Abstract
Aims
The effectiveness of pulmonary vein isolation (PVI) guided by VISITAG SURPOINT (VS) has been demonstrated in Western populations. However, data for Asian populations are limited. VS settings may differ for Asians, given their smaller body size. This study aimed to describe outcomes of radiofrequency atrial fibrillation (AF) ablation guided by VS in a large Asian population.
Methods and results
The prospective, observational, multicentre MIYABI registry collected real-world data from patients undergoing VS-guided AF ablation using ThermoCool SmartTouch and ThermoCool SmartTouch SF catheters from 50 Japanese centres. All patients had paroxysmal AF or persistent AF for <6 months. Primary adverse events (PAEs) were evaluated for safety. The primary efficacy endpoint was the proportion of patients with PVI at the end of the procedure. Mid-term effectiveness (up to 12 months) was evaluated by freedom from documented atrial arrhythmias. Of the 1011 patients enrolled, 1002 completed AF ablation. The mean number of VS values per procedure was 428.8 on the anterior wall and 400.4 on the posterior wall. Nine patients (0.9%) experienced PAEs. Upon procedure completion, 99.7% of patients had PVI. Twelve-month freedom from atrial arrhythmia recurrence was 88.5%; 5.7% of patients were re-ablated. At repeat ablation, 54% of RSPV, 73% of RIPV, 70% of LSPV, and 86% of LIPV evaluated remained durably isolated.
Conclusion
Despite lower anterior wall VS values compared with the CLOSE protocol (≥550), the present study demonstrated comparable efficacy outcomes, indicating that a VS of ≥550 for the anterior wall may not be necessary for Asian patients.
Keywords: VISITAG SURPOINT, Ablation index, Asian, Atrial fibrillation, Pulmonary vein isolation
Graphical Abstract
Graphical Abstract.
What’s new?
To the authors’ knowledge, this is the largest prospective, multicentre, mid-term (12-month follow-up) study on VISITAG SURPOINT (VS)-guided atrial fibrillation ablation in Japanese patients.
Given that there are no recommended VS values in Japan, this study did not set a unified VS value but rather collected data from real-world clinical practice.
The VS values used in Japan are lower than those used in Europe and the USA.
The safety and efficacy of these lower VS values, as used in real-world clinical practice, were confirmed in Japanese patients undergoing atrial fibrillation ablation.
Introduction
Atrial fibrillation (AF) is the most common paroxysmal/sustained arrhythmia. Radiofrequency (RF) catheter ablation provides excellent results for treating many types of supraventricular arrhythmias.1 Electrical isolation of the pulmonary veins (PVs) from the left atrium is the cornerstone of all AF ablation procedures.2 The creation of durable lesions during PV isolation (PVI) for AF is of critical importance for preventing late PV reconnection and subsequent recurrence of arrhythmia.3–5 Pulmonary vein reconnections may result from failure to create transmural, continuous lesions followed by the generation of conduction gaps in the PV-encircling ablation lines.
The combined use of VISITAG SURPOINT (VS) values (also called ‘ablation index’) and VISITAG guidance could potentially lead to more durable PVI and may be associated with the prevention of AF recurrence. The effectiveness of PVI guided by VS (posterior wall, ≥400; anterior wall, ≥550) and interlesion distance (≤6 mm) was demonstrated in the VISTAX multicentre study conducted in Europe amongst patients with paroxysmal AF.6 That study demonstrated 12-month freedom from atrial arrhythmia of 78.3% and 12-month freedom from repeat ablations of 90.4%. A similar robust multicentre study conducted in the USA used VS target values of 380 for the posterior wall and 550 for the anterior wall; the target interlesion distance was the same (≤6 mm). The US study reported values comparable to the European study for 12-month freedom from atrial arrhythmia and 12-month freedom from repeat ablation (81.5% and 94.0%, respectively).7
Compared with Europe and the USA, data on the effectiveness of PVI guided by VS in Asian patients are limited. Given that Asians tend to be smaller in body size than Europeans and Americans, optimal VS settings may differ between the two populations. Thus, identifying the most favourable VS values for Asian patients may improve treatment outcomes. A small retrospective study in Japan (N = 100) investigated the utility of PVI guided by VS in Asian patients using lower VS targets (posterior wall, 375; anterior wall, 425) and a shorter target interlesion distance (4 mm) compared with both European and US studies.8 Using these conditions, 12-month freedom from any atrial arrhythmia was 87.2%, and 12-month freedom from repeat ablation was 91.9%. Whilst data from the Asian study are encouraging, the findings are limited by the small study size. To date, favourable VS values (i.e. VS targets) for Asian patients with AF remain unknown.
The prospective Multicenter Registry of AF Ablation with Ablation Index (MIYABI) registry study aimed to obtain real-world clinical outcomes of AF ablation in a large Asian population using RF ablation catheters guided by VS with contact force (CF) technology in Japan.
Methods
Study design
The MIYABI registry was a prospective, observational, multicentre study that collected real-world clinical outcomes of AF ablation using RF ablation catheters guided by VS with CF technology and included patients from 50 participating centres in Japan (see Supplementary material online, Table S1). Patients in this study received AF ablation using ThermoCool SmartTouch (n = 9, 0.9%) and ThermoCool SmartTouch SF (n = 993, 99.1%) catheters with CF-sensing capability. The VS module used as part of the CARTO 3 VISITAG module preference provides visual indications based on the evaluation of power, CF, and time parameters. In this study, no VS cut-off values were predefined for PVI throughout the participating centres. Rather, each centre determined their own VS values for anterior and posterior wall of the left atrium and for the oesophageal region based on the retrospective studies in which force-time integrals (FTIs) were used for PVI and VS values were calculated from the data of power, CF, and time parameters, achieving a higher rate of first-pass isolation (FPI).8,9
Medical information obtained as part of the standard of care using electronic case report forms and a site questionnaire was collected. Assessments, drug administration, and any other medical practices conducted for diagnosis and/or treatment as part of routine medical practice were not limited by the study protocol. In addition to the data obtained through electronic case report forms and the site questionnaire, data obtained from CARTO 3 records for each patient were used as a primary data source. Anonymized data were collected and sent to the research office (IQVIA Services Japan K.K.) using an electronic storage device. For follow-up, available data obtained through routine clinical practice at the 12-month (±30 days) post-operative visit were collected.
The study was conducted in accordance with ethical principles that have their origin in the Declaration of Helsinki, the Guidelines for Good Pharmacoepidemiology Practices, and all national regulations. The study protocol was approved by the ethics committee at each participating study site prior to patient enrolment. This study was registered at the UMIN Clinical Trials Registry (UMIN000038608). All patients provided written informed consent for the collection of their data for this study.
Patients
Patients who were ≥20 years old, who had drug-refractory symptomatic paroxysmal AF or persistent AF for <6 months and were considered candidates for catheter ablation by the study investigators per the standard of care, who planned to have PVI that was amenable to VS, and who had not previously undergone AF ablation were eligible for study inclusion. Key exclusion criteria were persistent AF with a continuous episode lasting ≥6 months, a previous AF ablation procedure, patients who were or planned to become pregnant during the study, and those with a life expectancy of <12 months.
Endpoints
Safety outcomes included primary adverse events (PAEs), defined as device- or procedure-related serious adverse events that occurred within the 7 days following the procedure. Pulmonary vein stenosis and atrio-oesophageal fistula were also considered PAEs even if they occurred during >7-day post-procedure. Predefined PAEs included death, thromboembolism, PV stenosis, atrio-oesophageal fistula, transient ischaemic attack, pulmonary oedema, cardiac tamponade/perforation, diaphragmatic paralysis, pericarditis/pericardial effusion, myocardial infarction, pneumothorax, major vascular access complication/bleeding, stroke/cerebrovascular accident, and heart block.
The primary effectiveness endpoint was the proportion of patients with PVI at the end of the procedure. Mid-term effectiveness at 12 months after the procedure was evaluated by freedom from documented atrial arrhythmias [AF, atrial tachycardia (AT), and atrial flutter (AFL)] lasting ≥30 s during the effectiveness evaluation period (post-90-day blanking period, i.e. Days 91–365). The recurrence of atrial arrhythmias was checked by periodic 12-lead electrocardiogram (ECG), 24-h Holter ECG monitoring, and/or mobile ECG at the discretion of the treating physician; any recurrence data within 90 days after the procedure were excluded. The proportions of patients with FPI (i.e. PVI after first encirclement) and with FPI that remained isolated until the end of the procedure were determined. The incidence of repeat ablation procedures during the 12-month post-procedure period was described.
Statistical methods
No formal hypothesis was tested; therefore, the sample size was not formally calculated. The sample size was set at 1000 patients and was based on enrolling ∼20 patients per participating site to reflect diverse real-world clinical practice for generalizability of the results.
The safety analysis set included all patients who met the eligibility criteria and underwent catheter insertion. Procedural effectiveness endpoints were evaluated based on subjects who underwent PVI with VS-guided ablation. The analysis set for mid-term effectiveness consisted of subjects who completed 12-month follow-up, whilst subjects with missing recurrence monitoring data were excluded. Subjects with recurrence before 12 months were included in the mid-term effectiveness analysis, even if they did not complete 12-month follow-up.
N (%) and the 95% exact binomial confidence interval (CI) were calculated for categorical variables, and descriptive statistics were used to describe continuous variables. Subgroup analysis according to AF category (paroxysmal or persistent) was performed using the Student’s t-test for continuous variables, the Fisher’s exact test for binomial variables, and the chi-square test for categorical variables. The Tukey–Kramer test was performed for multiple comparisons of VS amongst the segments (posterior wall, anterior wall, and oesophageal region).
All data analyses were conducted using SAS statistical software, version 9.4 (SAS Institute Japan Ltd.; Tokyo, Japan).
Results
Patients
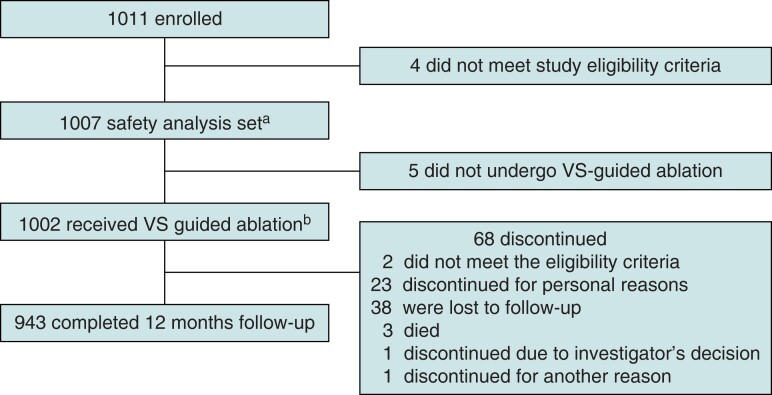
This study was conducted between January 2020 and May 2022, and 1011 patients were enrolled from 50 sites (Figure 1). The safety analysis set included 1007 patients who underwent catheter insertion, and the population that did undergo VS-guided ablation included 1002 patients. In total, 943/1011 patients (93.3% of enrolled patients) completed 12-month follow-up. The mean (SD) follow-up duration was 353.7 (70.9) days for the 1007 population. In total, 68 patients (6.7%) discontinued the study; the two most common reasons for discontinuation were loss to follow-up (55.9%; 38/68) and personal reasons (33.8%; 23/68).
Figure 1.
Patient disposition. PVI, pulmonary vein isolation; VS, VISITAG SURPOINT. aPopulation who met the eligibility criteria and had a catheter inserted. bThose in the total population who underwent PVI with VS -guided ablation.
For the safety analysis set, the mean age was 66.7 years (range, 21–90 years), 67.7% of patients were male, most patients had a known medical history (82.1%), most (62.2%) patients did not have heart failure based on the New York Heart Association class, the mean left ventricular ejection fraction was 62.4%, the mean left atrium diameter was 39.2 mm, and the mean left atrium volume was 68.3 mL (Table 1). There were 801 patients (79.9%) with paroxysmal AF and 201 patients (20.1%) with persistent AF (<6 months). The mean age was similar between patients with paroxysmal AF and persistent AF. Compared with the paroxysmal AF group, the persistent AF group consisted of more male patients and more patients with heart failure.
Table 1.
Patient demographic and background characteristics
| Received VS-guided ablation (N = 1002) |
Paroxysmal AF (n = 801) |
Persistent AF (n = 201) |
P-valuea | |
|---|---|---|---|---|
| Age (year) | 66.7 ± 10.8 | 66.5 ± 11.0 | 67.5 ± 9.6 | 0.197 |
| Male | 678 (67.7) | 522 (65.2) | 156 (77.6) | <0.001 |
| BMI (kg/m2) | 24.06 ± 3.64 | 23.92 ± 3.51 | 24.64 ± 4.06 | 0.022 |
| CHA2DS2-VASc score | 2.4 ± 1.6 | 2.3 ± 1.6 | 2.7 ± 1.7 | 0.003 |
| NYHA class | ||||
| No heart failure | 621 (62.2) (n = 999) |
545 (68.3) (n = 798) |
76 (37.8) | <0.001 |
| Class I | 190 (19.0) (n = 999) |
142 (17.8) (n = 798) |
48 (23.9) | |
| Class II | 130 (13.0) (n = 999) |
75 (9.4) (n = 798) |
55 (27.4) | |
| Class III | 21 (2.1) (n = 999) |
9 (1.1) (n = 798) |
12 (6.0) | |
| LV ejection fraction (%) | 62.4 ± 10.0 (n = 945) |
63.7 ± 8.7 (n = 752) |
57.4 ± 12.9 (n = 193) |
<0.001 |
| LA diameter (mm) | 39.2 ± 6.9 (n = 939) |
38.3 ± 6.9 (n = 747) |
42.9 ± 5.8 (n = 192) |
<0.001 |
| LA volume (mL) | 68.3 ± 33.9 (n = 684) |
64.2 ± 32.6 (n = 545) |
84.0 ± 34.7 (n = 139) |
<0.001 |
| BNP (pg/mL) | 53.9 (19.0, 139.0) (n = 647) |
41.2 (16.1, 102.8) (n = 516) |
146.6 (81.7, 245.3) (n = 131) |
<0.001 |
| NT-pro BNP (pg/mL) | 199.4 (72.2, 529.5) (n = 328) |
134.0 (59.6, 330.0) (n = 263) |
699.0 (397.0, 1357.0) (n = 65) |
0.260 |
| AF-related symptoms | 846 (84.4) | 688 (85.9) | 158 (78.6) | 0.011 |
| Hypertension | 567 (56.6) | 443 (55.3) | 124 (61.7) | 0.112 |
| Congestive HF or LV dysfunction | 161 (16.1) | 89 (11.1) | 72 (35.8) | <0.001 |
| Diabetes mellitus | 140 (14.0) | 106 (13.2) | 34 (16.9) | 0.210 |
| Stroke, TIA, or thromboembolism | 100 (10.0) | 74 (9.2) | 26 (12.9) | 0.147 |
| Vascular diseaseb | 91 (9.1) | 57 (7.1) | 34 (16.9) | <0.001 |
| Sleep apnoea | 51 (5.1) | 39 (4.9) | 12 (6.0) | 0.590 |
Completed population (N = 1002). Data are n (%), mean ± SD, or median (Q1, Q3).
AF, atrial fibrillation; BNP, brain natriuretic peptide; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years (doubled), diabetes, stroke (doubled), vascular disease, age 65 to 74 years, and sex category (female); HF, heart failure; LA, left atrium; LV, left ventricle; NT-proBNP, N terminal-pro brain natriuretic peptide; NYHA, New York Heart Association; Q, quartile; TIA, transient ischaemic attack.
P-values were determined using t-test for continuous variables, Fisher’s exact test for binomial variables, and chi-square test for other categorical variables.
Prior myocardial infarction, peripheral artery disease, or aortic plaque.
Procedure characteristics
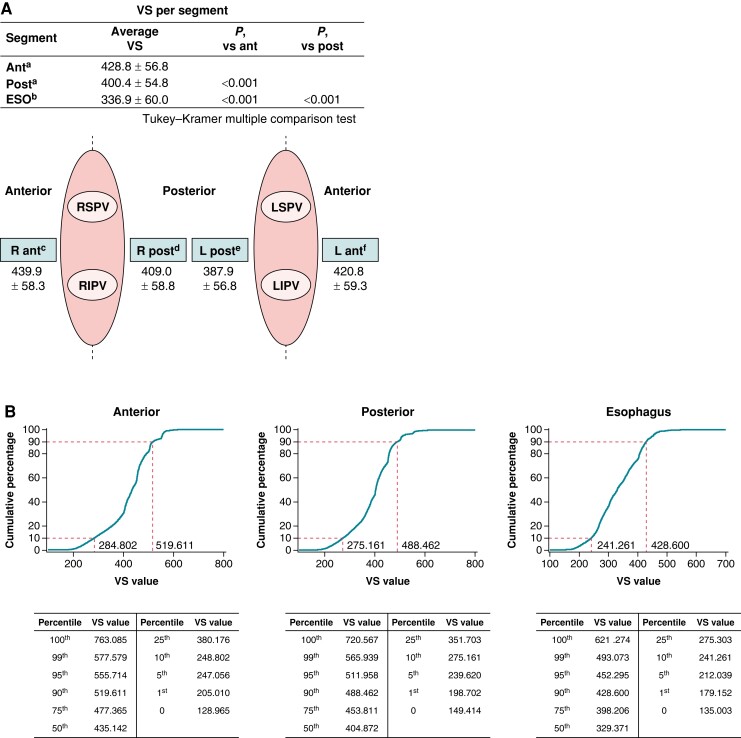
There were 108 685 RF applications for PVI amongst 1002 patients. Detailed information regarding the ablation procedures can be found in Table 2. The mean (SD) number of VS values per application by segment was 428.8 (56.8) on the anterior wall of the right and left veins and 400.4 (54.8) on the posterior wall of the right and left veins in 965 patients. The Tukey–Kramer test was performed for multiple comparisons of VS amongst the segments and demonstrated significant differences in the anterior wall vs. the posterior wall (P < 0.0001), the anterior wall vs. the oesophageal region (P < 0.0001), and the posterior wall vs. the oesophageal region (P < 0.0001) (Figure 2A). The cumulative distribution of VS values showed that the range of the 10th to 90th percentiles was 355.9 to 499.1 in the anterior regions, 331.3 to 471.8 in the posterior regions, and 259.4 to 418.3 in the oesophageal regions (Figure 2B). The VS values in ThermoCool SmartTouch and ThermoCool SmartTouch SF catheter groups are shown in Supplementary material online, Table S2.
Table 2.
Ablation procedure information
| Received VS-guided ablation (N = 1002) |
|
|---|---|
| Ablation catheter inserted, n (%) | |
| ThermoCool SmartTouch | 9 (0.9) |
| ThermoCool SmartTouch SF | 993 (99.1) |
| Total procedure time (min) | 142.9 ± 51.2 |
| Total ablation timea (min) | 86.3 ± 42.3 |
| Total PVI time (min) | 60.6 ± 30.1 |
| Total fluoroscopy timeb (min) | 22.4 ± 19.7 |
| Total mapping timec (min) | 32.3 ± 41.3 |
| CARTO data | |
| Total RF application timea (min) | 29.8 ± 11.7 |
| Number of RF applicationsa | 95.5 ± 36.4 |
| Mean contact forcea (g) | 13.4 ± 3.2 |
| Mean powera (W) | 38.4 ± 6.1 |
| Mean temperaturea (°C) | 27.4 ± 2.0 |
| Mean impedancea (Ω) | 128.3 ± 15.1 |
| Max impedance dropa (Ω) | 9.6 ± 2.9 |
Completed population (N = 1002).
Data are mean ± SD unless stated otherwise.
PVI, pulmonary vein isolation; RF, radiofrequency.
n = 998.
n = 962.
n = 868.
Figure 2.
Details of the ablation procedure (A) and cumulative distribution of VS values in the anterior, posterior, and oesophagus regions (B). Ant, anterior; ESO, oesophagus; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; post, posterior; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; VS, VISITAG SURPOINT. an = 965, bn = 651, cn = 954, dn = 955, en = 952, and fn = 960.
Safety in the acute stage
Ten PAEs occurred in nine patients (0.9%) and included cardiac tamponade/perforation (n = 3, 0.3%), major vascular access complication/bleeding (n = 3, 0.3%), pericarditis or pericardial effusion (n = 2, 0.2%), and pulmonary oedema (n = 1, 0.1%) (Table 3). There was no occurrence of device- or procedure-related deaths, atrio-oesophageal fistula, stroke, transient ischaemic attack, PV stenosis, or myocardial infarction.
Table 3.
Safety endpoints
| Safety analysis set (N = 1007) | ||||
|---|---|---|---|---|
| n (%) | 95% CI | Device-related (n) |
Procedure-related (n) |
|
| Primary safety endpoints | 9 (0.9) | 0.4–1.7 | ||
| Cardiac tamponade/perforation | 3 (0.3) | Related (3) | Related (3) | |
| Major vascular access Complication/bleeding | 3 (0.3) | Not related (3) | Related (3) | |
| Pericarditis/pericardial effusion | 2 (0.2) | Not related (2) | Related (1) Not related (1) |
|
| Pulmonary oedema (respiratory insufficiency) | 1 (0.1) | Not related (1) | Not related (1) | |
| Atrio-oesophageal fistula | 0 (0.0) | |||
| Death | 0 (0.0) | |||
| Diaphragmatic paralysis | 0 (0.0) | |||
| Heart block | 0 (0.0) | |||
| Myocardial infarction | 0 (0.0) | |||
| Pneumothorax | 0 (0.0) | |||
| PV stenosis | 0 (0.0) | |||
| Stroke/cerebrovascular accident | 0 (0.0) | |||
| Thromboembolism | 0 (0.0) | |||
| Transient ischaemic attack | 0 (0.0) | |||
Total population (N = 1007).
Device-related primary adverse events were cardiac tamponade/perforation in three patients. Procedure-related primary adverse events were cardiac tamponade/perforation in three patients, major vascular access complication/bleeding in three patients, and pericarditis/pericardial effusion in one patient.
Acute effectiveness
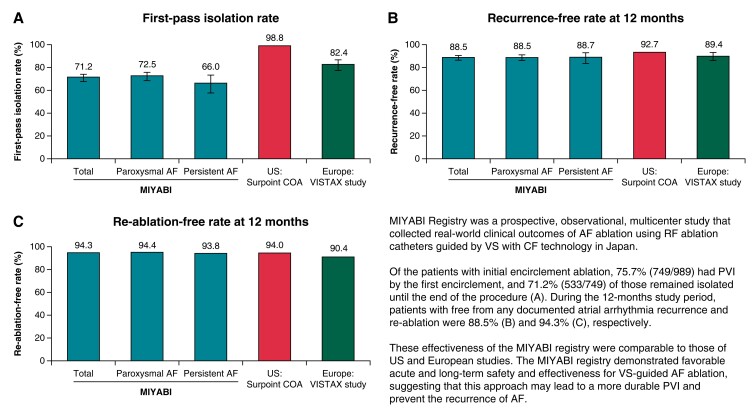
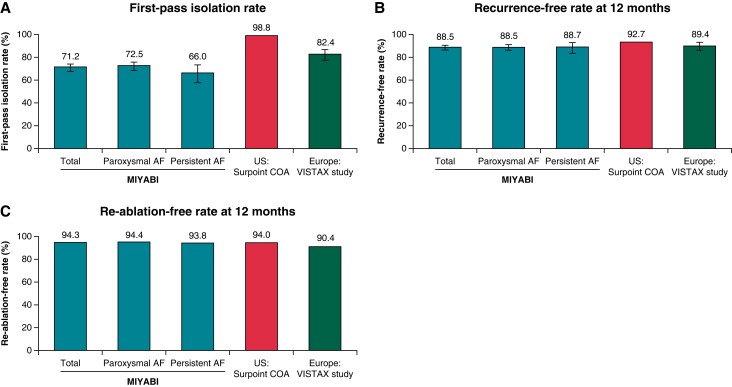
The population that underwent VS-guided ablation was used to determine the primary effectiveness endpoint (Table 4). At the end of the procedure, 99.7% (999/1002; 95% CI, 99.1–99.9%) of patients had PVI. Of note, 749 patients had FPI, of whom 71.2% (533/749) remained isolated at the end of the procedure after the waiting period/adenosine challenge (Figure 3A). The proportions of reconnection after the waiting period and pharmacological challenge were 16.1% (161/1002; 95% CI, 13.9–18.5%) and 11.5% (115/1002; 95% CI, 9.6–13.6%), respectively. When categorized by AF type, acute success rates were similar between paroxysmal AF [99.9% (800/801); 95% CI, 99.3–100.0%] and persistent AF [99.0% (199/201); 95% CI, 96.5–99.9%] patients.
Table 4.
Acute effectiveness endpoints
| Received VS-guided ablation (N = 1002) |
Paroxysmal AF (n = 801) |
Persistent AF (n = 201) |
P-valuea | |
|---|---|---|---|---|
| Primary acute effectiveness endpoint | ||||
| Patients with PVI at the end of the procedure | 99.7 (99.1–99.9) |
99.9 (99.3–100.0) |
99.0 (96.5–99.9) |
0.104 |
| Secondary acute effectiveness endpoints | ||||
| Patients with PVI by first encirclement | 75.7 (72.9–78.4) |
75.7 (72.6–78.7) |
75.8 (69.2–81.6) |
1.000 |
| Patients with PVI by first encirclement that remained isolated until the end of the procedure | 71.2 (67.8–74.4) |
72.5 (68.7–76.0) |
66.0 (57.8–73.5) |
0.131 |
Completed population (N = 1002).
Data are (%) (95% CI).
AF, atrial fibrillation; PVI, pulmonary vein isolation.
Difference between paroxysmal and persistent AF groups by Fisher’s exact test.
Figure 3.
First-pass isolation rate (A), recurrence-free rates at 12 months (B), and re-ablation-free rates at 12 months (C) in MIYABI, US SURPOINT COA7 and Europe VISTAX6 studies. Definition of first-pass isolation rate for each study: MIYABI, PVI after a first encirclement that remained isolated until the end of the procedure; US SURPOINT COA, PVI toward the end of the procedure confirmed by an entrance block; Europe VISTAX, first-pass isolation proof to a 30-min wait period and adenosine challenge. Rates were determined by standard-of-care monitoring. AF, atrial fibrillation; PVI, pulmonary vein isolation.
Mid-term effectiveness
Freedom from documented atrial arrhythmias (AF, AT, and AFL) in the subjects who completed 12-month follow-up is presented in Figure 3B. Amongst 940 patients for whom data were available, 88.5% (95% CI, 86.3–90.5%) were free from any documented atrial arrhythmia recurrence. The proportion of patients with freedom from documented atrial arrhythmias was similar for patients with paroxysmal AF and persistent AF. Mean VS values were similar regardless of atrial arrhythmia recurrence.
Repeat ablation procedures during the 12-month post-procedure period
At 12-month follow-up, 5.7% (54/942) of patients received repeat procedures (total number of repeat procedures, n = 56; one repeat procedure, n = 52; and two repeat procedures, n = 2) (Figure 3C and Table 5). Out of the 56 re-ablation procedures, 53.6% (30/56) of right superior PV (RSPV), 73.2% (41/56) of right inferior PV (RIPV), 69.6% (39/56) of left superior PV (LSPV), and 85.7% (48/56) of left inferior PV (LIPV) evaluated in repeat procedures remained durably isolated, whilst 36 procedures required re-isolation of at least 1 PV. All the reconnected PVs were successfully re-isolated during the repeat procedure. A total of 69.6% of repeat procedures required interventions in a location other than the initial PV encirclement.
Table 5.
Repeat ablation procedures during the 12-month follow-up perioda
| Variable | Received VS-guided ablation (N = 1002) |
|---|---|
| Freedom from repeat ablation | 888/942 (94.3) |
| Patients who underwent repeat ablation procedures | 54/942 (5.7) |
| No. repeat ablation proceduresb | |
| 1 | 52/54 (96.3) |
| 2 | 2/54 (3.7) |
| >2 | 0/54 (0) |
| Timing of repeat ablation proceduresb | |
| ≤90 | 1/54 (1.9) |
| >90 | 53/54 (98.2) |
| Targeted location at repeat procedurec | 56/56 (100) |
| RSPV | 26/56 (46.4) |
| RIPV | 15/56 (26.8) |
| LSPV | 17/56 (30.4) |
| LIPV | 8/56 (14.3) |
| Other | 36/56 (64.3) |
| PVs re-isolated amongst all targeted PVs at repeat procedured | 66/66 (100) |
| Repeat ablation procedures for other location outside of first encirclement (Visit 1) amongst repeat ablation procedurese | 39/56 (69.6) |
AF, atrial fibrillation; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; PV, pulmonary vein; PVI, pulmonary vein isolation; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Follow-up was performed by confirming available data obtained from routine practice at the 12-month (±30 days) post-operative visit. Mid-term data are from Day 91 after the procedure.
Denominator was the number of patients who underwent a repeat ablation procedure.
Denominator was the number of repeat ablation procedures.
Percentage was calculated as (number of PVs re-isolated amongst targeted PVs at repeat procedure/number of all targeted PVs at repeat procedure) × 100.
Percentage was calculated as (number of repeat ablation procedures identifying new foci outside of the initially isolated area amongst repeat ablation procedures/number of all repeat ablation procedures) × 100.
Discussion
This prospective registry study described the real-world clinical outcomes of AF ablation guided by VS with CF technologies in Japan. To the best of our knowledge, this is the largest prospective, multicentre, mid-term (12-month follow-up) study of VS-guided AF ablation in an Asian population, demonstrating favourable safety (0.9%) and 1-year effectiveness (89%) outcomes.
The present study reported an overall incidence of PAEs of 0.9%, which is lower than that reported in the European VISTAX study (3.6%).6 The 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement2 summarizes the incidence of AF-ablation-related complications, with incidence rates ranging from 0% to 50%. The statement notes that incidence of cardiac tamponade and pericarditis ranges from 0.2% to 5% and 0% to 50%, respectively. The incidence in our study was on the low end of that range (0.3% and 0.2%, respectively) and was similar to that reported in the Japanese Catheter Ablation registry (0.64% and 0.14%, respectively).10 Given that there was no oral anticoagulant-related bleeding in the present study, anticoagulants appeared to be well controlled. Taken together, our findings demonstrate a favourable safety profile for VS-guided AF ablation in Japanese patients. Recently, higher-power, short-duration ablation has become popular.9,11 The results of this study, in which the mean power was 38.4 ± 6.1 W, also support the safety benefit of VS-guided ablation.
A meta-analysis of the efficacy of VS-guided catheter ablation for AF reported a 12-month effectiveness of 88.2% for freedom from documented atrial arrhythmia,12 which is comparable to the 88.5% shown in the present study. A similar level of effectiveness was also observed in patients with early persistent AF (88.7%), indicating that VS-guided catheter ablation is similarly effective for both paroxysmal and persistent AF (duration <6 months). The effectiveness reported in our study was notably higher than that reported in studies evaluating other approaches to catheter ablation in Japan (61.4%)13 and elsewhere (46–59%) in patients with persistent AF14,15 and comparable to studies conducted in the USA using the ThermoCool SmartTouch SF catheter (92.7%).7 A comparison of outcomes for the present study (Asia) and the SURPOINT COA (USA)7 and VISTAX (Europe)6 studies is shown in Figure 3 and Supplementary material online, Table S3. The mean VS value per procedure used in the anterior wall in the present study (428.8) was lower than those used in Europe (target, ≥550)6,16,17 and in the USA (mean, 519.3)7 but was similar to previous studies in Asia.18–20 The mean VS value used in the posterior wall (400.4) was similar to that in Europe6 and the USA.7 Since no recommended values were available at the time when the VS module was first introduced to Japan, each facility determined VS values to achieve a higher FPI rate.8,9 Consequently, lower VS values in the anterior wall than those in the CLOSE protocol may have been used.20 The decision to use lower VS values in some procedures was likely dependent on the experience of each facility, including for safety reasons due to excessive energy delivery.
Although VS values were lower, especially in the anterior wall, in the present study than in the CLOSE protocol,21 the total procedure time and total RF ablation procedure times in the present study were comparable to those in the European study.6 The mean (SD) total procedure time was similar, with the main difference between the present study and the European study6 being a 20-min vs. a 30-min waiting period [present study, 142.9 (51.2) min, including a 20-min waiting period; European study, 156.2 (37.0) min, including a 30-min waiting period]. Total RF ablation procedure times were also similar [29.8 (11.7) min vs. 35.2 (11.1) min]. Although the mean (SD) total fluoroscopy time was longer in the present study vs. the European study [22.4 (19.7) min vs. 7.9 (6.9) min], it was comparable to the time reported for AF ablation in Japan.22,23 The number of applications is not available from the European study, but the number in the study was close to that of the US study7 [95.5 (36.4) vs. 89.2 (30.6)]. Pulmonary vein isolation was accomplished in almost all patients at the end of the procedure (99.7%). Recent studies have shown that the VS values were tailored by the left atrial wall thickness measured by enhanced computed tomography, and a low VS value may be sufficient to safely ablate an area with a thin wall.24,25 The results suggest the possibility that effective and safe ablation can be performed by using relatively low VS values that take wall thickness into account.
A suggestion from a previous study that a smaller interlesion distance could yield better outcomes even with lower VS values may partially explain the reason why the long-term outcome was maintained at a high level in this study with a low VS value.26 Previous reports have focused mainly on Caucasian populations, and given that Asians typically have a smaller body stature and weight than Caucasians, a lower VS value and smaller interlesion distance may be more effective, especially for Asians. An ablation protocol with a low VS value and smaller interlesion distance may be second-most optimal next to CLOSE for Asians, which needs further investigation. We note that the rate of first-pass circumferential PVI was lower compared with rates in VS ablation studies from the USA7 and Europe6 (Figure 3A and Supplementary material online, Table S3). There was no significant difference in mean VS values between subgroups with and without FPI [408.98 (51.45) vs. 408.81 (55.71)]. The reason for the difference in the FPI rate is unclear and requires further investigation.
This study had several limitations. The definitions for each PAE and the causal relationships between adverse events and the devices and procedures were described in the study protocol; however, a safety review committee was not involved in the study, leaving it up to the investigators at each study site to determine causal relationships. Arrhythmia recurrence was monitored under the standard of care following the Japanese Guidelines27 at the discretion of each treating physician; therefore, there was the possibility of under-detection compared with studies that had stricter monitoring procedures. Furthermore, the details of follow-up were determined by each participating institution, so there may have been variation in these procedures. For an accurate evaluation of arrhythmia recurrence, a more sensitive analysis method, such as an implantable loop recorder, may be required. There were no restrictions on postoperative antiarrhythmic drug administration during the study. Given that the VS target values and procedures were determined at the investigator’s discretion, additional studies are needed to validate the optimal VS setting observed in this study. Finally, ablation therapy, particularly PVI, is considered mandatory; however, the use of other ablation procedures in addition to PVI was determined at the discretion of each treating physician.
The present study demonstrated favourable acute and mid-term safety and effectiveness for VS-guided AF ablation, evident by a high level of durable PVI and a low recurrence rate.
Supplementary Material
Acknowledgements
The authors thank all participating patients, physicians, and institutions. The authors thank Sarah Bubeck, PhD, of Edanz (www.edanz.com) for providing medical writing support, which was funded by Johnson & Johnson K.K. Medical Company, in accordance with Good Publication Practice (GPP) 2022 guidelines (http://www.ismpp.org/gpp-2022).
Contributor Information
Ken Okumura, Division of Cardiology, Saiseikai Kumamoto Hospital, 5-3-1 Chikami, Minami-ku, Kumamoto 861-4193, Japan.
Koichi Inoue, Division of Cardiology, National Hospital Organization Osaka National Hospital, 2-1-14 Houenzaka, Chuo-ku, Osaka 540-0006, Japan; Cardiovascular Center, Sakurabashi Watanabe Hospital, 2-4-32 Umeda, Kita-ku, Osaka 530-0001, Japan.
Masahiko Goya, Department of Cardiovascular Medicine, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyo-ku, Tokyo 113-8510, Japan.
Hideki Origasa, The Institute of Statistical Mathematics, 10-3 Midori-cho, Tachikawa, Tokyo 190-8562, Japan.
Makiho Yamazaki, Department of Clinical Research, Johnson & Johnson K.K. Medical Company, Chiyoda First Building West Tower, 3-5-2 Nishi-Kanda, Chiyoda-ku, Tokyo 101-0065, Japan.
Akihiko Nogami, Department of Cardiology, Institute of Medicine, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8575, Japan.
Supplementary material
Supplementary material is available at Europace online.
Funding
This study was funded by Johnson & Johnson K.K. Medical Company.
Data availability
The data that support the findings of this study are available from the authors and Johnson & Johnson K.K. upon reasonable request.
References
- 1. Eitel C, Ince H, Brachmann J, Kuck KH, Willems S, Spitzer SGet al. Catheter ablation of supraventricular tachycardia in patients with and without structural heart disease: insights from the German ablation registry. Clin Res Cardiol 2022;111:522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga Let al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SAet al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528–606. [DOI] [PubMed] [Google Scholar]
- 4. Neuzil P, Reddy VY, Kautzner J, Petru J, Wichterle D, Shah Det al. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study. Circ Arrhythm Electrophysiol 2013;6:327–33. [DOI] [PubMed] [Google Scholar]
- 5. Kautzner J, Neuzil P, Lambert H, Peichl P, Petru J, Cihak Ret al. EFFICAS II: optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. Europace 2015;17:1229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duytschaever M, Vijgen J, De Potter T, Scherr D, Van Herendael H, Knecht Set al. Standardized pulmonary vein isolation workflow to enclose veins with contiguous lesions: the multicentre VISTAX trial. Europace 2020;22:1645–52. [DOI] [PubMed] [Google Scholar]
- 7. Di Biase L, Monir G, Melby D, Tabereaux P, Natale A, Manyam Het al. Composite index tagging for PVI in paroxysmal AF: a prospective, multicenter postapproval study. JACC Clin Electrophysiol 2022;8:1077–89. [DOI] [PubMed] [Google Scholar]
- 8. Inoue K, Tanaka N, Ikada Y, Mizutani A, Yamamoto K, Matsuhira Het al. Characterizing clinical outcomes and factors associated with conduction gaps in VISITAG SURPOINT-guided catheter ablation for atrial fibrillation. J Arrhythm 2021;37:574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okamatsu H, Koyama J, Sakai Y, Negishi K, Hayashi K, Tsurugi Tet al. High-power application is associated with shorter procedure time and higher rate of first-pass pulmonary vein isolation in ablation index-guided atrial fibrillation ablation. J Cardiovasc Electrophysiol 2019;30:2751–8. [DOI] [PubMed] [Google Scholar]
- 10. Kusano K, Yamane T, Inoue K, Takegami M, Nakao YM, Nakai Met al. The Japanese Catheter Ablation Registry (J-AB): annual report in 2019. J Arrhythm 2021;37:1443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chieng D, Segan L, Sugumar H, Al-Kaisey A, Hawson J, Moore BMet al. Higher power short duration vs. lower power longer duration posterior wall ablation for atrial fibrillation and oesophageal injury outcomes: a prospective multi-centre randomized controlled study (Hi-Lo HEAT trial). Europace 2023;25:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ioannou A, Papageorgiou N, Lim WY, Wongwarawipat T, Hunter RJ, Dhillon Get al. Efficacy and safety of ablation index-guided catheter ablation for atrial fibrillation: an updated meta-analysis. Europace 2020;22:1659–71. [DOI] [PubMed] [Google Scholar]
- 13. Murakawa Y, Nogami A, Shoda M, Inoue K, Naito S, Kumagai Ket al. Nationwide survey of catheter ablation for atrial fibrillation: the Japanese Catheter Ablation Registry of Atrial Fibrillation (J-CARAF)—report of 1-year follow-up -. Circ J 2014;78:1091–6. [DOI] [PubMed] [Google Scholar]
- 14. Verma A, Jiang C, Betts TR, Chen J, Deisenhofer I, Mantovan Ret al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 15. Mansour M, Calkins H, Osorio J, Pollak SJ, Melby D, Marchlinski FEet al. Persistent atrial fibrillation ablation with contact force-sensing catheter: the prospective multicenter PRECEPT trial. JACC Clin Electrophysiol 2020;6:958–69. [DOI] [PubMed] [Google Scholar]
- 16. Taghji P, El Haddad M, Phlips T, Wolf M, Knecht S, Vandekerckhove Yet al. Evaluation of a strategy aiming to enclose the pulmonary veins with contiguous and optimized radiofrequency lesions in paroxysmal atrial fibrillation: a pilot study. JACC Clin Electrophysiol 2018;4:99–108. [DOI] [PubMed] [Google Scholar]
- 17. El Haddad M, Taghji P, Phlips T, Wolf M, Demolder A, Choudhury Ret al. Determinants of acute and late pulmonary vein reconnection in contact force-guided pulmonary vein isolation: identifying the weakest link in the ablation chain. Circ Arrhythm Electrophysiol 2017;10:e004867. [DOI] [PubMed] [Google Scholar]
- 18. Xiong Q, Liao J, Chen W, Xiao P, Du H, He Qet al. Tailored target ablation index guided pulmonary vein isolation in treating paroxysmal atrial fibrillation: a single center randomized study in Asian population (AI-Asian-I). Front Cardiovasc Med 2022;9:937913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ninomiya Y, Inoue K, Tanaka N, Okada M, Tanaka K, Onishi Tet al. Absence of first-pass isolation is associated with poor pulmonary vein isolation durability and atrial fibrillation ablation outcomes. J Arrhythm 2021;37:1468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kobayashi S, Fukaya H, Oikawa J, Saito D, Sato T, Matsuura Get al. Optimal interlesion distance in ablation index-guided pulmonary vein isolation for atrial fibrillation. J Interv Card Electrophysiol 2021;62:123–31. [DOI] [PubMed] [Google Scholar]
- 21. Phlips T, Taghji P, El Haddad M, Wolf M, Knecht S, Vandekerckhove Yet al. Improving procedural and one-year outcome after contact force-guided pulmonary vein isolation: the role of interlesion distance, ablation index, and contact force variability in the ‘CLOSE'-protocol. Europace 2018;20:f419–27. [DOI] [PubMed] [Google Scholar]
- 22. Miwa Y, Ueda A, Komeda M, Takeuchi S, Nagaoka M, Momose Yet al. Reducing radiation exposure during atrial fibrillation ablation using lectures to promote awareness. Open Heart 2019;6:e000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inoue K, Murakawa Y, Nogami A, Shoda M, Naito S, Kumagai Ket al. Current status of catheter ablation of atrial fibrillation in Japan: summary of the 4th survey of the Japanese Catheter Ablation Registry of Atrial Fibrillation (J-CARAF). J Cardiol 2016;68:83–8. [DOI] [PubMed] [Google Scholar]
- 24. Teres C, Soto-Iglesias D, Penela D, Jáuregui B, Ordoñez A, Chauca Aet al. Personalized paroxysmal atrial fibrillation ablation by tailoring ablation index to the left atrial wall thickness: the ‘Ablate by-LAW’ single-centre study-a pilot study. Europace 2022;24:390–9. [DOI] [PubMed] [Google Scholar]
- 25. Falasconi G, Penela D, Soto-Iglesias D, Francia P, Teres C, Saglietto Aet al. Personalized pulmonary vein antrum isolation guided by left atrial wall thickness for persistent atrial fibrillation. Europace 2023 May 19;25:euad118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoffmann P, Diaz Ramirez I, Baldenhofer G, Stangl K, Mont L, Althoff TF. Randomized study defining the optimum target interlesion distance in ablation index-guided atrial fibrillation ablation. Europace 2020;22:1480–6. [DOI] [PubMed] [Google Scholar]
- 27. Nogami A, Kurita T, Abe H, Ando K, Ishikawa T, Imai Ket al. JCS/JHRS 2019 guideline on non-pharmacotherapy of cardiac arrhythmias. Circ J 2021;85:1104–244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the authors and Johnson & Johnson K.K. upon reasonable request.