Abstract
Neuromyelitis optica (NMO) is an autoimmune inflammatory disease of the central nervous system (CNS) characterized by transverse myelitis and optic neuritis. The pathogenic serum IgG antibody against the aquaporin-4 (AQP4) on astrocytes triggers the activation of the complement cascade, causing astrocyte injury, followed by oligodendrocyte injury, demyelination, and neuronal loss. Complement C3 is positioned as a central player that relays upstream initiation signals to activate downstream effectors, potentially stimulating and amplifying host immune and inflammatory responses. However, whether targeting the inhibition of C3 signaling could ameliorate tissue injury, locomotor defects, and visual impairments in NMO remains to be investigated. In this study, using the targeted C3 inhibitor CR2-Crry led to a significant decrease in complement deposition and demyelination in both slice cultures and focal intracerebral injection models. Moreover, the treatment downregulated the expression of inflammatory cytokines and improved motor dysfunction in a systemic NMO mouse model. Similarly, employing serotype 2/9 adeno-associated virus (AAV2/9) to induce permanent expression of CR2-Crry resulted in a reduction in visual dysfunction by attenuating NMO-like lesions. Our findings reveal the therapeutic value of inhibiting the complement C3 signaling pathway in NMO.
Keywords: neuromyelitis optica, AQP4, astrocyte, C3 signaling, AAV2/9.CR2-Crry, neuroinflammation, motor and visual dysfunction
Graphical abstract

Tang and colleagues demonstrate that the complement C3 signaling contributes to NMO-like pathology and functional deficits in different types of NMO models. Targeting the inhibition of C3 signaling ameliorates motor and visual impairments associated with NMO.
Introduction
Neuromyelitis optica (NMO) is a neuroinflammatory demyelinating central nervous system (CNS) disease that can cause severe disability including blindness and paralysis.1 The majority of NMO patients are seropositive for autoantibodies against the water channel aquaporin-4 (AQP4), which is highly expressed on the surface of astrocytes. Accordingly, the presence of these autoantibodies is a major diagnostic criterion.2,3 The IgG antibodies bind to astrocyte AQP4 channels and activate the classical complement cascade. This leads to granulocyte, eosinophil, and lymphocyte infiltration, which subsequently trigger injury to astrocytes then oligodendrocytes. This is followed by major demyelination, neuronal loss, and neurodegeneration.3,4,5,6 Treatment for NMO typically includes immunosuppressants, B cell depletion, and plasma exchange.7 New treatments, such as aquaporumab, eculizumab, satralizumab, and sivelestat, are now under consideration for NMO.6,7 However, the optimal first-line treatment to reduce the relapse rate remains unclear. Furthermore, no drug has been shown to improve neurological disability in NMO patients.
The complement pathway is essential for immune regulation, recognition and elimination of pathogens, development, regeneration, and tissue homeostasis in the peripheral system.8,9 However, because it is an early initiator of the inflammatory response, inappropriate or unregulated complement activity can lead to many human diseases that differ in severity, location, and duration. Numerous complement proteins, receptors, and regulators are upregulated in CNS diseases. Analysis of cerebrospinal fluid, plasma, and postmortem tissues have revealed dysregulation in the complement pathway in CNS diseases such as neuroimmune diseases (e.g., NMO and multiple sclerosis [MS]) and neurodegenerative diseases (e.g., Alzheimer’s disease [AD], Parkinson’s disease, and Huntington’s disease).10,11,12
Full complement activation involves the coordinated actions of over 30 proteins that participate in 3 distinct pathways: classical, alternative, and mannose-binding lectin.13 These pathways converge on the cleavage of the central complement protein C3. Inhibition of C3 activation abrogates the formation of the C3 and C5 convertases, prevents the amplification of complement deposition on the target surface via the alternative pathway, and attenuates the generation of downstream proinflammatory effectors.13 Thus far, clinical research has demonstrated that the sole complement-specific drug, eculizumab, a therapeutic antibody that targets C5 activation, can block the generation of downstream effectors.14 C5 blockade has not only provided an effective therapeutic approach for treating diseases with complement-mediated pathophysiology, but has also revealed new pathogenic mechanisms that remain unaddressed by this clinically available anti-complement therapy. Because it is strategically positioned at the intersection of all three complement activation pathways, C3 is an ideal candidate for comprehensive complement modulation.11,13,15 Similarly, the pivotal role of C3 in coordinating crosstalk with multiple immune and inflammatory networks have galvanized effects to develop C3-based therapies. Accumulating evidence suggests that C3-targeted intervention may hold clinical promise as a viable therapeutic entity alongside anti-therapeutics.
Inhibitors affecting the key step of C3 convertase formation, cleavage, and activation have become a focus for the development of treatments that effectively modulate complement activation, regardless of the initiation pathway. The deposition of C3 activation fragments iC3b, C3dg, and C3d, which are covalently bound to cell surfaces, serves as a long-lived indicator of local complement activation. This phenomenon is amenable to imaging in living animals or ex vivo.16,17,18 Complement receptor-1-related gene/protein Y (Crry) plays a critical role in the regulation of complement activation in mice since its deletion results in complement-dependent fetal lethality.19 Crry inhibits all three complement pathways at the central C3 activation stage. The fusion protein CR2-Crry has an inhibitory effect on C3 that is 10-fold stronger than that of Crry due to the targeted binding of C3d as one of C3 cleaved fragments.18,20,21 Several studies have demonstrated that CR2-Crry could reduce inflammation by inhibiting C3 activation, thereby lessening tissue injury and dysfunction in various disease models including MS, which is also a demyelinating disease.22,23,24,25,26 Whether inhibition of C3 signaling could improve locomotor or visual function in NMO is still unclear.
Here, we first confirmed induction of C3 in NMO models in vivo and in vitro. Importantly, the C3a of C3 split products was significantly elevated in patients with NMO, and the levels of C3a had a strong correlation with clinical disability measures (Expanded Disability Status Scale [EDSS]). To investigate whether inhibition of C3 signaling could reduce the NMO-like lesions, we used mouse models of NMO, including ex vivo cerebellar slice cultures and a focal intracerebral injection model, in addition to a systemic NMO mouse model. Our findings indicate that CR2-Crry could significantly reduce complement deposition and lessen demyelination injury by reducing inflammation in organotypic slice cultures and focal passive-transfer mouse models of NMO. In addition, treatment ameliorated motor deficits in a systemic mouse model of NMO by alleviating neuron loss and inflammation. When AAV2/9.CR2-Crry was used as a gene transfer strategy, it resulted in marked improvement of the NMO eyes model. These discoveries may be highly valuable for the development of much-needed therapeutics to prevent neuroinflammatory toxicity in NMO and other similar autoimmune CNS diseases.
Results
Complement C3 is induced in primary mouse astrocytes treated with hsAQP4-IgG
Previous studies have shown that there is a significant correlation between the EDSS score and C3a protein levels or the C3a/C3 ratio.27 These correlations were as strong as the correlation between EDSS score and titers of AQP4-IgG. To further investigate the clinical relevance of C3a levels in NMO patients with myelitis, we analyzed a cohort of 45 patients with complete disease records, as well as 46 age-matched healthy controls. All patients were positive for autoantibodies against AQP4, and most were women (mean age, 38.84 ± 7.73 years; male/female = 5/40) (Table S1). We observed that C3a protein levels were significantly increased in serum samples from NMO patients as compared with healthy controls (Figures S1A and S1B). Importantly, we also found a significant positive correlation between the EDSS score and C3a protein levels in serum of NMO patients (Figure S1C). Hence, the increased expression of C3a may contribute to motor dysfunction observed in NMO patients.
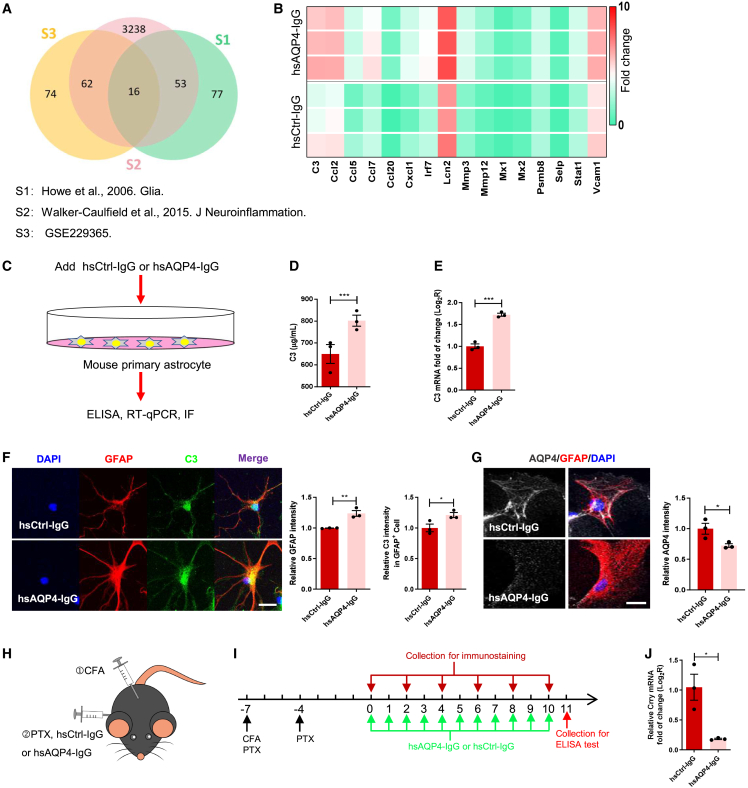
Since complement activation is one of the central pathological features of active NMO,6,11,28 we first performed RNA sequencing (RNA-seq) on primary astrocytes from mice stimulated with human anti-AQP4 autoantibodies that were purified from NMO patient plasma (hsAQP4-IgG, 100 ng/mL), in addition to human control IgG (hsCtrl-IgG, 100 ng/mL), to identify specific transcriptomic changes that may drive pathogenesis. Compared with control cells, a total of 152 genes were significantly altered by hsAQP4-IgG (Figure S1D), suggesting a strong transcriptional response to stimulation in glia. To characterize expression of key genes involved in the immune response in primary astrocytes stimulated with hsAQP4-IgG, we compared our RNA-seq dataset with that of two previous studies.29,30 We identified 16 significant differentially expressed genes that overlapped across the datasets (Figure 1A). Among these, we chose to focus on complement C3, the central component of the complement system (Figure 1B).
Figure 1.
C3 signaling was reactivated in the mouse primary astrocytes with hsAQP4-IgG treatment
(A) Venn diagrams showing the number of overlapping genes among immune response-related genes of mouse astrocytes stimulated by hsAQP4-IgG from the RNA sequencing analysis of three studies (p < 0.05, fold change >1.5). (B) Heatmap for the expression of genes in the term "immune response" in mouse primary astrocytes treated with hsAQP4-IgG compared with mouse primary astrocytes treated with hsCtrl-IgG. (C) Schematic representation of in vitro mouse primary astrocyte assay. (D) Comparison of C3 protein levels in the culture medium of mouse primary astrocytes treated with hsCtrl-IgG or hsAQP4-IgG for 24 h using ELISA. n = 3 biological replicates per group. (E) Comparison of C3 mRNA levels in mouse primary astrocytes treated with hsCtrl-IgG or hsAQP4-IgG for 24 h using qRT-PCR. n = 3 biological replicates per group. (F) Representative coimmunostaining images of DAPI (blue), GFAP (red), and C3 (green) (left) and quantification of GFAP (middle) and astrocytic C3 (right) relative immunofluorescence intensity in mouse primary astrocytes treated with hsCtrl-IgG or hsAQP4-IgG for 24 h. n = 3 slides (6 sections/slide) per group. Scale bars, 20 μm. (G) Representative coimmunostaining images of AQP4 (silver), GFAP (red), and DAPI (blue) (left) and quantification of AQP4 relative immunofluorescence intensity (right) in mouse primary astrocytes treated with hsCtrl-IgG or hsAQP4-IgG. n = 3 slides (6 sections/slide) per group. Scale bars, 20 μm. (H and I) Schematic diagram (H) and timeline (I) for experimental procedures in a systemic NMO mouse model. (J) Comparison of Crry mRNA levels in spinal cord homogenates between the two groups using qRT-PCR. n = 3 mice per group. All data except (A and B) are presented as mean ± SEM. Statistical analysis was performed with Student’s t test. Non-significant comparisons are not identified. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Recent research showed that C3 activation is implicated in the pathogenesis of NMO and may therefore represent a disease biomarker.27,28 To investigate this link in our model, we cultured primary astrocytes from neonatal mice as previously described31 and treated them with hsAQP4-IgG (100 ng/mL) or hsCtrl-IgG (100 ng/mL) for 24 h (Figure 1C). Compared with cells treated with hsCtrl-IgG, ELISA analysis showed that the levels of C3 increased significantly in supernatant of astrocytes stimulated with hsAQP4-IgG (Figure 1D). Similarly, mRNA expression of C3 was significantly upregulated in the stimulated astrocytes (Figure 1E). Moreover, immunostaining analysis showed that hsAQP4-IgG treatment caused a surge in immunoreactivity of glial fibrillary acidic protein (GFAP) and high degree of C3 protein in GFAP+ cells (Figure 1F), suggesting that C3 was mainly derived from astrocytes, which was consistent with a previous finding.32 While it decreased immunoreactivity of AQP4 compared with that in the control (Figure 1G), which was recognized as neuropathology induced by CNS application of hsAQP4-IgG without complement in rodents described as AQP4 loss and astrocytic activation (enhanced expression of GFAP and C3).28,33 Collectively, these results indicate that C3 was induced in active astrocytes via hsAQP4-IgG stimulation.
Complement C3 is highly expressed in GFAP+ cells in a systemic model of NMO
To investigate whether C3 contributes to histopathological damage of NMO in vivo, we utilized a systemic mouse model of NMO with motor impairment (Figures 1H and 1I).34 Because limb movement is mainly dependent on motor neurons in the spinal cord, we used NeuN, a neuronal nuclear marker, to assesses pathological changes in this region. We found that the number of NeuN+ neurons was gradually decreased in gray matter of hsAQP4-IgG group mice compared with that of control group mice, especially in ventral horns (Figure S2A). Besides, immunostaining analysis also revealed a marked decrease in AQP4 levels, myelin basic protein (MBP) levels (a myelin marker), and neurofilament H (NFH) levels (an axonal marker), along with a robust increase in the number of ionized calcium-binding adaptor molecule 1 (Iba1)-positive microglia in the spinal cord of mice treated with hsAQP4-IgG (Figures S2B–S2D). These results consisted with pathological characteristics of active NMO, including AQP4 loss, demyelination, and microglial activation.1 Importantly, in spinal cord cross-sections, we found that the percentage of C3+ GFAP+ astrocytes in NMO group mice was approximately 2-fold higher than that in control group mice on day 11 (Figures S2F and S2G). This indicates that numerous astrocytes were activated by hsAQP4-IgG stimulation and concurrently secreted C3 in vivo. We further examined deposition of complement by immunofluorescence staining of C5b-9 (the terminal membrane attack complex of complement). There was no significant change in the spinal cord between the two groups (data not shown), which was consistent with a previous study.28 AQP4-IgG has been shown to cause astrocytes to release a broad spectrum of proinflammatory mediators.29 Accordingly, we performed quantitative real-time PCR (qRT-PCR) analysis and found that mRNA levels of various proinflammatory cytokines in spinal cords of hsAQP4-IgG mice were significantly upregulated compared with those of hsCtrl-IgG mice (Figure S2E). In summary, C3 was highly expressed in GFAP+ astrocytes in the spinal cord with NMO-like lesions, hinting toward the role of C3 as an effector of neuronal dysfunction underlying behavioral impairment that follows IgG binding to astrocytic AQP4.
CR2-Crry improves motor dysfunction in a systemic NMO mouse model by reducing spinal cord motor neuron loss and demyelination
The fusion protein CR2-Crry has been shown to confer neuroprotection against closed head injury (CHI), traumatic brain injury (TBI), and spinal cord injury in MS through inhibiting the C3 activation step.22,23,25 Most importantly, we found Crry mRNA levels were remarkably decreased in spinal cords of mice treated with hsAQP4-IgG compared with those in mice treated with hsCtrl-IgG (Figure 1J), suggesting that endogenous C3 inhibitor Crry may be downregulated during NMO progression. To enhance recovery in a systemic NMO mouse model, we administered CR2-Crry (0.1 mg/mouse) via intraperitoneal injection once daily on the second and sixth day after IgG injection (Figures 2A and 2B). As expected, mice receiving hsAQP4-IgG and CR2-Crry exhibited better motor performance characterized by longer latency in the rotarod test and longer stride length in the gait test than mice receiving hsAQP4-IgG alone (Figures 2C and 2D). Moreover, performance in the rotarod task gradually improved when compared with baseline in mice receiving hsAQP4-IgG and CR2-Crry (Figure 2C). Consequently, CR2-Crry could prevent motor deficits in NMO mice. Similarly, the loss of spinal cord NeuN+ neurons after hsAQP4-IgG infusion were weaker in mice administered CR2-Crry (Figure 2E). In addition, while spinal cords of NMO mice displayed patchy loss of AQP4, MBP, and NFH immunofluorescence, these changes were rescued in the spinal cord of NMO mice treated with CR2-Crry (Figures 2F and 2G). Hence, CR2-Crry relieved neuronal damage, AQP4 loss, demyelination, and axonal loss in the spinal cord of NMO mice.
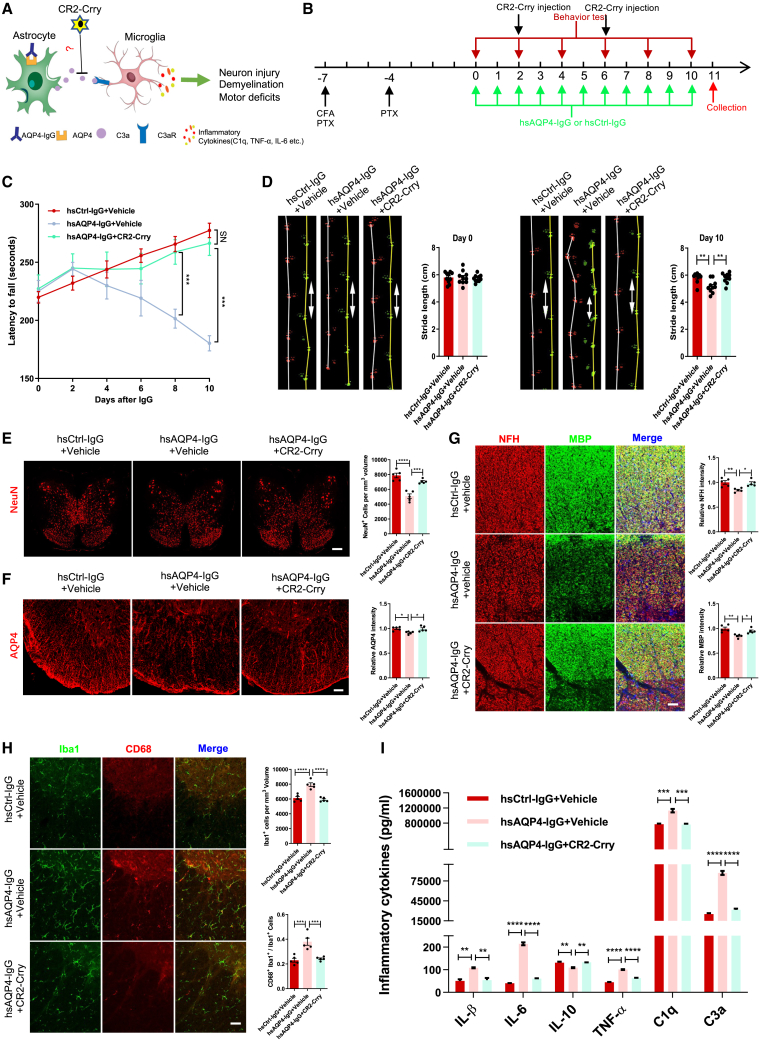
Figure 2.
CR2-Crry ameliorates locomotor deficits in NMO mouse model by reducing NMO-related lesions and inflammation
(A) Schematic diagram illustrating that CR2-Crry may prevent NMO-like lesions through targeting the inhibition of C3. (B) Timeline for experimental procedures in a systemic NMO mouse model with CR2-Crry or PBS (vehicle) treatment. (C) Quantification of latency in rotarod test for the three groups of mice. Data show that CR2-Crry treatment group mice have increased latency to fall compared with NMO group mice. n = 10 mice per group. (D) Representative images and quantification of stride length on day 0 and day 10 in gait test for the three groups of mice. Data show that CR2-Crry treatment group mice have longer stride length on day 10 compared with NMO group mice, indicating improvement in motor impairment. n = 10 mice per group. (E) Representative images (left) and quantification (right) of NeuN+ cells (per mm³) (red) in spinal cord transections of the three groups. n = 5 mice (3 sections/mouse) per group. Scale bars, 400 μm. (F) Representative images (left) and quantification (right) of AQP4 (red) relative immunofluorescence intensity in spinal cord transections of the three groups. n = 5 mice (3 sections/mouse) per group. Scale bars, 100 μm. (G) Representative images (left) and quantification of NFH (right upper, red) and MBP (right lower, green) relative immunofluorescence intensity in white matter of spinal cord transections of the three groups. n = 5 mice (3 sections/mouse) per group. Scale bars, 100 μm. (H) Representative images (left) and quantification of Iba1+ microglia (per mm³) (right upper, green) and CD68+ Iba1+ microglia percentage (right lower, red) in spinal cord transections of the three groups. n = 5 mice (3 sections/mouse) per group. Scale bars, 100 μm. (I) Quantification of six inflammatory cytokine (IL-1β, IL-6, IL-10, TNF-α, C1q, and C3a) protein levels in spinal cord homogenates among the three groups using ELISA. n = 3 mice per group. All data are presented as mean ± SEM. Statistical analysis was performed with two-way ANOVA plus a Tukey’s multiple comparisons test for (C) and one-way ANOVA and Tukey’s post hoc multiple comparisons for the others. Non-significant comparisons are not identified. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Microglial activation plays a pivotal role in NMO pathogenesis, which is regulated by C3-C3aR signaling.28 We examined microglial phagocytic activity as measured by CD68, a marker that distinguishes between resting versus activated microglia. The number of Iba1+ microglia and the percentage of CD68+ Iba1+ microglia were significantly increased in the spinal cord of NMO mice, whereas these changes were rescued in CR2-Crry-treated NMO mice (Figure 2H). This indicates that CR2-Crry could inhibit microglial proliferation and CD68 upregulation during NMO progression. Furthermore, activated microglia release proinflammatory cytokines, such as IL-1β, IL-6, TNF-α, and C1q,22,35 while inhibiting the release of anti-inflammatory cytokines such as IL-10.36 We evaluated levels of these cytokines in spinal cord homogenates of mice by ELISA. C3a protein levels were highly upregulated, in addition to significant increases in expression of IL-1β, IL-6, TNF-α, and C1q in NMO mice. In contrast, IL-10 expression was significantly decreased in NMO group mice compared with controls and CR2-Crry treatment mice (Figure 2I), suggesting that C3 has a regulatory role in inflammatory cytokine release in NMO. Taken together, these observations demonstrated that CR2-Crry inhibited activation of C3 and rescued spinal cord lesions caused by hsAQP4-IgG stimulation.
CR2-Crry inhibits NMO-like lesions in cerebellar slice cultures
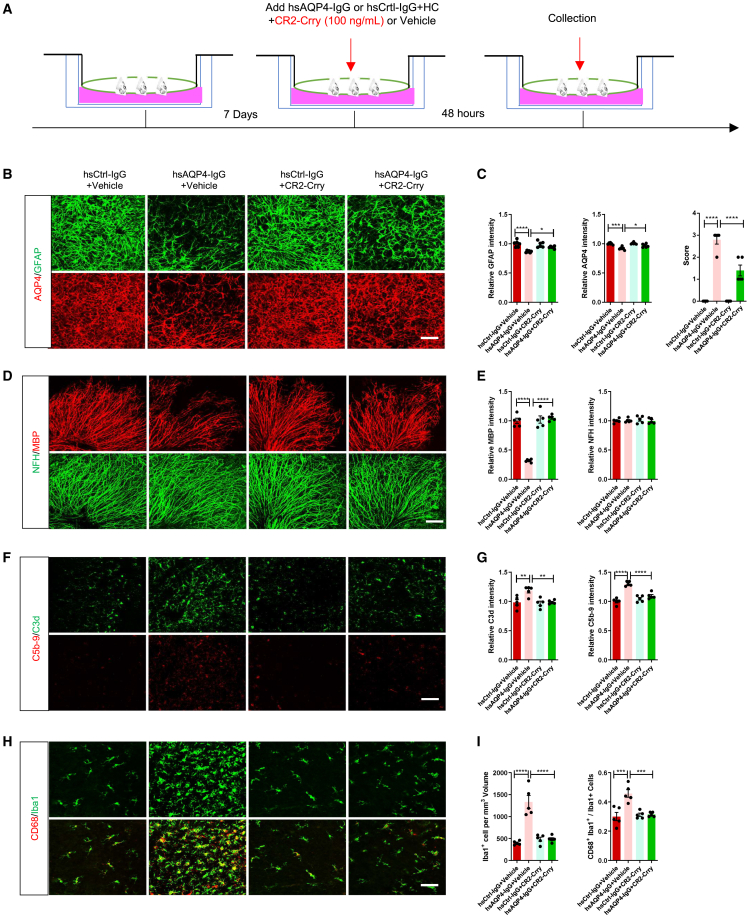
To further confirm the therapeutic effect of CR2-Crry on NMO, we tested CR2-Crry in a cerebellar slice culture model, which is commonly used to study mechanisms underlying NMO disease pathogenesis.1 As described previously,34 cerebellar slices from postnatal day 7 (P7)-day-old mice were cultured for 7 days. Next, hsAQP4-IgG or hsCtrl-IgG and human complement (HC) were added with or without CR2-Crry protein (100 ng/mL) for 48 h (Figure 3A). Consistent with other studies,1,34 we found a marked loss of AQP4, GFAP, and MBP immunoreactivity, but no change in NFH in cerebellar slices treated with hsAQP4-IgG and HC (NMO slice group), reflecting that the NMO model was successful as it showed astrocyte damage, myelin loss, and axonal preservation (Figures 3B–3E). Furthermore, abundant complement disposition was detected by C3d (C3 activation fragments) and C5b-9, in addition to a high degree of colocalization of CD68 in Iba1+ microglia in the NMO slice group, suggesting that C3 and microglia were strongly activated under hsAQP4-IgG and HC stimulation (Figures 3F–3I). However, when cerebellar slices treated with hsAQP4-IgG and HC were simultaneously incubated with CR2-Crry supplements (CR2-Crry treatment slice group), fluorescence intensity of AQP4, GFAP, and MBP were remarkably increased in comparison with NMO slices (Figures 3B–3E). Likewise, fewer activated microglia and complement disposition were observed in CR2-Crry-treated slices versus NMO slices (Figures 3F–3I). These results indicated that CR2-Crry could alleviate astrocyte injury and myelinolysis, while also reducing microglial activation and complement activation. Consequently, CR2-Crry inhibited C3 activation to reduce inflammation and therefore ameliorated NMO-like lesions in cerebellar slice cultures.
Figure 3.
CR2-Crry inhibits astrocytopathy, demyelination, and inflammation ex vivo
(A) Schematic diagram and timeline for experimental procedures of NMO cerebellar slice culture model with CR2-Crry or PBS (vehicle) treatment. (B) Representative immunostaining images of GFAP (upper, green) and AQP4 (lower, red) in cerebellar slices of the four groups. Scale bars, 100 μm. (C) Quantification of GFAP (left) and AQP4 (middle) relative immunofluorescence intensity and NMO-like lesion score (right) in cerebellar slices of the four groups. n = 5 or 6 slices (3 sections/slice) per group. (D) Representative immunostaining images of MBP (upper, red) and NFH (lower, green) in cerebellar slices of the four groups. Scale bars, 100 μm. (E) Quantification of MBP (left) and NFH (right) relative immunofluorescence intensity in cerebellar slices of the four groups. n = 5 slices (3 sections/slice) per group. (F) Representative immunostaining images of C3d (upper, green) and C5b-9 (lower, red) in cerebellar slices of the four groups. Scale bars, 100 μm. (G) Quantification of C3d (left) and C5b-9 (right) relative immunofluorescence intensity in cerebellar slices of the four groups. n = 5 slices (3 sections/slice) per group. (H) Representative immunostaining images of Iba1 (upper, green) and CD68 (lower, red) in cerebellar slices of the four groups. Scale bars, 100 μm. (I) Quantification of Iba1+ microglia (per mm³) (left) and CD68+ Iba1+ microglia percentage (right) in cerebellar slices of the four groups. n = 5 slices (3 sections/slice) per group. All data are presented as mean ± SEM. Statistical analysis was performed with one-way ANOVA and Tukey’s post hoc multiple comparisons. Non-significant comparisons are not identified. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
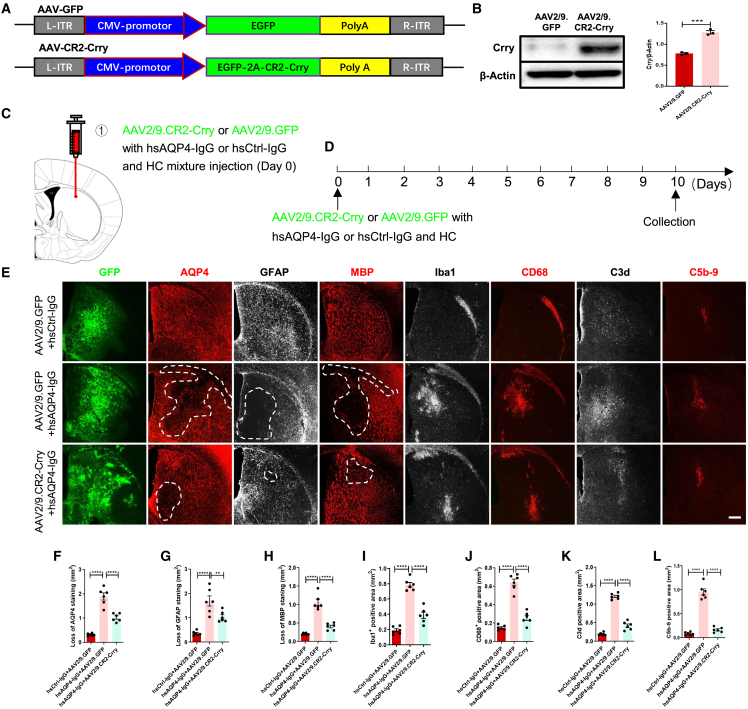
AAV2/9.CR2-Crry gene therapy limits complement deposition and demyelination in a focal intracerebral injection mouse model of NMO
Recombinant adeno-associated virus (AAV)-based treatment is commonly applied in experimental and pre-clinical gene therapy settings due to the versatility, efficacy, and permanence of these vectors in transferring genes to the local lesions.24,37 A recent study demonstrated that unilateral transduction of cortical motoneurons with an AAV-expressing hyper-IL-6 led to significant locomotor recovery of both hindlimbs after complete spinal cord crushing.38 Thus, we asked whether using AAV2/9 to deliver CR2-Crry could inhibit local C3 activation and ameliorate NMO-like lesions in a focal intracerebral injection mouse model of NMO that we described previously.34 To address this, we first constructed AAV2/9.CR2-Crry (with GFP co-expression) and verified its high efficiency of upregulating Crry protein synthesis in HEK293 cells (Figures 4A and 4B). Then, we performed bilateral, intracerebral injections of AAV2/9.CR2-Crry (or AAV2/9.GFP) with hsAQP4-IgG (or hsCtrl-IgG) and HC, and we confirmed efficient transduction by detecting GFP expression in the corpus striatum 10 days later (Figures 4C and 4D). As shown in Figures 4E–4L, we found that mice receiving AAV2/9.GFP with hsAQP4-IgG and HC (NMO group) exhibited typical manifestations of NMO that were mentioned above (Figures 3B–3I). However, these observations were significantly reversed in mice that received AAV2/9.CR2-Crry with hsAQP4-IgG and HC (CR2-Crry treatment group). In addition, we found a much higher concentration of CD45+ leukocytes in the corpus striatum of NMO mice compared with CR2-Crry treatment mice, suggesting that CR2-Crry treatment caused a reduction in granulocyte infiltration (Figures S3A and S3B). We did not detect significant differences in any NFH immunofluorescence intensity among the three groups (Figures S3A and S3C), which was consistent with our previous study,34 indicating that the axon was maintained in this model. These findings show that AAV2/9.CR2-Crry gene therapy limited NMO-like lesions in a focal intracerebral injection mouse model of NMO.
Figure 4.
AAV2/9.CR2-Crry prevents NMO-related lesions, inflammation, and complement deposition in vivo
(A) Linear map of the AAV2/9.CR2-Crry construct. ITR, inverted terminal repeat; CMV, human cytomegalovirus promoter; 2A, autocleavage site; Poly A, polyadenylation signal. (B) Representative immunoblots (left) and quantification (right) of relative Crry levels in homogenates from AAV2/9.GFP and AAV2/9.CR2-Crry transduced HEK293T cells. n = 3 biological replicates per group. (C and D) Schematic diagram (C) and timeline (D) for experimental procedures of a focal intracerebral injection mouse model of NMO with AAV2/9.CR2-Crry or AAV2/9.GFP treatment. (E) Representative immunostaining images of AQP4 (red), GFAP (silver), MBP (red), Iba1 (silver), CD68 (red), C3d (silver), and C5b-9 (red) in the injection site with GFP (green) of coronal corpus striatum sections in the three groups of mice. Areas with lesions are outlined with white dotted lines. Scale bars, 100 μm. (F–L) Quantification of the loss area of AQP4 (F), GFAP (G), and MBP (H), and the positive area of Iba1 (I), CD68 (J), C3d (K), and C5b-9 (L) in the coronal corpus striatum sections of the three groups of mice. n = 6 mice (3 sections/mouse) per group. All data are presented as mean ± SEM. Statistical analysis was performed with one-way ANOVA and Tukey’s post hoc multiple comparisons. Non-significant comparisons are not identified. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
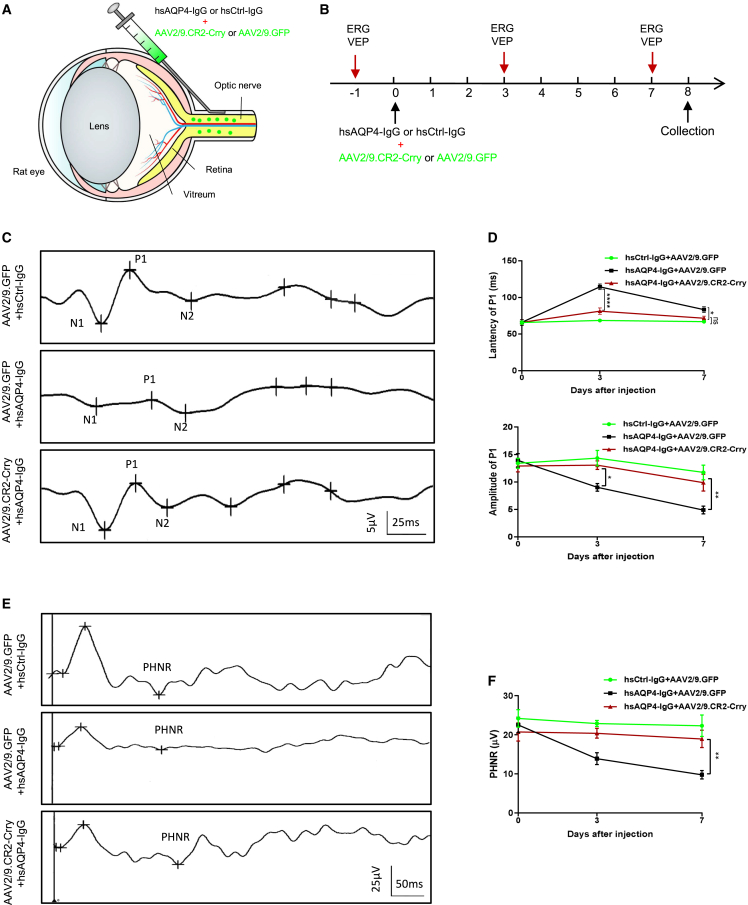
AAV2/9.CR2-Crry gene therapy ameliorates visual function deficits in an NMO optic neuritis rat model
Besides longitudinally extensive transverse myelitis resulting in motor impairment, optic neuritis leading to visual disability is the other typical manifestation of NMO.1 Recent studies have demonstrated that AAV2.CR2-Crry is a viable therapeutic strategy that can target pathogenic C3-mediated complement activation in the glaucomatous retina.24 Therefore, we speculated that AAV2/9.CR2-Crry could attenuate complement activation to ameliorate visual deficits in an NMO rat model. To test this hypothesis, a solution containing AAV2/9.CR2-Crry or AAV2/9.GFP with hsAQP4-IgG or hsCtrl-IgG was injected slowly underneath the optic nerve (ON) sheath and visual electrophysiological examinations (visual evoked potentials [VEP] and electroretinography [ERG]) were conducted before surgery, 3 days, and 7 days after operation (Figures 5A and 5B). Prolonged latency and decreased amplitude of VEP recordings reflect demyelination and axonal damage in the ON.39 VEP analysis showed that the average latency to the P1 wave increased significantly and the average amplitude of P1 decreased quickly within 7 days in NMO eyes (rat eyes receiving AAV2/9.GFP and hsAQP4-IgG mixture), while these measures remained stable in rat eyes treated with AAV2/9.CR2-Crry and hsAQP4-IgG mixture (AAV2/9.CR2-Crry group) and control group eyes (rat eyes receiving AAV2/9.GFP and hsCtrl-IgG mixture) (Figures 5C and 5D). Retinal function is evaluated using flash ERG and the PhNR amplitude is positively correlated with the functional status of retinal ganglion cells (RGCs).40 ERG analysis showed that in the NMO group, PhNR amplitude decreased gradually and there was a significant reduction in the average PhNR amplitude (51.5%) at the last test compared with that of the AAV2/9.CR2-Crry group (Figures 5E and 5F). These findings suggested that gene therapy with AAV2/9.CR2-Crry ameliorated visual dysfunction in NMO-affected eyes.
Figure 5.
AAV2/9.CR2-Crry improves visual dysfunction in NMO eye model
(A and B) Schematic diagram (A) and timeline (B) for experimental procedures of an NMO optic neuritis rat model with AAV2/9.CR2-Crry or AAV2/9.GFP treatment. (C) Representative waveforms of VEP recording on day 7 after operation. (D) Quantification of P1 latency (upper) and P1 amplitude (lower) over time for the three groups. Data show that the AAV2/9.CR2-Crry group performed better in VEP examinations compared with the NMO group (eyes receiving hs-AQP4-IgG and AAV2/9.GFP). n = 6 eyes per group. (E) Representative waveforms of ERG recording on day 7 after operation. (F) Quantification of PhNR amplitude over time for the three groups. Data show that the AAV2/9.CR2-Crry group performed better in ERG examinations compared with the NMO group. n = 6 eyes per group. All data are presented as mean ± SEM. Statistical analysis was performed with two-way ANOVA plus a Tukey’s multiple comparisons test. Non-significant comparisons are not identified. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
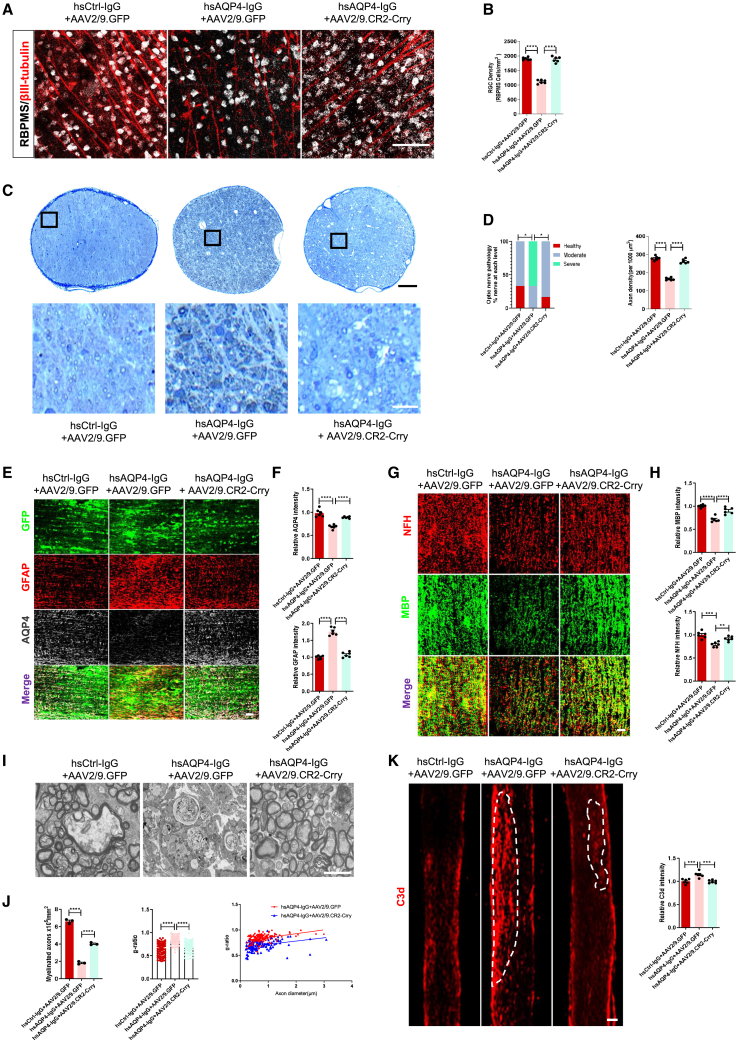
We further searched for retinal and ON alteration in the different groups. Compared with control eyes, the number of RGCs (labeled by double staining βIII tubulin and RBPMS) from whole-mount retina was significantly lower in NMO eyes (Figures 6A and 6B). Furthermore, toluidine blue staining of ON cross-sections revealed significant axonal loss in NMO eyes (Figures 6C and 6D). With AAV2/9.CR2-Crry gene therapy, more surviving RGCs and intact axons were detected in NMO eyes (Figures 6A–6D), highlighting its protective effect in this model.
Figure 6.
AAV2/9.CR2-Crry prevents RGC loss, axon damage, and NMO-like lesions in NMO eye model
(A and B) Representative immunostaining images (A) and quantification (B) of RBPMS (silver) and βIII tubulin (red) in the rat flat-mount retina of the three groups’ eyes. n = 6 eyes (12 sections/eye) per group. Scale bars, 100 μm. (C) Representative images of ON semithin transections showing axonal density by toluidine blue staining. Insets at higher magnification show that intact axons were recognizable by a clear axoplasm and intact myelin sheath, whereas dystrophic axons were detectable by a dark axoplasm and/or thick, delaminated myelin sheath. Scale bars, 100 μm (black) and 20 μm (white). (D) Quantification of the distribution of rat optic nerve damage degree based on myelin sheath damage: healthy (red), moderate (blue), and severe (cyan) (left). Quantification of axon density in optic nerves of the three groups (right). n = 6 eyes (3 sections/eye) per group. (E) Representative immunostaining images of GFAP (red), AQP4 (silver) in the injection site with GFP (green) in longitudinal sections of optic nerves of the three groups. Scale bars, 100 μm. (F) Quantification of AQP4 (upper) and GFAP (lower) relative immunofluorescence intensity in optic nerves of the three groups. n = 6 eyes (3 sections/eye) per group. (G) Representative immunostaining images of MBP (green) and NFH (red) in the injection site in longitudinal sections of optic nerves of the three groups. Scale bars, 100 μm. (H) Quantification of MBP (upper) and NFH (lower) relative immunofluorescence intensity in optic nerves of the three groups. n = 6 eyes (3 sections/eye) per group. (I) Representative electron microscopy (EM) images of the optic nerve from the three groups of rats. Scale bars, 2 μm. (J) Quantification of myelinated axons (left) and G-ratio (ratio between axon and fiber diameters, middle) in transverse sections of optic nerves of the three groups. Scatterplot showing individual G-ratio values and axonal size distribution (right). G-ratio manually calculated on 150 axons per group. n = 3 eyes per group. (K) Representative immunostaining images (left) and quantification (right) of C3d relative immunofluorescence intensity in optic nerves of the three groups. n = 6 eyes (3 sections/eye) per group. Scale bars, 200 μm. All data except (D, left and J, right) are presented as mean ± SEM. Statistical analysis was performed with Kruskal-Wallis test for (D left), scatterplot for (J, right) and one-way ANOVA and Tukey’s post hoc multiple comparisons for the others. Non-significant comparisons are not identified. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
On the other hand, evaluation of AQP4, GFAP, MBP, and NFH staining intensity in the ONs indicated significant decreases in NMO eyes compared with those of control eyes and the AAV2/9.CR2-Crry eyes (Figures 6E–6H). This suggests that AAV2/9.CR2-Crry therapy prevented ON stimulated by hsAQP4-IgG from astrocyte damage, myelin disorganization, and axonal loss. In addition, electron microscopy analysis revealed the myelin sheaths in NMO eyes displayed remarkable less myelinated axons and a higher G-ratio than those of AAV2/9.CR2-Crry treatment group eyes (Figures 6I and 6J), indicating a great reduction in the myelin thickness of ON by hsAQP4-IgG stimulation.
Remarkably, C3 activation resulted in significantly greater C3d deposition in ONs in the NMO group compared with the AAV2/9.CR2-Crry group (Figure 6K), and this was accompanied by significant elevation in microglial (Figure S4A) and C5b-9 staining (Figure S4B), indicating that AAV2/9.CR2-Crry decreased activation of C3 to inhibit microglial activation and reduce complement deposition. These data suggest that stable expression of CR2-Crry via AAV gene therapy could be a viable approach to attenuate NMO-like lesions in ONs during disease progression.
Discussion
NMO is considered a complement-mediated astrocytopathy disease. Our study and others have demonstrated that hsAQP4-IgG can elicit significant production of C3 by astrocytes.28,41 In patients, we found a significant correlation between the levels of C3a and the EDSS score. We provide clinical, serological, and immunohistochemical data suggesting that C3 activation is implicated in the pathogenesis of NMO and might represent a biomarker of disease activity. Importantly, our findings support the potential of AAV2/9.CR2-Crry as an effective gene therapy to locally correct pathogenic C3 overactivation and restrict onset and progression of NMO, especially for optic neuritis. In summary, our findings show that inhibition of C3 signaling is sufficient to rescue functional impairments in NMO. These findings advance our knowledge of astrocytic C3 signaling changes in NMO-like pathology and functional deficits in different types of NMO models.
Abundant evidence supports a pathogenic role for complement activation following AQP4-IgG binding to AQP4, which leads to inflammation and demyelination.11 We found that C3 expression was significantly elevated in astrocytes with hsAQP4-IgG stimulation both in vitro and in vivo, as previous studies have also reported.28,42 A hallmark histopathological feature of NMO lesions is astrocytopathy characterized by prominent loss of AQP4 and GFAP.41 Interestingly, the protein levels of AQP4 decreased while those of GFAP and C3 were significantly increased in vitro and in vivo NMO models without HC supplement, which is consistent with recent research reports.28 These results confirm that, without HC’s assistant effect, NMO models exhibit earlier pathological stage of NMO, presenting as astrocytic activation (enhanced expression both of GFAP and C3) and AQP4 downregulation.28,33 It has been demonstrated that the organotypic slice model and focal intracerebral injection model of NMO did not exhibit typical NMO-like lesions without HC supplement.43,44 In line with this, we observed a striking loss of GFAP in lesions of two NMO models, which is inconsistent with the previous results without HC stimulation in vitro and in vivo, since the complex factors in HC may modulate lesion severity.43 In addition, astrocytes were exposed to a high titer of local antibodies in these two different models therefore astrocytes may tend to skip the cell activation phase and exhibit cell damage. However, in NMO models with HC supplement, there is abundant C3d deposition in NMO-like lesions, indicating intense C3 activation. Hence, we speculate that C3 activation is involved in the whole pathological process of NMO, while the pathological manifestations of astrocytes in NMO-like lesions are affected by the concentration of AQP4-IgG and the supplement of HC. On the other hand, ex vivo and in vivo NMO models exhibited progressive AQP4 loss, demyelination, and microglial activation. In addition, mice receiving hsAQP4-IgG exhibited worse motor performance. These active NMO-like lesions and behavioral deficits were in accordance with other studies.28,35,43,44
CR2-Crry has been shown to be effective in ameliorating nervous system pathology across a spectrum of disease models, such as CHI, TBI, and MS.22,23,25,26 As expected, CR2-Crry treatment reduced spinal cord NMO-like lesions and improved motor dysfunction, which is similar to the effect observed in C3 gene knockout in mice in a previous study.28 Viral gene delivery is widely used in experimental and pre-clinical tests, especially AAV2 and AAV9 which have affinity for the nervous system.24,37,38,45 AAV2/9.CR2-Crry gene therapy alleviated NMO-like lesions of intracerebral injection NMO mouse model. We also found that mice receiving AAV2/9.CR2-Crry treatment had a smaller CD45+ area, suggesting that AAV2/9.CR2-Crry could also prevent neuroinflammation by reducing granulocyte infiltration in NMO progression.11 In fact, AAV gene therapy is more widely used in neurodegenerative diseases that elicit ophthalmological defects.24,37 Moreover, NMO optic neuritis is the other core symptom commonly leading to severe visual deficits.5,6 We transferred AAV2/9.CR2-Crry into the ON sheath of Sprague-Dawley (SD) rats with hsAQP4-IgG as reported previously.46,47 Without HC supplement, expression of both GFAP and C3d significantly increase, representing intense astroglial and C3 activation in NMO eyes at 7 days after injection, which is consistent with recent studies.47 However, other studies have shown a reduction of GFAP immunoreactivity in NMO eyes, in the context of NMO serum injection (containing HC) after 21 days, whereas GFAP+ astrocytes are overactivated surrounding the GFAP loss lesions.46 In addition to a difference in the components used for stimulation, we speculate that the duration is also a crucial factor affecting pathology changes. Previous studies indicate that the disease process of NMO can be initiated by nonspecific astrocytic activation, followed by astrocytic injury, and finally astrocytic apoptosis.48 These pathological states are not independent of each other, as they can exist simultaneously. Thus, after hsAQP4-IgG combining to the endfeet of astrocytes, most astrocytes may be activated within a short period of 7 days in our study, whereas the majority develop cell injury and apoptosis and only a few astrocytes remain in activation over time after injection in the study by Zhang et al.46 Overall, our findings show that the delivery of CR2-Crry via AAV2/9 induces sustained Crry overexpression to regulating local pathogenic C3 overactivation, which provides a potential neuroprotective effect on both retina and ON pathology to ameliorate visual dysfunction in NMO, similar to observations in other ophthalmic disease models.24,26,46,47
Recently, research has highlighted the vital role of C3 in astrocyte-microglia crosstalk in neuroinflammation of NMO, MS, AD, and perioperative neurocognitive disorders, which results in microglial activation.28,49,50 Consistent with a previous study,28 our findings show that inhibiting C3 by CR2-Crry administration can reduce microglial activation in various NMO models. Previous studies have proposed that microglial activation is secondary to C3aR signaling after binding to C3a, and that active C3-C3aR signaling plays a crucial role in the pathogenesis of demyelination and neurodegeneration by regulating the complement alternative pathway.16,28,51 In addition, activated M1 microglia (proinflammatory microglia) could release proinflammatory cytokines such as IL-1β, IL-6, C1q, and TNF-α.28,52,53 Consistent with previous studies, CR2-Crry in our study could reduce proinflammatory cytokine release in spinal cord homogenates of NMO mice.29,54 This indicates that targeting C3 signaling could block the downstream inflammatory response in NMO through prevent microglial activation.
Importantly, the clinical translation of our discoveries would be relatively simple given the complex protective roles of focusing on C3 signaling. We demonstrated that the complement C3 contributes to motor and visual impairments after hsAQP4-IgG stimulation. Our study would provide a new therapeutic strategy by targeting C3 inhibition to improve NMO-like lesions and dysfunction due to local or systematic inflammatory reactions. Notably, to our knowledge, we are the first to use AAV gene therapy as a treatment for optic neuritis associated with NMO, which may be therapeutically efficacious for ameliorating NMO attacks. There has been an evolving interest in developing therapeutic strategies aimed to reduce complement C3 activation in pathological conditions involving locomotor and cognition impairments, such as MS and AD. In the context of our findings, this interest is further underscored and warranted. We anticipate that the impact of developing a therapy targeting C3 signaling to improve motor and visual impairments will be far-reaching.
Materials and methods
Animals
Wild-type C57BL/6 mice and SD rats were purchased from Vital River and housed in the Guangdong Laboratory Animals Monitoring Institute on a 12-h light/dark cycle and given free access to food and water. We used 8-week-old female mice and female rats weighing between 230 and 250 g for all experiments. All mouse and rat husbandry and experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University (no. SYSU-IACUC-2023-B0123).
Primary astrocyte isolation and culture
Primary astrocyte isolation and culture were performed and modified based on a previous study.31 Whole brains were obtained from mouse pups at P0. After removal of the meninges and bilateral hippocampi, the brain was washed with Dulbecco’s modification of Eagle’s medium-12 (DMEM-F12) (Gibco, C11330500BT) supplemented with 10% fetal bovine serum (FBS) PANTM SERATECH, ST30-3302) and 1% penicillin-streptomycin (PS) (Gibco, 15140-122). Brains were transferred to 0.25% trypsin EDTA for 20 min and DMEM-F12 complete medium was used to stop the trypsinization. Cell debris and aggregates were removed by passing the single-cell suspension through a 40-μm nylon mesh. After 5 min of centrifugation at 1,000 × g, the single-cell suspension was collected and cultured with astrocyte medium in T-75 flasks for 14 days, during which medium was replaced every 3 days. The astrocyte medium contained the following: DMEM-F12 with 10% FBS, 1% non-essential amino acid, 1% PS, 1% glutamine (Gibco, 25030-081), and 1 mM sodium pyruvate (Sigma, S8636). Characterization of the astrocytes was done by immunofluorescence using GFAP (1:1,000, CST, 12389S) as a biomarker.
Cell lines
Human embryonic kidney (HEK293T) cells (ATCC, CRL-11268) were maintained at 37°C in a 5% CO2 atmosphere in DMEM (Wisent Bioproducts, 319-005-CL) containing 10% FBS, and 1% PS.
IgG purification
IgG was purified from the plasma of seropositive NMO patients who received plasmapheresis and from healthy volunteers. All participants provided their consent and this procedure was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University (no. 02-405-01). The specific purification method was consistent with our previous studies.34 In brief, total IgG was purified using protein A beads (GE Healthcare, 71149800-EG). The beads were eluted with 0.1 mol/L glycine-HCl (pH 2.5) and the eluent was then concentrated using Amicon Ultra15 centrifugal filter units (100 kDa, Millipore, Billerica, MA). The concentrated IgG was sterilely filtered through 0.22 μm and working liquids were stored at −80°C. The purified IgG samples were termed hsAQP4-IgG (20.0–25.5 mg/mL) and hsCtrl-IgG (21.5–27.0 mg/mL). HC was purchased from Innovative Research (Novil, MI).
Organotypic cerebellar slice cultures
Organotypic cerebellar slice cultures were prepared according to previous studies.34,55 In brief, P7 mouse pups were decapitated, and the whole cerebellum was rapidly removed and placed in ice-cold Hank’s balanced salt solution (HBSS) (pH 7.2, Invitrogen). Transverse sections of the cerebellum were sliced at a thickness of 350 μm using the McIlwain Tissue Chopper (Cavey Laboratory Engineering, England). Each slice was placed on transparent, non-coated membrane inserts in 6-well plates (Corning 3450 Transwell, 0.4 μm pores) containing 1 mL of culture medium. The culture medium consisted of 50% minimum essential medium, 25% HBSS, 25% horse serum, 200 mg/mL D-glucose, 2 mM glutamine, and 1% PS. Slices were cultured in 5% CO2 at 37°C for 7 days and the medium was changed every other day. A mixture of hsAQP4-IgG or hsCtrl-IgG, HC and CR2-Crry, or phosphate-buffered saline (PBS) as vehicle was added to the culture medium on day 7 for 48 h and then slices were fixed in 4% paraformaldehyde (PFA) for immunostaining. NMO-like lesions of cerebellar slices were scored as follows: 0, intact slice with normal GFAP and AQP4 staining; 1, mild astrocyte swelling and/or reduced AQP4 staining; 2, at least one lesion with loss of GFAP and AQP4 staining; 3, multiple lesions affecting >30% of slice area; and 4, lesions affecting >80% of slice area.43
Focal intracerebral injection
The procedure for the focal intracerebral injection mouse model of NMO has been described in our previous work.34 In brief, mice were anesthetized via inhalation of isoflurane and mounted in a stereotaxic frame (RWD Life Science, Shenzhen, China). A midline scalp incision was made, and a burr hole (1 mm diameter) was drilled on each side of the skull 0.5 mm anterior and 2 mm lateral to bregma using a high-speed drill (RWD Life Science). A 26-gauge needle attached to a 10-μL gas-tight glass syringe (Hamilton) was inserted 3 mm deep to infuse a mixture (hsAQP4-IgG or hsCtrl-IgG, HC, and AAVs) in a total volume of 6 μL over 6 min by pressure injection (KDS/USA, Legato 130). After injection, the glass pipette remained in place for 10 min and was then slowly withdrawn to prevent leaking. Mice were then allowed to recover in a heated chamber and were ambulating normally after awakening from anesthesia.
NMO-related optic neuritis rat model
The NMO-related optic neuritis rat model was established according to previous reports.46 In brief, rats were anesthetized via inhalation of isoflurane before surgery. Then, the ON was exposed from the superior conjunctiva by blunt dissection. At about 2 mm posterior to the globe, a 33-gauge needle attached to a 10-μL gas-tight glass syringe (Hamilton) was inserted into the ON sheath without touching the ON. A total of 8 μL of solution (hsAQP4-IgG or hsCtrl-IgG and AAVs) was injected in each side slowly underneath the ON sheath for 2 min to avoid excessive force and minimize damage to the ON. After injection, the needle was kept underneath the sheath for 1 min to prevent leakage upon withdrawal. Preservation of the retinal circulation was confirmed by checking the color of the retinal blood vessels, and antibiotic ointment was applied.
NMO systemic mouse model
The NMO systemic mouse model was established according to the procedures in our previous study.34 Mice were anesthetized through inhalation of isoflurane and received subcutaneous injections of complete Freund’s adjuvant (CFA) (Sigma-Aldrich) containing heat-killed H37Ra Mycobacterium tuberculosis (BD Difco) at four sites (50 μg in 50 μL of CFA per site) on the hind flank once 7 days before IgG transfer. Animals were subsequently intraperitoneally injected with pertussis toxin (200 ng in 0.2 mL of ddH2O, Enzo Life Science) 7 and 4 days before IgG administration to disrupt the blood-brain barrier (BBB). Finally, mice were randomly assigned to different subgroups following BBB disruption and received intraperitoneal injection of hsAQP4-IgG or hsCtrl-IgG for 10 days (4.0 mg IgG in 0.2 mL PBS daily).
CR2-Crry protein treatment
Recombinant complement inhibitor CR2-Crry was prepared as described previously21 and the final drug concentration was 1 mg/mL. In experiments on the NMO systemic mouse model, each mouse was treated via intraperitoneal injection at a dose of 0.1 mg CR2-Crry in PBS once daily on days 2 and 6 after hsAQP4-IgG administration. In organotypic cerebellar slice culture experiments, we added CR2-Crry at a dose of 100 ng with hsAQP4-IgG or hsCtrl-IgG at a concentration of 1 mg/mL and 10% HC simultaneously and slices were subsequently incubated for 48 h.
Treatment with AAV expressing CR2-Crry
AAV2/9-CMV-EGFP and AAV2/9-CMV-CR2-Crry-P2A-EGFP viruses were composed and purchased from Obio Technology (Shanghai, China). In experiments on the focal intracerebral injection mouse model of NMO, each mouse received an intracerebral injection with a total of 6 μL solution containing 1 μL of AAV2/9.CR2-Crry or AAV2/9.GFP, 3 μL of hsAQP4-IgG or hsCtrl-IgG, and 2 μL of HC. Mice were sacrificed 10 days after the surgery. In experiments on NMO-related optic neuritis the rat model, a total of 8 μL of solution containing 6 μL of hsAQP4-IgG or hsCtrl-IgG and 2 μL of AAV2/9.CR2-Crry or AAV2/9.GFP was injected slowly underneath the ON sheath on each side. Rats were sacrificed 8 days after surgery.
Motor behavior analyses
A systemic mouse model of NMO with motor impairment was established as described previously.34,35 Behavioral tests were performed on days 0, 2, 4, 6, 8, and 10 following IgG systemic transfer. The rotarod task was performed using a six-lane apparatus. Mice needed to maintain balance and walk on a cylinder that started rotating at 10 rounds per min and uniformly accelerated over a 5-min period to reach 40 rounds per min. Each mouse was tested three times with 15-min intervals. The time of latency to fall was calculated as the average of three times. In the gait task, mice walked freely across a narrow strip, and performance was analyzed using the CatWalk-assisted gait analysis system (XR-FP101, Shxinruan Corporation, China). The length of the hindlimb stride was calculated as the median of five sequential steps.
Electrophysiology assays
We assessed the visual function of rats with NMO-related optic neuritis through flash VEP and ERG 1 day before surgery and on day 3 and day 7 after injection. VEP examination was performed as reported previously.46 In brief, with a stereotaxic instrument, positive electrodes (stainless steel screws) were implanted in the skull above the visual cortex area 7 mm behind the bregma and 3 mm lateral of the midline. Reference electrodes were implanted in the area of the midline 3 mm anterior to the bregma, as described previously.39 A needle electrode was inserted into the tail as the ground electrode. The electrodes were covered with dental cement for fixation. Antibiotics were administered topically after closing the skin on the skull. During VEP recording, rats were dark adapted for 2 h and then anesthetized with 2% pentobarbital sodium in a dark room illuminated with dim red light. The pupils were dilated with one drop of compound tropicamide (0.5% tropicamide and 0.5% phenylephrine hydrochloride, Xing Qi Ophthalmic, Shenyang, China) and artificial tears were intermittently applied to the corneas to prevent corneal dryness. The skin over the skull was opened and the screws were connected to the amplifier. Visual stimuli (9.49 cd·s/m2) consisting of 1 Hz white light were generated by a full-field Ganzfeld stimulator (Roland Consult, Brandenburg, Germany) under a dark-adapted condition. A stimulus of 100 flashes was repeated three times with 5-min intervals. The results for each eye were recorded individually. The contralateral eye was occluded by a homemade black blinder. After recording, the wound was closed, and antibiotics were administered. Signals were bandpass filtered between 0.5 and 50 Hz. All data were measured using Roland software (Roland Consult). Latency of the P1 wave was measured from the time of light onset to the peak of the first positive wave (P1), and the amplitude of P1 was measured from the trough of the first negative peak after light onset (N1) to the peak of P1. Each eye was measured three times to obtain an average value.
For ERG examination, an ERG recording system (Roland Consult) was used to induce retinal potential in rats by flashing light stimulation. Before the experiment, rats were prepared for ERG recording using overnight dark adaption. They were then anesthetized with 2% pentobarbital sodium, and the pupils were dilated and lubricated with 1% methylcellulose. Two positive electrodes were gently placed on the corneal surface of the eye and two reference electrodes were inserted into the hypodermis between the ears. A needle electrode was inserted into the tail as the ground electrode. Photopic light adaption with a white background of 25 cd·s/m2 was applied for 10 min to suppress rod-cell photosensitivity, and the photopic negative response (PhNR) was recorded using white flashes of 10 cd·s/m2. The stimulus frequency was 0.03 Hz. The results for each eye were recorded individually, and the contralateral eye was occluded using a homemade black blinder. The statistical indicator was the amplitude of PhNR, which was defined as the amplitude of the largest negative wave after baseline to the b wave. All statistical values were represented as absolute values.
Immunofluorescence staining
At the indicated time points, cerebellar slices were washed with PBS three times and then fixed in 4% PFA for 1 h at room temperature (RT). Mice were euthanized by intraperitoneal injection of 2% pentobarbital sodium, and transcardially perfused with PBS followed by 4% PFA. Brains and spinal cords were dissected and post-fixed in 4% PFA at 4°C overnight, and then balanced in 30% sucrose until submerged. Brains and spinal cords were cut into 40-μm-thick coronal sections and transversal sections, respectively, using a microtome (Thermo Fisher Scientific) and placed in a 96-well plate. The slices were kept in cryoprotectant solutions (glycerol, ethylene glycol, and 0.1 M phosphate buffer [pH 7.4], 1:1:2 by volume) at 4°C for further experiments. Rats were deeply anesthetized and intracardially perfused with 0.9% normal saline, followed by 4% PFA in 0.01 M PBS. Segments of ON were extracted from the ON head to the optic chiasm and post-fixed for 1 h at 4°C. After several washes with PBS, the ON samples were dehydrated in 0.01 M PBS containing 15% and 30% sucrose. Then, samples were embedded in optimal cutting temperature compound (Tissue Tek, Torrance, CA) and cryosectioned using a Leica microtome (Leica Microsystems, Buenos Aires, Argentina) longitudinally and at a thickness of 10 μm. All ON sections were mounted on glass slides and stored at −80°C for future procedures.
For immunofluorescence staining, cerebellar slices were blocked with 0.5% Triton X-100 for 30 min followed by blocking buffer containing PBS with 3% donkey serum or goat serum (Sigma-Aldrich, D9663) at RT for 1 h and then incubated with primary antibodies overnight at 4°C. Brain, spinal cord, and ON sections were blocked with 300 μL/10 mL goat serum or donkey serum and 250 μL/10 mL 10% Triton X-100 for 1 h at RT and then incubated with primary antibodies overnight at 4°C. Samples were washed with PBS three times and were then incubated with secondary antibody at RT for 1 h. The nuclei were stained with 4′,6-dimethyl-2′-phenylindole dihydrochloride (Sigma Aldrich, no. b2261). Each set of immunofluorescence experiments were repeated at least three times. The primary antibodies used were: rabbit anti-AQP4 (1:1,000, Santa Cruz Biotechnology), mouse anti-GFAP (1:1,000, CST), rabbit anti-C3 (1:200, Thermo Fisher Scientific), rabbit anti-Iba1 (1:1,000, Wako Chemicals), rat anti-CD68 (1:1,000, Abcam), goat anti C3d (1:300, R&D), rabbit anti-C5b-9 (1:500, Abcam), rabbit anti-MBP (1:500, CST), chicken anti-NFH (1:400, Millipore), mouse anti-NeuN (1:500, Abcam), and rabbit anti-CD45 (1:500, CST). The following fluorescent secondary antibodies were used: goat anti-mouse 488 (1:1,000, Invitrogen), goat anti-mouse 568 (1:1,000, Invitrogen), goat anti-chicken 488 (1:1,000, Invitrogen), goat anti-chicken 647 (1:1,000, Invitrogen), goat anti-rabbit 488 (1:1,000, Invitrogen), goat anti-rabbit 568 (1:1,000, Invitrogen), goat anti-rabbit 647 (1:1,000, Invitrogen), donkey anti-goat 488 (1:1,000, Invitrogen), donkey anti-goat 647 (1:1,000, Invitrogen), and donkey anti-rat 568 (1:1,000, Invitrogen). Except original brain section, images were captured at 10× magnification by an upright fluorescent microscope (Nikon, Japan, Eclipse E100), confocal single-plane images and z stacks were captured at 10× and 20× magnification using a laser confocal microscope (Leica, Germany, TCS SP8) for the other samples.
Immunofluorescence image analysis
For the brain images, we used the loss area of GFAP, AQP4, MBP, and NFH, and the positive area of C3d and C5b-9, for comparison among different groups of mice in the focal intracerebral injection mouse model of NMO. Because microglial activation in the corpus callosum of the focal intracerebral injection mouse model of NMO often manifested as large aggregations in sheets and it was difficult to distinguish individual cells, we also used both Iba1+ and CD68+areas to compare among different groups as in our previous study.34 For images of the other samples from different models, we used relative fluorescence intensities of GFAP, AQP4, C3, MBP, NFH, C3d, C5b-9, and CD45, and the number of NeuN+ neurons and Iba1+ microglia per cubic millimeter volume for intergroup comparison. Besides, we used C3+ GFAP+ astrocytes percentage and CD68+ Iba1+ microglia percentage representing astrocytic activation and microglial activation, respectively, to find differences among different groups.
Counting of RGCs
Rats were sacrificed by overdose anesthesia and the eyes were enucleated to assess the RGCs as described in our previous study.46 Whole-mount retinas were fixed in 4% PFA in 0.01 M PBS for 1 h. They were then washed and placed in CAS-Block blocking buffer (Life Technologies, 00-8120) with 3% Triton X-100 for 1 h and then incubated in rabbit anti-RBPMS (1:500, Santa Cruz Biotechnology) and mouse anti-βIII tubulin (1:1,000, Promega) for 48 h at 4°C on a shaker. After several washes, retinas were incubated with secondary antibodies donkey anti-rabbit 647 (1:1,000, Invitrogen) and donkey anti-mouse 568 (1:1,000, Invitrogen) for 2 h at RT. After several washes, retinas were flat-mounted on glass slides and cover slipped. Survival of RGCs was assessed by counting the number of RBPMS+ cells in the flat whole-mount retina using a laser confocal microscope (Leica, TCS SP8). We divided each retina into four quadrants, and for each quadrant we acquired three images of 0.06 mm2 that were separated by 1 mm, which represented the peripheral, medial, and central parts of the retina. Thus, the average of 12 images were sampled for each retina.
For RGC axonal counts, ONs were processed as previously described.56 In brief, ONs were removed and post-fixed in ice-cold 5% glutaraldehyde in 0.1 M PBS for 48 h at 4°C, immersed in 1% osmium tetroxide (pH 7.4) for 3 h, dehydrated in ascending grades of ethanol (75%, 95%, and 100%), and finally embedded in epoxy resin. Semi-thin (1 μm) ON coronal sections were collected using an ultramicrotome (Leica), dried on slides, and stained with 1% toluidine blue (Sigma-Aldrich). Imaging was performed on 3 to 5 cryosections (2–3 mm distal from the eye globe) per biological sample. Images were captured at 10× and 63× magnification using a bright-field microscope (Nikon, Eclipse E100). Axonal survival was assessed by automated axonal count using ImageJ (National Institutes of Health) and recorded as axon density (the number of axons per 1,000 μm2). In addition, whole images were graded by three independent investigators on a scale of 1–5 and then further classified as healthy, moderate, or severe damaged as previously described.57 Healthy ONs (grade 1) had few degenerating axons. Moderate damage (grades 2 and 3) was characterized by numerous degenerating and swelling axons with some normal axons. Severely damaged ONs (grades 4 and 5) exhibited gliosis with degenerating axons across the entire ON.24,57
Electron microscopy and G-ratio determination
Electron microscopy on ON was performed as described in our previous study.34 Rats were perfused with glutaraldehyde fixative (Servicebio), and the ON was isolated and cut into 1-mm3 pieces. The region (2 mm posterior to the globe) was post-fixed in 2.5% glutaraldehyde solution for 2 h at 4°C. Then, the ON tissue was washed, fixed in 1% osmium tetroxide (Ted Pella) in 0.1 M PBS (pH 7.4) for 2 h at RT, dehydrated in graded concentrations of ethyl alcohol and acetone (Sinaopharm Group Chemical Reagent), and embedded in pure EMBed 812 (SPI). Transverse ultrathin sections were cut (60–80 nm) on an ultramicrotome (Leica UC7) and stained with uranyl acetate and lead citrate for examination using an HT7800 transmission electron microscope (Hitachi, Japan). The G-ratio (axon diameter/fiber diameter) of the myelin sheath was analyzed using ImageJ.
Primary astrocytes in vitro assay
Primary astrocytes were plated onto poly-L-lysine-coated glass coverslips in 24-well plates at a density of 1 × 105 cells per well. Cells were then treated with hsAQP4-IgG or hsCtrl-IgG at a concentration of 100 ng/mL for 24 h. The conditional medium was collected for ELISA and astrocytes were harvested by fixation with 4% PFA for immunostaining and by lysis buffers for RNA-seq. Cells were fixed with 4% PFA for 45 min at RT. The subsequent steps were similar to those described for immunofluorescence staining of brain sections. All cell slides were examined with a confocal microscope. This procedure was repeated at least three times for each experimental group.
RNA-seq
Total messenger RNA (mRNA) was extracted from primary astrocytes treated with hsAQP4-IgG or hsCtrl-IgG at a concentration of 100 ng/mL. High-throughput RNA-seq was performed using Illumina HiSeq 2500 (Illumina, San Diego, CA) at CapitalBio (Beijing, China). The raw sequencing data were aligned to the mouse reference genome (GRCm38, mm10). All RNA-seq results of samples were assessed in triplicate. For quantification, Fisher’s exact test was used for comparing hsAQP4-IgG and hsCtrl-IgG groups. Genes were classified depending on a p value <0.05 and a fold change cutoff of a log2 ratio ≥0.5.
Quantitative real-time PCR
Total mRNA from primary astrocyte cells and spinal cord homogenates was extracted using a miRNeasy kit (QIAGEN) following the manufacturer’s instructions. RNA reverse transcription was performed using the SureScript First-Strand cDNA Synthesis Kit (GeneCopoeia) according to the manufacturer’s instructions. Real time qPCR was performed using BlazeTaq SYBR Green qPCR Mix (GeneCopoeia) with Applied Biosystems (Thermo Fisher Scientific). The expression of each gene relative to GAPDH mRNA was determined using the 2−ΔΔCT method. Each sample was assessed in triplicate. The primer sequences are listed in Table 1.
Table 1.
The primers were used in this study
| Genes | Forward primer | Reverse primer |
|---|---|---|
| IL-1β | CGAAGACTACAGTTCTGCCATT | GACGTTTCAGAGGTTCTCAGAG |
| IL-6 | GCTTAATTACACATGTTCTCTGGGAAA | CAAGTGCATCATCGTTGTTCATAC |
| IL-10 | GCTGGACAACATACTGCTAACC | ATTTCCGATAAGGCTTGGCAA |
| CXCL1 | AACGGAGATCAAACCTGCCT | AGATTCAGGGTGCTTGTTGGT |
| CXCL5 | GCCCTACGGTGGAAGTCATA | GTGCATTCCGCTTAGCTTTC |
| CXCL10 | CATCCCTGCGAGCCTATCC | CATCTCTGCTCATCATTCTTTTTC |
| CCL2 | TGAGTAGGCTGGAGAGCTACAAG | TGTATGTCTGGACCCATTCCTTC |
| CCL5 | CCTCACCATCCTCACTG | TCTTCTCTGGGTTGGCACAC |
| CCL7 | ATCTCTGCCACGCTTCTGTG | CCTCTTGGGGATCTTTTGTTT |
| CCL20 | ATGGCCGATGAAGCTTGTGA | CTCCTTGGGCTGTGTCCAAT |
| NF-κB | CATGAAGCAGCTGACAGAAG | TTCAATAGGTCCTTCCTGCC |
| TNF-α | GACTCAAATGGGCTTTCCGA | TCCAGCCTCATTCTGAGACAGAG |
| G-CSF | GCTTAATTACACATGTTCTCTGGGAAA | CAAGTGCATCATCGTTGTTCATAC |
| GM-CSF | GGCCTTGGAAGCATGTAGAGG | GGAGAACTCGTTAGAGACGACTT |
| C3 | CCAGCTCCCCATTAGCTCTG | GCACTTGCCTCTTTAGGAAGTC |
| Crry | CCAGCAGTGTGCATTGTCAGTCC | CCCCTTCTGGAATCCACTCATCTC |
| GAPDH | GGGTGTGAACCACGAGAAAT | ACTGTGGTCATGAGCCCTTC |
Western blot
HEK293T cells were lysed using radioimmunoprecipitation assay lysis buffer with proteinase and phosphatase inhibitors. Protein concentrations were determined using a bicinchoninic acid protein assay kit (KeyGen Biotech, KGP902). The protein samples were separated on 10% sodium dodecyl sulfate-polyacrylamide gel by electrophoresis, transferred to ethanol-activated polyvinylidene fluoride membranes, blocked in 5% skim milk for 1 h at RT, and then incubated with the primary antibody (rabbit anti-Crry, 1:1,000, CST) overnight at 4°C. The membranes were washed with TBST three times and then incubated with horseradish peroxidase secondary antibodies for 1 h at RT. Protein bands were detected using SuperSignal West Pico PLUS chemiluminescent substrate (Thermo Scientific, 34580). The intensity of the bands was normalized to that of mouse anti-β-actin (HuABio, H01217) and quantified using ImageJ software.
ELISA
Samples were collected from serum of NMO patients and healthy controls, the conditional medium of mouse primary astrocytes, and mouse spinal cord homogenates. Levels of inflammatory factors were measured with commercial ELISA kits according to the manufacturer’s instructions. The following inflammatory factors were tested.
C3: (MEIMIAN, MM-44699M1); C3a: (MEIMIAN, MM-0402M1)
IL1β: (MEIMIAN, MM-0040M1); IL6:(MEIMIAN, MM-0163M1)
Il-10: (MEIMIAN, MM-46420M2); C1q: (MEIMIAN, MM-44729M1)
TNF-α: (MEIMIAN, MM-0132M1); C3a: (MEIMIAN, MM-0906H1)
Clinical data and serum samples collection
Ninety-one participants were included in this study from the Third Affiliated Hospital of Sun Yat-sen University in 2019–2020, among whom 45 NMO patients were diagnosed based on the 2015 International Panel for NMO Diagnosis criteria58 and the other 46 participants were appointed as healthy controls. Consent was obtained from all participants and this study was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University (no. 02-405-01). Clinical data, demographic characteristics, magnetic resonance imaging reports, and EDSS score were collected and recorded. Serum samples were collected from NMO patients experiencing symptom onset within 7 days (active state) or being relapse-free for at least 3 months (inactive state). Each participant provided a serum sample once.
Statistical analysis
Clinical data were presented as descriptive statistics. Correction for EDSS score and C3a protein levels in serum were performed and analyzed using the Pearson correlation coefficient. Scatterplot was used to show the individual G-ratio values and axonal size distribution. All numerical data were expressed as the mean ± standard error (SEM) from at least three independent replicates for each experimental group. Normality of the data distribution was assessed by a Shapiro-Wilk normality test (p < 0.05 indicating a non-normal distribution). Two-tailed Student’s t test was used for comparing two groups, and one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison post hoc test was used for comparing multiple groups. Levels of ON degeneration in different groups were compared by Kruskal-Wallis test. Data of the tendency to fall in the rotarod test, the latency of P1 and the amplitude of P1 in VEP test, and the amplitude of PhNR in ERG test were analyzed using two-way ANOVA with Tukey’s multiple comparisons test. Values of ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 were considered statistically significant. All statistical analyses were performed using GraphPad Prism software (GraphPad, La Jolla, CA).
Acknowledgments
We thank Songqing He for providing the primary CR2-Crry plasmid, Dr. Weixiang Guo and Dr. Qingfeng Wu for helpful discussion, and Xiaoyan Han for technical assistance. This research was supported by grants from the National Natural Science Foundation of China (grant 32100787), the Science and Technology Plan Project of Guangzhou City (grant 202201020489), the Guangzhou Science and Technology Talent Project (201909020006), and the Basic and Applied Research Foundation of Guangdong Province (Key Program) (2022B1515120042).
Author contributions
C.T. and W.Q. conceived the experiments. L.X. and H.X. performed the experiments with help from S.C., W.J., Y.W., X.R., Y.Z., S.L., X.Q., Y.-W.A.H., Y.C., and H.Y. L.X., H.X., and W.J. analyzed the data. C.T., L.X., and S.K.A. wrote the manuscript. All authors read, revised, and approved the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.07.017.
Contributor Information
Wei Qiu, Email: qiuwei120@vip.163.com.
Changyong Tang, Email: tangchy23@mail.sysu.edu.cn.
Supplemental information
Data and code availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
- 1.Duan T., Verkman A.S. Experimental animal models of aquaporin-4-IgG-seropositive neuromyelitis optica spectrum disorders: progress and shortcomings. Brain Pathol. 2020;30:13–25. doi: 10.1111/bpa.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lennon V.A., Wingerchuk D.M., Kryzer T.J., Pittock S.J., Lucchinetti C.F., Fujihara K., Nakashima I., Weinshenker B.G. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 3.Papadopoulos M.C., Verkman A.S. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 2012;11:535–544. doi: 10.1016/S1474-4422(12)70133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawachi I., Lassmann H. Neurodegeneration in multiple sclerosis and neuromyelitis optica. J. Neurol. Neurosurg. Psychiatry. 2017;88:137–145. doi: 10.1136/jnnp-2016-313300. [DOI] [PubMed] [Google Scholar]
- 5.Graber D.J., Levy M., Kerr D., Wade W.F. Neuromyelitis optica pathogenesis and aquaporin 4. J. Neuroinflammation. 2008;5:22. doi: 10.1186/1742-2094-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carnero Contentti E., Correale J. Neuromyelitis optica spectrum disorders: from pathophysiology to therapeutic strategies. J. Neuroinflammation. 2021;18:208. doi: 10.1186/s12974-021-02249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collongues N., Ayme-Dietrich E., Monassier L., de Seze J. Pharmacotherapy for Neuromyelitis Optica Spectrum Disorders: Current Management and Future Options. Drugs. 2019;79:125–142. doi: 10.1007/s40265-018-1039-7. [DOI] [PubMed] [Google Scholar]
- 8.Chen M., Daha M.R., Kallenberg C.G.M. The complement system in systemic autoimmune disease. J. Autoimmun. 2010;34:J276–J286. doi: 10.1016/j.jaut.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Killick J., Morisse G., Sieger D., Astier A.L. Complement as a regulator of adaptive immunity. Semin. Immunopathol. 2018;40:37–48. doi: 10.1007/s00281-017-0644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J.D., Coulthard L.G., Woodruff T.M. Complement dysregulation in the central nervous system during development and disease. Semin. Immunol. 2019;45 doi: 10.1016/j.smim.2019.101340. [DOI] [PubMed] [Google Scholar]
- 11.Asavapanumas N., Tradtrantip L., Verkman A.S. Targeting the complement system in neuromyelitis optica spectrum disorder. Expert Opin. Biol. Ther. 2021;21:1073–1086. doi: 10.1080/14712598.2021.1884223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingram G., Hakobyan S., Robertson N.P., Morgan B.P. Complement in multiple sclerosis: its role in disease and potential as a biomarker. Clin. Exp. Immunol. 2009;155:128–139. doi: 10.1111/j.1365-2249.2008.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zipfel P.F., Skerka C. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 14.Pittock S.J., Berthele A., Fujihara K., Kim H.J., Levy M., Palace J., Nakashima I., Terzi M., Totolyan N., Viswanathan S., et al. Eculizumab in Aquaporin-4-Positive Neuromyelitis Optica Spectrum Disorder. N. Engl. J. Med. 2019;381:614–625. doi: 10.1056/NEJMoa1900866. [DOI] [PubMed] [Google Scholar]
- 15.Dalakas M.C., Alexopoulos H., Spaeth P.J. Complement in neurological disorders and emerging complement-targeted therapeutics. Nat. Rev. Neurol. 2020;16:601–617. doi: 10.1038/s41582-020-0400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricklin D., Reis E.S., Mastellos D.C., Gros P., Lambris J.D. Complement component C3 - The "Swiss Army Knife" of innate immunity and host defense. Immunol. Rev. 2016;274:33–58. doi: 10.1111/imr.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alawieh A., Tomlinson S. Injury site-specific targeting of complement inhibitors for treating stroke. Immunol. Rev. 2016;274:270–280. doi: 10.1111/imr.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holers V.M., Tomlinson S., Kulik L., Atkinson C., Rohrer B., Banda N., Thurman J.M. New therapeutic and diagnostic opportunities for injured tissue-specific targeting of complement inhibitors and imaging modalities. Semin. Immunol. 2016;28:260–267. doi: 10.1016/j.smim.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banadakoppa M., Pennington K., Balakrishnan M., Yallampalli C. Complement inhibitor Crry expression in mouse placenta is essential for maintaining normal blood pressure and fetal growth. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song H., He C., Knaak C., Guthridge J.M., Holers V.M., Tomlinson S. Complement receptor 2-mediated targeting of complement inhibitors to sites of complement activation. J. Clin. Invest. 2003;111:1875–1885. doi: 10.1172/JCI17348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atkinson C., Song H., Lu B., Qiao F., Burns T.A., Holers V.M., Tsokos G.C., Tomlinson S. Targeted complement inhibition by C3d recognition ameliorates tissue injury without apparent increase in susceptibility to infection. J. Clin. Invest. 2005;115:2444–2453. doi: 10.1172/JCI25208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao M., Dong Q., Wang W., Yang Z., Guo L., Lu Y., Ding B., Chen L., Zhang J., Xu R. Induced neural stem cell grafts exert neuroprotection through an interaction between Crry and Akt in a mouse model of closed head injury. Stem Cell Res. Ther. 2021;12:128. doi: 10.1186/s13287-021-02186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallah K., Couch C., Alshareef M., Borucki D., Yang X., Alawieh A., Tomlinson S. Complement mediates neuroinflammation and cognitive decline at extended chronic time points after traumatic brain injury. Acta Neuropathol. Commun. 2021;9:72. doi: 10.1186/s40478-021-01179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bosco A., Anderson S.R., Breen K.T., Romero C.O., Steele M.R., Chiodo V.A., Boye S.L., Hauswirth W.W., Tomlinson S., Vetter M.L. Complement C3-Targeted Gene Therapy Restricts Onset and Progression of Neurodegeneration in Chronic Mouse Glaucoma. Mol. Ther. 2018;26:2379–2396. doi: 10.1016/j.ymthe.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu X., Tomlinson S., Barnum S.R. Targeted inhibition of complement using complement receptor 2-conjugated inhibitors attenuates EAE. Neurosci. Lett. 2012;531:35–39. doi: 10.1016/j.neulet.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werneburg S., Jung J., Kunjamma R.B., Ha S.-K., Luciano N.J., Willis C.M., Gao G., Biscola N.P., Havton L.A., Crocker S.J., et al. Targeted Complement Inhibition at Synapses Prevents Microglial Synaptic Engulfment and Synapse Loss in Demyelinating Disease. Immunity. 2020;52:167–182.e7. doi: 10.1016/j.immuni.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nytrova P., Potlukova E., Kemlink D., Woodhall M., Horakova D., Waters P., Havrdova E., Zivorova D., Vincent A., Trendelenburg M. Complement activation in patients with neuromyelitis optica. J. Neuroimmunol. 2014;274:185–191. doi: 10.1016/j.jneuroim.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Chen T., Lennon V.A., Liu Y.U., Bosco D.B., Li Y., Yi M.-H., Zhu J., Wei S., Wu L.-J. Astrocyte-microglia interaction drives evolving neuromyelitis optica lesion. J. Clin. Invest. 2020;130:4025–4038. doi: 10.1172/JCI134816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howe C.L., Kaptzan T., Magaña S.M., Ayers-Ringler J.R., LaFrance-Corey R.G., Lucchinetti C.F. Neuromyelitis optica IgG stimulates an immunological response in rat astrocyte cultures. Glia. 2014;62:692–708. doi: 10.1002/glia.22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker-Caulfield M.E., Guo Y., Johnson R.K., McCarthy C.B., Fitz-Gibbon P.D., Lucchinetti C.F., Howe C.L. NFkappaB signaling drives pro-granulocytic astroglial responses to neuromyelitis optica patient IgG. J. Neuroinflammation. 2015;12:185. doi: 10.1186/s12974-015-0403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xian P., Hei Y., Wang R., Wang T., Yang J., Li J., Di Z., Liu Z., Baskys A., Liu W., et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. 2019;9:5956–5975. doi: 10.7150/thno.33872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S.M., Li B., Zhang L., Zhang G.F., Sun J., Ji M.H., Yang J.J. A complement-microglial axis driving inhibitory synapse related protein loss might contribute to systemic inflammation-induced cognitive impairment. Int. Immunopharmacol. 2020;87 doi: 10.1016/j.intimp.2020.106814. [DOI] [PubMed] [Google Scholar]
- 33.Geis C., Ritter C., Ruschil C., Weishaupt A., Grünewald B., Stoll G., Holmoy T., Misu T., Fujihara K., Hemmer B., et al. The intrinsic pathogenic role of autoantibodies to aquaporin 4 mediating spinal cord disease in a rat passive-transfer model. Exp. Neurol. 2015;265:8–21. doi: 10.1016/j.expneurol.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo W., Xu H., Xu L., Jiang W., Chen C., Chang Y., Liu C., Tian Z., Qiu X., Xie C., et al. Remyelination in neuromyelitis optica spectrum disorder is promoted by edaravone through mTORC1 signaling activation. Glia. 2023;71:284–304. doi: 10.1002/glia.24271. [DOI] [PubMed] [Google Scholar]
- 35.Yick L.-W., Tang C.-H., Ma O.K.-F., Kwan J.S.-C., Chan K.-H. Memantine ameliorates motor impairments and pathologies in a mouse model of neuromyelitis optica spectrum disorders. J. Neuroinflammation. 2020;17:236. doi: 10.1186/s12974-020-01913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C., Zhang L., Ndong J.D.L.C., Hettinghouse A., Sun G., Chen C., Zhang C., Liu R., Liu C.-J. Progranulin deficiency exacerbates spinal cord injury by promoting neuroinflammation and cell apoptosis in mice. J. Neuroinflammation. 2019;16:238. doi: 10.1186/s12974-019-1630-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett J., Ashtari M., Wellman J., Marshall K.A., Cyckowski L.L., Chung D.C., McCague S., Pierce E.A., Chen Y., Bennicelli J.L., et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci. Transl. Med. 2012;4:120ra15. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leibinger M., Zeitler C., Gobrecht P., Andreadaki A., Gisselmann G., Fischer D. Transneuronal delivery of hyper-interleukin-6 enables functional recovery after severe spinal cord injury in mice. Nat. Commun. 2021;12:391. doi: 10.1038/s41467-020-20112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.You Y., Klistorner A., Thie J., Graham S.L. Latency delay of visual evoked potential is a real measurement of demyelination in a rat model of optic neuritis. Invest. Ophthalmol. Vis. Sci. 2011;52:6911–6918. doi: 10.1167/iovs.11-7434. [DOI] [PubMed] [Google Scholar]
- 40.Niyadurupola N., Luu C.D., Nguyen D.Q., Geddes K., Tan G.X.V., Wong C.C.W., Tran T., Coote M.A., Crowston J.G. Intraocular pressure lowering is associated with an increase in the photopic negative response (PhNR) amplitude in glaucoma and ocular hypertensive eyes. Invest. Ophthalmol. Vis. Sci. 2013;54:1913–1919. doi: 10.1167/iovs.12-10869. [DOI] [PubMed] [Google Scholar]
- 41.Lucchinetti C.F., Guo Y., Popescu B.F.G., Fujihara K., Itoyama Y., Misu T. The pathology of an autoimmune astrocytopathy: lessons learned from neuromyelitis optica. Brain Pathol. 2014;24:83–97. doi: 10.1111/bpa.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davoust N., Nataf S., Reiman R., Holers M.V., Campbell I.L., Barnum S.R. Central nervous system-targeted expression of the complement inhibitor sCrry prevents experimental allergic encephalomyelitis. J. Immunol. 1999;163:6551–6556. [PubMed] [Google Scholar]
- 43.Zhang H., Bennett J.L., Verkman A.S. Ex vivo spinal cord slice model of neuromyelitis optica reveals novel immunopathogenic mechanisms. Ann. Neurol. 2011;70:943–954. doi: 10.1002/ana.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saadoun S., Waters P., Bell B.A., Vincent A., Verkman A.S., Papadopoulos M.C. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133:349–361. doi: 10.1093/brain/awp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foust K.D., Nurre E., Montgomery C.L., Hernandez A., Chan C.M., Kaspar B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y., Bao Y., Qiu W., Peng L., Fang L., Xu Y., Yang H. Structural and visual functional deficits in a rat model of neuromyelitis optica spectrum disorders related optic neuritis. Exp. Eye Res. 2018;175:124–132. doi: 10.1016/j.exer.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Felix C.M., Levin M.H., Verkman A.S. Complement-independent retinal pathology produced by intravitreal injection of neuromyelitis optica immunoglobulin G. J. Neuroinflammation. 2016;13:275. doi: 10.1186/s12974-016-0746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaknin-Dembinsky A., Karussis D., Avichzer J., Abramsky O. NMO spectrum of disorders: a paradigm for astrocyte-targeting autoimmunity and its implications for MS and other CNS inflammatory diseases. J. Autoimmun. 2014;54:93–99. doi: 10.1016/j.jaut.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Domingues H.S., Portugal C.C., Socodato R., Relvas J.B. Corrigendum: Oligodendrocyte, Astrocyte and Microglia Crosstalk in Myelin Development, Damage, and Repair. Front. Cell Dev. Biol. 2016;4:79. doi: 10.3389/fcell.2016.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong C., Liu J., Lin D., Zhang J., Terrando N., Wu A. Complement activation contributes to perioperative neurocognitive disorders in mice. J. Neuroinflammation. 2018;15:254. doi: 10.1186/s12974-018-1292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lian H., Litvinchuk A., Chiang A.C.A., Aithmitti N., Jankowsky J.L., Zheng H. Astrocyte-Microglia Cross Talk through Complement Activation Modulates Amyloid Pathology in Mouse Models of Alzheimer's Disease. J. Neurosci. 2016;36:577–589. doi: 10.1523/JNEUROSCI.2117-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dubbelaar M.L., Kracht L., Eggen B.J.L., Boddeke E.W.G.M. The Kaleidoscope of Microglial Phenotypes. Front. Immunol. 2018;9:1753. doi: 10.3389/fimmu.2018.01753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colonna M., Butovsky O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017;35:441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yick L.W., Ma O.K.F., Chan E.Y.Y., Yau K.X., Kwan J.S.C., Chan K.H. T follicular helper cells contribute to pathophysiology in a model of neuromyelitis optica spectrum disorders. JCI Insight. 2023;8 doi: 10.1172/jci.insight.161003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Najm F.J., Madhavan M., Zaremba A., Shick E., Karl R.T., Factor D.C., Miller T.E., Nevin Z.S., Kantor C., Sargent A., et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature. 2015;522:216–220. doi: 10.1038/nature14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marina N., Bull N.D., Martin K.R. A semiautomated targeted sampling method to assess optic nerve axonal loss in a rat model of glaucoma. Nat. Protoc. 2010;5:1642–1651. doi: 10.1038/nprot.2010.128. [DOI] [PubMed] [Google Scholar]
- 57.Jia L., Cepurna W.O., Johnson E.C., Morrison J.C. Patterns of intraocular pressure elevation after aqueous humor outflow obstruction in rats. Invest. Ophthalmol. Vis. Sci. 2000;41:1380–1385. [PubMed] [Google Scholar]
- 58.Wingerchuk D.M., Banwell B., Bennett J.L., Cabre P., Carroll W., Chitnis T., de Seze J., Fujihara K., Greenberg B., Jacob A., et al. International Panel for NMO Diagnosis International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.