Abstract
Cancer metastatic organotropism is still a mystery. The liver is known to be susceptible to cancer metastasis and alcoholic injury. However, it is unclear whether and how alcohol facilitates liver metastasis and how to intervene. Here, we show that alcohol preferentially promotes liver metastasis in colon-cancer-bearing mice and post-surgery pancreatic cancer patients. The mechanism is that alcohol triggers an extra- and intrahepatic crosstalk to reshape an immunosuppressive liver microenvironment. In detail, alcohol upregulates extrahepatic IL-6 and hepatocellular IL-6 receptor expression, resulting in hepatocyte STAT3 signaling activation and downstream lipocalin-2 (Lcn2) upregulation. Furthermore, LCN2 promotes T cell-exhaustion neutrophil recruitment and cancer cell epithelial plasticity. In contrast, knocking out hepatocellular Stat3 or systemic Il6 in alcohol-treated mice preserves the liver microenvironment and suppresses liver metastasis. This mechanism is reflected in hepatocellular carcinoma patients, in that alcohol-associated signaling elevation in noncancerous liver tissue indicates adverse prognosis. Accordingly, we discover a novel application for BBI608, a small molecular STAT3 inhibitor that can prevent liver metastasis. BBI608 pretreatment protects the liver and suppresses alcohol-triggered premetastatic niche formation. In conclusion, under extra- and intrahepatic crosstalk, the alcoholic injured liver forms a favorable niche for cancer cell metastasis, while BBI608 is a promising anti-metastatic agent targeting such microenvironments.
Keywords: alcohol, BBI608, liver, lipocalin-2, premetastatic niche, STAT3, tumor targeting strategy
Graphical abstract

The mechanism of liver metastasis susceptibility is unclear. Lin and colleagues show that the liver microenvironment with alcoholic injury characteristics, which is driven by the extra- and intrahepatic immunosuppressive crosstalk-activated IL-6/STAT3/LCN2 axis, is favorable for cancer cell metastasis. Targeting this microenvironment, BBI608 is a promising agent for liver metastasis prevention.
Introduction
The liver is one of the most common destination organs for cancer metastasis. Cancer patients with liver metastasis typically have more adverse prognoses and weaker therapeutic responses than those without metastasis.1 Previous studies on metastasis organotropism have mainly focused on tumor cell heterogenicity.2,3,4 However, how changes in the somatic microenvironment influence liver metastasis and its treatment remain unclear.
On the other hand, the liver is mainly responsible for alcohol metabolism and is vulnerable to alcoholic injury. Alcohol is undoubtedly a risk factor associated with causing liver carcinoma and many other cancers and has been listed as a group 1 carcinogen by the World Health Organization.5 However, the burdens of genetic mutations require years, or even decades, to accumulate to lead to carcinogenesis. Before that, can relatively short-term alcohol intake influence cancer progression? Can alcohol change the somatic microenvironment and promote malignancy? Solid clinical and basic research evidence is still lacking. Furthermore, although it has been suggested that alcohol intake promotes colorectal cancer liver metastasis in mouse models,6 the clinical relevance and the detailed underlying biological mechanisms of how alcohol triggers liver metastasis organotropism are still unclear. Thus, understanding these mechanisms has great significance in cancer prevention and optimizing therapeutic strategies.
Alcohol-induced liver injury is known to be accompanied by inflammatory factor secretion, immunocyte infiltration, and microenvironment remodeling.7 Levels of many cytokines, including interleukin-6 (IL-6), can be elevated in the serum after alcohol consumption. In colorectal cancer patients, high serum IL-6 levels are associated with a large liver metastasis volume.8 By binding to the IL-6 receptor (IL-6R) and subsequently dimerizing with the coreceptor IL-6 signal transducer (IL-6st), IL-6 activates the signal transducer and activator of transcription 3 (STAT3) signaling pathway.9 STAT3 activation triggers secretion of many downstream inflammatory factors, including lipocalin-2 (LCN2).10,11 LCN2, a hepatocyte- and neutrophil-secreted inflammatory glycoprotein, has been shown to play an important role in liver tumorigenesis.12,13 However, its role in metastasis is still not entirely clear, due to some deceptively conflicting reports from different cancer models.13,14,15,16,17,18 In alcoholic liver disease, elevated LCN2 level in the liver drives neutrophilic inflammation.14,19 In the colon cancer microenvironment, neutrophils suppress tumor-infiltrating T cell immunity.20,21 Our previous study also found that a high circulating neutrophil-to-lymphocyte ratio indicates an adverse cancer outcome.22
These clues suggest that alcohol induces extra- and intrahepatic abnormalities, some of which are cancer promoting. Nevertheless, before cancer cells accumulate and metastasize to the liver, it is still unclear whether, and if so, how, alcohol-induced inflammatory changes engage in microenvironment formation. Accordingly, we hypothesize that some alcohol-induced inflammatory alterations (e.g., IL-6/STAT3 signaling activation or LCN2 secretion) may disrupt the liver immune microenvironment (e.g., neutrophil infiltration or T cell exhaustion) and lead to the formation of a premetastatic niche, thereby facilitating cancer cell settlement. To address this, we used a mouse alcohol consumption model together with clinical data to explore how alcohol promotes liver metastasis. This study aimed to recognize the danger of alcohol consumption from a new perspective, specifically for cancer patients, and to explore therapeutic strategies.
Results
Alcohol exposure preferentially promotes liver metastasis
To study the influence of alcohol on the liver microenvironment and to avoid involving cancer cells, we adopted an alcohol-pretreated mouse model. Mice were daily gavaged with 20% alcohol per volume (alc/vol) for 2 weeks, and different colon cancer cell lines (CT26 for BALB/c and MC38 for C57BL/6 mice) were then injected into the spleen to induce liver metastasis via the portal vein. Ten days later, the tumor growth was finally assessed. Under the naked eye, the difference in metastases on the liver surface was already obvious, in that the tumors were more likely to grow in the alcohol-affected liver. Then, the tumor growth was quantified by using the gross weights of livers and spleens with tumors and the net weights of tumors that separated from the liver. As a result, alcohol pretreatment significantly facilitated liver metastasis. However, tumor growth in the spleen (the primary site) of the alcohol-treated group showed no significant difference compared with that in the control group. Furthermore, no significant correlation between the tumor burden in the spleen and the liver was observed (Figure 1A). To further confirm the effect of alcohol on liver metastasis, another batch of experiments were performed using the indicator of the relative metastatic area on the liver histopathological sections. Consistently, the alcohol pretreatment group showed a larger metastatic burden (Figure 1B). However, in the subcutaneous xenograft mouse model, alcohol pretreatment did not accelerate tumor growth (Figure 1C). These results suggest that alcohol promotes liver metastasis in an organotropic manner.
Figure 1.
Alcohol facilitates liver metastasis
(A) Alcohol pretreatment promoted colon cancer cell metastasis to the liver in BALB/c (Ctrl, n = 8; Alco, n = 7) and C57BL/6 (n = 5) mice, but did not influence tumor growth in the spleen or the correlation between tumor burdens in liver and spleen. The metastases on liver were quantified by the gross liver weight, the tumor net weight, and (B) the relative metastatic area on the liver histopathological sections (n = 5). (C) Alcohol pretreatment had no effect on subcutaneous MC38 tumor growth in C57BL/6 mice (n = 7). (D) Analysis workflow for post-surgery patients with pancreatic cancer. (E) Alcohol exposure resulted in higher rates of liver metastasis. (F) Patients with liver metastasis had a higher proportion of alcohol exposure than those with other metastatic sites and those free of metastasis. (G) Alcohol exposure and liver metastasis influenced patient pancreatic cancer progression-free time. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Few clinical studies have focused on the relationship between alcohol intake and liver metastasis. Here, we analyzed 185 cases of post-surgery pancreatic cancer from The Cancer Genome Atlas (TCGA), of which 99 patients had documented alcohol exposure status (Figure 1D). Of these, 38 cases were free of the occurrence until the end of the follow-up, while 31 of 61 cases were documented with new site occurrences. Compared with patients without alcohol exposure, the alcohol exposure group showed a higher metastasis proportion, which was almost 40% higher for liver metastasis and 23.5% higher for the other metastasis sites (Figure 1E). In patients with liver metastasis, 78.6% were alcohol exposed, which is higher than the 76.5% and 68.4% of those with metastasis in other sites and those free of metastasis, respectively (Figure 1F). In Kaplan-Meier analysis, patients with alcohol exposure and liver metastasis showed a much shorter progression-free interval than those free of alcohol exposure and liver metastasis (Figure 1G).
Alcohol-triggered liver premetastatic niche is dependent on hepatocyte STAT3 activation
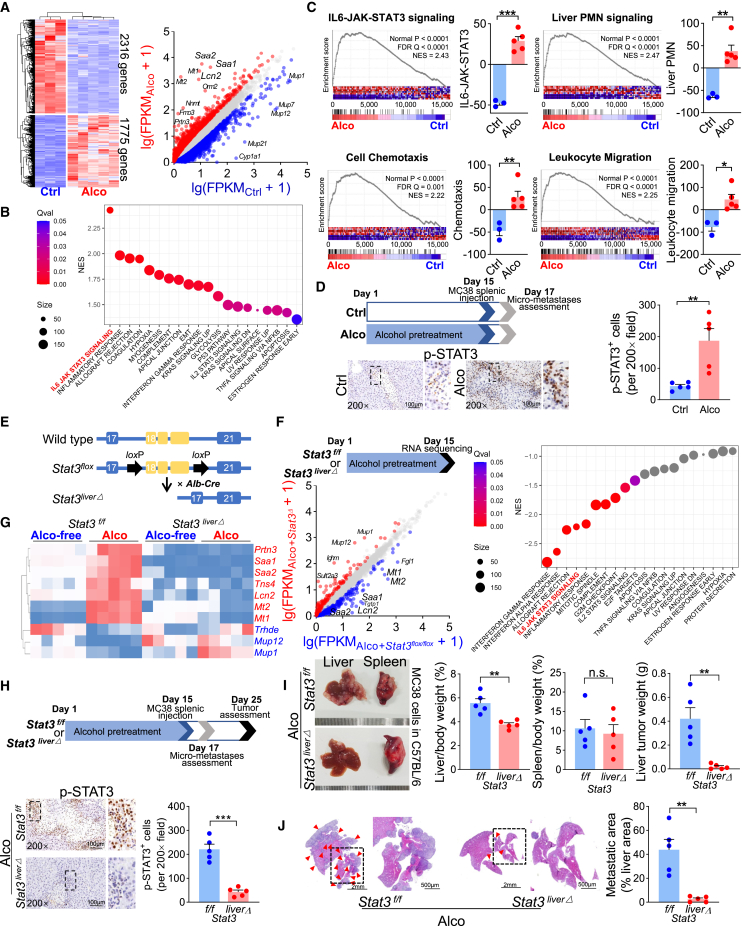
Next, we focused on how alcohol changed the pro-liver metastatic niche. After 2 weeks of 20% alc/vol exposure, lipids accumulated in hepatocytes, wherein cholesterol and triglyceride levels were increased. Serum alanine transaminase (ALT) and aspartate transaminase (AST) levels were elevated as well. However, liver collagen deposition was not obvious, indicating hepatic steatosis before fibrosis in this model (Figures S1A and S1B). RNA sequencing was performed under these conditions, identifying 1,775 upregulated and 2,316 downregulated genes. Remarkably, Lcn2, Saa1, Saa2, Mt1, and Mt2 were dramatically upregulated (Figure 2A). In Hallmark gene sets, gene set enrichment analysis (GSEA) revealed that IL-6-JAK-STAT3 signaling was the most significantly enriched pathway (Figure 2B). In addition, alcohol-associated signaling was also highly enriched in the liver premetastatic niche,10 as well as cell chemotaxis and leukocyte migration (Figure 2C).
Figure 2.
Alcohol promotes liver metastasis by activating hepatocyte STAT3 signaling
(A) RNA sequencing revealed the differentially expressed genes in the alcohol-treated mouse liver (Ctrl, n = 3; Alco, n = 5). (B) Upregulated genes were highly enriched in IL-6-JAK-STAT3 signaling. (C) GSEA of the upregulated genes (Ctrl, n = 3; Alco, n = 5). (D) Analysis of mouse liver early micrometastases 48 h after splenic injection of MC38 cells. p-STAT3-positive hepatocytes around the tumor clones increased following alcohol pretreatment (n = 5). (E) Schematic gene organization of the Stat3liverΔ mouse. (F) By Stat3 conditional knockout, RNA sequencing revealed differentially expressed genes and negatively enriched pathways from alcohol-treated mouse liver tissue (n = 4). (G) The qRT-PCR validation of the most dramatically changed genes modulated by alcohol and STAT3. (H) Suppressing STAT3 phosphorylation in hepatocyte Stat3 knockout restricted alcohol-induced liver metastasis, which is quantified by (I) the gross liver weight and the tumor net weight (n = 5) and (J) the relative liver metastatic area (n = 5). Red arrowheads indicate metastases. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Consistent with the sequencing results (Figure S2A), liver Il6ra mRNA was upregulated by alcohol pretreatment (Figure S2B). Through immunohistochemical (IHC) staining, we found that the IL-6R protein was mainly expressed on the hepatocyte membrane, suggesting “classical IL-6 signaling” transduction (Figure S2C). Consequently, downstream STAT3 phosphorylation was greatly enhanced in alcohol-treated liver tissue (Figures S2D and S2E). To explore the influence of alcohol-induced hepatocyte STAT3 phosphorylation on cancer metastasis, the C57BL/6 mouse liver was dissected 48 h after MC38 cell injection into the spleen (Figure 2D). Under microscopy, early liver micrometastatic colonization was observed in both the alcohol and the control groups. Phosphorylated STAT3 (p-STAT3)-positive hepatocytes were still more abundant in the alcohol group. Interestingly, in alcohol-pretreated mice, metastases were usually close to the p-STAT3-positive hepatocytes (Figure 2D), suggesting the presence of some mechanical connections.
Next, we used a transgenic mouse model, Stat3flox/flox;Alb-Cre (Stat3liverΔ) (Figures 2E and S3A), in which Stat3 was specifically knocked out in hepatocytes (Figure S3B). Through RNA sequencing of the alcohol-treated liver tissue, we found that the expression of many alcohol-upregulated genes, such as Lcn2, Saa1, Saa2, Mt1, Mt2, Trpn3, and Tns4, together with inflammatory-related pathways, was significantly suppressed by Stat3 genetic ablation in hepatocytes (Figure 2F). Based on this, the mRNA expression of these genes, together with the genes Mup1, Mup12, and Trhde, remarkably upregulated by Stat3 knockout, was further validated by qRT-PCR. Generally, the alcohol-induced expression alterations were reversed by hepatocellular Stat3 knockout (Figure 2G), suggesting these genes share a common background of both alcoholic injury and STAT3 modulation. As the consequence of hepatocellular STAT3 phosphorylation abrogation (Figure 2H), alcohol-facilitated liver metastasis was significantly eliminated (Figures 2I and 2J). These results implied that alcohol-induced liver metastasis was greatly dependent on hepatocellular STAT3 activation.
Extrahepatic IL-6 elevation is necessary for alcohol-induced liver metastasis
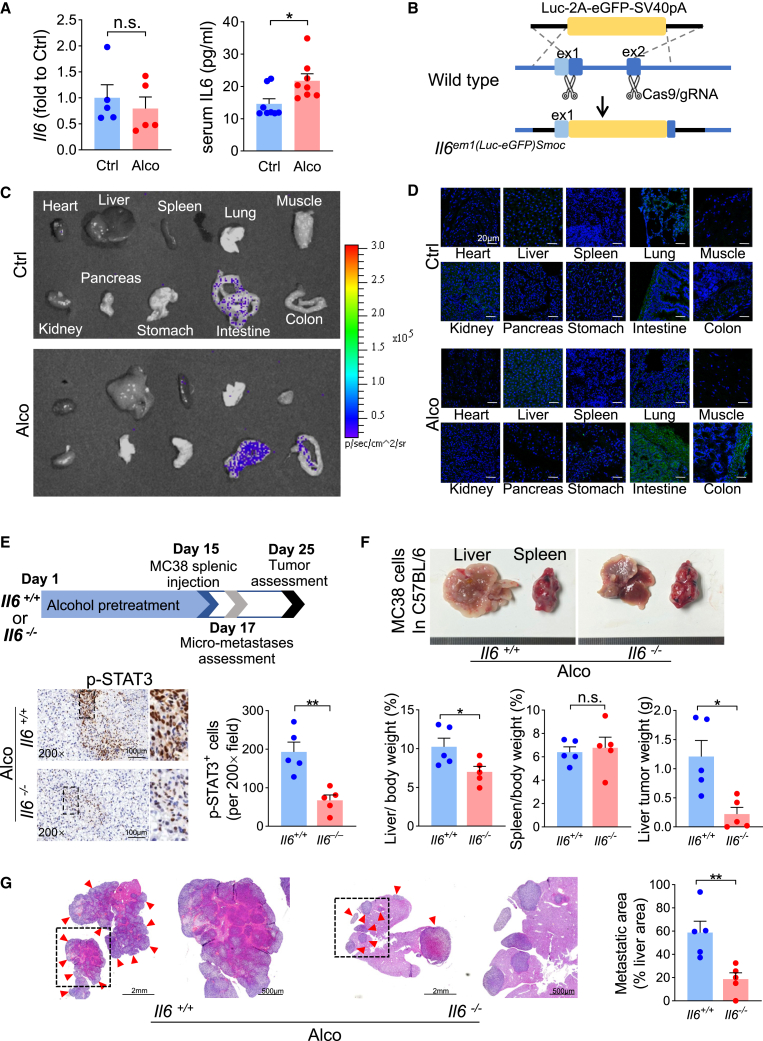
Interestingly, in the IL-6-JAK-STAT3 pathway, the FPKM (Fragments Per Kilobase of exon model per Million mapped fragments) value (Figure S2A) and mRNA expression (Figure 3A) of Il6 in alcohol-treated liver remained almost unchanged. However, serum IL-6 levels were elevated (Figure 3A), indicating that the elevated IL-6 might be generated from some extrahepatic organs. To determine the source of IL-6, we adopted an Il6 reporter transgenic mouse model (Il6em1(Luc-eGFP)Smoc). Through the knocking in of the luciferase-2A-egfp-pA sequence at the ATG site of the Il6 gene initiation codon, the Il6em1(Luc-eGFP)Smoc mouse expressed luciferase and enhanced green fluorescent protein (eGFP) when Il6 transcription was initiated (Figures 3B and S3C). After alcohol stimulation, luciferase (Figure 3C) and eGFP (Figure 3D) signals were enhanced in the intestine and colon, but not in the liver. Thus, it is alcohol-upregulated extra- and intrahepatic factors that both synergistically contribute to hepatic STAT3 signaling activation.
Figure 3.
Extrahepatic IL-6 elevation is necessary for alcohol-induced liver metastasis
(A) Liver Il6 mRNA (n = 5) and serum IL-6 protein (n = 8) levels in the alcohol-treated mouse. (B) Schematic gene organization of the Il6em1(Luc-eGFP)Smoc mouse. (C) Luciferase and (D) GFP signals in different organs. (E) Alcohol-induced STAT3 phosphorylation and (F and G) liver metastasis were restricted by systemic Il6 knockout (n = 5). Red arrowheads indicate metastases. ∗p < 0.05, ∗∗p < 0.01.
Since Il6 was systemically knocked out in Il6em1(Luc-eGFP)Smoc homozygous mice, we used these Il6-deficient mice to assess the influence of alcohol-induced systemic IL-6 elevation on liver metastasis. Consequently, alcohol pretreatment did not increase p-STAT3 levels in hepatocytes (Figure 3E). These Il6-deficient mice showed greatly decreased liver metastases compared with the wild-type controls (Figures 3F and 3G), suggesting that systemic IL-6 elevation is necessary for STAT3 activation and alcohol-induced liver metastasis.
Alcohol reshapes a liver premetastatic niche with LCN2 elevation and neutrophil infiltration
As suggested by the sequencing data, Lcn2 was one of the most remarkably alcohol-upregulated STAT3 target genes; this was further confirmed by qRT-PCR at the mRNA level. In vivo, alcohol treatment significantly upregulated Lcn2 mRNA expression in C57BL/6 mouse liver tissue (Figure 4A). In vitro, culturing wild-type C57BL/6 mouse primary hepatocytes with alcohol and/or recombinant mouse IL-6 (rmIL-6) significantly upregulated Lcn2 mRNA expression (Figure 4B), showing a synergistic modulation. However, unlike that observed in the Stat3flox/flox mouse hepatocytes, alcohol and rmIL-6 stimulation did not elevate Lcn2 mRNA expression in the Stat3liverΔ mouse hepatocytes (Figure 4C). This once again confirmed the regulation of the IL-6/STAT3/LCN2 axis by alcohol. It was reported that, in addition to hepatocytes, LCN2 was more abundant in neutrophils.11,14 However, by adding alcohol to in vitro-cultured neutrophils, Lcn2 mRNA was not further upregulated (Figure 4D), suggesting that hepatocytes are the major source of alcohol-elevated LCN2.
Figure 4.
Alcohol enhances hepatocyte Lcn2 expression and intrahepatic neutrophil infiltration via the IL-6/STAT3 axis
(A) Lcn2 mRNA expression in alcohol-treated C57BL/6 mouse liver tissue (n = 5). (B) Lcn2 mRNA levels in primary C57BL/6 mouse hepatocytes treated with alcohol (50 mM), rmIL-6 (50 ng/mL), and BBI608 (0.1 mM) (n = 4). (C) Lcn2 mRNA levels in primary Stat3flox/flox and Stat3liverΔ mouse hepatocytes treated with alcohol and rmIL-6 (n = 4). (D) Neutrophil Lcn2 mRNA expression by alcohol treatment in vitro (n = 6). (E) Immunofluorescence of C57BL/6 mouse liver tissue sections under low-power field. (F) Alcohol-promoted liver LCN2 expression and neutrophil (labeled by Ly6G) infiltration (n = 5), which was reversed by knocking out (G) hepatocellular Stat3 (n = 5) and (H) systemic Il6 (n = 5), respectively. LCN2 expression and neutrophil infiltration were positively correlated. ‘L’ and ‘M’ denote liver and metastases, respectively. IOD, integral optical density. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Forty-eight hours after MC38 cell injection into the spleen, liver micrometastases were observed via microscopy. In addition to hepatocellular LCN2 upregulation, alcohol pretreatment promoted infiltration of LCN2 strongly positive cells. These cells were confirmed as neutrophils by identifying the neutrophil marker lymphocyte antigen 6 complex locus G6D (Ly6G) (Figures 4E and 4F). Like the case of p-STAT3 staining, metastatic colonization tended to occur at sites where hepatocyte LCN2 was elevated and neutrophils were accumulated (Figure 4E). In addition, neutrophil infiltration was positively correlated with hepatocellular LCN2 elevation (Figure 4F). In contrast, by knocking out hepatocellular Stat3 or systemic Il6, alcohol-induced hepatocyte LCN2 upregulation and neutrophil infiltration were restrained, implying that regulatory relationships were present. Moreover, a positive correlation was maintained between LCN2 expression and neutrophil infiltration (Figures 4G and 4H). Collectively, these data suggest that, under STAT3 signaling activation, the increased neutrophil and LCN2 levels may participate in niche remodeling.
Alcohol-induced liver niche is immunosuppressive with T cell exhaustion
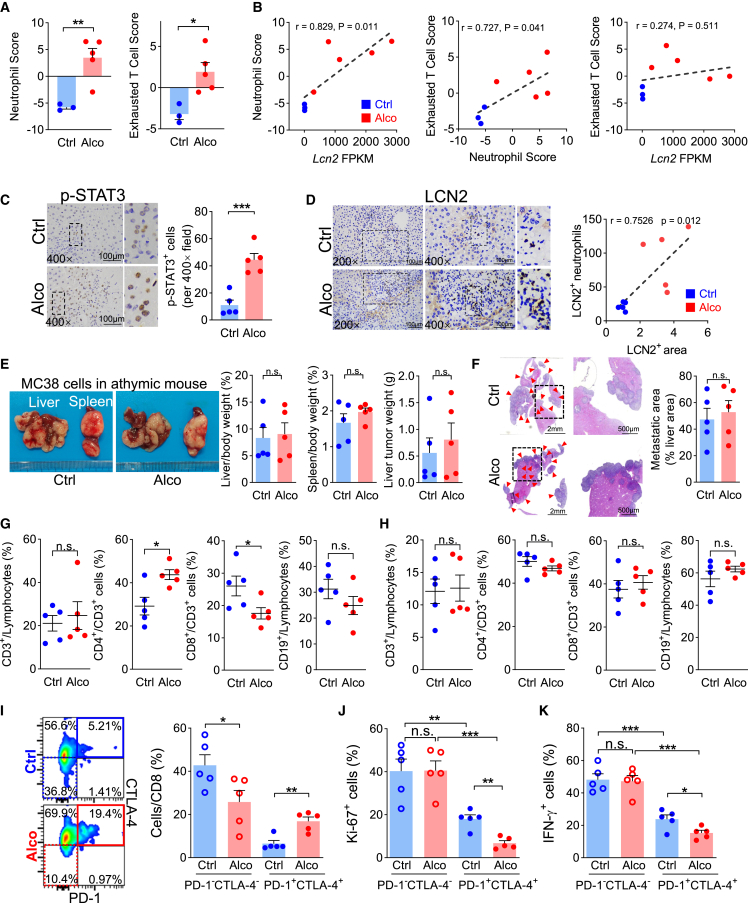
To explore the relationships between immune cells in the alcohol-related liver microenvironment, we adopted a computational algorithm to estimate 29 immune cell subtype signatures based on corresponding characteristic gene sets (Figure S4). As a result, the neutrophil signature was negatively correlated with most of the anti-tumor cell signatures, including activated CD8 T cells and γδ T cells. In contrast, it was positively correlated with the exhausted T cell signature. When comparing the two groups, the signature scores of neutrophils and exhausted T cells were significantly higher in the alcohol group than in the alcohol-free control (Figure 5A). The correlations of the Lcn2 FPKM-neutrophil score and neutrophil-exhausted T cell scores were significantly positive (Figure 5B).
Figure 5.
Alcohol induces liver CD8 T cell exhaustion in the liver
(A) Neutrophil and exhausted T cell signatures were enhanced by alcohol treatment. (B) Correlation of Lcn2 FPKM value, neutrophil signature, and exhausted T cell signatures. In athymic mice, alcohol enhanced hepatocyte (C) p-STAT3 and (D) LCN2 expression and LCN2-positive neutrophil liver infiltration (n = 5), but (E and F) did not promote liver metastasis (n = 5). Red arrowheads indicate metastases. In immune-competent C57BL/6 mice, (G) CD8 T cell proportion was decreased by alcohol treatment in the liver, but (H) not influenced in the spleen (n = 5). (I) In vivo (n = 5), alcohol increased the hepatic PD-1+CTLA-4+CD8 cell proportion but decreased the PD-1−CTLA-4− proportion. PD-1+CTLA-4+ cells showed low (J) Ki-67 and (K) IFN-γ expression, and alcohol further decreased it. (G), (H), (J), and (K) correspond to Figures S6A–S6D, respectively. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Unlike the immune-competent mice, although alcohol successfully increased liver p-STAT3 (Figure 5C) and LCN2 expression (Figures 5D and S5) in the athymic mouse models, its influence on liver metastasis was eliminated (Figures 5E and 5F). This suggests that defects in T cell immunity are essential during alcohol-associated liver metastasis. Thus, we focused on T cell immunity in the niche. In alcohol-treated immune-competent C57BL/6 mice, the proportion of CD8-positive cells decreased in the liver (Figures 5G and S6A), whereas it remained almost unchanged in the spleen (Figures 5H and S6B). In liver CD8 T cells, the proportion of programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) double-negative subgroups was significantly higher in the control group. In contrast, the alcohol group contained more PD-1 and CTLA-4 double-positive T cells (Figure 5I). Compared with the PD-1 and CTLA-4 double-negative CD8 cells, the double-positive cells showed significantly lower markers of proliferation antigen Ki-67 (Figures 5J and S6C) and interferon γ (IFN-γ) (Figures 5K and S6D) expression, indicating T cell exhaustion. This T cell exhaustion was more evident in the alcohol group than in the control group. These results showed that the alcohol-related liver microenvironment was immunosuppressive.
LCN2 promotes T cell exhaustion via neutrophil recruitment
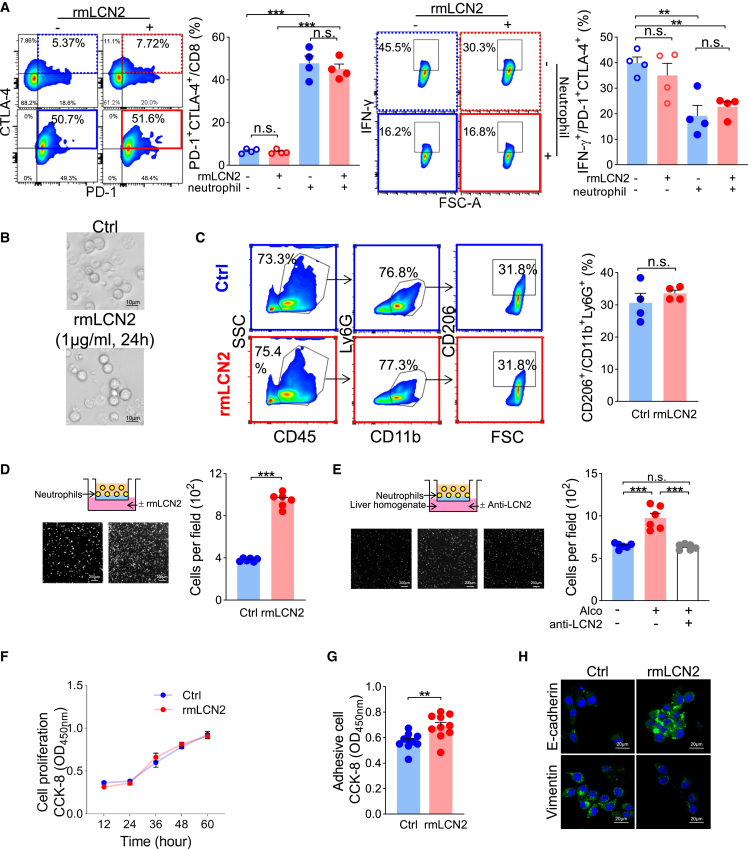
Considering the close relationships among LCN2, neutrophils, and exhausted T cells, we performed in vitro experiments by culturing T lymphocytes with or without neutrophils or recombinant mouse LCN2 (rmLCN2). rmLCN2 alone did not induce T lymphocyte exhaustion. However, coculturing with neutrophils dramatically elevated T lymphocyte expression of PD-1 and CTLA-4 and suppressed their IFN-γ expression (Figure 6A). Given the significant correlations of Lcn2 FPKM-neutrophil score and neutrophil-exhausted T cell scores but not Lcn2 FPKM-exhausted T cell score (Figure 5B), we supposed that the effect of LCN2 on T lymphocytes may be indirect and require neutrophil assistance. Thus, we focused on the neutrophil phenotype induced by LCN2 stimulation. As a result, rmLCN2 did not influence the neutrophil morphology or polarization (Figures 6B and 6C) but functioned as a chemoattractant for neutrophils (Figure 6D). Furthermore, the alcohol-pretreated mouse liver homogenate consistently attracted neutrophils; however, it was suppressed by adding an anti-LCN2 neutralizing antibody (Figure 6E). These data suggest that alcohol-upregulated liver LCN2 promotes T cell exhaustion via neutrophil recruitment.
Figure 6.
LCN2 promotes T cell-exhausting neutrophil recruitment and cancer cell epithelial plasticity
(A) Lymphocytes cocultured with neutrophils showed elevated PD-1 and CTLA-4 levels, characterized by IFN-γ downregulation. (B) Neutrophil morphology and (C) polarization (n = 4) were not altered by rmLCN2. (D) Neutrophil chemotaxis was enhanced by rmLCN2 and (E) alcohol-treated liver tissue homogenate, which was ameliorated by anti-LCN2 neutralizing antibodies. (F) MC38 cell proliferation was not altered by rmLCN2 treatment, but (G) adhesiveness and (H) epithelial plasticity were enhanced. ∗∗p < 0.01, ∗∗∗p < 0.001.
It seems contradictory that LCN2 in the lung promotes breast cancer cell lung metastasis,16 while in hepatocellular carcinoma, LCN2 inhibits distant metastasis.13 Thus, we investigated the effect of LCN2 on MC38 cells. rmLCN2 did not promote MC38 cell proliferation (Figure 6F). However, rmLCN2 enhanced MC38 cell adhesion to Matrigel (Figure 6G), suggesting that LCN2 might assist cancer cell settlement rather than cell proliferation. As expected, rmLCN2 upregulated MC38 cells with the epithelial marker E-cadherin but downregulated the mesenchymal marker vimentin (Figure 6H). Therefore, in addition to neutrophil recruitment, LCN2 also helps circulating cancer cells gain epithelial plasticity, which facilitates their integration into the liver.
Alcohol-characteristic signaling elevation in noncancerous liver tissue indicates an adverse cancer prognosis
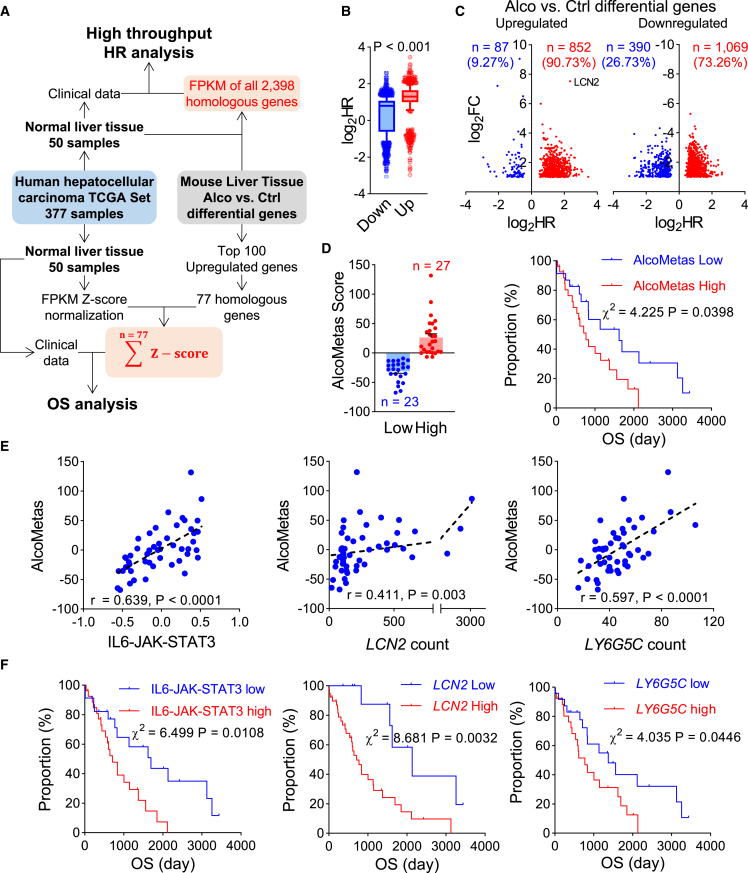
Given the above experimental findings, we assumed that the liver niche with molecular alterations similar to alcoholic liver injury might also influence cancer progression. Thus, to demonstrate the significance of the alcohol-characteristic liver niche in cancer patients, we analyzed the TCGA human liver cancer dataset, which contained 50 cases of normal liver tissue sequencing data and corresponding patient survival information (Figure 7A). First, we performed a high-throughput hazard ratio (HR) analysis. In the mouse liver tissue of the alcohol-treated group vs. the control differential gene set, 2,398 genes were homologous to those in humans and mice. An overall survival (OS) analysis was performed for each homologous gene. As a result, the average HR of the upregulated genes was higher than that of the downregulated genes (Figure 7B). More than 90% of upregulated genes were positively associated with cancer progression, of which LCN2 was the most prominent (Figure 7C). Such gene upregulation in noncancerous liver tissues may form an unfavorable niche.
Figure 7.
Alcohol-characteristic signaling activation in liver tissue promotes cancer progression
(A–D) Survival analysis of hepatocellular carcinoma patients by alcohol-characteristic signaling activation in noncancerous liver tissue. (A) Schematic analysis workflow. (B) The human-homologous genes that were upregulated by alcohol in the mouse liver showed a higher average overall survival hazard ratio (HR) than the downregulated homologous genes. (C) HR distribution of the upregulated and downregulated genes. (D) The patients were categorized using the AlcoMetas score. High AlcoMetas score indicated adverse overall survival. (E) Correlation analysis of the IL-6-JAK-STAT3 signature and the LCN2 and LY6GC count values each with the AlcoMetas Score. (F) Kaplan-Meier overall survival analysis by the IL-6-JAK-STAT3 signature and LCN2 and LY6GC count values.
Hence, we focused on the top 100 upregulated genes and identified 77 homologous genes in humans (Table S1). By adding the normalized FPKM Z score, we generated the AlcoMetas score (Figure 7A). The patients were categorized using this AlcoMetas Score. The higher the AlcoMetas score in the noncancerous liver tissue, the greater the shared similarity with the microenvironment of alcohol-associated signaling activation characteristics. As expected, the OS time of the high-AlcoMetas-score group was significantly shorter than that of the low-score group. There was a more than 2-fold HR increment (Figure 7D). In addition, the AlcoMetas score positively correlated with the IL-6-JAK-STAT3 signature, as well as LCN2 and LY6G5C mRNA expression in liver tissue (Figure 7E). Moreover, enhanced IL-6-JAK-STAT3 signature and high LCN2 and LY6G5C mRNA expression also indicated adverse cancer prognosis (Figure 7F). Collectively, these clinical data further support our experimental findings.
Alcoholic liver premetastatic niche crosstalk can be interrupted by BBI608
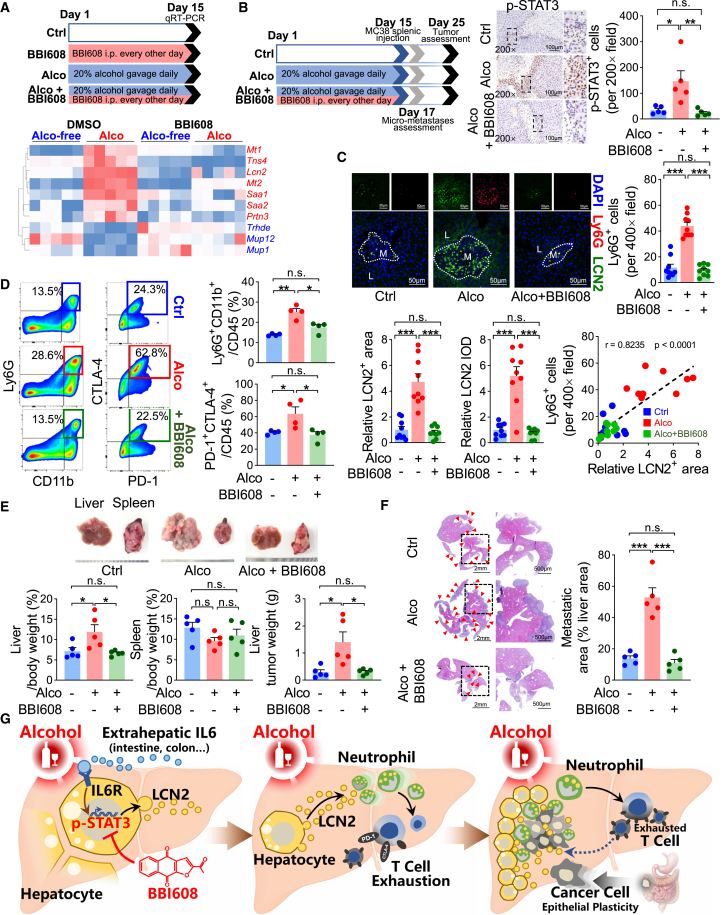
BBI608 (also called napabucasin) is a small-molecule inhibitor of STAT3, for which human safety has been confirmed in previous clinical trials.23,24,25 Given our experimental findings that hepatocyte STAT3 activation facilitates liver metastasis, we hypothesized that BBI608 might prevent the alcoholic pro-liver metastatic niche formation. BBI608 was given every other day in advance of the daily alcohol treatment to the mice, and the mouse liver genes that share the common backgrounds of both alcoholic injury and STAT3 modulation were assessed by qRT-PCR. Similar to the case of the hepatocellular Stat3 knockout, BBI608 pretreatment generally reversed the gene expression alterations by alcohol exposure in vivo (Figure 8A). As confirmed in vitro, BBI608 pretreatment ameliorated alcohol- and rmIL-6-induced Lcn2 mRNA expression in primary mouse hepatocytes (Figure 4B).
Figure 8.
BBI608 prevents liver metastasis by targeting the alcohol-induced premetastatic liver niche
(A) The expression of mouse liver genes sharing the backgrounds of both alcoholic injury and STAT3 modulation was assessed under the conditions of alcohol and/or BBI608. Analyses of (B) p-STAT3 expression by IHC, (C) LCN2 and Ly6G expression by immunofluorescence, and (D) infiltrated neutrophil and exhausted T lymphocyte proportions by flow cytometry (B, n = 5; C, n = 9; D, n = 4) in mouse liver treated with BBI608 and alcohol. (E and F) BBI608 administration against alcohol pretreatment prevented liver metastasis quantified by (E) the liver and tumor weight (n = 5) and (F) the liver metastatic area. Red arrowheads indicate metastases. (G) A schematic summarizing the current study. Alcohol induces hepatocyte STAT3 phosphorylation by upregulating hepatocellular IL-6R and extrahepatic IL-6. Consequently, hepatocytes upregulate and secrete LCN2, which induces neutrophil recruitment and cancer cell mesenchymal-to-epithelial transition. Neutrophils direct a T cell-exhausted immunosuppressive niche for cancer cell settlement. BBI608 is demonstrated as a promising drug against the alcohol-induced liver premetastatic niche. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
After BBI608 and alcohol pretreatment, MC38 cells were injected into the spleen, then neither alcohol nor BBI608 was administered; the liver tissue was analyzed 48 h after the implantation. Near the metastatic lesions, we found that BBI608 pretreatment significantly suppressed alcohol-promoted STAT3 phosphorylation (Figure 8B). As a result, the alcohol-associated live premetastatic niche formation was inhibited by BBI608 pretreatment. Both hepatocyte LCN2 expression and neutrophil infiltration were greatly restrained yet maintained the positive correlation between them (Figure 8C). As a consequence of neutrophil infiltration, BBI608 protected CD8 lymphocytes from alcohol-induced exhaustion (Figure 8D). Finally, the preventive effect of BBI608 on liver metastasis was evaluated. Ten days after tumor implantation, the mice were dissected for tumor assessment. Strikingly, the protective effect of BBI608 against alcohol-related STAT3 signaling activation was liver tendentious: alcohol-promoted liver metastasis was dramatically inhibited (Figures 8E and 8F), whereas tumor growth in the spleen was not influenced (Figure 8E), suggesting that it is a promising agent against liver premetastatic niche formation.
Discussion
The prognostic outcome of liver damage caused by alcohol consumption in cancer patients is not clear. Although alcohol abstinence may help in cancer prevention (primary prevention), those already diagnosed with cancer may be less concerned with it. In this study, we revealed the harm caused by alcohol consumption in cancer patients from a novel perspective (secondary and tertiary prevention). Alcohol facilitates liver metastasis by reshaping a liver premetastatic niche via extra- and intrahepatic crosstalk-mediated immune evasion. Moreover, we discovered a novel therapeutic strategy using BBI608 for liver metastasis prevention by targeting such microenvironment (Figure 8G).
Generally, alcohol beverages can be categorized as fermented drinks (ethanol content below 20% alc/vol, such as wine and beer) and distilled drinks (over 20% alc/vol, such as liquor). The current study used 20% alc/vol for alcohol induction, which led to steatosis but without fibrosis in mouse liver. In humans with alcoholic diseases, more than 95% of heavy drinkers develop fatty liver, but only up to 35% of this population develops more severe forms (i.e., fibrosis, alcoholic hepatitis, cirrhosis, and hepatocellular carcinoma).26 Thus, the current model is representative of the majority. Previously, many studies have addressed the influence of alcohol on liver disease, but few have examined cancer patients. This study showed that pancreatic cancer patients with an alcohol exposure history displayed increased susceptibility to liver metastasis. The essence of the liver premetastatic niche is the alcohol-triggered molecular alterations. Thus, we generated an AlcoMetas score to represent a gene expression background similar to our alcohol model. Since it is ethically infeasible to perform a liver biopsy in patients who have not developed liver lesions, we analyzed the noncancerous liver tissue from hepatocellular carcinoma patients. Patients with a high AlcoMetas score in the liver tissue showed an adverse prognosis. These data suggest that the liver niche with a genetic background similar to that of alcoholic liver is detrimental to cancer survival. Therefore, corresponding prevention and treatment strategies targeting such characteristic niches are urgently needed.
We showed that the alcohol-induced liver premetastatic niche was characterized by IL-6/STAT3 signaling activation. Interestingly, IL-6 elevation in serum and IL-6Rα upregulation in hepatocytes were observed, indicating that alcohol induced IL-6/STAT3 signaling activation in an extra- and intrahepatic synergistic manner. It is known that the primary sources of IL-6 production are monocytes and macrophages, while muscle and stromal cells are also reported to produce IL-6 in response to distinct stimulation.27 Here, we used the Il6 reporter transgenic mouse to trace the source and found that it was extrahepatic organs, other than the liver, that generated IL-6 under alcohol stimulation. Unlike IL-6, transmembrane IL-6R is expressed only on the surface of a few cell types, such as hepatocytes. The interaction of IL-6 and IL-6R is necessary for initiating the “classical IL-6 signaling.”27,28 This tissue specificity of IL-6R elevation explains why alcohol promotes liver metastasis, but not tumor growth, in the subcutaneous or spleen.
Although serum IL-6 elevation might protect hepatocytes against alcohol-induced apoptosis,29 its downstream STAT3 activation in hepatocytes could direct the formation of a pro-metastatic niche in the liver.10 In this study, systemic Il6 knockout and specific Stat3 knockout in hepatocytes showed significant inhibitory effects on alcohol-induced liver metastasis, as well as on the niche alterations. As a transcriptional factor, STAT3 activation in the liver upregulates several inflammatory factors.10,30 Here, we found that the acute-phase protein LCN2 was among the secretory factors that were most remarkably upregulated by alcohol exposure. Originally discovered in human neutrophils, LCN2 is also known as neutrophil gelatinase-associated lipocalin. However, upon IL-6/STAT3 signaling activation, hepatocytes, rather than neutrophils, are the major source of elevated serum LCN2.11 Consistently, in this study, alcohol increased both hepatocyte Lcn2 expression and LCN2-positive neutrophil infiltration, in a hepatocyte IL-6/STAT3 signaling-dependent manner. In vitro, alcohol stimulation did not further upregulate neutrophil Lcn2 expression.
Extracellular LCN2 acts as a chemoattractant31 and migration activator32 for neutrophils. In severe alcohol-induced hepatitis and other liver diseases, neutrophil-derived LCN2 participates in the neutrophil extracellular trap formation.33 For cancer cells, LCN2 was reported to be highly expressed in hepatocellular carcinoma but acted as a distant metastasis suppressor.13 Consistently, the metastatic suppressive effect was also reported when LCN2 was highly expressed in pancreatic cancer and colon cancer cells.17,18 However, in nicotine-treated mouse models, LCN2-secreting neutrophil numbers were higher in the lungs, facilitating lung metastasis of breast cancer.16 The reason for this apparent contradiction is that LCN2 negatively modulates the epithelial-to-mesenchymal transition, facilitating cancer cell settlement. Moreover, in leptomeningeal metastasis, IL-6 from the cerebrospinal spinal fluid induces cancer cell LCN2 expression, supporting cancer growth in the hypoxic microenvironment.15 Hence, it seems that the expression site of LCN2 affects the tendency of metastasis. Taken together with our findings that both hepatocyte-derived LCN2 and LCN2-positive neutrophil infiltration increased in the liver, it is not difficult to understand why cancer cells are more likely to metastasize to the alcohol-remodeled liver.
Nevertheless, LCN2 elevation alone is not sufficient to increase metastases. We found that alcohol affected liver metastasis of only immune-competent mice, rather than that of athymic mice, indicating that T cell immune abnormalities are necessary for pro-metastasis niche remodeling. It was reported that exhausted T cells increased in alcoholic liver disease,34 while increased circulating IL-6 was associated with CD8 T cell exhaustion by neutrophil recruitment.35 Here, we show that, in C57BL/6 immune-competent mice, CD8 T cell levels decreased and gained exhausted T cell characteristics. In vitro, we demonstrated that LCN2 did not directly influence T cell immunity; however, LCN2 influenced the immunity in a neutrophil-dependent manner. Hence, with the addition of LCN2-driven neutrophil recruitment in the liver, T cell immune surveillance breaches provide the necessary key conditions for tumor cell colonization.
Based on this newly discovered mechanism, we provide a novel application prospect of BBI608 in cancer liver metastasis prevention. BBI608 is a STAT3 inhibitor proven safe humans in several clinical trials.23,24,25 Although the development of BBI608 has attracted great attention, some clinical trial data from recent years are not that satisfactory. However, the positive results achieved are enlightening. In a phase III trial of metastatic colorectal cancer, BBI608 monotherapy showed OS benefits in the subgroup of p-STAT3-positive tumors.23 In another phase II trial, BBI608 in combination with pembrolizumab, an anti-PD-1 antibody, provided an improved response in some microsatellite stable metastatic colorectal cancers,24 which previously proved insensitive to the anti-PD-1 antibody alone.36,37 Hence, we believe that trials of BBI608 must be optimized to screen suitable patient populations. Based on the findings that alcohol-treated hepatocytes are p-STAT3 positive with PD-1 lymphocyte infiltration, we demonstrated the preventive effect of BBI608 in improving the immunity of alcohol-treated mouse livers, thus restraining liver metastasis. Interestingly, the protective effect was significantly more obvious in liver metastasis, but not regarding tumor growth in the spleen. This evidence further shows that alcohol-induced metastasis is liver tendentious by preferentially activating hepatocyte STAT3 signaling. Our current findings may provide a new theoretical basis for the future clinical trial design of BBI608 and propose a new prospect for BBI608 in liver metastasis prevention.
We acknowledge that there still remain some issues not identified sufficiently in the current study. For example, we mainly focused on the IL-6/STAT3/LCN2 signaling, while the sequencing result suggests that alcohol may also aberrantly activate many other signaling pathways (e.g., NF-κB signaling), which have also been reported to be involved in alcoholic liver injury26 and a premetastatic niche formation.38,39 Indeed, we believe that there may well be more than one mechanism that participates in the alcohol-induced premetastatic niche formation; however, the IL-6/STAT3/LCN2 signaling activation in hepatocytes is one of the most important. Furthermore, the small-molecule STAT3 inhibitor BBI608 may affect whole systems more than the liver. A recent study reported that BBI608 could suppress myeloid-derived suppressor cells in mice to enhance anti-tumor immunity,40 which also reveals the prospect of anti-STAT3 therapy from different perspectives. In the mouse model of the current study, STAT3 was highly activated by alcohol in hepatocytes. Thus, the hepatocytes are theoretically much more likely to be and efficient as the target of BBI608 under alcoholic stress, but the mechanisms of BBI608 under many other conditions are still worth further exploration.
In conclusion, alcohol is detrimental to cancer patients. It initiates an immune evasion cascade via the extra- and intrahepatic crosstalk along the IL-6/STAT3/LCN2 axis, forming a liver premetastatic niche. BBI608 is a promising agent targeting the niche, and further clinical exploration may help to optimize preventive and therapeutic strategies on liver metastasis.
Materials and methods
Animals
All animal studies were approved by the Institutional Animal Care and Use Committee of Nanfang Hospital (ethics no. L2019251). All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” and the institutional guidelines. The mice were raised under specific-pathogen-free conditions at our center. Wild-type C57BL/6, BALB/c, and athymic mice were obtained from the Southern Medical University Laboratory Animal Center.
Commercial transgenic Stat3flox/flox (strain 016923) and Albumin-Cre mice (strain 016832) were from The Jackson Laboratory. The original mouse model construction process was published previously.41,42 For Stat3flox/flox, a loxP site-flanked targeting vector was inserted upstream of exon 18 of Stat3, and another loxP site was inserted downstream of exon 20. When Stat3flox/flox mice are bred to Albumin-Cre mice that express Cre recombinase under the control of albumin (Alb) promoter/enhancer elements, the offspring will have exons 18, 19, and 20 deleted in the Alb and Cre-expressing hepatocytes.10
The Il6em1(Luc-eGFP)Smoc mice (NM-KI-00011) were commercially obtained from the Shanghai Model Organisms Center (Shanghai, China). The luciferase-2a-eGFP-pA expression fragment was inserted into the Il6 translation start site ATG. Il6 gene expression in this mouse is lacking, while the endogenous promoter drives the expression of the luciferase reporter gene and eGFP. Homozygous Il6em1(Luc-eGFP)Smoc mice were used in the experiments.
The primers used to verify the genotypes are presented in Table S2. All models were created using 4-week-old male mice and grouped by randomization.
Alcohol induction
For alcohol induction, mice were administered edible alcohol (15 μL/g) by gavage daily for 2 weeks. It is worth noting that the neurological symptoms of acute alcohol intoxication in mice were obvious at a higher concentration (40% alc/vol), while the liver pathological changes were not significant enough at lower concentration (10% alc/vol) with the same induction time (Figures S1A and S1B); thus, we finally adopted 20% alc/vol for all the formal experiments.6,43 To better simulate the physiological condition, a normal saline solution was used as the control fluid. Using the normal saline solution for gavage did not show a significance difference in liver metastases compared with water (Figure S7).
BBI608 administration
By referring to previous studies,44,45 BBI608 (SelleckChem, Shanghai, China), which blocked STAT3 signaling, was administered at 20 mg/kg by intraperitoneal injection every other day for 2 weeks and was given 1 h ahead of alcohol induction.
Tumor implantation and evaluation
To induce liver metastasis, cancer cells were injected into the spleen as previously described.46 Mice were anesthetized using pentobarbital sodium (50 mg/kg) by intraperitoneal injection. In brief, an incision of about 1 cm in the midline of the left abdomen was cut after depilation and disinfection. The spleen was pulled out gently and injected with 100 μL cell suspension (about 1 × 106/mL) through a 1 mL insulin needle. After the blood was adequately stopped, the spleen was returned to the abdominal cavity and the peritoneum closed layer by layer.
To evaluate the tumor burden on liver and spleen, indicators of gross liver and spleen weight, liver tumor net weight, and tumor metastatic area were used.6,47 After pentobarbital sodium (50 mg/kg) anesthesia, mice were sacrificed by cervical dislocation. For liver metastasis weights, the tumors were separated from the liver. Due to the textural differences, the tumor tissue could be easily separated from the liver by blunt dissection. The tissue and the separated tumors were weighed on an electronic scale. For tumor metastatic area evaluation, the liver tissue with metastases was paraffin embedded and cut into 4 μm sections for hematoxylin and eosin staining. The metastatic areas on the section were analyzed using Image-Pro Plus software (Media Cybernetics, Rockville, MA, USA).
To evaluate subcutaneous tumor growth, cancer cells (about 1 × 106/mL) were implanted at the right flank.46 Tumor nodule volumes were monitored every other day according to the formula volume = width2 × length/2.48
Cell culture and reagents
All cells were cultured at 37°C in a 5% CO2 atmosphere.
Mouse colon carcinoma cell lines MC38 and CT26 were obtained from the American Type Culture Collection. Cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS), streptomycin (100 μg/mL), and penicillin (100 units/mL).
C57BL/6 mouse primary hepatocytes were isolated as described.49 In brief, after anesthesia, the vena cava was cannulated. The liver was perfused with chelate calcium to wash out the blood and then collagenase for extracellular matrix dissociation, and then the liver was dissected. Hepatocytes were purified by density-based separation. Hepatocytes were cultured in 10% FBS DMEM. After adherence for 48 h, hepatocytes were incubated in medium with additional alcohol (50 mM), rmIL-6 (50 ng/mL; MedChemExpress, Monmouth Junction, NJ, USA), and/or BBI608 (0.1 mM) for 72 h.
To isolate mouse primary neutrophils, the femur and tibia of euthanized mice were removed and flushed using a 25G needle filled with PBS. Cells were further purified using a mouse bone marrow neutrophil isolation kit (Solarbio, Beijing, China) following the manufacturer’s recommendation. Neutrophils were cultured in 10% FBS RPMI 1640.
RNA sequencing and analysis
RNA sequencing of mouse liver tissues was carried out using the Illumina HiSeq X10 platform (San Diego, CA, USA). Genes of average FPKM change >2-fold and p < 0.05 (by Student’s t test) were considered differentially expressed.
GSEA was performed using GSEA v.3.0 based on the Molecular Signatures Database (MSigDB) (https://www.gsea-msigdb.org/) and the characteristic gene of the pro-liver metastasis niche (GSE109480).10
Histological analysis
After being fixed in 4% paraformaldehyde and embedded in paraffin, tissues were cut into 4 μm sections. Hematoxylin and eosin staining, immunohistochemistry staining, and immunofluorescence staining were performed following the conventional protocols.46,50 For immunohistochemistry staining, primary antibodies to IL-6Ra (Bioss, Beijing, China; 1:300), LCN2 (Abcam, Cambridge, MA, USA; 1:2,000), and p-STAT3 (Abcam; 1:100) were used. Slices were incubated with secondary antibody and DAB solution (ZSGB-BIO OriGene, Beijing, China) for color development. For immunofluorescence staining, slices were stained by primary antibodies to LCN2 (Abcam; 1:800) and Ly6G (BioLegend, San Diego, USA; 1:80) and subsequent secondary antibody and DAPI. Sudan Black B (0.3%) in 70% ethanol was used to reduce autofluorescence. Immunofluorescence images were captured by confocal microscope analysis (Nikon, Tokyo, Japan). Images were analyzed using Image-Pro Plus software to calculate the positively stained area and integrated optical density (IOD).
ELISA
Mouse serum IL-6 level was tested with an ELISA kit according to the instructions (MultiScience, Hangzhou, China).
Flow cytometry
Mouse liver was separated and dissected by cutting off the portal vein and the inferior vena cava. After being thoroughly washed in PBS, tissues were immersed in an enzymatic hydrolysis solution containing type IV collagenase (5 mg/mL; Invitrogen) and DNase I (0.1 mg/mL; Solarbio) (37°C, Hanks solution) and fully ground with a tissue dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany). To obtain a single-cell suspension, the tissue homogenate was passed through 70 μm cell strainer and centrifuged to remove hepatocytes and impurities. Afterward, it was resuspended in red blood cell lysis buffer at room temperature for 10 min to remove red blood cells.
To identify immune cell subsets, cells were first incubated with extracellular antibodies including APC-CD3 (Biolegend; 100236), PerCP/Cyanine5.5-CD4 (MultiScience; 70-AM00407-100), PE-CD19 (MultiScience; AM01904), FITC-CD8a (eBioscience; 11-0081-82), PerCP/Cyanine5.5-CD8a (Biolegend; 100734), eFluor 506-CD45 (eBioscience; 69-0451-82), PE-CD279 (PD-1) (Biolegend; 135205), Brilliant Violet 421TM-CD152 (Biolegend; 106312), Brilliant Violet 650TM-Ly6G (Biolegend; 127641), and Alexa Fluor 700-CD11b (Biolegend; 101222), and then incubated with intracellular antibodies, including PE/Cyanine7-IFN-γ (Biolegend; 505826) and APC-Ki-67 (Biolegend; 652405), after fixation and rupture. Flow cytometry was performed on BD platforms and stasis was analyzed by FlowJo software version 10.62 (TreeStar, Ashland, OR, USA).
Cell adhesion and cell viability assays
MC38 cells (4 × 104 cells per well) were seeded into 96 well plates coated with Matrigel (50 μL/well, 0.04 μg/μL). After forming a monolayer, the cells were cultured for 28 h in the presence and absence of rmLcn2 (1 μg/mL). The nonadherent MC38 cells were removed from the plate by washing with PBS. The remaining cells were quantified by adding CCK8 solution. After 1 h incubation, the absorbance at 450 nm was measured. Cell viability at different time points was also analyzed using CCK8 solution without adding Matrigel.
Chemotaxis assay
Chemotaxis assay was performed using the 5-μm-pore Boyden chamber system with uncoated polyester membrane (Corning, Tewksbury, MA, USA). Neutrophils were seeded on the upper chamber with 0.1% FBS RPMI 1640, while the lower chamber additionally contained rmLCN2 (1 μg/mL) or liver homogenate (10 μL/mL). The liver homogenate LCN2 was neutralized by monoclonal mouse anti-LCN2 (clone 228416; R&D Systems) for 1 h, 37°C, preincubation. After incubating at 37°C for 12 h, the cells in the lower chambers were observed under microscope (Nikon, Tokyo, Japan).
Real-time quantitative PCR
Total RNA was extracted using TRIzol reagent (Vazyme, Nanjing, China) according to the manufacturer’s protocols and reverse transcribed using the First Strand cDNA Synthesis kit (Vazyme). The amplification of cDNA was performed in 10 μL reactions on a LightCycler 480 system (Roche, Rotkreuz, Switzerland) with SYBR Premix Ex Taq II (Tli RNaseH Plus) (Vazyme). Gapdh was used as the internal control gene for qPCR. The primers used are listed in Table S3. The mean cycle threshold was determined by triplicate PCR runs, and the relative expression was normalized to that of the internal control via the 2−ΔΔCt method.
Western blotting
Western blotting procedures were as previously described.50 Primary antibodies of anti-STAT3 (ABclonal, Wuhan, China; 1:1,000), anti-p-STAT3 (Cell Signaling Technology, Beverly, MA, USA; 1:2,000), and anti-GAPDH (Sigma, Darmstadt, Germany; 1:5,000) were used.
Immune cell correlation analysis
The ingle-sample GSEA (ssGSEA) was used to quantify the relative infiltration of 29 immune cell types in the microenvironment according to the expression levels of immune cell-specific marker genes. Feature gene panels of the immune cell types were obtained from recent publications.51,52,53 The relative abundance of each immune cell type was represented by an enrichment score. The ssGSEA was performed based on the R “GSVA” package. The Spearman correlation coefficients of these immune cells’ infiltration abundance were calculated.
High-throughput HR analysis and AlcoMetas score of hepatocellular carcinomas
The analysis of hepatocellular carcinoma was based on the data from TCGA and FPKM data, together with the clinical information of 50 adjacent normal tissues from the liver hepatocellular carcinoma (LIHC) set. Alcohol vs. control differentially expressed mouse genes (DEGs) were converted to homologous genes of Homo sapiens using the R package “homologene.” The survival HR for each homologous gene was calculated based on the FPKM value.
From the top 100 upregulated DEGs, 77 genes homologous to Homo sapiens were identified. The AlcoMetas score was established based on the combined expression Z scores of the 77 homologous genes.
The optimal cutoff value for Kaplan-Meier survival analysis was determined by the receiver-operating characteristic (ROC) method. The SuvivalROC R package was adopted with the predict.time of 3 years.
Statistics
Statistical analyses were conducted using GraphPad Prism (version 8.0.2) (La Jolla, CA, USA). The results are presented as the mean ± SEM. For the comparison of means, the significance of differences was analyzed using one-way ANOVA or Student’s t test. Correlations between two continuous variables were examined using Pearson’s test. Survival curves were constructed using the Kaplan-Meier method and compared with the log-rank test. A two-tailed p < 0.05 was considered statistically significant.
Data availability
Data are available in a public, open access repository. The alcohol-pretreated wild-type mouse liver and hepatocellular Stat3 knockout mouse liver sequencing data were deposited in the Gene Expression Omnibus database, under accession nos. GSE189277 and GSE224602, respectively.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81972319 to L.L.) and the Natural Science Foundation of Guangdong Province (2021A1515010003 to L.L.). We acknowledge Zhongyi Dong from the Department of Radiation Oncology, Yongfeng Liu and Bing Huang from the Department of Gastroenterology, and Zhizhang Wang from the Department of Pathology, Nanfang Hospital, Southern Medical University, for their kind help and constructive advice.
Author contributions
L.L. designed the project. X.Q., J. Zhou, and L.L. performed the animal experiments. J. Zhou, Y. Li, and Q.W. performed the cellular experiments. H.X., Yuanhan Liu, and L.L. performed the bioinformatics analyses. K.Z., Yantan Liu, J.O., and J. Zhang performed the molecular biology experiments. S.M., M.S., and L.L. analyzed the clinical data. L.L., H.Q., J.D., W.L., and Y. Liao wrote the manuscript. J. Zhou, Q.W., Yuanhan Liu, and K.Z. accessed and verified the original data. X.Q., J. Zhou, H.X., Y. Li, and S.M. share the co-first authorship based on their contributions, and the order was determined by when they joined the project.
Declaration of interests
The authors declare that no competing interests exist.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.07.012.
Supplemental information
References
- 1.Tumeh P.C., Hellmann M.D., Hamid O., Tsai K.K., Loo K.L., Gubens M.A., Rosenblum M., Harview C.L., Taube J.M., Handley N., et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol. Res. 2017;5:417–424. doi: 10.1158/2326-6066.CIR-16-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie R., Chen X., Cheng L., Huang M., Zhou Q., Zhang J., Chen Y., Peng S., Chen Z., Dong W., et al. NONO Inhibits Lymphatic Metastasis of Bladder Cancer via Alternative Splicing of SETMAR. Mol. Ther. 2021;29:291–307. doi: 10.1016/j.ymthe.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reichert M., Bakir B., Moreira L., Pitarresi J.R., Feldmann K., Simon L., Suzuki K., Maddipati R., Rhim A.D., Schlitter A.M., et al. Regulation of Epithelial Plasticity Determines Metastatic Organotropism in Pancreatic Cancer. Dev. Cell. 2018;45:696–711.e8. doi: 10.1016/j.devcel.2018.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nimmakayala R.K., Leon F., Rachagani S., Rauth S., Nallasamy P., Marimuthu S., Shailendra G.K., Chhonker Y.S., Chugh S., Chirravuri R., et al. Metabolic programming of distinct cancer stem cells promotes metastasis of pancreatic ductal adenocarcinoma. Oncogene. 2021;40:215–231. doi: 10.1038/s41388-020-01518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer Alcohol drinking. IARC Monogr. Eval. Carcinog. Risks Hum. 1988;44 [PMC free article] [PubMed] [Google Scholar]
- 6.Im H.J., Kim H.G., Lee J.S., Kim H.S., Cho J.H., Jo I.J., Park S.J., Son C.G. A Preclinical Model of Chronic Alcohol Consumption Reveals Increased Metastatic Seeding of Colon Cancer Cells in the Liver. Cancer Res. 2016;76:1698–1704. doi: 10.1158/0008-5472.CAN-15-2114. [DOI] [PubMed] [Google Scholar]
- 7.Torok N.J. Update on Alcoholic Hepatitis. Biomolecules. 2015;5:2978–2986. doi: 10.3390/biom5042978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen-Mersh T.G., Glover C., Fordy C., Henderson D.C., Davies M. Relation between depression and circulating immune products in patients with advanced colorectal cancer. J. R. Soc. Med. 1998;91:408–413. doi: 10.1177/014107689809100803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson D.E., O'Keefe R.A., Grandis J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018;15:234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J.W., Stone M.L., Porrett P.M., Thomas S.K., Komar C.A., Li J.H., Delman D., Graham K., Gladney W.L., Hua X., et al. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature. 2019;567:249–252. doi: 10.1038/s41586-019-1004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu M.J., Feng D., Wu H., Wang H., Chan Y., Kolls J., Borregaard N., Porse B., Berger T., Mak T.W., et al. Liver is the major source of elevated serum lipocalin-2 levels after bacterial infection or partial hepatectomy: a critical role for IL-6/STAT3. Hepatology. 2015;61:692–702. doi: 10.1002/hep.27447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao F., Deng Y., Zhao Y., Mei Y., Zhang Y., Liu X., Martinez C., Su X., Rosato R.R., Teng H., et al. A targetable LIFR-NF-kappaB-LCN2 axis controls liver tumorigenesis and vulnerability to ferroptosis. Nat. Commun. 2021;12:7333. doi: 10.1038/s41467-021-27452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y.P., Yu G.R., Lee M.J., Lee S.Y., Chu I.S., Leem S.H., Kim D.G. Lipocalin-2 negatively modulates the epithelial-to-mesenchymal transition in hepatocellular carcinoma through the epidermal growth factor (TGF-beta1)/Lcn2/Twist1 pathway. Hepatology. 2013;58:1349–1361. doi: 10.1002/hep.26467. [DOI] [PubMed] [Google Scholar]
- 14.Wieser V., Tymoszuk P., Adolph T.E., Grander C., Grabherr F., Enrich B., Pfister A., Lichtmanegger L., Gerner R., Drach M., et al. Lipocalin 2 drives neutrophilic inflammation in alcoholic liver disease. J. Hepatol. 2016;64:872–880. doi: 10.1016/j.jhep.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 15.Chi Y., Remsik J., Kiseliovas V., Derderian C., Sener U., Alghader M., Saadeh F., Nikishina K., Bale T., Iacobuzio-Donahue C., et al. Cancer cells deploy lipocalin-2 to collect limiting iron in leptomeningeal metastasis. Science. 2020;369:276–282. doi: 10.1126/science.aaz2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyagi A., Sharma S., Wu K., Wu S.Y., Xing F., Liu Y., Zhao D., Deshpande R.P., D'Agostino R.B., Jr., Watabe K. Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating pre-metastatic niche in lung. Nat. Commun. 2021;12:474. doi: 10.1038/s41467-020-20733-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Tong Z., Kunnumakkara A.B., Wang H., Matsuo Y., Diagaradjane P., Harikumar K.B., Ramachandran V., Sung B., Chakraborty A., Bresalier R.S., et al. Neutrophil gelatinase-associated lipocalin: a novel suppressor of invasion and angiogenesis in pancreatic cancer. Cancer Res. 2008;68:6100–6108. doi: 10.1158/0008-5472.CAN-08-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H.J., Lee E.K., Lee K.J., Hong S.W., Yoon Y., Kim J.S. Ectopic expression of neutrophil gelatinase-associated lipocalin suppresses the invasion and liver metastasis of colon cancer cells. Int. J. Cancer. 2006;118:2490–2497. doi: 10.1002/ijc.21657. [DOI] [PubMed] [Google Scholar]
- 19.Kim A., Wu X., Allende D.S., Nagy L.E. Gene Deconvolution Reveals Aberrant Liver Regeneration and Immune Cell Infiltration in Alcohol-Associated Hepatitis. Hepatology. 2021;74:987–1002. doi: 10.1002/hep.31759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y., Diao N., Lee C.K., Chu H.W., Bai L., Li L. Neutrophils Deficient in Innate Suppressor IRAK-M Enhances Anti-tumor Immune Responses. Mol. Ther. 2020;28:89–99. doi: 10.1016/j.ymthe.2019.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Germann M., Zangger N., Sauvain M.O., Sempoux C., Bowler A.D., Wirapati P., Kandalaft L.E., Delorenzi M., Tejpar S., Coukos G., Radtke F. Neutrophils suppress tumor-infiltrating T cells in colon cancer via matrix metalloproteinase-mediated activation of TGFbeta. EMBO Mol. Med. 2020;12:e10681. doi: 10.15252/emmm.201910681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Z., Liu Y., Yang C., Li X., Pan C., Rao J., Li N., Liao W., Lin L. Combined neutrophil/platelet/lymphocyte/differentiation score predicts chemosensitivity in advanced gastric cancer. BMC Cancer. 2018;18:515. doi: 10.1186/s12885-018-4414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonker D.J., Nott L., Yoshino T., Gill S., Shapiro J., Ohtsu A., Zalcberg J., Vickers M.M., Wei A.C., Gao Y., et al. Napabucasin versus placebo in refractory advanced colorectal cancer: a randomised phase 3 trial. Lancet Gastroenterol. Hepatol. 2018;3:263–270. doi: 10.1016/S2468-1253(18)30009-8. [DOI] [PubMed] [Google Scholar]
- 24.Kawazoe A., Kuboki Y., Shinozaki E., Hara H., Nishina T., Komatsu Y., Yuki S., Wakabayashi M., Nomura S., Sato A., et al. Multicenter Phase I/II Trial of Napabucasin and Pembrolizumab in Patients with Metastatic Colorectal Cancer (EPOC1503/SCOOP Trial) Clin. Cancer Res. 2020;26:5887–5894. doi: 10.1158/1078-0432.CCR-20-1803. [DOI] [PubMed] [Google Scholar]
- 25.Noda N., Takagaki T., Yodo Y., Horibuchi Y., Iino S., Matsuki S., Ogama Y., Kakuyama H. Effects of a reactive oxygen species generator, napabucasin (BBI608), on tolerability, safety, pharmacokinetics, and QT/QTc interval in healthy volunteers. Pharmacol. Res. Perspect. 2021;9:e00874. doi: 10.1002/prp2.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao B., Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones S.A., Jenkins B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018;18:773–789. doi: 10.1038/s41577-018-0066-7. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt-Arras D., Rose-John S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016;64:1403–1415. doi: 10.1016/j.jhep.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Hong F., Kim W.H., Tian Z., Jaruga B., Ishac E., Shen X., Gao B. Elevated interleukin-6 during ethanol consumption acts as a potential endogenous protective cytokine against ethanol-induced apoptosis in the liver: involvement of induction of Bcl-2 and Bcl-x(L) proteins. Oncogene. 2002;21:32–43. doi: 10.1038/sj.onc.1205016. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L., Kuca K., You L., Zhao Y., Musilek K., Nepovimova E., Wu Q., Wu W., Adam V. Signal transducer and activator of transcription 3 signaling in tumor immune evasion. Pharmacol. Ther. 2022;230:107969. doi: 10.1016/j.pharmthera.2021.107969. [DOI] [PubMed] [Google Scholar]
- 31.Schroll A., Eller K., Feistritzer C., Nairz M., Sonnweber T., Moser P.A., Rosenkranz A.R., Theurl I., Weiss G. Lipocalin-2 ameliorates granulocyte functionality. Eur. J. Immunol. 2012;42:3346–3357. doi: 10.1002/eji.201142351. [DOI] [PubMed] [Google Scholar]
- 32.Ye D., Yang K., Zang S., Lin Z., Chau H.T., Wang Y., Zhang J., Shi J., Xu A., Lin S., Wang Y. Lipocalin-2 mediates non-alcoholic steatohepatitis by promoting neutrophil-macrophage crosstalk via the induction of CXCR2. J. Hepatol. 2016;65:988–997. doi: 10.1016/j.jhep.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 33.Li H., Feng D., Cai Y., Liu Y., Xu M., Xiang X., Zhou Z., Xia Q., Kaplan M.J., Kong X., Gao B. Hepatocytes and neutrophils cooperatively suppress bacterial infection by differentially regulating lipocalin-2 and neutrophil extracellular traps. Hepatology. 2018;68:1604–1620. doi: 10.1002/hep.29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markwick L.J.L., Riva A., Ryan J.M., Cooksley H., Palma E., Tranah T.H., Manakkat Vijay G.K., Vergis N., Thursz M., Evans A., et al. Blockade of PD1 and TIM3 restores innate and adaptive immunity in patients with acute alcoholic hepatitis. Gastroenterology. 2015;148:590–602.e10. doi: 10.1053/j.gastro.2014.11.041. [DOI] [PubMed] [Google Scholar]
- 35.Koyama S., Akbay E.A., Li Y.Y., Aref A.R., Skoulidis F., Herter-Sprie G.S., Buczkowski K.A., Liu Y., Awad M.M., Denning W.L., et al. STK11/LKB1 Deficiency Promotes Neutrophil Recruitment and Proinflammatory Cytokine Production to Suppress T-cell Activity in the Lung Tumor Microenvironment. Cancer Res. 2016;76:999–1008. doi: 10.1158/0008-5472.CAN-15-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Neil B.H., Wallmark J.M., Lorente D., Elez E., Raimbourg J., Gomez-Roca C., Ejadi S., Piha-Paul S.A., Stein M.N., Abdul Razak A.R., et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One. 2017;12:e0189848. doi: 10.1371/journal.pone.0189848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Overman M.J., McDermott R., Leach J.L., Lonardi S., Lenz H.J., Morse M.A., Desai J., Hill A., Axelson M., Moss R.A., et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrissey S.M., Zhang F., Ding C., Montoya-Durango D.E., Hu X., Yang C., Wang Z., Yuan F., Fox M., Zhang H.G., et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 2021;33:2040–2058.e10. doi: 10.1016/j.cmet.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M., Liu Z.Z., Aoshima K., Cai W.L., Sun H., Xu T., Zhang Y., An Y., Chen J.F., Chan L.H., et al. CECR2 drives breast cancer metastasis by promoting NF-kappaB signaling and macrophage-mediated immune suppression. Sci. Transl. Med. 2022;14:eabf5473. doi: 10.1126/scitranslmed.abf5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bitsch R., Kurzay A., Özbay Kurt F., De La Torre C., Lasser S., Lepper A., Siebenmorgen A., Müller V., Altevogt P., Utikal J., Umansky V. STAT3 inhibitor Napabucasin abrogates MDSC immunosuppressive capacity and prolongs survival of melanoma-bearing mice. J. Immunother. Cancer. 2022;10:e004384. doi: 10.1136/jitc-2021-004384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moh A., Iwamoto Y., Chai G.X., Zhang S.S.M., Kano A., Yang D.D., Zhang W., Wang J., Jacoby J.J., Gao B., et al. Role of STAT3 in liver regeneration: survival, DNA synthesis, inflammatory reaction and liver mass recovery. Lab. Invest. 2007;87:1018–1028. doi: 10.1038/labinvest.3700630. [DOI] [PubMed] [Google Scholar]
- 42.Yakar S., Liu J.L., Stannard B., Butler A., Accili D., Sauer B., LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. USA. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li D., He Q., Yang H., Du Y., Yu K., Yang J., Tong X., Guo Y., Xu J., Qin L. Daily Dose of Bovine Lactoferrin Prevents Ethanol-Induced Liver Injury and Death in Male Mice by Regulating Hepatic Alcohol Metabolism and Modulating Gut Microbiota. Mol. Nutr. Food Res. 2021;65:e2100253. doi: 10.1002/mnfr.202100253. [DOI] [PubMed] [Google Scholar]
- 44.Li Y., Rogoff H.A., Keates S., Gao Y., Murikipudi S., Mikule K., Leggett D., Li W., Pardee A.B., Li C.J. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc. Natl. Acad. Sci. USA. 2015;112:1839–1844. doi: 10.1073/pnas.1424171112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang D., Zheng X., Fu B., Nian Z., Qian Y., Sun R., Tian Z., Wei H. Hepatectomy promotes recurrence of liver cancer by enhancing IL-11-STAT3 signaling. EBioMedicine. 2019;46:119–132. doi: 10.1016/j.ebiom.2019.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin L., Liu Y., Pan C., Zhang J., Zhao Y., Shao R., Huang Z., Su Y., Shi M., Bin J., et al. Gastric cancer cells escape metabolic stress via the DLC3/MACC1 axis. Theranostics. 2019;9:2100–2114. doi: 10.7150/thno.29538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou M., Yang H., Learned R.M., Tian H., Ling L. Non-cell-autonomous activation of IL-6/STAT3 signaling mediates FGF19-driven hepatocarcinogenesis. Nat. Commun. 2017;8:15433. doi: 10.1038/ncomms15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie L., Shi F., Li Y., Li W., Yu X., Zhao L., Zhou M., Hu J., Luo X., Tang M., et al. Drp1-dependent remodeling of mitochondrial morphology triggered by EBV-LMP1 increases cisplatin resistance. Signal Transduct. Target. Ther. 2020;5:56. doi: 10.1038/s41392-020-0151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charni-Natan M., Goldstein I. Protocol for Primary Mouse Hepatocyte Isolation. STAR Protoc. 2020;1:100086. doi: 10.1016/j.xpro.2020.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin L., Li X., Pan C., Lin W., Shao R., Liu Y., Zhang J., Luo Y., Qian K., Shi M., et al. ATXN2L upregulated by epidermal growth factor promotes gastric cancer cell invasiveness and oxaliplatin resistance. Cell Death Dis. 2019;10:173. doi: 10.1038/s41419-019-1362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller B.C., Sen D.R., Al Abosy R., Bi K., Virkud Y.V., LaFleur M.W., Yates K.B., Lako A., Felt K., Naik G.S., et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 2019;20:326–336. doi: 10.1038/s41590-019-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wherry E.J., Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charoentong P., Finotello F., Angelova M., Mayer C., Efremova M., Rieder D., Hackl H., Trajanoski Z. Pan-cancer Immunogenomic Analyses Reveal Genotype-Immunophenotype Relationships and Predictors of Response to Checkpoint Blockade. Cell Rep. 2017;18:248–262. doi: 10.1016/j.celrep.2016.12.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in a public, open access repository. The alcohol-pretreated wild-type mouse liver and hepatocellular Stat3 knockout mouse liver sequencing data were deposited in the Gene Expression Omnibus database, under accession nos. GSE189277 and GSE224602, respectively.