Abstract
As the major intracellular anion, chloride plays an important role in maintaining intracellular and extracellular ion homeostasis, osmotic pressure, and cell volume. Intracellular chloride channel 1, which has the physiological role of forming membrane proteins in the lipid bilayer and playing ion channels, is a hot research topic in recent years. It has been found that CLIC1 does not only act as an ion channel but also participates in cell cycle regulation, apoptosis, and intracellular oxidation; thus, it participates in the proliferation, invasion, and migration of various tumor cells in various systems throughout the body. At the same time, CLIC1 is highly expressed in tumor cells and is associated with malignancy and a poor prognosis. This paper reviews the pathological mechanisms of CLIC1 in systemic diseases, which is important for the early diagnosis, treatment, and prognosis of systemic diseases associated with CLIC1 expression.
Keywords: CLIC1, tumor, proliferation, invasion, migration
Introduction
Chloride ions, the most abundant intracellular anions, are involved in the control of salt secretion and uptake, membrane potential regulation, organelle acidification, and cell volume homeostasis [1]. In recent years, more and more studies have demonstrated the involvement of chloride channels in tumor development. A member of the chloride intracellular channel protein (CLIC1) family, an ion channel protein consisting of 241 amino acids, has been focused on [2]. It plays an important role in various physiological functions, including cell volume regulation, organelle acidification, transepithelial transport, and ion homeostasis [3-7]. CLIC1 regulates the cell cycle and participates in cell proliferation, apoptosis, and differentiation, which may be closely related to tumor invasion and migration [8,9]. It has been demonstrated that CLIC1 regulates the invasion, migration, and apoptosis of various tumor cells, including gastric, colorectal, and hepatocellular carcinomas, by participating in various signaling pathways, such as MAPK/ERK, ROS/ERK, and MAPK/AKT [10,11]. This article reviews some of the physiological functions of CLIC1, pathological conditions, and studies on the development of tumors in various systems.
CLIC1 structure
The chloride ion intracellular channel protein (CLIC) family, which consists of six highly conserved members (CLIC1-6) [12], has a highly variable distribution despite their gene sequences having 60%-75% identity [13,14]. These proteins are expressed in the cytoplasm, cell membranes, organelles, and nuclei of all bodily tissues and are involved in a variety of physiological processes in the body [1] (Table 1). One of the first intracellular chloride channel proteins to be identified contains 241 amino acids and is widely expressed in human tissues and cellular anion channels; however, it is mainly expressed in the nucleus [8].
Table 1.
Expression and function of CLICs
| Protein | Subcellular localization | Expression | Function | Refs |
|---|---|---|---|---|
| CLIC1 | Cytoplasm, plasm membrane, intracellular membrane, nucleoplasm | Universally expression | Ion channel, cell cycle, Osteoblast differentiation | [2,8,20,22-27] |
| CLIC2 | Adult muscles, fetal liver | Modulates activity of RyR | [23,28-32] | |
| CLIC3 | Nucleus and plasm membranes | Placenta, lung, heart, kidney, pancreas, and skeletal muscles | Cellular growth control | [23,33-35] |
| CLIC4 | Cytoplasm, nucleus, intracellular organelles, plasm membrane, and intracellular membranes | Brain, liver, testis, kidney, lungs, and skeletal muscles | Ion channel, angiogenesis, acidification, apoptosis, and cancer, endothelial proliferation and morphogenesis, cytoskeletal components | [14,20,23,36-49] |
| CLIC5A | Cytoplasm | Heart, kidney, lung, placenta, and skeletal muscles | Ion channel, associated with cytoskeletal elements and promote actin polymerization | [20,50-53] |
| CLIC5B | Cytoplasm, plasm membrane, intracellular membranes, and secretory vesicles | Heart, kidney, lung, placenta, and skeletal muscles | Ion channel, ion absorption and secretion, formation of stereocilia, and development of the organ of corti | [54,55] |
| CLIC6 | Cytoplasm and plasma membrane | Choroid plexus, gastric mucosa, brain, and kidney | Water transport and interacts with dopamine receptors | [23,56-58] |
CLIC1, Intracellular Chloride Channel 1; CLIC2, Intracellular Chloride Channel 2; CLIC3, Intracellular Chloride Channel 3; CLIC4, Intracellular Chloride Channel 4; CLIC5, Intracellular Chloride Channel 5; CLIC6, Intracellular Chloride Channel 6.
Currently, the three main inhibitors of chloride intracellular channel proteins are dihydro-4,4’ diisothiocyanostilbene-2,2’-disulphonic acid (DIDS), 5-nitro-2-(phenylpropylamino)-benzoate (NPPB) and 2’,4’,6’-trihydroxy-3-(4-hydroxyphenyl)-propiophenone (phloretin). The major chloride channel inhibitor DIDS has been previously documented to prevent apoptosis of cardiomyocytes induced by staurosporine [15,16]. NPPB, DIDS, and phloretin all prevented ischemia-reperfusion-induced apoptosis in mouse cardiomyocytes [17]. And H2O2-induced cardiomyocyte apoptosis in mitochondrial and lysosomal vesicles in rat hearts [18], suggesting that DIDS, NPPB, and phloretin, inhibitors of chloride intracellular channel proteins, have cardioprotective effects. However, there are no reports in the literature on whether inhibitors of CLIC1 affect the normal physiological functions of healthy tissues or cells.
One feature that distinguishes members of the CLIC family from other ion channel proteins is that they have both soluble and membrane-bound forms and can exist as soluble monomeric proteins, soluble dimeric proteins, or membrane-bound forms [19]. It exists mainly in the form of soluble proteins, with a small proportion forming transmembrane structures [2]. Except for CLIC 5B and CLIC 6, which have an extended N-terminal region, the other CLICs consist of an N-terminal thioredoxin-like structural domain and an α-helical C-terminal structural domain (Figure 1) [20]. The soluble protein form of CLIC1 is a monomeric protein with high structural homology to the glutathione S-transferase (GST) superfamily, with a glutoxigenin-like redox-active site [21]. This soluble form of CLIC1 consists of two structural domains; namely, the N-terminal structural domain, which is highly conserved and contains a glutaredoxin-like enzymatic active site that closely resembles the glutaredoxin thioredoxin fold, and the C-terminal structural domain, which is an all-alpha helical structure [19,21]. Harrop et al. [1] determined the high-resolution x-ray crystal structure of the CLIC1 soluble protein; however, there is no clear literature on the specific transmembrane structure of CLIC1.
Figure 1.
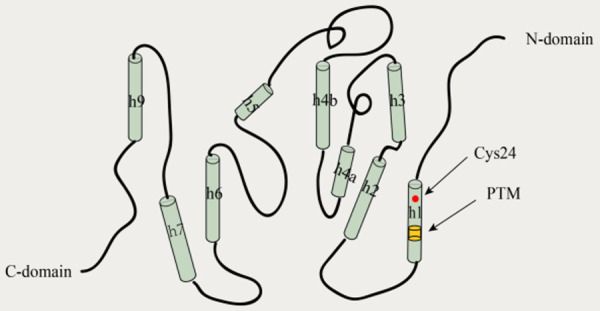
Putative structural model of the CLIC1 protein in the soluble, reduced monomeric form. CLIC1 has an active-site cysteine residue (Cys24). The N-terminus and C-terminus of CLIC1 are both cytoplasmic. The N-terminus domain is on the right (helices h1, h2, and h3), while the all-helical C-terminus domain is on the left (helices h4-h9). CLIC1, chloride intracellular channel 1; PTM, putative transmembrane region.
CLIC1’s function
Physiology
CLIC1 is involved in important physiological processes in the organism. In addition to its well-known functions such as the maintenance of cell membrane stability, intercellular transmembrane material transport, organelle acidification, intracellular pH stability, and cell volume regulation, CLIC1 has also been discovered to have some less-known special functions in recent years. Previous studies have demonstrated that CLIC1 promotes platelet and endothelial cell adhesion, which has important implications for angiogenesis [59]. Meanwhile, CLIC1 plays an important role in the regulation of osteoblast differentiation [60]. CLIC1 has also been found to play a role in sperm function and to be involved in the fertilization process [61]. Decreased CLIC1 levels lead to the impaired acidification of phagocytes such as macrophages and dendritic cells, thereby inhibiting T-cell activity by regulating antigen presentation by dendritic cells [62], suggesting that CLIC1 has a role in regulating immune cell function [63]. Furthermore, CLIC1 has been shown to be a receptor for cellular oxidation and to be involved in the inflammatory response [64]. It has also been shown that CLIC1 is an important downstream molecule of the insulin transduction pathway in hematopoietic cells [65]. Kagiali et al. first proposed that CLIC1 plays an important role in mammalian cytoplasmic division, enabling daughter cells to successfully undergo cytoplasmic division without inducing significant multinucleated cell aggregation by shedding [66]. This physiological function of CLIC1 to promote cortical stabilization and the successful completion of cytoplasmic division is important for further studies on the role CLIC1 plays at the cellular level. Although CLIC1 has been involved in a variety of important physiological processes in the human body, a broader physiological role needs to be explored for discovery.
Pathology
Previous studies on CLIC1 have focused on its physiological functions regarding non-neoplastic diseases [67], such as neurodegenerative diseases [22], rheumatic diseases (including rheumatoid arthritis and psoriatic arthritis) [68], Alzheimer’s disease [69], etc. It is well-known that the pathogenesis of cancer is closely related to the imbalance of the cellular redox status [70]. CLIC1 can act as a “sensor” and an “effector” of the oxidative stress-induced redox state in cells [64].
CLIC1 also promotes the proliferation, migration, and invasion of certain cancer cells [71,72]. Moreover, during cell proliferation, CLIC1 can be automatically transferred from the cytoplasm to the plasma membrane in the absence of transport vesicles [23]. This could be the reason why CLIC1 promotes tumor cell migration and invasion. CLIC1 can also promote tumor metastasis by recruiting PIP5K1A and PIP5K1C from the cytoplasm to the leading edge of the plasma membrane [12]. In addition, elevated CLIC1 expression was strongly associated with lymph node metastasis, perineural and lymphatic infiltration, pathological staging, and low survival rates [73].
CLIC1 upregulation in certain malignancies is also associated with poor prognoses, suggesting that it is a potential prognostic marker and a potential target for antitumor therapy [74,75].
CLIC1 and tumors
Nervous system
Glioma is the most common primary brain tumor [76]. The disease is characterized by extensive local tumor migration and invasion, which results in rapid disease progression and a poor prognosis. In recent years, despite the ongoing efforts to target gliomas for treatment, the results remain unsatisfactory. Previous studies have demonstrated that CLIC1 is highly activated in cancer cells in a highly proliferative state and plays an important role in tumor invasion and migration [12]. CLIC1 mRNA and protein expression are upregulated in human glioma tissues compared to glioma-free tissues, and increased CLIC1 expression correlates with the histopathological grading of gliomas [77]. Its expression was found to be higher in high-grade glioma tissues than in low-grade glioma tissues and to increase with the WHO (World Health Organization) grade of the tumor, suggesting that this protein may serve as an indicator of the aggressiveness of gliomas. It was also demonstrated that CLIC1 is an independent predictor of short overall survival in patients with glioma.
Therefore, CLIC1 may be associated with glioma development, migration invasion and prognosis. The findings of previous experiments suggested that high CLIC1 expression is a valuable prognostic marker for this disease, and these findings will lead to new directions in the treatment and prognosis of glioma.
Respiratory system
Lung adenocarcinoma, the most common subtype of lung cancer, has the highest mortality rate of all cancers [78]. Lung adenocarcinoma is usually asymptomatic in its early stages, and many patients have advanced forms of the disease by the time it is detected, which makes its prognosis poor. Previous studies have demonstrated that CLIC1 is more highly expressed in lung adenocarcinoma compared to paraneoplastic tissue and that CLIC1 expression is negatively correlated with patients’ overall survival [79]. They also found that the knockdown of CLIC1 inhibited the p38 MAPK signaling pathway in the A549 and PC9 lung adenocarcinoma cell lines, thereby suppressing cell proliferation and migration (Figure 2). Therefore, they concluded that CLIC1 expression is an independent determinant of the prognosis of lung adenocarcinoma. CLIC1 is a promising target for cancer therapy because of its important role in the cell cycle and its overexpression in a variety of tumors. Ca2+ and reactive oxygen species (ROS) are important signaling molecules that regulate various cellular functions such as cell death; e.g., a surge in Ca2+ and ROS levels is required for the initiation of apoptosis at the mitochondria-endoplasmic reticulum interface [80]. Lee et al. [81] investigated the function of CLIC1 in Ca2+ metabolism and the link between CLIC1 and ROS signaling in the A549 human lung cancer cell line. Their results demonstrated that CLIC1 expression was reduced by L-type Ca2+ channel blockers (LTCCs) to induce an increase in intracellular Ca2+ levels and that ROS levels increased when Ca2+ signaling was significantly enhanced and its expression was increased [82]. This activates the JNK signaling pathway, which plays an important role in various physiological and pathological processes such as the cell cycle, reproduction, apoptosis, and cellular stress [83]. In conclusion, their study found that CLIC1 is involved in the proliferation of lung adenocarcinoma, migration, and has an important role in the prognosis of lung adenocarcinoma. Although the role of CLIC1 in human lung cancer cells A549 and its molecular mechanism has been demonstrated, it has not been confirmed in animal models, and further studies are needed to confirm as well as to explore the deeper mechanisms bringing new therapeutic targets for the treatment of lung cancer. Thus, CLIC1 is a key regulator of calcium ion signaling and controls the survival of lung cancer cells. In conclusion, their study identified the role of CLIC1 in A549 human lung cancer cells and its molecular mechanism; however, this finding has not been confirmed in animal models, and further studies are needed for confirmation and more in-depth exploration of mechanisms involved to identify new therapeutic targets for the treatment of lung cancer.
Figure 2.
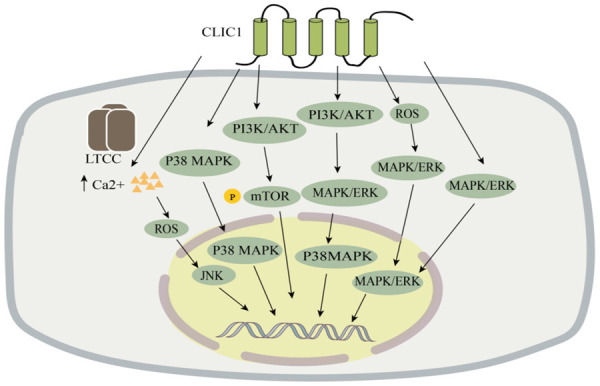
CLIC1 is involved in the pathogenesis of lung, esophageal squamous, gastric, liver, colon, prostate, and other cancers. LTCC, L-type Ca2+ channel blockergai.
Digestive system
Esophageal squamous cell carcinoma
Esophageal cancer is the eighth most common cancer in the world and the sixth leading cause of cancer-related death [84]. It is a gastrointestinal tumor characterized by high incidence, poor prognosis, and high mortality. Huiwu Geng et al. [85] explored the clinical significance of chloride intracellular channel 1 (CLIC1) expression in esophageal squamous cell carcinoma (ESCC) and its molecular mechanism. They reduced CLIC1 expression by silencing CLIC1 in an esophageal epithelial cell line and found that CLIC1 silencing inhibited the proliferation of ESCC cells. In addition, they found that CLIC1 expression was positively correlated with the TNM stage in advanced tumors and that CLIC1 expression was higher in these tumors. To further search for specific signaling pathways through which CLIC1 affects ESCC cell proliferation, they used ESCC proteomics technology to find a strong correlation between CLIC1 expression and PI3K-Akt signaling regulation in ESCC. Their subsequent experiments also confirmed that CLIC1, although not directly affecting PI3K/Akt, promotes the activation of the phosphorylation of mTOR downstream of PI3K/Akt in ESCC cells, thereby functionally promoting ESCC progression. Therefore, CLIC1 is a candidate molecular biomarker for ESCC.
Gastric cancer
Gastric cancer, a malignant tumor of the gastrointestinal tract that originates from epithelial cells, is the fifth most common cancer worldwide and the third leading cause of cancer-related death [86]. However, the conventional biomarkers of gastric cancer, carcinoembryonic antigen (CEA) and glycoconjugate antigen (CA) 199, are not sensitive enough to diagnose the condition. It has previously been demonstrated that the CLIC1 protein is highly expressed in gastric cancer tissues and that the upregulation of CLIC1 expression is strongly associated with lymph node metastasis, pathological staging, lymphatic infiltration, and low survival rates [87]. It has also been shown that CLIC1 overexpression promotes gastric cancer cell migration and invasion [88]. These findings suggest that CLIC1 is a potential diagnostic gastric cancer marker. The results of a study by Li et al. [10] showed that CLIC1 silencing promoted apoptosis and inhibited migration and invasion in gastric cancer cells in vitro. They further found, in animal experiments, that CLIC1 inhibition affected the AKT and MAPK pathways in in situ xenograft tumors in nude mice, thereby inhibiting the in-situ xenograft tumor volume, weight, metabolism, and lymphatic infiltration capacity while promoting apoptosis. This also confirms that CLIC1 may play an important role in tracking the progression of gastric cancer by regulating integrin family proteins and subsequently affecting the PI3K/AKT, MAPK/ERK, and MAPK/p38 pathways. That is, CLIC1 may promote the development of gastric carcinogenesis through the molecular mechanism of AKT and MAPK signaling pathways. This discovery is a major step forward in the exploration of therapeutic targets for gastric cancer. As far as the downregulation of CLIC1 expression can effectively inhibit the growth of tumor cells, new treatments for gastric cancer can be explored by targeting CLIC1 in the future; however, more research needs to be conducted to investigate whether CLIC1 may also preserve tumor growth in mouse models of gastric cancer and to explore the mechanisms involved more deeply.
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC), which originates from hepatocytes, is the most common primary liver cancer (90%), the sixth most common cancer worldwide, and the third most common cause of cancer-related death [89]. Therefore, to improve the survival rate of patients with hepatocellular carcinoma, it is particularly important to detect tumors at an early stage through screening programs. Although serum tumor markers are reliable tools for the surveillance and early diagnosis of hepatocellular carcinoma, the most common serologic test, methemoglobin, has low sensitivity and specificity, which predisposes it to missed diagnoses [90,91]. Therefore, in recent years, researchers have been actively searching for new liver cancer markers, and they have discovered through proteomics that certain proteins are highly expressed in cancer and are associated with disease migration and invasion, such as CLIC1 [12]. In their experiments, Wei et al. [92] found that CLIC1 expression was significantly increased in HCC compared with paraneoplastic tissues, and CLIC1 expression was positively correlated with vascular infiltration in hepatocellular carcinoma. In contrast, after CLIC1 knockdown, hepatocellular carcinoma cell viability and invasiveness were inhibited to some extent, and apoptosis was induced. In contrast, CLIC1 overexpression promoted the viability and invasiveness of hepatocellular carcinoma cells and also inhibited apoptosis. They previously performed proteomic assays on CLIC1 and demonstrated that increased CLIC1 expression correlated with the aggressiveness of hepatocellular carcinoma. In this regard, they selected some HCC tissues and paraneoplastic tissues for immunohistochemical analyses of CLIC1 protein expression and its association with HCC aggressiveness. These analyses confirmed the correlation between high CLIC1 expression and hepatocellular carcinoma aggressiveness, suggesting that CLIC1 promotes HCC invasion. Meanwhile, they found that Maspin (an oncogene) was closely related to CLIC1 via the gene expression profiling technique, and after detecting Maspin and its related proteins, they found that CLIC1 expression was negatively correlated with the Maspin protein level. In conclusion, their study identified an unknown possible mechanism by which CLIC1 controls HCC invasion, and CLIC1 could serve as a potential therapeutic target. Their latest study found that CLIC1 regulates cell-matrix adhesion and membrane protrusion by recruiting PIP5Ks to the plasma membrane [12]. After comparing proteomics, they found that CLIC1 was upregulated in human hepatocellular carcinoma (HCC) and was associated with tumor invasion, metastasis, and a poor prognosis. Then, they further validated this in a mouse model of HCC cancer and found that silencing CLIC1 inhibited the suppression of lung metastasis in mice. This study reveals that CLIC1 plays an important role in the metastasis of hepatocellular carcinoma cells. The upregulation of CLIC1 in hepatocellular carcinoma cells and its unique membrane-targeting properties could make it an excellent target for the treatment of tumor metastasis.
Colon cancer
Colorectal cancer is one of the most common malignancies causing death in humans [93]. The 5-year survival rate for patients with early-stage colorectal cancer is higher than 90%; however, in patients with distant metastases, the 5-year survival rate is less than 10% [94]. Therefore, for patients with colorectal cancer, early diagnosis and treatment are critical. Previous studies have demonstrated that CLIC1 is highly expressed in colon cancer, and Wang et al. further found that CLIC1 promotes metastasis in rectal cancer [95], however, the exact mechanism of transfer remains unclear. Studies have shown that tumor cells under hypoxic conditions are more aggressive, which promotes cancer cell metastasis [96]. Due to the irregular microvascular network and blood flow pattern of tumor cells, cancer cells are in a hypoxia-reoxygenation (H-R) microenvironment [97]. The irregularity of blood perfusion during H-R promotes the migration and invasion of cancer cells [98,99]. ROS in cancer cells can play a role in their proliferation, apoptosis, differentiation, migration, and invasion [70,96]. On the basis of this theory, Wang et al. [100] investigated the relationship between CLIC1 and ROS under H-R conditions. It was found that ROS production was associated with the H-R process in CLIC1 colon cancer cells and that the functional inhibition of CLIC1 downregulated ROS production in colon cancer cells. Also, they found that CLIC1 was involved in the migration of colon cancer cells under H-R conditions, and the inhibition of CLIC1 hindered colon cancer cell invasion. Their further study found that H-R treatment increased intracellular ROS levels and activated the MAPK/ERK pathway, thereby promoting the migration and invasion of colon cancer cells. In conclusion, their study demonstrated that the CLIC1 protein is involved in the metastasis of colon cancer cells by regulating the ROS/ERK pathway in the H-R process. This study illustrates that CLIC1 is an important candidate protein that may act as a potent regulator of colon cancer metastasis and a possible molecular mechanism to promote colon cancer metastasis.
Gallbladder cancer
Gallbladder cancer (GBC) is the most common biliary tract malignancy and a common type of gastrointestinal cancer, with over 10,000 new cases being diagnosed each year [101]. Its early clinical manifestations are not obvious, and in most patients, GBC is diagnosed at a late stage with extensive metastases and other-organ invasion [102]. Currently, cholecystectomy is the only effective treatment strategy for 10% of patients with early GBC [103,104]. Therefore, finding novel and effective biomarkers associated with GBC progression for early diagnosis and treatment may improve the prognosis of patients with GBC by facilitating the selection of the most appropriate treatment options. Considering the characteristics of the CLIC1 gene in cell regulation and tumorigenesis, the CLIC1 gene may have some influence on the biological behavior of GBC cells. He et al. [72] found that CLIC1 mRNA and protein expression were significantly upregulated in GBC tissues by comparing GBC tissues with normal gallbladder tissues. Then, they silenced the CLIC1 gene and found that CLIC1 gene silencing inhibited the proliferation, migration, and invasion of GBC cells and promoted apoptosis. In contrast, in a subsequent study by Zhou et al. [105], hsa-miR-372 expression was found to be reduced in GBC tissues, and the degree of reduction was associated with poor prognosis in GBC patients Also, hsa-miR-372 was found to play a key role in tumorigenesis and tumor progression [106]. Their further experiments identified CLIC1 as a direct target of hsa-miR-372. This also implies that hsa-miR-372 may regulate the progression and development of GBC by directly targeting CLIC1. Therefore, CLIC1 may be an effective and promising treatment for GBC patients in the future.
Pancreatic cancer
Pancreatic cancer has the eighth highest mortality rate of all cancers and a low 5-year survival rate [107]. Because pancreatic cancer is prone to local invasion, early metastasis, and resistance to chemotherapy, patient survival rates remain low despite recent advances in surgical techniques and medical management [108,109]. Therefore, finding effective early markers is of great clinical value for the diagnosis and prognosis of this disease, as well as for new therapeutic targets. Lu et al. [9] also confirmed by immunohistochemistry that CLIC1 was highly expressed in pancreatic cancer tissues relative to normal tissues. Meanwhile, CLIC1 can promote pancreatic cancer cell growth and proliferation. They also found that CLIC1 overexpression was significantly and positively correlated with the histopathological subtype, TNM stage, and tumor size, suggesting that CLIC1 may play an important role in pancreatic cancer invasion. In conclusion, their study suggests that CLIC1 may be involved in the progression and invasion of pancreatic cancer.
Reproductive system
Breast cancer
In recent years, the incidence of breast cancer in women has been growing rapidly, and breast cancer has overtaken lung cancer as the most common cancer [110]. Currently, both surgery and radiotherapy have affected the quality of life of patients with breast cancer to varying degrees in clinical practice. Aggressive triple-negative breast cancer is also prone to distant metastasis in the short term, and there is still a lack of effective chemotherapy or targeted therapy in clinical practice. Therefore, it is necessary to find new biomarkers and therapeutic targets. In Xia’s study [111], CLIC1 was shown to be highly expressed in breast cancer tissues by both qRT-PCR and WB. The results of IHC analyses confirmed that CLIC1 expression was higher at higher pathological grades and higher TNM stages. They also found that positive CLIC1 expression was associated with ki67 overexpression, lymph node metastasis, and vascular and nerve invasion, suggesting that CLIC1 may be involved in the metastasis and invasion of breast cancer and that breast cancer patients with high CLIC1 expression have a poorer prognosis. The above results suggest that CLIC1 is expected to be an independent prognostic and metastatic indicator of breast cancer as well as a new potential biomarker. Therefore, CLIC1 may play a role in the diagnosis and treatment of breast cancer, and its molecular mechanisms affecting the development of breast cancer deserve to be studied and explored.
Epithelial ovarian cancer
Ovarian cancer is a serious gynecological malignancy, of which epithelial ovarian cancer is the most dominant pathological type [112]. Its incidence is increasing each year [113]. Late detection, easy metastasis, and a poor prognosis are its characteristics [114]. Although CA125 can monitor epithelial ovarian cancer in its early stages, it has its limitations. Therefore, it is particularly important to screen for specific and sensitive markers for the early detection of ovarian cancer. Yu et al. demonstrated that CLIC1 mRNA and protein levels were significantly upregulated in ovarian cancer tissues compared to normal ovaries [6]. Moreover, CLIC1 expression was significantly higher in high-grade tumors than in low-grade tumors. In addition, they found that CLIC1 overexpression was associated with cisplatin resistance. These findings suggest that CLIC1, which is overexpressed in patients with epithelial ovarian cancer, is associated with a poor prognosis. This means it has the potential to be a marker for the early detection of epithelial ovarian cancer. Blocking CLIC1 overexpression may improve the prognosis of epithelial ovarian cancer by improving cisplatin resistance.
Human choriocarcinoma
Staphylomas are usually benign, and only a few of them can progress to malignant disease, including invasive staphylomas, choriocarcinoma, and placental trophoblastic tumors that require chemotherapy [115]. Serum human chorionic gonadotropin (hCG) concentrations are used to determine the malignant transformation status of staphylomas. However, it takes time for elevated hCG titers to be detected in the serum, leaving little time for clinical intervention and treatment; therefore, this affects the prognosis of the disease. Currently, there are no available prognostic markers to determine which intact gravida will convert to highly invasive disease or choriocarcinoma at the time of uterine evacuation [115]. Then, they applied proteomic techniques to identify differentially expressed proteins in human malignantly transformed staphylomas and found that CLIC1 was expressed at higher levels in choriocarcinoma tissue than intact staphyloma tissue. Meanwhile, CLIC1 expression was increased in malignant-transformed staphyloma tissue compared to non-transformed staphyloma tissue [115]. This finding will contribute to the early diagnosis and early intervention of malignant-transformed staphyloma.
Urinary system
Kidney cancer
Renal cell carcinoma is one of the most common malignancies in humans, and renal clear cell carcinoma (ccRCC), its most common histological type, accounts for more than 80% of renal cancers [116]. ccRCC is highly malignant, insensitive to radiotherapy and chemotherapy, and is associated with high patient mortality rates [117,118], there is no effective treatment for this condition [119,120]. Therefore, we need new prognostic biological markers for the reliable, early detection of ccRCC and its early treatment to improve the prognosis of patients with the condition. The role of ion channels in tumors has attracted a lot of attention in the last two decades. In recent years, researchers have found that chloride channels seem to play a vital role in cancer and have a more prominent position [121]. It has been reported in the literature that the abnormal triggering of ion channel activity contributes to the high proliferation rate of tumor cells [122]. CLIC1, which is expressed in a variety of cancers, is a potential biomarker for a number of malignancies [123,124]. It can sometimes be detected in the plasma of patients; therefore, it is highly useful in clinical practice [125]. Nesiu et al. [126] first concluded that CLIC1 was highly expressed in the renal vesicles of several patients with renal clear cell carcinoma after an immunohistochemical examination of this patient. CLIC1 was also found to be highly expressed in the surrounding connective tissue and blood vessels invaded by ccRCC cells. This experimental study is of great clinical importance because CLIC1 is able to detect not only aggressive cell populations but also isolated cells spreading around the tumor and cells associated with aggressive tumors. The finding that CLIC1 was detectable in the surrounding tissues of ccRCC was also confirmed in other experiments [127]. This suggests that CLIC1 could be involved in tumor invasion and metastasis. Although the specific role played by CLIC1 in renal clear cell carcinoma has not been elucidated, it does represent a promising therapeutic target for ccRCC and other malignancies.
Prostate cancer
Prostate cancer is the most common cancer in men and the second leading cause of cancer-related deaths in men worldwide [128,129]. The key to prostate cancer treatment is early diagnosis and treatment. While localized prostate cancer can be cured, aggressive and metastatic prostate cancer has no effective treatment [130,131]. Although certain markers for prostate cancer screening, diagnosis, and prognosis, such as prostate-specific antigen (PSA), have emerged in recent years, they are not specific. Therefore, there is a need to develop new diagnostic methods to enhance the early detection of prostate cancer, and the identification of new molecules with diagnostic and therapeutic implications for prostate cancer remains a major research focus. Recent studies have shown that CLIC1 is upregulated in prostate cancer [128] and might be a potential biomarker for prostate cancer diagnosis. On this basis, Tian et al. [132] investigated whether CLIC1 is involved in the biological functions associated with prostate cancer. They used interference techniques in prostate cancer PC-3 and DU145 cell lines to downregulate CLIC1 expression. The relationship between CLIC1 and cell proliferation was measured by the MTT method, and the results showed that CLIC1 inhibited the growth of cells in a time-dependent manner. The transwell assay demonstrated that the downregulation of CLIC1 significantly reduced cancer cell migration. The authors further investigated whether CLIC1 induces apoptosis in human PC-3 and DU145 prostate cancer cells. Flow cytometry assay data demonstrated that the downregulation of CLIC1 had no effect on PC-3 and DU145 cell apoptosis. These results suggest that the downregulation of CLIC1 can inhibit the proliferation and migration of PC-3 and DU145 cells but has no significant effect on apoptosis. The authors further explored the molecular signaling pathways that could be involved in the proliferation and migration of prostate cancer cell lines. They found that the MAPK/ERK pathway was inhibited in PC-3 cells in the CLIC1 siRNA group, suggesting that CLIC1 promotes prostate cancer proliferation and migration by regulating the MAPK/ERK pathway. This pathway provides a candidate molecular target for the prevention and treatment of prostate cancer.
Conclusions
CLIC1, as the major intracellular anion channel, plays an important role in various human physiological functions, including cell volume regulation, organelle acidification, trans-epithelial transport, and ion homeostasis. Meanwhile, the functions of CLIC1 in cell cycle regulation, as well as its participation in cell proliferation, apoptosis, and differentiation may be closely related to the pathogenesis of tumors. This review provides a basic and systematic summary of the simple physiological roles of CLIC1 and the pathological roles of CLIC1 in relation to tumors in various bodily systems, with the aim of providing an up-to-date background of the current literature. However, the detailed physiological and pathological mechanisms involving CLIC1 in humans have not been elucidated and need to be further investigated. The exact physiological and pathological significance of CLIC1 should be further investigated, and its relevance as a molecular marker for the diagnosis and treatment of cancer-related diseases should be assessed.
Acknowledgements
The present study was supported by a grant from the National Natural Science Foundation of China (grant No. 82073087), the National Natural Science Foundation of China (grant No. 81960507) and the Science and Technology Bureau fund of Zunyi city [grant No. ZUN SHI KE HE HZ ZI(2019)93-HAO] Collaborative Innovation Center of Chinese Ministry of Education (2020-39).
Disclosure of conflict of interest
None.
References
- 1.Harrop SJ, DeMaere MZ, Fairlie WD, Reztsova T, Valenzuela SM, Mazzanti M, Tonini R, Qiu MR, Jankova L, Warton K, Bauskin AR, Wu WM, Pankhurst S, Campbell TJ, Breit SN, Curmi PM. Crystal structure of a soluble form of the intracellular chloride ion channel CLIC1 (NCC27) at 1.4-A resolution. J Biol Chem. 2001;276:44993–45000. doi: 10.1074/jbc.M107804200. [DOI] [PubMed] [Google Scholar]
- 2.Warton K, Tonini R, Fairlie WD, Matthews JM, Valenzuela SM, Qiu MR, Wu WM, Pankhurst S, Bauskin AR, Harrop SJ, Campbell TJ, Curmi PM, Breit SN, Mazzanti M. Recombinant CLIC1 (NCC27) assembles in lipid bilayers via a pH-dependent two-state process to form chloride ion channels with identical characteristics to those observed in Chinese hamster ovary cells expressing CLIC1. J Biol Chem. 2002;277:26003–26011. doi: 10.1074/jbc.M203666200. [DOI] [PubMed] [Google Scholar]
- 3.Okada Y. Ion channels and transporters involved in cell volume regulation and sensor mechanisms. Cell Biochem Biophys. 2004;41:233–258. doi: 10.1385/CBB:41:2:233. [DOI] [PubMed] [Google Scholar]
- 4.Jiang L, Salao K, Li H, Rybicka JM, Yates RM, Luo XW, Shi XX, Kuffner T, Tsai VW, Husaini Y, Wu L, Brown DA, Grewal T, Brown LJ, Curmi PM, Breit SN. Intracellular chloride channel protein CLIC1 regulates macrophage function through modulation of phagosomal acidification. J Cell Sci. 2012;125:5479–5488. doi: 10.1242/jcs.110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 6.Yu W, Cui R, Qu H, Liu C, Deng H, Zhang Z. Expression and prognostic value of CLIC1 in epithelial ovarian cancer. Exp Ther Med. 2018;15:4943–4949. doi: 10.3892/etm.2018.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haddad P, Beck JS, Boyer JL, Graf J. Role of chloride ions in liver cell volume regulation. Am J Physiol. 1991;261:G340–G348. doi: 10.1152/ajpgi.1991.261.2.G340. [DOI] [PubMed] [Google Scholar]
- 8.Valenzuela SM, Mazzanti M, Tonini R, Qiu MR, Warton K, Musgrove EA, Campbell TJ, Breit SN. The nuclear chloride ion channel NCC27 is involved in regulation of the cell cycle. J Physiol. 2000;529:541–552. doi: 10.1111/j.1469-7793.2000.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu J, Dong Q, Zhang B, Wang X, Ye B, Zhang F, Song X, Gao G, Mu J, Wang Z, Ma F, Gu J. Chloride intracellular channel 1 (CLIC1) is activated and functions as an oncogene in pancreatic cancer. Med Oncol. 2015;32:616. doi: 10.1007/s12032-015-0616-9. [DOI] [PubMed] [Google Scholar]
- 10.Li BP, Mao YT, Wang Z, Chen YY, Wang Y, Zhai CY, Shi B, Liu SY, Liu JL, Chen JQ. CLIC1 promotes the progression of gastric cancer by regulating the MAPK/AKT pathways. Cell Physiol Biochem. 2018;46:907–924. doi: 10.1159/000488822. [DOI] [PubMed] [Google Scholar]
- 11.Ding Q, Li M, Wu X, Zhang L, Wu W, Ding Q, Weng H, Wang X, Liu Y. CLIC1 overexpression is associated with poor prognosis in gallbladder cancer. Tumour Biol. 2015;36:193–198. doi: 10.1007/s13277-014-2606-5. [DOI] [PubMed] [Google Scholar]
- 12.Peng JM, Lin SH, Yu MC, Hsieh SY. CLIC1 recruits PIP5K1A/C to induce cell-matrix adhesions for tumor metastasis. J Clin Invest. 2021;131:e133525. doi: 10.1172/JCI133525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Astrid E, Andreas H, Christian S, Brigitte H, Elke FS, Ulrich L, Anna M, Gerhard T. A plant homolog of animal chloride intracellular channels (CLICs) generates an ion conductance in heterologous systems. J Biol Chem. 2007;282:8786–8792. doi: 10.1074/jbc.M607241200. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Salas E, Sagar M, Cheng C, Yuspa SH, Weinberg WC. p53 and tumor necrosis factor alpha regulate the expression of a mitochondrial chloride channel protein. J Biol Chem. 1999;274:36488–36497. doi: 10.1074/jbc.274.51.36488. [DOI] [PubMed] [Google Scholar]
- 15.Fujita H, Ishizaki Y, Yanagisawa A, Morita I, Murota SI, Ishikawa K. Possible involvement of a chloride-bicarbonate exchanger in apoptosis of endothelial cells and cardiomyocytes. Cell Biol Int. 1999;23:241–249. doi: 10.1006/cbir.1999.0342. [DOI] [PubMed] [Google Scholar]
- 16.Tanabe S, Wang X, Takahashi N, Uramoto H, Okada Y. HCO(3)(-)-independent rescue from apoptosis by stilbene derivatives in rat cardiomyocytes. FEBS Lett. 2005;579:517–522. doi: 10.1016/j.febslet.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Takahashi N, Uramoto H, Okada Y. Chloride channel inhibition prevents ROS-dependent apoptosis induced by ischemia-reperfusion in mouse cardiomyocytes. Cell Physiol Biochem. 2005;16:147–154. doi: 10.1159/000089840. [DOI] [PubMed] [Google Scholar]
- 18.Malekova L, Tomaskova J, Novakova M, Stefanik P, Kopacek J, Lakatos B, Pastorekova S, Krizanova O, Breier A, Ondrias K. Inhibitory effect of DIDS, NPPB, and phloretin on intracellular chloride channels. Pflugers Arch. 2007;455:349–357. doi: 10.1007/s00424-007-0300-9. [DOI] [PubMed] [Google Scholar]
- 19.Peter B, Ngubane NC, Fanucchi S, Dirr HW. Membrane mimetics induce helix formation and oligomerization of the chloride intracellular channel protein 1 transmembrane domain. Biochemistry. 2013;52:2739–2749. doi: 10.1021/bi4002776. [DOI] [PubMed] [Google Scholar]
- 20.Singh H, Cousin MA, Ashley RH. Functional reconstitution of mammalian ‘chloride intracellular channels’ CLIC1, CLIC4 and CLIC5 reveals differential regulation by cytoskeletal actin. FEBS J. 2007;274:6306–6316. doi: 10.1111/j.1742-4658.2007.06145.x. [DOI] [PubMed] [Google Scholar]
- 21.Jones PM, Curmi PM, Valenzuela SM, George AM. Computational analysis of the soluble form of the intracellular chloride ion channel protein CLIC1. Biomed Res Int. 2013;2013:170586. doi: 10.1155/2013/170586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novarino G, Fabrizi C, Tonini R, Denti MA, Malchiodi-Albedi F, Lauro GM, Sacchetti B, Paradisi S, Ferroni A, Curmi PM, Breit SN, Mazzanti M. Involvement of the intracellular ion channel CLIC1 in microglia-mediated beta-amyloid-induced neurotoxicity. J Neurosci. 2004;24:5322–5330. doi: 10.1523/JNEUROSCI.1170-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashley RH. Challenging accepted ion channel biology: p64 and the CLIC family of putative intracellular anion channel proteins (Review) Mol Membr Biol. 2003;20:1–11. doi: 10.1080/09687680210042746. [DOI] [PubMed] [Google Scholar]
- 24.Singh H, Ashley RH. Redox regulation of CLIC1 by cysteine residues associated with the putative channel pore. Biophys J. 2006;90:1628–1638. doi: 10.1529/biophysj.105.072678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milton RH, Abeti R, Averaimo S, DeBiasi S, Vitellaro L, Jiang L, Curmi PM, Breit SN, Duchen MR, Mazzanti M. CLIC1 function is required for beta-amyloid-induced generation of reactive oxygen species by microglia. J Neurosci. 2008;28:11488–11499. doi: 10.1523/JNEUROSCI.2431-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tulk BM, Kapadia S, Edwards JC. CLIC1 inserts from the aqueous phase into phospholipid membranes, where it functions as an anion channel. Am J Physiol Cell Physiol. 2002;282:C1103–1112. doi: 10.1152/ajpcell.00402.2001. [DOI] [PubMed] [Google Scholar]
- 27.Ulmasov B, Bruno J, Woost PG, Edwards JC. Tissue and subcellular distribution of CLIC1. BMC Cell Biol. 2007;8:8. doi: 10.1186/1471-2121-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Board PG, Coggan M, Watson S, Gage PW, Dulhunty AF. CLIC-2 modulates cardiac ryanodine receptor Ca2+ release channels. Int J Biochem Cell Biol. 2004;36:1599–1612. doi: 10.1016/j.biocel.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 29.Cromer BA, Gorman MA, Hansen G, Adams JJ, Coggan M, Littler DR, Brown LJ, Mazzanti M, Breit SN, Curmi PM, Dulhunty AF, Board PG, Parker MW. Structure of the Janus protein human CLIC2. J Mol Biol. 2007;374:719–731. doi: 10.1016/j.jmb.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 30.Dulhunty A, Gage P, Curtis S, Chelvanayagam G, Board P. The glutathione transferase structural family includes a nuclear chloride channel and a ryanodine receptor calcium release channel modulator. J Biol Chem. 2001;276:3319–3323. doi: 10.1074/jbc.M007874200. [DOI] [PubMed] [Google Scholar]
- 31.Dulhunty AF, Pouliquin P, Coggan M, Gage PW, Board PG. A recently identified member of the glutathione transferase structural family modifies cardiac RyR2 substate activity, coupled gating and activation by Ca2+ and ATP. Biochem J. 2005;390:333–343. doi: 10.1042/BJ20042113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heiss NS, Poustka A. Genomic structure of a novel chloride channel gene, CLIC2, in Xq28. Genomics. 1997;45:224–228. doi: 10.1006/geno.1997.4922. [DOI] [PubMed] [Google Scholar]
- 33.Littler DR, Brown LJ, Breit SN, Perrakis A, Curmi PM. Structure of human CLIC3 at 2 A resolution. Proteins. 2010;78:1594–1600. doi: 10.1002/prot.22675. [DOI] [PubMed] [Google Scholar]
- 34.Money TT, King RG, Wong MH, Stevenson JL, Kalionis B, Erwich JJ, Huisman MA, Timmer A, Hiden U, Desoye G, Gude NM. Expression and cellular localisation of chloride intracellular channel 3 in human placenta and fetal membranes. Placenta. 2007;28:429–436. doi: 10.1016/j.placenta.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Qian Z, Okuhara D, Abe MK, Rosner MR. Molecular cloning and characterization of a mitogen-activated protein kinase-associated intracellular chloride channel. J Biol Chem. 1999;274:1621–1627. doi: 10.1074/jbc.274.3.1621. [DOI] [PubMed] [Google Scholar]
- 36.Duncan RR, Westwood PK, Boyd A, Ashley RH. Rat brain p64H1, expression of a new member of the p64 chloride channel protein family in endoplasmic reticulum. J Biol Chem. 1997;272:23880–23886. doi: 10.1074/jbc.272.38.23880. [DOI] [PubMed] [Google Scholar]
- 37.Singh H, Ashley RH. CLIC4 (p64H1) and its putative transmembrane domain form poorly selective, redox-regulated ion channels. Mol Membr Biol. 2007;24:41–52. doi: 10.1080/09687860600927907. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez-Salas E, Suh KS, Speransky VV, Bowers WL, Levy JM, Adams T, Pathak KR, Edwards LE, Hayes DD, Cheng C, Steven AC, Weinberg WC, Yuspa SH. mtCLIC/CLIC4, an organellular chloride channel protein, is increased by DNA damage and participates in the apoptotic response to p53. Mol Cell Biol. 2002;22:3610–3620. doi: 10.1128/MCB.22.11.3610-3620.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suh KS, Mutoh M, Nagashima K, Fernandez-Salas E, Edwards LE, Hayes DD, Crutchley JM, Marin KG, Dumont RA, Levy JM, Cheng C, Garfield S, Yuspa SH. The organellular chloride channel protein CLIC4/mtCLIC translocates to the nucleus in response to cellular stress and accelerates apoptosis. J Biol Chem. 2004;279:4632–4641. doi: 10.1074/jbc.M311632200. [DOI] [PubMed] [Google Scholar]
- 40.Suh KS, Mutoh M, Gerdes M, Yuspa SH. CLIC4, an intracellular chloride channel protein, is a novel molecular target for cancer therapy. J Investig Dermatol Symp Proc. 2005;10:105–109. doi: 10.1111/j.1087-0024.2005.200402.x. [DOI] [PubMed] [Google Scholar]
- 41.Berryman MA, Goldenring JR. CLIC4 is enriched at cell-cell junctions and colocalizes with AKAP350 at the centrosome and midbody of cultured mammalian cells. Cell Motil Cytoskeleton. 2003;56:159–172. doi: 10.1002/cm.10141. [DOI] [PubMed] [Google Scholar]
- 42.Chuang JZ, Milner TA, Zhu M, Sung CH. A 29 kDa intracellular chloride channel p64H1 is associated with large dense-core vesicles in rat hippocampal neurons. J Neurosci. 1999;19:2919–2928. doi: 10.1523/JNEUROSCI.19-08-02919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Littler DR, Assaad NN, Harrop SJ, Brown LJ, Pankhurst GJ, Luciani P, Aguilar MI, Mazzanti M, Berryman MA, Breit SN, Curmi PM. Crystal structure of the soluble form of the redox-regulated chloride ion channel protein CLIC4. FEBS J. 2005;272:4996–5007. doi: 10.1111/j.1742-4658.2005.04909.x. [DOI] [PubMed] [Google Scholar]
- 44.Proutski I, Karoulias N, Ashley RH. Overexpressed chloride intracellular channel protein CLIC4 (p64H1) is an essential component of novel plasma membrane anion channels. Biochem Biophys Res Commun. 2002;297:317–322. doi: 10.1016/s0006-291x(02)02199-x. [DOI] [PubMed] [Google Scholar]
- 45.Shukla A, Malik M, Cataisson C, Ho Y, Friesen T, Suh KS, Yuspa SH. TGF-beta signalling is regulated by Schnurri-2-dependent nuclear translocation of CLIC4 and consequent stabilization of phospho-Smad2 and 3. Nat Cell Biol. 2009;11:777–784. doi: 10.1038/ncb1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suginta W, Karoulias N, Aitken A, Ashley RH. Chloride intracellular channel protein CLIC4 (p64H1) binds directly to brain dynamin I in a complex containing actin, tubulin and 14-3-3 isoforms. Biochem J. 2001;359:55–64. doi: 10.1042/0264-6021:3590055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suh KS, Mutoh M, Mutoh T, Li L, Ryscavage A, Crutchley JM, Dumont RA, Cheng C, Yuspa SH. CLIC4 mediates and is required for Ca2+-induced keratinocyte differentiation. J Cell Sci. 2007;120:2631–2640. doi: 10.1242/jcs.002741. [DOI] [PubMed] [Google Scholar]
- 48.Suh KS, Crutchley JM, Koochek A, Ryscavage A, Bhat K, Tanaka T, Oshima A, Fitzgerald P, Yuspa SH. Reciprocal modifications of CLIC4 in tumor epithelium and stroma mark malignant progression of multiple human cancers. Clin Cancer Res. 2007;13:121–131. doi: 10.1158/1078-0432.CCR-06-1562. [DOI] [PubMed] [Google Scholar]
- 49.Tung JJ, Hobert O, Berryman M, Kitajewski J. Chloride intracellular channel 4 is involved in endothelial proliferation and morphogenesis in vitro. Angiogenesis. 2009;12:209–220. doi: 10.1007/s10456-009-9139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shanks RA, Larocca MC, Berryman M, Edwards JC, Urushidani T, Navarre J, Goldenring JR. AKAP350 at the Golgi apparatus. II. Association of AKAP350 with a novel chloride intracellular channel (CLIC) family member. J Biol Chem. 2002;277:40973–40980. doi: 10.1074/jbc.M112277200. [DOI] [PubMed] [Google Scholar]
- 51.Gagnon LH, Longo-Guess CM, Berryman M, Shin JB, Saylor KW, Yu H, Gillespie PG, Johnson KR. The chloride intracellular channel protein CLIC5 is expressed at high levels in hair cell stereocilia and is essential for normal inner ear function. J Neurosci. 2006;26:10188–10198. doi: 10.1523/JNEUROSCI.2166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berryman M, Bretscher A. Identification of a novel member of the chloride intracellular channel gene family (CLIC5) that associates with the actin cytoskeleton of placental microvilli. Mol Biol Cell. 2000;11:1509–1521. doi: 10.1091/mbc.11.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berryman M, Bruno J, Price J, Edwards JC. CLIC-5A functions as a chloride channel in vitro and associates with the cortical actin cytoskeleton in vitro and in vivo. J Biol Chem. 2004;279:34794–34801. doi: 10.1074/jbc.M402835200. [DOI] [PubMed] [Google Scholar]
- 54.Redhead C, Sullivan SK, Koseki C, Fujiwara K, Edwards JC. Subcellular distribution and targeting of the intracellular chloride channel p64. Mol Biol Cell. 1997;8:691–704. doi: 10.1091/mbc.8.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Landry D, Sullivan S, Nicolaides M, Redhead C, Edelman A, Field M, al-Awqati Q, Edwards J. Molecular cloning and characterization of p64, a chloride channel protein from kidney microsomes. J Biol Chem. 1993;268:14948–14955. [PubMed] [Google Scholar]
- 56.Friedli M, Guipponi M, Bertrand S, Bertrand D, Neerman-Arbez M, Scott HS, Antonarakis SE, Reymond A. Identification of a novel member of the CLIC family, CLIC6, mapping to 21q22.12. Gene. 2003;320:31–40. doi: 10.1016/s0378-1119(03)00830-8. [DOI] [PubMed] [Google Scholar]
- 57.Griffon N, Jeanneteau F, Prieur F, Diaz J, Sokoloff P. CLIC6, a member of the intracellular chloride channel family, interacts with dopamine D(2)-like receptors. Brain Res Mol Brain Res. 2003;117:47–57. doi: 10.1016/s0169-328x(03)00283-3. [DOI] [PubMed] [Google Scholar]
- 58.Nishizawa T, Nagao T, Iwatsubo T, Forte JG, Urushidani T. Molecular cloning and characterization of a novel chloride intracellular channel-related protein, parchorin, expressed in water-secreting cells. J Biol Chem. 2000;275:11164–11173. doi: 10.1074/jbc.275.15.11164. [DOI] [PubMed] [Google Scholar]
- 59.Piersma SR, Broxterman HJ, Kapci M, de Haas RR, Hoekman K, Verheul HM, Jimenez CR. Proteomics of the TRAP-induced platelet releasate. J Proteomics. 2009;72:91–109. doi: 10.1016/j.jprot.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 60.Yang JY, Jung JY, Cho SW, Choi HJ, Kim SW, Kim SY, Kim HJ, Jang CH, Lee MG, Han J, Shin CS. Chloride intracellular channel 1 regulates osteoblast differentiation. Bone. 2009;45:1175–1185. doi: 10.1016/j.bone.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 61.Myers K, Somanath PR, Berryman M, Vijayaraghavan S. Identification of chloride intracellular channel proteins in spermatozoa. FEBS Lett. 2004;566:136–140. doi: 10.1016/j.febslet.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 62.Salao K, Jiang L, Li H, Tsai VW, Husaini Y, Curmi PM, Brown LJ, Brown DA, Breit SN. CLIC1 regulates dendritic cell antigen processing and presentation by modulating phagosome acidification and proteolysis. Biol Open. 2016;5:620–630. doi: 10.1242/bio.018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qiu MR, Jiang L, Matthaei KI, Schoenwaelder SM, Kuffner T, Mangin P, Joseph JE, Low J, Connor D, Valenzuela SM, Curmi PM, Brown LJ, Mahaut-Smith M, Jackson SP, Breit SN. Generation and characterization of mice with null mutation of the chloride intracellular channel 1 gene. Genesis. 2010;48:127–136. doi: 10.1002/dvg.20590. [DOI] [PubMed] [Google Scholar]
- 64.Averaimo S, Milton RH, Duchen MR, Mazzanti M. Chloride intracellular channel 1 (CLIC1): sensor and effector during oxidative stress. FEBS Lett. 2010;584:2076–2084. doi: 10.1016/j.febslet.2010.02.073. [DOI] [PubMed] [Google Scholar]
- 65.Saeki K, Yasugi E, Okuma E, Breit SN, Nakamura M, Toda T, Kaburagi Y, Yuo A. Proteomic analysis on insulin signaling in human hematopoietic cells: identification of CLIC1 and SRp20 as novel downstream effectors of insulin. Am J Physiol Endocrinol Metab. 2005;289:E419–E428. doi: 10.1152/ajpendo.00512.2004. [DOI] [PubMed] [Google Scholar]
- 66.Uretmen Kagiali ZC, Saner N, Akdag M, Sanal E, Degirmenci BS, Mollaoglu G, Ozlu N. CLIC4 and CLIC1 bridge plasma membrane and cortical actin network for a successful cytokinesis. Life Sci Alliance. 2019;3:e201900558. doi: 10.26508/lsa.201900558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh H. Two decades with dimorphic chloride intracellular channels (CLICs) FEBS Lett. 2010;584:2112–2121. doi: 10.1016/j.febslet.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 68.Bordean L, Chis M, Raica M, Cotoi OS, Ceausu AR, Avram C, Cimpean AM. CLIC1 expression in skin biopsies from patients with rheumatoid and psoriatic arthritis as a potential tool to predict therapy response. In Vivo. 2021;35:2559–2567. doi: 10.21873/invivo.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen X, Deng S, Wang W, Castiglione S, Duan Z, Luo L, Cianci F, Zhang X, Xu J, Li H, Zhao J, Kamau PM, Zhang Z, Mwangi J, Li J, Shu Y, Hu X, Mazzanti M, Lai R. Human antimicrobial peptide LL-37 contributes to Alzheimer’s disease progression. Mol Psychiatry. 2022;27:4790–4799. doi: 10.1038/s41380-022-01790-6. [DOI] [PubMed] [Google Scholar]
- 70.Clerkin JS, Naughton R, Quiney C, Cotter TG. Mechanisms of ROS modulated cell survival during carcinogenesis. Cancer Lett. 2008;266:30–36. doi: 10.1016/j.canlet.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 71.Tung JJ, Kitajewski J. Chloride intracellular channel 1 functions in endothelial cell growth and migration. J Angiogenes Res. 2010;2:23. doi: 10.1186/2040-2384-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He YM, Zhang ZL, Liu QY, Xiao YS, Wei L, Xi C, Nan X. Effect of CLIC1 gene silencing on proliferation, migration, invasion and apoptosis of human gallbladder cancer cells. J Cell Mol Med. 2018;22:2569–2579. doi: 10.1111/jcmm.13499. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Zhang S, Wang XM, Yin ZY, Zhao WX, Zhou JY, Zhao BX, Liu PG. Chloride intracellular channel 1 is overexpression in hepatic tumor and correlates with a poor prognosis. APMIS. 2013;121:1047–1053. doi: 10.1111/apm.12093. [DOI] [PubMed] [Google Scholar]
- 74.Cianci F, Verduci I. Transmembrane chloride intracellular channel 1 (tmCLIC1) as a potential biomarker for personalized medicine. J Pers Med. 2021;11:635. doi: 10.3390/jpm11070635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang X, Liu Y, Wang G, Yao Y, Mei C, Wu X, Ma W, Yuan Y. Up-regulation of CLIC1 activates MYC signaling and forms a positive feedback regulatory loop with MYC in hepatocellular carcinoma. Am J Cancer Res. 2020;10:2355–2370. [PMC free article] [PubMed] [Google Scholar]
- 76.Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh KM, Wrensch MR, Barnholtz-Sloan JS. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16:896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang L, He S, Tu Y, Ji P, Zong J, Zhang J, Feng F, Zhao J, Zhang Y, Gao G. Elevated expression of chloride intracellular channel 1 is correlated with poor prognosis in human gliomas. J Exp Clin Cancer Res. 2012;31:44. doi: 10.1186/1756-9966-31-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Succony L, Rassl DM, Barker AP, McCaughan FM, Rintoul RC. Adenocarcinoma spectrum lesions of the lung: detection, pathology and treatment strategies. Cancer Treat Rev. 2021;99:102237. doi: 10.1016/j.ctrv.2021.102237. [DOI] [PubMed] [Google Scholar]
- 79.Yasuda Y, Nagano T, Jimbo N, Kiriu T, Suraya R, Hazama D, Yamamoto M, Maniwa Y, Nishimura Y, Kobayashi K. Chloride intracellular channel 1 expression is associated with poor prognosis of lung adenocarcinoma. Anticancer Res. 2022;42:271–277. doi: 10.21873/anticanres.15482. [DOI] [PubMed] [Google Scholar]
- 80.Hempel N, Trebak M. Crosstalk between calcium and reactive oxygen species signaling in cancer. Cell Calcium. 2017;63:70–96. doi: 10.1016/j.ceca.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee JR, Lee JY, Kim HJ, Hahn MJ, Kang JS, Cho H. The inhibition of chloride intracellular channel 1 enhances Ca(2+) and reactive oxygen species signaling in A549 human lung cancer cells. Exp Mol Med. 2019;51:1–11. doi: 10.1038/s12276-019-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gordeeva AV, Zvyagilskaya RA, Labas YA. Cross-talk between reactive oxygen species and calcium in living cells. Biochemistry (Mosc) 2003;68:1077–1080. doi: 10.1023/a:1026398310003. [DOI] [PubMed] [Google Scholar]
- 83.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 84.Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, Laversanne M, Ferlay J, Arnold M. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163:649–658. e642. doi: 10.1053/j.gastro.2022.05.054. [DOI] [PubMed] [Google Scholar]
- 85.Geng H, Feng C, Sun Z, Fan X, Xie Y, Gu J, Fan L, Liu G, Li C, Thorne RF, Zhang XD, Li X, Liu X. Chloride intracellular channel 1 promotes esophageal squamous cell carcinoma proliferation via mTOR signalling. Transl Oncol. 2023;27:101560. doi: 10.1016/j.tranon.2022.101560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 87.Chen CD, Wang CS, Huang YH, Chien KY, Liang Y, Chen WJ, Lin KH. Overexpression of CLIC1 in human gastric carcinoma and its clinicopathological significance. Proteomics. 2007;7:155–167. doi: 10.1002/pmic.200600663. [DOI] [PubMed] [Google Scholar]
- 88.Zheng DL, Huang QL, Zhou F, Huang QJ, Lin JY, Lin X. PA28beta regulates cell invasion of gastric cancer via modulating the expression of chloride intracellular channel 1. J Cell Biochem. 2012;113:1537–1546. doi: 10.1002/jcb.24022. [DOI] [PubMed] [Google Scholar]
- 89.Chakraborty E, Sarkar D. Emerging therapies for hepatocellular carcinoma (HCC) Cancers (Basel) 2022;14:2789. doi: 10.3390/cancers14112798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, Reddy KR, Harnois D, Llovet JM, Normolle D, Dalhgren J, Chia D, Lok AS, Wagner PD, Srivastava S, Schwartz M. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110–118. doi: 10.1053/j.gastro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Bisceglie AM, Morgan TR, Kim HY, Lee WM, Bonkovsky HL, Dienstag JL HALT-C Trial Group. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wei X, Li J, Xie H, Wang H, Wang J, Zhang X, Zhuang R, Lu D, Ling Q, Zhou L, Xu X, Zheng S. Chloride intracellular channel 1 participates in migration and invasion of hepatocellular carcinoma by targeting maspin. J Gastroenterol Hepatol. 2015;30:208–216. doi: 10.1111/jgh.12668. [DOI] [PubMed] [Google Scholar]
- 93.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 94.O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–1425. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 95.Wang P, Zhang C, Yu P, Tang B, Liu T, Cui H, Xu J. Regulation of colon cancer cell migration and invasion by CLIC1-mediated RVD. Mol Cell Biochem. 2012;365:313–321. doi: 10.1007/s11010-012-1271-5. [DOI] [PubMed] [Google Scholar]
- 96.Law AY, Wong CK. Stanniocalcin-2 promotes epithelial-mesenchymal transition and invasiveness in hypoxic human ovarian cancer cells. Exp Cell Res. 2010;316:3425–3434. doi: 10.1016/j.yexcr.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 97.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Binker MG, Binker-Cosen AA, Richards D, Gaisano HY, de Cosen RH, Cosen-Binker LI. Hypoxia-reoxygenation increase invasiveness of PANC-1 cells through Rac1/MMP-2. Biochem Biophys Res Commun. 2010;393:371–376. doi: 10.1016/j.bbrc.2010.01.125. [DOI] [PubMed] [Google Scholar]
- 99.Kokura S, Yoshida N, Imamoto E, Ueda M, Ishikawa T, Uchiyama K, Kuchide M, Naito Y, Okanoue T, Yoshikawa T. Anoxia/reoxygenation down-regulates the expression of E-cadherin in human colon cancer cell lines. Cancer Lett. 2004;211:79–87. doi: 10.1016/j.canlet.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 100.Wang P, Zeng Y, Liu T, Zhang C, Yu PW, Hao YX, Luo HX, Liu G. Chloride intracellular channel 1 regulates colon cancer cell migration and invasion through ROS/ERK pathway. World J Gastroenterol. 2014;20:2071–2078. doi: 10.3748/wjg.v20.i8.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591–1602. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]
- 102.Wistuba II, Gazdar AF. Gallbladder cancer: lessons from a rare tumour. Nat Rev Cancer. 2004;4:695–706. doi: 10.1038/nrc1429. [DOI] [PubMed] [Google Scholar]
- 103.Balachandran P, Agarwal S, Krishnani N, Pandey CM, Kumar A, Sikora SS, Saxena R, Kapoor VK. Predictors of long-term survival in patients with gallbladder cancer. J Gastrointest Surg. 2006;10:848–854. doi: 10.1016/j.gassur.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 104.Kim WS, Choi DW, You DD, Ho CY, Heo JS, Choi SH. Risk factors influencing recurrence, patterns of recurrence, and the efficacy of adjuvant therapy after radical resection for gallbladder carcinoma. J Gastrointest Surg. 2010;14:679–687. doi: 10.1007/s11605-009-1140-z. [DOI] [PubMed] [Google Scholar]
- 105.Zhou N, Cheng W, Peng C, Liu Y, Jiang B. Decreased expression of hsa-miR-372 predicts poor prognosis in patients with gallbladder cancer by affecting chloride intracellular channel 1. Mol Med Rep. 2017;16:7848–7854. doi: 10.3892/mmr.2017.7520. [DOI] [PubMed] [Google Scholar]
- 106.Nikaki A, Piperi C, Papavassiliou AG. Role of microRNAs in gliomagenesis: targeting miRNAs in glioblastoma multiforme therapy. Expert Opin Investig Drugs. 2012;21:1475–1488. doi: 10.1517/13543784.2012.710199. [DOI] [PubMed] [Google Scholar]
- 107.International Agency for Research on Cancer: Pancreas. GLOBOCAN. 2020. https://gco.iarc.fr/today/data/factsheets/cancers/39-All-Cancers-fact-sheet.pdf.
- 108.Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sanchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V, Kost-Alimova M, Muller F, Colla S, Nezi L, Genovese G, Deem AK, Kapoor A, Yao W, Brunetto E, Kang Y, Yuan M, Asara JM, Wang YA, Heffernan TP, Kimmelman AC, Wang H, Fleming JB, Cantley LC, DePinho RA, Draetta GF. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rosenfeldt MT, O’Prey J, Morton JP, Nixon C, MacKay G, Mrowinska A, Au A, Rai TS, Zheng L, Ridgway R, Adams PD, Anderson KI, Gottlieb E, Sansom OJ, Ryan KM. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504:296–300. doi: 10.1038/nature12865. [DOI] [PubMed] [Google Scholar]
- 110.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 111.Xia J, Wang Q, Ju F, Luo X, Wang F, Zhou Y, Huang H, Wang H, Bao X. Chloride intracellular channel 1 is a potential biomarker for breast cancer. Breast Cancer (Dove Med Press) 2022;14:247–258. doi: 10.2147/BCTT.S367519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jessmon P, Boulanger T, Zhou W, Patwardhan P. Epidemiology and treatment patterns of epithelial ovarian cancer. Expert Rev Anticancer Ther. 2017;17:427–437. doi: 10.1080/14737140.2017.1299575. [DOI] [PubMed] [Google Scholar]
- 113.Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69:280–304. doi: 10.3322/caac.21559. [DOI] [PubMed] [Google Scholar]
- 114.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 115.Shi ZH, Zhao C, Wu H, Wang W, Liu XM. CLIC1 protein: a candidate prognostic biomarker for malignant-transformed hydatidiform moles. Int J Gynecol Cancer. 2011;21:153–160. doi: 10.1097/IGC.0b013e3182022997. [DOI] [PubMed] [Google Scholar]
- 116.Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, Heng DY, Larkin J, Ficarra V. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. doi: 10.1038/nrdp.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Q, Tang H, Luo X, Chen J, Zhang X, Li X, Li Y, Chen Y, Xu Y, Han S. Immune-associated gene signatures serve as a promising biomarker of immunotherapeutic prognosis for renal clear cell carcinoma. Front Immunol. 2022;13:890150. doi: 10.3389/fimmu.2022.890150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ye Y, Yin M, Huang B, Wang Y, Li X, Lou G. CLIC1 a novel biomarker of intraperitoneal metastasis in serous epithelial ovarian cancer. Tumour Biol. 2015;36:4175–9. doi: 10.1007/s13277-015-3052-8. [DOI] [PubMed] [Google Scholar]
- 119.Makhov P, Joshi S, Ghatalia P, Kutikov A, Uzzo RG, Kolenko VM. Resistance to systemic therapies in clear cell renal cell carcinoma: mechanisms and management strategies. Mol Cancer Ther. 2018;17:1355–1364. doi: 10.1158/1535-7163.MCT-17-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Duran I, Lambea J, Maroto P, González-Larriba JL, Flores L, Granados-Principal S, Graupera M, Sáez B, Vivancos A, Casanovas O. Resistance to targeted therapies in renal cancer: the importance of changing the mechanism of action. Target Oncol. 2017;12:19–35. doi: 10.1007/s11523-016-0463-4. [DOI] [PubMed] [Google Scholar]
- 121.Cuddapah VA, Sontheimer H. Ion channels and transporters [corrected] in cancer. 2. Ion channels and the control of cancer cell migration. Am J Physiol Cell Physiol. 2011;301:C541–9. doi: 10.1152/ajpcell.00102.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pardo LA, Stühmer W. The roles of K(+) channels in cancer. Nat Rev Cancer. 2014;14:39–48. doi: 10.1038/nrc3635. [DOI] [PubMed] [Google Scholar]
- 123.Xu Y, Xu J, Feng J, Li J, Jiang C, Li X, Zou S, Wang Q, Li Y. Expression of CLIC1 as a potential biomarker for oral squamous cell carcinoma: a preliminary study. Onco Targets Ther. 2018;11:8073–8081. doi: 10.2147/OTT.S181936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Singha B, Harper SL, Goldman AR, Bitler BG, Aird KM, Borowsky ME, Cadungog MG, Liu Q, Zhang R, Jean S, Drapkin R, Speicher DW. CLIC1 and CLIC4 complement CA125 as a diagnostic biomarker panel for all subtypes of epithelial ovarian cancer. Sci Rep. 2018;8:14725. doi: 10.1038/s41598-018-32885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Peretti M, Angelini M, Savalli N, Florio T, Yuspa SH, Mazzanti M. Chloride channels in cancer: focus on chloride intracellular channel 1 and 4 (CLIC1 AND CLIC4) proteins in tumor development and as novel therapeutic targets. Biochim Biophys Acta. 2015;1848:2523–2531. doi: 10.1016/j.bbamem.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 126.Nesiu A, Cimpean AM, Ceausu RA, Adile A, Ioiart I, Porta C, Mazzanti M, Camerota TC, Raica M. Intracellular chloride ion channel protein-1 expression in clear cell renal cell carcinoma. Cancer Genomics Proteomics. 2019;16:299–307. doi: 10.21873/cgp.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gurski LA, Knowles LM, Basse PH, Maranchie JK, Watkins SC, Pilch J. Relocation of CLIC1 promotes tumor cell invasion and colonization of fibrin. Mol Cancer Res. 2015;13:273–280. doi: 10.1158/1541-7786.MCR-14-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ummanni R, Junker H, Zimmermann U, Venz S, Teller S, Giebel J, Scharf C, Woenckhaus C, Dombrowski F, Walther R. Prohibitin identified by proteomic analysis of prostate biopsies distinguishes hyperplasia and cancer. Cancer Lett. 2008;266:171–185. doi: 10.1016/j.canlet.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 129.Pugh TJ, Choi S, Nguyen QN, Gillin MT, Ron Zhu X, Palmer MB, Lee AK. Proton beam therapy for the treatment of prostate cancer. Pract Radiat Oncol. 2013;3:e87–94. doi: 10.1016/j.prro.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 130.Shukla S, Fu P, Gupta S. Apigenin induces apoptosis by targeting inhibitor of apoptosis proteins and Ku70-Bax interaction in prostate cancer. Apoptosis. 2014;19:883–894. doi: 10.1007/s10495-014-0971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moul JW, Mouraviev V, Sun L, Schroeck FR, Polascik TJ. Prostate cancer: the new landscape. Curr Opin Urol. 2009;19:154–160. doi: 10.1097/mou.0b013e328323f5d6. [DOI] [PubMed] [Google Scholar]
- 132.Tian Y, Guan Y, Jia Y, Meng Q, Yang J. Chloride intracellular channel 1 regulates prostate cancer cell proliferation and migration through the MAPK/ERK pathway. Cancer Biother Radiopharm. 2014;29:339–344. doi: 10.1089/cbr.2014.1666. [DOI] [PubMed] [Google Scholar]


