Abstract
Purpose: To clarify the association of serum alpha-fetoprotein (AFP) with inflammatory markers interleukin (IL)-6 and tumor necrosis factor (TNF)-α in patients with chronic hepatitis B (CHB) during the immune-clearance phase in Eastern China. Methods: This research selected 60 CHB patients during the immune clearance phase who tested positive for AFP, including 32 cases treated by non-antiviral therapy (experimental group) and 28 cases treated by antiviral therapy (positive control group). Another 30 cases tested negative for AFP were set as a negative control group. The correlations of serum AFP with IL-6 and TNF-α in patients were analyzed. Results: HBV DNA clearance in patients receiving antiviral therapy, in both the positive or negative control groups, was not significantly related to other clinical data. In the experimental group, a positive correlation of HBV DNA clearance with serum AFP level (r=0.5126, P=0.0027), alanine aminotransferase (r=0.3924, P=0.0263), and total bilirubin (r=0.5126, P=0.0027) was found. The experimental and positive control groups exhibited elevated serum IL-6 and TNF-α contents versus the negative control group (P<0.05). A positive association of AFP with IL-6 and TNF-α was also identified. Conclusion: Serum AFP level is positively related to IL-6 and TNF-α levels in CHB patients during the immune-clearance phase.
Keywords: Immune-clearance phase, alpha-fetoprotein, HBV DNA, IL-6, TNF-α
Introduction
Hepatitis B refers to a liver infection induced by hepatitis B virus (HBV) - a double-stranded DNA virus of the family Hepeviridae [1]. Based on World Health Organization (WHO) statistics, there are over 2 billion HBV-infected cases globally, 350 million of which were chronic, and an estimated 65 million of the chronically infected die from chronic hepatitis B (CHB)-induced liver disease [2]. According to the “Guidelines for the Prevention and Treatment of Chronic Hepatitis B (2022 edition)” [3] revised by the Hepatology and Infectious Diseases Branch of the Chinese Medical Association, CHB can be divided into four phases based on the integration of virological, biochemical and histological features, namely hepatitis B e antigen (HBeAg)-positive chronic HBV infection (previously known as immune tolerance, chronic HBV carrier status), HBeAg-positive CHB (previously known as immune-clearance [IC], immune activity), HBeAg-negative chronic HBV infection (previously known as immune control, inactive hepatitis B surface antigen [HBsAg] carrier status), and HBeAg-negative CHB (also known as reactivation). The IC phase begins with the host’s immune response to the infected hepatocytes (HCs). At this time, elevated serum alanine aminotransferase (ALT: higher levels indicate more intense responses and more severe HC injury) and chronic active hepatitis visible on liver ultrasound scan (USS) or biopsy can be observed, while the immune system can gradually clear HBV DNA along with increased levels of liver inflammation [4,5].
Once they are CHB-infected, patients will develop chronic inflammation that is linked to certain liver complications [6]. The immune system consists of various molecules and cells involved in the induction of inflammation [7], among which interleukin (IL)-6 [8] and tumor necrosis factor (TNF)-α [9] are the major innate immunocytokines that trigger liver inflammation and immunoreactions. Inflammation in the liver leads to extracellular matrix accumulation, causing fibrosis. Therefore, it is crucial to identify inflammation in time for early antiviral therapy [10]. As an embryo-specific glycoprotein comprising nearly 580 amino acids and 3-5% carbohydrates, alpha-fetoprotein (AFP) is the most extensively used serum biomarker for cirrhosis, which is synthesized by the yolk sac and liver (1-2 months) and then predominates in the human liver [11]. AFP is the most extensively applied serum biomarker for hepatocellular carcinoma (HCC) surveillance in China [12,13], with its elevated serum levels associated with acute HCC. Antiviral drugs are known to be effective in inhibiting not only HBV replication but also hepatitis activity, which may reduce the false-positive rate of AFP testing. However, some patients still develop HCC during antiviral therapy [14-16]. Therefore, it is clinically important to distinguish between the high AFP level associated with HBV infection and that which occurs with early-stage HCC, especially in those on antiviral therapy for elevated AFP.
Moreover, studies have demonstrated increased serum AFP levels with the worsening of pathologic inflammation and fibrosis in CHB [17]. However, a relationship between serum levels of AFP and inflammation-related factors in different phases of CHB, especially the IC phase, has not been reported. Therefore, in this study, IC-phase CHB patients were enrolled to clarify the correlation of AFP with inflammatory indicators.
Materials and methods
Participants
In this retrospective study, the clinical files of initially infected IC-phase CHB inpatients at the Taizhou People’s Hospital or Taizhou Hospital of Traditional Chinese Medicine between January, 2019 and January, 2021 were analyzed. Inclusion criteria: (1) All cases met the diagnostic standards for IC phase CHB [18]; (2) All cases were serum HBsAg positive and HBeAg positive for more than 6 months; (3) All cases had a serum HBV DNA level of >2000 IU/mL for more than 6 months; (4) All cases with ALT >5× ULN or persistently elevated (>2× ULN for more than 3 months); (5) All cases with complete clinical files and follow-up data. Exclusion criteria: (1) Patients with other viral hepatitis, fatty/alcoholic/drug-induced hepatitis, or autoimmune hepatitis; (2) Patients with primary hepatic carcinoma; (3) Patients receiving nucleotides, interferon or thymidine; (4) Patients with incomplete clinical files and follow-up data. The patients were classified as either AFP negative (≤20 ng/mL) or positive (AFP >20 ng/mL) with the acknowledged upper limit of normal AFP level of 20 ng/mL as the threshold [19]. A total of 60 AFP positive cases were included with 32 cases treated by non-antiviral therapy (experimental group) and 28 cases treated with antiviral therapy (positive control group). Besides, another 30 AFP negative CHB cases were used as a negative control group. No statistical differences were identified in sex, age, height, or weight among the groups. This study was approved by the ethics committee of Taizhou Hospital of Traditional Chinese Medicine.
Data collection
After hospitalization, patients were examined once every two weeks for ALT, total bilirubin (TBil), HBsAg, HBeAg titer, HBV DNA, AFP quota, IL-6 and TNF-α. The positive and negative control groups received antiviral therapy with nucleotides analogues (Entecavir, Tenofovir disoproxil fumarate, telbivudina, adefovir dipiboxil, and Lamivudine), while the experimental group received none. Patients were closely observed for their treatment and conditions throughout their hospitalization and underwent a ≥2-year follow-up after hospital discharge.
Outcome measures
The primary outcome measure was the AFP level. The secondary outcomes measures were the differences in the AFP level and other indicators (ALT, total bilirubin [TBil], HBsAg, HBeAg titer, HBV DNA, HBV DNA AFP quota, IL-6 and TNF-α).
Statistical analyses
SPSS 20.0 software was used for statistical analyses. Measured data were expressed by mean ± standard deviation (SD); Inter-group and multi-group comparisons were conducted using the Student’s t-test and the variance analysis (ANOVA) following with Bonferroni post-hoc test, respectively. Counted data were described as number (percentage) and analyzed using the Chi-square test. Pearson correlation analysis was used for two-factor correlation analysis. P<0.05 was considered significant.
Results
Baseline information and prognosis
There were 32, 28, and 30 cases in the experimental, positive control, and negative control group, respectively (Table 1). The enrolled patients were all discharged from hospital after their condition improved without death. There was no proof for liver tumors in patients with increased AFP. Three patients from the control group were excluded due to no apparent drop in virus after four weeks of liver protection treatment. The auto clearance ratio reached 90.32% (28/31). The average length of stay and hospitalization costs of each group are listed in Table 2, which showed no significant difference among the groups.
Table 1.
Baseline information
| Group | Experimental group (n=32) | Positive control group (n=28) | Negative control group (n=30) | F/χ2 | P |
|---|---|---|---|---|---|
| Age (year) | 41.6±13.2 | 40.3±18.3 | 43.1±17.5 | 0.2126 | 0.8089 |
| Gender, male (%) | 18 (56.3) | 16 (57.1) | 17 (56.7) | 0.0048 | 0.9976 |
| BMI (kg/m2) | 22.6±2.4 | 23.1±2.8 | 22.9±3.0 | 0.2553 | 0.7753 |
| TBil (µmol/L) | 172.6±71.3 | 179.3±68.3 | 176.1±77.5 | 0.0640 | 0.9381 |
| ALT (U/L) | 573.2±249.1 | 527.6±310.9 | 601.9±191.2 | 0.6300 | 0.5350 |
| AFP (ng/mL) | 369.1±184.1 | 348.6±176.6 | 24.1±2.8*,# | 51.8600 | <0.0001 |
| HBV DNA (log) | 5.72±2.87 | 5.96±2.13 | 5.94±2.76 | 0.0794 | 0.9237 |
| HBsAg (ng/mL) | 3437.1±1964.1 | 3988.6±2276.2 | 3816.5±2862.0 | 0.4236 | 0.6560 |
| HBeAg (IU/mL) | 756.7±591.8 | 693.5±669.1 | 782.1±610.4 | 0.1546 | 0.8570 |
Notes: BMI, body mass index; TBil, total bilirubin; ALT, alanine aminotransferase; AFP, alpha-fetoprotein; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen;
P<0.05 vs experimental group;
P<0.05 vs positive control group.
Table 2.
Comparison of average length of day and hospitalization costs
| Group | Average length of day | Average hospitalization costs |
|---|---|---|
| Experimental group (n=32) | 45.3±14.2 | 22178.4±3670.9 |
| Positive control group (n=28) | 46.7±13.1 | 21712.3±4460.1 |
| Negative control group (n=30) | 44.1±14.8 | 22809.8±3955.9 |
| F | 0.2472 | 0.5453 |
| P | 0.7815 | 0.5817 |
The association of HBV DNA clearance with clinical data
The correlation of HBV DNA concentration with all the concerned clinical data are presented in Tables 3, 4 and Figure 1, which only identified a correlation of HBV DNA clearance with TBil (r=0.5126, P=0.0027) and ALT (r=0.3924, P=0.0263).
Table 3.
HBV DNA, AFP, TBil, ALT, HBsAg and HBeAg of experimental group
| HBV DNA (log) | AFP (ng/mL) | TBil (µmol/L) | ALT (U/L) | HBsAg (ng/mL) | HBeAg (IU/mL) | |
|---|---|---|---|---|---|---|
| 0 w | 5.72±2.87 | 369.1±184.1 | 172.6±71.2 | 573.2±249.0 | 3437.8±1964.3 | 756.7±591.2 |
| 2 w | 5.86±2.40 | 1083.1±591.2a | 203.1±87.7 | 221.7±165.6a | 3675.4±2016.0 | 741.6±513.5 |
| 4 w | 4.45±3.02b | 689.6±100.9a,b | 143.7±91.5b | 40.3±5.8a,b | 3859.9±2103.5 | 801.2±555.4 |
| 6 w | 3.57±1.58a,b | 355.2±173.4b,c | 50.6±45.4a,b,c | 27.7±3.9a,b,c | 3457.1±2045.8 | 792.8±590.0 |
| 8 w | 3.24±1.66a,b | 108.3±65.5a,b,c,d | 30.1±17.3a,b,c,d | 26.5±4.8a,b,c | 3760.5±1962.0 | 789.6±603.3. |
| 16 w | 3.01±0.64a,b | 31.1±10.3a,b,c,d,e | 16.1±9.8a,b,c,d,e | 28.1±6.2a,b,c | 3578.1±1960.7 | 748.7±619.2 |
| 20 w | 2.97±0.71a,b | 6.5±2.0a,b,c,d,e,f | 14.6±5.9a,b,c,d,e | 21.5±3.0a,b,c,d,e,f | 3428.9±1994.1 | 785.5±590.3 |
| 24 w | 2.59±0.42a,b,c,d,f,g | 3.2±2.1a,b,c,d,e,f,g | 8.1±6.0a,b,c,d,e,f,g | 21.6±3.2a,b,c,d,e,f | 3635.6±1987.6 | 761.9±568.2 |
Notes: HBV, hepatitis B virus; AFP, alpha-fetoprotein; TBil, total bilirubin; ALT, alanine aminotransferase; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen.
P<0.05 vs 0 w in the same group;
P<0.05 vs 2 w in the same group;
P<0.05 vs 4 w in the same group;
P<0.05 vs 6 w in the same group;
P<0.05 vs 8 w in the same group;
P<0.05 vs 16 w in the same group;
P<0.05 vs 20 w in the same group.
Table 4.
Significant changes in HBV DNA, AFP, TBil, ALT, HBsAg, and HBeAg over time
| Source | Type III Sum of Square | Mean Square | F | P value |
|---|---|---|---|---|
| HBV DNA | 3947.61 | 3947.61 | 1156.52 | <0.001 |
| Error | 105.81 | 3.413 | ||
| AFP | 28016365.07 | 28016365.07 | 723.827 | <0.001 |
| Error | 1199882.15 | 38705.88 | ||
| TBil | 1639508.99 | 1639508.99 | 550.539 | <0.001 |
| Error | 92318.230 | 2978.01 | ||
| ALT | 3688368.26 | 3688368.26 | 317.399 | <0.001 |
| Error | 360239.22 | 11620.62 | ||
| HBsAg | 3338269767.82 | 3338269767.82 | 1935.409 | <0.001 |
| Error | 53387267.76 | 1722169.93 | ||
| HBeAg | 153036893.67 | 153036893.67 | 647.823 | <0.001 |
| Error | 7323208.62 | 236232.536 |
Figure 1.
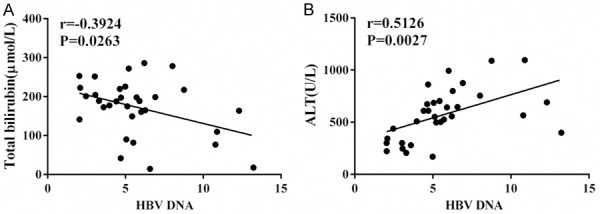
Correlation of HBV DNA level and other indicators. A: Correlation of HBV DNA level and TBil level; B: Correlation of HBV DNA level and ALT level. Notes: HBV, hepatitis B virus; TBil, total bilirubin; ALT, alanine aminotransferase.
Association of AFP with inflammatory indicators
Markedly elevated serum IL-6 and TNF-α levels were determined in the experimental and positive control groups compared to the negative control group (P<0.05; Table 5). AFP was statistically correlated with IL-6, TNF-α and HBV DNA (Figure 2).
Table 5.
Comparison of HBV DNA, AFP, IL-6 and TNF-α levels
| Group | AFP (ng/mL) | IL-6 (ng/L) | TNF-α (ng/L) |
|---|---|---|---|
| Experimental group (n=32) | 369.1±184.1 | 42.6±7.2 | 73.1±24.9 |
| Positive control group (n=28) | 348.6±176.6 | 43.1±7.7 | 71.7±19.6 |
| Negative control group (n=30) | 24.1±2.8*,# | 27.7±9.5*,# | 40.3±10.6*,# |
| F | 51.86 | 34.23 | 27.41 |
| P | <0.0001 | <0.0001 | <0.0001 |
Notes: AFP, alpha-fetoprotein; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α;
P<0.05 vs experimental group;
P<0.05 vs positive control group.
Figure 2.
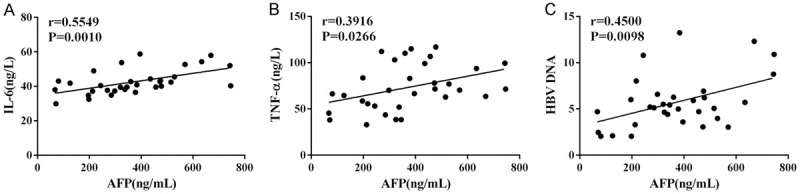
Correlation of AFP level and other indicators. A: Correlation of AFP level with IL-6 level; B: Correlation of AFP level with TNF-α level; C: Correlation of AFP level with HBV DNA level. Notes: AFP, alpha-fetoprotein; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; HBV, hepatitis B virus.
Discussion
The IC phase is the second phase of HBV infection [4]. It is characterized by high serum HBV DNA concentration, persistent or indirect increase of ALT/AST, and necro-inflammatory changes of the liver [20]. Due to the multiple replications of HBV in the body, the immune system is activated and launches an immune attack against HBV, resulting in an active immune response of the organism. When the immune system attacks HBsAg on the liver cell membrane, a large number of HCs are damaged or even become necrotic, accompanied by liver cell reproduction [21]. At the same time, there may be a rise in jaundice and abnormalities of aminotransferase clinically. CHB is a persistent HBV infection-induced chronic necroinflammatory liver disease [22]. The difference in the CHB phase may depend on the dynamic interplay between the virus and the hepatic microenvironment composed of hepatic parenchymal, non-parenchymal and local immune cells. Lifelong quiescence may occur in some patients, but others can experience severe complications featuring HBV DNA and ALT level fluctuations [23]. In our study, HBV DNA was also in direct proportion to ALT levels in IC-phase CHB.
AFP is a bioprotein that is generated under pathologic state during the embryonic phase [24]. When cell division enters an exuberant state, the body grows mature, resulting in a decrease in AFP secretion. However, in the case of liver cancer or liver damage, HCs will secrete AFP in great quantities. Since the identification of the correlation between AFP and liver cancer in the 1960s, AFP has been widely used in HCC screening, diagnosing, early diagnosing, therapeutic evaluation, and prognosis prediction, [25]. However, analyses in recent years have shown that dramatic increases in AFP during acute or chronic hepatitis outbreaks may not be associated with tumors. In hepatocirrhosis patients with acute liver injury, AFP is elevated but tumors do not develop [26]. The research by Ahn [27] and He [28] came to the conclusion that AFP level elevation was strongly linked to myofibrosis progression in hepatitis C patients without hepatocarcinogenesis. Uslu [29] and his collaborators reached the same conclusion in hepatitis B patients. Our research found that the decrease in HBV DNA concentration had a significant relationship with AFP levels in IC-phase CHB patients with rising AFP, possibly due to the reproduction of HCs when virus-infected HCs are damaged or necrotic or because ALT and TBil are significantly associated with decreased HBV DNA concentrations. Our research did not find a high correlation of HBV DNA and HBeAg with decreasing HBV DNA concentrations.
The immune system is composed of several molecules and cells involved in inflammation induction [30]. IL-6 and TNF-α affect inflammatory responses [31] and participate in the regulation of important pathophysiologic processes [32]. Increased IL-6 production is associated with the pathogenesis of HCC in animal models. Previous studies have shown that high serum IL-6 levels predate HCC carcinogenesis in CHB patients and are moderately accurate in predicting future cancer [33]. Short-term IL-6 production may be the body’s natural response to liver injury and may even be protective, but long-term exposure to high levels of IL-6 may increase the likelihood of developing liver damage and HCC. TNF-α system activity gets elevated in cirrhosisand, is acknowledged to be linked to several known cirrhosis-associated adverse events, such as hyperkinetic circulation, infection predisposition, and hepatic encephalopathy [34,35]. As has been observed in multiple chronic inflammatory disorders including CHB, activation of the cytokine system may lead to energy expenditure enhancement and nutrient intake reduction [36]. The endogenous TNF-α elevation in advanced hepatopathy patients is widely acknowledged to be a result of chronic liver failure, which is related to endotoxin-dependent macrophage stimulation and reduced cytokine clearance [37]. TNF-α and IL-6 may be critical mediators of viral mass processes, although this association has yet to be fully established [38]. Our data revealed evidently higher serum IL-6 and TNF-α in the experimental and positive control groups versus the negative control group, demonstrating their roles as two important risk factors for CHB infection. In addition, a close relationship was determined between serum AFP and IL-6 and TNF-α levels.
In recent years, antiviral therapy for CHB has received increasing attention. It is believed that hepatitis B should be given positive antiviral therapy [39,40]. Our research revealed that the probability of HBV DNA clearance in quite higher in IC-phase CHB patients tested positive for AFP (28/31), with no effect on patient prognosis, length of stay,orhospitalization costs. The condition of such patients should be closely observed, or antivirus therapy can be given when needed. Because this clinical research followed up the patients only short term, further research is needed to observe long-term outcomes.
Acknowledgements
The work was supported in part by Taizhou Science & Technology Bureau of Jiangsu Province for basic research (TS02012, SML).
Disclosure of conflict of interest
None.
References
- 1.Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 2.Lee HM, Banini BA. Updates on chronic HBV: current challenges and future goals. Curr Treat Options Gastroenterol. 2019;17:271–291. doi: 10.1007/s11938-019-00236-3. [DOI] [PubMed] [Google Scholar]
- 3.Guidelines for the prevention and treatment of chronic hepatitis B (version 2022) Zhonghua Gan Zang Bing Za Zhi. 2022:1309–1331. doi: 10.3760/cma.j.cn501113-20221204-00607. [DOI] [PubMed] [Google Scholar]
- 4.Asselah T, Loureiro D, Boyer N, Mansouri A. Targets and future direct-acting antiviral approaches to achieve hepatitis B virus cure. Lancet Gastroenterol Hepatol. 2019;4:883–892. doi: 10.1016/S2468-1253(19)30190-6. [DOI] [PubMed] [Google Scholar]
- 5.Idilman R. Management of special patient groups with hepatitis B virus infection: the EASL 2017 clinical practice guidelines. Turk J Gastroenterol. 2017;28:518–521. doi: 10.5152/tjg.2017.171017. [DOI] [PubMed] [Google Scholar]
- 6.Yang F, Yu X, Zhou C, Mao R, Zhu M, Zhu H, Ma Z, Mitra B, Zhao G, Huang Y, Guo H, Wang B, Zhang J. Hepatitis B e antigen induces the expansion of monocytic myeloid-derived suppressor cells to dampen T-cell function in chronic hepatitis B virus infection. PLoS Pathog. 2019;15:e1007690. doi: 10.1371/journal.ppat.1007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xun Z, Liu C, Yu QQ, Lin JP, Huang JL, Yang TW, Wu WN, Wu SH, Ou QS. Albumin-bilirubin score is associated with response to pegylated interferon and nucleos(t)ide analogues in chronic hepatitis B patients. Clin Chim Acta. 2020;502:120–127. doi: 10.1016/j.cca.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Lu B, Zhang B, Wang L, Ma C, Liu X, Zhao Y, Jiao Y. Hepatitis B virus e antigen regulates monocyte function and promotes B lymphocyte activation. Viral Immunol. 2017;30:35–44. doi: 10.1089/vim.2016.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Townsend EC, Zhang GY, Ali R, Firke M, Moon MS, Han MAT, Fram B, Glenn JS, Kleiner DE, Koh C, Heller T. The balance of type 1 and type 2 immune responses in the contexts of hepatitis B infection and hepatitis D infection. J Gastroenterol Hepatol. 2019;34:764–775. doi: 10.1111/jgh.14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151–166. doi: 10.1038/s41575-020-00372-7. [DOI] [PubMed] [Google Scholar]
- 11.Manuc D, Preda CM, Sandra I, Baicus C, Cerban R, Constantinescu I, Olteanu AO, Ciora CA, Manuc T, Chiriac DE, Chifulescu AE, Diculescu M, Tieranu C, Negreanu L, Oprea-Calin G, Manuc M. Signification of serum alpha-fetoprotein levels in cases of compensated cirrhosis and hepatitis C virus without hepatocellular carcinoma. J Med Life. 2020;13:68–74. doi: 10.25122/jml-2019-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo P, Yin P, Hua R, Tan Y, Li Z, Qiu G, Yin Z, Xie X, Wang X, Chen W, Zhou L, Wang X, Li Y, Chen H, Gao L, Lu X, Wu T, Wang H, Niu J, Xu G. A large-scale, multicenter serum metabolite biomarker identification study for the early detection of hepatocellular carcinoma. Hepatology. 2018;67:662–675. doi: 10.1002/hep.29561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong GL, Chan HL, Tse YK, Chan HY, Tse CH, Lo AO, Wong VW. On-treatment alpha-fetoprotein is a specific tumor marker for hepatocellular carcinoma in patients with chronic hepatitis B receiving entecavir. Hepatology. 2014;59:986–995. doi: 10.1002/hep.26739. [DOI] [PubMed] [Google Scholar]
- 14.Lee M, Chung GE, Lee JH, Oh S, Nam JY, Chang Y, Cho H, Ahn H, Cho YY, Yoo JJ, Cho Y, Lee DH, Cho EJ, Yu SJ, Lee DH, Lee JM, Kim YJ, Yoon JH. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology. 2017;66:1556–1569. doi: 10.1002/hep.29318. [DOI] [PubMed] [Google Scholar]
- 15.Nam JY, Chang Y, Cho H, Kang SH, Cho YY, Cho EJ, Lee JH, Yu SJ, Yoon JH, Kim YJ. Delayed viral suppression during antiviral therapy is associated with increased hepatocellular carcinoma rates in HBeAg-positive high viral load chronic hepatitis B. J Viral Hepat. 2018;25:552–560. doi: 10.1111/jvh.12838. [DOI] [PubMed] [Google Scholar]
- 16.Chen XX, Cheng JW, Huang A, Zhang X, Wang J, Fan J, Zhou J, Yang XR. The effect of antiviral therapy on patients with hepatitis B virus-related hepatocellular carcinoma after curative resection: a systematic review and meta-analysis. Onco Targets Ther. 2017;10:5363–5375. doi: 10.2147/OTT.S150281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu YR, Lin BB, Zeng DW, Zhu YY, Chen J, Zheng Q, Dong J, Jiang JJ. Alpha-fetoprotein level as a biomarker of liver fibrosis status: a cross-sectional study of 619 consecutive patients with chronic hepatitis B. BMC Gastroenterol. 2014;14:145. doi: 10.1186/1471-230X-14-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. The guidelines of prevention and treatment for chronic hepatitis B (2019 version) Zhonghua Gan Zang Bing Za Zhi. 2019;27:938–961. doi: 10.3760/cma.j.issn.1007-3418.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 19.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 20.Lee HW, Kim EH, Lee J, Kim SU, Park JY, Kim DY, Ahn SH, Han KH, Kim BK. Natural history of untreated HBeAg-positive chronic HBV infection with persistently elevated HBV DNA but normal alanine aminotransferase. Clin Transl Gastroenterol. 2020;11:e00140. doi: 10.14309/ctg.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaongo SD, Ouyang J, Chen Y, Jiao YM, Wu H, Chen Y. HIV infection predisposes to increased chances of hBV infection: current understanding of the mechanisms favoring HBV infection at each clinical stage of HIV infection. Front Immunol. 2022;13:853346. doi: 10.3389/fimmu.2022.853346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fung S, Choi HSJ, Gehring A, Janssen HLA. Getting to HBV cure: the promising paths forward. Hepatology. 2022;76:233–250. doi: 10.1002/hep.32314. [DOI] [PubMed] [Google Scholar]
- 23.Gish RG, Given BD, Lai CL, Locarnini SA, Lau JY, Lewis DL, Schluep T. Chronic hepatitis B: virology, natural history, current management and a glimpse at future opportunities. Antiviral Res. 2015;121:47–58. doi: 10.1016/j.antiviral.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Sim HW, Knox J. Hepatocellular carcinoma in the era of immunotherapy. Curr Probl Cancer. 2018;42:40–48. doi: 10.1016/j.currproblcancer.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Yang N, Li Z, Yan M, Xiao W, Zhang W, Long Y, Cheng Y, Ming K, Xu B. Evaluation of serum alpha-fetoprotein level in chronic hepatitis C patients. Clin Lab. 2019;65 doi: 10.7754/Clin.Lab.2018.180607. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Zhang Y, Wang Y, Xu L, Xu W. Alpha-fetoprotein-L3 and Golgi protein 73 may serve as candidate biomarkers for diagnosing alpha-fetoprotein-negative hepatocellular carcinoma. Onco Targets Ther. 2015;9:123–129. doi: 10.2147/OTT.S90732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn DG, Kim HJ, Kang H, Lee HW, Bae SH, Lee JH, Paik YH, Lee JS. Feasibility of α-fetoprotein as a diagnostic tool for hepatocellular carcinoma in Korea. Korean J Intern Med. 2016;31:46–53. doi: 10.3904/kjim.2016.31.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He C, Zhang X, Li C, Peng W, Wen TF, Yan LN, Yang J, Lu W. Changes of alpha-fetoprotein levels could predict recurrent hepatocellular carcinoma survival after trans-arterial chemoembolization. Oncotarget. 2017;8:85599–85611. doi: 10.18632/oncotarget.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uslu S, Alaca N, Kilic KD, Uysal A, Kurtel H. The effects of aerobic exercise frequencies on liver fibrosis, α-fetoprotein and cytokeratin 19 in experimental type 2 diabetes-induced rats: an immunohistochemistry study. Biotech Histochem. 2018;93:615–622. doi: 10.1080/10520295.2018.1517898. [DOI] [PubMed] [Google Scholar]
- 30.Zhen S, Qiang R, Lu J, Tuo X, Yang X, Li X. Enhanced antiviral benefit of combination therapy with anti-HBV and anti-PD1 gRNA/cas9 produces a synergistic antiviral effect in HBV infection. Mol Immunol. 2021;130:7–13. doi: 10.1016/j.molimm.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Lu YX, He CZ, Wang YX, Ai ZS, Liang P, Yang CQ. Effect of entecavir on the intestinal microflora in patients with chronic hepatitis B: a controlled cross-sectional and longitudinal real-world study. Infect Dis Ther. 2021;10:241–252. doi: 10.1007/s40121-020-00355-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wungu CDK, Amin M, Ruslan SEN, Purwono PB, Kholili U, Maimunah U, Setiawan PB, Lusida MI, Soetjipto S, Handajani R. Association between host TNF-α, TGF-β1, p53 polymorphisms, HBV X gene mutation, HBV viral load and the progression of HBV-associated chronic liver disease in Indonesian patients. Biomed Rep. 2019;11:145–153. doi: 10.3892/br.2019.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong VW, Yu J, Cheng AS, Wong GL, Chan HY, Chu ES, Ng EK, Chan FK, Sung JJ, Chan HL. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 2009;124:2766–2770. doi: 10.1002/ijc.24281. [DOI] [PubMed] [Google Scholar]
- 34.Wang SS, Lee FY, Chan CC, Lu RH, Chao Y, Lin HC, Wu SL, Tsai YT, Lee SD. Sequential changes in plasma cytokine and endotoxin levels in cirrhotic patients with bacterial infection. Clin Sci (Lond) 2000;98:419–425. [PubMed] [Google Scholar]
- 35.Odeh M, Sabo E, Srugo I, Oliven A. Serum levels of tumor necrosis factor-alpha correlate with severity of hepatic encephalopathy due to chronic liver failure. Liver Int. 2004;24:110–116. doi: 10.1111/j.1478-3231.2004.0894.x. [DOI] [PubMed] [Google Scholar]
- 36.Gerstner C, Schuetz T, Von Baehr V, Roske A, Volk H, Lochs H, Plauth M. Correlation between energy expenditure, nutrient intake, malnutrition and activation of the inflammatory system in patients with hepatic cirrhosis. J Hepatol. 2001:196. [Google Scholar]
- 37.Tilg H, Wilmer A, Vogel W, Herold M, Nölchen B, Judmaier G, Huber C. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992;103:264–274. doi: 10.1016/0016-5085(92)91122-k. [DOI] [PubMed] [Google Scholar]
- 38.Inui A. Cancer anorexia-cachexia syndrome: are neuropeptides the key? Cancer Res. 1999;59:4493–4501. [PubMed] [Google Scholar]
- 39.Xing H, Zheng YJ, Han J, Zhang H, Li ZL, Lau WY, Shen F, Yang T. Protein induced by vitamin K absence or antagonist-II versus alpha-fetoprotein in the diagnosis of hepatocellular carcinoma: a systematic review with meta-analysis. Hepatobiliary Pancreat Dis Int. 2018;17:487–495. doi: 10.1016/j.hbpd.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Sinn DH, Kim SE, Kim BK, Kim JH, Choi MS. The risk of hepatocellular carcinoma among chronic hepatitis B virus-infected patients outside current treatment criteria. J Viral Hepat. 2019;26:1465–1472. doi: 10.1111/jvh.13185. [DOI] [PubMed] [Google Scholar]


