Abstract
As is well understood that malignant tumour progression requires additional blood vessels to provide the nutrients necessary for growth. Many patients with advanced hepatocellular carcinoma (aHCC) experience disease progression after treatment with lenvatinib (Lenva) and immune checkpoint inhibitors (ICIs). Therefore, we designed a double-arm retrospective study to evaluate the antitumour activity of additional bevacizumab (Beva, an anti-vascular endothelial growth factor-targeting drug) as a means to reduce the blood vessels needed for tumour growth. Compared with the control group, the group that received Beva had prolonged progression-free survival (PFS) and a trend toward a benefit for overall survival duration. This study aimed to evaluate the anticancer effect of Beva in patients with aHCC who experienced tumour progression after treatment with Lenva+ICIs. From April 2021 to March 2023, we retrospectively included 20 patients as the experimental group and 21 patients as the control group. The patients in the experimental group experienced disease progression after receiving targeted therapy and ICIs, after which we added Beva to the treatment. The patients in the control group only received targeted therapy and ICIs. The efficacy endpoints were overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR), which were evaluated according to RECIST v1.1. Adverse events were assessed using NCI-CTCAE v5.0. Ultimately, 20 patients with aHCC in the experimental group of received Beva after disease progression, compared with 21 patients in the control group. The median OS was 12.6 mo (95% CI: 6.8-18.7) vs. 9.3 mo (95% CI: 4.3-14.4), and the median PFS was 6.9 mo (95% CI: 6.4-7.4) vs. 4.1 mo (95% CI: 2.4-5.8). The ORR for all patients was 5%, and the DCR for all patients was 70.0%. The median follow-up time for all patients was 7.5 mo (95% CI: 5.0-10.0). All patients had adverse events, but no fatal adverse events were observed. In conclusion, Bevacizumab is a drug resistant treatment option for patients with advanced hepatocellular carcinoma after Lenva+PD-1/PD-L1 treatment.
Keywords: Bevacizumab, immune checkpoint inhibitors, lenvatinib, advanced hepatocellular carcinoma, targeted immunotherapy, tumor resistance
Introduction
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, accounting for approximately 90% of all primary liver cancers [1]. In China, HCC has risen to the second position among all neoplasms [2] and exhibits malignant behaviour, including rapid metastasis, fast development, and transient free-tumour survival [3,4]. Moreover, more than 80% of HCC cases occur in patients with hepatitis and liver cirrhosis. For the treatment of HCC, only 20% of HCC patients can be treated with surgical resection, liver transplantation, or radiofrequency ablation, while patients with advanced HCC cannot be treated with radical treatment, and their survival rate is gradually declining [5]. Since the multitarget kinase inhibitor lenvatinib (Lenva) replaced sorafenib as the first-line treatment for patients with unresectable HCC, it has brought new hope for the overall survival (OS) of patients with advanced HCC [6]. The use of immune checkpoint inhibitors (ICIs) has achieved a relatively high objective response rate (ORR) and disease control rate (DCR) [7]. However, due to the heterogeneity and drug resistance of tumours [8,9], many patients still experience progressive disease (PD) after targeted immunotherapy, so it is urgent to find a new combination treatment after disease progression.
In recent years, China has extensively explored the clinical treatment of hepatobiliary tumours and proposed an ideal model (stereoscopic phase) [10]. HCC is a highly heterogeneous tumour. Many therapeutic options have been established, such as tyrosine kinase inhibitors (TKIs) plus PD-1 inhibitors combined with stereotactic therapy for extrahepatic metastasis, which may allow patients with extrahepatic metastasis who are not suitable for surgical intervention to become suitable for surgical treatment [11]. Alternatively, conversion surgery for unresectable HCC may be attempted after treatment in the new era of targeted therapy plus ICIs [12].
Lenva is a multitarget receptor tyrosine kinase (RTK) inhibitor [13]. In the REFLECT study, Lenva was compared head-to-head with sorafenib as first-line treatment for advanced liver cancer. The effective ORR was more than twice that of sorafenib (24.1% vs. 9.2%), OS was comparable between the two drugs (13.6 vs. 12.3 months), and PFS was significantly better than that of sorafenib (7.4 vs. 3.7 months) [6]. Moreover, according to the population subgroup analysis, the OS advantage of using Lenva in Chinese patients was more prominent, with OS times of 15.0 and 10.2 months, respectively. It is a kind of targeted drug suitable for the treatment of Chinese HCC patients. PD-1 and PD-L1 are type I transmembrane proteins [14]. The ligands of PD-1 include PD-L1 and PD-L2. PD-1 can inhibit the activity of T lymphocytes, induce antigen tolerance, and promote the apoptosis of T lymphocytes, thus inhibiting or terminating the immune response and preventing autoimmune diseases. Therefore, the application of specific monoclonal antibodies to block the binding of PD-1 and PD-L1 can enhance the proliferation and killing function of T lymphocytes and exert an antitumour effect [15]. Antiangiogenic targeted therapy can act on different links of the tumour immune cycle, normalize tumour vasculature and increase T-cell infiltration in tumour cells. Inhibition of immunosuppressive cell activity can reprogram the tumour microenvironment from an immunosuppressive state to an immune-activated state to provide a suitable tumour microenvironment for immunotherapy, and combination treatment with immunotherapy can synergistically enhance the antitumour effect [16].
Bevacizumab (Beva) is a human monoclonal antibody IgG1 produced by recombinant DNA technology [17,18]. In the IMbrave150 study, atezolizumab plus Beva (T+A) combination therapy significantly extended OS and PFS and directly improved the 12-month survival rate to 67.2% in patients with advanced liver cancer compared with sorafenib alone, which had a 54.6% survival rate [19,20]. This shows that the use of Beva can enhance the effect of immunotherapy, prolong the survival time of patients, and reduce drug resistance after treatment.
Material and methods
Research design
This was a single-centre, dual-arm retrospective real-world study. All patients were admitted to the Peking Union Liver Surgery Department and were regularly followed up by our team. Since this was a retrospective study, all patients without informed consent were exempted from informed consent by the ethics committee of Peking Union Medical College Hospital (PUMCH). This study complied with the Declaration of Helsinki and was approved by the abovementioned ethics committee.
Study participants
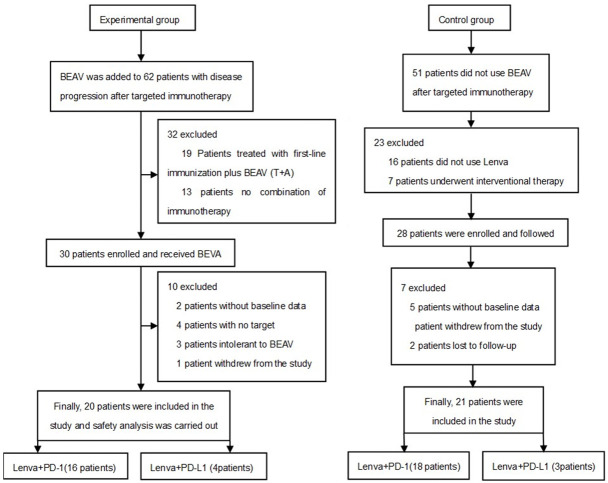
From April 2021 to March 2023, a total of 20 patients were included in the experimental group, while 21 patients were included in the control group. The patient screening process is shown in Figure 1.
Figure 1.
Research workflow.
First-line treatment
Based on the data on sorafenib in the SHARP and Oriental studies [21], 3 patients chose to receive sorafenib as first-line treatment, and 17 patients chose to receive Lenva because of the promising data from the REFLECT study [6]. All 21 patients in the control group were treated with Lenva as first-line treatment.
Second-line treatment
Due to the development of resistance to sorafenib and Lenva monotherapy over time, which results in PD, we added PD-1/PD-L1-targeting agents as second-line treatment. In total, 16 patients in the experimental group received PD-1-targeting therapy, and 4 patients received PD-L1-targeting therapy due to positive genetic testing. In the control group, 18 patients received PD-1-targeting therapy, and 3 patients received PD-L1-targeting therapy due to positive genetic testing.
Third-line treatment
Since the data of Lenva were better than those of sorafenib, 6 we replaced sorafenib with Lenva for three patients in the experimental group. All of these patients developed disease progression after treatment with Lenva+PD-1/PD-L1-targeting therapy. The RECIST v1.1 criteria were used to rigidly evaluate disease progression [22], and we added Beva as a third-line treatment to the existing regimen. Every three weeks, 15 mg/kg was administered via intravenous infusion. The control group of patients received best supportive treatment.
Endpoints and follow-up
In order to ensure the reliability of the data, the study team conducted regular follow-up evaluations of the enrolled patients every two cycles (41 days). The primary endpoint was overall survival (OS, defined as the time between the start of Beva treatment and death). Secondary endpoints were progression-free survival (PFS, defined as the time between the start of Beva treatment and disease recurrence or progression or death), objective response rate (ORR), and disease control rate (DCR). Complete response (CR), partial response (PR), or stable disease (SD) for six consecutive months or more was defined as a clinically beneficial response (CBR). All secondary endpoints were rigorously evaluated using RECIST V1.5, and adverse events (AEs) were evaluated for safety using NCI-CTCAE v5.0 [23].
Statistical analysis
Kaplan-Meier analysis was used for survival analysis, and R v4.2.2 was used for statistical analysis. Plots were generated using R v4.2.2 and Excel 2019.
Results
Baseline features
The median age in the experimental group was 56 years; 14 (70.0%) patients were male, and 15 patients were infected with hepatitis B virus (HBV), 5 patients were not infected with HBV. The median age in the control group was 58 years; 18 (85.7%) patients were male, and 18 (85.7%) patients were infected with hepatitis B virus. Other baseline data are shown in Table 1.
Table 1.
Patient and treatment characteristics
| Characteristics | Varieties | BEAV | Non BEAV | P value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| N=20 N% | N=21 N% | |||||
| Median age | 56 [35-67] | 58 [35-80] | ||||
| Gender | 0.4021 | |||||
| Male | 14 | 70.0% | 18 | 85.7% | ||
| Female | 6 | 30.0% | 3 | 14.3% | ||
| Hepatitis virus | 0.6381 | |||||
| HBV | 15 | 75.0% | 18 | 85.7% | ||
| N | 5 | 25.0% | 3 | 14.3% | ||
| AFP (ng/mL) | 0.6571 | |||||
| <400 | 13 | 65.0% | 16 | 76.2% | ||
| ≥400 | 7 | 35.0% | 5 | 23.8% | ||
| Child-Pugh (class) | 0.2381 | |||||
| A | 13 | 65.0% | 18 | 85.7% | ||
| B | 7 | 35.0% | 3 | 14.3% | ||
| Tumor distribution | 0.0411 | |||||
| Solitary | 7 | 35.0% | 1 | 4.8% | ||
| Multifocal | 13 | 65.0% | 20 | 95.2% | ||
| ECOG score | 0.9061 | |||||
| 0 | 8 | 40.0% | 7 | 33.3% | ||
| 1 | 12 | 60.0% | 14 | 66.7% | ||
| TNM (stage) | 0.0691 | |||||
| IVA | 9 | 45.0% | 3 | 14.3% | ||
| IVB | 11 | 55.0% | 18 | 85.7% | ||
| Previous therapy | First-line treatment | |||||
| Sorafenib | 3 | 15.0% | ||||
| Lenvatinib | 17 | 85.0% | 21 | 100% | ||
| Second-line therapy | ||||||
| PD-1 (Tirelizumab) | 16 | 80.0% | 18 | 85.7% | ||
| PDL1 (Durvalumab) | 4 | 20.0% | 3 | 14.3% | ||
| Third-line treatment | ||||||
| Bevacizumab | 20 | 100.0% | 0 | 0% | ||
| Metastatic site | Intrahepatic | 8 | 40.0% | 20 | 95.2% | |
| Lymph nodes | 8 | 40.0% | 7 | 33.3% | ||
| Lung | 6 | 30.0% | 3 | 14.3% | ||
| Bone | 5 | 25.0% | 3 | 14.3% | ||
χ 2 test.
HBV, hepatitis B virus; N, no HBV infection; AFP, alpha-fetoprotein; TNM (stage), American Joint Committee on Cancer-Tumor Node Metastasis staging; ECOG score, American Joint Committee on Cancer-Tumor Node Metastasis staging.
Overall efficacy
As of March 2023, in the aHCC experimental group, 16 patients received Lenva+tislelizumab (Tis), and 3 patients received Lenva+durvalumab (Durva) and then Beva. However, 21 patients in the control group experienced disease progression after receiving Lenva+Tis treatment, after which they received only best supportive care due to personal reasons. The median follow-up time for all patients was 7.5 mo (95% CI: 5.0-10.0), the median OS for all patients was 9.4 mo (95% CI: 6.4-12.4) (Figure 2A), the median PFS for all patients was 5.6 mo (95% CI: 4.7-6.5) (Figure 2B), the median OS in the experimental group was 12.6 mo (95% CI: 6.5-18.7), the median OS in the control group was 9.3 mo (95% CI: 4.3-14.4) (Figure 2C), the median PFS in the experimental group was 6.9 mo (95% CI: 6.4-7.4), and the median PFS in the control group was 4.1 mo (95% CI: 2.4-5.8) (Figure 2D). The treatment duration of all patients in the experimental group is shown in Figure 3. The tumour size of 9 (45.0%) patients decreased from baseline (Figure 4), with 1 (5.0%) patient achieving partial response (PR), 13 (65.0%) patients achieving stable disease (SD), and 6 (30.0%) patients having progressive disease (PD) (Table 2).
Figure 2.
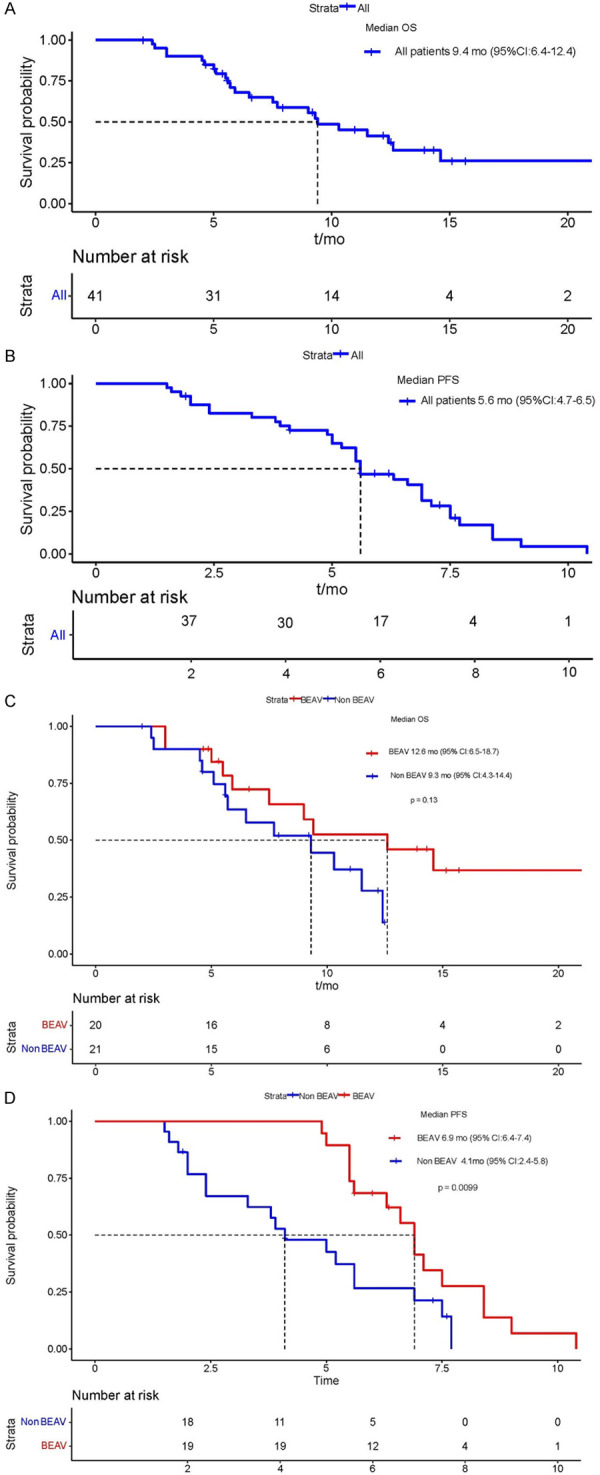
A: Median OS time for all patients; B: Median PFS time for all patients; C: The OS times of Beav group and Non Beav group; D: The PFS times of Beav group and Non Beav group.
Figure 3.
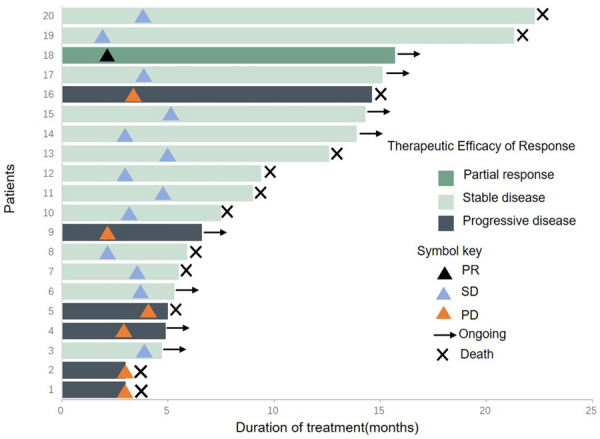
Duration of treatment and optimal evaluation time for experimental group patients. Complete response (CR), partial response (PR), or stable disease (SD).
Figure 4.
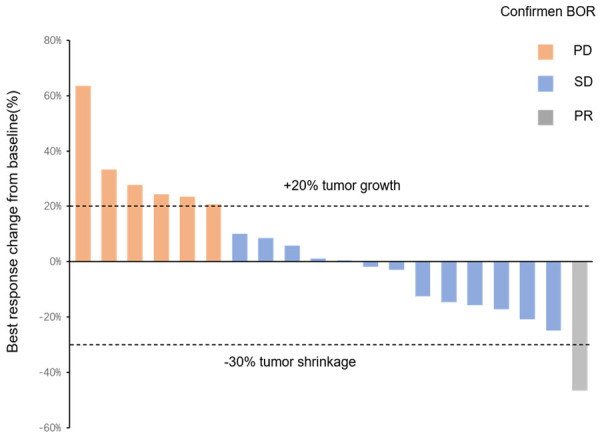
The maximum percentage change between the total diameter of the target lesion and baseline, BOR, best overall response; Complete response (CR), partial response (PR), or stable disease (SD).
Table 2.
Confirmed best overall response rates according to RECIST v1.1 (FAS)
| Bevacizumab | |
|---|---|
| N=20 | |
| Best overall response, n (%) | |
| CR | 0 (0%) |
| PR | 1 (5.0%) |
| SD | 13 (65.0%) |
| PD | 6 (30.0%) |
| ORR (CR+PR), n (%) | 1 (5.0%) |
| Disease control rate (CR+PR+SD), n (%) | 14 (70.0%) |
Abbreviations: CR, Complete reaction; PR, partial reaction; SD, stable disease; PD, progressive disease.
Adverse events
All patients experienced adverse events: 55.0% of patients in the experimental group experienced grade 3-4 adverse events, but no deaths related to adverse events occurred. The most common adverse event was hypertension (60%), and one patient experienced gastric perforation but continued to receive Beva treatment. The most common adverse event in the control group was liver dysfunction (42.9); 38.1% of patients experienced grade 3-4 adverse reactions, but there were no deaths related to adverse events (Table 3).
Table 3.
Summary of adverse events
| Toxicity | Number of patients (BEAV) N=20 (%) | Number of patients (Non BEAV) N=21 (%) | ||
|---|---|---|---|---|
|
|
|
|||
| Any grade | Grade 3-4 | Any grade | Grade 3-4 | |
| Summary | 20 (100%) | 11 (55.0%) | 21 (100%) | 8 (38.1%) |
| Hypertension | 12 (60.0%) | 1 (5.0%) | 7 (33.3%) | 1 (4.8%) |
| Thrombocytopenia | 10 (50.0%) | 1 (5.0%) | 6 (28.6%) | 0 (0%) |
| Fatigue | 9 (45.0%) | 0 (0%) | 6 (28.6%) | 1 (4.8%) |
| Abnormal liver function | 8 (40.0%) | 1 (5.0%) | 9 (42.9%) | 1 (4.8%) |
| Nausea | 7 (35.0%) | 0 (0%) | 5 (23.8%) | 0 (0%) |
| Mucosal inflammation | 7 (35.0%) | 1 (5.0%) | 7 (33.3%) | 0 (0%) |
| Anemia | 7 (35.0%) | 1 (5.0%) | 6 (28.6%) | 1 (4.8%) |
| Proteinuria | 6 (30.0%) | 1 (5.0%) | 5 (23.8%) | 0 (0%) |
| Thrombocytopenia | 6 (30.0%) | 0 (0%) | 6 (28.6%) | 0 (0%) |
| Diarrhea | 6 (30.0%) | 1 (5.0%) | 5 (23.8%) | 1 (4.8%) |
| Vomiting | 6 (30.0%) | 0 (0%) | 5 (23.8%) | 1 (4.8%) |
| Asthenia | 6 (30.0%) | 1 (5.0%) | 7 (33.3%) | 0 (0%) |
| Abdominal pain | 6 (30.0%) | 1 (5.0%) | 8 (38.1%) | 0 (0%) |
| Constipation | 5 (25.0%) | 0 (0%) | 4 (19.0%) | 1 (4.8%) |
| Skin rash | 5 (25.0%) | 1 (5.0%) | 4 (19.0%) | 0 (0%) |
| Leukopenia | 5 (25.0%) | 0 (5.0%) | 4 (19.0%) | 0 (0%) |
| Pain in extremity | 5 (25.0%) | 0 (0%) | 3 (12.8%) | 0 (0%) |
| Epistaxis | 4 (20.0%) | 0 (0%) | 2 (9.5%) | 1 (4.8%) |
| Gastrointestinal perforation | 1 (5.0%) | 1 (5.0%) | 0 (0%) | 0 (0%) |
Discussion
Our study added Beva as a third-line therapy for advanced HCC patients with disease progression after treatment with Lenva+PD-1/PD-L1-targeting therapy. The ORR was 5.0%, and the DCR was 70.0%, indicating that the treatment was effective. This is the only cohort analysis of adding Beva after disease progression following targeted immunotherapy. All patients experienced AEs, the most common of which was hypertension (12/20, 60.0%). A total of 55.0% (11/20) patients had grade 3/4 AEs, and no grade 5 AEs occurred. All AEs were reversible, and there was no risk of death for patients regarding safety.
The REFLECT study enrolled 288 patients (approximately 83% were HBV-related liver cancer patients) in the Chinese subgroup. Further analysis of the Chinese subgroup showed that the median overall survival (mOS) was 15.0 months in the Lenva group compared with 10.2 months in the sorafenib group. Of note, the mOS of the Lenva group was five months longer in HBV-associated liver cancer than the sorafenib group in Chinese patients (14.9 months versus 9.9 months) [6]. Since HBV infection is China’s leading cause of liver cancer, we switched sorafenib to Lenva in 3 patients.
The Keynote-240 phase III trial failed to show a statistically significant OS benefit [24], as did the phase III CheckMate-459 study [25]. This indicates that PD-1 monotherapy cannot bring long-term benefits to patients. In contrast, in the Keynote-524 study [26], the ORR was 46%. Therefore, we believe that Lenva changes the immune microenvironment of tumours, leading to sensitized immunotherapy and prolonging the survival of patients.
For the choice of PD-1, we did not directly choose first-line drug treatment but instead chose Tis because, the ORR and DCR were 47.7% (21/44) and 84.1% (37/44) for combination treatment with Tis in aHCC in the 141P clinical trial [27]. The final reports of the RATIONALE-301 of LBA36 trials showed that the mOS of the groups treated with Tis and sorafenib were 15.9 months and 14.1 months, respectively [28,29]. Therefore, we believe Tis is more suitable for Chinese people. However, 4 patients chose Deva treatment because of PD-L1-positive gene detection [30].
In the Keynote-240 and CheckMate-459 clinical trials, the benefit of PD-1 inhibitor monotherapy in patients with HCC was less [24,25], but in the IMbrave150 clinical trial, the December OS rate of A+T was 67.2% vs. 54.6%. The median PFS was 6.8 months vs. 4.3 months [19]. Based on the above data, we believe that Beva changed the immune microenvironment of tumours and allowed patients to gain survival benefits. Therefore, Beva was added to the treatment of the included patients to change the immune microenvironment and provide better survival benefits.
Our study was a single-centre, double-arm retrospective study, so the data were limited. However, our study design and protocol showed promising antitumour activity in patients with advanced HCC and a tendency to prolong OS in patients. HCC is an immune cold tumour. In the case of progression in the treatment of frontline lenva+PD-1/PD-L1, we added Beva to sensitize the immune microenvironment again. It is hoped that more verification can be obtained in future treatment programs.
Acknowledgements
We acknowledge the work of all authors who participated in this study. This work was supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-061) (2021-I2M-1-003), CSCO-hengrui Cancer Research Fund (Y-HR2019-0239) (Y-HR2020MS-0415) (Y-HR2020QN-0414), CSCO-MSD Cancer Research Fund (Y-MSDZD2021-0213), National Ten-thousand Talent Program, International Natural Science Foundation (81960125) and Guizhou Provincial Department of Science and Technology ([2020] 1Y302).
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:7. doi: 10.1038/s41572-021-00245-6. [DOI] [PubMed] [Google Scholar]
- 4.Zheng R, Qu C, Zhang S, Zeng H, Sun K, Gu X, Xia C, Yang Z, Li H, Wei W, Chen W, He J. Liver cancer incidence and mortality in China: temporal trends and projections to 2030. Chin J Cancer Res. 2018;30:571–579. doi: 10.21147/j.issn.1000-9604.2018.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang B, Zhang B, Zhang Z, Huang Z, Chen Y, Chen M, Bie P, Peng B, Wu L, Wang Z, Li B, Fan J, Qin L, Chen P, Liu J, Tang Z, Niu J, Yin X, Li D, He S, Jiang B, Mao Y, Zhou W, Chen X. 42,573 cases of hepatectomy in China: a multicenter retrospective investigation. Sci China Life Sci. 2018;61:660–670. doi: 10.1007/s11427-017-9259-9. [DOI] [PubMed] [Google Scholar]
- 6.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 7.Xu F, Jin T, Zhu Y, Dai C. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37:110. doi: 10.1186/s13046-018-0777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y, Liu Z, Xu X. Molecular subtyping of hepatocellular carcinoma: a step toward precision medicine. Cancer Commun (Lond) 2020;40:681–693. doi: 10.1002/cac2.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei CY, Zhu MX, Zhang PF, Huang XY, Wan JK, Yao XZ, Hu ZT, Chai XQ, Peng R, Yang X, Gao C, Gao J, Wang SW, Zheng YM, Tang Z, Gao Q, Zhou J, Fan JB, Ke AW, Fan J. PKCalpha/ZFP64/CSF1 axis resets the tumor microenvironment and fuels anti-PD1 resistance in hepatocellular carcinoma. J Hepatol. 2022;77:163–176. doi: 10.1016/j.jhep.2022.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Lin J, Zhao H. Systemic management for patients with hepatobiliary tumors in a multi-dimensional view. Hepatobiliary Surg Nutr. 2019;8:626–628. doi: 10.21037/hbsn.2019.07.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X, Xu H, Zuo B, Yang X, Bian J, Long J, Wang D, Zhang J, Ning C, Wang Y, Xun Z, Wang Y, Lu X, Mao Y, Sang X, Zhao H. Downstaging and resection of hepatocellular carcinoma in patients with extrahepatic metastases after stereotactic therapy. Hepatobiliary Surg Nutr. 2021;10:434–442. doi: 10.21037/hbsn-21-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao L, Zhao H. Conversion surgery for hepatocellular carcinoma in the new era of targeted and immune checkpoint inhibitor therapies. Hepatobiliary Surg Nutr. 2020;9:809–811. doi: 10.21037/hbsn-20-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Salama ZT, Syed YY, Scott LJ. Lenvatinib: a review in hepatocellular carcinoma. Drugs. 2019;79:665–674. doi: 10.1007/s40265-019-01116-x. [DOI] [PubMed] [Google Scholar]
- 14.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol. 2019;12:92. doi: 10.1186/s13045-019-0779-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes PE, Caenepeel S, Wu LC. Targeted therapy and checkpoint immunotherapy combinations for the treatment of cancer. Trends Immunol. 2016;37:462–476. doi: 10.1016/j.it.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, Chinot OL. Bevacizumab (Avastin(R)) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. doi: 10.1016/j.ctrv.2020.102017. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 19.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 20.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 21.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the common terminology criteria for adverse events (CTCAE - Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed) 2021;112:90–92. doi: 10.1016/j.ad.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL KEYNOTE-240 investigators. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J. Clin. Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 25.Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Han KH, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Begic D, Chen G, Neely J, Anderson J, Sangro B. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC) Ann Oncol. 2019;30:v874–v875. [Google Scholar]
- 26.Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 2020;38:2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu L, Chen J, Yang J, Gong W, Zhang Y, Zhao H, Yan S, Jia W, Wu Z, Liu C, Song X, Ma Y, Yang X, Gao Z, Zhang N, Zheng X, Li M, Zhang X, Chen M. 165P efficacy and safety of tislelizumab (TIS) plus lenvatinib (LEN) as first-line treatment in patients (pts) with unresectable hepatocellular carcinoma (uHCC): a single-arm, multicenter, phase II trial. Immuno-Oncology and Technology. 2022:16. [Google Scholar]
- 28.Qin S, Finn RS, Kudo M, Meyer T, Vogel A, Ducreux M, Macarulla TM, Tomasello G, Boisserie F, Hou J, Li X, Song J, Zhu AX. RATIONALE 301 study: tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol. 2019;15:1811–1822. doi: 10.2217/fon-2019-0097. [DOI] [PubMed] [Google Scholar]
- 29.Qin S, Kudo M, Meyer T, Finn RS, Vogel A, Bai Y, Guo Y, Meng Z, Zhang T, Satoh T, Hiraoka A, Marino D, Assenat E, Wyrwicz L, Campos MC, Hsing-Tao K, Boisserie F, Li S, Chen Y, Zhu AX. LBA36 final analysis of RATIONALE-301: randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann Oncol. 2022;33:S1402–S1403. [Google Scholar]
- 30.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]