nishio A., Ingham N.J., Chandra R., Fitzgerald T., Martelletti E., Borck G., Wilson E., Riordan G.P., Wangemann P., et al. ILDR1 null mice, a model of human deafness DFNB42, show structural aberrations of tricellular tight junctions and degeneration of auditory hair cells. Hum. Mol. Genet. 2015;24:609–624. doi: 10.1093/hmg/ddu474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borck G., Ur Rehman A., Lee K., Pogoda H.M., Kakar N., von Ameln S., Grillet N., Hildebrand M.S., Ahmed Z.M., Nürnberg G., et al. Loss-of-function mutations of ILDR1 cause autosomal-recessive hearing impairment DFNB42. Am. J. Hum. Genet. 2011;88:127–137. doi: 10.1016/j.ajhg.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sang Q., Li W., Xu Y., Qu R., Xu Z., Feng R., Jin L., He L., Li H., Wang L. ILDR1 deficiency causes degeneration of cochlear outer hair cells and disrupts the structure of the organ of Corti: a mouse model for human DFNB42. Biol. Open. 2015;4:411–418. doi: 10.1242/bio.201410876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lesica N.A. Why Do Hearing Aids Fail to Restore Normal Auditory Perception? Trends Neurosci. 2018;41:174–185. doi: 10.1016/j.tins.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiam N.T., Caldwell M.T., Limb C.J. What Does Music Sound Like for a Cochlear Implant User? Otol. Neurotol. 2017;38:e240–e247. doi: 10.1097/MAO.0000000000001448. [DOI] [PubMed] [Google Scholar]
- 21.Askew C., Chien W.W. Adeno-associated virus gene replacement for recessive inner ear dysfunction: Progress and challenges. Hear. Res. 2020;394 doi: 10.1016/j.heares.2020.107947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed H., Shubina-Oleinik O., Holt J.R. Emerging Gene Therapies for Genetic Hearing Loss. J. Assoc. Res. Otolaryngol. 2017;18:649–670. doi: 10.1007/s10162-017-0634-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akil O., Seal R.P., Burke K., Wang C., Alemi A., During M., Edwards R.H., Lustig L.R. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron. 2012;75:283–293. doi: 10.1016/j.neuron.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao X., Liu H., Liu H., Cai R., Wu H. Gene Therapy Restores Auditory Functions in an Adult Vglut3 Knockout Mouse Model. Hum. Gene Ther. 2022;33:729–739. doi: 10.1089/hum.2022.062. [DOI] [PubMed] [Google Scholar]
- 25.Yu Q., Li Y., Mu K., Li Z., Meng Q., Wu X., Wang Y., Li L. Virally expressed connexin26 restores gap junction function in the cochlea of conditional Gjb2 knockout mice. Gene Ther. 2014;9:71–80. doi: 10.1038/gt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isgrig K., McDougald D.S., Zhu J., Wang H.J., Bennett J., Chien W.W. AAV2.7m8 is a powerful viral vector for inner ear gene therapy. Nat. Commun. 2019;10:427. doi: 10.1038/s41467-018-08243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isgrig K., Ishibashi Y., Lee H.J., Zhu J., Grati M., Bennett J., Griffith A.J., Roux I., Chien W.W. AAV8BP2 and AAV8 transduce the mammalian cochlear lateral wall and endolymphatic sac with high efficiency. Mol. Ther. Methods Clin. Dev. 2022;26:371–383. doi: 10.1016/j.omtm.2022.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gavara N., Chadwick R.S. Noncontact microrheology at acoustic frequencies using frequency-modulated atomic force microscopy. Nat. Methods. 2010;7:650–654. doi: 10.1038/nmeth.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szarama K.B., Gavara N., Petralia R.S., Kelley M.W., Chadwick R.S. Cytoskeletal changes in actin and microtubules underlie the developing surface mechanical properties of sensory and supporting cells in the mouse cochlea. Development. 2012;139:2187–2197. doi: 10.1242/dev.073734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cartagena-Rivera A.X., Le Gal S., Richards K., Verpy E., Chadwick R.S. Cochlear outer hair cell horizontal top connectors mediate mature stereocilia bundle mechanics. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aat9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delmaghani S., El-Amraoui A. Inner Ear Gene Therapies Take Off: Current Promises and Future Challenges. J. Clin. Med. 2020;9 doi: 10.3390/jcm9072309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.György B., Meijer E.J., Ivanchenko M.V., Tenneson K., Emond F., Hanlon K.S., Indzhykulian A.A., Volak A., Karavitaki K.D., Tamvakologos P.I., et al. Gene Transfer with AAV9-PHP.B Rescues Hearing in a Mouse Model of Usher Syndrome 3A and Transduces Hair Cells in a Non-human Primate. Mol. Ther. Methods Clin. Dev. 2019;13:1–13. doi: 10.1016/j.omtm.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivanchenko M.V., Hanlon K.S., Hathaway D.M., Klein A.J., Peters C.W., Li Y., Tamvakologos P.I., Nammour J., Maguire C.A., Corey D.P. AAV-S: A versatile capsid variant for transduction of mouse and primate inner ear. Mol. Ther. Methods Clin. Dev. 2021;21:382–398. doi: 10.1016/j.omtm.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pickles J.O., Ebooks Corporation . 4th ed. Emerald Group Pub; 2012. An Introduction to the Physiology of Hearing. [Google Scholar]
- 35.Chandra R., Wang Y., Shahid R.A., Vigna S.R., Freedman N.J., Liddle R.A. Immunoglobulin-like domain containing receptor 1 mediates fat-stimulated cholecystokinin secretion. J. Clin. Invest. 2013;123:3343–3352. doi: 10.1172/JCI68587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hauge H., Patzke S., Delabie J., Aasheim H.C. Characterization of a novel immunoglobulin-like domain containing receptor. Biochem. Biophys. Res. Commun. 2004;323:970–978. doi: 10.1016/j.bbrc.2004.08.188. [DOI] [PubMed] [Google Scholar]
- 37.Nayak G., Lee S.I., Yousaf R., Edelmann S.E., Trincot C., Van Itallie C.M., Sinha G.P., Rafeeq M., Jones S.M., Belyantseva I.A., et al. Tricellulin deficiency affects tight junction architecture and cochlear hair cells. J. Clin. Invest. 2013;123:4036–4049. doi: 10.1172/JCI69031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikenouchi J., Furuse M., Furuse K., Sasaki H., Tsukita S., Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J. Cell Biol. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cartagena-Rivera A.X., Van Itallie C.M., Anderson J.M., Chadwick R.S. Apical surface supracellular mechanical properties in polarized epithelium using noninvasive acoustic force spectroscopy. Nat. Commun. 2017;8:1030. doi: 10.1038/s41467-017-01145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babahosseini H., Belyantseva I.A., Yousaf R., Tona R., Hadi S., Inagaki S., Wilson E., Kitajiri S.I., Frolenkov G.I., Friedman T.B., Cartagena-Rivera A.X. Unbalanced bidirectional radial stiffness gradients within the organ of Corti promoted by TRIOBP. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2115190119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ben-Yosef T., Belyantseva I.A., Saunders T.L., Hughes E.D., Kawamoto K., Van Itallie C.M., Beyer L.A., Halsey K., Gardner D.J., Wilcox E.R., et al. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum. Mol. Genet. 2003;12:2049–2061. doi: 10.1093/hmg/ddg210. [DOI] [PubMed] [Google Scholar]
- 42.Krug S.M., Amasheh S., Richter J.F., Milatz S., Günzel D., Westphal J.K., Huber O., Schulzke J.D., Fromm M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol. Biol. Cell. 2009;20:3713–3724. doi: 10.1091/mbc.e09-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gale J.E., Piazza V., Ciubotaru C.D., Mammano F. A mechanism for sensing noise damage in the inner ear. Curr. Biol. 2004;14:526–529. doi: 10.1016/j.cub.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Ramachandran P.S., Lee V., Wei Z., Song J.Y., Casal G., Cronin T., Willett K., Huckfeldt R., Morgan J.I.W., Aleman T.S., et al. Evaluation of Dose and Safety of AAV7m8 and AAV8BP2 in the Non-Human Primate Retina. Hum. Gene Ther. 2017;28:154–167. doi: 10.1089/hum.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennicelli J., Wright J.F., Komaromy A., Jacobs J.B., Hauck B., Zelenaia O., Mingozzi F., Hui D., Chung D., Rex T.S., et al. Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol. Ther. 2008;16:458–465. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isgrig K., Chien W.W. Surgical Methods for Inner Ear Gene Delivery in Neonatal Mouse. Methods Mol. Biol. 2019;1937:221–226. doi: 10.1007/978-1-4939-9065-8_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katsuno T., Belyantseva I.A., Cartagena-Rivera A.X., Ohta K., Crump S.M., Petralia R.S., Ono K., Tona R., Imtiaz A., Rehman A., et al. TRIOBP-5 sculpts stereocilia rootlets and stiffens supporting cells enabling hearing. JCI Insight. 2019;4 doi: 10.1172/jci.insight.128561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon request.