Abstract
Background
Diabetes mellitus is a widespread metabolic disorder affecting global populations. Lavandula stoechas from Moroccan traditional medicine is used for its potential anti-diabetic effects.
Objective
This study aims to evaluate the antihyperglycemic impact of the aqueous extract of L. stoechas (AqLs) and explore its mechanisms.
Methods
The study employed a glucose tolerance test (OGTT) on normal and diabetic Wistar rats, administering AqLs at 150 mg/kg. In vitro, AqLs was tested against α-glucosidase and α-amylase activities, confirmed in vivo using normal and Allx-diabetic rats. The extract’s impact on intestinal d-glucose absorption was assessed using the jejunum segment perfusion technique at 250 mg/kg in situ. Albino mice were used to assess toxicity.
Results
AqLs significantly reduced postprandial hyperglycemia (P < 0.001) due to glucose overload. It inhibited pancreatic α-amylase (IC50: 0.485 mg/mL) and intestinal α-glucosidase (IC50: 168 µg/mL) in vitro. Oral AqLs at 150 mg/kg reduced hyperglycemia induced by sucrose and starch in normal and diabetic rats. It also lowered (P < 0.001) intestinal glucose absorption in situ at 250 mg/kg. Oral acute toxicity tests on Albino mice indicated no adverse effects at different doses.
Conclusion
to summarize, L. stoechas has evident antihyperglycemic effects attributed to inhibiting intestinal glucose absorption and key monosaccharide digestion enzymes like α-amylase and α-glucosidase.
Keywords: Lavandula stoechas, Antihyperglycemic, α-glucosidase, α-amylase, In vitro, In vivo
1. Introduction
Diabetes is a metabolic disorder caused due to elevated blood insulin levels and/or insulin insensitivity in the targeted organs [1]. This state of permanent hyperglycemia is greater than or equal to 1.26 g/L (7 mmol) on an empty stomach and greater than or equal to 2 g/L (11 mmol) at any time of day [2]. It can be divided into two forms; diabetes mellitus type 1, which is the product of a total insulin deficiency, and the more commonly found diabetes mellitus type 2 which is caused by insulin resistance [3]. In 2021, approximately 8 million people worldwide were diagnosed with type 1 diabetes: of these, 1.5 million were under the age of 20 years, 5.4 million were between the ages of 20 and 59, and 1.6 million were 60 years of age or older. A total of 0.5 million new cases were identified in that year, and 35,000 undiagnosed people passed away within a year of exhibiting symptoms [4]. Among the therapies used to decrease postprandial hyperglycemia, responsible for the chronic complication presence, are digestive enzymes (α-amylase and α-glucosidase) inhibition linked to diabetes, and glucose intestinal absorption reduction [5]. Despite the appearance of new therapeutic molecules such as insulin or oral hypoglycemic agents such as biguanides and sulfonylureas, their regular administration causes several side effects [6].
Therefore, in developing nations, medicinal plants are the usual form of therapy [7]. In the Indian system of medicine and other traditional healing systems around the world, several plants have been used to treat diabetes mellitus [8]. Studies have shown that there are over 400 species of plants with antidiabetic activity [9]. Herbal remedies have proven to be effective in treating diseases using essential oils, alcoholic, aqueous extract, and fruit juices extracted from aromatic and medicinal plants [10]. Lavandula stoechas is an important medicinal and aromatic plant from the Lamiaceae family [11], Currently, it's cultivated globally for a variety of uses, including ornamental, soil stabilization, culinary, and cosmetic uses [11]. This perennial shrub can be found in the following continents: Africa, Asia, and Europe [12]. It also spreads across the Mediterranean, including Morocco [13]. The pharmacological properties of L. stoechas essential oils and extracts have attracted the attention of several researchers, who have assessed their anti-leishmanial, insecticidal, anti-fungal [14] antioxidant [15] as well as anti-inflammatory properties [16,17]. L. stoechas is also used in Morocco to treat rheumatoid arthritis, nephrotic syndrome, as an antispasmodic, to relieve pain [18,19], and for its antidiabetic effect as a decoction or infusion [14,20]. This genus contains a large number of phenolic compounds; such as protocatechuic, ferulic, caffeic, rosmarinic, and chlorogenic acids, as well as pinocembrin, pinobanksin, quercetin, and luteolin [21]. We used mice and rats in this experiment because they have long been the preferred animal models in biomedical research, owing to their anatomical, physiological, and genetic similarities to humans. Additionally, rodents offer several advantages as experimental animals, including their small size, ease of maintenance, short lifespan, and abundant genetic resources [22].
In this study, we report the antihyperglycemic activity of L. stoechas aqueous extract in order to assess its potential use for diabetic treatment. As far as we know, this is the first report on the antihyperglycemic activity of L. stoechas aqueous extract.
2. Material and methods
2.1. Chemicals and reagents
Alloxan monohydrate 98%, glucose oxidase-peroxidase reagent, sucrose, starch powder, and magnesium chloride-6-hydrate [MgCl2, 6H2O] were acquired from Sigma-Aldrich, Steinheim, Germany, as for the α-glucosidase enzyme, and acarbose were imported from Sigma-Aldrich, China, furthermore, the α-amylase enzyme and phlorizin dehydrate were both purchased from Sigma-Aldrich, USA, and Pentobarbital was imported from France. Dimethyl-sulfoxide (DMSO), Sodium chloride (NaCl) and potassium chloride (KCl) were purchased from Sigma Aldrich, Riedel-de Haen, Denmark. Ether and glibenclamide have been both acquired from Casablanca and Oujda respectively in Morocco, as for the Calcium chloride dihydrate [CaCl2, 2H2O] was imported from Spain, Scharlau Chemie, s.a. The sodium bicarbonate [NaHCO3] was purchased from Puerto Rico, (Farco Chemical Supply), and finally Sodium phosphate monobasic dihydrate [NaH2PO4 · 2H2O] from Panreac Spain.
2.2. Plant identification and preparation
Lavandula stoechas was first obtained from Tafoughalt (Oriental Morocco) in the autumn of November 2019. The plant sample was deposited in the Herbarium of Mohamed First University's Faculty of Sciences in Oujda, Morocco, and identified by a botanist using the reference number (HUMPOM77). The plant had to pass through two important methods in order to achieve its intended final form which is the L. stoechas powder. The first process was to carefully clean the aerial part of the plant from dust, and from any undesirable particles using distilled water, air dry it afterward at 40 °C in an oven, and then cut the plant into small pieces. For the next process, the aqueous extract of the plant was made into decoctions, as a result of boiling 80 g of the used plant for 20 min in 800 mL of distilled water. Finally, the decoction was filtered and dried at 40 °C to transform it into a powder.
2.3. Experimental animals
Three-month-old rats weighing under 250 g were housed in standard polypropylene cages in groups of 3 or 4 under normal laboratory conditions and fed a standard diet composed of cereals, oilseed meals, vegetable by-products, alfalfa, carob, mineral materials, mineral compounds, and vitamins, with access to fresh tap water ad libitum. The animals were maintained under a natural 12-h light/dark cycle, with a temperature of 24 ± 2 °C and 60% humidity in Mohammed First University, Faculty of Sciences, Oujda, Morocco. All of the animals were handled according to the standards imposed by the National Institutes of Health's Guide for the Treatment and Use of Laboratory Animals [23]. The studies were carried out in accordance with a protocol approved by the Institutional Care and Use Committee at the Faculty of Sciences in Oujda, under the certification reference 15/19-LBBEH-06.
2.4. Acute toxicity test
To look at any potential toxic effects or changes in normal behavior; a total of 24 female and male albino mice were used that weigh between 20 and 30 g. They were split randomly into four experimental groups of 6 mice (3 males and 3 females) each for use in this experiment. Different doses of AqLs diluted with distilled water, were administered to various groups of mice using two different methods. Specifically, three groups of mice were orally administered doses of AqLs (0.5, 1, and 2 g/kg of body weight) using intragastric gavage (i.g.), with each group receiving one dose. Additionally, three other groups were administered other doses of AqLs (0.1, 0.3, and 0.5 g/kg) using an intraperitoneal injection (i.p.). The control group, on the other hand, was administered only distilled water. Different doses of AqLs (0.5, 1, 2 g/kg of body weight) were orally administered by intragastric gavage (i.g.) and other doses (0.1, 0.3, 0.5 g/kg) were administered by intraperitoneal injection (i.p.), to these groups of mice, while the control group received only distilled water. Changes in general behavior in mice and mortality were observed regularly in all animals every 30 min, then 2 h, 4 h, 6 h, 10 h, and finally 24 h on the first day after sample administration. Later we used the latter routine daily for a total of 14 days, including body weight, breathing difficulty, and hypo-activity. The acute oral toxicity study was performed following the recommendations of OECD Guidelines (425) [24].
2.5. Diabetes induction
A single intraperitoneal (i.p) injection of Alloxan monohydrate 98% (120 mg/kg) dissolved in fresh and cold phosphate citrate buffer with pH = 4.5 induced diabetes in fasting rats (14 h). For this analysis, rats with blood sugar levels greater than 1.26 g/L were selected for the study.
2.6. Oral glucose tolerance test
The aqueous extract's antihyperglycemic impact on L. stoechas was evaluated in normal and diabetic rats, as defined by Bouhrim [25] with some modifications. Before experimentation, the animals in the study were denied food for 16 h and only had access to water which was critical for their survival, three classes of animals were divided, so that the normal rats were randomly divided into groups, whereas the diabetic rats were separated into groups based on their glycemic levels, each class consisting of six rats. The first one which is the control group was treated only with distilled water (10 mL/kg) by intragastric gavage (i.g.). The second group was treated with the AqLs diluted with distilled water at a dose of 150 mg/kg as it was mentioned in previous studies [26], and the last group was treated orally by intragastric gavage (i.g.) with Glibenclamide also diluted with distilled water at a dose of 2 mg/kg. Thirty minutes after treatments, rats were orally supplied with glucose (2 g/kg). After 30, 60, 90, and 150 min, the tail end of a rat was used to collect blood, being anesthetized with light ethanol, the rats were placed in a cage with a piece of cotton soaked in the anesthesia solution for 2–3 min to maintain anesthesia and ensure that the animals did not experience any pain or distress during the experimental procedures. The blood samples were then centrifuged in a hematocrit centrifuge for 10 min to separate the serum and measure the amount of glucose in the blood using the glucose oxidase–peroxidase assay (GOP-POD). (Hermle Z230H, Gosheim, Germany) (Glucose, SGM Italia).
2.7. Intestinal glucose absorption assay, In situ
The effect of AqLs on d-glucose uptake in the intestine was measured according to the protocol defined by Bouhrim et al. [25]. Before the experiment, normal Wistar rats weighing between 150 and 250 g, were fasted for 36 h with free access to water, and then a single intramuscular (i.m.) injection using a needle of 50 mg/kg of the pentobarbital with a volume of 0.2 mL for each rat weighing 200 g, was given to anesthetize the animals. Typically it takes 5–10 min for a rat to be fully anesthetized by pentobarbital administered intraperitoneally. The rats were then divided randomly into three groups, then 10 cm of the jejunum segment of each was perfused with an adequate solution. The first one (control group) received the perfusion solution that contains: (7.37 g/L of NaCl, 0.2 g/L of KCl, 0.065 g/L of NaH2PO4·2H2O, 0.213 g/L of MgCl2·6H2O, 0.6 g/L of NaHCO3 and 1.02 g/L of CaCl2·2H2O, with a pH: 7.5, and 1 g/L of glucose), the second one (positive control group); phlorizin was added to the perfusion solution (0.1 mM), and the last group received the perfusion solution plus 250 mg/kg of AqLs as it was indicated in previous ethnopharmacological studies [27], using a roller pump (Fisher Scientific Inc., Waltham, MA) at 0.53 mL/min. One hour later, the perfusate was collected using glucose oxidase–peroxidase to measure the quantity of glucose in the final solution, the perfused jejunal segment's length was measured in cm, and the intestinal uptake of glucose was calculated in mg/10 cm/1 h.
2.8. In vitro, pancreatic α-amylase inhibitory assay
The inhibition activity of α-amylase was studied using the procedure defined by Daoudi [28] with some modifications. The test mixtures contained 200 μl of phosphate buffer (0.02 M; pH = 6.9), 200 μL of α-amylase enzyme solution, and 200 μL of AqLs (0.23, 0.45, 0.91, 1.36, 1.82, 2.23 mg/mL). The mixtures were pre-incubated for 10 min at 37 °C, and then to each tube, 200 μL of starch (1%) was added, and the tubes were incubated at 37 °C for 15 min. Furthermore, the tubes were supplemented with a volume of 600 μL of DNSA (3,5-dinitrosalicylic acid), in order to stop the enzymatic reaction. The tubes were then incubated at 100 °C for 8 min. This reaction was then stopped by a thermal shock, by placing the tubes in an ice-water bath, before adding 1 mL of distilled water to dilute the mixtures. Finally, at 540 nm, the absorbance was measured using a spectrophotometer. The blank was produced by swapping out the extract for 200 μL of phosphate buffer for 100% enzyme activity. In the absence of the enzyme solution, a blank reaction was likewise generated using the plant extract at each dose. Acarbose (0.060, 0.11, 0.23, 0.45, 0.91, 2.27, 4.54 et 6.82 mg/mL), was used as a positive control sample, and the reaction was carried out in the same manner as the previously mentioned reaction with plant extract. The inhibition ratio was determined using the formula below:
| % Inhibition = [(Acontrol − Asample)/Acontrol] × 100. |
AControl: Absorbance of enzymatic activity without inhibitor.
ASample: Absorbance of enzymatic activity in the presence of AqLs or acarbose.
The concentration of samples that inhibit 50% (IC50) of α-amylase enzyme activity is determined graphically by function: inhibition percentage = f (log sample concentration).
2.9. In vivo, pancreatic α-amylase inhibitory assay
In this study, normal and diabetic rats weighing between 250 and 350 g were used, they were fasted for 16 h, and then divided, so that the normal rats were randomly divided into groups, whereas the diabetic rats were separated into groups based on their glycemic levels, into three experimental groups of 6 rats; only distilled water was provided to the control group (10 mL/kg), the other group received a single dose of Acarbose by gavage (i.g.) (10 mg/kg), and the last one received a single dose of the AqLs (150 mg/kg) [26], by intragastric gavage (i.g.) for all groups. The animals were loaded orally with starch (2 g/kg) after 30 min of solution administration. And then, the concentration of blood glucose was estimated using the glucose-peroxidase method, at various times: 0, 30, 60, 90, and 150 min.
2.10. In vitro, intestinal α-glucosidase inhibitory assay
The inhibition of the activity of α-glucosidase was studied using the procedure defined by Hayat [29]. Mixtures used contained 1 mL of phosphate buffer (pH = 7.5), 0.1 mL of α-glucosidase enzyme solution (10 IU), and 200 μL of AqLs (80, 170, 250, 330, 650 μg/mL) and the same volume of distilled water and acarbose (41, 82, 165, 328, 656 μg/mL), as a control and negative and positive controls were used respectively. Moreover, the mixtures were pre-incubated for 20 min at 37 °C, and then to each tube, 0.1 mL of sucrose was added, and an incubation of the tubes was done at 100 °C for 5 min to stop the reaction. Furthermore, 1 mL of GOD-POD (Glucose oxidase–peroxidase) was added and then an incubation was done at 37 °C for 10 min Finally, at 500 nm, the absorbance was measured using a spectrophotometer, And the inhibition ratio was determined using the formula below:
| % Inhibition = [(Acontrol − Asample)/Acontrol] × 100. |
AControl: Absorbance of enzymatic activity without inhibitor.
ASample: Absorbance of enzymatic activity in the presence of extract or acarbose.
The concentration of samples that inhibit 50% (IC50) of α-amylase enzyme activity is determined graphically by function: inhibition percentage = f (log sample concentration).
2.11. In vivo, intestinal α-glucosidase inhibitory assay
In this study, normal and diabetic rats weighing between 250 and 350 g were used, they were fasted for 16 h, and then divided so that the normal rats were randomly divided into groups, whereas the diabetic rats were separated into groups based on their glycemic levels, into three experimental groups of 6 rats; only distilled water was provided to the control group (10 mL/kg), the second one received a single dose of Acarbose by gavage (i.g.) (10 mg/kg), and the last one received a single dose of the AqLs (150 mg/kg) [26], by intragastric gavage (i.g.) for all groups. Sucrose was given to the animals orally (2 g/kg) after 30 min of solution administration. And then, the concentration of blood glucose was estimated using the glucose-peroxidase method, at various times: 0, 30, 60, 90, and 150 min.
3. Statistical analysis
The obtained results were analyzed using GraphPad Prism 5 and expressed as the mean ± standard error of the mean (SEM). One-way ANOVA test was conducted to analyze the results, and Turkey's multiple comparison test was performed for multiple comparisons. Statistical significance was considered when P < 0.05. The sample size was n = 6, and by comparing the means of different groups using appropriate statistical tests, statistically significant results were obtained.
4. Results
4.1. Acute toxicity
The AqLs showed no symptoms of toxicity, and no improvements in the general behavior of mice in the acute toxicity test with oral and intraperitoneal administration. In contrast to the normal control group, no major differences were found in the daily intake of food in the treated mice. During the experiment, both normal and treated mice appeared healthy.
4.2. Effect of AqLs on oral glucose tolerance test
4.2.1. Healthy rats
The oral glucose tolerance test performed in normal rats at a concentration of 2 g/kg gave the results shown in Fig. 1. At first, rats in the control group, treated only with distilled water, have a basal blood sugar level (0.8 g/L), after glucose administration the postprandial hyperglycemia reached 1.5 ± 0.03 g/L at 60 min and 1.53 ± 0.20 g/L at 90 min, after which it was reduced to an average of 1.44 ± 0.08 g/L at 150 min of glucose overload. However, the postprandial hyperglycemia level has been significantly suppressed by AqLs at a dose of 150 mg/kg (P < 0.001) compared to the control group (1.15 ± 0.18 g/L, 1.04 ± 0.17 g/L, 0.69 ± 0.08 g/L at 60, 90, and 150 min respectively). And the glibenclamide significantly (P < 0.001) decreased the level of blood glucose (0.95 ± 0.10 g/L, 0.82 ± 0.06 g/L, 0.78 ± 0.14 g/L by 60, 90, and 150 min).
Fig. 1.
Effect of AqLs and glibenclamide on postprandial glycemia in normal rats after glucose overload (2 g/kg). AqLs: Aqueous extract of Lavandula stoechas. The values are the means ± SEM (n= 6). ∗∗∗ P < 0.001, compared to the control.
The area under a curve (AUC) of glucose sensitivity for the normal and diabetic rats treated with the AqLs was significantly (P < 0.001) lower than the normal control group's AUC and similar to the glibenclamide one (Table 1).
Table 1.
The glucose levels of three groups of normal rats (control group, AqLs group, and glib group) measured at three different time points after glucose overload.
| Time | Glucose level (control) | Glucose level (AqLs) | Glucose level (Glib) |
|---|---|---|---|
| 60 min | 1.5 ± 0.03 g/L | 1.15 ± 0.18 g/L | 0.95 ± 0.10 g/L |
| 90 min | 1.53 ± 0.20 g/L | 1.04 ± 0.17 g/L | 0.82 ± 0.06 g/L |
| 150 min | 1.44 ± 0.08 g/L | 0.69 ± 0.08 g/L | 0.78 ± 0.14 g/L |
4.2.2. Diabetic rats
In diabetic rats, the oral glucose tolerance test was conducted in the same way (Fig. 2); for this reason, distilled water (control group), aqueous extract (plant group), and glibenclamide (drug group) were used to pretreat the animals. Rats in the control group, treated only with distilled water, have a high blood sugar level (1.36 g/L), after glucose administration, the postprandial hyperglycemia reached 1.83 ± 0.10 g/L at 60 min, 1.94 ± 0.12 g/L at 90 min, and 1.99 ± 0.07 g/L at 150 min of glucose overload. The administration of the aqueous extract of Lavandula stoechas (AqLs) significantly (P < 0.001) inhibited the rise of blood glucose levels after the overload of 2 g/kg of glucose, (1.36 ± 0.06 g/L at 60 min; 1.37 ± 0.07 g/L at 90 min and 1.36 ± 0.11 g/L at 150 min). Relative to the diabetic control group. The pretreatment with Glibenclamide (2 mg/kg) significantly (P < 0.001) prevented the rise of blood sugar after glucose overload relative to diabetic rats (1.46 ± 0.14 g/L at 60 min; 1.48 ± 0.15 g/L at 90 min and 1.44 ± 0.19 g/L at 150 min).
Fig. 2.
Effect of AqLs and glibenclamide on postprandial glycemia in diabetic rats after glucose overload (2 g/kg) (A) and with a representation of the area under curves (B). AqLs: Aqueous extract of Lavandula stoechas. The values are the means ± SEM (n = 6). ∗∗∗P < 0.001, ∗∗P < 0.01 compared to the control.
The area under a curve (AUC) of glucose sensitivity for the normal and diabetic rats treated with the AqLs was significantly (P < 0.01) lower than that of normal control group and glibenclamide group (Table 2).
Table 2.
The glucose levels of three groups of diabetic rats (control group, AqLs group, and glib group) measured at three different time points after glucose overload.
| Time | Glucose level (control) | Glucose level (AqLs) | Glucose level (Glib) |
|---|---|---|---|
| 60 min | 1.83 ± 0.10 g/L | 1.36 ± 0.06 g/L | 1.46 ± 0.14 g/L |
| 90 min | 1.94 ± 0.12 g/L | 1.37 ± 0.07 g/L | 1.48 ± 0.15 g/L |
| 150 min | 1.99 ± 0.07 g/L | 1.36 ± 0.11 g/L | 1.44 ± 0.19 g/L |
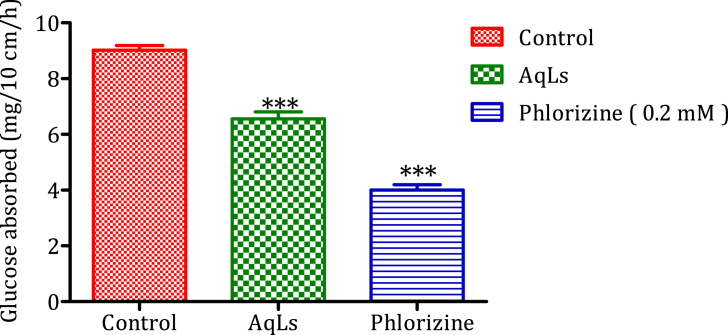
4.3. Intestinal glucose absorption
According to the obtained results in Fig. 3; the quantity of glucose absorbed in the jejunum in the absence of AqLs (control group) is 9.02 mg/10 cm/h. However, in the presence of AqLs, the amount of glucose absorbed significantly decreased (P < 0.001) to 6.55 mg/10 cm/h, in comparison to the control group. Phlorizin (0.1 mM) also decreased significantly (P < 0.001) the amount of glucose absorbed to 4 mg/10 cm/h, in comparison to the control group. The effect of AqLs is statistically similar to that of phlorizin.
Fig. 3.
Impact of the aqueous extract of Lavandula stoechas (AqLs) on the amount of glucose absorbed by a 10 cm segment of rats' jejunum (n = 6), in-situ. ∗∗∗P < 0.001.
4.4. In vitro, α-amylase inhibitory effect
Based on results obtained for the inhibitory activity of AqLs on pancreatic α-amylase activity in vitro, shown in Fig. 4, different doses (0.23, 0.45, 0.91, 1.36, 1.82, 2.23 mg/mL) of the AqLs induce the inhibition of α-amylase activity with IC50 value of 0.485 ± 0.13 mg/mL. Acarbose has also shown a strong inhibitory activity against α-amylase with IC50 value of 0.446 ± 0.01 mg/mL, which is statistically similar to the inhibitory activity of AqLs.
Fig. 4.
Inhibitory effect of pancreatic α-amylase activity by AqLs and acarbose in vitro. The values are the means ± SEM. AqLs: Aqueous extract of Lavandula stoechas.
4.5. In vivo, α-amylase inhibitory effect
4.5.1. Healthy rats
After starch administration (2 g/kg), glycemia increased to reach a peak of 1.48 ± 0.02 g/L, shown in Fig. 5, which gradually decreases to reach a value of 1.18 ± 0.07 g/L at 60 min. While, in the presence of AqLs (150 mg/kg), the postprandial glycemia decreased significantly (P < 0.001 at 30 min and P < 0.05 at 60 min) to reach values of 1.03 ± 0.06 g/L and 0.91 ± 0.004 g/L compared to the control. However, glycemia at 150 min showed no significant difference. Moreover, the administration of 10 mg/kg of acarbose, significantly inhibited starch-induced hyperglycemia (P < 0.001 and P < 0.05) at 30 and 60 min, to reach a value of 0.97 ± 0.08 g/L and 0.97 ± 0.05 g/L respectively.
Fig. 5.
Effect of aqueous extract of Lavandula stoechas and acarbose on glycemia after starch overload (2 g/kg) in normal rats (n = 6). ∗P < 0.05; ∗∗∗P < 0.001 as compared to the control group. B: Area under curve (AUC) for rats' overload with starch in the presence of the aqueous extract of Lavandula stoechas or acarbose. ∗∗P < 0.01 compared to the control group AqLs; Aqueous extract of Lavandula stoechas.
After starch overload to the normal rats, the area under the curve (AUC) in control group equals to (68.22 ± 0.01 g/L/h). While the pretreatment with the AqLs (150 mg/kg) inhibited (P < 0.001) the glycemic load with an area of (58.33 ± 0.01 g/L/h). Moreover, acarbose (10 mg/kg) showed similar inhibitory activity to that of AqLs with an area of (57.54 ± 0.01 g/L/h) (Table 3).
Table 3.
The glucose levels of three groups of normal rats (control group, AqLs group, and glib group) measured at two different time points after starch overload.
| Time | Glucose level (control) | Glucose level (AqLs) | Glucose level (Glib) |
|---|---|---|---|
| 30 min | 1.48 ± 0.02 g/L | 1.03 ± 0.06 g/L | 0.97 ± 0.08 g/L |
| 60 min | 1.18 ± 0.07 g/L | 0.91 ± 0.004 g/L | 0.97 ± 0.05 g/L |
4.5.2. Diabetic rats
After starch administration (2 g/kg) in diabetic rats (Fig. 6), an increase in glucose level (1.79 ± 0.07 g/L) was observed 30 min after the administration of starch (2 g/kg) in the control group, which gradually increases to reach a value of 1.88 ± 0.07 g/L at 90 min, and 1.97 ± 0.09 g/L at 150 min. While, in the presence of AqLs (150 mg/kg), the postprandial glycemia decreased significantly (P < 0,001 at 30, 60 min and at 150 min) to reach values of 1.42 ± 0.05 g/L, 1.45 ± 0.04 g/L, and 1.40 ± 0.05 g/L when compared to the control group. Moreover, an administration of 10 mg/kg of acarbose, significantly inhibited starch-induced hyperglycemia (P < 0.001) at 30 and 60 min and 150 min, to reach values of 1.05 ± 0.05 g/L, 1.06 ± 0.04 g/L, and 1.17 ± 0.07 g/L when compared to the control group.
Fig. 6.
Effect of aqueous extract of Lavandula stoechas and acarbose on glycemia after starch overload (2 g/kg) in diabetic rats (n = 6). ∗∗∗P < 0.001 as compared to the control group. AqLs: Aqueous extract of Lavandula stoechas.
The area under a curve in diabetic control group equals to (172.36 ± 0.01 g/L/h). While, the pretreatment with the AqLs (150 mg/kg) inhibited significantly (P < 0.001) the glycemic load with an area of (141.74 ± 0.01 g/L/h). Moreover, acarbose (10 mg/kg) showed stronger inhibitory activity than that of AqLs with an area of (113.77 ± 0.01 g/L/h) (Table 4).
Table 4.
The glucose levels of three groups of diabetic rats (control group, AqLs group, and glib group) measured at three different time points after starch overload.
| Time | Glucose level (control) | Glucose level (AqLs) | Glucose level (Glib) |
|---|---|---|---|
| 60 min | 1.79 ± 0.07 g/L | 1.42 ± 0.05 g/L | 1.05 ± 0.05 g/L |
| 90 min | 1.88 ± 0.07 g/L | 1.45 ± 0.04 g/L | 1.06 ± 0.04 g/L |
| 150 min | 1.97 ± 0.09 g/L | 1.40 ± 0.05 g/L | 1.17 ± 0.07 g/L |
4.6. α-glucosidase inhibitory effect in vitro
Based on results obtained for the inhibitory activity of AqLs on intestinal α-glucosidase activity in vitro, shown in Fig. 7. Different doses (80, 170, 250, 330, 650 μg/mL) of the AqLs induce the inhibition of α-glucosidase activity with IC50 value of 168 ± 40.10 μg/mL. However, acarbose has also shown a very strong inhibitory activity against α-glucosidase with IC50 value of 52.5 ± 2.67 μg/mL.
Fig. 7.
Inhibitory effect of intestinal α-lucosidase activity by AqLs and acarbose in vitro. The values are the means ± SEM. AqLs: Aqueous extract of Lavandula stoechas.
4.7. In vivo, α-glucosidase inhibitory effect
4.7.1. Healthy rats
After sucrose administration (2 g/kg), glycemia increased to reach a value of 1.40 ± 0.03 g/L, at 60 min and 1.5 ± 0.01 g/L at 90 min, and 1.34 ± 0.19 g/L at 150 min, shown in Fig. 8. While, in the presence of AqLs (150 mg/kg), the postprandial glycemia decreased significantly ((P < 0.01, P < 0.001 and P < 0.05) at 60, 90 and 150 mins respectively), to reach values of 0.98 ± 0.08 g/L at 60 min, 0.97 ± 0.04 g/L at 90 min and 0.99 ± 0.03 g/L at 150 min, when compared to control group. Moreover, an administration of 10 mg/kg of acarbose, significantly (P < 0.01, P < 0.01 and P < 0.05) inhibited starch-induced hyperglycemia at 60, 90, and 150 min respectively, to reach values of 0.99 ± 0.06 g/L at 60 min, 1.10 ± 0.03 g/L at 90 min and 1.05 ± 0.06 g/L at 150 min.
Fig. 8.
Effect of aqueous extract of Lavandula stoechas and acarbose on glycemia after sucrose overload (2 g/kg) in normal rats (n = 6). ∗P < 0.05; ∗∗P < 0.01 as compared to the control group. B: Area under curve (AUC) for rats' overload with starch in the presence of the aqueous extract of Lavandula stoechas or acarbose. ∗∗∗P < 0.001 compared to the control group. AqLs; Aqueous extract of Lavandula stoechas.
After sucrose overload, the area under curve (AUC) in control group equals to (81.10 ± 0.01 g/L/h). While, the pretreatment with the AqLs (150 mg/kg) inhibited (P < 0.001) the glycemic load with an area of (41.87 ± 0.01 g/L/h). Moreover, acarbose (10 mg/kg) showed similar inhibitory activity (P < 0.001) to that of AqLs with an area of (62.05 ± 0.01 g/L/h) (Table 5).
Table 5.
The glucose levels of three groups of normal rats (control group, AqLs group, and glib group) measured at three different time points after sucrose overload.
| Time | Glucose level (control) | Glucose level (AqLs) | Glucose level (Glib) |
|---|---|---|---|
| 60 min | 1.40 ± 0.03 g/L | 0.98 ± 0.08 g/L | 0.99 ± 0.06 g/L |
| 90 min | 1.5 ± 0.01 g/L | 0.97 ± 0.04 g/L | 1.10 ± 0.03 g/L |
| 150 min | 1.34 ± 0.19 g/L | 0.99 ± 0.03 g/L | 1.05 ± 0.06 g/L |
4.7.2. Diabetic rats
After sucrose administration (2 g/kg) to diabetic rats (Fig. 9), an increase in glucose level (1.80 ± 0.07 g/L) was observed 30 min after the administration of sucrose (2 g/kg) in the control group, which gradually increases to reach a value of 1.84 ± 0.08 g/L at 90 min, and 1.86 ± 0.23 g/L at 150 min. While, in the presence of AqLs (150 mg/kg), the postprandial glycemia decreased significantly (P < 0.05 at 30, 60 min and at 150 min) to reach values of 1.62 ± 0.07 g/L, 1.67 ± 0.08 g/L, and 1.46 ± 0.10 g/L when compared to the control group.
Fig. 9.
Effect of aqueous extract of Lavandula stoechas and acarbose on glycemia after sucrose overload (2 g/kg) in diabetic rats (n = 6). ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 as compared to the control group. B: Area under curve (AUC) for rats' overload with starch in the presence of the aqueous extract of Lavandula stoechas or acarbose. ∗∗P < 0.01 compared to the control group. AqLs: Aqueous extract of Lavandula stoechas.
Moreover, an administration of 10 mg/kg of acarbose, significantly inhibited sucrose-induced hyperglycemia (P < 0.01, P < 0.001 and P < 0.05) at 60, 90 and 150 min respectively, to reach values of 1.50 ± 0.14 g/L at 60 min, 1.47 ± 0.12 g/L at 90 min and 1.31 ± 0.12 g/L at 150 min.
After sucrose overload to diabetic rats, the area under curve (AUC) in control group equals to (171.41 ± 0.01 g/L/h). While, the pretreatment with the AqLs (150 mg/kg) inhibited the glycemic load with an area of (153.16 ± 0.01 g/L/h). Moreover, acarbose (10 mg/kg) showed similar inhibitory activity (P < 0.01) to that of AqLs with an area of (141.88 ± 0.01 g/L/h) (Table 6).
Table 6.
The glucose levels of three groups of diabetic rats (control group, AqLs group, and glib group) measured at three different time points after sucrose overload.
| Time | Glucose level (control) | Glucose level (AqLs) | Glucose level (Glib) |
|---|---|---|---|
| 60 min | 1.80 ± 0.07 g/L | 1.62 ± 0.07 g/L | 1.50 ± 0.14 g/L |
| 90 min | 1.84 ± 0.08 g/L | 1.67 ± 0.08 g/L | 1.47 ± 0.12 g/L |
| 150 min | 1.86 ± 0.23 g/L | 1.46 ± 0.10 g/L | 1.31 ± 0.12 g/L |
5. Discussion
Before being absorbed, the disaccharides go through basic digestion, both intraluminal and simple, to create natural oligosaccharides with the action of α-amylase, which are then cleaved into monosaccharides with the action of α-glucosidase and then transported through the SGLT1 to be absorbed in the brush border of the enterocytes [30]. The inhibition of sugar-digesting enzymes such as intestinal α-glucosidase and pancreatic α-amylase, as well as a slowing of intestinal d-glucose absorption and a decrease in postprandial d-glucose, are some of the targeted pathways for treating diabetes mellitus [31]. So that the objective of treating patients with diabetes is to regulate glycemic control levels to be as close to normal as possible during both fasting and postprandial states. Numerous natural resources have been examined to determine their ability to inhibit glucose production from carbohydrates in the gut or the absorption of glucose from the intestine [22] A potential therapeutic strategy involves slowing down the absorption of glucose in the human digestive tract by inhibiting carbohydrate hydrolyzing enzymes such as α-amylases and α-glucosidases [32,33].
The primary aim of this research was to determine the antihyperglycemic effect of the aqueous extract of Lavandula stoechas, and the mechanism of action, based on tests performed in vitro, in situ, and in vivo, by looking at how it influences the absorption of glucose from the intestine or the glucose production in the gut from carbohydrates, Which are considered to be the best choices for managing postprandial hyperglycemia and one of the diabetes control strategies [34,35].
The aqueous extract of Lavandula stoechas inhibits the activities of α-amylase and α-glucosidase in vitro and in vivo significantly, and can reduce intestinal glucose uptake, according to the results of this report. Indeed, in non-diabetic and alloxan-diabetic rats overloaded with d-glucose, oral administration of AqLs has a major antihyperglycemic impact. This impact was close to that of glibenclamide in terms of statistics. The oral administration of the aqueous extract of L. stoechas also showed a significant decrease in postprandial hyperglycemia after oral starch overload in normal and alloxan-induced diabetes rats, demonstrating the inhibitory effect of α-amylase in vivo. It also has an inhibitory effect on α-glucosidase, which it exerts after sucrose overloading on normoglycemic and alloxan-diabetic rats. This effect was statistically similar to that of acarbose. According to the literature, it was reported that the aqueous extract of Lavandula stoechas from Turkey showed an important inhibition activity of α-glucosidase [36]. In situ, AqLs inhibited the intestinal absorption of liberated d-glucose; however, the inhibitory effect of d-glucose intestinal absorption was statistically similar to that of phlorizin (a specific inhibitor of intestinal SGLT2 (The sodium-glucose cotransporter 2)).
Blocking the passage of d-glucose from the intestine to the bloodstream could explain the antihyperglycemic effect of the AqLs. In vitro and in vivo, AqLs also inhibited sucrose degradation by intestinal α-glucosidase and starch degradation by pancreatic α-amylase. The extract is not toxic even at a dose of 2 g/kg, according to the acute toxicity test. These results suggest that the antihyperglycemic activity of Lavandula stoechas is attributable to the presence of multiple compounds which may be important for its therapeutic action. This genus contains a large number of phenolic compounds; such as tannins, catechic tannins, flavonoids, sterols, coumarins, leucoanthocyans, and mucilage compounds [19]. In previous findings, on α-glucosidase blockers extracted from traditional medicines, some authors propose that several potential α-glucosidase inhibitors belong to the flavonoids glycosides category and have the characteristics to suppress the α-glucosidase enzyme [37]. Likewise, tannins have been found to increase glucose uptake by activating insulin-signaling mediators such as PI3K (Phosphoinositide 3-Kinase) and p38 MAPK (Mitogen-Activated Protein Kinase), and GLUT-4 translocation. The reduction in glycemia caused by phenolic compounds has been due to a variety of mechanisms, including reduced nutrient absorption, reduced food intake [38], activation of cell regeneration [39], and direct action on adipose cells that increases insulin activity [40]. Plant sterols' dual lipid-lowering effect can also be beneficial to people who have or are at risk of type 2 diabetes [41]. Plant extracts containing coumarins have also been discovered to have antidiabetic activity [42], and they have been confirmed to have a promising therapeutic impact on diabetes and its complications due to their ability to repair pancreatic cell damage, improve insulin signaling [43,44], and provide antioxidative safety [45]. As a result, we conclude that the existence of some of these constituents, as well as many other phytochemical components that can act individually or synergistically, is responsible for Lavandula stoechas' antihyperglycemic impact. This study is the first to confirm the antihyperglycemic effect of Lavandula stoechas aqueous extract in vivo, in vitro, and in situ, despite the fact that other studies have shown the antihyperglycemic effect of Lavandula stoechas hydroalcoholic extract [46], essential oil [47], ethanolic extract and materials obtained from the aerial parts of Lavandula stoechas [36].
During the course of our study, we encountered a challenge with diabetes induction, whereby only 19 out of the 25 rats injected with alloxan developed diabetes within 3–5 days, while the others remained normal. Despite this challenge, it is worth noting that our sample size was still statistically significant for the purposes of our research, as we worked with six rats in each group. This allowed us to conduct robust statistical analyses and draw meaningful conclusions from our data, despite the unexpected variation in response to alloxan induction. We have outlined our sample size and statistical methods in the Methods section of our research paper, in order to provide transparency and clarity to our research approach.
The findings of our study endorse the conventional usage of Lavandula stoechas for treating DM. Our results propose that the plant extract may have an inhibitory effect on digestive enzymes, which in turn could support its hypoglycemic properties. Furthermore, the extract and/or its derived phytochemicals may hold promise for the prevention and treatment of T2DM. To confirm the plant's anti-hyperglycemic effect and demonstrate its anti-diabetic properties, several tests can be performed, including testing its antioxidant activity, analyzing its chemical composition, assessing its effect on glycation of hemoglobin and albumin, and conducting long-term treatments to evaluate its impact on organs and biochemical parameters of rats. Based on our findings, which indicate that Lavandula stoechas has antihyperglycemic and antidiabetic effects in rats, we can potentially extrapolate these results to human populations. However, it is important to note that the generalisability of our findings may be limited by factors such as differences in physiology and metabolism between rats and humans. Additionally, our study was conducted under specific experimental conditions and with a limited sample size, so further research is needed to confirm and extend our findings. Despite these limitations, we believe that our findings have important implications for the potential use of Lavandula stoechas as a treatment for diabetes in humans. We have shown that the plant extract is not toxic at high doses, and our research provides a basis for future studies to explore the safety and efficacy of Lavandula stoechas in larger animal models and human clinical trials. Ultimately, the potential translation of our findings to human populations will depend on further research and validation, but we believe that our work represents an important step towards understanding the antidiabetic properties of this traditional medicinal plant.
6. Conclusion
The antidiabetic effect of the AqLs was investigated in this study. This extract had an antihyperglycemic activity in both normal and alloxan-diabetic rats. The inhibition of intestinal α-glucosidase and pancreatic α-amylase enzymes, as well as the inhibition of intestinal d-glucose absorption, were some of the causes of this result. Additional research is required to determine the other pathways by which this antihyperglycemic action passes.
Funding
The department's facilities were used for all of the tests. Therefore, no financing was needed for this investigation.
Author contributions
Amal Elrherabi; conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing original draft, visualization, supervision, project administration, funding acquisition.
Mohamed Bouhrim: conceptualization, software, supervision, validation, writing – review and editing, data curation.
Ali Berraaouan: software, formal analysis, investigation, Writing – review and editing, funding acquisition.
Abdnim Ghizlane: conceptualization, software, resources, data curation, funding acquisition.
Abderrahim Ziyyat: software, data curation, writing – review and editing, visualization.
Hassane Mekhfi: software, data curation, funding acquisition.
Abdelkhaleq Legssyer: software, formal analysis, data curation, Writing – review and editing.
Mohamed Bnouham: conceptualization, methodology/study design, validation, investigation, resources, data curation, writing – review and editing, supervision, project administration, funding acquisition.
Data availability
On request, the data used to support the findings of this study may be obtained from the corresponding author.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The participants in this project would like to thank Ramdaoui Karim and Badraoui Mustapha for their technical assistance.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Organization WH . World Health Organization; 1999. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1, diagnosis and classification of diabetes mellitus. [Google Scholar]
- 2.Guillausseau P.J. Type II diabetes in children and adults. Rev Prat. 2003;53(13):1463. [PubMed] [Google Scholar]
- 3.Bahmani M., Golshahi H., Saki K., Rafieian-Kopaei M., Delfan B., Mohammadi T. Medicinal plants and secondary metabolites for diabetes mellitus control. Asian Pacific J Trop Dis. 2014;4:S687–S692. [Google Scholar]
- 4.Gregory G.A., Robinson T.I.G., Linklater S.E., Wang F., Colagiuri S., de Beaufort C., et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. 2022;10(10):741–760. doi: 10.1016/S2213-8587(22)00218-2. [DOI] [PubMed] [Google Scholar]
- 5.Ceriello A., Hanefeld M., Leiter L., Monnier L., Moses A., Owens D., et al. Postprandial glucose regulation and diabetic complications. Arch Intern Med. 2004;164(19):2090–2095. doi: 10.1001/archinte.164.19.2090. [DOI] [PubMed] [Google Scholar]
- 6.Bailey C.J. Metformin: effects on micro and macrovascular complications in type 2 diabetes. Cardiovasc Drugs Ther. 2008;22(3):215–224. doi: 10.1007/s10557-008-6092-0. [DOI] [PubMed] [Google Scholar]
- 7.Marghich M., Daoudi N.E., Amrani O., Addi M., Hano C., Chen J.-T., et al. Antioxidant activity and inhibition of carbohydrate digestive enzymes activities of Artemisia campestris L. Front Biosci. 2022;14(4):25. doi: 10.31083/j.fbs1404025. [DOI] [PubMed] [Google Scholar]
- 8.Shukia R., Sharma S.B., Puri D., Prabhu K.M., Murthy P.S. Medicinal plants for treatment of diabetes mellitus. Indian J Clin Biochem. 2000;15(1):169–177. doi: 10.1007/BF02867556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grover J.K., Yadav S., Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81(1):81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 10.Dunning T. Aromatherapy: overview, safety and quality issues. OA Altern Med. 2013;1(1):6. [Google Scholar]
- 11.Sierra M.J., Millán R., Esteban E. Mercury uptake and distribution in Lavandula stoechas plants grown in soil from Almadén mining district (Spain) Food Chem Toxicol. 2009;47(11):2761–2767. doi: 10.1016/j.fct.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Uritu C.M., Mihai C.T., Stanciu G.-D., Dodi G., Alexa-Stratulat T., Luca A., et al. Medicinal plants of the family Lamiaceae in pain therapy: a review. Pain Res Manag. 2018;2018 doi: 10.1155/2018/7801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Upson T.M., Jury S.L. A revision of native Moroccan species of Lavandula L. section Pterostoechas Ging.(Lamiaceae) Taxon. 2002;51(2):309–327. [Google Scholar]
- 14.Bouyahya A., Et-Touys A., Abrini J., Talbaoui A., Fellah H., Bakri Y., et al. Lavandula stoechas essential oil from Morocco as novel source of antileishmanial, antibacterial and antioxidant activities. Biocatal Agric Biotechnol. 2017;12:179–184. [Google Scholar]
- 15.Messaoud C., Chograni H., Boussaid M. Chemical composition and antioxidant activities of essential oils and methanol extracts of three wild Lavandula L. species. Nat Prod Res. 2012;26(21):1976–1984. doi: 10.1080/14786419.2011.635343. [DOI] [PubMed] [Google Scholar]
- 16.Benabdelkader T., Zitouni A., Guitton Y., Jullien F., Maitre D., Casabianca H., et al. Essential oils from wild populations of Algerian Lavandula stoechas L.: composition, chemical variability, and in vitro biological properties. Chem Biodivers. 2011;8(5):937–953. doi: 10.1002/cbdv.201000301. [DOI] [PubMed] [Google Scholar]
- 17.Maganga A. South Thompson Org Prod Assoc; 2004. Influence of variety and organic cultural practices on yield and essential oil content of lavender and rosemary in interior BC. [Google Scholar]
- 18.El-Hilaly J., Hmammouchi M., Lyoussi B. Ethnobotanical studies and economic evaluation of medicinal plants in Taounate province (Northern Morocco) J Ethnopharmacol. 2003;86(2–3):149–158. doi: 10.1016/s0378-8741(03)00012-6. [DOI] [PubMed] [Google Scholar]
- 19.Yassine E.Z., Dalila B., Latifa E.M., Smahan B., Lebtar S., Sanae A., et al. Phytochemical screening, anti-inflammatory activity and acute toxicity of hydro-ethanolic, flavonoid, tannin and mucilage extracts of Lavandula stoechas L. from Morocco. Int J Pharm Phytochem Res. 2016;8(1):31–37. [Google Scholar]
- 20.Benkhnigue O., Ben Akka F., Salhi S., Fadli M., Douira A., Zidane L. Catalogue des plantes médicinales utilisées dans le traitement du diabète dans la région d'Al Haouz-Rhamna (Maroc) J Anim Plant Sci. 2014;23(1):3539–3568. [Google Scholar]
- 21.Lee C.-J., Chen L.-G., Chang T.-L., Ke W.-M., Lo Y.-F., Wang C.-C. The correlation between skin-care effects and phytochemical contents in Lamiaceae plants. Food Chem. 2011;124(3):833–841. [Google Scholar]
- 22.Bryda E.C. The Mighty Mouse: the impact of rodents on advances in biomedical research. Mo Med. 2013;110(3):207. [PMC free article] [PubMed] [Google Scholar]
- 23.Council N.R. The National Academies Press; Washington. DC: 2011. Guide for the care and use of laboratory animals. [PubMed] [Google Scholar]
- 24.Tchoumtchoua J., Mouchili O.R., Ateba S.B., Zingue S., Halabalaki M., Mbanya J.C., et al. Safetyassessment of the methanol extract of the stem bark of Amphimaspterocarpoides Harms: Acute and subchronic oral toxicitystudies in Wistar rats. Toxicol Rep. 2014;1:877–884. doi: 10.1016/j.toxrep.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouhrim M., Ouassou H., Boutahiri S., Daoudi N.E., Mechchate H., Gressier B., et al. Opuntia dillenii (ker Gawl.) Haw., seeds oil antidiabetic potential using in vivo, in vitro, in situ, and ex vivo approaches to reveal its underlying mechanism of action. Molecules. 2021;26(6):1677. doi: 10.3390/molecules26061677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouknana S., Daoudi N.E., Bouhrim M., Ziyyat A., Legssyer A., Mekhfi H., et al. Ammodaucus leucotrichus Coss. & Durieu: antihyperglycemic activity via the inhibition of α-amylase, α-glucosidase, and intestinal glucose absorption activities and its chemical composition. J Pharm Pharmacogn Res. 2022;10:94–103. [Google Scholar]
- 27.Ouassou H., Bouhrim M., Bencheikh N., Addi M., Hano C., Mekhfi H., et al. In vitro antioxidant properties, glucose-diffusion effects, α-amylase inhibitory activity, and antidiabetogenic effects of C. Europaea extracts in experimental animals. Antioxidants. 2021;10(11):1747. doi: 10.3390/antiox10111747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daoudi N.E., Bouhrim M., Ouassou H., Legssyer A., Mekhfi H., Ziyyat A., et al. Inhibitory effect of roasted/unroasted Argania spinosa seeds oil on α-glucosidase, α-amylase and intestinal glucose absorption activities. South African J Bot. 2020;135:413–420. [Google Scholar]
- 29.Ouassou H., Zahidi T., Bouknana S., Bouhrim M., Mekhfi H., Ziyyat A., et al. Inhibition of α-glucosidase, intestinal glucose absorption, and antidiabetic properties by Caralluma europaea. Evidence-Based Complement Altern Med. 2018;2018 doi: 10.1155/2018/9589472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tortora G.J., Grabowski S.R. De Boeck Supérieur; 2001. Principes d'anatomie et de physiologie. [Google Scholar]
- 31.Shai L.J., Masoko P., Mokgotho M.P., Magano S.R., Mogale A.M., Boaduo N., et al. Yeast alpha glucosidase inhibitory and antioxidant activities of six medicinal plants collected in Phalaborwa, South Africa. South African J Bot. 2010;76(3):465–470. [Google Scholar]
- 32.Hara Y., Honda M. The inhibition of α-amylase by tea polyphenols. Agric Biol Chem. 1990;54(8):1939–1945. [Google Scholar]
- 33.Deshpande M.C., Venkateswarlu V., Babu R.K., Trivedi R.K. Design and evaluation of oral bioadhesive controlled release formulations of miglitol, intended for prolonged inhibition of intestinal α-glucosidases and enhancement of plasma glucagon like peptide-1 levels. Int J Pharm. 2009;380(1–2):16–24. doi: 10.1016/j.ijpharm.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 34.Mccue P., Kwon Y., Shetty K. Anti-amylase, anti-glucosidase and anti-angiotensin i-converting enzyme potential of selected foods. J Food Biochem. 2005;29(3):278–294. [Google Scholar]
- 35.Telagari M., Hullatti K. In-vitro α-amylase and α-glucosidase inhibitory activity of Adiantum caudatum Linn. and Celosia argentea Linn. extracts and fractions. Indian J Pharmacol. 2015;47(4):425. doi: 10.4103/0253-7613.161270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.AYAZ11 APDF Lavandula stoechas ssp. stoechas: in vitro antioxidant, and enzyme inhibitory activities. Res Med Aromat Plants. 2020;25 [Google Scholar]
- 37.Mahomoodally M.F., Subratty A.H., Gurib-Fakim A., Choudhary M.I., Nahar Khan S. Traditional medicinal herbs and food plants have the potential to inhibit key carbohydrate hydrolyzing enzymes in vitro and reduce postprandial blood glucose peaks in vivo. Sci World J. 2012;2012 doi: 10.1100/2012/285284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumari M., Tannins Jain S. An antinutrient with positive effect to manage diabetes. Res J Recent Sci ISSN. 2012;2277:2502. [Google Scholar]
- 39.Kim M.-J., Ryu G.R., Chung J.-S., Sim S.S., Rhie D.-J., Yoon S.H., et al. Protective effects of epicatechin against the toxic effects of streptozotocin on rat pancreatic islets: in vivo and in vitro. Pancreas. 2003;26(3):292–299. doi: 10.1097/00006676-200304000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Anderson R.A., Polansky M.M. Tea enhances insulin activity. J Agric Food Chem. 2002;50(24):7182–7186. doi: 10.1021/jf020514c. [DOI] [PubMed] [Google Scholar]
- 41.Trautwein E.A., Koppenol W.P., De Jong A., Hiemstra H., Vermeer M.A., Noakes M., et al. Plant sterols lower LDL-cholesterol and triglycerides in dyslipidemic individuals with or at risk of developing type 2 diabetes; a randomized, double-blind, placebo-controlled study. Nutr Diabetes. 2018;8(1):1–13. doi: 10.1038/s41387-018-0039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao H., Ji Y., Li W., Liu Y., Fu R., Xiang M. Protective effects of the total coumarin fraction of Urtica dentata on experimental diabetic nephropathy in vitro and in vivo. Planta Med. 2015;81(15):1353–1360. doi: 10.1055/s-0035-1557866. [DOI] [PubMed] [Google Scholar]
- 43.Kang K.S., Lee W., Jung Y., Lee J.H., Lee S., Eom D.-W., et al. Protective effect of esculin on streptozotocin-induced diabetic renal damage in mice. J Agric Food Chem. 2014;62(9):2069–2076. doi: 10.1021/jf403840c. [DOI] [PubMed] [Google Scholar]
- 44.Islam M.N., Jung H.A., Sohn H.S., Kim H.M., Choi J.S. Potent α-glucosidase and protein tyrosine phosphatase 1B inhibitors from Artemisia capillaris. Arch Pharm Res. 2013;36(5):542–552. doi: 10.1007/s12272-013-0069-7. [DOI] [PubMed] [Google Scholar]
- 45.Borges Bubols G., da Rocha Vianna D., Medina-Remon A., von Poser G., Maria Lamuela-Raventos R., Lucia Eifler-Lima V., et al. The antioxidant activity of coumarins and flavonoids. Mini Rev Med Chem. 2013;13(3):318–334. doi: 10.2174/138955713804999775. [DOI] [PubMed] [Google Scholar]
- 46.Mustafa S.B., Akram M., Muhammad Asif H., Qayyum I., Hashmi A.M., Munir N., et al. Antihyperglycemic activity of hydroalcoholic extracts of selective medicinal plants Curcuma longa, Lavandula stoechas, Aegle marmelos, and Glycyrrhiza glabra and their polyherbal preparation in Alloxan-induced diabetic mice. Dose Response. 2019;17(2) doi: 10.1177/1559325819852503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sebai H., Selmi S., Rtibi K., Souli A., Gharbi N., Sakly M. Lavender (Lavandula stoechas L.) essential oils attenuate hyperglycemia and protect against oxidative stress in alloxan-induced diabetic rats. Lipids Health Dis. 2013;12(1):1–9. doi: 10.1186/1476-511X-12-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
On request, the data used to support the findings of this study may be obtained from the corresponding author.