Summary
Circular RNA (circRNA) is a special category of non-coding RNA that has garnered increasing attention in the exploration of lipid metabolism. However, the functional regulation mechanisms of circRNAs in obesity diseases remain unclear. By whole transcriptome sequencing, a total of 164 circular RNAs were found to exhibit differential expression between lean and obese individuals. RT-qPCR was used to detect significant expression of circMAPK9 in obese individuals, and it was closely related to BMI. Western blot, triglyceride detection, and Oil Red O staining were employed to investigate the role of circMAPK9/hsa-miR-1322/FTO in adipogenesis. In adipocytes, the connection between hsa-miR-1322 and circMAPK9 was verified using fluorescence in situ hybridization, luciferase reporter assay, and RNA immunoprecipitation. It was found that circMAPK9 competed for binding hsa-miR-1322 in the cytoplasm, weakening the inhibitory effect on FTO and promoting adipogenesis. Our study revealed the regulatory mechanism and important role of circMAPK9 in the process of adipogenesis.
Subject areas: Molecular biology experimental approach, Molecular network, Specialized functions of cells, Transcriptomics
Graphical abstract

Highlights
-
•
CircMAPK9 expression level was significantly correlated with BMI in obese patients
-
•
CircMAPK9 is involved in adipogenesis and acts as a key regulator
-
•
CircMAPK9 sponges hsa-miR-1322 to regulate FTO expression through a ceRNA mechanism
Molecular biology experimental approach; Molecular network; Specialized functions of cells; Transcriptomics
Introduction
Over the past 50 years, the prevalence of obesity as a chronic disorder has almost tripled throughout the world’s population, greatly increasing the risk of developing disorders including myocardial infarction, type 2 diabetes, hypertension, fatty liver, and several cancers, resulting in a decrease in life expectancy and life quality.1 Two years into the global coronavirus epidemic, evidence is growing that obesity is likewise a risk factor for serious complications from coronavirus.2 Therefore, studying obesity-related modulator molecules to prevent metabolic syndrome is an important task.
Adipogenesis, which differentiates from preadipocytes and matures into functional adipocytes, has received widespread attention due to its close association with the global epidemic of obesity and associated metabolic disorders.3 PPARγ and CEBPα are the two main fat-generating transcription factors at the core of this differentiation-regulating network, in a coordinated manner, they drive the whole process of final differentiation and regulate the transcription activation of numerous mature adipocytes markers including fatty acid binding proteins (FABP4), insulin receptors, and adiponectin.4 Working with these transcription factors, including DNA demethylase, histone modifiers, and a variety of epigenetic regulatory factors involved in the fat formation, their role can be to promote or limit lipogenesis.5 Fat accumulation is a major manifestation of obesity. The greatest fat pool forms in obesity because white adipocytes predominantly store energy, whereas bigger adipocytes may encounter hypoxic and mechanical stress and have higher levels of inflammatory cytokine and adipolysis release.6 Hence, it might be advantageous to control the overproduction of white fat.
The emergence of high-throughput sequencing technology has led to the revelation of the involvement of non-coding RNAs (ncRNAs) in lipogenesis, and the correlation between microRNA (miRNA)7 and long non-coding RNA (lncRNA)8 has increased. miRNA is a class of endogenous non-coding small RNA molecules with an average length of 18–24 nucleotides, it plays an important role in molecular genetic changes and post-translational modifications during biological development.9 Different miRNA play different functions during adipogenesis.10 In addition, has-miR-1322 related studies have found that it is mainly involved in the development of tumors through ceRNA mechanism.11,12 However, its function in adipogenesis is unknown.
The exploration of circular RNAs (circRNAs) in various biological processes for their functionality has recently gained attention, such as tumor development,13 neuron development,14 and blood vessel reconstruction.15 In addition, a large number of investigations have demonstrated that circRNAs have a part in regulating the metabolic activities of adipocytes. Zhang’s study identified 77 circRNAs upregulated and 40 circRNAs downregulated during brown fat formation.16 Additionally, their research displayed that the production of white fat was accompanied by significant numbers of circRNAs.17 According to reports, circPTK2 acts as a sponge for miR-182-5p, thereby regulating JAZF1 expression and influencing adipogenesis.18 In addition, studies have found hsa_circH19 lipogenic differentiation of adipose mesenchymal stem cells can be facilitated by direct targeting PTBP1.19 Moreover, circMAPK9 has been reported to be involved in the process of rheumatoid arthritis exacerbation as a sponge of miRNA,20 and the function of circMAPK9 in other life activities has not been reported. These studies provide compelling evidence for the crucial functionality of circRNAs in adipocyte differentiation. Nonetheless, the role of circRNAs in the context of obesity is yet to be comprehensively investigated.
In our study, the differences in circRNA expression in obese and lean individuals were analyzed by visceral adipose tissues (VATs) samples, and the important regulatory effects of circMAPK9 in adipogenesis were determined. The mechanism of action of circMAPK9 was further analyzed, and it was found that circMAPK9 acted as a sponge of has-miR-1322 through the ceRNA mechanism, which weakened the regulation of fat mass and obesity-associated protein (FTO). In conclusion, this study shows that circMAPK9 promotes adipogenesis and further offers new insights into the function of circRNA in lipid metabolism.
Results
Screening, identification, and clinical significance of circMAPK9
According to the background of the study, the expression profile of circRNA was first obtained by the whole transcriptome sequencing of obese and lean VATs (n = 8). A total of 164 differential expression circRNAs were obtained, of which 125 were raised among obese VAT samples and 39 were lowered (|Log2FC| > 0.5, p < 0.05, Figure 1A). The top four circRNAs highly expressed in obese VATs were selected for validation. Validate 20 pairs of tissues with RT-qPCR. hsa_circ_0008861, hsa_circ_0001566 and hsa_circ_0004039 were found to be significantly high in obese VATs, consistent with sequencing results. However, the hsa_circ_0008179 expression level was not considerably different (Figure 1B). Based on hsa_circ_0001566 parent gene MAPK9 important role in obesity development,21 hsa_circ_0001566 has drawn attention. A review of the human reference genome (GRCh37/hg19) pointed out that hsa_circ_0001566 (chr5:179688683-179707608) originated from chromosome 5q35.3 MAPK9 gene, formed from exons 2–5 after transcription by back-splicing, and hsa_circ_0001566 sequence confirmed by Sanger sequencing (Figure 1C). Thus, hsa_circ_0001566 was named circMAPK9. Besides, convergent and divergent primers were devised to amplify circMAPK9 from adipocytes using cDNA and genomic DNA (gDNA). According to the findings, the divergent primer could detect circMAPK9 in cDNA but not gDNA, thereby indicating that the back-splicing site was not formed by trans-splicing or genomic rearrangement. (Figure 1D). To further evaluate its properties, total RNA is treated with RNase R. CircMAPK9 resistance to RNase R is higher than that of linear MAPK9 mRNA, showing the circular configuration of circMAPK9 in adipocytes (Figure 1E). CircMAPK9 was shown by fluorescence in site hybridization (FISH) assay to be primarily expressed in the cytoplasm of preadipocytes (Figure 1F).
Figure 1.
Screening and identification of circMAPK9
(A) 164 differentially expressed circRNAs obtained by whole transcriptome sequencing of Visceral adipose tissues (VATs) from obese and lean individuals.
(B) The top 4 circRNAs highly expressed in obese individuals were validated in 20 pairs of VATs of obese and lean individuals.
(C) CircMAPK9 is looped by MAPK9 exons 2, 3, 4, and 5. Verified by Sanger sequencing, the red arrow indicates the splice site of circMAPK9 in the head and tail.
(D) The divergent and convergent primers of circMAPK9 were designed and amplified in cDNA and gDNA, respectively. The divergent primers detected circMAPK9 from cDNA but not from gDNA.
(E) Expression levels of circMAPK9 and MAPK9 in RNase R treated preadipocytes were detected by RT-qPCR. The amounts of circMAPK9 and MAPK9 mRNA were normalized to the amounts of the mock-treated samples.
(F) The localization of circMAPK9 in preadipocytes was detected by fluorescence in situ hybridization (FISH). Scale bar, 20 μm. ∗∗p < 0.01; ns (not significant). Data are represented as mean ± SD.
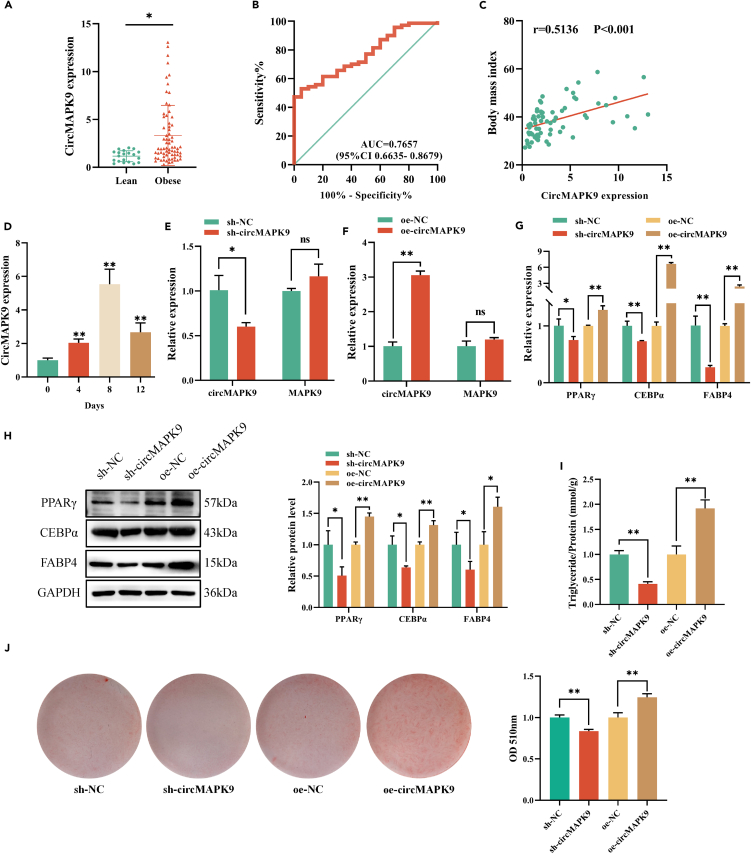
To further understand the clinical significance of circMAPK9 in obese individuals, we analyzed the expression level of circMAPK9 in VATs using RT-qPCR in a cohort of 70 obese and 20 lean individuals. The findings indicated that the circMAPK9 expression level was substantially more in obese VATs than in lean VATs (Figure 2A). ROC analysis showed that circMAPK9 had a good diagnostic value (AUC = 0.7657, 95%CI 0.6635–0.8679, Figure 2B). Additionally, using Spearman’s correlation coefficient method found that circMAPK9 expression levels were positively associated with BMI in 70 obese individuals (r = 0.513, p < 0.001, Figure 2C). Other outcomes suggested that circMAPK9 expression was associated with triglycerides (TG) levels and Homeostasis model assessment of insulin resistance (HOMA-IR, Table 1). At the same time, circMAPK9 may be expressed in conjunction with the formation of non-alcoholic fatty liver disease (NAFLD), Type II Diabetes, and Hyperuricemia (Table 1). After that, the association among NAFLD and circMAPK9 expression levels in 70 obese individuals were examined, and it is discovered that circMAPK9 expression level in patients with NAFLD was considerably more in comparison to that in those without the disease (p < 0.01, Figure S1). The ROC curve indicated that the circMAPK9 expression was clinically significant for the diagnosis of NAFLD (AUC = 0.7947, 95%CI0.6693–0.9200, Figure S2).
Figure 2.
CircMAPK9 promotes lipogenic differentiation of preadipocytes
(A) Differential expression of circMAPK9 between obese (n = 70) and lean (n = 20) individuals.
(B) ROC curve circMAPK9 indicates the diagnostic value for obese patients.
(C) Correlation between circMAPK9 expression and BMI in 70 obese patients.
(D) Expression of circMAPK9 at days 0, 4, 8 and 12 during preadipocyte differentiation.
(E and F) RT-qPCR was used to detect the expression levels of circMAPK9 and MAPK9 after infecting preadipocytes with lentiviruses that silenced and overexpressed circMAPK9.
(G) Compared with the control group, the mRNA levels of the markers PPARγ, CEBPα, and FABP4 in the sh-circMAPK9 group were significantly decreased, while the levels of markers in the oe-circMAPK9 group were significantly up-regulated on the 8th day of adipogenesis.
(H) Western blot analysis showed that the protein levels of PPARγ, CEBPα, and FABP4 were significantly impaired in the sh-circMAPK9 group compared with the control group at day 8 of adipogenesis, while oe-circMAPK9 group significantly promoted their expression.
(I) Triglyceride levels were decreased and increased in sh-circMAPK9 and oe-circMAPK9 groups, respectively, compared with the control group at day 8 of adipogenesis.
(J) Oil Red O staining was used to evaluate the effect of circMAPK9 expression on lipid droplet accumulation at day 8 of adipogenesis. ∗p < 0.05; ∗∗p < 0.01; ns (not significant). Data are represented as mean ± SD.
Table 1.
CircMAPK9 expression in the obese sample
| Clinical or molecular feature | CircMAPK9 down-expression (35) | CircMAPK9 over-expression (35) | P |
|---|---|---|---|
| Age (y) | 33 (29, 39) | 30 (26, 40) | 0.445 |
| BMI (kg/m2) | 35.26 ± 4.92 | 41.66 ± 7.28 | < 0.001 |
| HbA1c (%) | 5.9 (5.5, 6.25) | 6 (5.55, 6.55) | 0.441 |
| HOMA-IR | 3.11 (2.21, 4.22) | 4.4 (3.28, 5.69) | 0.004 |
| TC (mmol/L) | 4.46 (4.06, 5.4) | 4.64 (4.29, 5.37) | 0.356 |
| TG (mmol/L) | 1.24 (0.86, 1.96) | 1.81 (1.36, 3) | 0.018 |
| LDL-C (mmol/L) | 2.52 (2.33, 3.41) | 3.11 (2.71, 3.45) | 0.096 |
| HDL-C (mmol/L) | 1.19 (1.02, 1.36) | 1.06 (0.96, 1.24) | 0.055 |
| Hypertension | 51% (18/35) | 57% (20/35) | 0.810 |
| Type Ⅱ Diabetes | 34% (12/35) | 66%(23/35) | 0.017 |
| Hyperlipemia | 34% (12/35) | 51% (18/35) | 0.227 |
| Hyperuricemia | 17% (6/35) | 49% (17/35) | 0.011 |
| OSAS | 23% (8/35) | 29% (10/35) | 0.784 |
| NAFLD | 60% (21/35) | 91% (32/35) | 0.005 |
| Cholelithiasis | 6% (2/35) | 9% (3/35) | 1.000 |
BMI, Body mass index; HbA1c, Glycated hemoglobin; HOMA-IR, Homeostasis model assessment of insulin resistance; TC, Total cholesterol; TG, Triglycerides; LDL-C, Low-density lipoprotein cholesterol; HDL-C, High-density lipoprotein cholesterol; OSAS, Obstructive sleep apnea syndrome; NAFLD, Non-alcoholic fatty liver disease.
CircMAPK9 promotes lipogenic differentiation of preadipocytes
We extract primary preadipocytes from VATs and induce them to differentiate into mature adipocytes, as in previous studies.22 It was found that in the process of inducing lipogenesis of preadipocytes, the expression level of circMAPK9 increased significantly in the 4, 8, and 12 days compared with the 0 days (Figure 2D). Based on the circMAPK9 sequence, we designed silencing and overexpressing vectors that were transfected into preadipocytes to silence and overexpress circMAPK9 expression levels. Under fluorescence microscope, the positive proportion of green fluorescent positive cells reached more than 90% (Figure S3), and RT-qPCR was employed for verifying transfection efficiency. When circMAPK9 expression was silenced, circMAPK9 expression level was considerably reduced, whereas that of MAPK9 was not significantly different (Figure 2E). Moreover, when circMAPK9 was overexpressed, circMAPK9 expression level increased significantly, while the expression level of MAPK9 did not change (Figure 2F), indicating that the expression level of MAPK9 was not regulated by circMAPK9. On day eight of adipogenesis, PPARγ, CEBPα, and FABP4 expression levels in stably silenced and overexpressed circMAPK9 adipocytes were detected. The outcomes of RT-qPCR and Western blot findings indicated that when circMAPK9 was silenced, PPARγ, CEBPα, and FABP4 expression levels were considerably decreased compared with the control group. When circMAPK9 was overexpressed, PPARγ, CEBPα, and FABP4 expression levels were considerably increased compared to the control group (Figures 2G and 2H). We further examined intracellular triglyceride levels and found that intracellular triglyceride levels were reduced when circMAPK9 was silenced and enhanced when circMAPK9 was overexpressed (Figure 2I). In addition, Oil Red O staining findings revealed that lipid droplet production was reduced when circMAPK9 was silenced, and enhanced when circMAPK9 was overexpressed (Figure 2J). These findings suggest that circMAPK9 is a part of the process of adipogenesis and affects the formation of triglycerides.
CircMAPK9 acts as a sponge for hsa-miR-1322
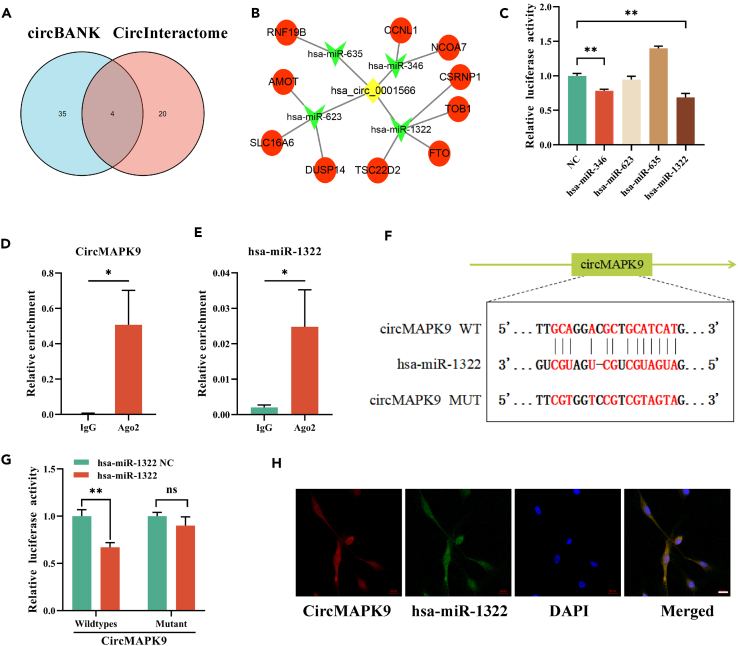
According to prior investigations, circRNA can act as a sponge for miRNA in the cytoplasm.23 Based on the fact that circMAPK9 was primarily localized in the cytoplasm of preadipocyte, we further explored whether circMAPK9 could serve as a miRNA sponge to regulate downstream target genes through the ceRNA mechanism. circBANK and CircInteractome databases were employed for predicting miRNAs that circMAPK9 might bind to. The outcomes presented that four miRNAs were hsa-miR-346, hsa-miR-623, hsa-miR-635 and hsa-miR-1322 (Figure 3A). Then, the circMAPK9-miRNA-mRNA interaction network was constructed (Figure 3B). We first screened bound miRNAs by dual luciferase assay. hsa-miR-346 mimic, hsa-miR-623 mimic, hsa-miR-635 mimic, and hsa-miR-1322 mimic were co-transfected into 293T cells with wild-type circMAPK9 reporter plasmid, respectively. The luciferase activity of hsa-miR-1322 group was significantly decreased (Figure 3C). In the mechanism of ceRNA, AGO2 is generally involved in the binding of circRNA and miRNA.24 RNA immunoprecipitation (RIP) outcomes exhibited that circMAPK9 and hsa-miR-1322 were significantly enriched by AGO2 antibody (Figures 3D and 3E), which proved the binding relationship between them. To detect the predicted binding sites of circMAPK9 and hsa-miR-1322 (Figure 3F), we carried out a dual luciferase assay. The outcomes indicated that the luciferase activity was significantly reduced following the co-transfection of wild-type circMAPK9 reporter plasmid and hsa-miR-1322 mimic, and no substantial change exists in luciferase activity when the target site of hsa-miR-1322 was mutated (Figure 3G). In addition, the findings of FISH assay highlighted that circMAPK9 and hsa-miR-1322 were co-localized in the cytoplasm of preadipocytes (Figure 3H). These findings proved that circMAPK9 could bind to hsa-miR-1322 directly.
Figure 3.
CircMAPK9 acts as a sponge for hsa-miR-1322
(A) The circBANK and CircInteractom databases predict the miRNAs that circMAPK9 may bind.
(B) CircMAPK9-miRNA-mRNA interaction network.
(C) 293T cells were co-transfected with miRNA mimic and luciferase reporter plasmid of wild-type circMAPK9 to detect luciferase activity.
(D and E) AGO2 RIP method was used to detect the enrichment of circMAPK9 and hsa-miR-1322.
(F) Schematic diagram of the predicted hsa-miR-1322 binding site on circMAPK9.
(G) Luciferase activity was detected in 293T cells after the co-transfection of wild-type or mutant circMAPK9 reporter plasmid with hsa-miR-1322 or miRNA controls.
(H) Colocalization between circMAPK9 and hsa-miR-1322 was observed in preadipocytes by FISH. Scale bar, 20 μm. ∗p < 0.05; ∗∗p < 0.01; ns (not significant). Data are represented as mean ± SD.
hsa-miR-1322 inhibited adipogenesis
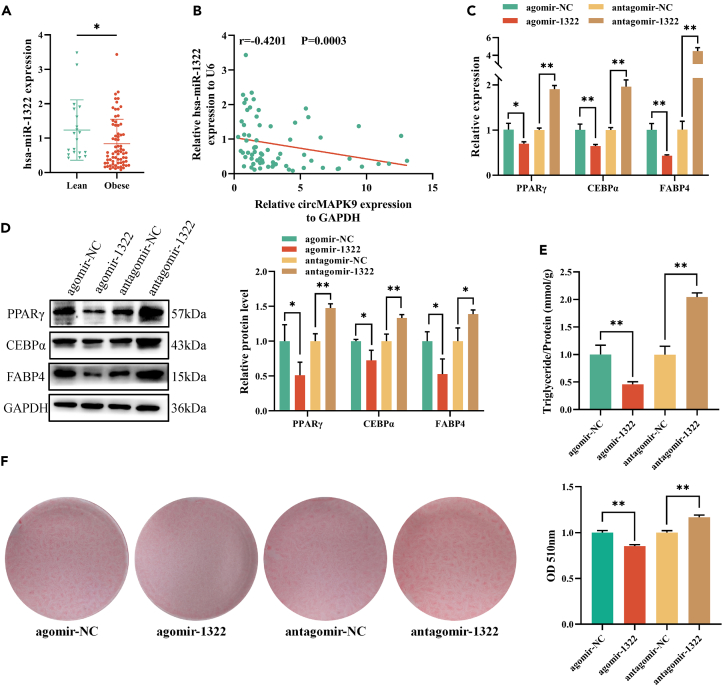
In comparison to the lean group, we discovered that the expression level of hsa-miR-1322 was very lower in the obese group (Figure 4A). Meanwhile, hsa-miR-1322 was significantly down-regulated on day 8 of adipogenic differentiation (Figure S4). Moreover, hsa-miR-1322 expression level was considerably negatively linked to the circMAPK9 expression level in 70 obese patients (r = −0.4201, p = 0.0003, Figure 4B). miRNA agomir and antagomir are specially chemically modified miRNA agonists and antagonists, which can specifically enhance or inhibit the function of hsa-miR-1322. RT-qPCR and Western blot outcomes presented that PPARγ, CEBPα, and FABP4 expression levels were significantly lowered after transfection with agomir-1322 in contrast to the control group (Figures 4C and 4D). Besides, after antagomir-1322 transfection, the PPARγ, CEBPα, and FABP4 expression levels were considerably increased compared with the control group (Figures 4C and 4D). Intracellular triglyceride levels were further examined and found to be decreased after the transfection of agomir-1322 and increased after the transfection of antagomir-1322 (Figure 4E). Oil Red O staining outcomes suggested that lipid droplets production decreased after the transfection of agomir-1322, and increased after the transfection of antagomir-1322 (Figure 4F). These results indicated that hsa-miR-1322 was involved in the process of adipose maturation and affected the formation of triglyceride.
Figure 4.
hsa-miR-1322 inhibited adipogenesis
(A) Differential expression of hsa-miR-1322 between obese (n = 70) and lean (n = 20) individuals.
(B) Correlation between expression levels of circMAPK9 and hsa-miR-1322 in 70 obese patients.
(C) mRNA expression levels of the markers PPARγ, CEBPα, and FABP4 at day 8 of adipogenesis after agomir-1322 and antagomir-1322 transfection compared with control group.
(D) Western blot was used to evaluate the protein expression levels of PPARγ, CEBPα, and FABP4 at day 8 of adipogenesis after agomir-1322 and antagomir-1322 transfection.
(E) Triglyceride levels of agomir-1322 and antagomir-1322 groups compared with control group at day 8 of adipogenesis.
(F) Oil Red O staining was used to detect lipid accumulation in adipocytes after treatment with agomir-1322 and antagomir-132. ∗p < 0.05; ∗∗p < 0.01. Data are represented as mean ± SD.
Fat mass and obesity-associated protein is the downstream target of hsa-miR-1322 and is involved in adipogenesis
In this study, miRDB, TargetScan, and miRWalk databases were employed for predicting the downstream target genes of hsa-miR-1322, and 46 possible targets were found (Figure 5A). We found that FTO is one of the targets and that FTO can promote autophagy and participate in adipogenesis.25 First, we verified the expression of FTO in tissue samples. FTO was considerably more in the VAT of obese patients than in the lean group (Figure 5B). And it was considerably negatively associated with the expression level of hsa-miR-1322 in 70 obese VATs (r = −3237, p = 0.0063, Figure 5C). We further built a luciferase reporter vector with wild-type or mutant FTO 3′ untranslated region (UTR) binding site of hsa-miR-1322 (Figure 5D). The FTO 3′ UTR wild-type reporter’s luciferase activity was considerably decreased in 293T cells transfected with hsa-miR-1322 mimic (Figure 5E). However, when transfected into the mutant FTO 3′ UTR reporter, no discernible variation in luciferase activity was seen.
Figure 5.
FTO is the downstream target of hsa-miR-1322 and is involved in adipogenesis
(A) The downstream target genes of hsa-miR-1322 were predicted by miRDB, TargetScan, and miRWalk databases.
(B) Differential expression of FTO between obese (n = 70) and lean (n = 20) individuals.
(C) Correlation between the expression levels of hsa-miR-1322 and FTO in 70 obese patients.
(D) Scheme exhibiting the complementary sequence between FTO and hsa-miR-1322.
(E) Luciferase activity of wild-type or mutant FTO 3′ UTR in 293T cells after co-transfection with hsa-miR-1322 or miRNA control.
(F) RT-qPCR was used to detect the silencing efficiency of FTO by siRNA.
(G) RT-qPCR was used to detect the mRNA expression levels of PPARγ, CEBPα, and FABP4 after silencing FTO at day 8 of adipogenesis.
(H and I) Western blot was used to evaluate the protein expression levels of PPARγ, CEBPα, and FABP4 after silencing FTO at day 8 of adipogenesis.
(J) Changes in triglyceride level after silencing FTO at day 8 of adipogenesis.
(K) Correlation between the expression levels of FTO and circMAPK9 in 70 obese patients. ∗p < 0.05; ∗∗p < 0.01; ns (not significant). Data are represented as mean ± SD.
We created and produced siRNA that specifically targets FTO and transfected it into adipocytes to verify the silencing expression efficiency for investigating the function of FTO in adipocytes. si-FTO#1 and si-FTO#2 were selected to interfere with the expression of FTO for further experiments (Figure 5F). At day 8 of adipogenesis, cells were harvested for RT-qPCR and Western blot, and the expression levels of PPARγ, CEBPα, and FABP4 were considerably reduced when FTO was down-regulated (Figures 5G–5I). Meanwhile, triglyceride levels were significantly decreased after silencing FTO expression (Figure 5J). In 70 obese VATs, it was found that the FTO expression level was positively associated with circMAPK9 expression level (r = 0.3644, p = 0.0019, Figure 5K).
CircMAPK9 promoted adipogenesis by alleviating the inhibitory impact of hsa-miR-1322 on fat mass and obesity-associated protein
To confirm whether circMAPK9 acts on adipocytes through hsa-miR-1322, we observed that silencing circMAPK9 significantly inhibited adipogenesis. In addition, when the hsa-miR-1322 inhibitor was transfected into the cells with stably down-regulated circMAPK9 expression, the hsa-miR-1322 inhibitor lowered the inhibitory impact of sh-circMAPK9 on adipogenesis (Figures 6A–6D). As a result, these outcomes point out that circMAPK9 regulates adipogenesis by regulating FTO expression through hsa-miR-1322. Then, we looked into whether FTO is required for circMAPK9/hsa-miR-1322 axis regulation in adipocytes. The findings demonstrated that circMAPK9 overexpression significantly enhanced adipogenesis, while in the absence of FTO, the adipogenesis induced by circMAPK9 overexpression was restored (Figures 6E–6H). In summary, circMAPK9 acts as a sponge for hsa-miR-1322 for regulating FTO expression through the ceRNA mechanism and regulating adipogenesis.
Figure 6.
CircMAPK9 promoted adipogenesis by alleviating the inhibitory impact of hsa-miR-1322 on FTO
(A–D) Western blot, RT-qPCR, Oil Red O staining, and triglycerides measurement were performed in mature adipocytes treated with negative control, sh-circMAPK9, and sh-circMAPK9 + antagomir-1322.
(E–H) Western blot, RT-qPCR, Oil Red O staining, and triglycerides measurement were performed in mature adipocytes treated with negative control, oe-circMAPK9, and oe-circMAPK9 + si-FTO. ∗p < 0.05; ∗∗p < 0.01; ns (not significant). Data are represented as mean ± SD.
CircMAPK9 regulates lipid deposition in vivo
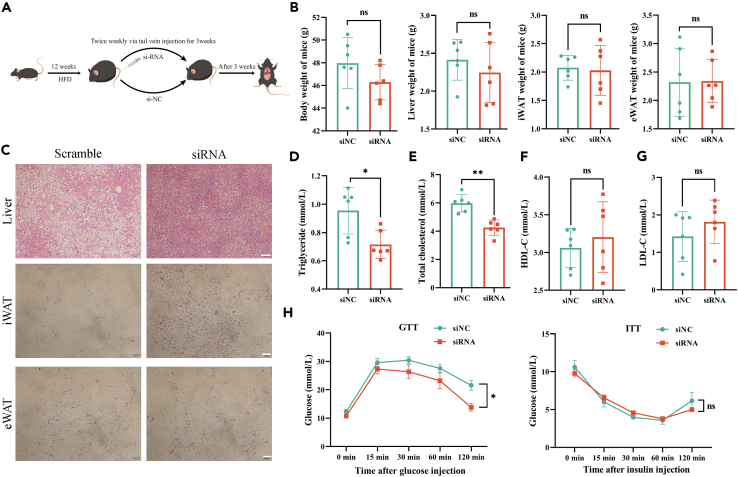
To further confirm whether circMAPK9 plays a similar function in vivo, we first searched for sequences homologous to human circMAPK9, murine chr11:49,863,543-49,869,310 (hereafter referred to as mmu-circMAPK9) was found to share 91.2% identity with human-derived circMAPK9 (Figure S5A). Back-splice site and primer uniqueness were determined by Sanger sequencing and agarose gel electrophoresis (Figures S5B and S5C). siRNA was designed and knockdown efficiency was verified in mouse cells, and it was found that si-mmu-circMAPK9#1 could significantly silence mmu-circMAPK9 expression (Figure S6). Then, we created an obese mouse model by feeding it an HFD for 12 weeks, 40nmol siRNA (si-mmu-circMAPK9#1) was injected each time through the tail vein 2 times in a week for three weeks, and mouse were sacrificed 3 weeks after the end of treatment (Figure 7A). No discernible disparity exists in body weight, liver, inguinal white adipose tissue (iWAT) and epididymal white adipose tissue (eWAT) between the treatment group and the control group (Figure 7B). However, hematoxylin/eosin staining of liver, iWAT, and eWAT tissues revealed that obese mice had reduced ectopic lipid buildup in the liver and smaller adipocytes in iWAT and eWAT after siRNA injection compared with control mice (Figure 7C).
Figure 7.
CircMAPK9 regulates lipid deposition in vivo
(A) Mice fed a high-fat diet for 12 weeks were treated with siNC (n = 6) and siRNA (n = 6) by tail vein injection twice a week for 3 weeks.
(B) Mice were sacrificed after week 16, and total body weight and liver, inguinal white adipose tissue (iWAT), and epididymal white adipose tissue (eWAT) weights were measured.
(C) Hematoxylin/Eosin staining of liver (Scale bar, 50 μm), iWAT (Scale bar, 200 μm), and eWAT (Scale bar, 200 μm).
(D–G) Plasma levels of triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were measured.
(H) Glucose and insulin were injected intraperitoneally, and glucose tolerance and insulin sensitivity of the two groups of mice were detected. ∗p < 0.05; ∗∗p < 0.01; ns (not significant). Data are represented as mean ± SD.
In addition, we examined the changes in TG, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels in obese mice after siRNA injection. According to the findings, TG and TC plasma levels in the treated group were extremely lower in comparison to those in the control group (Figures 7D and 7E). While the HDL-C and LDL-C plasma level was not significantly changed (Figures 7F and 7G). And the siRNA-treated obese mice had considerably higher glucose tolerance when compared to the control group, whereas there was no discernible disparity in insulin sensitivity among the two groups (Figure 7H).
Discussion
The rapid advancement of high-throughput sequencing illustrates the changes in genomics in the process of disease development and provides new ideas for disease treatment. Numerous data indicate that ncRNAs are key regulators of genes encoded at transcriptional or post-transcriptional levels in a wide range of metabolic disorders, including lipid metabolism.26,27 By whole genome sequencing, it is discovered that circMAPK9 was significantly expressed in obese patients, played a role in the regulation of adipogenesis and affected the formation of triglyceride. Therefore, circMAPK9 plays a ceRNA mechanism and alleviates the inhibitory effect on FTO by competitive binding with hsa-miR-1322. Our study demonstrates the important role and mechanism of circMAPK9 in adipogenesis and illustrates the therapeutic and diagnostic significance of circMAPK9 in obese patients.
CircRNAs are closed-loop non-coding RNAs that lack 5 ′-cap structure and poly (a) tail, are not impacted by RNA exonucleases, and can exist stably in body fluids. They are uniquely expressed in various developmental stages, tissues, and diseases, making them a suitable targeted analysis for disease diagnosis and treatment.28,29 Many investigations have demonstrated that circRNA has a crucial part in the regulation mechanism of lipid metabolism.30 Zhang’s study found that circSAMD4A regulated the differentiation of preadipocytes by increasing the expression of EZH2 by acting as a miR-138-5p sponge, and expression level circSAMD4A expression level was strongly connected with the weight gain of obese patients after bariatric surgery.31 We demonstrated for the first time that silencing circMAPK9 expression could affect adipogenesis and subsequently reduce triglyceride levels. In addition, circMAPK9 is involved in adipogenesis through the ceRNA network. Silencing circMAPK9 could inhibit FTO expression and has an impact on the PPARγ, CEBPα, and FABP4 expression levels in adipocytes in vitro. Meanwhile, hsa-miR-1322 inhibitor could partially rescue this effect through circMAPK9/hsa-miR-1322 sponge effect. And in vivo intervention of circMAPK9 expression affected triglyceride levels and improved fat accumulation. CircMAPK9 may therefore be a possible target for the therapy of illnesses associated with obesity. Nevertheless, more studies are needed in the future to apply it to the treatment of obesity.
One of the most common functional models for circRNAs to be found to exert functional effects is that circRNAs act as ceRNAs to sponge miRNAs, competing with linear targets bound by RNA-induced silencing complex (RISC). AGO2, a crucial element of RISC, has demonstrated that it is essential for mediating the interaction among miRNAs and circRNAs.32,33 Studies have found that AGO2 is involved in the interaction between circRNF111 and miR-143-3p to affect lipid deposition.34 Moreover, circArhgap5-2 was not significantly enriched in the process of RIP by AGO2 antibody, considering that circArhgap5-2 is unlikely to play a regulatory role in adipogenesis by sponge miRNA.35 In our study, four miRNAs that could bind to circMAPK9 were predicted by the database, and hsa-miR-1322 was screened out by dual luciferase assays experiment. Further, RIP experiments showed that AGO2 antibodies could significantly enrich circMAPK9 and hsa-miR-1322. Meanwhile, dual-luciferase assays demonstrated binding sites of both. FISH outcomes showed that circMAPK9 and hsa-miR-1322 were significantly co-localized in the cytoplasm. The experimental findings revealed that circMAPK9 served as a sponge for hsa-miR-1322 to further regulate adipogenesis. Studies have found that hsa-miR-1322 can not only be adsorbed by circMAPK9 sponge, but also in the regulation of tumor, circSMARCC1 binds hsa-miR-1322 to promote CCL20 secretion and affect the development of prostate cancer.12 And, circ_0000291 regulates UBE2T expression by targeting hsa-miR-1322 and promotes the progression of hepatocellular carcinoma.36 It indicates that hsa-miR-1322 is involved in ceRNA mechanism and is significant in biological processes. Furthermore, numerous investigations have discovered that miRNA plays a role in the regulation of lipid metabolism. Wang’s study found that microRNA-138 could inhibit the expression of lipoprotein lipase and reduce adipogenesis.37 miR-142-3p affects the adipogenesis process by targeting KLF9 to inhibit the expression of autophagy-related genes.38
FTO is a demethylase encoding m6A, which regulates RNA methylation levels and participates in the control of lipid metabolism and glucose, and has a deep relationship with metabolic diseases.39,40,41 In this study, circMAPK9 could significantly promote FTO expression and promote adipogenesis by sponge adsorption of hsa-miR-1322. Myrte’s study found that FTO affected adipogenesis through the regulation of mitotic clonal expansion.42 Meanwhile, FTO-deficient mice foster the conversion of white fat to brown and beige fat.43 This demonstrates how crucial a part FTO plays in the regulation of lipid metabolism. Additionally, FTO promotes SREBP1C’s nuclear translocation, mRNA processing, and translation and promotes hepatic lipid synthesis.44 In this study, it was found in animal experiments that the injection of siRNA affected the accumulation of liver fat, which may be one of the main reasons for the function of circMAPK9.
In summary, our data suggest a novel regulatory role of circMAPK9 during adipogenesis through the hsa-miR-1322/FTO pathway and that silencing circMAPK9 expression in vivo can significantly improve triglyceride levels. The current study offers novel perspectives into the function and governing mechanisms of circRNA in adipogenesis.
Limitations of the study
CircRNAs may have multiple functions, and our study only explored the experimental verification of circMAPK9 as a ceRNA mechanism. There may be other regulatory mechanisms of circMAPK9 that need to be further explored. In addition, the types of adipose tissue include white adipose tissue, brown adipose tissue, and beige adipose tissue. Our study only induced preadipocytes to differentiate into white adipocytes to explore the function of circMAPK9. It is also necessary to further clarify whether circMAPK9 participates in the differentiation process of other types of adipocytes. Finally, our clinical sample size was relatively small, and it is necessary to further expand the sample size for further analysis.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-PPARγ antibody | Abmart | Cat# PA4778; RRID: AB_2940815 |
| Anti-CEBPα antibody | Abmart | Cat# TA6333; RRID: AB_2940813 |
| Anti-FABP4 antibody | Abmart | Cat# T56768; RRID: AB_2940814 |
| Anti-GAPDH antibody | Proteintech | Cat# 10494-1-AP; RRID: AB_2263076 |
| HRP-conjugated Affinipure Goat Anti-Rabbit IgG | Proteintech | Cat# SA00001-2; RRID: AB_2722564 |
| Biological samples | ||
| Omental adipose tissue | The Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Dexamethasone | Aladdin | D137736 |
| 3-Isobutyl-1-methylxanthine (IBMX) | Aladdin | I106812 |
| Insulin | Aladdin | I189675 |
| Oil Red O | Sigma | O0625 |
| Collagen Type Ⅰ | Gibco | 17100-017 |
| Critical commercial assays | ||
| HiScript II Q Select RT SuperMix for qPCR | Vazyme | R233-01 |
| AceQ qPCR SYBR Green Master Mix | Vazyme | Q131-02 |
| Ribonuclease R (RNase R) | Geneseed | R0301 |
| Magna RIP kit | Millipore | merck.17-700 |
| miDETECT A TrackTM miRNA RT-qPCR Starter Kit | RiboBio | R110068.5 |
| Ribo™ Fluorescent In Situ Hybridization Kit | RiboBio | R11060.7 |
| Triglyceride assay | Jiancheng Bioengineering Institute | A110-1-1 |
| Lipo6000™ Transfection Reagent | Beyotime | C0526 |
| Deposited data | ||
| Raw and analyzed data | This paper | GEO: GSE235696 |
| Experimental models: Cell lines | ||
| Preadipocytes | Isolated from omental adipose tissue | N/A |
| Experimental models: Organisms/strains | ||
| C57BL/6 mice | Cavens Laboratory Animal Co., Ltd. | N/A |
| Oligonucleotides | ||
| hsa_circ_0008861 Forward (5’ to 3’): TGGCAGCAGTTGGCAAAGAA | This paper | N/A |
| hsa_circ_0008861 Reverse (5’ to 3’): TCACCAGCAGATGCAAATCA | This paper | N/A |
| hsa_circ_0008179 Forward (5’ to 3’): CATGTTCCTCTTTTCCTACC | This paper | N/A |
| hsa_circ_0008179 Reverse (5’ to 3’): AGGGGCTTCTTGAAATAGCA | This paper | N/A |
| hsa_circ_0001566 Forward (5’ to 3’): AGCTGGATCATGAAAGAATGT | This paper | N/A |
| hsa_circ_0001566 Reverse (5’ to 3’): GGCAAGTTTCAGATCCTCTA | This paper | N/A |
| hsa_circ_0004039 Forward (5’ to 3’): TGGAGAACCACTCTGGAATA | This paper | N/A |
| hsa_circ_0004039 Reverse (5’ to 3’): CCAAGTGTTCAAGGCCATAG | This paper | N/A |
| GAPDH Forward (5’ to 3’): CATGTTCCAATATGATTCCAC | This paper | N/A |
| GAPDH Reverse (5’ to 3’): CCTGGAAGATGGTGATG | This paper | N/A |
| MAPK9 Forward (5’ to 3’): GTCATCCTGGGTATGGGCTACAAAG | This paper | N/A |
| MAPK9 Reverse (5’ to 3’): ATCACACAACCTTTCACCAGCTCTC | This paper | N/A |
| PPARγ Forward (5’ to 3’): GCCCTTCACTACTGTTGACTTCTCC | This paper | N/A |
| PPARγ Reverse (5’ to 3’): CAGGCTCCACTTTGATTGCACTTTG | This paper | N/A |
| CEBPα Forward (5’ to 3’): TCGGTGGACAAGAACAGCAACG | This paper | N/A |
| CEBPα Reverse (5’ to 3’): GGCGGTCATTGTCACTGGTCAG | This paper | N/A |
| FABP4 Forward (5’ to 3’): TGGTACATGTGCAGAAATGGGATGG | This paper | N/A |
| FABP4 Reverse (5’ to 3’): TGACGCATTCCACCACCAGTTTATC | This paper | N/A |
| FTO Forward (5’ to 3’): TCAACTGGAAGCACTGTGGAAGAAG | This paper | N/A |
| FTO Reverse (5’ to 3’): CGAGGCAAGGATGGCAGTCAAG | This paper | N/A |
| Mmu-circMAPK9 Forward (5’ to 3’): CAGGTTATTCATATGGAACTGGAC | This paper | N/A |
| Mmu-circMAPK9 Reverse (5’ to 3’): TGCCTTCGTCAGACCCTCT | This paper | N/A |
| sh-circMAPK9 (5’ to 3’): TAATTCATAGAGGATCTGA | This paper | N/A |
| si-FTO | RiboBio | N/A |
| si-mmu-circMAPK9#1 (5’ to 3’): GTATCATTCATAGAGGTCT | This paper | N/A |
| si-mmu-circMAPK9#2 (5’ to 3’): TTCATAGAGGTCTGACGAA | This paper | N/A |
| si-mmu-circMAPK9#3 (5’ to 3’): ATCATTCATAGAGGTCTGA | This paper | N/A |
| Agomir-NC | RiboBio | N/A |
| Agomir-1322 | RiboBio | N/A |
| Antagomir-NC | RiboBio | N/A |
| Antagomir-1322 | RiboBio | N/A |
| Software and algorithms | ||
| GraphPad Prism 8 | GraphPad | N/A |
| SPSS 19.0 | IBM | N/A |
Resource availability
Lead contact
The relevant experimental reagents, experimental methods and related data of this study can be obtained by contacting Liming Tang (drtangliming@163.com).
Materials availability
The study did not generate new unique reagents.
Experimental model and study participant details
Clinical samples
The VATs used in the experiment were derived from the Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University. The obese patients had VATs derived from bariatric surgery (BMI ≥ 28kg/m2, 18 men aged 22-40 years and 52 women aged 18-60 years).45 The control group had VATs derived from hernia repair surgery (18.5 < BMI ≤ 23.9kg/m2 without metabolic disease, 20 men aged 18-60 years). All the patients are of Asian ethnicity, primarily from the Jiangsu province in China. The Ethics Committee of the Second People's Hospital of Changzhou City (batch number: 2021YLA001) has approved this study, and all patients who have been specimens have been informed and signed an informed consent form before the operation. The specimens were treated with omentum adipose tissue of about 1cm3, which was then promptly frozen in liquid nitrogen, returned to the lab, kept in a -80°C refrigerator, and recorded the patient's relevant clinical information.
Preadipocytes extraction and culture
From the VAT of obese individuals undergoing bariatric surgery, 2-3cm3 of adipose tissue was removed. With the help of phosphate-buffered saline (PBS), the tissue is flushed repeatedly. Cut the connective tissue and blood vessels out before using scissors to treat adipose tissue into a melt. Then the tissue is fully soaked in 0.1% collagen type I (Gibco), slowly oscillating for thirty minutes in a 37°C constant temperature oscillating box. After 30 minutes, 200 mesh screen was used for filtration, then the liquid was removed centrifugally. The cells were then incubated at 37°C in a 5 percent CO2 incubator in DMEM/F12 media (Gibco) with 100 U/mL penicillin, 100 g/mL streptomycin complete medium, and 10 percent fetal bovine serum (FBS, Gibco).
In vivo experiments
We obtained 12 male C57BL/6 mice aged 8 weeks from Cavens Laboratory Animal Co., Ltd. In a pathogen-free environment with a 12-hour light/dark cycle, 22°C, and 40–50% humidity, all mice were housed. High-fat diet (HFD; D12492, Research Diets, Inc., USA) feeding for 12 weeks builds obese mouse models that measure weight weekly. After 12 weeks, two groups of six mice were split into control and siRNA groups at random. Each mouse received 40nmol siRNA intravenously and was treated two times a week for three weeks. In the third week after treatment, the mice were put to death, collecting blood, inguinal white adipose tissues (iWAT), epididymal white adipose tissues (eWAT), and liver. After being quickly frozen in liquid nitrogen and stored at −80°C, tissue and plasma samples are examined histologically and biochemically. The iWAT, liver, and eWAT are fixed with 4% formaldehyde, and buried with paraffin wax, then the tissue is cut into 4μm thick slices for Hematoxylin/Eosin staining, photoscopic evaluation. All animal investigations are carried out based on the directions of the Experimental Animal Care Committee (batch number: IACUC22-0104).
Method details
Sequencing of RNAs and data analysis
After the sample's total RNA has been extracted and ribosomal RNA is digested using the kit ribo-zero. Utilizing the interrupted RNA as a template, add the interrupt reagent to split the RNA into a short fragment. cDNA strand synthesis using a six-base random primer. Then, a two-stranded synthesis reaction system is created to synthesize two-stranded cDNA. dUTP instead of dTTP in cDNA two-stranded synthesis, then connect different joints and digest a chain containing dUTP using the UNG enzyme. Only one strand of cDNA that connects to different joints is preserved; Use of kits to purify one strand of cDNA; The purified cDNA was sequenced, repaired, and A-tailed, then fragment size was chosen, and PCR amplification was carried out. Built library with Agilent 2100 Bioanalyzer quality test, sequenced with Illumina sequencer.
CIRI (v2.0.3) was used to identify circRNAs, and spliced reads per millon mapping (RPM) was used to estimate their expression.46 The R package DESeq (2012) was utilized for conducting the differential expression analysis. CircRNAs showing Log2|fold change| > 0.5 and P value < 0.05 were considered to be significantly differentially expressed. The original sequencing dataset supporting the results of this study is stored in the NCBI GEO database. These data are available at GEO: GSE235696.
Total RNA isolation and quantitative real-time PCR (RT-qPCR)
Following trizol's instructions, total RNA was extracted, and Vazyme HiScript II Q RTSuper mix (Vazyme, China) was used for reverse transcription. The qPCR was conducted using the AceQ qPCR SYBR Green Master Mix (Vazyme, China). In addition, for miRNAs, miDETECT A Track™ miRNA RT-qPCR Starter Kit (RiboBio, China) was employed for performing reverse transcription and qPCR. GAPDH and U6 are employed as internal parameters for mRNAs and miRNAs, respectively. Key resources table contains a list of the primers that were utilized. By using the 2−(ΔΔCt) approach, the relative expression of each gene was computed.
RNase R treatment
For measuring stability of circMAPK9, 3 μg total RNA was initially subjected to a 3 U/mg RNase R treatment at 37°C. Thirty minutes later, by using RT-qPCR, circMAPK9, and linear MAPK9 expressions were detected.
Fluorescence in site hybridization (FISH)
FISH kit purchased at RiboBio (Guangzhou, China). RiboBio synthesized the circRNA-specific probe, and GENESEED synthesized the miRNA probe. 4,6-diamidino-2-phenylindole (DAPI) was used as a counterstain for nuclei All operations follow the manufacturer's instructions.
Lipogenic differentiation of preadipocytes
As previously described, differentiation methods are implemented.22 Firstly, the differentiation of preadipocytes was induced by 10 μg/mL insulin (Aladdin, China), 1 μmol Dexamethasone (Aladdin, China), and 0.5 mmol 3-isobutyl-1methylxanthine (IBMX, Aladdin, China) for 48 hours, after which 10 μg/mL insulin induces 48 hours. Observe cell morphology and conduct subsequent experiments.
Oil Red O staining
Oil Red O powder (Sigma) is soluble in isopropanol, diluted into a working solution with distilled water 3:2 and filtered with filter paper. The cells were treated with 4% polymethanol for 30 minutes followed by three washes with PBS. They were then stained in the dark for 30 minutes with Oil Red O working solution, after which they were examined and photographed under a microscope.
Triglyceride assay
Ultrasonic fragmentation of cell suspensions (power: 300W, 3 ∼ 5 seconds/time, 30 seconds interval, 3 ∼ 5 times repetition) under ice bath conditions, prepared homogeneous slurry without centrifugal direct determination, based on Triglyceride assay kit (Jiancheng Bioengineering Institute, Nanjing, China). Manufacturer's instructions were followed during the procedure.
Western blot assay
Total protein extraction was carried out using 1 mM PMSF-containing RIPA buffer (Beyotime, China). Protein extracts were analyzed using 10 percent SDS-PAGE before being applied to PVDF membranes (Beyotime, China). Western blot blocking buffer was used to block the membranes for two hours. After that, membranes were treated overnight at 4°C with the corresponding antibodies CEBPα (Abmart, China), PPARγ (Abmart, China), GAPDH (Proteintech, China), and FABP4 (Abmart, China). Subsequently, a secondary antibody (Proteintech, China) linked to horseradish peroxidase (HRP) was used to incubate the western blot membranes. The signals were visualized using ProteinSimple FluorChem E (ProteinSimple, USA).
RNA immunoprecipitation (RIP) assay
The Magna RIP kit from Millipore (USA) was used to carry out the RIP assay. In a nutshell, either negative control IgG or RIP buffer that contains magnetic beads conjugated with human anti-Ago2 antibody (Thermo Fisher) were used to treat cell lysate. Proteinase K/0.1% SDS was used to remove protein from complexes after beads had been washed with wash buffer. The assay was followed by RT-qPCR.
Dual-luciferase reporter assay
In 96-well plates, HEK293T cells were seeded and cultured. Then, utilizing Lipo6000™ (Beyotime, China), the pmirGLO reporter vector containing wild-type (WT) or mutant-type (MUT) circMAPK9 and FTO was transfected into HEK293T cells in conjunction with hsa-miR-1322 mimics or NC mimics.
Quantification and statistical analysis
GraphPad Prism 8.0 (GraphPad, San Diego, CA, USA) and SPSS 19.0 (SPSS, Chicago, IL, USA) software was used to analyze the data. The Student's t-test was applied in order to compare the two groups. To calculate the diagnostic sensitivity and specificity, ROC curve analysis was performed. Spearman’s correlation analysis was used for correlations. Chi-square test was applied to analyze the link among circMAPK9 expression and clinicopathological characteristics. P < 0.05 was considered statistically different.
Acknowledgments
Changzhou Medical Center of Nanjing Medical University Program (CZKYCMCM202204 and CZKYCMCB202212) and Changzhou Sci&Tech Program (CM20223008, CE20215039 and 2022CZLJ017) supported this work.
Author contributions
The article was drafted by SC and PS. The concept for this study’s design was created by SC, JZ, and LT. The experiment was finished and the data were examined by SC, JX, and ZW. The data were processed and ideas were supplemented by YJ and YW. The data as well as the article were corrected by YZ. The final article was read and approved by all authors..
Declaration of interests
The authors declare no conflict of interest.
Published: August 28, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107756.
Contributor Information
Jie Zhao, Email: zhaojie@njmu.edu.cn.
Liming Tang, Email: drtangliming@163.com.
Supplemental information
Data and code availability
-
•
The raw sequencing data for this study was stored in the GEO database. These data can be obtained at GEO: GSE235696.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Blüher M. Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 2.Luo X., Jiaerken Y., Shen Z., Wang Q., Liu B., Zhou H., Zheng H., Li Y., Gao Y., He S., et al. Obese COVID-19 patients show more severe pneumonia lesions on CT. Diabetes Obes. Metab. 2021;23:290–293. doi: 10.1111/dom.14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen E.D., Spiegelman B.M. What We Talk About When We Talk About Fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farmer S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao J., Zhou A., Qi W. The Potential to Fight Obesity with Adipogenesis Modulating Compounds. IJMS. 2022;23:2299. doi: 10.3390/ijms23042299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vishvanath L., Gupta R.K. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J. Clin. Invest. 2019;129:4022–4031. doi: 10.1172/JCI129191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanousková B., Vávrová G., Ambrož M., Boušová I., Karlsen T.A., Skálová L., Matoušková P. MicroRNAs mediated regulation of glutathione peroxidase 7 expression and its changes during adipogenesis. Biochim. Biophys. Acta. Gene Regul. Mech. 2021;1864:194734. doi: 10.1016/j.bbagrm.2021.194734. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y., Li K., Zhang X., Chen J., Li M., Liu L. The novel long noncoding RNA lncRNA-Adi regulates adipogenesis. Stem Cells Transl. Med. 2020;9:1053–1067. doi: 10.1002/sctm.19-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendell J.T. MicroRNAs: Critical Regulators of Development, Cellular Physiology and Malignancy. Cell Cycle. 2005;4:1179–1184. doi: 10.4161/cc.4.9.2032. [DOI] [PubMed] [Google Scholar]
- 10.Ambele M.A., Dhanraj P., Giles R., Pepper M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. IJMS. 2020;21:4283. doi: 10.3390/ijms21124283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao M., Dong G., Meng Q., Lin S., Li X. Circ-HOMER1 enhances the inhibition of miR-1322 on CXCL6 to regulate the growth and aggressiveness of hepatocellular carcinoma cells. J. Cell. Biochem. 2020;121:4440–4449. doi: 10.1002/jcb.29672. [DOI] [PubMed] [Google Scholar]
- 12.Xie T., Fu D.J., Li Z.M., Lv D.J., Song X.-L., Yu Y.Z., Wang C., Li K.J., Zhai B., Wu J., et al. CircSMARCC1 facilitates tumor progression by disrupting the crosstalk between prostate cancer cells and tumor-associated macrophages via miR-1322/CCL20/CCR6 signaling. Mol. Cancer. 2022;21:173. doi: 10.1186/s12943-022-01630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J., Zhao X., Wang Y., Ren F., Sun D., Yan Y., Kong X., Bu J., Liu M., Xu S. circRNA-002178 act as a ceRNA to promote PDL1/PD1 expression in lung adenocarcinoma. Cell Death Dis. 2020;11:32. doi: 10.1038/s41419-020-2230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y.-J., Chen C.-Y., Mai T.-L., Chuang C.-F., Chen Y.-C., Gupta S.K., Yen L., Wang Y.-D., Chuang T.-J. Genome-wide, integrative analysis of circular RNA dysregulation and the corresponding circular RNA-microRNA-mRNA regulatory axes in autism. Genome Res. 2020;30:375–391. doi: 10.1101/gr.255463.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong X., Tian M., Cao N., Yang P., Xu Z., Zheng S., Liao Q., Chen C., Zeng C., Jose P.A., et al. Circular RNA circEsyt2 regulates vascular smooth muscle cell remodeling via splicing regulation. J. Clin. Invest. 2021;131:e147031. doi: 10.1172/JCI147031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang P., Sheng M., Du C., Chao Z., Xu H., Cheng X., Li C., Xu Y. Assessment of CircRNA Expression Profiles and Potential Functions in Brown Adipogenesis. Front. Genet. 2021;12:769690. doi: 10.3389/fgene.2021.769690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang P.P., Han Q., Sheng M.X., Du C.Y., Wang Y.L., Cheng X.F., Xu H.X., Li C.C., Xu Y.J. Identification of Circular RNA Expression Profiles in White Adipocytes and Their Roles in Adipogenesis. Front. Physiol. 2021;12:728208. doi: 10.3389/fphys.2021.728208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding Z., Sun D., Han J., Shen L., Yang F., Sah S., Sui X., Wu G. Novel noncoding RNA CircPTK2 regulates lipolysis and adipogenesis in cachexia. Mol. Metabol. 2021;53:101310. doi: 10.1016/j.molmet.2021.101310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Y., Gui W., Lin X., Li H. Knock-down of circular RNA H19 induces human adipose-derived stem cells adipogenic differentiation via a mechanism involving the polypyrimidine tract-binding protein 1. Exp. Cell Res. 2020;387:111753. doi: 10.1016/j.yexcr.2019.111753. [DOI] [PubMed] [Google Scholar]
- 20.Luo Z., Chen S., Chen X. CircMAPK9 promotes the progression of fibroblast-like synoviocytes in rheumatoid arthritis via the miR-140-3p/PPM1A axis. J. Orthop. Surg. Res. 2021;16:395. doi: 10.1186/s13018-021-02550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pal M., Febbraio M.A., Lancaster G.I. The roles of c-Jun NH 2 -terminal kinases (JNKs) in obesity and insulin resistance: JNK and metabolic disease. J. Physiol. 2016;594:267–279. doi: 10.1113/JP271457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S., Jiang Y., Qi X., Song P., Tang L., Liu H. Bioinformatics analysis to obtain critical genes regulated in subcutaneous adipose tissue after bariatric surgery. Adipocyte. 2022;11:550–561. doi: 10.1080/21623945.2022.2115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haque S., Harries L.W. Circular RNAs (circRNAs) in Health and Disease. Genes. 2017;8:353. doi: 10.3390/genes8120353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou W.-Y., Cai Z.-R., Liu J., Wang D.-S., Ju H.-Q., Xu R.-H. Circular RNA: metabolism, functions and interactions with proteins. Mol. Cancer. 2020;19:172. doi: 10.1186/s12943-020-01286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X., Wu R., Liu Y., Zhao Y., Bi Z., Yao Y., Liu Q., Shi H., Wang F., Wang Y. m6 A mRNA methylation controls autophagy and adipogenesis by targeting Atg5 and Atg7. Autophagy. 2020;16:1221–1235. doi: 10.1080/15548627.2019.1659617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agbu P., Carthew R.W. MicroRNA-mediated regulation of glucose and lipid metabolism. Nat. Rev. Mol. Cell Biol. 2021;22:425–438. doi: 10.1038/s41580-021-00354-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C., Ni Y.-Q., Xu H., Xiang Q.-Y., Zhao Y., Zhan J.-K., He J.-Y., Li S., Liu Y.-S. Roles and mechanisms of exosomal non-coding RNAs in human health and diseases. Signal Transduct. Targeted Ther. 2021;6:383. doi: 10.1038/s41392-021-00779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vo J.N., Cieslik M., Zhang Y., Shukla S., Xiao L., Zhang Y., Wu Y.-M., Dhanasekaran S.M., Engelke C.G., Cao X., et al. The Landscape of Circular RNA in Cancer. Cell. 2019;176:869–881.e13. doi: 10.1016/j.cell.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kristensen L.S., Jakobsen T., Hager H., Kjems J. The emerging roles of circRNAs in cancer and oncology. Nat. Rev. Clin. Oncol. 2022;19:188–206. doi: 10.1038/s41571-021-00585-y. [DOI] [PubMed] [Google Scholar]
- 30.Yu G., Yang Z., Peng T., Lv Y. Circular RNAs: Rising stars in lipid metabolism and lipid disorders. J. Cell. Physiol. 2021;236:4797–4806. doi: 10.1002/jcp.30200. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y., Liu H., Li Y., Mao R., Yang H., Zhang Y., Zhang Y., Guo P., Zhan D., Zhang T. Circular RNA SAMD4A controls adipogenesis in obesity through the miR-138-5p/EZH2 axis. Theranostics. 2020;10:4705–4719. doi: 10.7150/thno.42417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 33.Rabin A., Zaffagni M., Ashwal-Fluss R., Patop I.L., Jajoo A., Shenzis S., Carmel L., Kadener S. SRCP: a comprehensive pipeline for accurate annotation and quantification of circRNAs. Genome Biol. 2021;22:277. doi: 10.1186/s13059-021-02497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin X., Du Y., Lu W., Gui W., Sun S., Zhu Y., Wang G., Eserberg D.T., Zheng F., Zhou J., et al. CircRNF111 Protects Against Insulin Resistance and Lipid Deposition via Regulating miR-143-3p/IGF2R Axis in Metabolic Syndrome. Front. Cell Dev. Biol. 2021;9:663148. doi: 10.3389/fcell.2021.663148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arcinas C., Tan W., Fang W., Desai T.P., Teh D.C.S., Degirmenci U., Xu D., Foo R., Sun L. Adipose circular RNAs exhibit dynamic regulation in obesity and functional role in adipogenesis. Nat. Metab. 2019;1:688–703. doi: 10.1038/s42255-019-0078-z. [DOI] [PubMed] [Google Scholar]
- 36.Wang F., Zhong S., Mao C., Jin J., Wang H. Circ_0000291 contributes to hepatocellular carcinoma tumorigenesis by binding to miR-1322 to up-regulate UBE2T. Ann. Hepatol. 2022;27:100722. doi: 10.1016/j.aohep.2022.100722. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y., Lin L., Huang Y., Sun J., Wang X., Wang P. MicroRNA-138 Suppresses Adipogenic Differentiation in Human Adipose Tissue-Derived Mesenchymal Stem Cells by Targeting Lipoprotein Lipase. Yonsei Med. J. 2019;60:1187–1194. doi: 10.3349/ymj.2019.60.12.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei Z., Qin X., Kang X., Zhou H., Wang S., Wei D. MiR-142-3p inhibits adipogenic differentiation and autophagy in obesity through targeting KLF9. Mol. Cell. Endocrinol. 2020;518:111028. doi: 10.1016/j.mce.2020.111028. [DOI] [PubMed] [Google Scholar]
- 39.Mizuno T.M. Fat Mass and Obesity Associated (FTO) Gene and Hepatic Glucose and Lipid Metabolism. Nutrients. 2018;10:1600. doi: 10.3390/nu10111600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L., Song C., Wang N., Li S., Liu Q., Sun Z., Wang K., Yu S.-C., Yang Q. NADP modulates RNA m6A methylation and adipogenesis via enhancing FTO activity. Nat. Chem. Biol. 2020;16:1394–1402. doi: 10.1038/s41589-020-0601-2. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y., Shen F., Huang W., Qin S., Huang J.-T., Sergi C., Yuan B.-F., Liu S.-M. Glucose Is Involved in the Dynamic Regulation of m6A in Patients With Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2019;104:665–673. doi: 10.1210/jc.2018-00619. [DOI] [PubMed] [Google Scholar]
- 42.Merkestein M., Laber S., McMurray F., Andrew D., Sachse G., Sanderson J., Li M., Usher S., Sellayah D., Ashcroft F.M., Cox R.D. FTO influences adipogenesis by regulating mitotic clonal expansion. Nat. Commun. 2015;6:6792. doi: 10.1038/ncomms7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ronkainen J., Huusko T.J., Soininen R., Mondini E., Cinti F., Mäkelä K.A., Kovalainen M., Herzig K.-H., Järvelin M.R., Sebert S., et al. Fat mass- and obesity-associated gene Fto affects the dietary response in mouse white adipose tissue. Sci. Rep. 2015;5:9233. doi: 10.1038/srep09233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Z., Yu G.L., Zhu X., Peng T.H., Lv Y.C. Critical roles of FTO-mediated mRNA m6A demethylation in regulating adipogenesis and lipid metabolism: Implications in lipid metabolic disorders. Genes Dis. 2022;9:51–61. doi: 10.1016/j.gendis.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H., Zhai F. Programme and policy options for preventing obesity in China: Options for preventing obesity in China. Obes. Rev. 2013;14(Suppl 2):134–140. doi: 10.1111/obr.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao Y., Wang J., Zhao F. CIRI: an efficient and unbiased algorithm for de novo circular RNA identification. Genome Biol. 2015;16:4. doi: 10.1186/s13059-014-0571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The raw sequencing data for this study was stored in the GEO database. These data can be obtained at GEO: GSE235696.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.