Abstract
Aims
Same-day discharge (SDD) following catheter ablation (CA) of atrial fibrillation (AF) was already introduced in selected facilities in Europe, but a widespread implementation has not yet succeeded. Data on patients’ perspectives are lacking. Therefore, we conducted a survey to address patients’ beliefs towards SDD and identify variables that are associated with their evaluation.
Methods and results
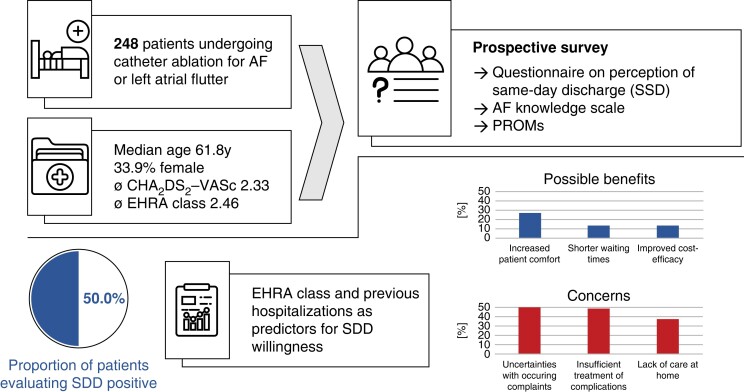
As part of the prospective, monocentric FAST AFA trial, patients aged ≥20 years undergoing left atrial CA for AF were asked to participate in the survey consisting of a study-specific questionnaire, the AF knowledge scale, and pre-defined patient-reported outcome measures. The study cohort was stratified based on SDD willingness, and a logistic regression analysis was used to identify predictors for patients’ valuation. Between 26 July 2021 and 01 July 2022, 256 of 376 screened patients consented to study participation of whom 248 (mean age 61.8 years, 33.9% female) completed the SDD survey. Of them, 50.0% were willing to have SDD concepts integrated into their clinical course with increased patient comfort (27.5%), shorter waiting times (14.6%), and a cost-efficient treatment (14.0%) being imaginable benefits. In contrast, expressed concerns included uncertainties with occurring complaints (50.6%), the insufficient recognition (47.8%), and treatment (48.9%) of complications. European Heart Rhythm Association class at baseline and inpatient treatments within the preceding year were predictors for SDD willingness whereas comorbidity burden or AF knowledge were not.
Conclusion
We provide a detailed survey expressing patients’ beliefs towards SDD following left atrial CA. Our findings may facilitate adequate patient selection to improve the future implementation of SDD programs in suitable cohorts.
Keywords: Same-day discharge, Patient survey, Atrial fibrillation, Catheter ablation
Graphical Abstract
Graphical abstract.
What’s new?
This is the first patient survey focusing on patients’ perception of same-day discharge (SDD) following catheter ablation of atrial fibrillation (AF) or left atrial flutter.
Of 248 patients responding, 50.0% were willing to have SDD concepts integrated into their clinical course.
Patients’ symptoms (assessed by the European Heart Rhythm Association classification) and the cumulative number of in-hospital treatments preceding the index case by 1 year were predictors for patients’ SDD willingness.
Age, sex, comorbidity burden, knowledge about AF, and patient-reported outcome measures were not associated with the perception of SDD.
Introduction
The role of catheter ablation (CA) as the most effective strategy of rhythm control for patients with atrial fibrillation (AF) has been underlined by the latest practice guideline provided by the European Society of Cardiology.1 Considering the projected increase in AF prevalence,1,2 the likewise growing need for AF CAs will put relevant demands on the health care system both from a financial and a structural perspective. Hand in hand with technical innovations that facilitate and shorten CA interventions,3,4 a growing body of evidence suggests the implementation of same-day discharge (SDD) protocols as a feasible and safe way to streamline patient pathways.5–8 Even though established in the context of other electrophysiological procedures,9,10 SDD has not yet become widely used for various reasons including expected patients’ reservations about it.11,12 As patient involvement and shared decision-making were highlighted as important by guidelines,1 aim of this study was (i) to evaluate the perception of SDD in patients who undergo CA for AF and (ii) identify factors to be associated with SDD willingness.
Methods
We conducted a prospective survey as part of the FAST AFA trial (Feasibility and safety of same-day discharge following atrial fibrillation catheter ablation). For this part of the study, patients with AF or atypical atrial flutter aged 20 years or older who were scheduled for left atrial CA in the Heart Center Leipzig were eligible and approached for survey participation without further selection criteria. Patients were screened after medical consultation with regard to their planned intervention at hospital admission. In case of consent to study participation, a study-specific questionnaire to assess beliefs towards SDD (binary assessment of SDD willingness, complete questionnaire provided in the Supplementary material online, Supplementary Material), the AF knowledge scale13 and pre-defined patient-reported outcome measures (PROMs) according to the ICHOM standard for outcome assessment in patients with AF14 were handed out before the planned CA by a study nurse as part of a structured interview. Patient-reported outcome measures included the patient-reported outcomes measurement information system (PROMIS) global health,15 patient health questionnaire-2 (PHQ-2),16 and the atrial fibrillation effect on quality-of-life questionnaire (AFEQT).17 All patients were informed in detail about the medical background, the practical implementation of SDD concepts (including selection criteria for SDD, see Supplementary material online, Supplementary Material), and the existing scientific data in this context. Handouts were collected from patients before regular hospital discharge and processed via an optical character recognition software (ABBYY, ic solutions GmbH, Leipzig, Germany). Clinical data were gathered using an electronic case report form via the software secuTrial (Interactive Systems GmbH, Berlin, Germany). The study database was expanded by data of electronic medical records and administrative data [based on the International Classification of Diseases and Related Health Problems, 10th Revision, German modification (ICD-10-GM)]. Elixhauser comorbidity score18 was used to assess comorbidity burden. Patients in whom CA has ultimately not been performed were excluded from the final analysis. All data were checked and validated by members of the local study team before final analysis. The study complied with the principles outlined in the Declaration of Helsinki and has been approved by the local ethics committee of the University of Leipzig (AZ287/20). Individual informed consent was obtained by all included patients prior to study inclusion.
Statistical analysis
Statistical analyses were executed using the R environment for statistical computing (version 4.0.2, 64-bit build).19 Descriptive baseline characteristics were described stratified for SDD willingness with means or medians (with interquartile ranges) depending on testing for Gaussian distribution. Percentages were computed using the number of valid responses per question. Variable comparisons were performed with Student’s t-test for continuous variables and Fisher’s exact test for categorical or binary variables including the computation of odds ratios (OR) and 95% confidence intervals (CI). Logistic regression was used for multivariable analysis after a variable transformation and backward selection based on Akaike’s information criterion.
Results
Cohort description
Between 26 July 2021 and 01 July 2022, 376 patients were screened and 256 patients consented to participate in the survey. Of them, 8 patients were excluded because questions on SDD willingness remained unanswered, resulting in a study population of 248 patients. Acute procedural success of CA was achieved in all cases and patients were discharged after a median length-of-stay of 1.2 ± 0.8 days. Only five patients were discharged on the day of CA. Mean age was 61.8 ± 10.5 years and 33.9% were female. Patients were symptomatic with a mean European Heart Rhythm Association (EHRA) symptom classification of 2.5 ± 0.6 and 49.6% had a history of prior or ongoing antiarrhythmic medication (Classes I or III according to Vaughan Williams). Based on the Elixhauser Comorbidity Score, the most prevalent comorbidities were hypertension (71.8%), heart failure (39.9%, unspecified for sub-type), obesity (33.5%), peripheral vascular disease (17.3%), and hypothyroidism (14.5%). Asked for cardiovascular care burden within the last 12 months prior to study enrolment, 52.8% of patients reported of at least one emergency room visit due to cardiac symptoms (number of visits, 1: 25.4%, 2: 15.3%, 3–4: 10.5%, and ≥ 5: 1.6%) and even more patients reported of any inpatient treatment (60.1%). Summarized PROM scores at study inclusion were 58.8 ± 19.2 for AFEQT, 1.3 ± 1.4 for PHQ2, and 29.7 ± 5.6 for PROMIS global health, respectively. Further baseline characteristics are presented in Table 1.
Table 1.
Baseline characteristics stratified for SDD willingness
| Variables | Overall cohorta | Existing SDD willingnessa | Absent SDD willingnessa | P-value |
|---|---|---|---|---|
| Number of patients | 248 | 124 | 124 | / |
| Baseline data | ||||
| Age (years) | 61.8 ± 10.5 | 61.8 ± 9.1 | 61.8 ± 11.8 | 0.981 |
| Gender (female) (%) | 33.9 | 29.8 | 37.9 | 0.180 |
| Physical activityb (days per week) | 4.6 ± 2.4 | 4.6 ± 2.5 | 4.6 ± 2.3 | 0.979 |
| Nicotine consumption (%) | 0.16 | |||
| Never smoked regularly | 50.0 | 43.5 | 56.5 | |
| Quit smoking >6 months ago | 41.1 | 46.0 | 36.3 | |
| Quit smoking <6 months ago | 0.4 | 0.8 | 0.0 | |
| Smokes regularly | 8.1 | 8.9 | 7.3 | |
| Distance to the nearest ER (km) | 6.7 ± 7.8 | 5.7 ± 6.9 | 7.6 ± 8.5 | 0.056 |
| Clinical presentation | ||||
| EHRA class | 2.5 ± 0.6 | 2.4 ± 0.5 | 2.6 ± 0.6 | <0.01 |
| Weight (kg) | 91.1 ± 18.5 | 92.4 ± 18.1 | 89.7 ± 18.8 | 0.247 |
| BMI (kg/m²) | 29.3 ± 5.3 | 29.6 ± 5.4 | 29.1 ± 5.3 | 0.399 |
| CHACSSUBSTART2CSSUBENDDSCSSUBSTART2CSSUBEND-VASc score | 2.3 ± 1.4 | 2.2 ± 1.4 | 2.4 ± 1.5 | 0.357 |
| Type of AF (%) | 0.294 | |||
| Paroxysmal | 40.3 | 42.7 | 37.9 | |
| Persistent | 58.9 | 55.6 | 62.1 | |
| Long-standing persistent | 0.8 | 1.6 | 0.0 | |
| Time-to-diagnosis of AF (months) | 60.1 ± 71.2 | 59.0 ± 63.5 | 61.1 ± 78.4 | 0.811 |
| Elixhauser comorbidity score | 3.3 ± 1.9 | 3.3 ± 1.9 | 3.3 ± 1.8 | 0.838 |
| Left ventricular ejection fractionc (%) | 56.0 ± 9.2 | 55.4 ± 10.4 | 56.7 ± 7.8 | 0.304 |
| PROMs | ||||
| AFEQT | 58.8 ± 19.2 | 60.0 ± 19.5 | 57.5 ± 18.9 | 0.315 |
| PHQ2 | 1.3 ± 1.4 | 1.2 ± 1.3 | 1.5 ± 1.5 | 0.186 |
| PROMIS global health (sum score) | 29.7 ± 5.6 | 30.3 ± 5.7 | 29.1 ± 5.5 | 0.110 |
| Previous AF-related interventions | ||||
| Previously performed CA (%)d | 40.7 | 44.4 | 37.1 | 0.245 |
| Pulmonary vein isolation | 33.1 | 33.1 | 33.1 | 1.000 |
| Ablation of the CTI | 11.7 | 8.9 | 14.5 | 0.167 |
| Surgical left atrial ablation | 0.4 | 0.0 | 0.8 | 1.000 |
| Previous CA-related adverse event (%) | 6.0 | 5.6 | 6.5 | 0.790 |
| Previous LAA closure procedure (%) | 2.0 | 3.2 | 0.8 | 0.370 |
| Previous CIED surgery (%) | 3.6 | 3.2 | 4.0 | 1.000 |
| Number of previous emergency visitse | 1.0 ± 1.2 | 0.8 ± 1.1 | 1.3 ± 1.4 | <0.01 |
| Number of previous inpatient treatmentse | 1.1 ± 1.2 | 0.9 ± 1.0 | 1.3 ± 1.1 | <0.01 |
Continuous variables are described with mean ± standard deviation.
Self-reported physical activity of at least 30 min moderate exercise per day.
Measured by echocardiography or cardiac MRI within 12 months before CA (latest available value).
As there might be more than one previous ablation procedure, the number of individual procedures adds up to more than the percentage of ‘previously performed CA’.
Within 12 months prior to study inclusion.
AF, atrial fibrillation; BMI, body mass index; CA, catheter ablation; CIED, cardiac implantable electronic device; CTI, cavotricuspid isthmus; EHRA, European Heart Rhythm Association; ER, emergency room; LAA, left atrial appendage; SDD, same-day discharge; PROM, patient-reported outcome measure.
Perception of same-day discharge
At baseline, 50.0% of patients expressed their willingness towards an implementation of SDD into clinical practice after left atrial CA. The vast majority of patients (91.9%) felt adequately informed about therapeutic options, and 84.3% of patients were very satisfied with their medical briefing/interview. Concerns with regard to the scheduled CA were expressed by 39.5% of participants and 89.9% of patients stated that they had somebody available who could take care of them for their post-discharge care supply. Asked for conceivable advantages, patients reported an increased patient comfort (27.5%), shortened waiting times (14.6%), and a cost-efficient treatment (14.0%), whereas 52.2% of patients could not imagine any positive effects (including patients with existing willingness of SDD). Potential disadvantages were seen in uncertainties associated with complaints (50.6%), a delayed recognition (47.8%) and response (48.9%) to occurring complications and a lack of care at home after discharge (37.6%). Only 12.4% of patients did not have any concerns related to SDD.
Stratifying patients according to their willingness towards SDD, age, gender distribution, and comorbidity burden did not differ between groups (Table 1). Moreover, there were no differences regarding the CHA2DS2-VASc score, AF type, or time to AF diagnosis. Comparing both groups (group with expressed SDD willingness is mentioned first), a similar proportion had undergone previous CA interventions (44.0% vs. 37.0%, P = 0.245) and experienced previous complications related to ablation procedures (5.6% vs. 6.5%, P = 0.790). There were no differences with regard to PROM scores or the AF knowledge scale at baseline. Both groups felt equally well informed (91.9% vs. 91.9%, P = 0.815), were satisfied with their medical interviews (very satisfied: 84.7% vs. 83.9%, P = 0.602) and a comparable proportion of patients reported concerns about their planned CA (34.7% vs. 44.4%, P = 0.186). There was no difference between groups with regard to the type of transportation home and the proportion of patients with a secured post-discharge care at home was similar (defined as at least one person who could look after them for the first day and night, 93.0% vs. 90.0%, P = 0.722). Acute procedural success defined as completed pulmonary vein isolation (100.0% vs. 100.0%, P = 1.0) and observed in-hospital complications requiring a prolongation on inpatient stay (4.0 vs. 6.5%, P = 0.328) were similar between groups with and without SDD willingness.
In univariable analysis, less AF-related symptoms according to EHRA class and fewer emergency medical visits as well as previous inpatient treatments within 12 months prior to study inclusion were associated with patients’ willingness towards SDD (Table 2). In the multivariable analysis, only two factors were identified as predictors for patients’ attitude towards SDD: EHRA class at baseline (OR for 1 point difference 0.67, 95% CI 0.50–0.91, P < 0.01) and previous inpatient treatments within 1 year (OR 0.72, 95% CI 0.53–0.97, P < 0.05).
Table 2.
Univariable analysis for SDD willingness
| Variables | Univariable analysis | P-value |
|---|---|---|
| OR (95% CI) | ||
| Distance to nearest ER (km) | 0.78 (0.59–1.00) | 0.059 |
| EHRA class | 0.67 (0.51–0.86) | 0.002 |
| Number of previous emergency visitsa | 0.66 (0.50–0.86) | 0.003 |
| Number of previous inpatient treatmentsa | 0.70 (0.53–0.91) | 0.008 |
Within 12 months prior to study inclusion.
CI, confidence interval; EHRA, European Heart Rhythm Association; ER, emergency room; OR, odds ratio.
Discussion
With this analysis, we provide the first prospective patient survey addressing the topic of SDD following left atrial CA. In this contemporary cohort of AF patients, half of the survey participants expressed their willingness towards a further implementation of SDD protocols. A lower pre-procedural burden of symptoms as indicated by EHRA class as well as a lower number of previous inpatient treatments were identified as relevant influencing factors towards this valuation.
There are no comparable investigations in the context of left atrial CA procedures assessing patients’ beliefs towards SDD. Though, studies that focused on the implementation of SDD protocols after CAs for AF did report that patients’ preferences are of relevance for failure of early discharges.8,20 Therefore, with our present analysis, we provide data that may facilitate adequate patient selection when planning a further rollout of SDD pathways. In the context of other cardiovascular procedures, namely following percutaneous coronary interventions (PCI), several groups found high satisfaction rates of patients who were discharged the same day and anxiety was low after adequate education.21–23 In contrast, one rather small investigation based on phone interviews of patients and family members reported of a negative perception of SDD following PCI as it was interpreted as a signal that the underlying cardiac disease was not taken seriously.24 However, considering the predominantly elective character of left atrial CAs, the latter misinterpretation should be circumventable with patient education and selection. Of note, the vast majority of patients from our cohort felt well informed about the planned treatment. Despite this, only half of the patients could envision SDD, which interestingly was not influenced by previous CA experiences. When asked for possible benefits and barriers regarding a further implementation of SDD concepts, patients evaluated SDD rather negative compared to health care professionals based on a recent EHRA survey. An increased patient comfort was considered an advantage only of less than one-third of patients in contrast to 63.0% of EHRA health care providers.11 In addition, the proportion of survey respondents that did not see any positive aspects in SDD implementation was increased more than seven-fold in the patient cohort. On the other hand, less patients were concerned about an inferior management of late-occurring complications when juxtaposed to the results of the EHRA survey.11 This fear of professionals is even more surprising since it cannot be substantiated with existing data, which did not show a meaningful difference in complication or rehospitalization rates between SDD and overnight stay cases.5,7,8,25–27 Of course, this mainly relates to observational and non-randomized data with an implicit potential selection bias. However, to date, there is no evidence of a higher rate of post-discharge adverse events associated with SDD except from one study with a numerical higher 30-day readmission rate.12,28
Symptom severity based on EHRA classification and the cumulative burden of health care visits preceding the CA procedure were relevant factors influencing SDD perception. The fact that EHRA class was relevantly associated with SDD willingness, and AFEQT score was not may be related to timeframe the symptom scores relate to in a sense that symptoms classified by EHRA class may reflect immediately existing symptoms whereas AFEQT explicitly queries symptoms within the last 4 weeks. Interestingly, those factors were not considered as eligibility criteria in the existing studies evaluating SDD following CA for AF.6 In contrast, infrastructural aspects as well as the post-procedural caring at home were taken into account for patient selection at least in selected trials.29,30 However, we only found a non-significant trend in univariable but not in multivariable analysis for the association of SDD perception and the distance between patients’ residence and an emergency medical facility. Neither surrogate parameters for general health like the PROMIS global health sum score nor factors like comorbidity burden were relevant to patients’ perception of SDD, meaning that not only frail or potentially vulnerable patients were not willing to be discharged the same day following left atrial CA. In order to prevent misconceptions, this could be understood as a call for a better SDD-related education of both patients and staff who are informing patients about different treatment options.
Limitations
The monocentric character of the performed patient survey carries the risk of a selection bias. Moreover, expanding the analysis to even larger case numbers could affect results. The study-specific questionnaire used for the assessment of SDD willingness has not been externally validated. However, there are no existing validated tools to address the objectives of this scientific investigation. It is possible that the questionnaires were filled out at different times in relation to the CA, which might have an influence on answers. Unfortunately, this could not be implemented in any other way in terms of infrastructure and there is no information when the questionnaires were processed in individual cases.
The evaluated data are in part based on administrative data that was not stored for research interests but for remuneration reasons, which potentially could affect the encoded information. Quality of the results depends to a large extent on the correct encoding of procedures and diagnoses at hospital discharge. Moreover, other parameters are measured subjectively by the treating physician like EHRA symptom class. Nevertheless, EHRA class is a tool to assess AF symptom severity that is used in several clinical trials and is also integral part of the current guidelines for the diagnosis and treatment of atrial fibrillation.1 In our study, EHRA class assessment has been performed before collecting questionnaires and was therefore blinded with regard to patient’s answer towards SDD willingness.
Conclusion
With this prospective survey in unselected patients who underwent CA for AF or left atrial flutter, we provide insights into patients’ perception of SDD and identified factors to be associated with patients’ willingness for early hospital discharge. This will facilitate adequate patient selection, which is critical for the successful implementation of SDD protocols in the future to respond to the increasing demands of AF patient care.
Supplementary Material
Acknowledgements
The authors thank Prof. Jeroen M. Hendricks for the permission to use the AF knowledge questionnaire.
Contributor Information
Sebastian König, Department of Electrophysiology, Heart Center Leipzig at University of Leipzig, Strümpellstraße 39, 04289 Leipzig, Germany; Real World Evidence & Health Technology Assessment, Helios Health Institute, Berlin, Germany.
Lisa Wohlrab, Real World Evidence & Health Technology Assessment, Helios Health Institute, Berlin, Germany.
Johannes Leiner, Department of Electrophysiology, Heart Center Leipzig at University of Leipzig, Strümpellstraße 39, 04289 Leipzig, Germany; Real World Evidence & Health Technology Assessment, Helios Health Institute, Berlin, Germany.
Vincent Pellissier, Real World Evidence & Health Technology Assessment, Helios Health Institute, Berlin, Germany.
Anne Nitsche, Real World Evidence & Health Technology Assessment, Helios Health Institute, Berlin, Germany.
Angeliki Darma, Department of Electrophysiology, Heart Center Leipzig at University of Leipzig, Strümpellstraße 39, 04289 Leipzig, Germany.
Sebastian Hilbert, Department of Electrophysiology, Heart Center Leipzig at University of Leipzig, Strümpellstraße 39, 04289 Leipzig, Germany.
Sotirios Nedios, Department of Electrophysiology, Heart Center Leipzig at University of Leipzig, Strümpellstraße 39, 04289 Leipzig, Germany.
Timm Seewöster, Department of Electrophysiology, Heart Center Leipzig at University of Leipzig, Strümpellstraße 39, 04289 Leipzig, Germany.
Borislav Dinov, Department of Electrophysiology, Heart Center Leipzig at University of Leipzig, Strümpellstraße 39, 04289 Leipzig, Germany.
Gerhard Hindricks, Department of Electrophysiology, Heart Center Leipzig at University of Leipzig, Strümpellstraße 39, 04289 Leipzig, Germany.
Andreas Bollmann, Department of Electrophysiology, Heart Center Leipzig at University of Leipzig, Strümpellstraße 39, 04289 Leipzig, Germany; Real World Evidence & Health Technology Assessment, Helios Health Institute, Berlin, Germany.
Supplementary material
Supplementary material is available at Europace online.
Funding
The study was funded by Biosense Webster (Biosense Webster, 31 Technology Drive, Suite 200, Irvine, CA 92618, USA) under the investigator-initiated study scheme (study ID: IIS-587) as an institutional grant. No author received personal/individual funding nor has any author other financial or other relations to Biosense Webster.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Ethics approval and patients’ individual consent
The study complied with the principles outlined in the Declaration of Helsinki and has been approved by the local ethics committee of the University of Leipzig (AZ287/20). Individual informed consent was obtained by all included patients prior to study inclusion.
Permissions information
The authors do hereby declare that all illustrations and figures in the manuscript are entirely original and do not require reprint permission.
Ethics/patient consent
The study was approved by the responsible ethics committee, and each patient provided written informed consent for study participation.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist Cet al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Di Carlo A, Bellino L, Consoli D, Mori F, Zaninelli A, Baldereschi Met al. Prevalence of atrial fibrillation in the Italian elderly population and projections from 2020 to 2060 for Italy and the European Union: the FAI Project. Europace 2019;21:1468–75. [DOI] [PubMed] [Google Scholar]
- 3. Ravi V, Poudyal A, Abid QU, Larsen T, Krishnan K, Sharma PSet al. High-power short duration vs. conventional radiofrequency ablation of atrial fibrillation: a systematic review and meta-analysis. Europace 2021;23:710–21. [DOI] [PubMed] [Google Scholar]
- 4. Reddy VY, Dukkipati SR, Neuzil P, Anic A, Petru J, Funasako Met al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol 2021;7:614–27. [DOI] [PubMed] [Google Scholar]
- 5. Jafry AH, Akhtar KH, Khan JA, Clifton S, Reese J, Sami KNet al. Safety and feasibility of same-day discharge for catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol 2022;65:803–11. [DOI] [PubMed] [Google Scholar]
- 6. Konig S, Richter S, Bollmann A, Hindricks G. Safety and feasibility of same-day discharge following catheter ablation of atrial fibrillation: what is known and what needs to be explored? Herz 2022;47:123–8. [DOI] [PubMed] [Google Scholar]
- 7. Prasitlumkum N, Cheungpasitporn W, Chokesuwattanaskul R, Kewcharoen J, Tokavanich N, Navaravong Let al. Comparison between same-day discharge and overnight stay after atrial fibrillation ablation: systematic review and meta-analysis. Pacing Clin Electrophysiol 2021;44:2054–66. [DOI] [PubMed] [Google Scholar]
- 8. Rashedi S, Tavolinejad H, Kazemian S, Mardani M, Masoudi M, Masoudkabir Fet al. Efficacy and safety of same-day discharge after atrial fibrillation ablation: a systematic review and meta-analysis. Clin Cardiol 2022;45:162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marijon E, Albenque JP, Boveda S, Jacob S, Schmutz M, Bortone Aet al. Feasibility and safety of same-day home discharge after radiofrequency catheter ablation. Am J Cardiol 2009;104:254–8. [DOI] [PubMed] [Google Scholar]
- 10. Theodoreson MD, Chohan BC, McAloon CJ, Sandhu A, Lancaster CJ, Yusuf Set al. Same-day cardiac catheter ablation is safe and cost-effective: experience from a UK tertiary center. Heart Rhythm 2015;12:1756–61. [DOI] [PubMed] [Google Scholar]
- 11. Konig S, Svetlosak M, Grabowski M, Duncker D, Nagy VK, Bogdan Set al. Utilization and perception of same-day discharge in electrophysiological procedures and device implantations: an EHRA survey. Europace 2021;23:149–56. [DOI] [PubMed] [Google Scholar]
- 12. Sahashi Y, Kuno T, Tanaka Y, Passman R, Briasoulis A, Malik AH. The 30-day readmission rate of same-day discharge protocol following catheter ablation for atrial fibrillation: a propensity score-matched analysis from National Readmission Database. Europace 2021;24:755–61. [DOI] [PubMed] [Google Scholar]
- 13. Hendriks JM, Crijns HJ, Tieleman RG, Vrijhoef HJ. The atrial fibrillation knowledge scale: development, validation and results. Int J Cardiol 2013;168:1422–8. [DOI] [PubMed] [Google Scholar]
- 14. Seligman WH, Das-Gupta Z, Jobi-Odeneye AO, Arbelo E, Banerjee A, Bollmann Aet al. Development of an international standard set of outcome measures for patients with atrial fibrillation: a report of the International Consortium for Health Outcomes Measurement (ICHOM) atrial fibrillation working group. Eur Heart J 2020;41:1132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res 2009;18:873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003;41:1284–92. [DOI] [PubMed] [Google Scholar]
- 17. Spertus J, Dorian P, Bubien R, Lewis S, Godejohn D, Reynolds MRet al. Development and validation of the Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) Questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol 2011;4:15–25. [DOI] [PubMed] [Google Scholar]
- 18. Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care 2017;55:698–705. [DOI] [PubMed] [Google Scholar]
- 19. R Core Team . R: A Language and Environment for Statistical Computing. .https://www.R-project.org/: R Foundation for Statistical Computing; 2019.
- 20. Akula DN, Mariam W, Luthra P, Edward F, Katz DJ, Levi SAet al. Safety of same day discharge after atrial fibrillation ablation. J Atr Fibrillation 2020;12:2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amin AP, Crimmins-Reda P, Miller S, Rahn B, Caruso M, Pierce Aet al. Novel patient-centered approach to facilitate same-day discharge in patients undergoing elective percutaneous coronary intervention. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perret X, Bergerot C, Rioufol G, Bonvini RF, Ovize M, Finet G. Same-day-discharge ad hoc percutaneous coronary intervention: initial single-centre experience. Arch Cardiovasc Dis 2009;102:743–8. [DOI] [PubMed] [Google Scholar]
- 23. Shroff A, Kupfer J, Gilchrist IC, Caputo R, Speiser B, Bertrand OFet al. Same-day discharge after percutaneous coronary intervention: current perspectives and strategies for implementation. JAMA Cardiol 2016;1:216–23. [DOI] [PubMed] [Google Scholar]
- 24. Chen Y, Lin FF, Marshall AP. Patient and family perceptions and experiences of same-day discharge following percutaneous coronary intervention and those kept overnight. Intensive Crit Care Nurs 2021;62:102947. [DOI] [PubMed] [Google Scholar]
- 25. Deyell MW, Hoskin K, Forman J, Laksman ZW, Hawkins NM, Bennett MTet al. Same-day discharge for atrial fibrillation ablation: outcomes and impact of ablation modality. Europace 2022;24:1112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sahashi Y, Kawamura I, Aikawa T, Takagi H, Briasoulis A, Kuno T. Safety and feasibility of same-day discharge in patients receiving pulmonary vein isolation-systematic review and a meta-analysis. J Interv Card Electrophysiol 2022;63:251–8. [DOI] [PubMed] [Google Scholar]
- 27. Tang PT, Davies M, Bashir Y, Betts TR, Pedersen M, Rajappan Ket al. Efficacy and safety of same-day discharge after atrial fibrillation ablation compared with post-procedural overnight stay: a systematic review and meta-analysis. Europace 2022;24:1569–84. [DOI] [PubMed] [Google Scholar]
- 28. Konig S, Andrade JG, Bollmann A. Administrative data confirm safety of same-day discharge following catheter ablation of atrial fibrillation: all good or is there a fly in the ointment? Europace 2022;24:701–2. [DOI] [PubMed] [Google Scholar]
- 29. Deyell MW, Leather RA, Macle L, Forman J, Khairy P, Zhang Ret al. Efficacy and safety of same-day discharge for atrial fibrillation ablation. JACC Clin Electrophysiol 2020;6:609–19. [DOI] [PubMed] [Google Scholar]
- 30. Rajendra A, Hunter TD, Morales G, Osorio J. Prospective implementation of a same-day discharge protocol for catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol 2021;62:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.