Abstract
Major advances in pulmonary arterial hypertension, pulmonary hypertension (PH) associated with lung disease, and chronic thromboembolic PH cast new light on the pathogenetic mechanisms, epidemiology, diagnostic approach, and therapeutic armamentarium for pulmonary vascular disease. Here, we summarize key basic, translational, and clinical PH reports, emphasizing findings that build on current state-of-the-art research. This review includes cutting-edge progress in translational pulmonary vascular biology, with a guide to the diagnosis of patients in clinical practice, incorporating recent PH definition revisions that continue emphasis on early detection of disease. PH management is reviewed including an overview of the evolving considerations for the approach to treatment of PH in patients with cardiopulmonary comorbidities, as well as a discussion of the groundbreaking sotatercept data for the treatment of pulmonary arterial hypertension.
Keywords: pulmonary hypertension, pulmonary arterial hypertension, pulmonary vascular disease
Contents
- Progress in Translational Science
- Epigenetic Mechanisms
- Transcription Factors in PAH
- BMPR2-mediated RV-PA Remodeling
- Tyrosine Kinase and Growth Factor Signaling
- Dysregulated Cellular Metabolism and Oxidant Stress
- Hypoxia and Metabolic Signaling
- Fibrosis, Thrombosis, and Extracellular Matrix Remodeling
- Inflammation and Immune Cell Infiltration
- Cutting-Edge Developments in Clinical PH
- Lowering the Thresholds—PH Redefined
- Novel Hemodynamic Parameters
- RV Pressure-Volume Analysis and RV-PA Coupling
- Approach to Diagnosis: Technique and Timing
- Exercise Hemodynamics
- Next-Generation Imaging in Pulmonary Vascular Disease
- Socioeconomic Parameters and Access to Health Care
- PH and Obesity
- PAH with Comorbidities versus Severe PH with Left Heart Disease
- PAH with Comorbidities versus Severe PH with Lung Disease
- Risk Stratification
- Serum Biomarkers
- Novel Assessment Tools
- Updates in the Approach to Treating Patients with PAH and CTEPH
- Sotatercept
- Updates on Combination Therapy in PAH
- Treating Mild PH and Mild PAH
- Balloon Pulmonary Angioplasty and CTEPH
- Physical Activity in PAH
Clinical Trial Design
Future Directions
Pulmonary hypertension (PH) is a heterogenous clinical disease characterized foremost by an abnormal increase in pulmonary artery pressure. Pulmonary vasculopathy, characterized by pathologic remodeling and vasoconstriction of the pulmonary arteries and (in the case of certain PH subtypes) veins, results in progressive dyspnea, exercise intolerance, right ventricular (RV) failure, and death. Earlier diagnosis and specialist referral is an emergent focus in the PH field (1), which is in line with a recent change in the hemodynamic criteria for diagnosing PH that now includes mean pulmonary artery pressure (mPAP) >20 mm Hg (from ⩾25 mm Hg) and pulmonary vascular resistance (PVR) >2.0 Wood units (WU) (from >3.0 WU) (2, 3). Major advances expanding the molecular mechanisms underpinning PH (Figure 1) dovetail progress advancing novel therapeutics for patients with pulmonary arterial hypertension (PAH), particularly the emergence of sotatercept, a novel first-in-class activin signaling inhibitor (4–6). Here, we summarize the most recent state-of-the-art research in PH, beginning with translational scientific discoveries and emphasizing promising therapeutic targets, and then we discuss key clinical advances and future goals for the PH field.
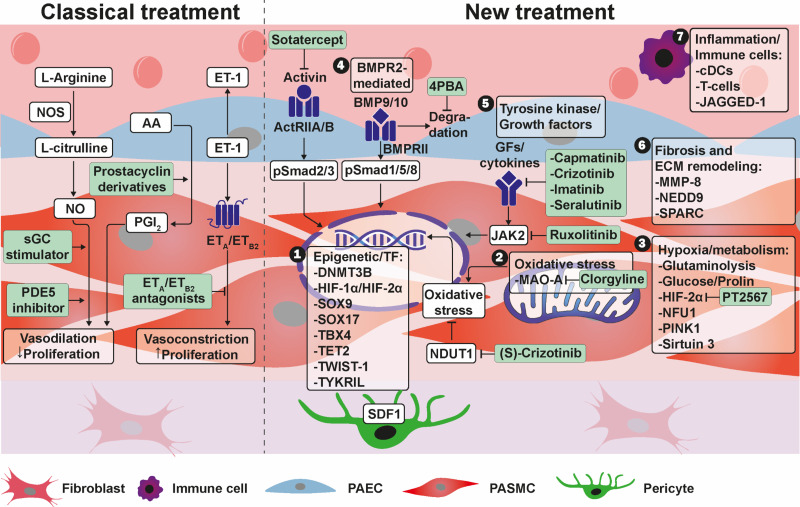
Figure 1.
Current treatment approaches and novel targets in pulmonary arterial hypertension. Current treatment approaches focus on endothelin, nitric oxide, and prostacyclin signaling. Novel treatment targets address 1) epigenetic mechanisms/transcriptional factors; 2) oxidative stress; 3) hypoxia and metabolic signaling; 4) BMPR2–mediated signaling; 5) tyrosine kinase and growth factor signaling; 6) fibrosis and extracellular matrix remodeling; and 7) inflammation and immune cell infiltration. 4PBA = 4-phenylbutyric acid; AA = arachidonic acid; ActRII = activin receptor type II; BMP = bone morphogenetic protein; BMPR2 = bone morphogenetic protein receptor type 2; cDC = conventional dendritic cell; DNMT3B = DNA methyltransferase 3 β; ET = endothelin; GDFs = growth and differentiation factors; HIF = hypoxia-inducible factor; JAGGED-1 = jagged canonical Notch ligand 1; JAK = Janus kinase; MAO-A = monoaminoxidase A; MMP = matrix metalloproteinase; NUDT1 = nudix hydrolase 1; NEDD9 = neural precursor cell–expressed developmentally downregulated protein 9; NFU1 = NFU1 iron-sulfur cluster scaffold; NO = nitric oxide; NOS = nitric oxide synthase; PAEC = pulmonary artery endothelial cell; PASMC = pulmonary arterial smooth muscle cell; PDE5 = phosphodiesterase 5; PGI2 = prostacyclin; PINK1 = phosphatase and tensin homolog–induced kinase 1; SDF1 = stromal cell–derived factor 1; sGC = soluble guanylate cyclase; SOX9 = SRY-box transcription factor 9; SOX17 = SRY-box transcription factor 17; SPARC = secreted protein acidic and rich in cysteine; TBX4 = T-box transcription factor 4; TET2 = Tet methylcytosine dioxygenase 2; TWIST1 = Twist family BHLH transcription factor 1; TYKRIL = tyrosine kinase receptor–inducing long noncoding RNA.
Progress in Translational Science
Proliferative, fibrotic, and plexogenic remodeling of distal pulmonary arterioles is the hallmark pathological feature of PAH. PAH vasculopathy is controlled by a heterogenous array of molecular events that differ subtly between patients and across cohorts. Despite this variability, certain recent advances have emerged with strong therapeutic relevance. For example, sotatercept is the first activin signaling inhibitor shown to improve outcomes in PAH clinical trials, which follows years of accumulating basic and translational data on the association between bone morphogenetic protein receptor type 2 (BMPR2) genetic variants, aberrant bone morphogenetic protein (BMP) signaling, and pulmonary artery (PA) remodeling. BMPR2 receptors belong to the large family of transforming growth factor-β (TGF-β) receptors that exert diverse and partially opposing effects through their variable receptor complex composition and affinity for different ligands (e.g., activins, inhibins, BMPs). The imbalance between proliferative and antiproliferative TGF-β receptor signaling in the development of PH has long been established; however, sotatercept is the first therapeutic approach to rebalance TGF-β signaling by the inhibition of proproliferative ligands—specifically, activins (7). This milestone establishes a new paradigm for developing disease-specific therapeutics and is aligned with progress in epigenetics, transcription factor biology, tyrosine kinase signaling, and pulmonary vascular immunology. Here, we summarize these and other recent advances, including progress in understanding unique molecular events that underpin pulmonary vascular and RV dysfunction and therapeutic prospects on the basis of these translational discoveries.
Epigenetic Mechanisms
Since the discovery of BMPR2 variants in PAH, knowledge about the genetic basis of PH has expanded significantly to now include information on novel epigenetic regulators of gene transcription and translation. The transcription factors T-box TF 4 and SRY-box TF 9 are normally expressed only prenatally; however, epigenetic derepression has been described in persistent PH in the newborn and adult PAH (8), implying an association between developmental gene programming and PH. Methylation and de-methylation epigenetic processes have also been addressed in PAH. For example, deleterious germline variants coding for the DNA methylation enzyme ten-eleven translocation methylcytosine dioxygenase 2 (or, TET2) are sixfold more common in patients with PAH compared with unaffected controls, and TET2−/− mice develop PAH spontaneously (9). Similarly, upregulation of DNA methyltransferase 3B (DNMT3B) is observed in pulmonary artery smooth muscle cells from PAH patients, and Dnmt3b−/− is associated with a severe pathophenotype in experimental (inflammatory) PAH in vivo (10).
Novel epigenetic regulators of gene transcription and translation include long noncoding RNAs, which belong to a group of transcripts that are not translated into proteins but regulate epigenetic, transcriptional, and posttranscriptional processes. Tyrosine kinase receptor–inducing long noncoding RNA (or, TYKRIL) has been shown to repress platelet-derived growth factor receptor β (or, PDGFRβ) signaling in idiopathic PAH, which regulates proliferation and apoptosis resistance in pulmonary artery smooth muscle cells and human pericytes, respectively (11). As PDGFRβ has emerged as a promising drug target in PAH—for example, imatinib (12) and seralutinib (13)—additional studies are needed to address the potential therapeutic and off-target effects of these epigenetic mechanisms (Figure 1).
Transcription Factors in PAH
Transcription factors may be more accessible to therapeutic intervention than genetic and epigenetic targets. Mutations of the transcription factor SRY-related HMG-box17 (SOX17) were recently identified as imposing a novel genetic predisposition to PAH (14). It is interesting that patients with PAH with SOX17 pathogenic variants show a severe phenotype that is frequently associated with congenital heart disease and malformations that affect pulmonary vessels as well as bronchial arteries (15). Large genome-wide association studies indicate that impairment of SOX17 because of genetic variations at loci in an enhancer near SOX17 might be more common in patients with PAH than anticipated (16). Subsequent animal studies confirm the functional relevance of SOX17 downregulation, which increases the susceptibility of mice to hypoxia-induced PH. It is interesting that these alterations are linked to overactivated c-Met hepatic growth factor signal transduction and are inhibited pharmacologically with the c-Met inhibitors crizotinib and capmatinib (17).
Other transcription factors hold promise as therapeutic targets in PAH; for example, inhibiting pulmonary endothelial Twist-related protein 1 (TWIST-1) expression inhibits endothelial-mesenchymal transition, which underlies vascular fibrosis in PAH (18). More recently, it was observed that TWIST-1 expression is increased in PAH pulmonary artery smooth muscle cells in situ and that TWIST-1–dependent degradation of GATA-binding protein 6 (or, GATA-6) disrupts BMPR2 signal transduction to promote cellular proliferation in vitro (19). In turn, smooth muscle cell–specific TWIST-1 downregulation normalizes BMPR2 to attenuate PH in animal models in vivo. This raises speculation that targeting E3-ligase murine double minute 2 pharmacologically, which mediates TWIST1-induced GATA-6 degradation and is already the focus of active clinical trials in oncology, could be important to consider in PAH (Figure 1).
BMPR2-mediated RV-PA Remodeling
In light of the recent sotatercept results (discussed later), the TGF-β/BMP pathway is perhaps the most promising target for novel therapeutic approaches for PH. A number of preclinical models are being used in current investigations of TGF-β/BMP signaling. Chaperone 4-phenylbutyrate (4PBA), which is a sodium salt of an aromatic fatty acid, rescues BMPR2 signaling in a murine model with the pathogenetic BMPR2 (C118W) variant to restore SMAD1/5 expression, among other effects (20). The administration of 4PBA to BMPR2C118W mice in vivo attenuated proliferative pulmonary vascular changes in situ. As 4PBA is a drug approved by the Food and Drug Administration (FDA) that could be repurposed for PH treatment, further studies are warranted. Other therapeutic approaches address the ligands of BMPR2, which are BMP9 and BMP10. Accordingly, heterozygous mutations in 4PBA (which encodes BMP9) are associated with decreased plasma levels of BMP9 and, surprisingly, also BMP10 in patients with PAH (21). Along these lines, exogenous BMP9 has been advanced as a potential therapeutic in pulmonary veno-occlusive disease (22). The complexity of BMP signaling has been investigated in a recent study showing that, at least under physiological conditions, BMP9/BMP10 supplementation promoted a switch from a synthetic to a contractile phenotype of pulmonary vascular smooth muscle cells, whereas a loss of both ligands caused a massive decrease of contractile vascular smooth muscle cells (23). Fresh data affirmed prior work (24), implicating circulating BMPs in the diagnoses of both portopulmonary hypertension and hepatopulmonary syndrome. Compared with cirrhotic controls awaiting liver transplant, levels of BMP9 (synthesized in the liver) in patients with portopulmonary hypertension were significantly lower, and those with hepatopulmonary syndrome had significantly reduced levels of both BMP9 and BMP10. These results perpetuate ongoing interest in the mechanistic roles of BMPs in cardiopulmonary complications of cirrhosis (25).
Tyrosine Kinase and Growth Factor Signaling
Opposing protective and pro-PAH effects have been reported for various tyrosine kinase inhibitor drugs, although the mechanisms by which to account for this finding are not fully known (26). Thus, the search for more precise tools targeting growth factor signaling is warranted. Findings from a tyrosine kinase–dependent phosphorylation screening assay (27) demonstrated that Janus kinase 2 (or, JAK2) is overactivated in PAH, leading to the discovery that ruxolitinib (which targets JAK2 and is approved for myelofibrosis among other diseases associated with PH) (28) inhibits cellular proliferation and partially reverses PH in animal models (29). Further research is needed to understand the therapeutic potential of specific tyrosine kinase inhibitors and kinase activity assay screening in patients with PAH before treatment.
Dysregulated Cellular Metabolism and Oxidant Stress
Reactive oxygen species (ROS) accumulation in vascular cells is known to dysregulate critical (and druggable) pathways that maintain normal pulmonary vascular reactivity and tone, including nitric oxide–soluble guanylyl cyclase signaling (30), endothelin receptor-B receptor-ligand interactions (31), and peroxisome proliferator-activated receptor gamma (PPAR-γ)–BMPR2 signaling (32), among numerous others. Contemporary observations identify upregulation of monoaminooxidase A, which generates the bioactive oxidant hydrogen peroxide, in experimental PAH. Treatment with the monoaminooxidase-selective antagonist clorgyline attenuated PAH in vivo, but not right heart dysfunction directly induced by mechanically induced RV afterload (through PA banding) (33).
However, currently, the relevance of ROS as a therapeutic target is controversial, as oxidant stress may also exert effects that attenuate PH. Building on prior evidence showing that remodeled pulmonary arterioles express DNA damage marker–positive cells (i.e., 53BP1 and γ-H2AX), Meloche and colleagues demonstrated that detoxifying DNA enzyme, nudrix hyrolase 1 (NUDT1), is increased in pulmonary artery smooth muscle cells isolated from patients with PAH (34, 35). Pharmacological inhibition of NUDT1 using (S)-crizotinib decreased PH in SU-5416/hypoxia (angioproliferative) and monocrotaline (inflammatory) PAH in rats, indicating that the protection of DNA against oxidative stress may allow the cell to escape apoptosis and promote proliferative remodeling. This study reinforces the diverse actions of ROS, which include proproliferative signaling molecules as well as toxins, depending on subcellular localization and concentration (36).
Hypoxia and Metabolic Signaling
The therapeutic potential of hypoxia-inducible factor (HIF)-2α was shown in experiments using the novel HIF-2α inhibitor PT2567, which improves PH and right heart remodeling in rodent models when administered using disease-prevention and -reversal protocols (37). The cell type–specific effects of HIF and derivative effects of HIF-1α versus those of HIF-2α, per se, likely need further empiric investigation, as there appear to be differential effects through each pathway on pulmonary vascular and RV remodeling.
Metabolic and mitochondrial dysregulation are key features of PAH. Recent work by Wertheim and colleagues has demonstrated a shift toward cellular glucose and proline avidity in PAH as a mechanism by which the highly synthetic pulmonary artery endothelial cell pathophenotype may induce vascular fibrosis (38). Other metabolic alterations, with similarities to solid tumor metabolism, include increased glutaminolysis, which may be therapeutically targeted (39). Saraji and colleagues showed that exaggerated phosphatase and tensin homolog–induced putative kinase 1 (or, PINK1)–dependent mitophagy may play a role in hypoxia-induced pulmonary vascular remodeling (40). In rats, engineered point mutations in NFU1, a mitochondrial protein essential for iron sulfate cluster biogenesis and mitochondrial function, resulted in aberrant hemodynamics and vascular rarefaction, as well as the sexual dimorphism that is characteristic of PAH (41). Further mitochondrial proteins that are involved in PAH are the uncoupling protein 2 and the NAD-dependent deacetylase sirtuin-3. Loss of their function was suggested to promote human PAH and shown to trigger development of PH in mice (42). Although there is one clinical study of dichloroacetate with mixed results (43), metabolic and mitochondrial dysfunction remains a desired druggable target in the field.
Fibrosis, Thrombosis, and Extracellular Matrix Remodeling
The mechanisms that regulate persistent fibrothrombotic remodeling after luminal pulmonary embolism are poorly characterized but could be important for developing disease-specific therapies in chronic thromboembolic PH (CTEPH). CTEPH is a disease that involves several pathogenetic factors, such as inflammation and abnormal coagulation (44), but also may be related to hypoxia in affected tissue (45, 46). Upregulation of HIF-1α increases pulmonary endothelial neural precursor cell expressed developmentally downregulated protein 9 (NEDD9), which interacts with P-Selectin expressed on activated platelets. This is a key event that controls platelet adhesion to pulmonary artery endothelial cells, with direct therapeutic implications for patients with CTEPH (47). It has been shown that NEDD9 also regulates extracellular matrix remodeling (48), which is consistent data with identifying key matrix metalloproteinase (MMP) subtypes in the pathogenesis of PAH (49). In this regard, the matricellular glycoprotein protein secreted protein acidic and rich in cysteine (or, SPARC), which regulates a variety of cellular functions such as extracellular matrix production and proliferation, is identified as a novel regulator of vascular cell function in PH. Its knockdown improves hemodynamic and cardiac function in mice with PH (50).
Not all matrix proteins are pathogenetic, however, as MMP-8 appears to be protective against PAH (51). Global MMP-8−/− mice exhibit exaggeration of hypoxia-induced PH, whereas genetic ablation of MMP-8 in pulmonary artery smooth muscle cells induces the pro-remodeling, mechanosensitive focal adhesion kinase–Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) pathway. These data, thus, imply endogenous negative regulation of YAP/TAZ, which is reported previously to affect cell adhesion and type IV (basement membrane) collagen in remodeled idiopathic PAH pulmonary arterioles (52–54). These findings emphasize the interaction between mechanical stress, extracellular matrix signaling, and pathways underlying the development of PH. The importance of extracellular matrix signaling is also addressed in a current clinical trial in healthy volunteers (ClinicalTrials.gov ID: NCT03522935) that aims to develop the elastase inhibitor elafin for PAH treatment.
Inflammation and Immune Cell Infiltration
Despite the widely accepted contributions of inflammation and immune cells in the development of PAH, currently, no approved therapy addressing inflammation is available. Thus, further studies to understand the inflammatory mechanisms in PH are warranted. In this regard, exposure to black mold (Stachybotrys chartarum) causes a time-dependent T cell response involving peribronchiolar and perivascular infiltration, resulting in PA remodeling (55). Repetitive intratracheal application of the common aeroallergen house dust mite induces perivascular inflammation and neointimal formation in mice by means of Notch-mediated signaling through JAGGED-1 (56). The extent to which vascular injury is directly caused by foreign (biological) particles or from an exaggerated immune response through IgE signaling (57) is not known and, thus, requires further investigation. T cell responses have also been a focus of attention; one study showed that activated conventional dendritic cells (cDCs), which regulate T cell responses and are increased in lungs with idiopathic PAH, induce development of PH in genetically altered mice with an increased portion of activated cDCs (58). In this regard, the monocrotaline PH model may better reflect the increase in cDCs and interstitial macrophages described in human PAH than the Sugen-5416/hypoxia model (59).
Pericytes are emergent players in PH pathology, possibly by promoting both vascular remodeling and pruning. Increased expression of the chemotactic chemokine stromal cell-derived factor 1 in neural/glial antigen 2–positive cells, including pericytes and pulmonary artery smooth muscle cells, promotes accumulation of these cells in muscularized microvessels leading to PH (60).
Stem cell therapy may provide another therapeutic approach to pulmonary vascular disease, at least in certain cases, as demonstrated recently in a case report with a 3-year-old female with severe heritable PAH who was treated with conditioned media from allogenic human umbilical cord mesenchymal stem cells (MSCs) and showed remarkable clinical improvement (61). Currently, the exact mechanism of stem cell–related treatment effects is unclear, but recent studies in preclinical PH models support the findings that MSCs exert most of their therapeutic effects through a paracrine mechanism (62); for example, extracellular vesicles released by MSCs (63).
Cutting-Edge Developments in Clinical PH
Lowering the Thresholds—PH Redefined
Regardless of PH subtype, there is a continuous relationship between all-cause mortality and mPAP that begins at ∼20 mm Hg (64–68) which is also the upper limit of normal (although a slight mPAP rise is expected with increasing age) (69, 70). Nonetheless, physiological conditions (e.g., pregnancy, reversible anemia, high cardiac output states) may induce flow-mediated elevation in mPAP, as described originally by Wood (71); thus, PVR has been added to enhance the specificity of PH diagnostic criteria compared with mPAP alone (72, 73). However, there was a need to clarify the classical threshold of PVR > 3.0 WU in contemporary terms along a spectrum of mPAP and PVR clinical risk (Table 1).
Table 1.
Overview of the 2022 ESC/ERS Definitions of PH
| Type of PH | Definition |
|---|---|
| Precapillary PH | mPAP > 20 mm Hg PAWP ⩽ 15 mm Hg PVR > 2 WU |
| Isolated postcapillary PH | mPAP > 20 mm Hg PAWP > 15 mm Hg PVR ⩽ 2 WU |
| Combined pre- and postcapillary PH | mPAP > 20 mm Hg PAWP > 15 mm Hg PVR > 2 WU |
Definition of abbreviations: ERS = European Respiratory Society; ESC = European Society of Cardiology; mPAP = mean pulmonary artery pressure; PAWP = pulmonary artery wedge pressure; PH = pulmonary hypertension; PVR = pulmonary vascular resistance; WU = Wood units.
A retrospective analysis of more than 32,000 patients with elevated mPAP found that PVR ⩾ 2.2 WU was associated with a 47% increase in mortality and a 17% increase in heart failure-associated hospitalizations, compared with PVR < 2.2 WU (74). In a cohort of 284 well-phenotyped patients with systemic sclerosis with mPAP > 20 mm Hg, PVR > 2.0 WU was associated with significant decreases in 6-minute-walk distance (6MWD), tricuspid annular plane of systolic excursion (TAPSE; a measure of RV systolic function on echocardiography), PA compliance, and long-term survival (75). Nevertheless, as discussed later, hemodynamics are only one criterion to be placed in context before the designation of PAH, which remains a clinical diagnosis.
Novel Hemodynamic Parameters
Pulmonary artery compliance (CPA) is a pulsatile measure of RV afterload calculated as stroke volume/pulmonary artery pulse pressure (ml/mm Hg) (76). Unlike PVR which represents static RV afterload, CPA captures the capacity for the PA bed to dilate and accommodate RV stroke volume (77). The prognostic significance of CPA has been established for over a decade (77), but there is interest in utilizing it as an RV workload surrogate to risk-stratify patients. For example, among patients with heart failure with preserved ejection fraction (HFpEF), each unit increase in CPA was associated with a significantly decreased risk of mortality and cardiac hospitalization by 23% and 13%, respectively (78).
RV Pressure-Volume Analysis and RV-PA Coupling
Pressure-volume (PV) analysis, the gold-standard method to assess dynamic ventricular function, is performed at end-expiration using a conductance catheter which approximates RV volume on the basis of the distance between electrodes and the cross-sectional area (79). Among patients with PH, a notched or trapezoidal relationship between beginning systolic pressure and end systolic pressure is representative of RV-PA uncoupling and associated with higher PVR, mPAP, and brain natriuretic peptide (BNP) and a lower CPA (80, 81). Catheter-based PV measurements depend on sophisticated equipment that is not readily available at most centers but do correlate with noninvasive measures of ventriculoarterial coupling and load-independent RV function (82). Initial studies utilized this method for characterization of ventriculoarterial coupling in patients with PH (83, 84) and to show the beneficial effects of iloprost treatment on RV contractility independent from its vasodilatory effects (85).
Approach to Diagnosis: Technique and Timing
PH is often suggested by findings on transthoracic echocardiography, including an increased tricuspid regurgitant jet velocity >2.8 m/s, elevated systolic pulmonary artery pressure, and dilated right heart chambers (86). However, accurate hemodynamic measurement during right heart catheterization (RHC) is essential for proper PH clinical classification and thereby informs appropriate treatment (Figure 2). A single-center study demonstrated the value of repeat hemodynamic measurements, especially for patients with borderline elevated PA wedge pressure (PAWP) (14–16 mm Hg) on first assessment, where the final diagnosis changed in 10% of patients across measurements (87). Similarly, a single-center, standard-of-care protocol to confirm the accuracy of PAWP > 15 mm Hg with suspected inaccurate waveform tracings revealed that confirmatory PAWP saturation measurements resulted in significantly lower PAWP and, therefore, higher PVR determinations. As a result, 12% of the N = 111 patients studied required reclassification (88).
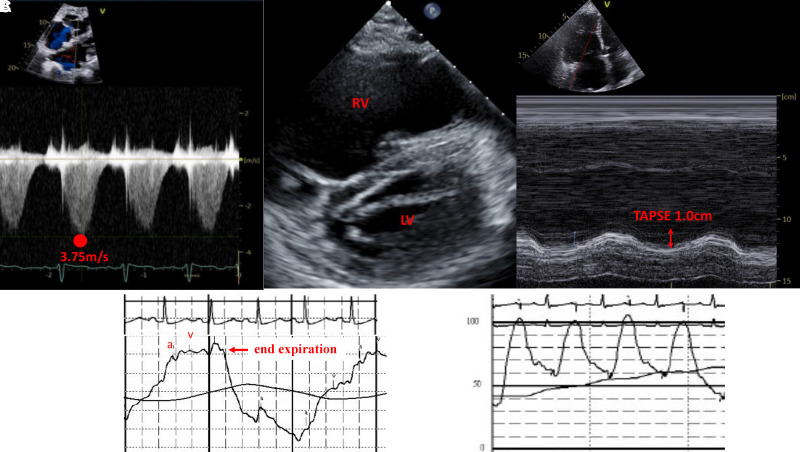
Figure 2.
Echocardiographic features supportive of pulmonary hypertension and characteristic confirmatory right heart catheterization tracings. (A) The velocity of the tricuspid regurgitant jet (3.75 m/s), measured with continuous‐wave Doppler imaging in the apical four‐chamber view, is used to calculate systolic pulmonary artery pressure (sPAP); sPAP = 4V2 + CVP. (B) In the short‐axis two‐chamber view, the interventricular septum imaged at end systole is flat (“D sign”), indicative of RV volume and pressure overload. (C) TAPSE, a measure of right ventricular function, represents the apical displacement of the tricuspid annulus between diastole and systole measured by M‐mode echocardiography. (D) Typical pulmonary artery wedge pressure tracing at end-expiration with black line representing average pressure over the respiratory cycle which can be reported in cases with significant respiratory variation, as opposed to typical end-expiration; a-wave and v-wave are noted in red, with the v-wave falling outside the T-wave on an associated ECG. (E) Example of an optimal pulmonary artery pressure waveform with preserved dichroic notch. 4V2 = V is the peak velocity (m/sec) of the tricuspid regurgitant jet; CVP = central venous pressure; RV = right ventricular; TAPSE = tricuspid annular plane of systolic excursion.
Standardizing RHC timing may be a major opportunity to limit misclassification of PH subtype. For example, RHC after full diuresis in patients with impaired left ventricular diastolic function at index presentation for heart failure often suggests precapillary PH but may simply reflect postcapillary PH after iatrogenic volume optimization. To improve diagnostic yield and capture patients earlier in the PH trajectory, proposed contemporary indications for RHC include unexplained dyspnea, unexplained (including asymptomatic) RV dysfunction, and estimated RV systolic pressure >30 mm Hg (without obvious left heart disease) (68, 89–93).
Exercise Hemodynamics
Invasive hemodynamics from healthy adults during exercise demonstrate a significant increase in mPAP and PAWP, with a slight but nonsignificant decrease in PVR, because of the recruitment and distensibility of a normal pulmonary vasculature (70). This relationship is influenced by age; an abnormal pulmonary hemodynamic response during exercise is characterized by an increased mPAP/cardiac output (CO) slope of 1.6 and 3.3 WU for subjects 30 and 70 years of age, respectively (94, 95). Exaggerated elevation of mPAP in response to increased CO during exercise is associated with impaired physical activity level and clinical events. Although variability between centers and studies is reported for the method used to collect exercise-hemodynamic data, an mPAP/CO slope between rest and exercise >3 mm Hg/L/min is generally viewed as pathogenic (2, 3, 96). Classification of PH remains a clinical diagnosis supported (or refuted) by hemodynamic values, and the implications of treating milder and exercise PH pharmacologically are largely unknown.
Next-Generation Imaging in Pulmonary Vascular Disease
Quantitating disease burden noninvasively may afford critical opportunities to monitor the effect of therapeutic interventions on disease trajectory (97). A study of 2,500 adults from the Framingham Heart Study utilizing noncontrast computed tomography (CT) to quantitate the distal pulmonary vessel loss (i.e., “vascular pruning”) characteristic of PH at autopsy identified a significant correlation between small-vessel volume and all-cause mortality (97). In fact, the quartile of individuals with the most severe vascular pruning, as measured by the cross-sectional area of small vessels (<5 mm2) compared with the total volume of intraparenchymal vessels, had a 2.64-fold higher rate of death (95% confidence interval [CI] = 1.30, 5.39; P = 0.0008). Similarly, using volumetric reconstruction of CT imaging, Rahaghi and colleagues found lower small-vessel venous volume in patients with normal resting hemodynamics and exercise-induced PH, compared with that of controls who did not demonstrate PH during cardiopulmonary exercise testing (98).
Among patients with established and advanced PAH, vascular reconstruction of high-resolution CT was used to estimate thoracic blood volume and explain the airway compression that occurs as a result of pulmonary artery dilation. These findings may account for the dyspnea and dynamic hyperinflation seen in pulmonary function testing in advanced PAH (99). Finally, CT may be helpful for further understanding the risk profile of patients who were previously thought to have isolated idiopathic IPAH (IPAH) or Group 3 PH. A recent study comparing 303 patients with IPAH with no evidence of parenchymal lung disease on a CT chest scan to 190 patients with IPAH with minor parenchymal abnormalities found a significant 5-year survival benefit among patients with no CT abnormalities (78%) compared with those with mild abnormalities (22%), P < 0.0001; there was no difference in those whose imaging findings were more consistent with emphysema as compared with interstitial lung abnormalities (100).
Socioeconomic Parameters and Access to Health Care
The health disparities that adversely impact patient access to medical care are especially burdensome to individuals with PAH, in which the disease trajectory can be affected by prompt identification, treatment, and follow-up at experienced centers. A recent prospective study of individuals enrolled in the Pulmonary Hypertension Association Registry (PHAR) found that Hispanic patients were more likely to have Medicaid or to be uninsured compared with non-Hispanic White patients (25% vs. 12% and 7.1% vs. 1.4%, respectively) and required more frequent hospitalizations despite similarly severe disease (101). Studies such as these emphasize the importance of the inclusion of underrepresented minorities, among others, in clinical trials and registries to adequately address the social determinants of health that influence disease course (101).
Additionally, therapeutic advances should be paralleled by discussions surrounding care access and affordability, especially in geographically isolated communities (102). A recent claims-based study of a national cohort of patients with PH identified an increased risk of all-cause mortality in patients living in rural counties as compared with patients living in metropolitan counties (hazard ratio [HR] = 1.48; 95% CI, 1.14–1.92; P = 0.003) (103).
Methamphetamine abuse, a practice more common among those with lower socioeconomic status, including lower income, level of education, and employment, is associated with a 42% increase in risk of developing PAH, compared with non-users (HR = 1.42; 95% CI = 1.26, 1.60) (104). A unique profile of methamphetamine-associated pulmonary arterial hypertension (meth-APAH) has emerged from the PHAR data, defined by younger age, lower cardiac index, more severe symptom burden (i.e., lower functional class), and lower health-related quality of life, compared with those with idiopathic PAH (105, 106).
PH and Obesity
It has long been recognized that PAH occurs more commonly in obese patients compared with lean counterparts (107); however, it is unclear whether adiposity directly regulates vascular remodeling or whether obesity is simply a highly prevalent comorbidity in at-risk patients. Higher thoracic visceral adipose tissue, but not subcutaneous adipose tissue, measured by CT, is associated with a decreased risk of PH (possibly as an autonomous source of vasoactive hormone synthesis, such as estrogen). Through the secretion of cardioprotective adipokines including vaspin, the visceral adipose tissue may exert favorable effects on the vascular endothelium, including potentiation of nitric oxide and inhibition of endothelial cell apoptosis (108). The association between fat distribution and PH risk was evaluated recently in a multicenter observational study of patients with advanced lung disease who were being evaluated for lung transplantation (109). In the PHAR, overweight and obese participants had significantly less favorable PH-specific, health-related quality-of-life scores and elevated rates of hospitalizations when compared with individuals of normal weight (110). However, overweight and obese individuals had longer transplant-free survival. This paradoxical observation may be due to adipose-related dimorphic effects or collider bias in a multipathogenic disease.
PAH with Comorbidities versus Severe PH with Left Heart Disease
Approximately 30% of patients diagnosed with PAH also harbor features of left heart disease (111). Owing to the association between severely elevated PVR (>5.0 WU) and mortality or hospitalization for heart failure in combined pre- and postcapillary PH (Cpc-PH) (74), referral to a specialty care center for affected patients for consideration of individualized care is reasonable (78, 112). However, the best approach to management in this subgroup is not known. At present, management of HFpEF (with or without PH) should focus on decongestion with diuretics as well as SGLT-2 inhibitor therapy (113, 114) and mineralocorticoid receptor antagonism therapy (115), which improve survival and cardiac remodeling in this population, respectively, and may also have salutary benefits in patients with Cpc-PH independent of volume-modulating effects (116–118).
Prospective studies evaluating approved therapies for PAH in patients with PH because of left heart disease are inconclusive or harmful, and contemporary data are lacking. A placebo-controlled trial of selective endothelin receptor antagonism in patients with HFpEF and CpcPH was associated with fluid retention with no significant change in hemodynamics (119). In a small study of patients with HFpEF and CpcPH (mean PVR, ∼3.5 WU), nitric oxide potentiation with the phosphodiesterase-5 inhibitor sildenafil demonstrated improvement in right heart hemodynamics and quality of life, although the results were not replicated in a similar prospective investigation (120, 121). Sildenafil improved exercise capacity at 12 weeks in a small study of patients with heart failure with reduced ejection fraction (HFrEF) and Cpc-PH; however, larger studies with longer follow-up are needed (122).
Additional prospective data from patients with overlapping phenotypes are necessary to refine the role for PAH therapies, if any, in patients with Cpc-PH. In the context of shared pulmonary vascular remodeling between patients with heart failure and those with PAH (123), it may be the case that the emergence of therapies with specificity toward endothelial dysfunction and cellular proliferation will lead to the identification of PAH treatments with efficacy in patients with combined precapillary and postcapillary disease. The actively enrolling CADENCE trial (ClinicalTrials.gov ID: NCT04945460) for sotatercept in HFpEF-associated Cpc-PH will address this question. At present, pulmonary vasodilator therapy is not recommended for patients with HFpEF-PH who are outside of individualized care plans that are developed at a PH center (2, 3).
PAH with Comorbidities versus Severe PH with Lung Disease
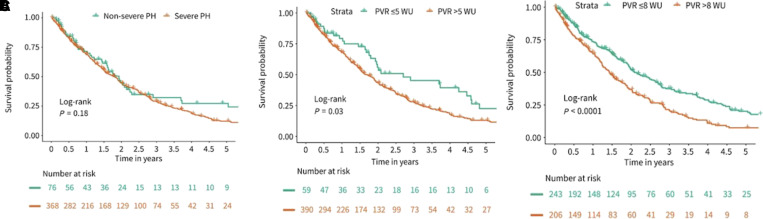
Chronic lung disease is the second most common cause of PH, affecting ∼50% of patients with chronic obstructive pulmonary disease (COPD) and two-thirds of patients with interstitial lung disease (ILD) (124, 125). Similar to patients with left heart disease, there is emerging interest in characterizing phenotypes beyond those classified by the underlying lung disease itself, especially as they may pertain to treatment-responsive subgroups. A retrospective review of 139 patients with COPD and PH (COPD-PH) identified PVR > 5 WU as the best predictor of adjusted all-cause mortality (HR = 2.59; 95% CI = 1.58, 427; P < 0.001). In a separate study of patients with ILD and PH (ILD-PH), higher PVR was independently associated with a modest increase in risk of death (HR = 1.09, 95% CI = 1.02, 1.17; P = 0.014) and PVR > 5 WU was similarly associated with a significantly worse survival compared with PVR ⩽ 5 WU (P = 0.03). A PVR cutoff of 8 WU provided even stronger discrimination between groups (P < 0.0001). In contrast, consideration of patients with ILD-PH on the basis of the current definition of severe disease (mPAP ⩾ 35 mm Hg or mPAP ⩾ 25 mm Hg with cardiac index <2.0 L/min/m2) failed to differentiate groups (Figure 3) (126, 127). An additional study of incident COPD-PH demonstrated the mortality risk associated with concomitant PH, where a cohort of 99 patients with a median PVR of 6.3 WU (interquartile range [IQR] = 4.2–7.9) experienced a mean survival of 15.0 months (IQR = 13.9–16.0) (128). In fact, multicenter prospective data tracking transplant-free survival identified incident Group 3 PH as a greater risk compared with Group 1 PH (HR = 4.26; 95% CI = 1.79, 10.17) (111).
Figure 3.
Prognostic value of pulmonary vascular resistance (PVR) in pulmonary hypertension (PH) caused by interstitial lung disease (ILD). (A) Kaplan-Meier survival curve stratified by the current hemodynamic definition of severe PH in lung disease: mean pulmonary arterial pressure (mPAP) ⩾ 35 mm Hg or mPAP ⩾ 25 mm Hg with cardiac index <2.0 L/min/m2. (B) Kaplan-Meier survival curve stratified by PVR ⩽ 5 WU and PVR > 5 WU. (C) Kaplan-Meier survival curve stratified by PVR ⩽ 8 WU and PVR > 8 WU. WU = Wood units. Reprinted by permission from Reference 126.
In a retrospective review of patients in the ASPIRE (Assessing the Spectrum of Pulmonary Hypertension Identified at a Referral Centre) registry previously characterized as isolated IPAH, the presence of mild lung disease on CT as well as a reduction in DlCO < 45% incurred independent prognostic significance. Among those with mild emphysema or fibrosis on imaging, 5-year survival was 22%, compared with 70% among those without radiographic lung disease (P < 0.0001) (100). Additionally, in the absence of lung disease on imaging, 5-year survival for patients with IPAH with reduced DlCO was 45%, compared with 84% among patients with DlCO ⩾ 45% (P < 0.0001). A subsequent study including patients from the Comparative Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA) found that patients with IPAH and a lung phenotype (defined by a DlCO < 45% and smoking history) experienced significantly reduced response to PAH therapy compared with those with classical IPAH, suggesting a role for prospective enrollment of overlapping World Symposium on Pulmonary Hypertension Group 1 and Group 3 subgroups in clinical trials to investigate whether treatment-responsive subgroups may emerge (129).
PH associated with neurofibromatosis type 1 (NF1-PH)—with NF1 being a systemic disease associated with vascular remodeling as well as ILD (characterized by panlobular cystic changes and ground class)—is a newly established risk factor for PH. Characteristics of patients with NF1-PH include severe PH at the time of diagnosis, including mean mPAP of 45 mm Hg, cardiac index of 2.3 L/min/m2, and PVR of 10.7 WU, with limited, if any, clinical improvement in response to PAH therapy, which may also worsen hypoxemia (130).
Cumulatively, these data support the importance of lung disease–associated PH assessment at expert centers and the development of individualized care plans, including early consideration for transplant referral. To date, investigations of oral therapies approved for PAH in patients with lung disease–associated PH are inconclusive or associated with harm (131, 132). However, encouraging results from the INCREASE trial in ILD-PH support the concept that inhaled prostacyclin drug formulations may be a preferable way to optimize ventilation/perfusion matching where patients with ILD-PH experienced a +31 m improvement in 6MWD compared with the placebo group (95% CI = 16.9–45.4 m; P < 0.001). The reproducibility of these benefits outside the tightly controlled framework of a clinical trial, particularly given the high cost of the medication and challenges with use compliance, are not known. Protocols to standardize patient evaluation and appropriate prescribing and, ultimately, pragmatic clinical studies in real-world populations may be helpful for addressing these lingering concerns (133).
Risk Stratification
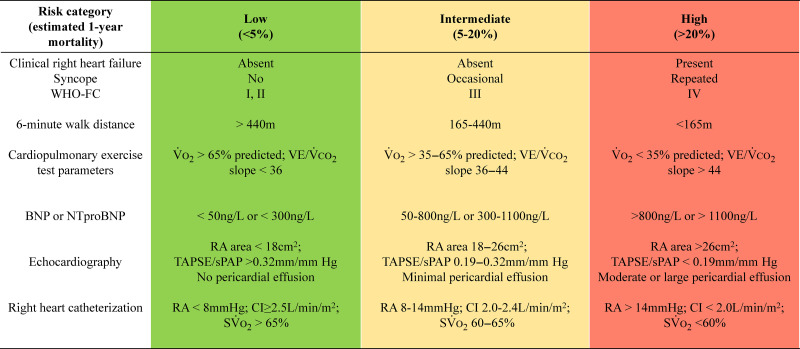
A multitude of point-of-care risk assessment scales exist to determine patient disease severity at presentation and to determine prognosis. These include COMPERA (134), REVEAL (the U.S. Registry to Evaluate Early and Long-Term PAH Disease Management) (135), and the European Society of Cardiology/European Respiratory Society risk stratification tables (Figure 4) (2, 3, 136). Higher risk calculations not only predict hospitalization but are associated with higher (worse) baseline and follow-up scores on the Emphasis-10 (e10), a PH-specific, health-related quality-of-life survey (137, 138). Risk scores require baseline evaluation of multiple clinical, hemodynamic, and exercise parameters, with the strongest emphasis on World Health Organization functional status (WHO-FC), N-terminal–pro hormone brain natriuretic peptide (NT-proBNP) levels, and 6MWD.
Figure 4.
Baseline pulmonary arterial hypertension (PAH) risk stratification. PAH treatment decisions should be made in the context of risk stratification. For example, the European Respiratory Society/European Society of Cardiology three-stratum risk stratification tool (presented here in an abbreviated form) is then paired with a treatment algorithm guided by risk (see Figure 5). BNP = brain natriuretic peptide; CI = cardiac index; NTproBNP = N-terminal–pro hormone brain natriuretic peptide; RA = right atrial; So2 = mixed venous oxygen saturation; sPAP = systolic pulmonary arterial pressure; TAPSE = tricuspid annular plane systolic excursion; VO2 = volume of oxygen consumption. Reprinted with permission from References 2 and 3.
Work by Hoeper and colleagues recently introduced the benefit of a simplified and non-invasive four-stratum follow-up risk assessment tool, as compared with a three-stratum tool (low, intermediate, and high risk). Among the 76% of patients falling into an intermediate-risk group at baseline, COMPERA 2.0 demonstrated that patients moving from the intermediate–high risk follow-up group to the intermediate–low risk follow-up group experienced a 20.1% decreased risk of death (HR = 0.799; 95% CI = 0.611, 1.046) (139). A validation of this four-stratum tool among patients with PAH in the French PH registry confirmed improved discrimination of 1-year mortality as compared with the three-stratum model (140).
Indeed, a minority of patients with PAH who are classified as intermediate or high risk (35–43%) will transition to low-risk status after 6 months of standard-of-care treatment with dual oral pulmonary vasodilatory therapy. Therefore, opportunities remain to add precision to risk stratification and to discriminate patients in the intermediate–low risk and intermediate–high risk range (141). Among responders, risk reduction appears to be a function of PVR reduction, whereas nonresponder status (i.e., blunted PVR improvement by <25.8% [the lower tertile of PVR change]) is associated with age >60 years, male sex, mPAP > 48 mm Hg, and TAPSE < 18 mm. In a study evaluating risk response to standard oral combination therapy (i.e., ambrisentan and tadalafil), as assessed by using REVEAL 2.0, at 2-year follow-up, 14% of patients remained high and 44% remained an intermediate risk. When evaluating risk score as a function of change in PVR (percent from baseline), favorable risk score response was most likely to be achieved among patients with PAH with >50% reduction in PVR at 24 months (142). These data emphasize the breadth of the intermediate-risk category, highlighting that subgroups remain clinically vulnerable despite dual oral therapy (143).
Incorporation of baseline risk assessment as a criterion for clinical trial enrollment may be helpful to ensure similar prognosis between groups. Despite the aim that a change in risk score may represent a more efficient clinical trial endpoint than events of clinical worsening (CW), recent data highlights that risk scores may not be valid as surrogate endpoints in clinical trials. In fact, Blette and colleagues found that while multicomponent risk scores predict outcomes, treatment-induced effects on low-risk status explained very little (7–13%, well below the desired threshold of 50%) of the effect of random treatment assignment on time to clinical worsening (144). Similar results were demonstrated in a post hoc analysis of FREEDOM-EV, a randomized clinical trial of oral treprostinil versus placebo added to background therapy, where 15–33% of the proportion of the treatment effect was explained by REVEAL Lite 2 scores (145). These recent studies highlight the need for continued efforts to validate endpoints in the field.
Serum Biomarkers
At this time, BNP and NT-proBNP are the only biomarkers considered useful in clinical practice. A recent study examining a novel risk score utilizing routine laboratory data with prognostic significance in left heart disease, including γ-glutamyl transferase and the ratio aspartate aminotransferase/alanine aminotransferase (both surrogates of inferior vena cava back flow and hepatic venous congestion), discriminated PH risk profiles at baseline and follow-up in a derivation and validation cohort of patients with either PAH or CTEPH, suggesting the potential for expanded utility of routine serum evaluations (146). Cartilage intermediate layer protein 1 was recently identified as a novel marker for RV remodeling and ventriculoarterial uncoupling in patients with precapillary PH; higher serum levels were seen in PH patients with maladaptive right ventricular remodeling as compared with patients with left heart disease (147). Overall, however, validated disease-specific biomarkers remain lacking for PAH.
Novel Assessment Tools
A recent study evaluated the relationship between uncoached daily activity (i.e., step counts measuring using Fitbit devices) and clinical outcomes in PAH. In a cohort of 41 subjects with PAH, an average daily step count of ⩽5,500 was associated with a significant increase in hospitalization rate compared with patients who averaged >5,500 steps (P = 0.04). A high daily step count was also associated with lower odds of worsening WHO-FC (odds ratio [OR] = 0.38; 95% CI = 0.16, 0.92; P = 0.03) (148). An additional study found that the incorporation of automated text-messaging reminders may improve daily activity for patients with PH. In a trial of 42 patients with PAH who were randomized to text-messaging versus usual care, the texting intervention resulted in a higher number of average daily steps, with a secondary benefit in subjective Emphasis 10 score as well as TAPSE measurements at 12-week follow-up (149). Last, peak o2 and E/CO2 slope obtained during noninvasive cardiopulmonary exercise testing are also useful in the risk stratification of patients. A recent study of intermediate-risk patients found a 3-year event-free survival rate of 41% among patients with a o2 peak < 10 ml/kg/min as compared with 79% among those with a o2 peak of 14 ml/kg/min (150).
Updates in the Approach to Treating Patients with PAH and CTEPH
Sotatercept
Sotatercept, a fusion protein of the Fc domain of human IgG and the extracellular domain of activin receptor type II A (ActRIIA), acts as an activin signaling inhibitor. Through this mechanism, pathogenetic members of the TGF-β superfamily rebalance proliferative ActRII ligands (activins A and B), which bind at the ActRII and activin receptor–like kinase (ALK) 4/5/7 receptor complex and trigger proliferative signaling by means of Smad 2/3 phosphorylation. Inhibition of proproliferative signaling is then balanced against antiproliferative signaling involving BMPs through the receptor complex consisting of BMPR2, ALK 1/2/3/6, and Smad 1/5/8 phosphorylation. The potential for imbalanced TGF-β signaling as a driver of PAH was suggested in 1994 (151), and later, BMPR2 loss-of-function mutations were linked to the penetrance of heritable PAH and identified in idiopathic PAH. Downregulation of BMP signaling is characteristic of PAH, regardless of the presence of a BMPR2 mutation. Notably, Guignabert and colleagues recently demonstrated the prognostic relevance of circulating levels of activin A and the ActRII ligand inhibitor follistatin-like 3 in patients with idiopathic, hereditary, or anorexigen-associated PAH (152). Although the precise mechanistic effects of sotatercept remain to be elucidated, inhibition of ALK 4/5/7-ActRIIA signal transduction derepresses (i.e., net increase) nongenomic and genomic antiproliferative targets that are regulated through the BMPR2 receptor (Figure 1) (153). Thus, the overall functional effect of sotatercept is to rebalance the TGF-β–BMPR2 axis, upregulating antiproliferative and inhibiting proproliferative pathways in pulmonary artery endothelial and smooth muscle cells.
The groundbreaking results of two recently published trials studying sotatercept in PAH are summarized here.
PULSAR, a large Phase-2 randomized placebo-controlled clinical trial tested the effect of sotatercept (administered every 3 weeks) versus placebo on changes in PVR from baseline at Week 24 in a cohort of 106 patients with prevalent PAH who were treated with standard-of-care background therapy (4). Nearly half of the patients enrolled continued to experience WHO-FC III dyspnea, despite triple-combination therapy, one-third of whom were managed with intravenous prostacyclin therapy. Compared with placebo, when added to standard PAH therapy, the least-squares mean PVR difference was −145.8 dyn ⋅ s ⋅ cm−5 (P < 0.001) and −239.5 dyn ⋅ s ⋅ cm−5 (P < 0.001) for sotatercept at 0.3 mg/kg and 0.7 mg/kg, respectively (4). The open-label extension study found that, over a period of 18–24 months, those who were initially in the placebo arm had significant improvements in PVR, 6MWD, and WHO-FC on sotatercept, and clinical benefit was maintained (but not improved further) in those who had been continued on the study drug (154). Of the entire cohort, 30.8% had treatment-emergent adverse events (∼10% with telangiectasia), 9.6% of which led to study discontinuation (Table 2).
Table 2.
Summary of Phase 2 and Phase 3 Randomized Placebo-controlled Clinical Trials of Sotatercept for the Treatment of PH
| Trial | Number of Patients | WHO Group | Risk Category | Background PAH Therapy | Target Dose | Endpoint | Trial Outcome | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| PULSAR* | 106 | Group 1† | WHO-FC II and III with minimum PVR ⩾ 5 WU | Yes | 0.3 mg/kg SQ or 0.7 mg/kg; or placebo, q3 wk | Change in PVR at 24 wk | Least-squares mean difference of −239.5 dyn ⋅ s ⋅ cm−5 (2.9 WU); 95% CI = −329.3, −149.7; P < 0.001 | Thrombocytopenia, increased hemoglobin, SOTERIA* study; open-label long-term follow-up to assess safety |
| STELLAR‡ | 324 | Group 1† | WHO-FC II and III with minimum PVR ⩾ 5 WU | Yes | 0.7 mg/kg SQ; or placebo, q3 wk | Change in 6MWD at 24 wk | Hodges-Lehmann location shift of +40.8 m; 95% CI = 27.5, 54.1; P < 0.001 | Epistaxis, telangiectasis, thrombocytopenia, increased hemoglobin |
| HYPERION‡ | 662 | Group 1† with new diagnosis of PAH | WHO-FC II and III with minimum PVR ⩾ 4 WU and REVEAL Lite 2 risk score ⩾ 6 | Yes, clinically stable doses of a double combination of PAH therapies and diuretics | 0.7 mg/kg SQ; or placebo, q3 wk | Time to clinical worsening§ | Actively enrolling | — |
| ZENITH‡ | 200 | Group 1† with high risk of mortality | WHO-FC III or FC IV at high risk of mortality with REVEAL Lite 2 risk score ⩾ 9 | Yes, clinically stable on maximally tolerated double or triple-combination therapy | 0.7 mg/kg SQ; or placebo, q3 wk | Time to first confirmed morbidity or mortality event | Actively enrolling | — |
| CADENCE* | 150 | Combined pre- and postcapillary PH because of HFpEF | NYHA-FC II or III with PAWP > 15 mm Hg but <30 mm Hg | — | 0.3 or 0.7 mg/kg; or placebo, q3 wk | Change in PVR at 24 wk | Actively enrolling | — |
| MOONBEAM* | 42 | Group 1†ǁ PH in children ⩾1 to <18 yr old | — | Yes, clinically stable on maximally tolerated double or triple combination therapy | 0.3 mg/kg SQ, q3 wk | Serum trough of drug before next dose, % of participants with adverse events¶ | Actively enrolling | — |
Definition of abbreviations: 6MWD = 6-minute walk distance; CI = confidence interval; HFpEF = heart failure with preserved ejection fraction; NYHA-FC = New York Heart Association functional class; PAH = pulmonary arterial hypertension; PAWP = pulmonary arterial wedge pressure; PH = pulmonary hypertension; PVR = pulmonary vascular resistance; q3 wk = once every 3 weeks; RCT = randomized clinical trial; SQ = subcutaneous; WHO-FC = World Health Organization functional class; WU = Wood units.
Phase-2 randomized, double-blind, placebo-controlled RCT.
Idiopathic or heritable, connective tissue disease, or drug induced.
Phase-3 randomized, double-blind, placebo-controlled RCT.
Defined as time from randomization to the first confirmed morbidity event or death.
PAH-congenital heart disease with shunt closure >6 mo before screening and PAH with coincidental shunt.
No formal hypothesis, several primary endpoints, including these.
STELLAR, the Phase-3 randomized placebo-controlled trial, evaluated the impact of 0.3 mg/kg (target dose, 0.7 mg/kg) sotatercept every 3 weeks versus placebo on change from baseline 6MWD at 24 weeks in patients with PAH and WHO-FC II or III symptoms despite background therapy (6). Two-thirds of patients were on triple-combination therapy, including parenteral prostacyclin in 40% of those enrolled. Patients treated with sotatercept experienced a mean change in 6MWD of +40 m (95% CI = 29.9, 50.2) compared with −1.4 m (95% CI = −13.2, 10.3) in the placebo group (P < 0.001). The study additionally met eight of the nine secondary hierarchical endpoints, including a three-tiered multicomponent endpoint of 1) ⩾30-m increase in 6MWD; 2) ⩾30% decrease in NT-proBNP (or an NT-proBNP < 300 pg/ml); and 3) improvement in WHO-FC to at least II, as well as reduction in PVR, shorter time to first occurrence of death or clinical worsening, and improvement in PAH quality-of-life scores. Telangiectasis occurred in 10.4% of the sotatercept group. Increased hemoglobin, thrombocytopenia, and bleeding events (including epistaxis and gingival bleeding) occurred more commonly in the treatment group. With a mean time from diagnosis to trial enrollment of 8.8 years, the results of the actively enrolling HYPERION study (ClinicalTrials.gov ID: NCT04811092) of sotatercept for newly diagnosed patients with PAH will lend insight into the potential for sotatercept to mitigate disease progression and associated morbidity and mortality (Table 2).
Updates on Combination Therapy in PAH
Cumulative data from clinical trials evaluating the efficacy of both sequential add-on or upfront combination PAH therapy demonstrate significantly decreased risk of clinical worsening compared with monotherapy (risk ratio = 0.65; 95% CI = 0.58, 0.72; P < 0.00001), as well as improvement in PH-specific outcomes, including WHO-FC and 6MWD (155). The recently published TRITON study (156) was the first to prospectively investigate upfront triple-combination therapy (selexipag added to macitentan/tadalafil) versus double-combination therapy (macitentan/tadalafil) for incident PAH. Dual therapy was started on Day 1 of the study, and the oral prostacyclin receptor agonist selexipag, versus placebo, was added on Day 15. The primary hemodynamic outcome of change in PVR at 26 weeks was nonsignificantly different between groups (157). Boucly and colleagues retrospectively evaluated risk of death on the basis of initial treatment strategy; in the overall population, 5-year survival for patients receiving triple-combination therapy, including intravenous or subcutaneous prostacyclins, was significantly improved (91%), compared with those receiving dual-combination therapy or monotherapy (61%) (158). Prospective randomized controlled trial data are needed to provide conclusive evidence of parenteral prostacyclin-based, triple-combination therapy superiority over dual-combination therapy (Figure 5) (159). Additionally, pending FDA approval, sotatercept is likely to be integrated into combination therapy regimens, noting significant 6MWD benefit in patients on background dual- as well as triple-combination therapy (6).
Figure 5.
Baseline pulmonary arterial hypertension (PAH) treatment algorithm. Dual-oral combination therapy is the standard of care for low- and intermediate-risk patients; parenteral prostacyclin analogue therapy should be added for high-risk patients. Treatment decisions for patients with PAH and cardiopulmonary comorbidities are different from those with isolated World Health Organization Group 1 PAH and should center around initial monotherapy. Cardiopulmonary comorbidities include left ventricular diastolic dysfunction, obesity, hypertension, diabetes mellitus, coronary artery disease, and parenchymal lung disease. Vasoreactivity testing° is recommended for patients with idiopathic, heritable, and drug-induced PAH. °Positive response, or “vasoreactive,” is defined as a mean pulmonary artery pressure reduction ⩾ 10 mm Hg to absolute value ⩽ 40 mm Hg with improved or unchanged cardiac output. ERA = endothelin receptor antagonist (either ambrisentan or macitentan); PDE5i = phosphodiesterase 5 inhibitor (either sildenafil or tadalafil); PH = pulmonary hypertension; I.V. = intravenous; S.Q. = subcutaneous, prostacyclin analog (either epoprostenol or treprostinil).
Treating Mild PH and Mild PAH
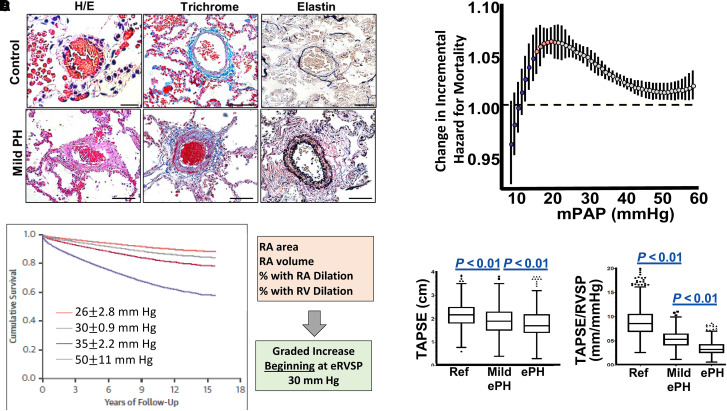
There are now data showing that mild PH is associated with substantial PA remodeling, pathogenic changes to right heart geometry and function, and mortality (Figure 6). However, at present, there are no data to warrant pulmonary vasodilator therapy in patients with mPAP of 20–25 mm Hg (2, 3). One recent retrospective study of 82 patients with PAH (mPAP = 27 mm Hg [IQR = 25–30]; PVR = 2.2 WU [IQR = 1.9–2.7]) (which did not require PVR > 3.0 WU for treatment) who were initiated on ERA or PDE5i monotherapy showed that, at first follow-up, there was an average +46 m improvement in 6MWD and that 35% of patients improved at least one New York Heart Association functional class; at 5 years, the survival rate was 84% (160). Populations with established PH risk where screening is recommended may serve as a starting point to prospectively study treatment of early disease. For example, in systemic sclerosis (161), where PAH-associated mortality is as high as 18% within 3 years and mild PH (mPAP, 21–24 mm Hg) predicts disease progression (162), studies such as the actively enrolling Sildenafil for Early Pulmonary Vascular Disease in Scleroderma study will evaluate the impact of sildenafil versus placebo on 6MWD in patients with mPAP of 21–24 mm Hg (ClinicalTrials.gov ID: NCT04797286). Large, adequately powered, prospective studies are necessary across World Symposium on Pulmonary Hypertension groups to understand the impact of treatment on early disease, although identifying at-risk patients may be challenging because most patients with PAH are diagnosed with advanced-stage disease. Thus, innovative strategies that leverage artificial intelligence applied to ECG testing as well as consideration to genetic profile to identify at-risk patients early may be helpful (163).
Figure 6.
The pathophysiology and risk associated with mild pulmonary hypertension (PH). (A) Compared with normal controls, pulmonary arterioles from patients with mild PH and hypertrophic cardiomyopathy demonstrate increased fibrosis and hypertrophy on Masson trichrome and elastin staining, respectively. Scale bar, 50 mm. Reproduced with permission from Reference 175. (B) When modeling mean pulmonary artery pressure (mPAP) as a continuous variable across a national cohort of 21,727 veterans, mortality risk that is associated with increases in mPAP emerges at 19 mm Hg. Subtle increases in mPAP between 19 and 24 mm Hg are associated with a much greater change in mortality, suggesting an opportunity to modulate clinical risk in the range of mild rather than severe PH. Reproduced with permission from Reference 65. (C) Increased mortality in an Australian national cohort of patients with mild PH suggested by echocardiography is associated with pathogenic changes to RA and right ventricular (RV) geometry. Reprinted with permission from Reference 92. (D) The pathogenicity of mild PH is suggested further by evidence of impaired tricuspid annular plane of systolic excursion (TAPSE) and surrogate for RV-pulmonary arterial coupling (TAPSE/RVSP). Reprinted with permission from Reference 91. ePH = echocardiographic PH; eRVSP = estimated right ventricular systolic pressure; H/E = hematoxylin and eosin; RA = right atrial; RVSP = right ventricular systolic pressure.
Balloon Pulmonary Angioplasty and CTEPH
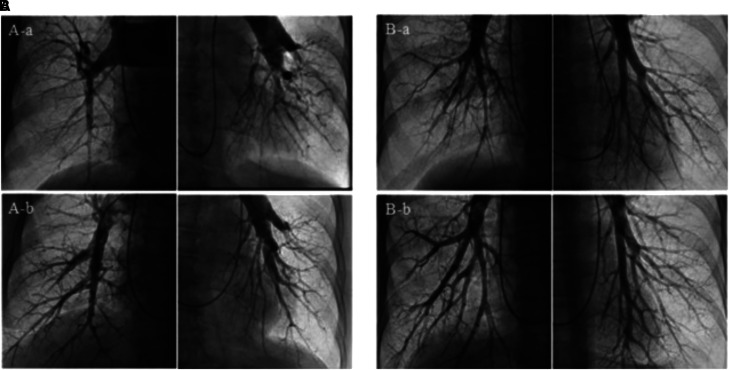
Pulmonary endarterectomy (PTE) remains the gold standard for operable disease but requires a high-volume center and careful patient selection for optimal results (i.e., anatomical clot considerations, few comorbidities, PVR < 12 WU, absence of right heart failure) (164). In patients with surgically accessible, proximal disease who either decline PTE or are ineligible for surgical intervention because of advanced age or significant comorbidities, retrospective data suggest that balloon pulmonary angioplasty (BPA) is an effective strategy to treat otherwise surgically accessible lesions (Figure 7) (165), although prospective data comparing PTE to BPA are needed.
Figure 7.
Balloon pulmonary angioplasty of proximal and distal chronic pulmonary emboli. Representative pulmonary angiogram before and after balloon pulmonary angioplasty. (A) Global pulmonary angiogram in a patient with surgically accessible proximal lesions (Group 1). (A-a) Pulmonary angiogram before balloon pulmonary angioplasty (BPA). (A-b) Pulmonary angiogram after four BPA procedures. (B) Global pulmonary angiogram in a patient with surgically inaccessible distal lesions (Group 2). (B-a) Pulmonary angiogram before BPA. (B-b) Pulmonary angiogram after four BPA procedures. Reprinted by permission from Reference 165.
Two recent studies have addressed the question of whether BPA is superior to medical therapy for inoperable CTEPH. A Phase-3, multicenter, open-label, randomized controlled trial including 105 patients receiving care at 23 French centers with inoperable CTEPH randomized to riociguat or BPA demonstrated that PVR reduction at 26 weeks was higher in the BPA group, but so were treatment-related serious adverse events; patients required an average of 7.7 BPA procedures (166). In an ancillary 52-week follow-up study of patients with residual PVR > 4 WU or WHO-FC II despite 26 weeks of riociguat, add-on BPA was associated with less adverse events in the combination therapy group—5 (2%) of 201 sessions—as compared with the initial BPA-alone group—33 (8%) of 33 sessions—suggesting a complementary role for riociguat in reducing BPA-related complications. A second multicenter, open-label, controlled trial at four Japanese centers randomized 61 patients with inoperable CTEPH to BPA or riociguat and corroborated that patients who underwent BPA had better hemodynamic improvement at 12 months, although it acknowledged the increased risk of procedural complications and the need for ∼5 BPA procedures (167).
Physical Activity in PAH
Clinically meaningful endpoints should robustly capture how a patient feels or functions and whether survival is affected. Given the importance of exercise limitation in PAH and the rise of mobile health technologies, actigraphy has been proposed as a novel study endpoint. A recent analysis of baseline accelerometry data from the recently completed PHANTOM trial (ClinicalTrials.gov ID: NCT03229499) identified physical activity phenotypes that tracked with 6MWD, RV function, and quality of life (168). Additionally, a large, randomized controlled, multicenter trial including 116 patients from different centers and European countries demonstrated that, compared with usual care, a PAH- and CTEPH-specific standardized rehabilitation program was safe, feasible, and associated with significant improvements in 6MWD, quality of life, WHO-FC, and peak oxygen consumption (169). As a result, exercise should be encouraged in patients with PAH stabilized on standard pharmacotherapy (2, 3).
Clinical Trial Design
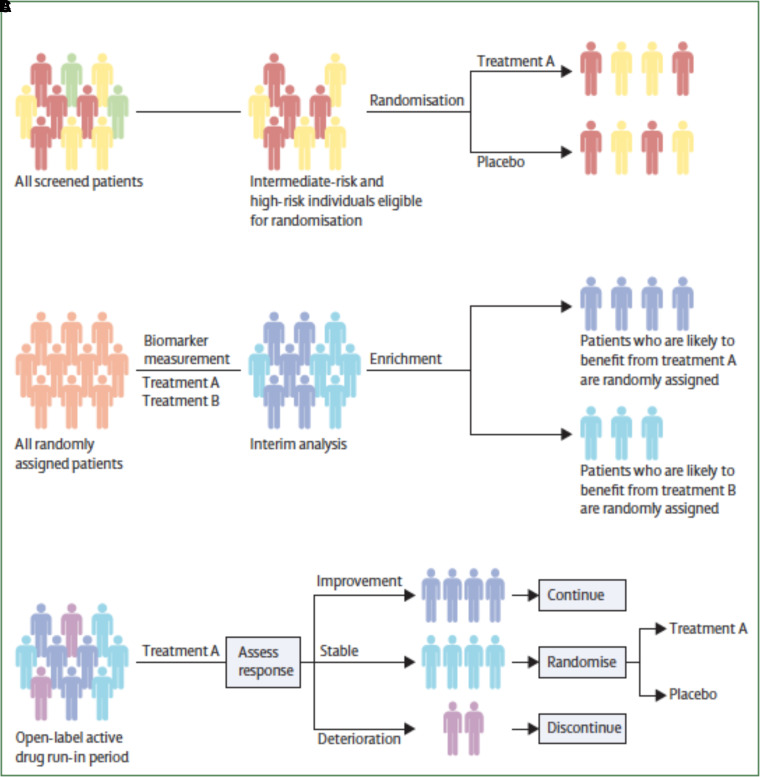
PAH clinical trials face several unique challenges, including low disease prevalence, indolent clinical course, and the heterogenous effect of various background treatment profiles. Future trial designs must adapt accordingly (Figure 8). For example, phenotypic and prognostic enrichment strategies may serve as a time- and cost-saving tool (170). Large-scale studies are underway that apply deep phenotyping techniques to discover molecular signatures that may more precisely refine enrichment (171). Use of adaptive trial design protocols with prespecified interim analyses and predetermined modifications may improve the efficiency of trials as well (172).
Figure 8.
Alternative clinical trial approaches. (A) Risk assessment–based eligibility criteria for enrichment. Green indicates low risk, yellow indicates intermediate risk, and red indicates high risk. (B) Adaptive enrichment recruitment based on interim analysis response. Dark blue indicates patients who might benefit from treatment A. Light blue indicates patients that might benefit from treatment B. All randomly assigned patients (light orange). (C) Randomized discontinuation study design. Dark blue indicates patients who improve with therapy, light blue indicates patients who are stable with treatment, and violet indicates patients who deteriorate with treatment. Reprinted by permission from Reference 174.
Enrichment strategies limit generalizability of results. Pragmatic clinical trials, which evaluate the effectiveness of therapy in real-world populations, including those with frequently co-occurring comorbidities for heart and lung disease, may improve generalizability in real-world practice and decrease the risk of slow enrollment caused by extensive exclusion criteria. Pragmatic trials provide a window into clinical efficacy of drugs outside highly controlled circumstances of (extremely) well-funded Phase 3 clinical trials. In addition, this approach decentralizes clinical trials to improve access to participation among cohorts that are generally underrepresented in PH clinical trials (see the previous section “Socioeconomic Parameters and Access to Health Care”) (173). Finally, pragmatic trials with Bayesian and/or adaptive designs allow for more rapid iteration with “go/no-go” decisions on therapeutics of interest.
There is a need to reexamine important clinical endpoints in PAH and shift from classical endpoints such as submaximal exercise capacity to novel endpoints including actigraphy, multiparametric risk assessments, and patient-reported outcomes, once adequately validated (174). We advocate for 1) universal protocols that allow for subsequent data harmonization, 2) accelerated conditional FDA approval of therapeutics found to be efficacious in Phase 3 clinical trials, 3) clinician support for enrollment including in nonindustry-sponsored trials, and 4) journal editorial support for publication of negative results.
Future Directions
Successes in preclinical, translational, and clinical pulmonary vascular research underscore the momentum achieved in the field as we move toward a refined understanding of pathophysiology, subphenotyping, and risk stratification. Enhanced appreciation for the heterogeneity of PH—particularly patient comorbidities, more nuanced utilization of existing drugs, and the emergence of promising novel therapeutics—will allow ongoing discoveries that promote improved outcomes for this highly morbid cardiopulmonary disease.
Acknowledgments
Acknowledgment
The authors acknowledge many excellent scientific contributions that, because of space constraints, could not be included in this article. The authors also thank the patients and their caregivers for participating in this human subject research. The authors thank Christine Veith (Justus Liebig University) for her suggestions and proofreading of the section “Progress in Translational Science.”
Footnotes
Supported by the German Research Foundation Project ID 268555672, SFB 1213; by grants from NIH (1F32HL164090-01 to S.J.; R01-HL141268 to C.E.V.; T32-HL134625 to K.C.-F.; 1-R01-HL139613-01, R01-HL153502, R01-HL155096-01, U54HL119145, and 2021A007243 to B.A.M.); and by the Broad Institute of Brigham and Women’s Hospital-Massachusetts Institute of Technology (to B.A.M.).
Originally Published in Press as DOI: 10.1164/rccm.202302-0327SO on July 14, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Maron BA, Humbert M. Finding pulmonary arterial hypertension-switching to offense to mitigate disease burden. JAMA Cardiol . 2022;7:369–370. doi: 10.1001/jamacardio.2022.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. ESC/ERS Scientific Document Group 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J . 2022;43:3618–3731. doi: 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 3. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J . 2023;61:2200879. doi: 10.1183/13993003.00879-2022. [DOI] [PubMed] [Google Scholar]
- 4. Humbert M, McLaughlin V, Gibbs JSR, Gomberg-Maitland M, Hoeper MM, Preston IR, et al. PULSAR Trial Investigators Sotatercept for the treatment of pulmonary arterial hypertension. N Engl J Med . 2021;384:1204–1215. doi: 10.1056/NEJMoa2024277. [DOI] [PubMed] [Google Scholar]
- 5. Humbert M, McLaughlin V, Gibbs JSR, Gomberg-Maitland M, Hoeper MM, Preston IR, et al. Sotatercept for the treatment of pulmonary arterial hypertension: PULSAR open-label extension. Eur Respir J . 2023;61:2201347. doi: 10.1183/13993003.01347-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoeper MM, Badesch DB, Ghofrani HA, Gibbs JSR, Gomberg-Maitland M, McLaughlin VV, et al. STELLAR Trial Investigators Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med . 2023;388:1478–1490. doi: 10.1056/NEJMoa2213558. [DOI] [PubMed] [Google Scholar]
- 7. Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, et al. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation . 2001;104:790–795. doi: 10.1161/hc3201.094152. [DOI] [PubMed] [Google Scholar]
- 8. Chelladurai P, Kuenne C, Bourgeois A, Günther S, Valasarajan C, Cherian AV, et al. Epigenetic reactivation of transcriptional programs orchestrating fetal lung development in human pulmonary hypertension. Sci Transl Med . 2022;14:eabe5407. doi: 10.1126/scitranslmed.abe5407. [DOI] [PubMed] [Google Scholar]
- 9. Potus F, Pauciulo MW, Cook EK, Zhu N, Hsieh A, Welch CL, et al. Novel mutations and decreased expression of the epigenetic regulator TET2 in pulmonary arterial hypertension. Circulation . 2020;141:1986–2000. doi: 10.1161/CIRCULATIONAHA.119.044320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yan Y, He YY, Jiang X, Wang Y, Chen JW, Zhao JH, et al. DNA methyltransferase 3B deficiency unveils a new pathological mechanism of pulmonary hypertension. Sci Adv . 2020;6:eaba2470. doi: 10.1126/sciadv.aba2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zehendner CM, Valasarajan C, Werner A, Boeckel JN, Bischoff FC, John D, et al. Long noncoding RNA TYKRIL plays a role in pulmonary hypertension via the p53-mediated regulation of PDGFRβ. Am J Respir Crit Care Med . 2020;202:1445–1457. doi: 10.1164/rccm.201910-2041OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quatredeniers M, Nakhleh MK, Dumas SJ, Courboulin A, Vinhas MC, Antigny F, et al. Functional interaction between PDGFβ and GluN2B-containing NMDA receptors in smooth muscle cell proliferation and migration in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol . 2019;316:L445–L455. doi: 10.1152/ajplung.00537.2017. [DOI] [PubMed] [Google Scholar]
- 13. Galkin A, Sitapara R, Clemons B, Garcia E, Kennedy M, Guimond D, et al. Inhaled seralutinib exhibits potent efficacy in models of pulmonary arterial hypertension. Eur Respir J . 2022;60:2102356. doi: 10.1183/13993003.02356-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gräf S, Haimel M, Bleda M, Hadinnapola C, Southgate L, Li W, et al. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat Commun . 2018;9:1416. doi: 10.1038/s41467-018-03672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Montani D, Lechartier B, Girerd B, Eyries M, Ghigna MR, Savale L, et al. An emerging phenotype of pulmonary arterial hypertension patients carrying SOX17 variants. Eur Respir J . 2022 doi: 10.1183/13993003.00656-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rhodes CJ, Batai K, Bleda M, Haimel M, Southgate L, Germain M, et al. UK NIHR BioResource Rare Diseases Consortium; UK PAH Cohort Study Consortium; US PAH Biobank Consortium Genetic determinants of risk in pulmonary arterial hypertension: international genome-wide association studies and meta-analysis. Lancet Respir Med . 2019;7:227–238. doi: 10.1016/S2213-2600(18)30409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park CS, Kim SH, Yang HY, Kim JH, Schermuly RT, Cho YS, et al. Sox17 deficiency promotes pulmonary arterial hypertension via HGF/c-Met signaling. Circ Res . 2022;131:792–806. doi: 10.1161/CIRCRESAHA.122.320845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mammoto T, Muyleart M, Konduri GG, Mammoto A. Twist1 in hypoxia-induced pulmonary hypertension through transforming growth factor-β-Smad signaling. Am J Respir Cell Mol Biol . 2018;58:194–207. doi: 10.1165/rcmb.2016-0323OC. [DOI] [PubMed] [Google Scholar]
- 19. Fan Y, Gu X, Zhang J, Sinn K, Klepetko W, Wu N, et al. TWIST1 drives smooth muscle cell proliferation in pulmonary hypertension via loss of GATA-6 and BMPR2. Am J Respir Crit Care Med . 2020;202:1283–1296. doi: 10.1164/rccm.201909-1884OC. [DOI] [PubMed] [Google Scholar]
- 20. Dunmore BJ, Yang X, Crosby A, Moore S, Long L, Huang C, et al. 4PBA restores signaling of a cysteine-substituted mutant BMPR2 receptor found in patients with pulmonary arterial hypertension. Am J Respir Cell Mol Biol . 2020;63:160–171. doi: 10.1165/rcmb.2019-0321OC. [DOI] [PubMed] [Google Scholar]
- 21. Hodgson J, Swietlik EM, Salmon RM, Hadinnapola C, Nikolic I, Wharton J, et al. Characterization of GDF2 mutations and levels of BMP9 and BMP10 in pulmonary arterial hypertension. Am J Respir Crit Care Med . 2020;201:575–585. doi: 10.1164/rccm.201906-1141OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manaud G, Nossent EJ, Lambert M, Ghigna MR, Boët A, Vinhas MC, et al. Comparison of human and experimental pulmonary veno-occlusive disease. Am J Respir Cell Mol Biol . 2020;63:118–131. doi: 10.1165/rcmb.2019-0015OC. [DOI] [PubMed] [Google Scholar]
- 23. Wang L, Rice M, Swist S, Kubin T, Wu F, Wang S, et al. BMP9 and BMP10 act directly on vascular smooth muscle cells for generation and maintenance of the contractile state. Circulation . 2021;143:1394–1410. doi: 10.1161/CIRCULATIONAHA.120.047375. [DOI] [PubMed] [Google Scholar]
- 24. Nikolic I, Yung LM, Yang P, Malhotra R, Paskin-Flerlage SD, Dinter T, et al. Bone morphogenetic protein 9 is a mechanistic biomarker of portopulmonary hypertension. Am J Respir Crit Care Med . 2019;199:891–902. doi: 10.1164/rccm.201807-1236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rochon ER, Krowka MJ, Bartolome S, Heresi GA, Bull T, Roberts K, et al. BMP9/10 in pulmonary vascular complications of liver disease. Am J Respir Crit Care Med . 2020;201:1575–1578. doi: 10.1164/rccm.201912-2514LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sommer N, Ghofrani HA, Pak O, Bonnet S, Provencher S, Sitbon O, et al. Current and future treatments of pulmonary arterial hypertension. Br J Pharmacol . 2021;178:6–30. doi: 10.1111/bph.15016. [DOI] [PubMed] [Google Scholar]
- 27. Yerabolu D, Weiss A, Kojonazarov B, Boehm M, Schlueter BC, Ruppert C, et al. Targeting Jak-Stat signaling in experimental pulmonary hypertension. Am J Respir Cell Mol Biol . 2021;64:100–114. doi: 10.1165/rcmb.2019-0431OC. [DOI] [PubMed] [Google Scholar]
- 28. Austin M, Quesenberry PJ, Ventetuolo CE, Liang O, Reagan JL. Prevalence and effect on survival of pulmonary hypertension in myelofibrosis. Clin Lymphoma Myeloma Leuk . 2019;19:593–597. doi: 10.1016/j.clml.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leopold JA. Inhibiting Jak2 ameliorates pulmonary hypertension: fulfilling the promise of precision medicine. Am J Respir Cell Mol Biol . 2021;64:12–13. doi: 10.1165/rcmb.2020-0384ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sandner P, Zimmer DP, Milne GT, Follmann M, Hobbs A, Stasch JP. Soluble guanylate cyclase stimulators and activators. Handb Exp Pharmacol . 2021;264:355–394. doi: 10.1007/164_2018_197. [DOI] [PubMed] [Google Scholar]
- 31. Maron BA, Zhang YY, White K, Chan SY, Handy DE, Mahoney CE, et al. Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation . 2012;126:963–974. doi: 10.1161/CIRCULATIONAHA.112.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hennigs JK, Cao A, Li CG, Shi M, Mienert J, Miyagawa K, et al. PPARγ-p53-mediated vasculoregenerative program to reverse pulmonary hypertension. Circ Res . 2021;128:401–418. doi: 10.1161/CIRCRESAHA.119.316339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sun XQ, Peters EL, Schalij I, Axelsen JB, Andersen S, Kurakula K, et al. Increased MAO-A activity promotes progression of pulmonary arterial hypertension. Am J Respir Cell Mol Biol . 2021;64:331–343. doi: 10.1165/rcmb.2020-0105OC. [DOI] [PubMed] [Google Scholar]
- 34. Vitry G, Paulin R, Grobs Y, Lampron MC, Shimauchi T, Lemay SE, et al. Oxidized DNA precursors cleanup by NUDT1 contributes to vascular remodeling in pulmonary arterial hypertension. Am J Respir Crit Care Med . 2021;203:614–627. doi: 10.1164/rccm.202003-0627OC. [DOI] [PubMed] [Google Scholar]
- 35. Meloche J, Pflieger A, Vaillancourt M, Paulin R, Potus F, Zervopoulos S, et al. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation . 2014;129:786–797. doi: 10.1161/CIRCULATIONAHA.113.006167. [DOI] [PubMed] [Google Scholar]
- 36. Maron BA, Michel T. Subcellular localization of oxidants and redox modulation of endothelial nitric oxide synthase. Circ J . 2012;76:2497–2512. doi: 10.1253/circj.cj-12-1207. [DOI] [PubMed] [Google Scholar]
- 37. Macias D, Moore S, Crosby A, Southwood M, Du X, Tan H, et al. Targeting HIF2α-ARNT hetero-dimerisation as a novel therapeutic strategy for pulmonary arterial hypertension. Eur Respir J . 2021;57:1902061. doi: 10.1183/13993003.02061-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wertheim BM, Wang RS, Guillermier C, Hütter CV, Oldham WM, Menche J, et al. Proline and glucose metabolic reprogramming supports vascular endothelial and medial biomass in pulmonary arterial hypertension. JCI Insight . 2023;8:e163932. doi: 10.1172/jci.insight.163932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Torrino S, Grasset EM, Audebert S, Belhadj I, Lacoux C, Haynes M, et al. Mechano-induced cell metabolism promotes microtubule glutamylation to force metastasis. Cell Metab . 2021;33:1342–1357.e10. doi: 10.1016/j.cmet.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 40. Saraji A, Sydykov A, Schäfer K, Garcia-Castro CF, Henneke I, Alebrahimdehkordi N, et al. PINK1-mediated mitophagy contributes to pulmonary vascular remodeling in pulmonary hypertension. Am J Respir Cell Mol Biol . 2021;65:226–228. doi: 10.1165/rcmb.2021-0082LE. [DOI] [PubMed] [Google Scholar]
- 41.Niihori M, Eccles CA, Kurdyukov S, Zemskova M, Varghese MV, Stepanova AA, et al. Rats with a human mutation of NFU1 develop pulmonary hypertension. Am J Respir Cell Mol Biol. 2020;62:231–242. doi: 10.1165/rcmb.2019-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang Y, Zervopoulos SD, Boukouris AE, Lorenzana-Carrillo MA, Saleme B, Webster L, et al. SNPs for genes encoding the mitochondrial proteins sirtuin3 and uncoupling protein 2 are associated with disease severity, type 2 diabetes, and outcomes in patients with pulmonary arterial hypertension and this is recapitulated in a new mouse model lacking both genes. J Am Heart Assoc . 2021;10:e020451. doi: 10.1161/JAHA.120.020451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Michelakis ED, Gurtu V, Webster L, Barnes G, Watson G, Howard L, et al. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci Transl Med . 2017;9:eaao4583. doi: 10.1126/scitranslmed.aao4583. [DOI] [PubMed] [Google Scholar]
- 44. Simonneau G, Torbicki A, Dorfmüller P, Kim N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev . 2017;26:160112. doi: 10.1183/16000617.0112-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu W, Zhang Y, Lu L, Wang L, Chen M, Hu T. Expression and correlation of hypoxia-inducible factor-1α (HIF-1α) with pulmonary artery remodeling and right ventricular hypertrophy in experimental pulmonary embolism. Med Sci Monit . 2017;23:2083–2088. doi: 10.12659/MSM.900354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Howden EJ, Ruiz-Carmona S, Claeys M, De Bosscher R, Willems R, Meyns B, et al. Oxygen pathway limitations in patients with chronic thromboembolic pulmonary hypertension. Circulation . 2021;143:2061–2073. doi: 10.1161/CIRCULATIONAHA.120.052899. [DOI] [PubMed] [Google Scholar]
- 47. Alba GA, Samokhin AO, Wang RS, Zhang YY, Wertheim BM, Arons E, et al. NEDD9 is a novel and modifiable mediator of platelet-endothelial adhesion in the pulmonary circulation. Am J Respir Crit Care Med . 2021;203:1533–1545. doi: 10.1164/rccm.202003-0719OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Samokhin AO, Stephens T, Wertheim BM, Wang RS, Vargas SO, Yung LM, et al. NEDD9 targets COL3A1 to promote endothelial fibrosis and pulmonary arterial hypertension. Sci Transl Med . 2018;10:eaap7294. doi: 10.1126/scitranslmed.aap7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goncharova EA, Kudryashova TV, Maroli G, Pullamsetti SS. Matrix metalloproteinase-8 in pulmonary hypertension: the sheep in the wolf’s skin? Am J Respir Crit Care Med . 2021;204:1361–1363. doi: 10.1164/rccm.202109-2144ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Veith C, Vartürk-Özcan I, Wujak M, Hadzic S, Wu CY, Knoepp F, et al. SPARC, a novel regulator of vascular cell function in pulmonary hypertension. Circulation . 2022;145:916–933. doi: 10.1161/CIRCULATIONAHA.121.057001. [DOI] [PubMed] [Google Scholar]
- 51. Dieffenbach PB, Mallarino Haeger C, Rehman R, Corcoran AM, Coronata AMF, Vellarikkal SK, et al. A novel protective role for matrix metalloproteinase-8 in the pulmonary vasculature. Am J Respir Crit Care Med . 2021;204:1433–1451. doi: 10.1164/rccm.202108-1863OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shen Y, Goncharov DA, Pena A, Baust J, Chavez Barragan A, Ray A, et al. Cross-talk between TSC2 and the extracellular matrix controls pulmonary vascular proliferation and pulmonary hypertension. Sci Signal . 2022;15:eabn2743. doi: 10.1126/scisignal.abn2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest . 2016;126:3313–3335. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jandl K, Marsh LM, Hoffmann J, Mutgan AC, Baum O, Bloch W, et al. Basement membrane remodeling controls endothelial function in idiopathic pulmonary arterial hypertension. Am J Respir Cell Mol Biol . 2020;63:104–117. doi: 10.1165/rcmb.2019-0303OC. [DOI] [PubMed] [Google Scholar]
- 55. Croston TL, Lemons AR, Barnes MA, Goldsmith WT, Orandle MS, Nayak AP, et al. Inhalation of Stachybotrys chartarum fragments induces pulmonary arterial remodeling. Am J Respir Cell Mol Biol . 2020;62:563–576. doi: 10.1165/rcmb.2019-0221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang Y, Hernandez M, Gower J, Winicki N, Morataya X, Alvarez S, et al. JAGGED-NOTCH3 signaling in vascular remodeling in pulmonary arterial hypertension. Sci Transl Med . 2022;14:eabl5471. doi: 10.1126/scitranslmed.abl5471. [DOI] [PubMed] [Google Scholar]
- 57. Shu T, Liu Y, Zhou Y, Zhou Z, Li B, Xing Y, et al. Inhibition of immunoglobulin E attenuates pulmonary hypertension. Nat Cardiovasc Res . 2022;1:665–678. doi: 10.1038/s44161-022-00095-9. [DOI] [PubMed] [Google Scholar]
- 58. Koudstaal T, van Hulst JAC, Das T, Neys SFH, Merkus D, Bergen IM, et al. DNGR1-Cre-mediated deletion of Tnfaip3/A20 in conventional dendritic cells induces pulmonary hypertension in mice. Am J Respir Cell Mol Biol . 2020;63:665–680. doi: 10.1165/rcmb.2019-0443OC. [DOI] [PubMed] [Google Scholar]
- 59. Hong J, Arneson D, Umar S, Ruffenach G, Cunningham CM, Ahn IS, et al. Single-cell study of two rat models of pulmonary arterial hypertension reveals connections to human pathobiology and drug repositioning. Am J Respir Crit Care Med . 2021;203:1006–1022. doi: 10.1164/rccm.202006-2169OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yuan K, Liu Y, Zhang Y, Nathan A, Tian W, Yu J, et al. Mural cell SDF1 signaling is associated with the pathogenesis of pulmonary arterial hypertension. Am J Respir Cell Mol Biol . 2020;62:747–759. doi: 10.1165/rcmb.2019-0401OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hansmann G, Chouvarine P, Diekmann F, Giera M, Ralser M, Mülleder M, et al. Human umbilical cord mesenchymal stem cell-derived treatment of severe pulmonary arterial hypertension. Nat Cardiovasc Res . 2022;1:568–576. doi: 10.1038/s44161-022-00083-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Huang J, Lu W, Ouyang H, Chen Y, Zhang C, Luo X, et al. Transplantation of mesenchymal stem cells attenuates pulmonary hypertension by normalizing the endothelial-to-mesenchymal transition. Am J Respir Cell Mol Biol . 2020;62:49–60. doi: 10.1165/rcmb.2018-0165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Klinger JR, Pereira M, Del Tatto M, Brodsky AS, Wu KQ, Dooner MS, et al. Mesenchymal stem cell extracellular vesicles reverse sugen/hypoxia pulmonary hypertension in rats. Am J Respir Cell Mol Biol . 2020;62:577–587. doi: 10.1165/rcmb.2019-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maron BA, Brittain EL, Choudhary G, Gladwin MT. Redefining pulmonary hypertension. Lancet Respir Med . 2018;6:168–170. doi: 10.1016/S2213-2600(17)30498-8. [DOI] [PubMed] [Google Scholar]
- 65. Maron BA, Hess E, Maddox TM, Opotowsky AR, Tedford RJ, Lahm T, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation . 2016;133:1240–1248. doi: 10.1161/CIRCULATIONAHA.115.020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Oliveira RKF, Faria-Urbina M, Maron BA, Santos M, Waxman AB, Systrom DM. Functional impact of exercise pulmonary hypertension in patients with borderline resting pulmonary arterial pressure. Pulm Circ . 2017;7:654–665. doi: 10.1177/2045893217709025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Assad TR, Maron BA, Robbins IM, Xu M, Huang S, Harrell FE, et al. Prognostic effect and longitudinal hemodynamic assessment of borderline pulmonary hypertension. JAMA Cardiol . 2017;2:1361–1368. doi: 10.1001/jamacardio.2017.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kolte D, Lakshmanan S, Jankowich MD, Brittain EL, Maron BA, Choudhary G. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc . 2018;7:e009729. doi: 10.1161/JAHA.118.009729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J . 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kovacs G, Herve P, Barbera JA, Chaouat A, Chemla D, Condliffe R, et al. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J . 2017;50:1700578. doi: 10.1183/13993003.00578-2017. [DOI] [PubMed] [Google Scholar]
- 71. Newman JH. Pulmonary hypertension. Am J Respir Crit Care Med . 2005;172:1072–1077. doi: 10.1164/rccm.200505-684OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Provencher S, Boucherat O, Potus F, Bonnet S. Pulmonary hypertension thresholds: time to lower further? Lancet Respir Med . 2020;8:834–836. doi: 10.1016/S2213-2600(20)30326-X. [DOI] [PubMed] [Google Scholar]
- 73. Johnson SW, Maron BA. COUNTERPOINT: did the World Symposium on Pulmonary Hypertension get it right in redefining abnormal pulmonary arterial pressure? No. Chest . 2022;161:313–315. doi: 10.1016/j.chest.2021.07.018. [DOI] [PubMed] [Google Scholar]
- 74. Maron BA, Brittain EL, Hess E, Waldo SW, Barón AE, Huang S, et al. Pulmonary vascular resistance and clinical outcomes in patients with pulmonary hypertension: a retrospective cohort study. Lancet Respir Med . 2020;8:873–884. doi: 10.1016/S2213-2600(20)30317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xanthouli P, Jordan S, Milde N, Marra A, Blank N, Egenlauf B, et al. Haemodynamic phenotypes and survival in patients with systemic sclerosis: the impact of the new definition of pulmonary arterial hypertension. Ann Rheum Dis . 2020;79:370–378. doi: 10.1136/annrheumdis-2019-216476. [DOI] [PubMed] [Google Scholar]
- 76. Thenappan T, Prins KW, Pritzker MR, Scandurra J, Volmers K, Weir EK. The critical role of pulmonary arterial compliance in pulmonary hypertension. Ann Am Thorac Soc . 2016;13:276–284. doi: 10.1513/AnnalsATS.201509-599FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol . 2006;47:799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 78. Vanderpool RR, Saul M, Nouraie M, Gladwin MT, Simon MA. Association between hemodynamic markers of pulmonary hypertension and outcomes in heart failure with preserved ejection fraction. JAMA Cardiol . 2018;3:298–306. doi: 10.1001/jamacardio.2018.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Brener MI, Masoumi A, Ng VG, Tello K, Bastos MB, Cornwell WK, III, et al. Invasive right ventricular pressure-volume analysis: basic principles, clinical applications, and practical recommendations. Circ Heart Fail . 2022;15:e009101. doi: 10.1161/CIRCHEARTFAILURE.121.009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Richter MJ, Hsu S, Yogeswaran A, Husain-Syed F, Vadász I, Ghofrani HA, et al. Right ventricular pressure-volume loop shape and systolic pressure change in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol . 2021;320:L715–L725. doi: 10.1152/ajplung.00583.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Houston BA, Brittain EL, Tedford RJ. Right ventricular failure. N Engl J Med . 2023;388:1111–1125. doi: 10.1056/NEJMra2207410. [DOI] [PubMed] [Google Scholar]
- 82. Richter MJ, Yogeswaran A, Husain-Syed F, Vadász I, Rako Z, Mohajerani E, et al. A novel non-invasive and echocardiography-derived method for quantification of right ventricular pressure-volume loops. Eur Heart J Cardiovasc Imaging . 2022;23:498–507. doi: 10.1093/ehjci/jeab038. [DOI] [PubMed] [Google Scholar]
- 83. Kremer N, Rako Z, Douschan P, Gall H, Ghofrani HA, Grimminger F, et al. Unmasking right ventricular-arterial uncoupling during fluid challenge in pulmonary hypertension. J Heart Lung Transplant . 2022;41:345–355. doi: 10.1016/j.healun.2021.11.019. [DOI] [PubMed] [Google Scholar]
- 84. Douschan P, Tello K, Rieth AJ, Wiedenroth CB, Sassmann T, Kovacs G, et al. Right ventricular-pulmonary arterial coupling and its relationship to exercise haemodynamics in a continuum of patients with pulmonary vascular disease due to chronic thromboembolism. Eur Respir J . 2022;60:2200450. doi: 10.1183/13993003.00450-2022. [DOI] [PubMed] [Google Scholar]
- 85. Tello K, Kremer N, Richter MJ, Gall H, Muenks J, Ghofrani A, et al. Inhaled iloprost improves right ventricular load-independent contractility in pulmonary hypertension. Am J Respir Crit Care Med . 2022;206:111–114. doi: 10.1164/rccm.202201-0095LE. [DOI] [PubMed] [Google Scholar]
- 86. Maron BA. Revised definition of pulmonary hypertension and approach to management: a clinical primer. J Am Heart Assoc . 2023;12:e029024. doi: 10.1161/JAHA.122.029024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Melillo CA, Lane JE, Aulak KS, Almoushref A, Dweik RA, Tonelli AR. Repeatability of pulmonary pressure measurements in patients with pulmonary hypertension. Ann Am Thorac Soc . 2020;17:1028–1030. doi: 10.1513/AnnalsATS.202002-182RL. [DOI] [PubMed] [Google Scholar]
- 88. Viray MC, Bonno EL, Gabrielle ND, Maron BA, Atkins J, Amoroso NS, et al. Role of pulmonary artery wedge pressure saturation during right heart catheterization: a prospective study. Circ Heart Fail . 2020;13:e007981. doi: 10.1161/CIRCHEARTFAILURE.120.007981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gall H, Yogeswaran A, Fuge J, Sommer N, Grimminger F, Seeger W, et al. Validity of echocardiographic tricuspid regurgitation gradient to screen for new definition of pulmonary hypertension. EClinicalMedicine . 2021;34:100822. doi: 10.1016/j.eclinm.2021.100822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Brittain EL, Thenappan T, Huston JH, Agrawal V, Lai YC, Dixon D, et al. American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; and Stroke Council Elucidating the clinical implications and pathophysiology of pulmonary hypertension in heart failure with preserved ejection fraction: a call to action: a science advisory from the American Heart Association. Circulation . 2022;146:e73–e88. doi: 10.1161/CIR.0000000000001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Huston JH, Maron BA, French J, Huang S, Thayer T, Farber-Eger EH, et al. Association of mild echocardiographic pulmonary hypertension with mortality and right ventricular function. JAMA Cardiol . 2019;4:1112–1121. doi: 10.1001/jamacardio.2019.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Strange G, Stewart S, Celermajer DS, Prior D, Scalia GM, Marwick TH, et al. NEDA Contributing Sites Threshold of pulmonary hypertension associated with increased mortality. J Am Coll Cardiol . 2019;73:2660–2672. doi: 10.1016/j.jacc.2019.03.482. [DOI] [PubMed] [Google Scholar]
- 93. Maron BA, Kovacs G, Vaidya A, Bhatt DL, Nishimura RA, Mak S, et al. Cardiopulmonary hemodynamics in pulmonary hypertension and heart failure: JACC review topic of the week. J Am Coll Cardiol . 2020;76:2671–2681. doi: 10.1016/j.jacc.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zeder K, Banfi C, Steinrisser-Allex G, Maron BA, Humbert M, Lewis GD, et al. Diagnostic, prognostic and differential-diagnostic relevance of pulmonary haemodynamic parameters during exercise: a systematic review. Eur Respir J . 2022;60:2103181. doi: 10.1183/13993003.03181-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wolsk E, Bakkestrøm R, Thomsen JH, Balling L, Andersen MJ, Dahl JS, et al. The influence of age on hemodynamic parameters during rest and exercise in healthy individuals. JACC Heart Fail . 2017;5:337–346. doi: 10.1016/j.jchf.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 96. Ho JE, Zern EK, Lau ES, Wooster L, Bailey CS, Cunningham T, et al. Exercise pulmonary hypertension predicts clinical outcomes in patients with dyspnea on effort. J Am Coll Cardiol . 2020;75:17–26. doi: 10.1016/j.jacc.2019.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Synn AJ, Li W, San José Estépar R, Washko GR, O’Connor GT, Tsao CW, et al. Pulmonary vascular pruning on computed tomography and risk of death in the Framingham Heart Study. Am J Respir Crit Care Med . 2021;203:251–254. doi: 10.1164/rccm.202005-1671LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rahaghi FN, Nardelli P, Harder E, Singh I, Sánchez-Ferrero GV, Ross JC, et al. Quantification of arterial and venous morphologic markers in pulmonary arterial hypertension using CT imaging. Chest . 2021;160:2220–2231. doi: 10.1016/j.chest.2021.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rahaghi FN, Trieu M, Shaikh F, Abtin F, Diaz AA, Liang LL, et al. Evolution of obstructive lung function in advanced pulmonary arterial hypertension. Am J Respir Crit Care Med . 2021;204:1478–1481. doi: 10.1164/rccm.202105-1169LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lewis RA, Thompson AAR, Billings CG, Charalampopoulos A, Elliot CA, Hamilton N, et al. Mild parenchymal lung disease and/or low diffusion capacity impacts survival and treatment response in patients diagnosed with idiopathic pulmonary arterial hypertension. Eur Respir J . 2020;55:2000041. doi: 10.1183/13993003.00041-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bernardo RJ, Lu D, Ramirez RL, III, Hedlin H, Kawut SM, Bull T, et al. Hispanic ethnicity and social determinants of health in pulmonary arterial hypertension: the Pulmonary Hypertension Association Registry. Ann Am Thorac Soc . 2022;19:1459–1468. doi: 10.1513/AnnalsATS.202109-1051OC. [DOI] [PubMed] [Google Scholar]
- 102. Gomberg-Maitland M, de Jesus Perez V. Health disparity is a global issue: understanding Latin America. Pulm Circ . 2022;12:e12049. doi: 10.1002/pul2.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Macías CG, Wharam JF, Maron BA, Ong MS. Urban-rural disparities in pulmonary hypertension mortality. Ann Am Thorac Soc . 2020;17:1168–1171. doi: 10.1513/AnnalsATS.202003-234RL. [DOI] [PubMed] [Google Scholar]
- 104. Curran L, Nah G, Marcus GM, Tseng Z, Crawford MH, Parikh NI. Clinical correlates and outcomes of methamphetamine-associated cardiovascular diseases in hospitalized patients in California. J Am Heart Assoc . 2022;11:e023663. doi: 10.1161/JAHA.121.023663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kolaitis NA, Zamanian RT, de Jesus Perez VA, Badesch DB, Benza RL, Burger CD, et al. Pulmonary Hypertension Association Registry Investigators Clinical differences and outcomes between methamphetamine-associated and idiopathic pulmonary arterial hypertension in the Pulmonary Hypertension Association Registry. Ann Am Thorac Soc . 2021;18:613–622. doi: 10.1513/AnnalsATS.202007-774OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kolaitis NA, Saggar R, De Marco T. Methamphetamine-associated pulmonary arterial hypertension. Curr Opin Pulm Med . 2022;28:352–360. doi: 10.1097/MCP.0000000000000888. [DOI] [PubMed] [Google Scholar]
- 107. Frank RC, Min J, Abdelghany M, Paniagua S, Bhattacharya R, Bhambhani V, et al. Obesity is associated with pulmonary hypertension and modifies outcomes. J Am Heart Assoc . 2020;9:e014195. doi: 10.1161/JAHA.119.014195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sakamoto Y, Kameshima S, Kakuda C, Okamura Y, Kodama T, Okada M, et al. Visceral adipose tissue-derived serine protease inhibitor prevents the development of monocrotaline-induced pulmonary arterial hypertension in rats. Pflugers Arch . 2017;469:1425–1432. doi: 10.1007/s00424-017-2043-6. [DOI] [PubMed] [Google Scholar]
- 109. Al-Naamani N, Pan HM, Anderson MR, Torigian DA, Tong Y, Oyster M, et al. Thoracic visceral adipose tissue area and pulmonary hypertension in lung transplant candidates. The Lung Transplant Body Composition Study. Ann Am Thorac Soc . 2020;17:1393–1400. doi: 10.1513/AnnalsATS.202003-247OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Min J, Feng R, Badesch D, Berman-Rosenzweig E, Burger C, Chakinala M, et al. PHAR Investigators Obesity in pulmonary arterial hypertension (PAH): the Pulmonary Hypertension Association Registry (PHAR) Ann Am Thorac Soc . 2020;18:229–237. doi: 10.1513/AnnalsATS.202006-612OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hemnes AR, Leopold JA, Radeva MK, Beck GJ, Abidov A, Aldred MA, et al. PVDOMICS Study Group Clinical characteristics and transplant-free survival across the spectrum of pulmonary vascular disease. J Am Coll Cardiol . 2022;80:697–718. doi: 10.1016/j.jacc.2022.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failure. JACC Heart Fail . 2013;1:290–299. doi: 10.1016/j.jchf.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 113. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. EMPEROR-Preserved Trial Investigators Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med . 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 114. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. DAPA-HF Trial Committees and Investigators Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med . 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 115.Ferreira JP, Cleland JG, Girerd N, Bozec E, Rossignol P, Pellicori P, et al. Spironolactone effect on cardiac structure and function of patients with heart failure and preserved ejection fraction: a pooled analysis of three randomized trials. Eur J Heart Fail. 2023;25:108–113. doi: 10.1002/ejhf.2726. [DOI] [PubMed] [Google Scholar]
- 116. Nassif ME, Qintar M, Windsor SL, Jermyn R, Shavelle DM, Tang F, et al. Empagliflozin effects on pulmonary artery pressure in patients with heart failure: results from the EMBRACE-HF trial. Circulation . 2021;143:1673–1686. doi: 10.1161/CIRCULATIONAHA.120.052503. [DOI] [PubMed] [Google Scholar]
- 117. Maron BA, Leopold JA. Aldosterone receptor antagonists: effective but often forgotten. Circulation . 2010;121:934–939. doi: 10.1161/CIRCULATIONAHA.109.895235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tu L, Thuillet R, Perrot J, Ottaviani M, Ponsardin E, Kolkhof P, et al. Mineralocorticoid receptor antagonism by finerenone attenuates established pulmonary hypertension in rats. Hypertension . 2022;79:2262–2273. doi: 10.1161/HYPERTENSIONAHA.122.19207. [DOI] [PubMed] [Google Scholar]
- 119. Vachiéry JL, Delcroix M, Al-Hiti H, Efficace M, Hutyra M, Lack G, et al. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J . 2018;51:1701886. doi: 10.1183/13993003.01886-2017. [DOI] [PubMed] [Google Scholar]
- 120. Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation . 2011;124:164–174. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 121. Hoendermis ES, Liu LC, Hummel YM, van der Meer P, de Boer RA, Berger RM, et al. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J . 2015;36:2565–2573. doi: 10.1093/eurheartj/ehv336. [DOI] [PubMed] [Google Scholar]
- 122. Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation . 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 123. Fayyaz AU, Edwards WD, Maleszewski JJ, Konik EA, DuBrock HM, Borlaug BA, et al. Global pulmonary vascular remodeling in pulmonary hypertension associated with heart failure and preserved or reduced ejection fraction. Circulation . 2018;137:1796–1810. doi: 10.1161/CIRCULATIONAHA.117.031608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Vizza CD, Hoeper MM, Huscher D, Pittrow D, Benjamin N, Olsson KM, et al. Pulmonary hypertension in patients with COPD: results from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA) Chest . 2021;160:678–689. doi: 10.1016/j.chest.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 125. Caminati A, Cassandro R, Harari S. Pulmonary hypertension in chronic interstitial lung diseases. Eur Respir Rev . 2013;22:292–301. doi: 10.1183/09059180.00002713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Olsson KM, Hoeper MM, Pausch C, Grünig E, Huscher D, Pittrow D, et al. Pulmonary vascular resistance predicts mortality in patients with pulmonary hypertension associated with interstitial lung disease: results from the COMPERA registry. Eur Respir J . 2021;58:2101483. doi: 10.1183/13993003.01483-2021. [DOI] [PubMed] [Google Scholar]
- 127. Zeder K, Avian A, Bachmaier G, Douschan P, Foris V, Sassmann T, et al. Elevated pulmonary vascular resistance predicts mortality in COPD patients. Eur Respir J . 2021;58:2100944. doi: 10.1183/13993003.00944-2021. [DOI] [PubMed] [Google Scholar]
- 128. Dauriat G, Reynaud-Gaubert M, Cottin V, Lamia B, Montani D, Canuet M, et al. Severe pulmonary hypertension associated with chronic obstructive pulmonary disease: a prospective French multicenter cohort. J Heart Lung Transplant . 2021;40:1009–1018. doi: 10.1016/j.healun.2021.04.021. [DOI] [PubMed] [Google Scholar]
- 129. Hoeper MM, Dwivedi K, Pausch C, Lewis RA, Olsson KM, Huscher D, et al. Phenotyping of idiopathic pulmonary arterial hypertension: a registry analysis. Lancet Respir Med . 2022;10:937–948. doi: 10.1016/S2213-2600(22)00097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Jutant EM, Jaïs X, Girerd B, Savale L, Ghigna MR, Perros F, et al. Phenotype and outcomes of pulmonary hypertension associated with neurofibromatosis type 1. Am J Respir Crit Care Med . 2020;202:843–852. doi: 10.1164/rccm.202001-0105OC. [DOI] [PubMed] [Google Scholar]
- 131. Prins KW, Duval S, Markowitz J, Pritzker M, Thenappan T. Chronic use of PAH-specific therapy in World Health Organization Group III pulmonary hypertension: a systematic review and meta-analysis. Pulm Circ . 2017;7:145–155. doi: 10.1086/690017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Farmakis IT, Vazakidis P, Doundoulakis I, Arvanitaki A, Zafeiropoulos S, Boutou A, et al. Haemodynamic effects of PAH-targeted therapies in pulmonary hypertension due to lung disease: a systematic review and meta-analysis. Pulm Pharmacol Ther . 2021;68:102036. doi: 10.1016/j.pupt.2021.102036. [DOI] [PubMed] [Google Scholar]
- 133. Johnson SW, Finlay L, Mathai SC, Goldstein RH, Maron BA. Real-world use of inhaled treprostinil for lung disease-pulmonary hypertension: a protocol for patient evaluation and prescribing. Pulm Circ . 2022;12:e12126. doi: 10.1002/pul2.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J . 2017;50:1700740. doi: 10.1183/13993003.00740-2017. [DOI] [PubMed] [Google Scholar]
- 135. Benza RL, Gomberg-Maitland M, Miller DP, Frost A, Frantz RP, Foreman AJ, et al. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest . 2012;141:354–362. doi: 10.1378/chest.11-0676. [DOI] [PubMed] [Google Scholar]
- 136. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J . 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 137. Benza RL, Gomberg-Maitland M, Elliott CG, Farber HW, Foreman AJ, Frost AE, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL Risk Score Calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest . 2019;156:323–337. doi: 10.1016/j.chest.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 138. Min J, Badesch D, Chakinala M, Elwing J, Frantz R, Horn E, et al. PHAR Investigators Prediction of health-related quality of life and hospitalization in pulmonary arterial hypertension: the Pulmonary Hypertension Association Registry. Am J Respir Crit Care Med . 2021;203:761–764. doi: 10.1164/rccm.202010-3967LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Hoeper MM, Pausch C, Olsson KM, Huscher D, Pittrow D, Grünig E, et al. COMPERA 2.0: a refined four-stratum risk assessment model for pulmonary arterial hypertension. Eur Respir J . 2022;60:2102311. doi: 10.1183/13993003.02311-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Boucly A, Weatherald J, Savale L, de Groote P, Cottin V, Prévot G, et al. External validation of a refined four-stratum risk assessment score from the French Pulmonary Hypertension Registry. Eur Respir J . 2022;59:2102419. doi: 10.1183/13993003.02419-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Badagliacca R, D’Alto M, Ghio S, Argiento P, Bellomo V, Brunetti ND, et al. Risk reduction and hemodynamics with initial combination therapy in pulmonary arterial hypertension. Am J Respir Crit Care Med . 2021;203:484–492. doi: 10.1164/rccm.202004-1006OC. [DOI] [PubMed] [Google Scholar]
- 142. D’Alto M, Badagliacca R, Lo Giudice F, Argiento P, Casu G, Corda M, et al. Hemodynamics and risk assessment 2 years after the initiation of upfront ambrisentan‒tadalafil in pulmonary arterial hypertension. J Heart Lung Transplant . 2020;39:1389–1397. doi: 10.1016/j.healun.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 143. Schoenberg NC, Farber HW. When “AMBITION” isn’t good enough: risk status and dual oral therapy in pulmonary arterial hypertension. Am J Respir Crit Care Med . 2021;203:410–411. doi: 10.1164/rccm.202008-3313ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Blette BS, Moutchia J, Al-Naamani N, Ventetuolo CE, Cheng C, Appleby D, et al. Is low-risk status a surrogate outcome in pulmonary arterial hypertension? An analysis of three randomised trials. Lancet Respir Med . 2023 doi: 10.1016/S2213-2600(23)00155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Benza RL, Gomberg-Maitland M, Farber HW, Vizza CD, Broderick M, Holdstock L, et al. Contemporary risk scores predict clinical worsening in pulmonary arterial hypertension – an analysis of FREEDOM-EV. J Heart Lung Transplant . 2022;41:1572–1580. doi: 10.1016/j.healun.2022.08.006. [DOI] [PubMed] [Google Scholar]
- 146. Yogeswaran A, Tello K, Lund J, Klose H, Harbaum L, Sommer N, et al. Risk assessment in pulmonary hypertension based on routinely measured laboratory parameters. J Heart Lung Transplant . 2022;41:400–410. doi: 10.1016/j.healun.2021.10.018. [DOI] [PubMed] [Google Scholar]
- 147. Keranov S, Dörr O, Jafari L, Troidl C, Liebetrau C, Kriechbaum S, et al. CILP1 as a biomarker for right ventricular maladaptation in pulmonary hypertension. Eur Respir J . 2021;57:1901192. doi: 10.1183/13993003.01192-2019. [DOI] [PubMed] [Google Scholar]
- 148. Marvin-Peek J, Hemnes A, Huang S, Silverman-Loyd L, MacKinnon G, Annis J, et al. Daily step counts are associated with hospitalization risk in pulmonary arterial hypertension. Am J Respir Crit Care Med . 2021;204:1338–1340. doi: 10.1164/rccm.202104-1035LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hemnes AR, Silverman-Lloyd LG, Huang S, MacKinnon G, Annis J, Whitmore CS, et al. A mobile health intervention to increase physical activity in pulmonary arterial hypertension. Chest . 2021;160:1042–1052. doi: 10.1016/j.chest.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Badagliacca R, Rischard F, Giudice FL, Howard L, Papa S, Valli G, et al. Incremental value of cardiopulmonary exercise testing in intermediate-risk pulmonary arterial hypertension. J Heart Lung Transplant . 2022;41:780–790. doi: 10.1016/j.healun.2022.02.021. [DOI] [PubMed] [Google Scholar]
- 151. Botney MD, Bahadori L, Gold LI. Vascular remodeling in primary pulmonary hypertension. Potential role for transforming growth factor-beta. Am J Pathol . 1994;144:286–295. [PMC free article] [PubMed] [Google Scholar]
- 152. Guignabert C, Savale L, Boucly A, Thuillet R, Tu L, Ottaviani M, et al. Serum and pulmonary expression profiles of the activin signaling system in pulmonary arterial hypertension. Circulation . 2023;147:1809–1822. doi: 10.1161/CIRCULATIONAHA.122.061501. [DOI] [PubMed] [Google Scholar]
- 153. Yung LM, Yang P, Joshi S, Augur ZM, Kim SSJ, Bocobo GA, et al. ACTRIIA-Fc rebalances activin/GDF versus BMP signaling in pulmonary hypertension. Sci Transl Med . 2020;12:eaaz5660. doi: 10.1126/scitranslmed.aaz5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Humbert M, McLaughlin V, Gibbs JSR, Gomberg-Maitland M, Hoeper MM, Preston IR, et al. Sotatercept for the treatment of pulmonary arterial hypertension: PULSAR open-label extension. Eur Respir J . 2022;61:2201347. doi: 10.1183/13993003.01347-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lajoie AC, Lauzière G, Lega JC, Lacasse Y, Martin S, Simard S, et al. Combination therapy versus monotherapy for pulmonary arterial hypertension: a meta-analysis. Lancet Respir Med. 2016;4:291–305. doi: 10.1016/S2213-2600(16)00027-8. [DOI] [PubMed] [Google Scholar]
- 156. Chin KM, Sitbon O, Doelberg M, Feldman J, Gibbs JSR, Grünig E, et al. Three- versus two-drug therapy for patients with newly diagnosed pulmonary arterial hypertension. J Am Coll Cardiol . 2021;78:1393–1403. doi: 10.1016/j.jacc.2021.07.057. [DOI] [PubMed] [Google Scholar]
- 157. Cascino TM, McLaughlin VV. Upfront combination therapy for pulmonary arterial hypertension: time to be more ambitious than AMBITION. Am J Respir Crit Care Med . 2021;204:756–759. doi: 10.1164/rccm.202107-1625ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Boucly A, Savale L, Jaïs X, Bauer F, Bergot E, Bertoletti L, et al. Association between initial treatment strategy and long-term survival in pulmonary arterial hypertension. Am J Respir Crit Care Med . 2021;204:842–854. doi: 10.1164/rccm.202009-3698OC. [DOI] [PubMed] [Google Scholar]
- 159. Vizza CD, Lang IM, Badagliacca R, Benza RL, Rosenkranz S, White RJ, et al. Aggressive afterload lowering to improve the right ventricle: a new target for medical therapy in pulmonary arterial hypertension? Am J Respir Crit Care Med . 2022;205:751–760. doi: 10.1164/rccm.202109-2079PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ratwatte S, Anderson J, Strange G, Corrigan C, Collins N, Celermajer DS, et al. PHSANZ Registry Pulmonary arterial hypertension with below threshold pulmonary vascular resistance. Eur Respir J . 2020;56:1901654. doi: 10.1183/13993003.01654-2019. [DOI] [PubMed] [Google Scholar]
- 161. Humbert M, Yaici A, de Groote P, Montani D, Sitbon O, Launay D, et al. Screening for pulmonary arterial hypertension in patients with systemic sclerosis: clinical characteristics at diagnosis and long-term survival. Arthritis Rheum . 2011;63:3522–3530. doi: 10.1002/art.30541. [DOI] [PubMed] [Google Scholar]
- 162. Valerio CJ, Schreiber BE, Handler CE, Denton CP, Coghlan JG. Borderline mean pulmonary artery pressure in patients with systemic sclerosis: transpulmonary gradient predicts risk of developing pulmonary hypertension. Arthritis Rheum . 2013;65:1074–1084. doi: 10.1002/art.37838. [DOI] [PubMed] [Google Scholar]
- 163. Montani D, Girerd B, Jaïs X, Laveneziana P, Lau EMT, Bouchachi A, et al. Screening for pulmonary arterial hypertension in adults carrying a BMPR2 mutation. Eur Respir J . 2021;58:2004229. doi: 10.1183/13993003.04229-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Kim NH, Delcroix M, Jais X, Madani MM, Matsubara H, Mayer E, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J . 2019;53:1801915. doi: 10.1183/13993003.01915-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Nishihara T, Shimokawahara H, Ogawa A, Naito T, Une D, Mukai T, et al. Comparison of the safety and efficacy of balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension patients with surgically accessible and inaccessible lesions. J Heart Lung Transplant . 2023;42:786–794. doi: 10.1016/j.healun.2023.01.003. [DOI] [PubMed] [Google Scholar]
- 166. Jaïs X, Brenot P, Bouvaist H, Jevnikar M, Canuet M, Chabanne C, et al. Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension (RACE): a multicentre, phase 3, open-label, randomised controlled trial and ancillary follow-up study. Lancet Respir Med . 2022;10:961–971. doi: 10.1016/S2213-2600(22)00214-4. [DOI] [PubMed] [Google Scholar]
- 167. Kawakami T, Matsubara H, Shinke T, Abe K, Kohsaka S, Hosokawa K, et al. Balloon pulmonary angioplasty versus riociguat in inoperable chronic thromboembolic pulmonary hypertension (MR BPA): an open-label, randomised controlled trial. Lancet Respir Med . 2022;10:949–960. doi: 10.1016/S2213-2600(22)00171-0. [DOI] [PubMed] [Google Scholar]
- 168. Minhas J, Shou H, Hershman S, Zamanian R, Ventetuolo CE, Bull TM, et al. Physical activity and its association with traditional outcome measures in pulmonary arterial hypertension. Ann Am Thorac Soc . 2022;19:572–582. doi: 10.1513/AnnalsATS.202105-560OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Grünig E, MacKenzie A, Peacock AJ, Eichstaedt CA, Benjamin N, Nechwatal R, et al. Standardized exercise training is feasible, safe, and effective in pulmonary arterial and chronic thromboembolic pulmonary hypertension: results from a large European multicentre randomized controlled trial. Eur Heart J . 2021;42:2284–2295. doi: 10.1093/eurheartj/ehaa696. [DOI] [PubMed] [Google Scholar]
- 170. Viele K, Girard TD. Risk, results, and costs: optimizing clinical trial efficiency through prognostic enrichment. Am J Respir Crit Care Med . 2021;203:671–672. doi: 10.1164/rccm.202009-3649ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Hemnes AR, Beck GJ, Newman JH, Abidov A, Aldred MA, Barnard J, et al. PVDOMICS Study Group PVDOMICS: a multi-center study to improve understanding of pulmonary vascular disease through phenomics. Circ Res . 2017;121:1136–1139. doi: 10.1161/CIRCRESAHA.117.311737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Bothwell LE, Avorn J, Khan NF, Kesselheim AS. Adaptive design clinical trials: a review of the literature and ClinicalTrials.gov. BMJ Open . 2018;8:e018320. doi: 10.1136/bmjopen-2017-018320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Ford I, Norrie J. Pragmatic trials. N Engl J Med . 2016;375:454–463. doi: 10.1056/NEJMra1510059. [DOI] [PubMed] [Google Scholar]
- 174. Weatherald J, Boucly A, Peters A, Montani D, Prasad K, Psotka MA, et al. Evolving Landscape of Pulmonary Arterial Hypertension and Redesigning Pulmonary Arterial Hypertension Clinical Trials Task Force of the 18th Global CardioVascular Clinical Trialists Forum The evolving landscape of pulmonary arterial hypertension clinical trials. Lancet . 2022;400:1884–1898. doi: 10.1016/S0140-6736(22)01601-4. [DOI] [PubMed] [Google Scholar]
- 175.Maron BA, Kleiner DE, Arons E, Wertheim BM, Sharma NS, Haley KJ, et al. Evidence of advanced pulmonary vascular remodeling in obstructive hypertrophic cardiomyopathy with pulmonary hypertension. Chest. 2023;163:678–686. doi: 10.1016/j.chest.2022.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]