Abstract
Drug repurposing, also known as drug repositioning, entails the application of pre-approved or formerly assessed drugs having potentially functional therapeutic amalgams for curing various disorders or disease conditions distinctive from their original remedial indication. It has surfaced as a substitute for the development of drugs for treating cancer, cardiovascular diseases, neurodegenerative disorders, and various infectious diseases like Covid-19. Although the earlier lines of findings in this area were serendipitous, recent advancements are based on patient centered approaches following systematic, translational, drug targeting practices that explore pathophysiological ailment mechanisms. The presence of definite information and numerous records with respect to beneficial properties, harmfulness, and pharmacologic characteristics of repurposed drugs increase the chances of approval in the clinical trial stages. The last few years have showcased the successful emergence of repurposed drug immunotherapy in treating various diseases. In this light, the present review emphasises on incorporation of drug repositioning with Immunotherapy targeted for several disorders.
Keywords: Cancer, infectious disease, Covid-19, cardiovascular diseases, drug discovery, drug repurposing, immunotherapy
Introduction
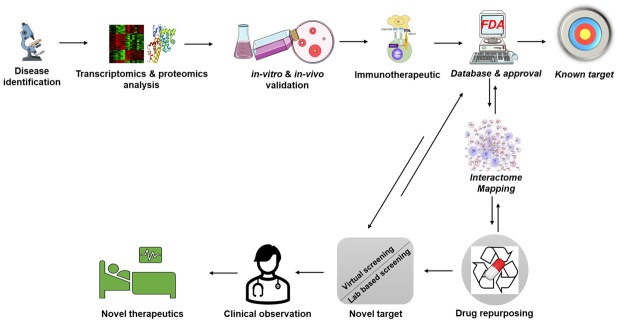
In highly competitive therapeutic and pharmaceutical industrial settings, drug repurposing immunotherapies are favored over the “de novo” approaches of drug discovery [1]. Drug repurposing is an approach which involves finding new indications for pre-existing, FDA approved, endorsed, vastly characterized medications used in different medical, experimental, or clinical backdrops [2]. These drugs could also be known to be failures in original indications but could hold a potential in curing various atypical and multifaceted terminal diseases depending on their structural as well as functional characteristics (Figure 1). The advancement of novel or new drugs is estimated to take approximately 15-20 years costing a valuation of USD ~3-5 billion to create and launch a drug into the market [3]. On the other hand, repurposed drugs are preapproved in terms of certified formulations, safety and preclinical examinations, with known pharmacokinetic reports from the primary stages of clinical tests. As a result, it is a more practical and efficient option with a reduced risk of failure [4].
Figure 1.
Drug repurposing strategy. This figure was created using the Servier Medical Art Commons Attribution 3.0 Unported Licence (http://smart.servier.com).
Many repurposed drugs were identified by a cause of unanticipated and serendipitous encounters. One of the well-known candidates is OnabotulinumtoxinA (BOTOX®; Allergan) which has eight distinctive sanctioned indications [5]. An unsuccessful chemotherapy drug, known as azidothymidine, worked well in curing human immunodeficiency virus [6]. With each passing year, a search for swifter, cost-effective and novel techniques is rising in the drug discovery and development sector. It demands advances in large data depositories and allied investigative techniques. This has gained attention in developing orderly approaches to drug repositioning. Diverse pioneering translational bioinformatics-based methods are empowering systematic repurposing screenings [7]. A research team has invented a progressive, commanding, and state-of-the-art artificial intelligence (AI) and network medicine technology that can accelerate remedial expansion [8]. The present review describes some of the main methodologies in drug repurposing immunotherapy, the means of successful applications of existing compounds to new symptoms, and their benefits to the society as well as the pharmaceutical industry.
Repurposing immunotherapy in cancer
One of the most advanced treatments in the cancer field is immunotherapy which aids the immune system to fight the disease [9]. The immune system which is composed of white blood cells and tissues of the lymphatic system identify and fight against cancer [10]. However, a dynamic microenvironment of malignant cells makes them unrecognizable and hides them from immune cells [11,12]. The M2 vs M1 macrophage recruitment paradigm executes a crucial function in tumor succession [13,14]. Conventional immunotherapy involved the use of remedies that either improved the cell’s defense mechanism against cancer or inhibited the tumor’s efficacy to disguise the antigens in the system [15]. In 1972, the very first case of drug repurposing was reported for treating leukemia with the help of a hypertoxic Arsenic trioxide which was used in traditional chinese medicine [16,17]. A drug previously used for morning sickness, the Thalidomide along with its analogues like thalidomide, lenalidomide and pomalidomide are repurposed for treating multiple myeloma [18,19]. Many clinical trials involving engineered T cells, natural killer (NK) cells, Adoptive cell therapy etc. have exhibited promising outcomes in a variety of malignant conditions [20-22]. However, the restrictions involved in these approaches open the room for improvement in terms of efficiency, cost effectiveness and time consumption. These limitations could be resolved by combining the repurposing of drugs with immunotherapy. It would not only decrease the expense and necessity of trial or testing but would also reduce the time that accompanies novel drug research [23]. Lately, numerous in silico advances and high-performance assessment techniques have been established to assist drug repurposing practice [24,25]. Based on the structure-activity relationship (SAR), various drugs have been evaluated to explore analogous clinical indications using electronic tools like Protein Data Bank and DrugPredict etc. [26-29]. Databases like the Library of Integrated Ntework-based Cellular Signatures are efficient in classifying drugs amongst similar transcriptional signatures for drug repositioning [30]. Human transcriptome and interactome data were combined in a recent study that took a network medicine strategy to screening diagnostic and prognostic biomarkers and exploring medication repurposing in human cancer [31]. One report presented a wide-ranging graphic analytics tool, ClinOmicsTrailbc, which examines epigenomics and transcriptomics datasets to distinguish as well as assess the tumor mutational burden, and biomarkers etc. [32]. The user-friendly databases like repoDB and repurposeDB combine data about clinical consequences of drug repurposing [33-35].
The immune checkpoint inhibitors (ICIs) are monoclonal antibodies that block receptors like TIM-3, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), LAG-3, programmed cell death-1 receptor (PD-1R) or anti-PD-L1 [36]. This augment T-cell activity, stemming an expansion in antineoplastic immunity which leads to demolition and eradication of tumor cells [37]. However, eventually, these immunotherapeutic means failed to control the disease due to the acquired resistance in cancer patients. When repositioned drugs were combined with ICIs, a remarkable improvement was observed with respect to antitumor immunity. For example, Metformin, a type 2 diabetes drug was reported to escalate CTL levels by destabilizing and misbalancing membrane localization of PD-L1 by triggring AMP-activated protein kinase (AMPK) and thereby inducing ER-associated protein degradation (ERAD) via S195 phosphorylation of PD-L1 [38]. Li et al. (2020) executed calcium flux blockade by Amlodipine which activate PD-L1 degradation and stimulated antitumor immunity. Cytokines, the ~30 KDa glycoprotein or polypeptide signaling molecules are paracrine facilitators [39]. Their pro-apoptotic and cytotoxic properties have been explored in cancer research as prospective drugs in combination with advanced immunotherapies to revitalize the immune system against cancer succession [40]. Mansurov et al., inactivated the immunotoxic properties of IL-12 by modifying its conformation using tumor-protease-cleavable linker [41]. Lately, studies have categorized novel T cell adapting remedies by phenotypical assessment of chemical libraries [42,43]. Marro et al., reported ingenol mebutate, a compound identified from a chemical library created on ReFRAME drug-repurposing collection, that expanded the endurance of wearied CD8+ T cells and provided immunity against LCMV infection and suppressed tumor progression inside the B16 sarcoma model [44]. These repurposed drugs possess the capacity to exhibit synergy with existing checkpoint-blockade immunotherapies.
In the tumor microenvironment (TME), the tumor puts the immune cells under metabolic stress by modulating the metabolic networks for its progression. Therefore, reprogramming the TME by means of drug repurposing may increase the efficiency of cancer immunotherapy [45]. Numerous examples of efficacious drug repurposing for Cancer immunotherapy are summarized in Table 1.
Table 1.
Drug repurposing cases for treating malignant systems
| Sr No. | Original indication | Name of the Drug | Repurposing immunotherapy used for treating type of cancer | Mode of action | Reference |
|---|---|---|---|---|---|
| 1 | Bone remodeling | Denosumab | Melanoma | M1 macrophage activation | [23,157] |
| T-cell activation | |||||
| Production of nuclear factor kappa B | |||||
| Dendritic cell survival and function | |||||
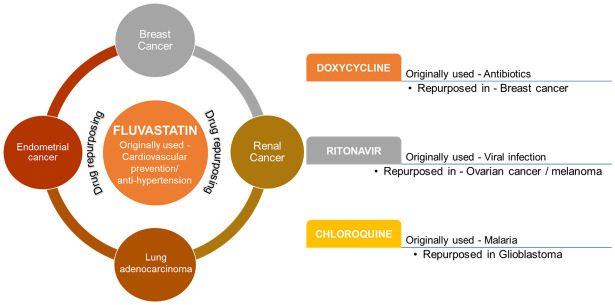
| 2 | Antibiotic | Doxycycline | Breast cancer | Suppression of stem cell marker | [158,159] |
| Inhibition of Autophagy | |||||
| Tigecycline | Ovarian cancer/Myeloid leukemia | Inhibition of mitochondrial translation | [160] | ||
| Suppression of MYC, HIFs, PI3K/AKT or AMPK-mediated mTOR, cytoplasmic p21 CIP1/Waf1, and Wnt/β-catenin signaling | |||||
| 3 | Viral Infection | Ritonavir | Ovarian cancer/Melanoma | AKT signaling Suppression | [161] |
| Apoptosis | |||||
| 4 | Antiretroviral Drug-HIV-1 integrase (IN) inhibitor | L-870810 | Cancer | Cytotoxicity | [162,163] |
| Blocking oncogenic kinases | |||||
| 5 | Anti-neurodegenerative agent | Benserazide | Colon cancer | Suppression of M2 splice isoform of pyruvate kinase (PKM2) | [164,165] |
| Melanoma | |||||
| Riluzole | Pancreatic Cancer | Suppressing the Wnt-β-catenin pathway | [166] | ||
| 6 | Anti-bacterial agent | Ciprofloxacin | Colon cancer | Reversal of MDR | [17,167] |
| 7 | Fungal infection | Enilconazole | Colorectal cancer | Suppression of PI3K/AKT pathways | [168] |
| 8 | Malaria | Chloroquine | Glioblastoma | Autophagy inhibition | [169] |
| Reduction of tumor hypoxia | |||||
| 9 | Antipsychotic drugs | Chlorpromazine | Glioblastoma | Inhibition of cytochrome c oxidase | [170] |
| Risperidone | Colorectal Cancer | Apoptosis | [171] | ||
| Anti-proliferative activity | |||||
| 10 | Antidepressants | All-trans retinoic acid (ATRA) | Acute myeloid leukemia | Suppression of PKCβ, MEK/ERK and Akt activity | [172] |
| 11 | Cardiovascular Prevention/antihypertensive drug | Losartan | Ovarian cancer | Apoptosis | [173] |
| Decrease in fibroblast infiltration | |||||
| Lower expression of collagen (Col)-I (Col)-III | |||||
| Lower expression of alpha smooth muscle actin (Acta2) | |||||
| Enalapril | Colorectal cancer | Activation of nuclear factor-κB (NF-κB) signaling proteins | [174] | ||
| Upregulation of vascular endothelial growth factor (VEGF) expression | |||||
| Anti-proliferative activity | |||||
| Apoptosis | |||||
| Valsartan | Gastric cancer | Regulation of PI3K/AKT Pathways | [175,176] | ||
| Telmisartan | Lung cancer/Gastric cancer | Induction of apoptosis | [177] | ||
| Inhibition of cadherin-mediated activation FGFR signaling | |||||
| Inhibition of the PI3K/AKT pathway | |||||
| Irbesartan | Prostate cancer | Renin-angiotensin blockade | [176] | ||
| Benazepril | Esophageal carcinoma | Inhibition of Ki-67 nuclear protein | [178] | ||
| Inhibition of angiogenesis | |||||
| Digoxin | Prostate cancer | Inhibition of VEGF | [179] | ||
| Inhibition of angiogenesis | |||||
| Fluvastatin | Breast cancer | Expression of Sirtuin 6 (SIRT6) | [180,181] | ||
| Renal cancer | Activation of mTOR pathway | ||||
| endometrial cancer (EC) | Endoplasmic reticulum (ER) stress leading to aggresome formation | ||||
| Lung adenocarcinoma | Anti-proliferative activity | ||||
| Apoptosis | |||||
| Propranolol | Malignant Melanoma | Suppression of ERK/Cyclin D1/Rb/Cyclin E pathway | [182] | ||
| Stimulation of G0/G1/S phase arrest | |||||
| Anti-inflammatory drugs | Ibuprofen | Gastric cancer | Apoptosis | [183,184] | |
| Inhibition of cell proliferation | |||||
| Inhibition of cyclooxygenase |
Abbreviations: MYC, MYC Proto-Oncogene; HIF, Hypoxia-inducible factor; PI3K, phosphoinositide 3-kinases; AMPK, AMP-activated protein kinase; mTOR, Mammalian target of rapamycin; p21 CIP1/Waf1, cyclin-dependent kinase inhibitor p21; Wnt, Wingless-Type; Akt, Ak strain transforming; PK, pyruvate kinase; MDR, Multidrug resistance; PKCβ, Protein kinase C-β; ERK, Extracellular signal-regulated kinase; MEK, Mitogen-activated protein kinase kinase MEK; Col, collagen; Acta, alpha smooth muscle actin; NF-κB, nuclear factor-κB; VEGF, vascular endothelial growth factor; FGFR, fibroblast growth factor receptor; SIRT6, Sirtuin 6; ER, Endoplasmic reticulum.
The Warburg effect postulates that cancer cells gain energy more efficiently via glycolysis than oxidative phosphorylation. This rises lactic acid levels and makes the pH of TME acidic and heightens immunosuppressive properties of TME by preventing the propagation of CTLs [13,46]. Lactate dehydrogenase (LDH), an enzyme involved in the glycolytic conversion of pyruvate to lactate, can be inhibited to slow tumor growth. Although, galloflavin was shown to disable LDH, it was also found to decrease interferon gamma (IFN-γ) levels by T-cells. In a glioma model, diclofenac an non-steroidal anti-inflammatory drug (NSAID) was reported to decrease the acidic levels of TME and obstruct cancer proliferation [47]. Inhibiting the PI3KeAKTemTOR network was found to downregulate the glycolysis. Amino acid metabolism stimulates the tumor’s growth as well as endurance with the help of building block synthesis, a decline in oxidative stress, and immune circumvention provokment [48]. Therefore, inhibition of indoleamine 2,3-dioxygenase (IDO), an enzyme responsible for the breakdown of tryptophan was found to be effective in augmenting antitumor immunity of the subjects in liver cancer [49,50]. In this regard, Imatinib was also reported to enhance antitumor immunity by activation of effector T cells. Leone et al., reported expansion of antitumor activity of T cells upon using glutamine antagonist 6-diazo-5-oxo-L-norleucin (DON) or its prodrug JHU-083 as a treatment for cancer therapy which supported tumor suppression [51]. Byun et al., also reported a synergic effect of Glutamine inhibition with ICI in supporting immunity against cancer [52]. Many studies have that adoptive T cell therapies are proven to be efficient in cancer treatment involving anti-CD19 chimeric antigen receptor (CAR) T cells and TILs. Many reviews have provided excellent information on these powerful treatment alternatives [53-55].
Oncolytic viruses were shown to work as an antigen-agnostic vaccine for various cancer conditions within the TME by activating innate immunity involving macrophages, dendritic cells, and NK cells [56]. As a response, OV-infected tumor cells get demolished. The expansion and accumulation of activated T cells within the TME results in elimination of cancer cells. Vijayakumar et al., showed the efficient synergy of Newcastle disease (ND) virus expressing anti-CTLA4 single chain variable fragment (scFv) with radiotherapy for boosting immune cell activity against murine melanoma [57]. Shekarian et al., provided a preclinical validation in support of intramural-attenuated rotavirus to prevent resistance towards immune checkpoint immunotherapies in pediatric cancers by expressing double-stranded RNA receptor retinoic acid-induced gene-I (RIG-I) [58]. In this regard, many advanced approaches are being explored to treat numerous cancer conditions [56,59-61]. Nanoparticle (NP)-centered drug transport schemes are being explored intensively for attaining targeted delivery of several antineoplastic mediators, counting small molecule drugs, monoclonal antibodies, DNAs, and siRNAs to the cancer sites [23]. Kadiyala et al., showed efficacy of a synthetic high-density lipoprotein nanodiscs for chemo-immunotherapy for treating glioblastoma [62]. Feng et al., evalutaed the efficiency of a prodrug nanoparticle in shunting the cancer proliferation and blocking metastasis in murine models of the breast as well as 4 colorectal cancer [63]. Figure 2 exhibits a schematic illustration of a few drugs that have been used to treat various malignancies.
Figure 2.
Schematic illustration of drugs that have been used to treat various malignancies.
Repurposing immunotherapy in cardiovascular diseases
Despite the development of novel therapeutics and medical innovations, cardiovascular diseases (CVD) continue to rank higher as a major contributor to the mortality index of the world. Drug repurposing can play a pivotal role to improve the treatment regime for the selection of the best drugs without or with minimum side effects. While the success rate of generating a new molecular entity is only 2.01%, the field of drug repurposing is gaining popularity [64], and the number of approved drugs has declined in the past three decades. In the pharmaceutical sector, drug repurposing accounts for roughly one-third of approvals over the past decade. It is considered as a direct application of polypharmacology where one drug molecules target multiple genes/proteins or disease pathway.
Initially, atherosclerosis was considered as an accumulation of lipoprotein in the arterial wall. With the advancement in decoding the disease pathophysiology, the role of inflammation was also recognized equally in cardiovascular diseases [65,66]. Inflammation is mediated by pro-inflammatory cytokines, chemokines, lipids, and adhesion molecules [67-69]. Fernandez-Gutierrez et al., highlighted the relevance between CVD and inflammation and suggested that the occurrences of inflammatory diseases like systemic lupus erythematous, arthritis, and psoriasis increase the risk of CVD [70]. Thus, a treatment involving the blockade of the inflammatory cytokines could hold a potential of health improvements in CVD patients, clinically [71] and the immunomodulatory effects of ketogenic diet reduces inflammation in various immune disorders including CVDs [72]. Pro-inflammatory cytokines include IL-1, IL-6, IL-18, and tumour necrosis factor (TNF), while IL-1R antagonists, IL-10, IL-19, and IL-33 antagonize inflammation [73]. Inflammation and disturbed immune system evokes occurrences of CVD and others. The increased level of inflammation is a challenge to clinicians as it predisposes an individual towards developing end organ comorbidities. Activated lymphocytes and monocytes run towards the endothelium, which penetrates the arterial wall and thus induce atherogesesis [74]. The healing of injury induced by the above cytokines favours the formation of atherosclerotic plaques which further increase the risk of plaque rupturing and may result in thromboembolic events [75]. Moreover, the availability of monoclonal antibody-based immunotherapies targeting pro-atherogenic cytokines paves a path to address the role of immunotherapy in CVD [76].
Interestingly, cancer and heart failure interact with each other in a bidirectional way [77,78]. It has been studied by Armenian et al., that 89% of lung cancer patients possess high risk of developing atherosclerosis [79]. In fact, the low grade inflammation is associated with the release of TNF-α, interlukin (IL)-1β, IL-6 and IFN-γ which increase the risk of heart disease. Anti-TNF-α therapy (Infliximab, Adalimumab, Certolizumab etc.) was associated with a reduced risk of all cardiovascular events [80]. Canakinumab (anti-IL-1), and Toclizumab (anti-IL-6) are the candidate of choice as immunotherapeutic agents to manage the inflammatory status. Canakinumab Anti-Inflmmatory Thrombosis Outcome Study (CANTOS) trial is one of the largest trials in the series of anti-cytokine immunotherapy which hints the roles of inflammation in triggering both CVD and cancer. The mature B lymphocytes are involved in the mobilization of inflammatory monocytes in the heart which leads to the declined heart function [81]. Rituximab was the first monoclonal antibody approved for cancer patients. It was developed in the form of anti-CD20 molecules [82] that caused depletion in normal and malignant B cells. Many studies are supporting CAR-T cell therapy as an interesting therapeutic option for treating several malignancies with respect to its anti-imflammatory effects on various functions of the heart [83]. Tocilizumab, a monoclonal antibody targeting IL-6 receptors used for the treatment in RA was found equally effective in myocardial injury [84]. Another monoclonal anti-IL-17 antibody, secukinumab, has been approved to treat arthritis and psoriasis, and it has been shown to improve myocardial function parameters like the global longitudinal strain rate during early diastole and left ventricular twisting, as well as the coronary flow reserve and pulse wave velocity.
The above facts strongly highlighted the key role of inflammation in the onset and progression of CVDs. However, the knowledge about the side effects of anti-inflammatory drugs limits the possibility to see their potential role in the cardiovascular field. With the support of various medical evidence, drug repurposing could be considered as a powerful strategy that offers a great hope in the treatment regime of the cardiac ailments within a clinically attainable safety range.
Repurposing immunotherapy in infectious disease
Infectious diseases continuously pose a significant burden to human health with a high rate of morbidity and mortality and are considered globally in the top 10 in mortality rate [85]. Globally, it is estimated that every third death results from an infectious disease and is predicted to acquire the highest contributor of mortality by the year 2050. The outspread of infectious diseases was majorly caused by viruses, bacteria, fungi, and protozoans which augmented weaker immunity. The WHO’s annual data estimated 300-500 million people infected with malaria, over 330 million with sexually transmitted diseases, 33 million cases of HIV/AIDS, and 14 million with tuberculosis. A recent survey carried out by the national sample survey organization (NSSO) estimated that over 30% of people in India are suffering from infectious diseases [86]. The prevalence of acute and chronic infectious diseases has been further challenging to mitigate after rapidly evolving resistance against frontline clinical therapies and most of the conventional treatments seem to be ineffective to work. The most recent, Covid-19 pandemic infection outbreak with higher morbidity and mortality rate throughout the different continents of the world, and no standalone therapy is available even today [87]. It is a serious concern to search for various strategies to resist such ailments. Much attention has been drawn to repurposing the existing therapies and drugs. Collectively, immunotherapies can be defined as the collection of treatments which able to boost the human immune system in such a precise way to promptly fight against infectious diseases. It is well-stated that infectious pathogens are not able to clear up, and remain present in the host when immunity gets weakened. Immunotherapy treatment can potentiate immune responses that will help to eradicate pathogens and fight against threats. Mechanistically, immunotherapy can either be passive which synthesizes ex-vivo and injected in the host for protection. In contrast, active immunotherapy induces immunological memories in the host using active effectors or virulence factors. Over a period, different immunotherapies have been investigated and tested to mitigate several infectious diseases. Immunomodulatory molecules, cell therapies, Monoclonal antibodies, and vaccines are such prominent examples of immunotherapy that have been successfully employed against infectious diseases. For a long time, vaccines symbolize the foremost immunotherapy used to protect hosts against different diseases. The success of the vaccine lies in an immunization program of vaccine run over a decade which has successfully eradicated polio and smallpox, as devastating diseases from India. Henceforth the research on vaccines has been shifted last few decades to find out their new activities (repurposed) against different infectious diseases. Some of the prominent examples of vaccines have been repurposed for the protection of infectious diseases. The promising BCG vaccine is a formulation of live attenuated Mycobacterium bovis. It is a standalone vaccine for the treatment of tuberculosis over the last 100 years. The efficacy of BCG was also repurposed for the treatment of non-muscular bladder cancer and melanoma. However, BCG has emerged as an adjuvant to cancer treatment without any successful completion of clinical trials.
The long-term phase-3 trial of the BCG vaccine was carried out on colon cancer patients and showed a promising result in overall survival when it was injected as an adjuvant after surgery [88,89]. Linezolid, marketed under the brand name Zyvox, is an antibiotic from the first generation. It works by blocking the production of proteins in bacteria. When bound to bacterial ribosomes, it prevents the production of functional 70S ribosomes and slows down the translation process [90,91]. In the past, linezolid was only used to treat infections caused by gram-positive bacteria like Staphylococcus aureus. Despite showing excellent antibacterial capabilities, linezolid’s usage against drug-resistant tuberculosis (DR-TB) is time-limited due to its neurological adverse effects [90]. Both Moxifloxacin and Gatifloxacin are fourth-generation antibiotics derived from fluroquinolone class of medicines. The major purpose of these medicines is to limit the enzymatic activity of DNA gyrases and topoisomerase-IV, therefore preventing the replication of DNA in bacteria and other microorganisms [92,93]. It was first approved for use against skin and stomach germs, but its encouraging effect led experts to conclude that it was also a safe candidate for treating tuberculosis [94]. While clofazimine’s approval [95] is limited to treating leprosy, the antibiotic’s antibacterial anti-inflammatory characteristics have been found to be highly effective in combating multidrug-resistant and extensively drug-resistant tuberculosis [96]. Similar to how the antibacterial drugs sanfetrinem cilexetil, spectinamide, meropenem, and faropenem were initially developed to treat various bacterial infections, they were later found to have a novel role against tuberculosis [97-99].
The Diptheria vaccine contains inactivated toxins that potentially protect the host from Corynebacterium diphtheriae infections. Different trials of the diphtheria vaccine combined with the tetanus vaccine (Td) have been found to induce immunogenic responses against the brain, prostate, pancreas, liver, breast, or lung cancer. Similarly, the influenza vaccine was reported to show antitumor activity if administered without adding any adjuvant against tumors on intratumoral administration. However, the Human Papillomavirus vaccine is formulated by viral-like particles (VLPs) of major capsid protein (L-1) effectively preventing Human Papillomavirus (HPV) infection and providing protection from cervical cancer [100]. The clinical trials of the HPV vaccine administered with sintilimab (anti-PD-1) showed promising results to prevent pre-cancerous anal or vulvar lesions. The 17D-204 strain of the yellow fever virus is transfected into a chicken embryo for preparation of yellow fever vaccine, used for the protection of travellers from this virus that transmits through a mosquito bite. The yellow fever vaccine also suppresses tumor progression in human and mouse cell lines via T-cell-mediated cell immunity [88]. In a recent study, the cancer approved drug Bruton’s tyrosine kinase (BTK) [101] inhibitor known as ibrutinib has been repurposed for the treatment of COVID-19 infection that promisingly reduces inflammation in the lungs. The use of ibrutinib is approved for clinical trials after receiving promising results against Covid-19 infections [102].
Repurposing immunotherapy in COVID-19 for targeting inflammatory pathway in disease progression
SARS-CoV-2, the new beta coronavirus responsible for the recent global public health disaster known as COVID-19, causes severe illness and millions of people have lost their lives due to COVID-19 infection. Acute respiratory distress syndrome (ARDS) is the most notable clinical symptom of COVID-19 infection among severely infected patients. Many of the extrapulmonary symptoms of COVID-19 are believed to have their origins in rapid virus replication and severe inflammatory response in the lung. Numerous tissues and nearly all bodily fluids have yielded SARS-Co-2 RNA [103]. Extrapulmonary involvement and systemic inflammatory symptoms are hallmarks of COVID-19, which can ultimately cause multiorgan failure and death [104,105]. Intriguingly, hospitalized patients with spiked inflammatory cytokines and persistent lung injury even after SARS-CoV-2 is under control or eradicated [105].
Immunotherapy refers to the use of medications comprised of immune cells or antibodies to modulate the immune system to treat SARS-CoV-2 infection and it is a relatively novel strategy to treat various cancers and infectious diseases [106,107]. Though, the toxicity outline of these treatment strategy, such as constrain in using CAR-T cells, despite the fact that immunotherapy has shown outstanding responses in patients with malignancies [108]. Cytokine storm, also known as cytokine release syndrome, is a potentially fatal consequence of immunotherapy that manifests with fever, hypotension, and respiratory failure alongside increased cytokine and inflammatory markers [109]. In the years following immunotherapy, many medications have proven effective in treating cytokine release syndrome, and numerous serologic markers are now accessible for both diagnosis and therapy response monitoring. It is possible that the pathophysiologic mechanisms underlying systemic symptoms of COVID-19 and toxicity after immunotherapy are identical. Therefore, immunotherapy may have an important role in COVID-19 treatment. Here in Table 2, we have summerized available immunotherapeutic targets for COVID-19.
Table 2.
Overview of available immunotherapeutic targets for COVID-19
| Sr No. | Target | Immunotherapeutic role | Available drugs | Intervention | Role of COVID-19 | Recommendation | Overall Conclusion | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | IL-6 | Elevated in cytokine restoration syndrome during CAR-T treatment | Tocilizumab | anti-IL-6 | Elevated with severity of diseases | Recommended for sever respiratory disease in combination of Dexamethasone | Beneficial | [185-187] |
| Sarilumab | ||||||||
| Otilimab | ||||||||
| 2 | JAK/STAT | Steroid refractory GVHD | Baricitinib | Jak kinase activate after interleukin stimulation | Interleukin receptor activation | Recommended for progressive ARDS in combination of Remdesivir | Partial beneficial | [188-190] |
| Ruxolitinib | ||||||||
| Tofacitinib | ||||||||
| Imatinib | ||||||||
| 3 | IL-1R | IL-1R inhibition to reduce inflammation and slower tissue damage | Anakinra | anti-IL-1 | IL-1b elevated during COVID-19 | Radiologically and PCR confirm sever hospitalize patients | Beneficial | [191-193] |
| Canakinumab | ||||||||
| 4 | IFN-γ | IFN blocker in familiar hemophagocytic lymphohistiocytosis | Emapalumab | anti-IFN-γ | Reduced inflammation | Radiologically and PCR confirm sever hospitalize patients | Partial beneficial | [194-196] |
| 5 | TNF-α | Inflammation suppression in autoimmune diseases | Infliximab | anti-TNF-α | Hyperactive immune status | Radiologically and PCR confirm sever hospitalize patients | Partial beneficial | [197,198] |
| Adalimumab | ||||||||
| 6 | Complement C5a | Hyperactivated during transplant associated thrombotic microangiopathy | Vilobelimab | anti-C5a | Anti-inflammatory effect and improved PaO2/FiO2 | Radiologically and PCR confirm sever hospitalize patients | No significant effect | [199-201] |
| 7 | Spike protein of SARS-CoV-2 | --- | Bamlanivimab | anti-spike protein mAb | Change is log viral load | Patients with mild to moderate severity | Beneficial | [202,203] |
| Casirivimab |
Abbreviations: IL, Interleukin; CAR-T, Chimeric antigen receptor; JAK/STAT, Janus kinase/signal transducers and activators of transcription; ARDS, Acute respiratory distress syndrome; GVHD, Graft-versus-host disease; IFN-γ, Interferon gamma; TNF-α, Tumor necrosis factor alpha; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; mAb, monoclonal antibody.
SARS-CoV-2 causes acute lung inflammation
SARS-CoV-2 detects ACE2 on respiratory epithelial cells. Spike protein mediates viral adherence and ACE2 recognition [110]. Most COVID-19 patients hospitalized had pneumonia or ARDS, viral replication in the respiratory tract might migrate to the lower respiratory tract and produce pneumonia [111]. Fewer, low oxygen saturation, shortness of breathing and dry cough are early SARS-CoV-2 lung symptoms, also 20% to 33% of patients need ICU hospitalization [112-114]. The most common histologic finding is diffuse alveolar injury, which damages the blood-air contact and causes inflammation and mucosal thickening during acute infection. Patients receiving mABs aiming TME by fetching PD-1 and CTLA4 receptors called checkpoint inhibitors (CPIs), to reverse cancer-induced T-cell anergy have similar pathophysiology (30546008). CPI, notably nivolumab, can cause severe inflammatory interstitial pneumonitis that resembles COVID-19 lung involvement [115]. PD-1-positive T cells may regulate pulmonary dendritic cells and macrophages in CPI-related pneumonitis [116]. CPI-related pneumonitis and COVID-19 pulmonary symptoms shares PD-1 and toll-like receptors (TLRs) mechanism. TLR stimulation on CD8+ T cells lowers PD-1 expression and SARS-Co-2 spike protein binds to TLR and stimulates inflammatory cytokines [117,118].
Endothelial impairment and systemic inflammation in COVID-19 and immunotherapy
COVID-19 patients observed with activated complement cascade that causes microvascular damage, thrombosis in the circulatory system, and intravenous catheters causes morbidity and mortality in COVID-19 patients [119-121]. ACE2-expressing endothelial cells in arteries and veins throughout the body may explain SARS-tropism CoV-2’s to renal, cardiac, and gastrointestinal organs outside the respiratory tract [122]. SARS-CoV-2 invades lung endothelial cells and is crucial to pneumonitis worsening and spreading to other organs. Endothelialitis can block fibrinolysis, stimulate the complement system, and cause microthrombi and microvascular dysfunction [123]. Thus, histopathology shows neutrophil extracellular traps, fibrin deposition, and/or microthrombi [124]. Many proinflammatory cytokines activate the coagulation system, making COVID-19 procoagulant. Acute inflammatory conditions were associated with high levels of tumor TNF-α, IL-6, and IL-1 and hypercoagulability, occasionally leading to diffuse intravascular coagulation [125,126]. A prelimilinary investigation recommends that SARS-CoV-2 directly promotes platelet adhesion and aggregation [127,128]. Thus, COVID-19 and post-CAT-T associated toxicity share endothelial involvement. Hay et al., showed that endothelial activation was associated with cytokine release syndrome and elevated levels of circulating endothelial-derived factors such as Von Willebrand and angiotensin-2 (28924019). SARS-CoV-2 may inhibit the host interferon response and downregulate major histocompatibility complex class I (MSC-I) molecules on many cells during early infection [129-131]. This prevents immune detection and slows viral clearance. COVID-19’s induced lymphopenia may cause uncontrolled viral replication [132].
IL-6, IL-1b, and TNF-α were considerably raised in severe COVID-19 patients and caused cytokine release syndrome, according to recent meta-analyses [133]. IL-6 has a key function in autoimmune disorders and is considered as a major proinflammatory cytokine, it affects IL-6R expressing cells like T cells, B cells, monocytes, and hepatocytes [134,135]. Multiple viral infections require IL-6, although the main source of IL-6 during COVID-19 remians unclear, upon IL-6R binding on the surface of target cells, intercellular signal leads to the activation of JAC/STAT3 axis [136]. Post-CAR-T therapy cytokine release syndrome has extremely elevated serum IL-6 levels [137]. In CAR-T-associated cytokine syndrome, release of IL-1 tends to precede that of IL-6; consequently, targeting IL-1 might attenuate or prevent cytokine release syndrome [138]. mABs (rituximab) and the bispecific antibody blinatumomab also produced IL-6 in B cell malignancies [139,140]. IL-6 concentrations correlated with pulmonary disease severity in 69 hospitalized SARS-CoV-2 patients, while IL-2 and IL-4 did not [141]. In conclusion, SARS-CoV-2 shares the rise of IL-6 with cytokines release syndrome due to CAR-T infusion and has an inflammatory profile more similar to other related cytokine release syndrome than other systemic inflammation.
Potential immunotherapeutic repurposing for COVID-19
Immunotherapy has the potential to treat COVID-19 by either eliminating the infected cells themselves or modulating the inflammatory responses that result in cytokines response syndrome. Treatment using SARS-CoV-2 specific T cells has not been documented in any investigations so far. T cell clones from recovering patients were used to prevent or treat SARS-CoV-2 infection in immunocompromised after bone marrow transplantation [142]. T cells with engineered tumor-recognizing T cell receptors can steer the immune system to target antigen (TCR). TCR-rngineered T cells have shown promise in acute myeloid leukemia, melanoma esophageal cancer, synovial sarcoma, and Wilms tumor [143]. Most clinical trials tolerated ex vivo expanded TCR-modified cell infusion. TCR-engineered T cells against SARS-CoV-2 antigen have been studied and may have similar downsides as CAR-T cells.
NK cells, part of the innate immune system are crucial to immunological surveillance and can be employed as adoptive immunotherapy [144]. Engineered CAR-NK cells may treat cancer, allogeneic NK cells from cord blood can be safely delivered without comprehensive human leukocyte antigen matching or CAR product customization [145]. Few in-vitro studies show, CAR-NK cells target anti-SARS-CoV-2 infected cells and show high efficacy in to abolishing them [146-148]. Mesenchymal stromal cells (MSCs) are a heterogeneous population of stromal cells that migrate to specific tissue in the setting of remodelling and regeneration. In some haematological malignancies, they immunomodulate the TME and increase local tumor aggressiveness [149,150]. Engineered MSCs to hyperexpress IFN-γ and injected tumor tissue [151,152]. MSC cell treatment is intriguing for targeting many inflammatory patterns. Many COVID-19 clinical trials use MSC from autologous or allogenic adipose tissue, dental pulp, bone marrow, or cord blood Wharton’s jelly.
Neutralizing antibodies can target SARS-CoV-2 spike protein to prevent virus-cell interaction and restrict SARS-CoV-2 from circulation. Several stabilized SARS-CoV-2 spike protein vaccines have shown potential efficacy in eliciting a protein-specific antibody response with an acceptable rate of anomalies [153,154]. In a recent randomized experiment, volunteered pooled plasma from recovered SARS-CoV-2 patients are proved to be helpful if administered within 72 hours of symptom start [155,156]. This approach is restricted by donor availability, allergic reaction safety, and blood-derived products. Conversely, B cell-produced non-neutralizing antibodies may accelerate SARS-CoV-2 infection through antibody-dependent augmentation and worsening organ damage.
Inflammation is the hallmark of COVID-19, a disease brought on by a virus. Global vaccination against SARS-CoV-2 is the best hope for the pandemic. Vaccines for both high- and low-risk populations are becoming increasingly accessible thanks to their recent approval in several nations. Treatment of individuals with COVID-19 may benefit from immunotherapeutic techniques that control the immune system, since these may help to reduce viral replication and stop the cascade of inflammatory processes triggered by SARS-CoV-2. Few immune-modulating effective against COVID-19 so far. The FDA-approved REGN-COV2 mAb combination is for newly infected COVID-19 and ARDS patients with limited symptoms and a high risk of deteriorating. Hospitalized patients are recommended for baricitinib, an anti-JAK medication, along with remdesivir. Tocilizumab and dexamethasone are now recommended for fast-progressing respiratory illness. Besides ruxolitinib and anakinra, other anti-IL6 drugs like sarilumab have shown promising outcomes, many clinical trials are testing cell therapy and inhibition of such defibrotide and eculizumab. If patients are classified by cytokines implicated in the COVID-19 inflammatory process, we may be able to learn more about the efficacy of the above drugs in specific subgroups.
Future perspective and conclusion
The ever increasing burden of the diseases in the present scenario warranted the need for the development of the quick and effective therapies to control the human diseases. The role of the immune response in the diverse diseases have been well established which makes the targeting of the immune system as an alternative tool for targeting the disease. Drug repurposing using immunotherapy could provide a quick and effective therapy against the diseases. In the recent years, several drugs have been repurposed either alone or in combination and have been found to be effective. Rigorous efforts in the drug repurposing may lead to the emergence of successful therapies against different diseases. Despite the development of the artificial intelligence and machine learning based drug designing, there are still road blocks which hinders and delays the human use. For this reason, drug repurposing provides the advantage over the AI designed drugs. In the near future, drug repurposing could develop into a cost effective and quick method for the diverse diseases. Further research is required to ensure the safety and efficacy of the patients with repurposed drugs. Overall, the future of the repurposing of the immunotherapy is promising.
Disclosure of conflict of interest
None.
References
- 1.Pillaiyar T, Meenakshisundaram S, Manickam M, Sankaranarayanan M. A medicinal chemistry perspective of drug repositioning: recent advances and challenges in drug discovery. Eur J Med Chem. 2020;195:112275. doi: 10.1016/j.ejmech.2020.112275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, Doig A, Guilliams T, Latimer J, McNamee C, Norris A, Sanseau P, Cavalla D, Pirmohamed M. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 3.Cong Y, Shintani M, Imanari F, Osada N, Endo T. A new approach to drug repurposing with two-stage prediction, machine learning, and unsupervised clustering of gene expression. OMICS. 2022;26:339–347. doi: 10.1089/omi.2022.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 5.Rupp D, Nicholson G, Canty D, Wang J, Rhéaume C, Le L, Steward LE, Washburn M, Jacky BP, Broide RS, Philipp-Dormston WG, Brin MF, Brideau-Andersen A. OnabotulinumtoxinA displays greater biological activity compared to incobotulinumtoxina, demonstrating non-interchangeability in both in vitro and in vivo assays. Toxins (Basel) 2020;12:393. doi: 10.3390/toxins12060393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cha Y, Erez T, Reynolds IJ, Kumar D, Ross J, Koytiger G, Kusko R, Zeskind B, Risso S, Kagan E, Papapetropoulos S, Grossman I, Laifenfeld D. Drug repurposing from the perspective of pharmaceutical companies. Br J Pharmacol. 2018;175:168–180. doi: 10.1111/bph.13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parvathaneni V, Kulkarni NS, Muth A, Gupta V. Drug repurposing: a promising tool to accelerate the drug discovery process. Drug Discov Today. 2019;24:2076–2085. doi: 10.1016/j.drudis.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y, Wang F, Tang J, Nussinov R, Cheng F. Artificial intelligence in COVID-19 drug repurposing. Lancet Digit Health. 2020;2:e667–e676. doi: 10.1016/S2589-7500(20)30192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 10.Abbott M, Ustoyev Y. Cancer and the immune system: the history and background of immunotherapy. Semin Oncol Nurs. 2019;35:150923. doi: 10.1016/j.soncn.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Singh A, Anang V, Kumari K, Kottarath SK, Verma C. Role of lymphocytes, macrophages and immune receptors in suppression of tumor immunity. Prog Mol Biol Transl Sci. 2023;194:269–310. doi: 10.1016/bs.pmbts.2022.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Anang V, Singh A, Kottarath SK, Verma C. Receptors of immune cells mediates recognition for tumors. Prog Mol Biol Transl Sci. 2023;194:219–267. doi: 10.1016/bs.pmbts.2022.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. 2021;22:6995. doi: 10.3390/ijms22136995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Geng X, Hou J, Wu G. New insights into M1/M2 macrophages: key modulators in cancer progression. Cancer Cell Int. 2021;21:389. doi: 10.1186/s12935-021-02089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swaminathan J, Gopalakrishnan V. In: Immunotherapy in Translational Cancer Research. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2018. Repurposing of Drugs for Immunotherapy; pp. 143–160. [Google Scholar]
- 16.Zhang P. On arsenic trioxide in the clinical treatment of acute promyelocytic leukemia. Leuk Res Rep. 2017;7:29–32. doi: 10.1016/j.lrr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu L, Jin W, Zhang J, Zhu L, Lu J, Zhen Y, Zhang L, Ouyang L, Liu B, Yu H. Repurposing non-oncology small-molecule drugs to improve cancer therapy: current situation and future directions. Acta Pharm Sin B. 2022;12:532–557. doi: 10.1016/j.apsb.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moehler TM, Hillengass J, Glasmacher A, Goldschmidt H. Thalidomide in multiple myeloma. Curr Pharm Biotechnol. 2006;7:431–440. doi: 10.2174/138920106779116919. [DOI] [PubMed] [Google Scholar]
- 19.Latif T, Chauhan N, Khan R, Moran A, Usmani SZ. Thalidomide and its analogues in the treatment of multiple myeloma. Exp Hematol Oncol. 2012;1:27. doi: 10.1186/2162-3619-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Levy CL, Li YF, El-Gamil M, Schwarz SL, Laurencot C, Rosenberg SA. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.To KKW, Cho WCS. In: Drug Repurposing in Cancer Therapy. Elsevier; 2020. Drugs repurposed to potentiate immunotherapy for cancer treatment; pp. 311–334. [Google Scholar]
- 24.Doshi S, Chepuri SP. A computational approach to drug repurposing using graph neural networks. Comput Biol Med. 2022;150:105992. doi: 10.1016/j.compbiomed.2022.105992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keenan AB, Jenkins SL, Jagodnik KM, Koplev S, He E, Torre D, Wang Z, Dohlman AB, Silverstein MC, Lachmann A, Kuleshov MV, Ma’ayan A, Stathias V, Terryn R, Cooper D, Forlin M, Koleti A, Vidovic D, Chung C, Schürer SC, Vasiliauskas J, Pilarczyk M, Shamsaei B, Fazel M, Ren Y, Niu W, Clark NA, White S, Mahi N, Zhang L, Kouril M, Reichard JF, Sivaganesan S, Medvedovic M, Meller J, Koch RJ, Birtwistle MR, Iyengar R, Sobie EA, Azeloglu EU, Kaye J, Osterloh J, Haston K, Kalra J, Finkbiener S, Li J, Milani P, Adam M, Escalante-Chong R, Sachs K, Lenail A, Ramamoorthy D, Fraenkel E, Daigle G, Hussain U, Coye A, Rothstein J, Sareen D, Ornelas L, Banuelos M, Mandefro B, Ho R, Svendsen CN, Lim RG, Stocksdale J, Casale MS, Thompson TG, Wu J, Thompson LM, Dardov V, Venkatraman V, Matlock A, Van Eyk JE, Jaffe JD, Papanastasiou M, Subramanian A, Golub TR, Erickson SD, Fallahi-Sichani M, Hafner M, Gray NS, Lin JR, Mills CE, Muhlich JL, Niepel M, Shamu CE, Williams EH, Wrobel D, Sorger PK, Heiser LM, Gray JW, Korkola JE, Mills GB, LaBarge M, Feiler HS, Dane MA, Bucher E, Nederlof M, Sudar D, Gross S, Kilburn DF, Smith R, Devlin K, Margolis R, Derr L, Lee A, Pillai A. The library of integrated network-based cellular signatures NIH program: system-level cataloging of human cells response to perturbations. Cell Syst. 2018;6:13–24. doi: 10.1016/j.cels.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown AC, Fraser TR. On the connection between chemical constitution and physiological action; with special reference to the physiological action of the salts of the ammonium bases derived from strychnia, brucia, thebaia, codeia, morphia, and nicotia. J Anat Physiol. 1868;2:224–42. [PMC free article] [PubMed] [Google Scholar]
- 27.Moura AS, Cordeiro MNDS. Got to write a classic: classical and perturbation-based QSAR methods, machine learning, and the monitoring of nanoparticle ecotoxicity. 2020. pp. 195–213. [Google Scholar]
- 28.Nagaraj AB, Wang QQ, Joseph P, Zheng C, Chen Y, Kovalenko O, Singh S, Armstrong A, Resnick K, Zanotti K, Waggoner S, Xu R, DiFeo A. Using a novel computational drug-repositioning approach (DrugPredict) to rapidly identify potent drug candidates for cancer treatment. Oncogene. 2018;37:403–414. doi: 10.1038/onc.2017.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sohraby F, Bagheri M, Aryapour H. Performing an in silico repurposing of existing drugs by combining virtual screening and molecular dynamics simulation. Methods Mol Biol. 2019;1903:23–43. doi: 10.1007/978-1-4939-8955-3_2. [DOI] [PubMed] [Google Scholar]
- 30.Koleti A, Terryn R, Stathias V, Chung C, Cooper DJ, Turner JP, Vidovic D, Forlin M, Kelley TT, D’Urso A, Allen BK, Torre D, Jagodnik KM, Wang L, Jenkins SL, Mader C, Niu W, Fazel M, Mahi N, Pilarczyk M, Clark N, Shamsaei B, Meller J, Vasiliauskas J, Reichard J, Medvedovic M, Ma’ayan A, Pillai A, Schürer SC. Data portal for the library of integrated network-based cellular signatures (LINCS) program: integrated access to diverse large-scale cellular perturbation response data. Nucleic Acids Res. 2018;46:D558–D566. doi: 10.1093/nar/gkx1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Fan S, Vera J, Lai X. A network medicine approach for identifying diagnostic and prognostic biomarkers and exploring drug repurposing in human cancer. Comput Struct Biotechnol J. 2022;21:34–45. doi: 10.1016/j.csbj.2022.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider L, Kehl T, Thedinga K, Grammes NL, Backes C, Mohr C, Schubert B, Lenhof K, Gerstner N, Hartkopf AD, Wallwiener M, Kohlbacher O, Keller A, Meese E, Graf N, Lenhof HP. ClinOmicsTrailbc: a visual analytics tool for breast cancer treatment stratification. Bioinformatics. 2019;35:5171–5181. doi: 10.1093/bioinformatics/btz302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown AS, Patel CJ. A standard database for drug repositioning. Sci Data. 2017;4:170029. doi: 10.1038/sdata.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shameer K, Glicksberg BS, Hodos R, Johnson KW, Badgeley MA, Readhead B, Tomlinson MS, O’Connor T, Miotto R, Kidd BA, Chen R, Ma’ayan A, Dudley JT. Systematic analyses of drugs and disease indications in RepurposeDB reveal pharmacological, biological and epidemiological factors influencing drug repositioning. Brief Bioinform. 2018;19:656–678. doi: 10.1093/bib/bbw136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernández-Lemus E, Martínez-García M. Pathway-based drug-repurposing schemes in cancer: the role of translational bioinformatics. Front Oncol. 2021;10:605680. doi: 10.3389/fonc.2020.605680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30:660–669. doi: 10.1038/s41422-020-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franzin R, Netti GS, Spadaccino F, Porta C, Gesualdo L, Stallone G, Castellano G, Ranieri E. The use of immune checkpoint inhibitors in oncology and the occurrence of AKI: where do we stand? Front Immunol. 2020;11:574271. doi: 10.3389/fimmu.2020.574271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cha JH, Yang WH, Xia W, Wei Y, Chan LC, Lim SO, Li CW, Kim T, Chang SS, Lee HH, Hsu JL, Wang HL, Kuo CW, Chang WC, Hadad S, Purdie CA, McCoy AM, Cai S, Tu Y, Litton JK, Mittendorf EA, Moulder SL, Symmans WF, Thompson AM, Piwnica-Worms H, Chen CH, Khoo KH, Hung MC. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Mol Cell. 2018;71:606–620. e7. doi: 10.1016/j.molcel.2018.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, Rodríguez-Ruiz ME, Ponz-Sarvise M, Castañón E, Melero I. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol. 2022;19:237–253. doi: 10.1038/s41571-021-00588-9. [DOI] [PubMed] [Google Scholar]
- 41.Mansurov A, Hosseinchi P, Chang K, Lauterbach AL, Gray LT, Alpar AT, Budina E, Slezak AJ, Kang S, Cao S, Solanki A, Gomes S, Williford JM, Swartz MA, Mendoza JL, Ishihara J, Hubbell JA. Masking the immunotoxicity of interleukin-12 by fusing it with a domain of its receptor via a tumour-protease-cleavable linker. Nat Biomed Eng. 2022;6:819–829. doi: 10.1038/s41551-022-00888-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, Chhabra S, Huang W, Liu H, Aref AR, Ivanova E, Paweletz CP, Bowden M, Zhou CW, Herter-Sprie GS, Sorrentino JA, Bisi JE, Lizotte PH, Merlino AA, Quinn MM, Bufe LE, Yang A, Zhang Y, Zhang H, Gao P, Chen T, Cavanaugh ME, Rode AJ, Haines E, Roberts PJ, Strum JC, Richards WG, Lorch JH, Parangi S, Gunda V, Boland GM, Bueno R, Palakurthi S, Freeman GJ, Ritz J, Haining WN, Sharpless NE, Arthanari H, Shapiro GI, Barbie DA, Gray NS, Wong KK. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 2018;8:216–233. doi: 10.1158/2159-8290.CD-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen EW, Brzostek J, Gascoigne NRJ, Rybakin V. Development of a screening strategy for new modulators of T cell receptor signaling and T cell activation. Sci Rep. 2018;8:10046. doi: 10.1038/s41598-018-28106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marro BS, Zak J, Zavareh RB, Teijaro JR, Lairson LL, Oldstone MBA. Discovery of small molecules for the reversal of T cell exhaustion. Cell Rep. 2019;29:3293–3302. e3. doi: 10.1016/j.celrep.2019.10.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bejarano L, Jordāo MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 2021;11:933–959. doi: 10.1158/2159-8290.CD-20-1808. [DOI] [PubMed] [Google Scholar]
- 46.Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020;5:166. doi: 10.1038/s41392-020-00280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sareddy GR, Kesanakurti D, Kirti PB, Babu PP. Nonsteroidal anti-inflammatory drugs diclofenac and celecoxib attenuates Wnt/β-catenin/Tcf signaling pathway in human glioblastoma cells. Neurochem Res. 2013;38:2313–2322. doi: 10.1007/s11064-013-1142-9. [DOI] [PubMed] [Google Scholar]
- 48.Muhammad N, Lee HM, Kim J. Oncology therapeutics targeting the metabolism of amino acids. Cells. 2020;9:1904. doi: 10.3390/cells9081904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang K, Wu YH, Song Y, Yu B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J Hematol Oncol. 2021;14:68. doi: 10.1186/s13045-021-01080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soliman H, Mediavilla-Varela M, Antonia S. Indoleamine 2,3-dioxygenase: is it an immune suppressor? Cancer J. 2010;16:354–9. doi: 10.1097/PPO.0b013e3181eb3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leone RD, Zhao L, Englert JM, Sun IM, Oh MH, Sun IH, Arwood ML, Bettencourt IA, Patel CH, Wen J, Tam A, Blosser RL, Prchalova E, Alt J, Rais R, Slusher BS, Powell JD. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366:1013–1021. doi: 10.1126/science.aav2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Byun JK, Park M, Lee S, Yun JW, Lee J, Kim JS, Cho SJ, Jeon HJ, Lee IK, Choi YK, Park KG. Inhibition of glutamine utilization synergizes with immune checkpoint inhibitor to promote antitumor immunity. Mol Cell. 2020;80:592–606. e8. doi: 10.1016/j.molcel.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 53.Wu R, Forget MA, Chacon J, Bernatchez C, Haymaker C, Chen JQ, Hwu P, Radvanyi LG. Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma. Cancer J. 2012;18:160–175. doi: 10.1097/PPO.0b013e31824d4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mills JK, Henderson MA, Giuffrida L, Petrone P, Westwood JA, Darcy PK, Neeson PJ, Kershaw MH, Gyorki DE. Generating CAR T cells from tumor-infiltrating lymphocytes. Ther Adv Vaccines Immunother. 2021;9:251513552110171. doi: 10.1177/25151355211017119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bell M, Gottschalk S. Engineered cytokine signaling to improve CAR T cell effector function. Front Immunol. 2021;12:684642. doi: 10.3389/fimmu.2021.684642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hemminki O, Dos Santos JM, Hemminki A. Oncolytic viruses for cancer immunotherapy. J Hematol Oncol. 2020;13:84. doi: 10.1186/s13045-020-00922-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vijayakumar G, Palese P, Goff PH. Oncolytic Newcastle disease virus expressing a checkpoint inhibitor as a radioenhancing agent for murine melanoma. EBioMedicine. 2019;49:96–105. doi: 10.1016/j.ebiom.2019.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shekarian T, Sivado E, Jallas AC, Depil S, Kielbassa J, Janoueix-Lerosey I, Hutter G, Goutagny N, Bergeron C, Viari A, Valsesia-Wittmann S, Caux C, Marabelle A. Repurposing rotavirus vaccines for intratumoral immunotherapy can overcome resistance to immune checkpoint blockade. Sci Transl Med. 2019;11:eaat5025. doi: 10.1126/scitranslmed.aat5025. [DOI] [PubMed] [Google Scholar]
- 59.Oh CM, Chon HJ, Kim C. Combination immunotherapy using oncolytic virus for the treatment of advanced solid tumors. Int J Mol Sci. 2020;21:7743. doi: 10.3390/ijms21207743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spiesschaert B, Angerer K, Park J, Wollmann G. Combining oncolytic viruses and small molecule therapeutics: mutual benefits. Cancers (Basel) 2021;13:3386. doi: 10.3390/cancers13143386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang B, Cheng P. Improving antitumor efficacy via combinatorial regimens of oncolytic virotherapy. Mol Cancer. 2020;19:158. doi: 10.1186/s12943-020-01275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kadiyala P, Li D, Nuñez FM, Altshuler D, Doherty R, Kuai R, Yu M, Kamran N, Edwards M, Moon JJ, Lowenstein PR, Castro MG, Schwendeman A. High-density lipoprotein-mimicking nanodiscs for chemo-immunotherapy against glioblastoma multiforme. ACS Nano. 2019;13:1365–1384. doi: 10.1021/acsnano.8b06842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feng B, Zhou F, Hou B, Wang D, Wang T, Fu Y, Ma Y, Yu H, Li Y. Binary cooperative prodrug nanoparticles improve immunotherapy by synergistically modulating immune tumor microenvironment. Adv Mater. 2018;30:1803001. doi: 10.1002/adma.201803001. [DOI] [PubMed] [Google Scholar]
- 64.Yeu Y, Yoon Y, Park S. Protein localization vector propagation: a method for improving the accuracy of drug repositioning. Mol Biosyst. 2015;11:2096–2102. doi: 10.1039/c5mb00306g. [DOI] [PubMed] [Google Scholar]
- 65.Kumar V, Gupta S, Rosenzweig R, Bansal SS. In: Immune Cells, Inflammation, and Cardiovascular Diseases. Boca Raton: CRC Press; 2022. Helper T-lymphocytes in cardiovascular diseases; pp. 25–46. [Google Scholar]
- 66.Kumar V, Rosenzweig R, Asalla S, Nehra S, Prabhu SD, Bansal SS. TNFR1 contributes to activation-induced cell death of pathological CD4+ T lymphocytes during ischemic heart failure. JACC Basic Transl Sci. 2022;7:1038–1049. doi: 10.1016/j.jacbts.2022.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosenzweig R, Gupta S, Kumar V, Gumina RJ, Bansal SS. Estrogenic bias in T-lymphocyte biology: implications for cardiovascular disease. Pharmacol Res. 2021;170:105606. doi: 10.1016/j.phrs.2021.105606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar V, Prabhu SD, Bansal SS. CD4+ T-lymphocytes exhibit biphasic kinetics post-myocardial infarction. Front Cardiovasc Med. 2022;9:992653. doi: 10.3389/fcvm.2022.992653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosenzweig R, Kumar V, Gupta S, Bermeo-Blanco O, Stratton MS, Gumina RJ, Bansal SS. Estrogen receptor-β agonists modulate T-lymphocyte activation and ameliorate left ventricular remodeling during chronic heart failure. Circ Heart Fail. 2022;15:e008997. doi: 10.1161/CIRCHEARTFAILURE.121.008997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fernández-Gutiérrez B, Perrotti PP, Gisbert JP, Domènech E, Fernández-Nebro A, Cañete JD, Ferrándiz C, Tornero J, García-Sánchez V, Panés J, Fonseca E, Blanco F, Rodríguez-Moreno J, Carreira P, Julià A, Marsal S, Rodriguez-Rodriguez L IMID Consortium. Cardiovascular disease in immune-mediated inflammatory diseases: a cross-sectional analysis of 6 cohorts. Medicine (Baltimore) 2017;96:e7308. doi: 10.1097/MD.0000000000007308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pawar VA, Srivastava S, Tyagi A, Tayal R, Shukla SK, Kumar V. Efficacy of bioactive compounds in the regulation of metabolism and pathophysiology in cardiovascular diseases. Curr Cardiol Rep. 2023:1–12. doi: 10.1007/s11886-023-01917-3. [DOI] [PubMed] [Google Scholar]
- 72.Srivastava S, Pawar VA, Tyagi A, Sharma KP, Kumar V, Shukla SK. Immune modulatory effects of ketogenic diet in different disease conditions. Immuno. 2022;3:1–15. [Google Scholar]
- 73.Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1. Circ Res. 2016;118:145–156. doi: 10.1161/CIRCRESAHA.115.306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chistiakov DA, Melnichenko AA, Grechko AV, Myasoedova VA, Orekhov AN. Potential of anti-inflammatory agents for treatment of atherosclerosis. Exp Mol Pathol. 2018;104:114–124. doi: 10.1016/j.yexmp.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 75.Kao TW, Huang CC. Inflammatory burden and immunomodulative therapeutics of cardiovascular diseases. Int J Mol Sci. 2022;23:804. doi: 10.3390/ijms23020804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gelosa P, Castiglioni L, Camera M, Sironi L. Drug repurposing in cardiovascular diseases: opportunity or hopeless dream? Biochem Pharmacol. 2020;177:113894. doi: 10.1016/j.bcp.2020.113894. [DOI] [PubMed] [Google Scholar]
- 77.Van Linthout S, Volk HD. Immuno-cardio-oncology: killing two birds with one stone? Front Immunol. 2022;13:1018772. doi: 10.3389/fimmu.2022.1018772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuan M, Li QG. Lung cancer and risk of cardiovascular disease: a meta-analysis of cohort studies. J Cardiothorac Vasc Anesth. 2018;32:e25–e27. doi: 10.1053/j.jvca.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 79.Armenian SH, Xu L, Ky B, Sun C, Farol LT, Pal SK, Douglas PS, Bhatia S, Chao C. Cardiovascular disease among survivors of adult-onset cancer: a community-based retrospective cohort study. J. Clin. Oncol. 2016;34:1122–1130. doi: 10.1200/JCO.2015.64.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ljung L, Rantapää-Dahlqvist S, Jacobsson LT, Askling J. Response to biological treatment and subsequent risk of coronary events in rheumatoid arthritis. Ann Rheum Dis. 2016;75:2087–2094. doi: 10.1136/annrheumdis-2015-208995. [DOI] [PubMed] [Google Scholar]
- 81.Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guérin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L, Dumeau E, Kotti S, Bruneval P, Charo IF, Binder CJ, Danchin N, Tedgui A, Tedder TF, Silvestre JS, Mallat Z. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013;19:1273–1280. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pierpont TM, Limper CB, Richards KL. Past, present, and future of rituximab-the world’s first oncology monoclonal antibody therapy. Front Oncol. 2018;8:163. doi: 10.3389/fonc.2018.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schroeder BA, Jess J, Sankaran H, Shah NN. Clinical trials for chimeric antigen receptor T-cell therapy: lessons learned and future directions. Curr Opin Hematol. 2022;29:225–232. doi: 10.1097/MOH.0000000000000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carroll MB, Haller C, Smith C. Short-term application of tocilizumab during myocardial infarction (STAT-MI) Rheumatol Int. 2018;38:59–66. doi: 10.1007/s00296-017-3842-y. [DOI] [PubMed] [Google Scholar]
- 85.Cloeckaert A, Kuchler K. Grand challenges in infectious diseases: are we prepared for worst-case scenarios? Front Microbiol. 2020;11:613383. doi: 10.3389/fmicb.2020.613383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ram B, Thakur R. Epidemiology and economic burden of continuing challenge of infectious diseases in India: analysis of socio-demographic differentials. Front Public Health. 2022;10:901276. doi: 10.3389/fpubh.2022.901276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mahalmani VM, Mahendru D, Semwal A, Kaur S, Kaur H, Sarma P, Prakash A, Medhi B. COVID-19 pandemic: a review based on current evidence. Indian J Pharmacol. 2020;52:117–129. doi: 10.4103/ijp.IJP_310_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Melero I, Gato M, Shekarian T, Aznar A, Valsesia-Wittmann S, Caux C, Etxeberrria I, Teijeira A, Marabelle A. Repurposing infectious disease vaccines for intratumoral immunotherapy. J Immunother Cancer. 2020;8:e000443. doi: 10.1136/jitc-2019-000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vandeborne L, Pantziarka P, Van Nuffel AMT, Bouche G. Repurposing infectious diseases vaccines against cancer. Front Oncol. 2021;11:688755. doi: 10.3389/fonc.2021.688755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tiberi S, Muñoz-Torrico M, Duarte R, Dalcolmo M, D’Ambrosio L, Migliori GB. New drugs and perspectives for new anti-tuberculosis regimens. Pulmonology. 2018;24:86–98. doi: 10.1016/j.rppnen.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 91.Adeniji AA, Knoll KE, Loots DT. Potential anti-TB investigational compounds and drugs with repurposing potential in TB therapy: a conspectus. Appl Microbiol Biotechnol. 2020;104:5633–5662. doi: 10.1007/s00253-020-10606-y. [DOI] [PubMed] [Google Scholar]
- 92.Maitra A, Bates S, Kolvekar T, Devarajan PV, Guzman JD, Bhakta S. Repurposing-a ray of hope in tackling extensively drug resistance in tuberculosis. Int J Infect Dis. 2015;32:50–5. doi: 10.1016/j.ijid.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 93.Stephanie F, Saragih M, Tambunan USF. Recent progress and challenges for drug-resistant tuberculosis treatment. Pharmaceutics. 2021;13:592. doi: 10.3390/pharmaceutics13050592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gillespie SH. The role of moxifloxacin in tuberculosis therapy. Eur Respir Rev. 2016;25:19–28. doi: 10.1183/16000617.0085-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cholo MC, Steel HC, Fourie PB, Germishuizen WA, Anderson R. Clofazimine: current status and future prospects. J Antimicrob Chemother. 2012;67:290–8. doi: 10.1093/jac/dkr444. [DOI] [PubMed] [Google Scholar]
- 96.Xu J, Wang B, Fu L, Zhu H, Guo S, Huang H, Yin D, Zhang Y, Lu Y. In vitro and in vivo activities of the riminophenazine TBI-166 against mycobacterium tuberculosis. Antimicrob Agents Chemother. 2019;63:e02155-18. doi: 10.1128/AAC.02155-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hugonnet JE, Tremblay LW, Boshoff HI, Barry CE 3rd, Blanchard JS. Meropenem-clavulanate is effective against extensively drug-resistant mycobacterium tuberculosis. Science. 2009;323:1215–8. doi: 10.1126/science.1167498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee RE, Hurdle JG, Liu J, Bruhn DF, Matt T, Scherman MS, Vaddady PK, Zheng Z, Qi J, Akbergenov R, Das S, Madhura DB, Rathi C, Trivedi A, Villellas C, Lee RB, Rakesh, Waidyarachchi SL, Sun D, McNeil MR, Ainsa JA, Boshoff HI, Gonzalez-Juarrero M, Meibohm B, Böttger EC, Lenaerts AJ. Spectinamides: a new class of semisynthetic antituberculosis agents that overcome native drug efflux. Nat Med. 2014;20:152–158. doi: 10.1038/nm.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roubert C, Fontaine E, Upton AM. “Upcycling” known molecules and targets for drug-resistant TB. Front Cell Infect Microbiol. 2022;12:1029044. doi: 10.3389/fcimb.2022.1029044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramamurthy D, Nundalall T, Cingo S, Mungra N, Karaan M, Naran K, Barth S. Recent advances in immunotherapies against infectious diseases. Immunother Adv. 2020;1:ltaa007. doi: 10.1093/immadv/ltaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Varikuti S, Singh B, Volpedo G, Ahirwar DK, Jha BK, Saljoughian N, Viana AG, Verma C, Hamza O, Halsey G, Holcomb EA, Maryala RJ, Oghumu S, Ganju RK, Satoskar AR. Ibrutinib treatment inhibits breast cancer progression and metastasis by inducing conversion of myeloid-derived suppressor cells to dendritic cells. Br J Cancer. 2020;122:1005–1013. doi: 10.1038/s41416-020-0743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Treon SP, Castillo JJ, Skarbnik AP, Soumerai JD, Ghobrial IM, Guerrera ML, Meid K, Yang G. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 2020;135:1912–1915. doi: 10.1182/blood.2020006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zheng KI, Feng G, Liu WY, Targher G, Byrne CD, Zheng MH. Extrapulmonary complications of COVID-19: a multisystem disease? J Med Virol. 2021;93:323–335. doi: 10.1002/jmv.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rosen HR, O’Connell C, Nadim MK, DeClerck B, Sheibani S, DePasquale E, Sanossian N, Blodget E, Angell T. Extrapulmonary manifestations of severe acute respiratory syndrome coronavirus-2 infection. J Med Virol. 2021;93:2645–2653. doi: 10.1002/jmv.26595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, Marron TU, Xie H, Patel M, Tuballes K, Van Oekelen O, Rahman A, Kovatch P, Aberg JA, Schadt E, Jagannath S, Mazumdar M, Charney AW, Firpo-Betancourt A, Mendu DR, Jhang J, Reich D, Sigel K, Cordon-Cardo C, Feldmann M, Parekh S, Merad M, Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zumla A, Rao M, Wallis RS, Kaufmann SH, Rustomjee R, Mwaba P, Vilaplana C, Yeboah-Manu D, Chakaya J, Ippolito G, Azhar E, Hoelscher M, Maeurer M Host-Directed Therapies Network consortium. Host-directed therapies for infectious diseases: current status, recent progress, and future prospects. Lancet Infect Dis. 2016;16:e47–63. doi: 10.1016/S1473-3099(16)00078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kroschinsky F, Stölzel F, von Bonin S, Beutel G, Kochanek M, Kiehl M, Schellongowski P Intensive Care in Hematological and Oncological Patients (iCHOP) Collaborative Group. New drugs, new toxicities: severe side effects of modern targeted and immunotherapy of cancer and their management. Crit Care. 2017;21:89. doi: 10.1186/s13054-017-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thakar MS, Kearl TJ, Malarkannan S. Controlling cytokine release syndrome to harness the full potential of CAR-based cellular therapy. Front Oncol. 2020;9:1529. doi: 10.3389/fonc.2019.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Quan C, Li C, Ma H, Li Y, Zhang H. Immunopathogenesis of coronavirus-induced acute respiratory distress syndrome (ARDS): potential infection-associated hemophagocytic lymphohistiocytosis. Clin Microbiol Rev. 2020;34:e00074-20. doi: 10.1128/CMR.00074-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Abate SM, Ahmed Ali S, Mantfardo B, Basu B. Rate of Intensive Care Unit admission and outcomes among patients with coronavirus: a systematic review and meta-analysis. PLoS One. 2020;15:e0235653. doi: 10.1371/journal.pone.0235653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 115.Koyama N, Iwase O, Nakashima E, Kishida K, Kondo T, Watanabe Y, Takahashi H, Umebayashi Y, Ogawa Y, Miura H. High incidence and early onset of nivolumab-induced pneumonitis: four case reports and literature review. BMC Pulm Med. 2018;18:23. doi: 10.1186/s12890-018-0592-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Y, Zugazagoitia J, Ahmed FS, Henick BS, Gettinger SN, Herbst RS, Schalper KA, Rimm DL. Immune cell PD-L1 colocalizes with macrophages and is associated with outcome in PD-1 pathway blockade therapy. Clin Cancer Res. 2020;26:970–977. doi: 10.1158/1078-0432.CCR-19-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shirato K, Kizaki T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via toll-like receptor 4 signaling in murine and human macrophages. Heliyon. 2021;7:e06187. doi: 10.1016/j.heliyon.2021.e06187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zahm CD, Colluru VT, McIlwain SJ, Ong IM, McNeel DG. TLR stimulation during T-cell activation lowers PD-1 expression on CD8+ T cells. Cancer Immunol Res. 2018;6:1364–1374. doi: 10.1158/2326-6066.CIR-18-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ghebrehiwet B, Peerschke EI. Complement and coagulation: key triggers of COVID-19-induced multiorgan pathology. J Clin Invest. 2020;130:5674–5676. doi: 10.1172/JCI142780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lazzaroni MG, Piantoni S, Masneri S, Garrafa E, Martini G, Tincani A, Andreoli L, Franceschini F. Coagulation dysfunction in COVID-19: the interplay between inflammation, viral infection and the coagulation system. Blood Rev. 2021;46:100745. doi: 10.1016/j.blre.2020.100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Karmouty-Quintana H, Thandavarayan RA, Keller SP, Sahay S, Pandit LM, Akkanti B. Emerging mechanisms of pulmonary vasoconstriction in SARS-CoV-2-induced acute respiratory distress syndrome (ARDS) and potential therapeutic targets. Int J Mol Sci. 2020;21:8081. doi: 10.3390/ijms21218081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Radermecker C, Detrembleur N, Guiot J, Cavalier E, Henket M, d’Emal C, Vanwinge C, Cataldo D, Oury C, Delvenne P, Marichal T. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J Exp Med. 2020;217:e20201012. doi: 10.1084/jem.20201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, Zheng M, Sundaram B, Banoth B, Malireddi RKS, Schreiner P, Neale G, Vogel P, Webby R, Jonsson CB, Kanneganti TD. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184:149–168. e17. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.AlSamman M, Caggiula A, Ganguli S, Misak M, Pourmand A. Non-respiratory presentations of COVID-19, a clinical review. Am J Emerg Med. 2020;38:2444–2454. doi: 10.1016/j.ajem.2020.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang S, Liu Y, Wang X, Yang L, Li H, Wang Y, Liu M, Zhao X, Xie Y, Yang Y, Zhang S, Fan Z, Dong J, Yuan Z, Ding Z, Zhang Y, Hu L. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13:120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Makowski L, Olson-Sidford W, W-Weisel J. Biological and clinical consequences of integrin binding via a rogue RGD motif in the SARS CoV-2 spike protein. Viruses. 2021;13:146. doi: 10.3390/v13020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Flower TG, Buffalo CZ, Hooy RM, Allaire M, Ren X, Hurley JH. Structure of SARS-CoV-2 ORF8, a rapidly evolving immune evasion protein. Proc Natl Acad Sci U S A. 2021;118:e2021785118. doi: 10.1073/pnas.2021785118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bastard P, Zhang Q, Zhang SY, Jouanguy E, Casanova JL. Type I interferons and SARS-CoV-2: from cells to organisms. Curr Opin Immunol. 2022;74:172–182. doi: 10.1016/j.coi.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lei X, Dong X, Ma R, Wang W, Xiao X, Tian Z, Wang C, Wang Y, Li L, Ren L, Guo F, Zhao Z, Zhou Z, Xiang Z, Wang J. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat Commun. 2020;11:3810. doi: 10.1038/s41467-020-17665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Carvalho T. Extrapulmonary SARS-CoV-2 manifestations. Nat Med. 2020;26:1806. doi: 10.1038/s41591-020-01162-z. [DOI] [PubMed] [Google Scholar]
- 133.Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, Calfee CS, Hirayama AV, Mastroiani F, Turtle CJ, Harhay MO, Legrand M, Deutschman CS. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Choy EH, De Benedetti F, Takeuchi T, Hashizume M, John MR, Kishimoto T. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020;16:335–345. doi: 10.1038/s41584-020-0419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mitsuyama K, Toyonaga A, Sasaki E, Ishida O, Ikeda H, Tsuruta O, Harada K, Tateishi H, Nishiyama T, Tanikawa K. Soluble interleukin-6 receptors in inflammatory bowel disease: relation to circulating interleukin-6. Gut. 1995;36:45–9. doi: 10.1136/gut.36.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hunter CA, Jones SA. Corrigendum: IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2017;18:1271. doi: 10.1038/ni1117-1271b. [DOI] [PubMed] [Google Scholar]
- 137.Kasakovski D, Xu L, Li Y. T cell senescence and CAR-T cell exhaustion in hematological malignancies. J Hematol Oncol. 2018;11:91. doi: 10.1186/s13045-018-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chau AS, Weber AG, Maria NI, Narain S, Liu A, Hajizadeh N, Malhotra P, Bloom O, Marder G, Kaplan B. The longitudinal immune response to coronavirus disease 2019: chasing the cytokine storm. Arthritis Rheumatol. 2021;73:23–35. doi: 10.1002/art.41526. [DOI] [PubMed] [Google Scholar]
- 139.Klinger M, Brandl C, Zugmaier G, Hijazi Y, Bargou RC, Topp MS, Gökbuget N, Neumann S, Goebeler M, Viardot A, Stelljes M, Brüggemann M, Hoelzer D, Degenhard E, Nagorsen D, Baeuerle PA, Wolf A, Kufer P. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119:6226–33. doi: 10.1182/blood-2012-01-400515. [DOI] [PubMed] [Google Scholar]
- 140.Burt R, Warcel D, Fielding AK. Blinatumomab, a bispecific B-cell and T-cell engaging antibody, in the treatment of B-cell malignancies. Hum Vaccin Immunother. 2019;15:594–602. doi: 10.1080/21645515.2018.1540828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang P, Shi L, Xu J, Wang Y, Yang H. Elevated interleukin-6 and adverse outcomes in COVID-19 patients: a meta-analysis based on adjusted effect estimates. Immunogenetics. 2020;72:431–437. doi: 10.1007/s00251-020-01179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Keller MD, Harris KM, Jensen-Wachspress MA, Kankate VV, Lang H, Lazarski CA, Durkee-Shock J, Lee PH, Chaudhry K, Webber K, Datar A, Terpilowski M, Reynolds EK, Stevenson EM, Val S, Shancer Z, Zhang N, Ulrey R, Ekanem U, Stanojevic M, Geiger A, Liang H, Hoq F, Abraham AA, Hanley PJ, Cruz CR, Ferrer K, Dropulic L, Gangler K, Burbelo PD, Jones RB, Cohen JI, Bollard CM. SARS-CoV-2-specific T cells are rapidly expanded for therapeutic use and target conserved regions of the membrane protein. Blood. 2020;136:2905–2917. doi: 10.1182/blood.2020008488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao L, Cao YJ. Engineered T cell therapy for cancer in the clinic. Front Immunol. 2019;10:2250. doi: 10.3389/fimmu.2019.02250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, Nassif Kerbauy L, Overman B, Thall P, Kaplan M, Nandivada V, Kaur I, Nunez Cortes A, Cao K, Daher M, Hosing C, Cohen EN, Kebriaei P, Mehta R, Neelapu S, Nieto Y, Wang M, Wierda W, Keating M, Champlin R, Shpall EJ, Rezvani K. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382:545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ma M, Badeti S, Geng K, Liu D. Efficacy of targeting SARS-CoV-2 by CAR-NK cells. bioRxiv. 2020 [Google Scholar]
- 147.Soleimanian S, Yaghobi R. Harnessing memory NK cell to protect against COVID-19. Front Pharmacol. 2020;11:1309. doi: 10.3389/fphar.2020.01309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ma MT, Badeti S, Chen CH, Kim J, Choudhary A, Honnen B, Reichman C, Calianese D, Pinter A, Jiang Q, Shi L, Zhou R, Xu H, Li Q, Gause W, Liu D. CAR-NK cells effectively target SARS-CoV-2-spike-expressing cell lines in vitro. Front Immunol. 2021;12:652223. doi: 10.3389/fimmu.2021.652223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rambaldi B, Diral E, Donsante S, Di Marzo N, Mottadelli F, Cardinale L, Dander E, Isimbaldi G, Pioltelli P, Biondi A, Riminucci M, D’Amico G, Elli EM, Pievani A, Serafini M. Heterogeneity of the bone marrow niche in patients with myeloproliferative neoplasms: activina secretion by mesenchymal stromal cells correlates with the degree of marrow fibrosis. Ann Hematol. 2021;100:105–116. doi: 10.1007/s00277-020-04306-w. [DOI] [PubMed] [Google Scholar]
- 150.Pacini S, Montali M, Mazziotta F, Schifone CP, Macchia L, Carnicelli V, Panvini FM, Barachini S, Notarfranchi L, Previti GB, Buda G, Petrini M. Mesangiogenic progenitor cells are forced toward the angiogenic fate, in multiple myeloma. Oncotarget. 2019;10:6781–6790. doi: 10.18632/oncotarget.27285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang T, Lee YW, Rui YF, Cheng TY, Jiang XH, Li G. Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res Ther. 2013;4:70. doi: 10.1186/scrt221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sartoris S, Mazzocco M, Tinelli M, Martini M, Mosna F, Lisi V, Indraccolo S, Moserle L, Cestari T, Riviera AP, Bifari F, Tridente G, Pizzolo G, Krampera M. Efficacy assessment of interferon-alpha-engineered mesenchymal stromal cells in a mouse plasmacytoma model. Stem Cells Dev. 2011;20:709–19. doi: 10.1089/scd.2010.0095. [DOI] [PubMed] [Google Scholar]
- 153.Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott AB, Flach B, Lin BC, Doria-Rose NA, O’Dell S, Schmidt SD, Corbett KS, Swanson PA 2nd, Padilla M, Neuzil KM, Bennett H, Leav B, Makowski M, Albert J, Cross K, Edara VV, Floyd K, Suthar MS, Martinez DR, Baric R, Buchanan W, Luke CJ, Phadke VK, Rostad CA, Ledgerwood JE, Graham BS, Beigel JH mRNA-1273 Study Group. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, Esteban I, Caballero MT, Wood C, Berrueta M, Rondan A, Lescano G, Cruz P, Ritou Y, Fernández Viña V, Álvarez Paggi D, Esperante S, Ferreti A, Ofman G, Ciganda Á, Rodriguez R, Lantos J, Valentini R, Itcovici N, Hintze A, Oyarvide ML, Etchegaray C, Neira A, Name I, Alfonso J, López Castelo R, Caruso G, Rapelius S, Alvez F, Etchenique F, Dimase F, Alvarez D, Aranda SS, Sánchez Yanotti C, De Luca J, Jares Baglivo S, Laudanno S, Nowogrodzki F, Larrea R, Silveyra M, Leberzstein G, Debonis A, Molinos J, González M, Perez E, Kreplak N, Pastor Argüello S, Gibbons L, Althabe F, Bergel E, Polack FP Fundación INFANT-COVID-19 Group. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu STH, Lin HM, Baine I, Wajnberg A, Gumprecht JP, Rahman F, Rodriguez D, Tandon P, Bassily-Marcus A, Bander J, Sanky C, Dupper A, Zheng A, Nguyen FT, Amanat F, Stadlbauer D, Altman DR, Chen BK, Krammer F, Mendu DR, Firpo-Betancourt A, Levin MA, Bagiella E, Casadevall A, Cordon-Cardo C, Jhang JS, Arinsburg SA, Reich DL, Aberg JA, Bouvier NM. Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat Med. 2020;26:1708–1713. doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 157.Angela Y, Haferkamp S, Weishaupt C, Ugurel S, Becker JC, Oberndörfer F, Alar V, Satzger I, Gutzmer R. Combination of denosumab and immune checkpoint inhibition: experience in 29 patients with metastatic melanoma and bone metastases. Cancer Immunol Immunother. 2019;68:1187–1194. doi: 10.1007/s00262-019-02353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Scatena C, Roncella M, Di Paolo A, Aretini P, Menicagli M, Fanelli G, Marini C, Mazzanti CM, Ghilli M, Sotgia F, Lisanti MP, Naccarato AG. Doxycycline, an inhibitor of mitochondrial biogenesis, effectively reduces cancer stem cells (CSCs) in early breast cancer patients: a clinical pilot study. Front Oncol. 2018;8:452. doi: 10.3389/fonc.2018.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang L, Xu L, Zhang F, Vlashi E. Doxycycline inhibits the cancer stem cell phenotype and epithelial-to-mesenchymal transition in breast cancer. Cell Cycle. 2017;16:737–745. doi: 10.1080/15384101.2016.1241929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dong Z, Abbas MN, Kausar S, Yang J, Li L, Tan L, Cui H. Biological functions and molecular mechanisms of antibiotic tigecycline in the treatment of cancers. Int J Mol Sci. 2019;20:3577. doi: 10.3390/ijms20143577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nunes M, Henriques Abreu M, Bartosch C, Ricardo S. Recycling the purpose of old drugs to treat ovarian cancer. Int J Mol Sci. 2020;21:7768. doi: 10.3390/ijms21207768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Daelemans D, Lu R, De Clercq E, Engelman A. Characterization of a replication-competent, integrase-defective human immunodeficiency virus (HIV)/simian virus 40 chimera as a powerful tool for the discovery and validation of HIV integrase inhibitors. J Virol. 2007;81:4381–4385. doi: 10.1128/JVI.02637-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zeng LF, Wang Y, Kazemi R, Xu S, Xu ZL, Sanchez TW, Yang LM, Debnath B, Odde S, Xie H, Zheng YT, Ding J, Neamati N, Long YQ. Repositioning HIV-1 integrase inhibitors for cancer therapeutics: 1,6-naphthyridine-7-carboxamide as a promising scaffold with drug-like properties. J Med Chem. 2012;55:9492–9509. doi: 10.1021/jm300667v. [DOI] [PubMed] [Google Scholar]
- 164.Li W, Zheng M, Wu S, Gao S, Yang M, Li Z, Min Q, Sun W, Chen L, Xiang G, Li H. Benserazide, a dopadecarboxylase inhibitor, suppresses tumor growth by targeting hexokinase 2. J Exp Clin Cancer Res. 2017;36:58. doi: 10.1186/s13046-017-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhou Y, Huang Z, Su J, Li J, Zhao S, Wu L, Zhang J, He Y, Zhang G, Tao J, Zhou J, Chen X, Peng C. Benserazide is a novel inhibitor targeting PKM2 for melanoma treatment. Int J Cancer. 2020;147:139–151. doi: 10.1002/ijc.32756. [DOI] [PubMed] [Google Scholar]
- 166.Roy SK, Ma Y, Lam BQ, Shrivastava A, Srivastav S, Shankar S, Srivastava RK. Riluzole regulates pancreatic cancer cell metabolism by suppressing the Wnt-β-catenin pathway. Sci Rep. 2022;12:11062. doi: 10.1038/s41598-022-13472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chrzanowska A, Roszkowski P, Bielenica A, Olejarz W, Stępień K, Struga M. Anticancer and antimicrobial effects of novel ciprofloxacin fatty acids conjugates. Eur J Med Chem. 2020;185:111810. doi: 10.1016/j.ejmech.2019.111810. [DOI] [PubMed] [Google Scholar]
- 168.Nowak-Sliwinska P, Scapozza L, Ruiz i Altaba A. Drug repurposing in oncology: compounds, pathways, phenotypes and computational approaches for colorectal cancer. Biochim Biophys Acta Rev Cancer. 2019;1871:434–454. doi: 10.1016/j.bbcan.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Compter I, Eekers DBP, Hoeben A, Rouschop KMA, Reymen B, Ackermans L, Beckervordersantforth J, Bauer NJC, Anten MM, Wesseling P, Postma AA, De Ruysscher D, Lambin P. Chloroquine combined with concurrent radiotherapy and temozolomide for newly diagnosed glioblastoma: a phase IB trial. Autophagy. 2021;17:2604–2612. doi: 10.1080/15548627.2020.1816343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Oliva CR, Zhang W, Langford C, Suto MJ, Griguer CE. Repositioning chlorpromazine for treating chemoresistant glioma through the inhibition of cytochrome c oxidase bearing the COX4-1 regulatory subunit. Oncotarget. 2017;8:37568–37583. doi: 10.18632/oncotarget.17247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen VC, Hsieh YH, Lin TC, Lu ML, Liao YT, Yang YH, Hsu TC, Stewart R, Weng JC, Lee MJ, Chiu WC, Tzang BS. New use for old drugs: the protective effect of risperidone on colorectal cancer. Cancers (Basel) 2020;12:1560. doi: 10.3390/cancers12061560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li ZY, Liang C, Ding M, Weng XQ, Sheng Y, Wu J, Lu H, Cai X. Enzastaurin enhances ATRA-induced differentiation of acute myeloid leukemia cells. Am J Transl Res. 2020;12:7836–7854. [PMC free article] [PubMed] [Google Scholar]
- 173.Zhao Y, Cao J, Melamed A, Worley M, Gockley A, Jones D, Nia HT, Zhang Y, Stylianopoulos T, Kumar AS, Mpekris F, Datta M, Sun Y, Wu L, Gao X, Yeku O, del Carmen MG, Spriggs DR, Jain RK, Xu L. Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc Natl Acad Sci U S A. 2019;116:2210–2219. doi: 10.1073/pnas.1818357116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yang Y, Ma L, Xu Y, Liu Y, Li W, Cai J, Zhang Y. Enalapril overcomes chemoresistance and potentiates antitumor efficacy of 5-FU in colorectal cancer by suppressing proliferation, angiogenesis, and NF-κB/STAT3-regulated proteins. Cell Death Dis. 2020;11:477. doi: 10.1038/s41419-020-2675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhou J, Duan X, Wang J, Feng Y, Yuan J. Valsartan regulates PI3K/AKT pathways through lncrna GASL1 to improve isoproterenol-induced heart failure. Dis Markers. 2022;2022:1447399. doi: 10.1155/2022/1447399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jones MR, Schrader KA, Shen Y, Pleasance E, Ch’ng C, Dar N, Yip S, Renouf DJ, Schein JE, Mungall AJ, Zhao Y, Moore R, Ma Y, Sheffield BS, Ng T, Jones SJ, Marra MA, Laskin J, Lim HJ. Response to angiotensin blockade with irbesartan in a patient with metastatic colorectal cancer. Ann Oncol. 2016;27:801–806. doi: 10.1093/annonc/mdw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Khorsand M, Khajeh S, Eslami M, Nezafat N, Ghasemi Y, Razban V, Mostafavi-Pour Z. Telmisartan anti-cancer activities mechanism through targeting N-cadherin by mimicking ADH-1 function. J Cell Mol Med. 2022;26:2392–2403. doi: 10.1111/jcmm.17259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang Q, Lei X, Zhu S, Zhang S. Angiotensin-I converting enzyme inhibitors suppress angiogenesis and growth of esophageal carcinoma xenografts. Dis Esophagus. 2012;25:757–763. doi: 10.1111/j.1442-2050.2011.01320.x. [DOI] [PubMed] [Google Scholar]
- 179.Osman MH, Farrag E, Selim M, Osman MS, Hasanine A, Selim A. Cardiac glycosides use and the risk and mortality of cancer; systematic review and meta-analysis of observational studies. PLoS One. 2017;12:e0178611. doi: 10.1371/journal.pone.0178611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cai Y, Zhao F. Fluvastatin suppresses the proliferation, invasion, and migration and promotes the apoptosis of endometrial cancer cells by upregulating Sirtuin 6 (SIRT6) Bioengineered. 2021;12:12509–12520. doi: 10.1080/21655979.2021.2009415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Okubo K, Isono M, Miyai K, Asano T, Sato A. Fluvastatin potentiates anticancer activity of vorinostat in renal cancer cells. Cancer Sci. 2020;111:112–126. doi: 10.1111/cas.14225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kuang X, Qi M, Peng C, Zhou C, Su J, Zeng W, Liu H, Zhang J, Chen M, Shen M, Xie X, Li F, Zhao S, Li Q, Luo Z, Chen J, Tao J, He Y, Chen X. Propranolol enhanced the anti-tumor effect of sunitinib by inhibiting proliferation and inducing G0/G1/S phase arrest in malignant melanoma. Oncotarget. 2017;9:802–811. doi: 10.18632/oncotarget.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yao M, Zhou W, Sangha S, Albert A, Chang AJ, Liu TC, Wolfe MM. Effects of nonselective cyclooxygenase inhibition with low-dose ibuprofen on tumor growth, angiogenesis, metastasis, and survival in a mouse model of colorectal cancer. Clin Cancer Res. 2005;11:1618–1628. doi: 10.1158/1078-0432.CCR-04-1696. [DOI] [PubMed] [Google Scholar]
- 184.Akrami H, Aminzadeh S, Fallahi H. Inhibitory effect of ibuprofen on tumor survival and angiogenesis in gastric cancer cell. Tumour Biol. 2015;36:3237–3243. doi: 10.1007/s13277-014-2952-3. [DOI] [PubMed] [Google Scholar]
- 185.Patel J, Bass D, Beishuizen A, Bocca Ruiz X, Boughanmi H, Cahn A, Colombo H, Criner GJ, Davy K, de-Miguel-Díez J, Doreski PA, Fernandes S, François B, Gupta A, Hanrott K, Hatlen T, Inman D, Isaacs JD, Jarvis E, Kostina N, Kropotina T, Lacherade JC, Lakshminarayanan D, Martinez-Ayala P, McEvoy C, Meziani F, Monchi M, Mukherjee S, Muñoz-Bermúdez R, Neisen J, O’Shea C, Plantefeve G, Schifano L, Schwab LE, Shahid Z, Shirano M, Smith JE, Sprinz E, Summers C, Terzi N, Tidswell MA, Trefilova Y, Williamson R, Wyncoll D, Layton M. A randomised trial of anti-GM-CSF otilimab in severe COVID-19 pneumonia (OSCAR) Eur Respir J. 2023;61:2101870. doi: 10.1183/13993003.01870-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.REMAP-CAP Investigators. Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Annane D, Beane A, van Bentum-Puijk W, Berry LR, Bhimani Z, Bonten MJM, Bradbury CA, Brunkhorst FM, Buzgau A, Cheng AC, Detry MA, Duffy EJ, Estcourt LJ, Fitzgerald M, Goossens H, Haniffa R, Higgins AM, Hills TE, Horvat CM, Lamontagne F, Lawler PR, Leavis HL, Linstrum KM, Litton E, Lorenzi E, Marshall JC, Mayr FB, McAuley DF, McGlothlin A, McGuinness SP, McVerry BJ, Montgomery SK, Morpeth SC, Murthy S, Orr K, Parke RL, Parker JC, Patanwala AE, Pettilä V, Rademaker E, Santos MS, Saunders CT, Seymour CW, Shankar-Hari M, Sligl WI, Turgeon AF, Turner AM, van de Veerdonk FL, Zarychanski R, Green C, Lewis RJ, Angus DC, McArthur CJ, Berry S, Webb SA, Derde LPG. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Aman J, Duijvelaar E, Botros L, Kianzad A, Schippers JR, Smeele PJ, Azhang S, Bartelink IH, Bayoumy AA, Bet PM, Boersma W, Bonta PI, Boomars KAT, Bos LDJ, van Bragt JJMH, Braunstahl GJ, Celant LR, Eger KAB, Geelhoed JJM, van Glabbeek YLE, Grotjohan HP, Hagens LA, Happe CM, Hazes BD, Heunks LMA, van den Heuvel M, Hoefsloot W, Hoek RJA, Hoekstra R, Hofstee HMA, Juffermans NP, Kemper EM, Kos R, Kunst PWA, Lammers A, van der Lee I, van der Lee EL, Maitland-van der Zee AH, Mau Asam PFM, Mieras A, Muller M, Neefjes ECW, Nossent EJ, Oswald LMA, Overbeek MJ, Pamplona CC, Paternotte N, Pronk N, de Raaf MA, van Raaij BFM, Reijrink M, Schultz MJ, Serpa Neto A, Slob EMA, Smeenk FWJM, Smit MR, Smits AJ, Stalenhoef JE, Tuinman PR, Vanhove ALEM, Wessels JN, van Wezenbeek JCC, Vonk Noordegraaf A, de Man FS, Bogaard HJ. Imatinib in patients with severe COVID-19: a randomised, double-blind, placebo-controlled, clinical trial. Lancet Respir Med. 2021;9:957–968. doi: 10.1016/S2213-2600(21)00237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Marconi VC, Ramanan AV, de Bono S, Kartman CE, Krishnan V, Liao R, Piruzeli MLB, Goldman JD, Alatorre-Alexander J, de Cassia Pellegrini R, Estrada V, Som M, Cardoso A, Chakladar S, Crowe B, Reis P, Zhang X, Adams DH, Ely EW COV-BARRIER Study Group. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9:1407–1418. doi: 10.1016/S2213-2600(21)00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz-Palacios GM, Hsieh L, Kline S, Tapson V, Iovine NM, Jain MK, Sweeney DA, El Sahly HM, Branche AR, Regalado Pineda J, Lye DC, Sandkovsky U, Luetkemeyer AF, Cohen SH, Finberg RW, Jackson PEH, Taiwo B, Paules CI, Arguinchona H, Erdmann N, Ahuja N, Frank M, Oh MD, Kim ES, Tan SY, Mularski RA, Nielsen H, Ponce PO, Taylor BS, Larson L, Rouphael NG, Saklawi Y, Cantos VD, Ko ER, Engemann JJ, Amin AN, Watanabe M, Billings J, Elie MC, Davey RT, Burgess TH, Ferreira J, Green M, Makowski M, Cardoso A, de Bono S, Bonnett T, Proschan M, Deye GA, Dempsey W, Nayak SU, Dodd LE, Beigel JH ACTT-2 Study Group Members. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mulchandani R, Lyngdoh T, Kakkar AK. Deciphering the COVID-19 cytokine storm: systematic review and meta-analysis. Eur J Clin Invest. 2021;51:e13429. doi: 10.1111/eci.13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Andaluz-Ojeda D, Vidal-Cortes P, Aparisi Sanz Á, Suberviola B, Del Río Carbajo L, Nogales Martín L, Prol Silva E, Nieto Del Olmo J, Barberán J, Cusacovich I. Immunomodulatory therapy for the management of critically ill patients with COVID-19: a narrative review. World J Crit Care Med. 2022;11:269–297. doi: 10.5492/wjccm.v11.i4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.CORIMUNO-19 Collaborative group. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir Med. 2021;9:295–304. doi: 10.1016/S2213-2600(20)30556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Locatelli F, Jordan MB, Allen C, Cesaro S, Rizzari C, Rao A, Degar B, Garrington TP, Sevilla J, Putti MC, Fagioli F, Ahlmann M, Dapena Diaz JL, Henry M, De Benedetti F, Grom A, Lapeyre G, Jacqmin P, Ballabio M, de Min C. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med. 2020;382:1811–1822. doi: 10.1056/NEJMoa1911326. [DOI] [PubMed] [Google Scholar]
- 195.Nguyen LS, Ait Hamou Z, Gastli N, Chapuis N, Pène F. Potential role for interferon gamma in the treatment of recurrent ventilator-acquired pneumonia in patients with COVID-19: a hypothesis. Intensive Care Med. 2021;47:619–621. doi: 10.1007/s00134-021-06377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.van Laarhoven A, Kurver L, Overheul GJ, Kooistra EJ, Abdo WF, van Crevel R, Duivenvoorden R, Kox M, Ten Oever J, Schouten J, van de Veerdonk FL, van der Hoeven H, Rahamat-Langendoen J, van Rij RP, Pickkers P, Netea MG. Interferon gamma immunotherapy in five critically ill COVID-19 patients with impaired cellular immunity: a case series. Med. 2021;2:1163–1170. e2. doi: 10.1016/j.medj.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kambas K, Markiewski MM, Pneumatikos IA, Rafail SS, Theodorou V, Konstantonis D, Kourtzelis I, Doumas MN, Magotti P, Deangelis RA, Lambris JD, Ritis KD. C5a and TNF-alpha up-regulate the expression of tissue factor in intra-alveolar neutrophils of patients with the acute respiratory distress syndrome. J Immunol. 2008;180:7368–75. doi: 10.4049/jimmunol.180.11.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Stallmach A, Kortgen A, Gonnert F, Coldewey SM, Reuken P, Bauer M. Infliximab against severe COVID-19-induced cytokine storm syndrome with organ failure-a cautionary case series. Crit Care. 2020;24:444. doi: 10.1186/s13054-020-03158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chi X, Yan R, Zhang J, Zhang G, Zhang Y, Hao M, Zhang Z, Fan P, Dong Y, Yang Y, Chen Z, Guo Y, Zhang J, Li Y, Song X, Chen Y, Xia L, Fu L, Hou L, Xu J, Yu C, Li J, Zhou Q, Chen W. A neutralizing human antibody binds to the N-terminal domain of the spike protein of SARS-CoV-2. Science. 2020;369:650–655. doi: 10.1126/science.abc6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mastellos DC, Pires da Silva BGP, Fonseca BAL, Fonseca NP, Auxiliadora-Martins M, Mastaglio S, Ruggeri A, Sironi M, Radermacher P, Chrysanthopoulou A, Skendros P, Ritis K, Manfra I, Iacobelli S, Huber-Lang M, Nilsson B, Yancopoulou D, Connolly ES, Garlanda C, Ciceri F, Risitano AM, Calado RT, Lambris JD. Complement C3 vs C5 inhibition in severe COVID-19: early clinical findings reveal differential biological efficacy. Clin Immunol. 2020;220:108598. doi: 10.1016/j.clim.2020.108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vlaar APJ, de Bruin S, Busch M, Timmermans SAMEG, van Zeggeren IE, Koning R, Ter Horst L, Bulle EB, van Baarle FEHP, van de Poll MCG, Kemper EM, van der Horst ICC, Schultz MJ, Horn J, Paulus F, Bos LD, Wiersinga WJ, Witzenrath M, Rueckinger S, Pilz K, Brouwer MC, Guo RF, Heunks L, van Paassen P, Riedemann NC, van de Beek D. Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): an exploratory, open-label, phase 2 randomised controlled trial. Lancet Rheumatol. 2020;2:e764–e773. doi: 10.1016/S2665-9913(20)30341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.RECOVERY Collaborative Group. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399:665–676. doi: 10.1016/S0140-6736(22)00163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, Shawa I, Kumar P, Adams AC, Van Naarden J, Custer KL, Durante M, Oakley G, Schade AE, Holzer TR, Ebert PJ, Higgs RE, Kallewaard NL, Sabo J, Patel DR, Klekotka P, Shen L, Skovronsky DM. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325:632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]