Abstract
Objective: To investigate the efficacy and safety of ultrasound-guided percutaneous intracystic deroofing for the treatment of simple renal cysts. Methods: A retrospective study was conducted to analyze the clinical data of 46 patients with dorsal exophytic simple renal cysts treated at the First Affiliated Hospital of Nanchang University between February 2017 and June 2022. The patients were divided into two groups according to the surgical method, with 20 cases undergoing ultrasound-guided percutaneous intracystic deroofing being assigned to the observation group and 26 cases treated by retroperitoneal laparoscopic renal cyst removal included in the control group. The operation time, blood loss, postoperative catheterization time, postoperative drainage tube indwelling time, postoperative hospital stay, and complications were compared. Results: None of the 46 patients converted to open surgery. The observation group showed significantly less blood loss, shorter operation time, drainage tube drainage time, postoperative hospital stay, and indwelling catheter time than the control group (all P<0.05). The two procedures had a success rate of 100%. There were no statistical significances in K+, Na+, or serum creatinine between the two groups (all P>0.05). All patients were followed up (3 to 6 months) after surgery, and no cyst recurrence was found by imaging examination. Conclusions: Ultrasound-guided percutaneous intracystic deroofing of renal cysts is worthy of clinical application in the treatment of simple renal cysts due to its significant advantages such as short operation time, less trauma, quick recovery, safety, effectiveness, and low cost.
Keywords: Percutaneous nephroscopy, renal cyst, intracapsular deroofing, laser
Introduction
Simple renal cyst is a common benign renal disease [1,2]. With increasing awareness of physical examination and advances in diagnostic equipment and techniques, the detection rate of renal cysts is getting higher [3]. Surgery is currently the main method to treat renal cysts, mainly including open surgery, retroperitoneal laparoscopic resection, percutaneous renal cyst aspiration plus sclerotherapy injection, and flexible ureteroscopic incision and internal drainage [4-7], among which retroperitoneal laparoscopic renal cyst decortication of the lower renal cysts, by virtue of less trauma, quick recovery, and good curative effects, is currently the preferred method for the treatment of simple renal cysts [8-10]. However, this method has limitations such as reduced operation field of the retroperitoneal approach and high requirements for the operator’s anatomic familiarity [11]. As medical technology constantly develops, percutaneous intracystic deroofing of renal cysts has been used in clinical practice and achieved significant curative effects [5]. This study retrospectively analyzed the clinical data of 20 cases of percutaneous renal cyst deroofing and 26 cases of laparoscopic renal cyst deroofing during the same time period to compare the efficacy of the two methods. The innovation of this study lies in the following points: the two surgical methods are confirmed to be equivalent in terms of clinical efficacy and postoperative safety; second, from the point of view of surgery-related recovery indicators, it was validated that ultrasound-guided percutaneous intracystic deroofing contributes to less operation time, intraoperative blood loss, postoperative drainage indwelling time, catheter retention time, and postoperative hospital stays, as well as fewer channels dilated intraoperatively and better surgical effect. Finally, it was confirmed from the perspective of electrolyte indicators that the two surgical modalities did not cause electrolyte disturbance. From the above points of view, we can see that ultrasound-guided percutaneous intracystic deroofing has advantages in surgical effects.
Materials and methods
Clinical data
The clinical data of 46 patients with renal cysts who were treated at the First Affiliated Hospital of Nanchang University between February 2017 and June 2022 were collected retrospectively. Among them, there were 20 cases undergoing ultrasound-guided percutaneous intracystic deroofing (observation group), including 12 males and 8 females, aged 32 to 98, with an average age of 52 years; the renal cysts were 4.1 to 9.7 cm in diameter, with an average of 5.7 cm. There were 26 cases treated by retroperitoneal laparoscopic renal cyst removal (control group), including 15 males and 11 females, aged 35 to 81, with an average age of 54 years; the diameter of renal cysts was 4.0 to 8.7 cm, with a mean of 5.5 cm.
Inclusion criteria: all patients were diagnosed with simple renal cysts by preoperative routine urinary B-ultrasound, intravenous urography (IVU), and enhanced bilateral renal Computed Tomography (CT) scan [12]; renal cyst diameter was >4 cm; cysts were not associated with the renal collection system; treatment-naive renal cyst patients; Bosniak grade was grade I with dorsal exophytic renal cysts. Exclusion criteria: patients with serious heart, liver, lung and brain diseases; surgical contraindications such as coagulation disorders; renal cystic renal cell carcinoma; renal cyst infection; or immune system disorders.
The study was approved by the ethics committee of the First Affiliated Hospital of Nanchang University.
Surgical methods
Percutaneous renal cyst deroofing (observation group)
After general intravenous anesthesia with the patient placed in a prone position and catheter indwelling, skin preparation and draping were routinely performed, and a surgical membrane was affixed. Under the guidance of a color Doppler ultrasound instrument (Shanghai Hanfei Medical Instrument Co., Ltd., E8), the renal cyst was punctured at the center of the renal cyst, the needle core was pulled out, and the cyst fluid discharged. The renal puncture guide wire was then inserted along the needle sheath, after which the needle sheath was withdrawn. The skin was cut at the puncture point with a sharp knife about 0.6~0.8 cm. Guided by a wire, the F8 fascia dilator was used to dilate the fascia, after which a Cook F20 tear sheath was directly inserted. After that, an ureteroscope was inserted into the sheath to check for tumors or hemorrhage in the cyst and to distinguish the boundary between the renal parenchyma and the cyst wall. Next, the outer sheath was withdrawn to the lateral side of the renal cyst, and the outer sheath and the endoscope were used to dissociate along the surface of the renal cyst to the junction of the cyst and the renal parenchyma and then re-entered the cyst using a 550 µm laser fiber (2.5 J/30 Hz) every 1 cm. The boundary between the renal parenchyma and the cyst wall was marked, and the cyst wall was completely excised along the marked point with an optical fiber and removed using a peeling sheath (Figure 1A-D). When there was no obvious active bleeding in the visual field, the F12 nephrostomy tube was indwelled and properly fixed, and was removed 1 to 2 days later.
Figure 1.
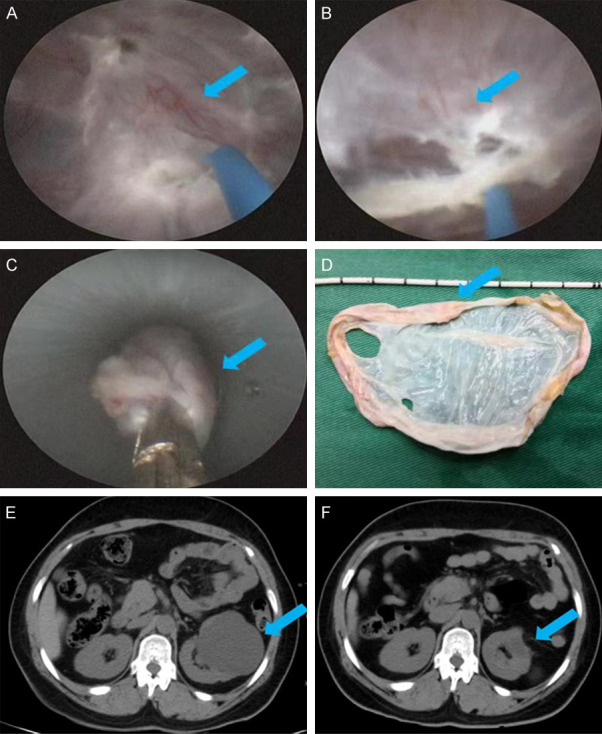
Cyst wall removal during percutaneous intracystic deroofing and reexamination three months after surgery. A. Marking of the renal parenchyma. B. Marking of the boundary of the cyst wall. C. Complete excision of the cyst wall along the marked points with optical fibers. D. Removal of the cyst wall by stripping the sheath. E. B-ultrasound review three months after surgery. F. CT plain scan of both kidneys three months after surgery. Note: The lesion site is indicated by the arrow.
Retroperitoneal laparoscopic renal cyst removal (control group)
After general intravenous anesthesia, the patient was placed in a jackknife position on the contralateral side. Catheterization was performed with the catheter being, 10 mm, 5 mm, and 5 mm at the iliac crest 2 cm above the midline of the contralateral subaxilla (10 mm catheter), below the costal margin on the anterior axillary line (5 mm catheter), and below the twelfth costal margin of the posterior axillary line (5 mm catheter), respectively. Pneumoperitoneum was then established with Trocar and the pressure was set at 10-14 mmHg. After that, a laparoscopic operating instrument was placed to separate the perirenal fascia and adipose tissue to reveal the renal cyst. After complete dissociation, the cyst wall was excised circularly at a distance of about 5 mm from the renal parenchyma using an ultrasonic knife.
Outcome measures and efficacy evaluation
The operation process, operation time, blood loss, urinary catheter retention time, postoperative drainage tube indwelling time, postoperative hospital stay, number of channels dilated intraoperatively, perioperative complications, perioperative serum electrolyte changes and prognosis were recorded and compared between the two groups. Treatment efficacy was evaluated as follows: Cured: The cyst disappeared or the diameter was less than 1 cm three months after ultrasound or CT review, with no recurrence or growth during the follow-up; Effective: The cyst diameter was reduced by more than 50% of the original diameter; Ineffective: The cyst did not shrink or grow.
In addition, except for perioperative serum electrolyte change, which was a secondary outcome measurement, the other indexes were the primary ones in this study.
Statistical methods
Data were processed using SPSS17.0 statistical software. Measured data were expressed by mean ± standard deviation (x̅ ± s), and t-test was used for comparison between groups. Counted data, represented by the number of cases (n), were compared by the chi-square test. P<0.05 was considered significant.
Results
Baseline data
As shown in Table 1, the observation group (n=20) and the control group (n=26) were not statistically different in baseline data such as age, sex, renal cyst diameter, smoking history, alcoholism history, or marital status (all P>0.05).
Table 1.
Comparison of baseline data between the two groups
| Observation group (n=20) | Control group (n=26) | χ2/t value | P | |
|---|---|---|---|---|
| Age (years) | 52.70±9.81 | 54.58±12.61 | 0.550 | 0.585 |
| Sex (male/female) | 12/8 | 15/11 | 0.025 | 0.875 |
| Renal cyst diameter (cm) | 5.71±1.13 | 5.50±1.26 | 0.586 | 0.561 |
| Smoking history (yes/no) | 6/14 | 9/17 | 0.110 | 0.741 |
| History of alcoholism (yes/no) | 5/15 | 6/20 | 0.023 | 0.880 |
| Marital status (married/single) | 14/6 | 20/6 | 0.281 | 0.596 |
Comparison of clinical efficacy
All the 46 patients underwent successful first-stage surgery, with stable intraoperative and postoperative vital signs, and no conversion to open surgery. Intraoperative and postoperative pathologic diagnosis were consistent with preoperative renal cysts. In the observation group, 18 cases were cured and 2 cases were effective, with a total effective rate of 100%. In the control group, the numbers of cured, effective, and ineffective cases were 23, 3, and 0, respectively, with an overall effective rate of 100%. There was no significant difference in the total effective rate between the two groups (P>0.05), as shown in Table 2.
Table 2.
Comparison of overall clinical efficacy between the two groups
| Group | No. | Cured | Efficient | Invalid | Total effective rate (%) |
|---|---|---|---|---|---|
| Observation group | 20 | 18 | 2 | 0 | 100 |
| Control group | 26 | 23 | 3 | 0 | 100 |
Note: Compared to the control group, P>0.05.
Comparison of intraoperative and postoperative indicators
In the observation group treated by percutaneous renal cyst deroofing, the operation time was (26.5±12.7) min, the intraoperative blood loss was (2.8±2.1) ml, the postoperative drainage tube indwelling time was (1.0±0.5) d, the urinary catheter retention time was (1.3±0.7) d, the postoperative hospital stay was (2.1±1.6) d, and the number of channels dilated intraoperatively was 1. In the control group treated by retroperitoneal laparoscopic renal cyst removal, the operation time was (56.3±13.8) min, the intraoperative blood loss was (10.9±3.5) ml, the postoperative drainage tube indwelling time was (2.6±1.8) d, the urinary catheter retention time was (2.3±1.4) d, the postoperative hospital stay was (3.4±1.7) d, and the number of channels dilated intraoperatively was 3. Statistical differences were observed in operation time, intraoperative blood loss, postoperative hospital stay, postoperative drainage tube indwelling time and postoperative catheterization time between the two groups (all P<0.05). Patients underwent B-ultrasound and double-renal CT plain scan re-examination 3 months after the operation. The results showed an effective rate of 100% in surgery of both groups, with significantly improved or even disappeared symptoms of low back pain. All patients were followed up for 3-24 months, and no case of recurrence of renal cyst was found, as shown in Table 3 and Figures 1E, 1F, 2.
Table 3.
Comparison of intraoperative and postoperative indicators between the two groups (x̅ ± s)
| Group | No. | Operation time (min) | Blood loss volume (ml) | Drain tube time (d) | Catheter retention time (d) | Postoperative hospital stay (d) | Number of channels dilated intraoperatively (n) |
|---|---|---|---|---|---|---|---|
| Observation group | 20 | 26.5±12.7* | 2.8±2.1* | 1.0±0.5* | 1.3±0.7* | 2.1±1.6* | 1 |
| Control group | 26 | 56.3±13.8 | 10.9±3.5 | 2.6±1.8 | 2.3±1.4 | 3.4±1.7 | 3 |
| χ2/t value | 7.513 | 9.147 | 3.853 | 0.006 | 0.012 | 0.609 | |
| P | <0.001 | <0.001 | <0.001 | 2.921 | 2.637 | 0.435 |
Note: Compared to the control group;
P<0.05.
Figure 2.
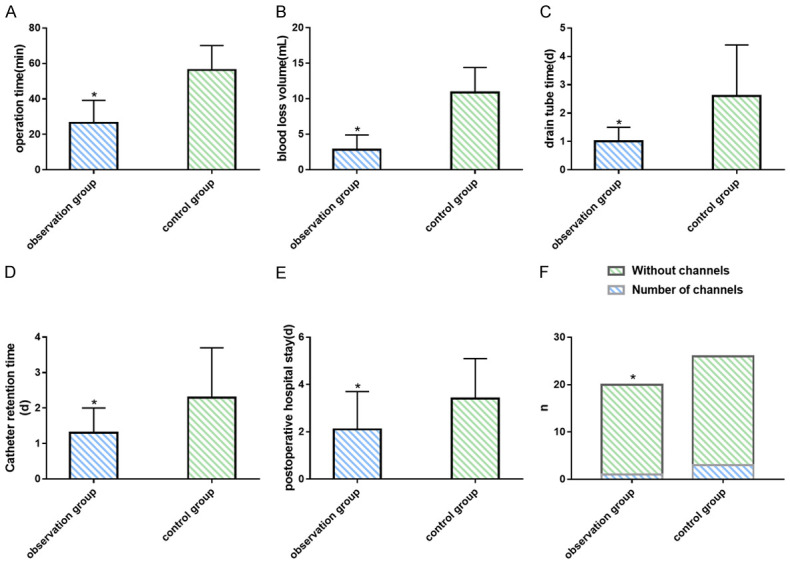
Comparison of intraoperative and postoperative indicators between the two groups. A. Comparison of operation time between groups. B. Comparison of blood loss volume between groups. C. Comparison of drain tube time between groups. D. Comparison of catheter retention time between groups. E. Comparison of postoperative hospital stay between groups. F. Comparison of number of channels dilated intraoperatively between groups. Note: Compared to the control group, *P<0.05.
Comparison of electrolyte indicators
There was no significant difference in electrolyte indicators serum potassium (K+), serum sodium (Na+) and serum creatinine (Scr) between the observation group and the control group (all P>0.05) as shown in Table 4 and Figure 3.
Table 4.
Comparison of electrolyte indicators between the two groups (x̅ ± s)
| Group | No. | K+ | Na+ | Scr |
|---|---|---|---|---|
| Observation group | 20 | 3.60±0.39 | 142.07±18.28 | 71.10±5.76 |
| Control group | 26 | 3.80±0.29 | 136.47±17.25 | 67.05±8.74 |
| t value | 1.996 | 1.064 | 1.792 | |
| P | 0.052 | 0.293 | 0.080 |
Note: K+ is serum potassium, Na+ is serum sodium, Scr is serum creatinine.
Figure 3.

Comparison of electrolyte indicators between the two groups. A. Comparison of K+ between groups. B. Comparison of Na+ between groups. C. Comparison of Scr between groups. Note: K+: serum potassium; Na+: serum sodium; Scr: serum creatinine.
Discussion
Simple renal cysts are a common benign renal disease [13]. Surgery can alleviate clinical symptoms and prevent the occurrence of complications, such as pain, bleeding, high blood pressure, infection and cyst compression of the renal parenchyma and collecting system [14-16]. How to achieve optimal therapeutic outcomes with minimal trauma is the focus of clinical research. There are many treatment methods for simple renal cysts [17], each with its own advantages and disadvantages. For example, traditional translumbar open roof decompression surgery has been gradually replaced by other minimally invasive procedures due to large trauma, slow postoperative recovery, and long hospital stays. Laparoscopic deroofing of renal cysts, by virtue of less trauma, quick recovery, and good curative effects, is currently the preferred method for the treatment of simple renal cysts, with a success rate of 96.3% [18]. However, this technique requires general anesthesia, long surgical preparation time, establishment of multiple channels, wide surgical anatomy, and skilled endoscopic operation techniques of the operator, which carries the risk of complications such as hypercapnia, subcutaneous emphysema, and postoperative adhesive intestinal obstruction during the operation [19-21]. The recurrence rate after simple percutaneous aspiration and drainage under B-ultrasound guidance is as high as 20% to 80% [22]. Percutaneous renal cyst puncture and sclerotherapy combined with injection sclerotherapy significantly improves the success rate of simple puncture and drainage and has the advantages of less trauma and fast recovery, which contributes to better treatment of small peripheral cysts; however, for endophytic, pararenal and larger cysts, it may result in many complications and a high possibility of recurrence [23].
At present, many scholars have carried out beneficial explorations on the minimally invasive treatment of renal cysts. Choi et al. [24] compared patient profiles, operation time, length of hospital stay, success rate of radiology, improvement of symptoms, treatment cost, and complication rate of the two groups through a controlled study of percutaneous aspirational sclerotherapy (PAS) and laparoscopic decapitation (LM). The results showed a significantly higher radiation success rate in LM group compared with PAS group at the 6-month follow-up (97.5% vs 60%; P<0.001); the symptom improvement rate was comparable (95% vs 90%; P=0.675), and no significant difference was identified in the treatment failure rate (5.0% vs 17.5%; P=0.154). The mean total cost for PAS and LM was $1256 and $2343, respectively (P=0.001). In a randomized study of percutaneous ureteroscopic plasma electrode removal (PCE) and laparoscopic deroofing for the treatment of simple renal cysts, Liu et al. [25] found that the mean operation time and average blood loss in the PCE group were significantly less than those of the laparoscopic group, while the average postoperative indwelling drainage tube time, average indwelling catheter time, and postoperative average hospital stay were comparable. Thus, PCE is a safe, minimally invasive and effective treatment for simple renal cysts. Busato et al. used percutaneous nephroscopic technique to treat 32 patients with Bosniak I and II renal cysts between 1995 and 2008, and the results showed that clinical success rate of symptom improvement was 100%, the mean hospital stay was (21.7±8.5) hours, and the mean operative time was (41.8±19.7) min, with no serious complications [26].
Through mastering percutaneous nephrolithotomy and learning from the successful experience of percutaneous nephroscopic technique in the treatment of simple renal cysts at home and abroad, we creatively proposed a new surgical method of percutaneous intracystic deroofing of renal cysts after careful research and repeated practice, a procedure with the following characteristics: 1. Within the cyst, it is easy to distinguish the boundary between the renal cyst and the renal parenchyma. The renal cyst is relatively transparent with pale yellow adipose tissue at the bottom, while the renal parenchyma is bright red and opaque with no adipose tissue at the bottom. 2. We retract the outer sheath to the outside of the cyst, and use the outer sheath and scope to dissociate along the surface of the renal cyst to the junction of the cyst and the renal parenchyma, so that the cyst is filled with water cushion and separated from other organs to increase the safety of the operation. 3. After removal from the outside of the cyst, the cyst wall is slightly shrunken. After re-entering the cyst, the cyst wall flutters slightly under the scouring of the water flow, making the boundary between the cyst and the renal parenchyma more clearly visible. 4. The boundary between the renal parenchyma and the cyst wall is marked with a laser beam at 1-cm intervals inside the cyst, and then a cut is made between the two marks. The cyst can be completely excised quickly and safely and removed through the outer sheath. 5. The use of laser cutting of the cyst wall avoids replacing perfusate, and the cutting effect is good. In addition, the isolation effect of the fat and water cushion on the outside of the cyst enables the cyst to be removed inside the cyst, with clear vision, good surgical safety, and comparable surgical effect to retroperitoneal laparoscopic deroofing.
Our results showed that compared to retroperitoneal laparoscopic renal cyst removal, percutaneous intracystic deroofing had significantly lower intraoperative blood loss, shorter operative time, postoperative drainage indwelling time, urinary catheter indwelling time and postoperative hospital stay, suggesting that percutaneous intracystic deroofing has lower surgical difficulty and postoperative risk, as well as faster postoperative recovery. This may be attributed to the above-mentioned characteristics, which can quickly and safely remove the cyst completely, improve visual field clarity, and effectively increase surgical safety [27]. Moreover, no significant differences were identified between groups in efficacy (total effective rate: both 100%), safety (no renal cyst recurrence), and postoperative electrolyte indicators (K+, Na+, Scr), suggesting that the efficacy, safety and influence on electrolytes of the two surgical methods are comparable.
In addition, the study has some limitations: first, due to the small number of cases studied in this project, it is necessary to increase the sample size to improve the accuracy of the research results; second, as a single-center study, there may be information collection bias, which can be addressed by conducting a multi-center study; third, it is limited by the sample size so research time, long-term effects, complications and related long-term prognosis need further observation and follow-up. The future research project will be supplemented and improved based on the above deficiencies.
To sum up, ultrasound-guided percutaneous intracystic deroofing has unique advantages in the treatment of simple renal cysts such as simple operation, high success rate, less trauma, fewer complications, and definite short-term efficacy.
Acknowledgements
The study was supported by the Science and Technology Plan of Jiangxi Provincial Health Commission (No. 202211637).
Disclosure of conflict of interest
None.
References
- 1.Koutlidis N, Joyeux L, Mejean N, Sapin E. Management of simple renal cyst in children: French multicenter experience of 36 cases and review of the literature. J Pediatr Urol. 2015;11:113–117. doi: 10.1016/j.jpurol.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Simms RJ, Ong AC. How simple are ‘simple renal cysts’? Nephrol Dial Transplant. 2014;29(Suppl 4):iv106–112. doi: 10.1093/ndt/gfu106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terada N, Ichioka K, Matsuta Y, Okubo K, Yoshimura K, Arai Y. The natural history of simple renal cysts. J Urol. 2002;167:21–23. [PubMed] [Google Scholar]
- 4.Vuruskan E, Ercil H, Anil H, Unal U, Ortoglu F, Karkin K, Ayhan L, Gurbuz ZG. Comparison of laparoscopic and open surgery in the treatment of renal hydatid cysts. J Laparoendosc Adv Surg Tech A. 2022;32:427–431. doi: 10.1089/lap.2021.0031. [DOI] [PubMed] [Google Scholar]
- 5.Maugeri A, Fanciulli G, Barchitta M, Agodi A, Basile G. Comparison of aspiration with sclerotherapy and laparoscopic deroofing for the treatment of symptomatic simple renal cysts: a systematic review and meta-analysis. Updates Surg. 2021;73:1691–1698. doi: 10.1007/s13304-021-01042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong Y, Chen X, Wu M, Xi H, Hu J. Percutaneous vs laparoscopic treatment for simple renal cysts: a meta-analysis. J Endourol. 2021;35:1793–1800. doi: 10.1089/end.2021.0264. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Wang R, Shen X, Tang J, Shen J, Fang Z, Shi Z, Jin X. Ultrasonography-assisted flexible ureteroscope for the treatment of parapelvic renal cysts: a comparison between the 1470-nm diode laser and the holmium laser. Exp Ther Med. 2021;21:172. doi: 10.3892/etm.2020.9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mai H, Liu J, Zhao L, Qu N, Wang Y, Huang C, Chen B, Li Y, Chen L, Zhang X. Efficacy investigation of transpostceliac single-port 3-channel laparoscope in the treatment of complex renal cyst. Int J Clin Exp Med. 2015;8:10031–10035. [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal MM, Hemal AK. Surgical management of renal cystic disease. Curr Urol Rep. 2011;12:3–10. doi: 10.1007/s11934-010-0152-2. [DOI] [PubMed] [Google Scholar]
- 10.Permpongkosol S, Ungbhakorn P, Leenanupunth C. Laparo-endoscopic single site (LESS) management of benign kidney diseases: evaluation of complications. J Med Assoc Thai. 2011;94:43–49. [PubMed] [Google Scholar]
- 11.Crisan N, Andras I, Telecan T, Szabo A, Popa A, Coman RT, Medan P, Coman I. Retroperitoneal laparoendoscopic single-site approach for renal cyst decortication - first experience and a review of literature. Clujul Med. 2018;91:346–350. doi: 10.15386/cjmed-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal M, Agrawal MS, Mittal R, Sachan V. A randomized study of aspiration and sclerotherapy versus laparoscopic deroofing in management of symptomatic simple renal cysts. J Endourol. 2012;26:561–565. doi: 10.1089/end.2011.0559. [DOI] [PubMed] [Google Scholar]
- 13.Eissa A, El Sherbiny A, Martorana E, Pirola GM, Puliatti S, Scialpi M, Micali S, Rocco B, Liatsikos E, Breda A, Porpiglia F, Bianchi G European Section of Uro-Technology (ESUT) Non-conservative management of simple renal cysts in adults: a comprehensive review of literature. Minerva Urol Nefrol. 2018;70:179–192. doi: 10.23736/S0393-2249.17.02985-X. [DOI] [PubMed] [Google Scholar]
- 14.Xiong L, Nguyen JK, Peng Y, Zhou Z, Ning K, Jia N, Nie J, Wen D, Wu Z, Roversi G, Palacios DA, Abramczyk E, Munoz-Lopez C, Campbell JA, Cao Y, Li W, Zhang X, He Z, Li X, Huang J, Shou J, Wu J, Chen M, Chen X, Zheng J, Xu C, Zhong W, Li Z, Dong W, Zhao J, Zhang H, Luo J, Liu J, Sun F, Han H, Guo S, Dong P, Zhou F, Yu C, Campbell SC, Zhang Z. What happens to the preserved renal parenchyma after clamped partial nephrectomy? Eur Urol. 2022;81:492–500. doi: 10.1016/j.eururo.2021.12.036. [DOI] [PubMed] [Google Scholar]
- 15.Kunwar AK, Upadhyay AM, Shrestha SB, Koirala U, Tiwari K, Dangal G. Initial experience of retroperitoneoscopic surgery in benign renal diseases. J Nepal Health Res Counc. 2019;17:94–99. doi: 10.33314/jnhrc.1772. [DOI] [PubMed] [Google Scholar]
- 16.Khanna A, Campbell SC, Murthy PB, Ericson KJ, Nyame YA, Abouassaly R. Unplanned conversion from minimally invasive to open kidney surgery: the impact of robotics. J Endourol. 2020;34:955–963. doi: 10.1089/end.2020.0357. [DOI] [PubMed] [Google Scholar]
- 17.Lai S, Xu X, Diao T, Jiao B, Jiang Z, Zhang G. The efficacy of retroperitoneal laparoscopic deroofing of simple renal cyst with perirenal fat tissue wadding technique: a retrospective study. Medicine (Baltimore) 2017;96:e8259. doi: 10.1097/MD.0000000000008259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nalagatla S, Manson R, McLennan R, Somani B, Aboumarzouk OM. Laparoscopic decortication of simple renal cysts: a systematic review and meta-analysis to determine efficacy and safety of this procedure. Urol Int. 2019;103:235–241. doi: 10.1159/000497313. [DOI] [PubMed] [Google Scholar]
- 19.Efesoy O, Tek M, Bozlu M, Doruk HE. Comparison of single-session aspiration and ethanol sclerotherapy with laparoscopic de-roofing in the management of symptomatic simple renal cysts. Turk J Urol. 2015;41:14–19. doi: 10.5152/tud.2015.77675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ngo TK, Le DB, Bui HT, Pham VK. Symptomatic pneumopericardium - a rare complication following retroperitoneal laparoscopic nephrectomy: a case report. Int J Surg Case Rep. 2021;79:299–301. doi: 10.1016/j.ijscr.2021.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Cao D, Han P, Ren Z, Wang J, Wei Q. Aspiration-sclerotherapy versus laparoscopic de-roofing in the treatment of renal cysts: which is better? BMC Nephrol. 2020;21:193. doi: 10.1186/s12882-020-01832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tu MQ, Li JH, Fu XC, Wang XL, Zhang J, Wang WZ, Shi GW. Clinical analysis of 28 cases of calculous pyonephrosis undergoing B-ultrasound-guided renal puncture and drainage followed by secondary percutaneous nephrolithotomy. Zhonghua Yi Xue Za Zhi. 2019;99:3005–3007. doi: 10.3760/cma.j.issn.0376-2491.2019.38.008. [DOI] [PubMed] [Google Scholar]
- 23.Brown D, Nalagatla S, Stonier T, Tsampoukas G, Al-Ansari A, Amer T, Aboumarzouk OM. Radiologically guided percutaneous aspiration and sclerotherapy of symptomatic simple renal cysts: a systematic review of outcomes. Abdom Radiol (NY) 2021;46:2875–2890. doi: 10.1007/s00261-021-02953-9. [DOI] [PubMed] [Google Scholar]
- 24.Choi JD, Yoo TK, Kang JY, Moon KT, Kim JH, Ahn SH, Lee JH, Cho JM. A comparative study of percutaneous aspiration with sclerotherapy and laparoscopic marsupialization for symptomatic simple renal cysts. J Laparoendosc Adv Surg Tech A. 2020;30:514–519. doi: 10.1089/lap.2019.0745. [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Zhang C, Wang B, Li B, Gao G, Sun G, Sun Y, Lin G. Randomized study of percutaneous ureteroscopic plasma column electrode decortication and laparoscopic decortication in managing simple renal cyst. Transl Androl Urol. 2018;7:260–265. doi: 10.21037/tau.2018.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busato WF Jr, Bettega LB. Percutaneous endocystolysis, a safe and minimally invasive treatment for renal cysts: a 13-year experience. J Endourol. 2010;24:1405–1410. doi: 10.1089/end.2009.0467. [DOI] [PubMed] [Google Scholar]
- 27.Hu J, Dirie NI, Yang J, Xia D, Lu Y, Yu X, Wang S. Percutaneous ureteroscopy laser unroofing-a minimally invasive approach for renal cyst treatment. Sci Rep. 2017;7:14445. doi: 10.1038/s41598-017-14605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


