Abstract
To evaluate the efficiency of pan-keratin immunostaining, tissue microarrays of 13,501 tumor samples from 121 different tumor types and subtypes as well as 608 samples of 76 different normal tissue types were analyzed by immunohistochemistry. In normal tissues, strong pan-keratin immunostaining was seen in epithelial cells. Staining intensity was lower in hepatocytes, islets of Langerhans, and pneumocytes but markedly reduced in the adrenal cortex. Pan-keratin was positive in ≥98% of samples in 62 (83%) of 75 epithelial tumor entities, including almost all adenocarcinomas, squamous cell and urothelial carcinomas. Only 17 of 121 tumor entities (13%) had a pan-keratin positivity rate between 25% and 98%, including tumors with mixed differentiation, endocrine/neuroendocrine tumors, renal cell carcinomas, adrenocortical tumors, and particularly poorly differentiated carcinoma subtypes. The 15 entities with pan-keratin positivity in 0.9%-25% were mostly of mesenchymal origin. Reduced/absent pan-keratin immunostaining was associated with high UICC stage (p = 0.0001), high Thoenes grade (p = 0.0183), high Fuhrman grade (p = 0.0049), advanced tumor stage (p < 0.0001) and lymph node metastasis (p = 0.0114) in clear cell renal cell carcinoma, advanced pT stage (p = 0.0007) in papillary renal cell carcinoma, and with advanced stage (p = 0.0023), high grade (p = 0.0005) as well as loss of ER and PR expression (each p < 0.0001) in invasive breast carcinoma of no special type (NST). In summary, pan-keratin can consistently be detected in the vast majority of epithelial tumors, although pan-keratin can be negative a fraction of renal cell, adrenocortical and neuroendocrine neoplasms. The data also link reduced pan-keratin immunostaining to unfavorable tumor phenotype in in epithelial neoplasms.
Keywords: pan-cytokeratin, pan-keratin, tissue microarray, immunohistochemistry, cancer
Introduction
Pan-keratin antibodies are mixtures of two or several antibodies that detect multiple low and high molecular weight keratins. These antibody cocktails have been designed to immunohistochemically detect all epithelial cell types irrespective of their tissues of origin with one single diagnostic tool. In surgical pathology they are typically employed to document the epithelial origin of neoplastic or non-neoplastic tissue or for detection of small metastases in lymph nodes. There are, however, limitations to the concept that pan-keratin antibodies stain all epithelial tumors and that non-epithelial tissues are “keratin negative”. For a large variety of different epithelial tumors, pan-keratin negative tumors have been described 1–6 and “keratin positive” mesenchymal tumors have also been reported across various mesenchymal tumor entities.7–12 The frequencies reported for pan-keratin negative carcinomas and pan-keratin positive non-epithelial tumors varies considerably in the literature, however. For example, pan-keratin positivity has been described in 15% to 45% of hepatocellular carcinoma carcinomas,3,5 0% to 95% of adrenocortical carcinomas,2,13,14 33% to 100% of clear cell15,16% and 73% to 100% of papillary renal cell carcinomas,17,18 20% to 78% of angiosarcomas,19,20 and 17% to 100% of leiomyosarcomas.21,22 These conflicting data may be caused by the use of different antibodies, immunostaining protocols, and criteria to determine “positivity” in these studies.
To generate a comprehensive dataset on the prevalence of pan-keratin positivity in epithelial and non-epithelial neoplasms, a set of tissue microarray (TMAs) was analyzed in this study that contained more than 15,500 tumor tissue samples from 121 different tumor types and subtypes as well as 76 different non-neoplastic tissue types.
Materials and Methods
Tissue Microarrays (TMAs). Our normal tissue TMA was composed of 8 samples from 8 different donors for each of 76 different normal tissue types (608 samples on one slide). The tumor TMAs contained a total of 15,940 primary tumors from 121 tumor types and subtypes. The composition of normal and tumor TMAs is described in the results section. Detailed histopathological and molecular data as well as clinical-follow up data were obtained from 1157 kidney and 1475 breast cancer patients. The median follow-up time was 39 months for kidney cancer (range 1-250) and 43 months for breast cancer patients (range 1-88). All samples were retrieved from the archives of the Institutes of Pathology, University Hospital of Hamburg, Germany, the Institute of Pathology, Clinical Center Osnabrueck, Germany, and Department of Pathology, Academic Hospital Fuerth, Germany. Tissues were fixed in 4% buffered formalin and then embedded in paraffin. The TMA manufacturing process was described earlier in detail.23,24 In brief, one tissue spot (diameter: 0.6 mm) was transmitted from a representative tumor containing donor block into an empty recipient paraffin block. The use of archived remnants of diagnostic tissues for TMA manufacturing, their analysis for research purposes, and use of patient data were according to local laws (HmbKHG, §12) and analysis had been approved by the local ethics committee (Ethics commission Hamburg, WF-049/09). All work has been carried out in compliance with the Helsinki Declaration.
Immunohistochemistry. Freshly cut TMA sections were immunostained on one day and in one experiment. Slides were deparaffinized and exposed to heat-induced antigen retrieval for 5 min in an autoclave at 121 °C in a pH 9.0 buffer. The pan-cytokeratin antibody pan-keratin (recombinant rabbit, MSVA-000R, MS Validated Antibodies, GmbH, Hamburg, Germany) was applied at 37 °C for 60 min at a dilution of 1:150. Bound antibody was then visualized using the EnVision Kit (Agilent, CA, USA; #K5007) according to the manufacturer's directions. For tumor tissues, the percentage of positive neoplastic cells was estimated, and the staining intensity was semiquantitatively recorded (0, 1 + , 2 + , 3 + ). For statistical analyses, the staining results were categorized into four groups. Tumors without any staining were considered negative. Tumors with 1 + staining intensity in ≤70% of cells and 2 + intensity in ≤30% of cells were considered weakly positive. Tumors with 1 + staining intensity in >70% of cells, 2 + intensity in 31-70%, or 3 + intensity in ≤30% were considered moderately positive. Tumors with 2 + intensity in >70% or 3 + intensity in >30% of cells werde considered strongly positive.
Statistics. Statistical calculations were performed with JMP 14 software (SAS Institute Inc., NC, USA). Contingency tables and the chi²-test were performed to search for associations between pan-keratin and tumor phenotype. Survival curves were calculated according to Kaplan-Meier. The Log-Rank test was applied to detect significant differences between groups.
Results
Technical issues. A total of 13,501 (85%) of 15,940 tumor samples were interpretable. The remaining 2439 (15%) samples were not analyzable due to the lack of unequivocal tumor cells or missing tissue spots on the TMA. For the normal tissue TMA, a sufficient number of samples was always interpretable per tissue to determine pan-keratin immunostaining.
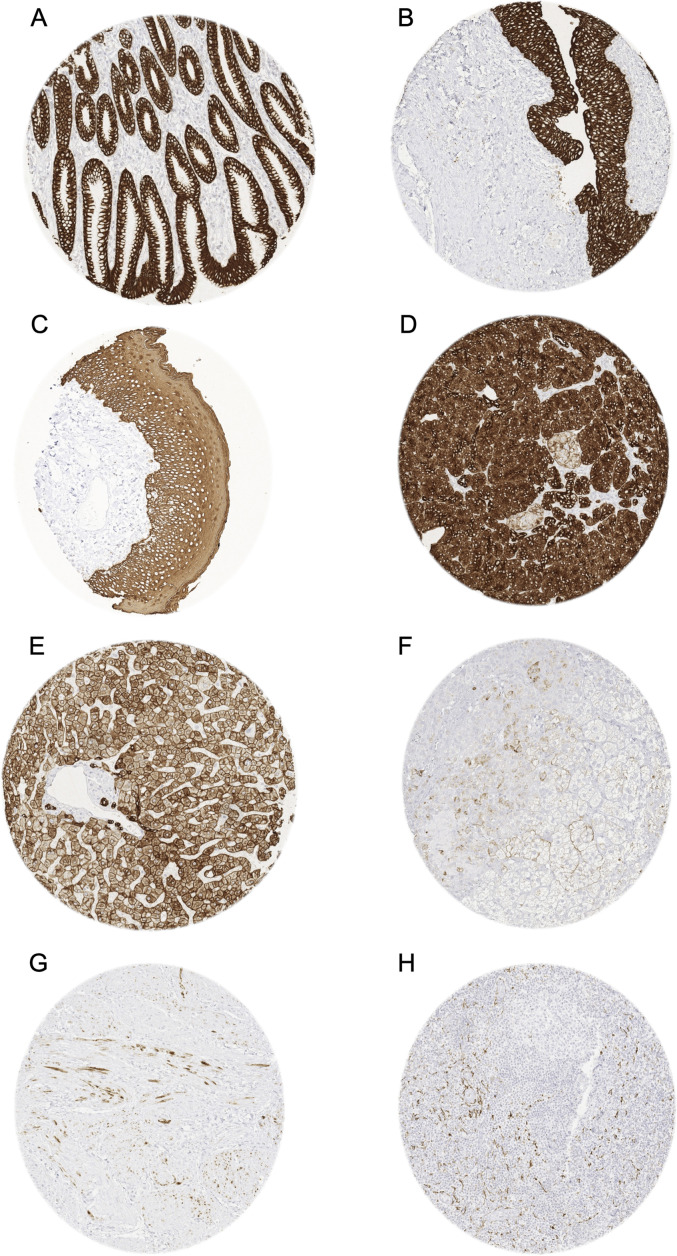
Pan-keratin in normal tissues. A positive pan-keratin immunostaining was seen in virtually all normal epithelial and mesothelial cells. The most significant exception was the adrenal cortex, where only a fraction of cells, typically arranged in groups, fascicles or sheets, stained weakly to moderately positive. A somewhat lower, but still moderate to strong staining intensity than in most other epithelial cells was seen in hepatocytes, Langerhans islets in the pancreas, and pneumocytes of the lung. In lymph nodes, tonsil, spleen, and the thymus, a delicate fibrillar staining caused by fibroblastic reticulum cells was regularly seen, mainly in the interfollicular area. Occasional pan-keratin positive spindle shaped myofibroblasts occurred in multiple organs, especially in case of degenerative or chronic inflammatory conditions. They were, for example, seen in the media of the aorta, muscular wall of the gallbladder, placental stroma, or in the ovary in the vicinity of a corpus luteum. Groups of spindle-shaped pan-keratin positive cells were also found in the myometrium. Pan-keratin immunostaining was absent in the testis, endothelial cells, the heart, striated muscle, muscular wall of the appendix, esophagus, stomach, ileum, colon, renal pelvis and urinary bladder, corpus spongiosum of the penis, ovarian stroma, fat, testis, neurohypophysis, cerebellum and cerebrum. Representative images of pan-keratin positive normal tissues are shown in Figure 1.
Figure 1.
Pan-keratin immunostaining of normal tissues. The panels show a strong pan-keratin positivity of epithelial cells of the stomach (A), the urothelium of the urinary bladder (B), and the squamous epithelium of the oral cavity (C). In the pancreas, acinar cells show a strong staining while cells of islets of Langerhans stain only weakly (D). In the liver pan-keratin staining is variable (weak to moderate) in hepatocytes but strong in bile ducts (E). In the adrenal gland, only a subset of cortical cells shows a weak staining (F). In the myometrium, groups of spindle-shaped pan-keratin positive cells are found (G). In lymph nodes, a delicate fibrillar staining caused by fibroblastic reticulum cells occurs mainly in the interfollicular area (H).
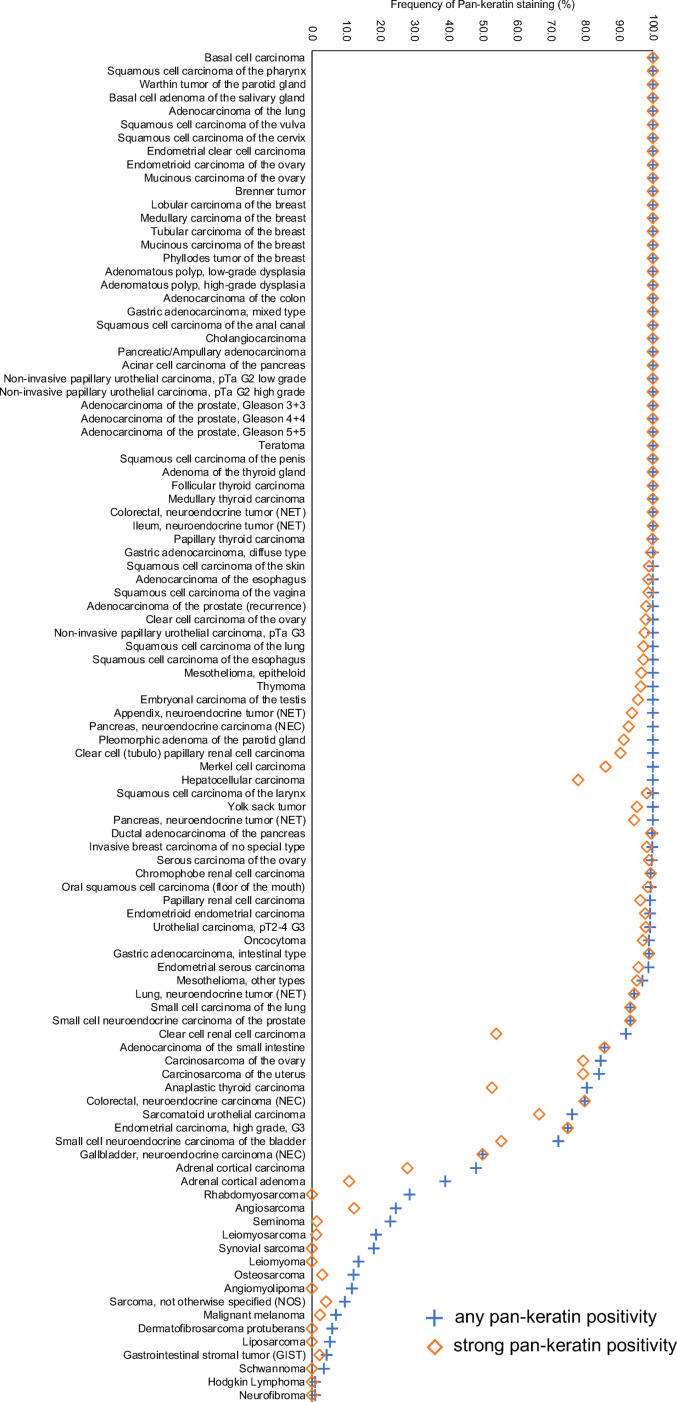
Pan-keratin in tumors. A membranous and cytoplasmic pan-keratin immunostaining was observed in 11,323 (84%) of 13,501 analyzable tumors, including 79% with strong, 2.1% with moderate, and 2.6% with weak staining intensity. At least an occasional weak pan-keratin positivity could be detected in 101 of 121 (84%) different tumor types and tumor subtypes (Table 1). Among 75 epithelial tumor entities, the pan-keratin positivity rate was 100% in 50 (67%) and 98 – 99.9% in 12 categories (16%). These tumors included almost all adenocarcinomas, squamous cell and urothelial carcinomas. Only 17 of 121 tumor entities (13%) had a pan-keratin positivity rate between 25% and 98%. This group mainly included tumors with mixed differentiation, endocrine/neuroendocrine tumors, renal cell carcinomas, adrenocortical tumors, and particularly poorly differentiated carcinoma subtypes (anaplastic, small cell, sarcomatoid). The tumors with a pan-keratin positivity in the range of 0.9%-25% included 15 tumor entities. Most of these were of mesenchymal origin and often showed a weaker staining than epithelial neoplasms. The 20 (17%) tumor entities that were always pan-keratin negative were all of mesenchymal and hemato-lymphatic origin. Representative images of pan-keratin positive tumors are shown in Figure 2. A graphical representation of a ranking order of pan-keratin positive tumors and the observed staining intensities in these tumors is given in Figure 3. These data also show, that most tumor entities with 90%-100% positive tumors showed a strong pan-keratin immunostaining while the staining intensity decreased in tumors entities with lower positivity rates. That significant pan-keratin staining can occur in sarcomas was also confirmed by using a second independent pan-keratin antibody (Supplementary Figure 1).
Table 1.
Pan-Keratin Immunostaining in Human Tumors.
| Pan-keratin immunostaining result | |||||||
|---|---|---|---|---|---|---|---|
| Tumor entity | on TMA (n) | analyzable (n) | neg. (%) | weak (%) | mod. (%) | strong (%) | |
| Tumors of the skin | Basal cell carcinoma | 88 | 82 | 0.0 | 0.0 | 0.0 | 100.0 |
| Benign nevus | 29 | 25 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Squamous cell carcinoma of the skin | 90 | 88 | 0.0 | 1.1 | 0.0 | 98.9 | |
| Malignant melanoma | 48 | 43 | 93.0 | 0.0 | 4.7 | 2.3 | |
| Merkel cell carcinoma | 46 | 43 | 0.0 | 2.3 | 11.6 | 86.0 | |
| Tumors of the head and neck | Squamous cell carcinoma of the larynx | 110 | 104 | 0.0 | 0.0 | 1.9 | 98.1 |
| Squamous cell carcinoma of the pharynx | 60 | 58 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Oral squamous cell carcinoma (floor of the mouth) | 130 | 125 | 0.8 | 0.8 | 0.0 | 98.4 | |
| Pleomorphic adenoma of the parotid gland | 50 | 35 | 0.0 | 0.0 | 8.6 | 91.4 | |
| Warthin tumor of the parotid gland | 49 | 45 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Basal cell adenoma of the salivary gland | 15 | 15 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Tumors of the lung, pleura and thymus | Adenocarcinoma of the lung | 246 | 176 | 0.0 | 0.0 | 0.0 | 100.0 |
| Squamous cell carcinoma of the lung | 130 | 73 | 0.0 | 0.0 | 2.7 | 97.3 | |
| Small cell carcinoma of the lung | 20 | 15 | 6.7 | 0.0 | 0.0 | 93.3 | |
| Mesothelioma, epitheloid | 39 | 29 | 0.0 | 0.0 | 3.4 | 96.6 | |
| Mesothelioma, other types | 76 | 63 | 3.2 | 0.0 | 1.6 | 95.2 | |
| Thymoma | 29 | 28 | 0.0 | 0.0 | 3.6 | 96.4 | |
| Tumors of the female genital tract | Squamous cell carcinoma of the vagina | 78 | 73 | 0.0 | 0.0 | 1.4 | 98.6 |
| Squamous cell carcinoma of the vulva | 130 | 124 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Squamous cell carcinoma of the cervix | 130 | 126 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Endometrioid endometrial carcinoma | 236 | 220 | 0.9 | 0.5 | 0.9 | 97.7 | |
| Endometrial serous carcinoma | 82 | 70 | 1.4 | 0.0 | 2.9 | 95.7 | |
| Carcinosarcoma of the uterus | 48 | 44 | 15.9 | 2.3 | 2.3 | 79.5 | |
| Endometrial carcinoma, high grade, G3 | 13 | 12 | 25.0 | 0.0 | 0.0 | 75.0 | |
| Endometrial clear cell carcinoma | 8 | 8 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Endometrioid carcinoma of the ovary | 110 | 102 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Serous carcinoma of the ovary | 559 | 509 | 0.4 | 0.2 | 0.6 | 98.8 | |
| Mucinous carcinoma of the ovary | 96 | 77 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Clear cell carcinoma of the ovary | 50 | 45 | 0.0 | 0.0 | 2.2 | 97.8 | |
| Carcinosarcoma of the ovary | 47 | 39 | 15.4 | 5.1 | 0.0 | 79.5 | |
| Brenner tumor | 9 | 9 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Tumors of the breast | Invasive breast carcinoma of no special type | 1391 | 1152 | 0.3 | 0.6 | 1.0 | 98.1 |
| Lobular carcinoma of the breast | 294 | 241 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Medullary carcinoma of the breast | 26 | 26 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Tubular carcinoma of the breast | 27 | 21 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Mucinous carcinoma of the breast | 58 | 44 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Phyllodes tumor of the breast | 50 | 43 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Tumors of the digestive system | Adenomatous polyp, low-grade dysplasia | 50 | 50 | 0.0 | 0.0 | 0.0 | 100.0 |
| Adenomatous polyp, high-grade dysplasia | 50 | 46 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Adenocarcinoma of the colon | 1932 | 1417 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Adenocarcinoma of the small intestine | 10 | 7 | 14.3 | 0.0 | 0.0 | 85.7 | |
| Gastric adenocarcinoma, diffuse type | 226 | 158 | 0.0 | 0.0 | 0.6 | 99.4 | |
| Gastric adenocarcinoma, intestinal type | 224 | 162 | 1.2 | 0.0 | 0.0 | 98.8 | |
| Gastric adenocarcinoma, mixed type | 62 | 59 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Adenocarcinoma of the esophagus | 133 | 77 | 0.0 | 0.0 | 1.3 | 98.7 | |
| Squamous cell carcinoma of the esophagus | 124 | 71 | 0.0 | 1.4 | 1.4 | 97.2 | |
| Squamous cell carcinoma of the anal canal | 91 | 87 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Cholangiocarcinoma | 114 | 106 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Hepatocellular carcinoma | 50 | 50 | 0.0 | 10.0 | 12.0 | 78.0 | |
| Ductal adenocarcinoma of the pancreas | 662 | 551 | 0.2 | 0.0 | 0.4 | 99.5 | |
| Pancreatic/Ampullary adenocarcinoma | 119 | 84 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Acinar cell carcinoma of the pancreas | 15 | 14 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Gastrointestinal stromal tumor (GIST) | 50 | 47 | 95.7 | 0.0 | 2.1 | 2.1 | |
| Tumors of the urinary system | Non-invasive papillary urothelial carcinoma, pTa G2 low grade | 177 | 147 | 0.0 | 0.0 | 0.0 | 100.0 |
| Non-invasive papillary urothelial carcinoma, pTa G2 high grade | 141 | 122 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Non-invasive papillary urothelial carcinoma, pTa G3 | 187 | 118 | 0.0 | 0.0 | 2.5 | 97.5 | |
| Urothelial carcinoma, pT2-4 G3 | 1207 | 862 | 0.9 | 0.3 | 0.9 | 97.8 | |
| Small cell neuroendocrine carcinoma of the bladder | 18 | 18 | 27.8 | 16.7 | 0.0 | 55.6 | |
| Sarcomatoid urothelial carcinoma | 25 | 21 | 23.8 | 4.8 | 4.8 | 66.7 | |
| Clear cell renal cell carcinoma | 858 | 791 | 8.0 | 18.6 | 19.5 | 54.0 | |
| Papillary renal cell carcinoma | 255 | 236 | 0.8 | 1.3 | 1.7 | 96.2 | |
| Clear cell (tubulo) papillary renal cell carcinoma | 21 | 21 | 0.0 | 4.8 | 4.8 | 90.5 | |
| Chromophobe renal cell carcinoma | 131 | 126 | 0.8 | 0.0 | 0.0 | 99.2 | |
| Oncocytoma | 177 | 168 | 1.2 | 0.6 | 1.2 | 97.0 | |
| Tumors of the male genital organs | Adenocarcinoma of the prostate, Gleason 3 + 3 | 83 | 83 | 0.0 | 0.0 | 0.0 | 100.0 |
| Adenocarcinoma of the prostate, Gleason 4 + 4 | 80 | 78 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Adenocarcinoma of the prostate, Gleason 5 + 5 | 85 | 85 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Adenocarcinoma of the prostate (recurrence) | 261 | 252 | 0.0 | 0.0 | 2.0 | 98.0 | |
| Small cell neuroendocrine carcinoma of the prostate | 17 | 15 | 6.7 | 0.0 | 0.0 | 93.3 | |
| Seminoma | 621 | 584 | 77.1 | 17.1 | 4.5 | 1.4 | |
| Embryonal carcinoma of the testis | 50 | 46 | 0.0 | 0.0 | 4.3 | 95.7 | |
| Yolk sac tumor | 50 | 42 | 0.0 | 0.0 | 4.8 | 95.2 | |
| Teratoma | 50 | 24 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Squamous cell carcinoma of the penis | 80 | 78 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Tumors of endocrine organs | Adenoma of the thyroid gland | 114 | 106 | 0.0 | 0.0 | 0.0 | 100.0 |
| Papillary thyroid carcinoma | 392 | 366 | 0.0 | 0.3 | 0.0 | 99.7 | |
| Follicular thyroid carcinoma | 158 | 152 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Medullary thyroid carcinoma | 107 | 103 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Anaplastic thyroid carcinoma | 45 | 36 | 19.4 | 13.9 | 13.9 | 52.8 | |
| Adrenal cortical adenoma | 50 | 46 | 60.9 | 13.0 | 15.2 | 10.9 | |
| Adrenal cortical carcinoma | 26 | 25 | 52.0 | 16.0 | 4.0 | 28.0 | |
| Phaeochromocytoma | 50 | 47 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Appendix, neuroendocrine tumor | 22 | 16 | 0.0 | 0.0 | 6.3 | 93.8 | |
| Colorectal, neuroendocrine tumor | 11 | 11 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Ileum, neuroendocrine tumor | 49 | 46 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Lung, neuroendocrine tumor | 19 | 18 | 5.6 | 0.0 | 0.0 | 94.4 | |
| Pancreas, neuroendocrine tumor | 98 | 91 | 0.0 | 1.1 | 4.4 | 94.5 | |
| Colorectal, neuroendocrine carcinoma | 12 | 10 | 20.0 | 0.0 | 0.0 | 80.0 | |
| Gallbladder, neuroendocrine carcinoma | 4 | 4 | 50.0 | 0.0 | 0.0 | 50.0 | |
| Pancreas, neuroendocrine carcinoma | 14 | 14 | 0.0 | 7.1 | 0.0 | 92.9 | |
| Tumors of haemotopoetic and lymphoid tissues | Hodgkin Lymphoma | 103 | 103 | 99.0 | 1.0 | 0.0 | 0.0 |
| Non-Hodgkin Lymphoma | 62 | 61 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Small lymphocytic lymphoma, B-cell type | 50 | 50 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Diffuse large B cell lymphoma | 114 | 114 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Follicular lymphoma | 88 | 88 | 100.0 | 0.0 | 0.0 | 0.0 | |
| T-cell Non Hodgkin lymphoma | 24 | 24 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Mantle cell lymphoma | 18 | 18 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Marginal zone lymphoma | 16 | 16 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Diffuse large B-cell lymphoma in the testis | 16 | 16 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Burkitt lymphoma | 5 | 3 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Tumors of soft tissue and bone | Tenosynovial giant cell tumor | 45 | 45 | 100.0 | 0.0 | 0.0 | 0.0 |
| Granular cell tumor | 53 | 46 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Leiomyoma | 50 | 44 | 86.4 | 13.6 | 0.0 | 0.0 | |
| Leiomyosarcoma | 87 | 85 | 81.2 | 12.9 | 4.7 | 1.2 | |
| Liposarcoma | 132 | 116 | 94.8 | 3.4 | 1.7 | 0.0 | |
| Malignant peripheral nerve sheath tumor | 13 | 13 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Myofibrosarcoma | 26 | 26 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Angiosarcoma | 73 | 65 | 75.4 | 9.2 | 3.1 | 12.3 | |
| Angiomyolipoma | 91 | 86 | 88.4 | 10.5 | 1.2 | 0.0 | |
| Dermatofibrosarcoma protuberans | 21 | 17 | 94.1 | 5.9 | 0.0 | 0.0 | |
| Ganglioneuroma | 14 | 12 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Kaposi sarcoma | 8 | 5 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Neurofibroma | 117 | 107 | 99.1 | 0.9 | 0.0 | 0.0 | |
| Sarcoma, not otherwise specified (NOS) | 75 | 73 | 90.4 | 4.1 | 1.4 | 4.1 | |
| Paraganglioma | 41 | 41 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Ewing Sarcoma | 23 | 15 | 100.0 | 0.0 | 0.0 | 0.0 | |
| Rhabdomyosarcoma | 7 | 7 | 71.4 | 28.6 | 0.0 | 0.0 | |
| Schwannoma | 121 | 117 | 96.6 | 3.4 | 0.0 | 0.0 | |
| Synovial sarcoma | 12 | 11 | 81.8 | 18.2 | 0.0 | 0.0 | |
| Osteosarcoma | 43 | 33 | 87.9 | 0.0 | 9.1 | 3.0 | |
| Chondrosarcoma | 38 | 18 | 100.0 | 0.0 | 0.0 | 0.0 | |
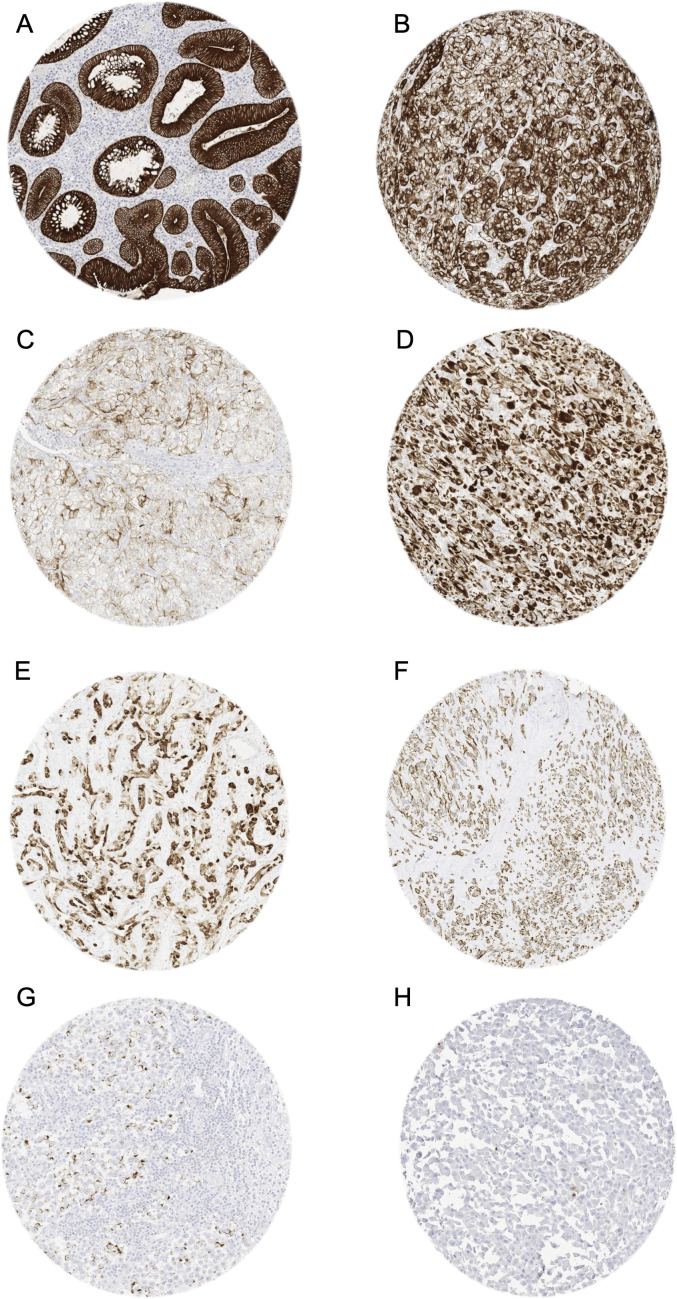
Figure 2.
Pan-keratin immunostaining in tumors. The panels show a cytoplasmatic pan-keratin immunostaining of variable intensity in samples from a colorectal adenoma (A: strong staining), two clear cell renal cell carcinomas (B: strong; C: weak), a sarcoma NOS (D: strong), an angiosarcoma (E: strong), a gastrointestinal stromal tumor (GIST) (F: moderate), and a seminoma (G: weak). Pan-keratin immunostaining is lacking in an adrenocortical carcinoma (H).
Figure 3.
Ranking order of pan-keratin immunostaining in tumors. Both the frequency of positive tumors (blue cross) and the frequency of strongly positive tumors (orange rhombus) are shown.
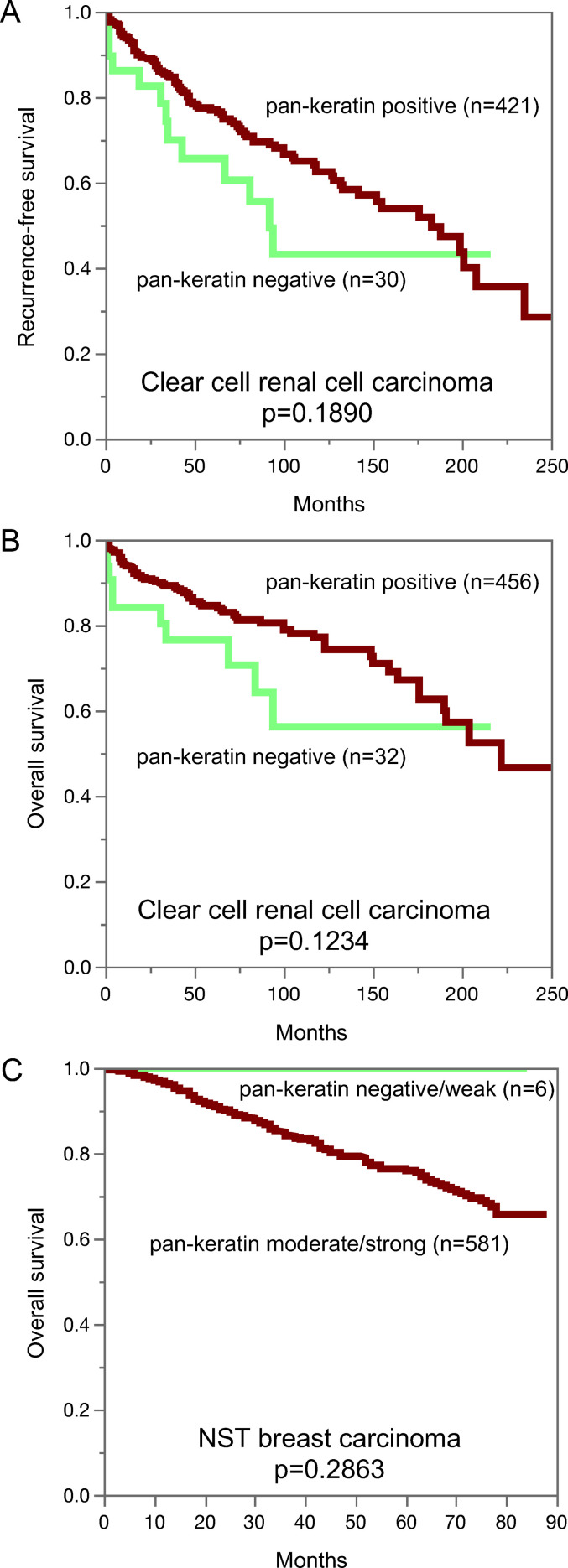
Pan-keratin immunostaining, tumor phenotype, and prognosis. The relationship between pan-keratin immunostaining and clinico-pathological data could be analyzed in breast and kidney cancer (Table 2). Reduced or absent pan-keratin immunostaining was associated with high UICC stage (p = 0.001), high Thoenes grade (p = 0.0183), high Fuhrman grade (p = 0.0049), advanced tumor stage (p < 0.0001) as well as lymph node metastasis (p = 0.0114) in clear cell renal cell carcinomas, with high UICC stage (p = 0.0062) and advanced pT stage (p = 0.0007) in papillary renal cell carcinoma, and with advanced stage (p = 0.0023), high grade (p = 0.0005) as well as loss of estrogen receptor (ER) and progesteron receptor (PR) expression and a triple-negative status (p < 0.0001 each) in invasive breast carcinoma of no special type (NST). Despite a tendency towards shortened recurrence free (Figure 4a; p = 0.1890) and overall survival (Figure 4b, p = 0.1234) for pan-keratin negative clear cell renal cell carcinomas, this difference did not reach statistical significance. Reduced pan-keratin immunostaining was not statistically linked to poor outcome in NST (Figure 4c, p = 0.2863) but there were only 6 patients with reduced pan-keratin staining for which clinical follow-up data were available.
Table 2.
Pan-Keratin Immunostaining and Tumor Phenotype in Breast and Clear Cell Renal and Papillary Renal Cell Cancer.
| Pan-keratin IHC result | ||||||||
|---|---|---|---|---|---|---|---|---|
| n | negative (%) | weak (%) | moderate (%) | strong (%) | P | |||
| Breast carcinoma of no special type | Primary Tumor | pT1 | 547 | 0.5 | 0.5 | 0.0 | 98.9 | 0.0023 |
| pT2 | 400 | 0.0 | 1.0 | 1.5 | 97.5 | |||
| pT3-4 | 89 | 0.0 | 0.0 | 3.4 | 96.6 | |||
| Grade | G1 | 171 | 0.6 | 0.6 | 0.0 | 98.8 | 0.0005 | |
| G2 | 538 | 0.2 | 0.4 | 0.0 | 99.4 | |||
| G3 | 366 | 0.3 | 1.1 | 2.7 | 95.9 | |||
| Regional Lymph Nodes | pN0 | 444 | 0.0 | 0.7 | 0.9 | 98.4 | 0.7401 | |
| pN1 | 217 | 0.0 | 0.5 | 0.9 | 98.6 | |||
| pN2 | 72 | 0.0 | 0.0 | 0.0 | 100.0 | |||
| pN3 | 50 | 0.0 | 0.0 | 0.0 | 100.0 | |||
| HER2 status | negative | 840 | 0.4 | 0.6 | 1.1 | 98.0 | 0.5544 | |
| positive | 114 | 0.0 | 0.0 | 0.9 | 99.1 | |||
| ER status | negative | 198 | 0.5 | 1.5 | 4.5 | 93.4 | <0.0001 | |
| positive | 713 | 0.1 | 0.3 | 0.1 | 99.4 | |||
| PR status | negative | 381 | 0.3 | 0.8 | 2.6 | 96.3 | <0.0001 | |
| positive | 564 | 0.2 | 0.4 | 0.0 | 99.5 | |||
| Triple negative | no | 749 | 0.1 | 0.3 | 0.3 | 99.3 | <0.0001 | |
| yes | 133 | 0.8 | 2.3 | 6.0 | 91.0 | |||
| Clear cell renal cell carcinoma | ||||||||
| ISUP grade | 1 | 236 | 7.6 | 17.8 | 21.2 | 53.4 | 0.1907 | |
| 2 | 248 | 7.7 | 17.3 | 21.0 | 54.0 | |||
| 3 | 206 | 7.8 | 20.9 | 19.4 | 51.9 | |||
| 4 | 42 | 21.4 | 26.2 | 9.5 | 42.9 | |||
| Fuhrmann grade | 1 | 38 | 7.9 | 5.3 | 15.8 | 71.1 | 0.0049 | |
| 2 | 443 | 7.4 | 18.1 | 21.2 | 53.3 | |||
| 3 | 210 | 7.6 | 21.0 | 21.0 | 50.5 | |||
| 4 | 50 | 20.0 | 26.0 | 6.0 | 48.0 | |||
| Thoenes grade | 1 | 268 | 6.3 | 17.2 | 20.1 | 56.3 | 0.0183 | |
| 2 | 406 | 8.6 | 18.0 | 21.4 | 52.0 | |||
| 3 | 67 | 14.9 | 29.9 | 9.0 | 46.3 | |||
| UICC stage | 1 | 340 | 3.5 | 15.0 | 17.9 | 63.5 | 0.001 | |
| 2 | 35 | 8.6 | 8.6 | 22.9 | 60.0 | |||
| 3 | 91 | 14.3 | 24.2 | 17.6 | 44.0 | |||
| 4 | 74 | 13.5 | 14.9 | 21.6 | 50.0 | |||
| Primary Tumor | 1 | 446 | 4.9 | 17.0 | 17.9 | 60.1 | <0.0001 | |
| 2 | 76 | 10.5 | 19.7 | 26.3 | 43.4 | |||
| 3-4 | 214 | 15.0 | 22.4 | 21.0 | 41.6 | |||
| Regional Lymph Nodes | 0 | 127 | 9.4 | 22.0 | 22.0 | 46.5 | 0.0114 | |
| ≥1 | 18 | 16.7 | 0.0 | 11.1 | 72.2 | |||
| Distant Metastasis | 0 | 113 | 6.2 | 15.9 | 22.1 | 55.8 | 0.192 | |
| ≥1 | 76 | 15.8 | 14.5 | 22.4 | 47.4 | |||
| Papillary renal cell carcinoma | ||||||||
| ISUP grade | 1 | 40 | 0.0 | 0.0 | 2.5 | 97.5 | 0.933 | |
| 2 | 92 | 1.1 | 2.2 | 1.1 | 95.7 | |||
| 3 | 58 | 1.7 | 1.7 | 3.4 | 93.1 | |||
| 4 | 1 | 0.0 | 0.0 | 0.0 | 100.0 | |||
| Fuhrmann grade | 1 | 2 | 0.0 | 0.0 | 0.0 | 100.0 | 0.9965 | |
| 2 | 130 | 0.8 | 1.5 | 1.5 | 96.2 | |||
| 3 | 56 | 1.8 | 1.8 | 3.6 | 92.9 | |||
| 4 | 3 | 0.0 | 0.0 | 0.0 | 100.0 | |||
| Thoenes grade | 1 | 49 | 0.0 | 0.0 | 2.0 | 98.0 | 0.664 | |
| 2 | 133 | 1.5 | 2.3 | 2.3 | 94.0 | |||
| 3 | 9 | 0.0 | 0.0 | 0.0 | 100.0 | |||
| UICC stage | 1 | 104 | 0.0 | 1.0 | 0.0 | 99.0 | 0.0062 | |
| 2 | 18 | 0.0 | 0.0 | 5.6 | 94.4 | |||
| 3 | 5 | 20.0 | 0.0 | 40.0 | 40.0 | |||
| 4 | 12 | 0.0 | 0.0 | 0.0 | 100.0 | |||
| Primary Tumor | 1 | 133 | 0.0 | 0.8 | 0.0 | 99.2 | 0.0007 | |
| 2 | 37 | 2.7 | 2.7 | 2.7 | 91.9 | |||
| 3-4 | 15 | 6.7 | 6.7 | 20.0 | 66.7 | |||
| Regional Lymph Nodes | 0 | 19 | 0.0 | 0.0 | 5.3 | 94.7 | 0.423 | |
| ≥1 | 7 | 0.0 | 0.0 | 0.0 | 100.0 | |||
| Distant Metastasis | 0 | 27 | 0.0 | 0.0 | 3.7 | 96.3 | 0.4935 | |
| ≥1 | 7 | 0.0 | 0.0 | 0.0 | 100.0 | |||
Figure 4.
Pan-keratin immunostaining and recurrence-free survival (A) and overall survival (B) in clear cell renal cancer. Pan-keratin immunostaining in NST breast cancer and overall survival (C).
Discussion
The standardized analysis of 13,501 tumors provided a comprehensive overview on pan-keratin immunostaining in different tumor types. That the graphical representation of the frequencies of pan-keratin immunostaining among 121 different tumor entities resulted in an S-shaped curve reflects the fact that intense pan-keratin immunostaining is common in epithelial neoplasms while non-epithelial tumors are usually pan-keratin negative.
Among epithelial tumor entities, 50 of 75 (67%) showed Pan-keratin positivity in 100% of tumors and 12 (16%) were positive in ≥98% of tumors. These entities include virtually all important types of adenocarcinomas and squamous cell carcinomas. We assume that a fraction of the few negative tumors in these cancer types may be caused by technical issues. Some unexpected negative immunostaining results always occur in TMAs because not all tissues are properly fixed in all areas. 25 Unequal immunostaining in tissues can results in an immunostaining gradient across a tissue block and can thus cause false negative immunostaining, if TMA cores are taken from areas with poor reactivity. 26 It is likely that – in at least a fraction of these tumors - some immunoreactive areas will be found if larger tissue samples, perhaps derived from different blocks, are analyzed.
The group of tumor entities with a pan-keratin positivity in 25% to 98% of analyzed tumors made up for only 17 (13%) of analyzed entities. Most of these tumors could be categorized into the following 5 groups: tumors with mixed differentiation, endocrine/neuroendocrine tumors, kidney tumors, adrenocortical tumors, and particularly poorly differentiated carcinoma subtypes. The group of tumors with mixed epithelial-mesenchymal differentiation includes carcinosarcoma of the uterus and the ovary, phyllodes tumor of the breast, teratoma of the testis, and malignant mesothelioma. In these tumors, epithelial but not mesenchymal tumor areas are pan-keratin positive. The pan-keratin TMA result therefore depends on whether epithelial components are present in the TMA spot or not. The rather low positivity rate in kidney cancers reflects the fact that these tumors have low cytokeratin levels and tend to express vimentin instead.27,28
For adrenocortical, neuroendocrine and endocrine tumors, the intermediate positivity rate appears to mirror the rather low pan-keratin immunostaining in corresponding normal cells. Hepatocellular carcinoma, another tumor for which reduced cytokeratin has often been reported 5,29,30 was always pan-keratin positive in this study, although staining was only weak or moderate in 22% of tumors.
Very poorly differentiated cancers, such as small cell and sarcomatoid cancers as well as anaplastic cancers of the thyroid, often showed lower pan-keratin immunostaining rates as compared to corresponding normal tissues and more differentiated tumors from these organs. This may reflect that a reduced expressions of keratins is a feature of tumors dedifferentiation that can occur during cancer progression. This interpretation is also in line with our findings in kidney and breast carcinomas showing significant associations between reduced pan-keratin immunostaining and several unfavorable phenotypic tumor features. Other investigators had previously also described significant correlations between reduced expression of specific keratins 31–34 or reduced pan-keratin immunostaining 35 and poor patient prognosis or unfavorable tumor phenotype in various tumor types. A reduced expression of keratins in tumors derived from keratin positive progenitor cells is likely to represent a feature of cellular dedifferentiation which regularly goes along with cancer progression. Our data also emphasize that pan-keratin immunostaining is not uncommon in mesenchymal tumors although the expression levels are usually lower in these neoplasms as compared to carcinomas. In this study, pan-keratin immunostaining was observed in 13 mesenchymal tumor entities with highest rates in rhabdomyosarcomas (29%) and angiosarcomas (25%). These findings are in line with earlier studies describing keratin expression in 20%, 36%, 100% of rhabdomyosarcomas 36–38 and in 20%, 33%, and 88% of angiosarcomas.19,20,39 Given the significant pan-keratin staining in the majority of cells is several sarcomas of different types, cytokeratin positivity – even if strong – should not automatically lead to a diagnosis of “sarcomatoid carcinoma”.
In summary, our data show that pan-keratin can consistently be detected in the vast majority of epithelial tumors but also identify renal cell, hepatocellular, adrenocortical and neuroendocrine neoplasms as tumors lacking pan-keratin immunostaining in a fraction of tumors. Moreover, pan-keratin immunostaining - usually at lower levels - can also occur in a broad range of mesenchymal tumors.
Supplemental Material
Supplemental material, sj-pdf-1-ijs-10.1177_10668969221117243 for Pan-keratin Immunostaining in Human Tumors: A Tissue Microarray Study of 15,940 Tumors by Anne Menz, Natalia Gorbokon, Florian Viehweger, Maximilian Lennartz, Claudia Hube-Magg, Lisa Hornsteiner, Martina Kluth, Cosima Völkel, Andreas M. Luebke, Christoph Fraune, Ria Uhlig, Sarah Minner, David Dum, Doris Höflmayer, Guido Sauter, Ronald Simon, Eike Burandt, Till S. Clauditz, Patrick Lebok, Frank Jacobsen, Stefan Steurer, Till Krech, Andreas H. Marx and Christian Bernreuther in International Journal of Surgical Pathology
Supplemental material, sj-docx-2-ijs-10.1177_10668969221117243 for Pan-keratin Immunostaining in Human Tumors: A Tissue Microarray Study of 15,940 Tumors by Anne Menz, Natalia Gorbokon, Florian Viehweger, Maximilian Lennartz, Claudia Hube-Magg, Lisa Hornsteiner, Martina Kluth, Cosima Völkel, Andreas M. Luebke, Christoph Fraune, Ria Uhlig, Sarah Minner, David Dum, Doris Höflmayer, Guido Sauter, Ronald Simon, Eike Burandt, Till S. Clauditz, Patrick Lebok, Frank Jacobsen, Stefan Steurer, Till Krech, Andreas H. Marx and Christian Bernreuther in International Journal of Surgical Pathology
Acknowledgements
We are grateful to Melanie Witt, Inge Brandt, Maren Eisenberg, and Sünje Seekamp for excellent technical assistance.
Footnotes
Contributiors: AM, RS, GS, CB: contributed to conception, design, data collection, data analysis and manuscript writing. NG, FV, ML, AML, EB, DH, CF, PL, RU, FJ, SM, TSC, DD: participated in pathology data analysis and data interpretation. TK, AHM collection of samples
AM, CB: immunohistochemistry analysis. RS, LH, MK, CHM: data analysis. AM, RS, GS, CB: study supervision. All authors agree to be accountable for the content of the work.
Data Availability Statement: All data generated or analyzed during this study are included in this published article
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The Pan-keratin antibody clone MSVA-000R was received from MS Validated Antibodies GmbH (owned by a family member of GS).
Ethical Approval: The use of archived remnants of diagnostic tissues for TMA manufacturing, their analysis for research purposes, and use of patient data were according to local laws (HmbKHG, §12) and analysis had been approved by the local ethics committee (Ethics commission Hamburg, WF-049/09). All work has been carried out in compliance with the Helsinki Declaration.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: Not applicable, because this article does not contain any studies with human or animal subjects.
Trial Registration: Not applicable, because this article does not contain any clinical trials.
ORCID iDs: Ronald Simon https://orcid.org/0000-0003-0158-4258
Eike Burandt https://orcid.org/0000-0002-5705-9084
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Badzio A, Czapiewski P, Gorczynski A, et al. Prognostic value of broad-spectrum keratin clones AE1/AE3 and CAM5.2 in small cell lung cancer patients undergoing pulmonary resection. Acta Biochim Pol. Feb 22 2019;66(1):111‐114. doi: 10.18388/abp.2018_2773 [DOI] [PubMed] [Google Scholar]
- 2.Sangoi AR, Fujiwara M, West RB, et al. Immunohistochemical distinction of primary adrenal cortical lesions from metastatic clear cell renal cell carcinoma: a study of 248 cases. Am J Surg Pathol. May 2011;35(5):678‐686. doi: 10.1097/PAS.0b013e3182152629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau SK, Prakash S, Geller SA, Alsabeh R. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum Pathol. Dec 2002;33(12):1175‐1181. doi: 10.1053/hupa.2002.130104 [DOI] [PubMed] [Google Scholar]
- 4.Cote RJ, Cordon-Cardo C, Reuter VE, Rosen PP. Immunopathology of adrenal and renal cortical tumors. Coordinated change in antigen expression is associated with neoplastic conversion in the adrenal cortex. Am J Pathol. May 1990;136(5):1077‐1084. [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen WN, Boitnott JK, Kuhajda FP. Immunoperoxidase staining as a diagnostic aid for hepatocellular carcinoma. Mod Pathol. Jan 1989;2(1):8‐12. [PubMed] [Google Scholar]
- 6.Wick MR, Cherwitz DL, McGlennen RC, Dehner LP. Adrenocortical carcinoma. An immunohistochemical comparison with renal cell carcinoma. Am J Pathol. Feb 1986;122(2):343‐352. [PMC free article] [PubMed] [Google Scholar]
- 7.Lin XY, Liu Y, Zhang Y, Yu JH, Wang EH. The co-expression of cytokeratin and p63 in epithelioid angiosarcoma of the parotid gland: a diagnostic pitfall. Diagn Pathol. Sep 3 2012;7(1):118. doi: 10.1186/1746-1596-7-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miettinen M, Fetsch JF. Distribution of keratins in normal endothelial cells and a spectrum of vascular tumors: implications in tumor diagnosis. Hum Pathol. Sep 2000;31(9):1062‐1067. doi: 10.1053/hupa.2000.9843 [DOI] [PubMed] [Google Scholar]
- 9.Gu M, Antonescu CR, Guiter G, Huvos AG, Ladanyi M, Zakowski MF. Cytokeratin immunoreactivity in Ewing's sarcoma: prevalence in 50 cases confirmed by molecular diagnostic studies. Am J Surg Pathol. Mar 2000;24(3):410‐416. doi: 10.1097/00000478-200003000-00010 [DOI] [PubMed] [Google Scholar]
- 10.Iwata J, Fletcher CD. Immunohistochemical detection of cytokeratin and epithelial membrane antigen in leiomyosarcoma: a systematic study of 100 cases. Pathol Int. Jan 2000;50(1):7‐14. doi: 10.1046/j.1440-1827.2000.01001.x [DOI] [PubMed] [Google Scholar]
- 11.Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. Jun 1998;22(6):683‐697. doi: 10.1097/00000478-199806000-00005 [DOI] [PubMed] [Google Scholar]
- 12.Miettinen M. Immunoreactivity for cytokeratin and epithelial membrane antigen in leiomyosarcoma. Arch Pathol Lab Med. Jun 1988;112(6):637‐640. [PubMed] [Google Scholar]
- 13.Lapinski JE, Chen L, Zhou M. Distinguishing clear cell renal cell carcinoma, retroperitoneal paraganglioma, and adrenal cortical lesions on limited biopsy material: utility of immunohistochemical markers. Appl Immunohistochem Mol Morphol. Oct 2010;18(5):414‐421. doi: 10.1097/PAI.0b013e3181ddf7b9 [DOI] [PubMed] [Google Scholar]
- 14.Pinkus GS, Etheridge CL, O'Connor EM. Are keratin proteins a better tumor marker than epithelial membrane antigen? A comparative immunohistochemical study of various paraffin-embedded neoplasms using monoclonal and polyclonal antibodies. Am J Clin Pathol. Mar 1986;85(3):269‐277. doi: 10.1093/ajcp/85.3.269 [DOI] [PubMed] [Google Scholar]
- 15.He H, Trpkov K, Martinek P, et al. "High-grade oncocytic renal tumor": morphologic, immunohistochemical, and molecular genetic study of 14 cases. Virchows Arch. Dec 2018;473(6):725‐738. doi: 10.1007/s00428-018-2456-4 [DOI] [PubMed] [Google Scholar]
- 16.Hayes M, Peckova K, Martinek P, et al. Molecular-genetic analysis is essential for accurate classification of renal carcinoma resembling Xp11.2 translocation carcinoma. Virchows Arch. Mar 2015;466(3):313‐322. doi: 10.1007/s00428-014-1702-7 [DOI] [PubMed] [Google Scholar]
- 17.Al-Obaidy KI, Eble JN, Cheng L, et al. Papillary renal neoplasm with reverse polarity: a morphologic, immunohistochemical, and molecular study. Am J Surg Pathol. Aug 2019;43(8):1099‐1111. doi: 10.1097/PAS.0000000000001288 [DOI] [PubMed] [Google Scholar]
- 18.Hes O, Brunelli M, Michal M, et al. Oncocytic papillary renal cell carcinoma: a clinicopathologic, immunohistochemical, ultrastructural, and interphase cytogenetic study of 12 cases. Ann Diagn Pathol. Jun 2006;10(3):133‐139. doi: 10.1016/j.anndiagpath.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 19.Nakashima Y, Inamura K, Ninomiya H, et al. Frequent expression of conventional endothelial markers in pleural mesothelioma: usefulness of claudin-5 as well as combined traditional markers to distinguish mesothelioma from angiosarcoma. Lung Cancer. Oct 2020;148:20‐27. doi: 10.1016/j.lungcan.2020.07.029 [DOI] [PubMed] [Google Scholar]
- 20.Allison KH, Yoder BJ, Bronner MP, Goldblum JR, Rubin BP. Angiosarcoma involving the gastrointestinal tract: a series of primary and metastatic cases. Am J Surg Pathol. Mar 2004;28(3):298‐307. doi: 10.1097/00000478-200403000-00002 [DOI] [PubMed] [Google Scholar]
- 21.Abeler VM, Nenodovic M. Diagnostic immunohistochemistry in uterine sarcomas: a study of 397 cases. Int J Gynecol Pathol. May 2011;30(3):236‐243. doi: 10.1097/PGP.0b013e318200caff [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Eddy JA, Pan Y, et al. Integrated proteomics and genomics analysis reveals a novel mesenchymal to epithelial reverting transition in leiomyosarcoma through regulation of slug. Mol Cell Proteomics. Nov 2010;9(11):2405‐2413. doi: 10.1074/mcp.M110.000240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dancau AM, Simon R, Mirlacher M, Sauter G. Tissue microarrays. Methods Mol Biol. 2016;1381:53‐65. doi: 10.1007/978-1-4939-3204-7_3 [DOI] [PubMed] [Google Scholar]
- 24.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. Jul 1998;4(7):844‐847. doi: 10.1038/nm0798-844 [DOI] [PubMed] [Google Scholar]
- 25.Tapia C, Schraml P, Simon R, et al. HER2 Analysis in breast cancer: reduced immunoreactivity in FISH non-informative cancer biopsies. Int J Oncol. Dec 2004;25(6):1551‐1557. [PubMed] [Google Scholar]
- 26.Fraune C, Simon R, Hube-Magg C, et al. MMR deficiency in urothelial carcinoma of the bladder presents with temporal and spatial homogeneity throughout the tumor mass. Urol Oncol. May 2020;38(5):488‐495. doi: 10.1016/j.urolonc.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 27.Skinnider BF, Folpe AL, Hennigar RA, et al. Distribution of cytokeratins and vimentin in adult renal neoplasms and normal renal tissue: potential utility of a cytokeratin antibody panel in the differential diagnosis of renal tumors. Am J Surg Pathol. Jun 2005;29(6):747‐754. doi: 10.1097/01.pas.0000163362.78475.63 [DOI] [PubMed] [Google Scholar]
- 28.Alexa A, Baderca F, Lighezan R, Izvernariu D, Raica M. The diagnostic value of cytokeratins expression in the renal parenchyma tumors. Rom J Morphol Embryol. 2010;51(1):27‐35. [PubMed] [Google Scholar]
- 29.Van Eyken P, Sciot R, Desmet VJ. Immunocytochemistry of cytokeratins in primary human liver tumors. APMIS Suppl. 1991;23:77‐85. [PubMed] [Google Scholar]
- 30.Van Eyken P, Sciot R, Paterson A, Callea F, Kew MC, Desmet VJ. Cytokeratin expression in hepatocellular carcinoma: an immunohistochemical study. Hum Pathol. May 1988;19(5):562‐568. doi: 10.1016/s0046-8177(88)80205-3 [DOI] [PubMed] [Google Scholar]
- 31.Pandey S, Soland TM, Bjerkli IH, et al. Combined loss of expression of involucrin and cytokeratin 13 is associated with poor prognosis in squamous cell carcinoma of mobile tongue. Head Neck. Nov 2021;43(11):3374‐3385. doi: 10.1002/hed.26826 [DOI] [PubMed] [Google Scholar]
- 32.Menz A, Bauer R, Kluth M, et al. Diagnostic and prognostic impact of cytokeratin 19 expression analysis in human tumors: a tissue microarray study of 13,172 tumors. Hum Pathol. Sep 2021;115:19‐36. doi: 10.1016/j.humpath.2021.05.012 [DOI] [PubMed] [Google Scholar]
- 33.Menz A, Weitbrecht T, Gorbokon N, et al. Diagnostic and prognostic impact of cytokeratin 18 expression in human tumors: a tissue microarray study on 11,952 tumors. Mol Med. Feb 15 2021;27(1):16. doi: 10.1186/s10020-021-00274-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vora HH, Patel NA, Rajvik KN, et al. Cytokeratin and vimentin expression in breast cancer. Int J Biol Markers. Jan-Mar 2009;24(1):38‐46. doi: 10.5301/jbm.2009.4965 [DOI] [PubMed] [Google Scholar]
- 35.Rajkovic N, Li X, Plataniotis KN, Kanjer K, Radulovic M, Milosevic NT. The pan-cytokeratin staining intensity and fractal computational analysis of breast tumor malignant growth patterns prognosticate the occurrence of distant metastasis. Front Oncol. 2018;8:348. doi: 10.3389/fonc.2018.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Loarer F, Cleven AHG, Bouvier C, et al. A subset of epithelioid and spindle cell rhabdomyosarcomas is associated with TFCP2 fusions and common ALK upregulation. Mod Pathol. Mar 2020;33(3):404‐419. doi: 10.1038/s41379-019-0323-8 [DOI] [PubMed] [Google Scholar]
- 37.Thompson LDR, Jo VY, Agaimy A, et al. Sinonasal tract alveolar rhabdomyosarcoma in adults: a clinicopathologic and immunophenotypic study of fifty-two cases with emphasis on epithelial immunoreactivity. Head Neck Pathol. Jun 2018;12(2):181‐192. doi: 10.1007/s12105-017-0851-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stock N, Chibon F, Binh MB, et al. Adult-type rhabdomyosarcoma: analysis of 57 cases with clinicopathologic description, identification of 3 morphologic patterns and prognosis. Am J Surg Pathol. Dec 2009;33(12):1850‐1859. doi: 10.1097/PAS.0b013e3181be6209 [DOI] [PubMed] [Google Scholar]
- 39.Matoso A, Epstein JI. Epithelioid angiosarcoma of the bladder: a series of 9 cases. Am J Surg Pathol. Oct 2015;39(10):1377‐1382. doi: 10.1097/PAS.0000000000000444 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ijs-10.1177_10668969221117243 for Pan-keratin Immunostaining in Human Tumors: A Tissue Microarray Study of 15,940 Tumors by Anne Menz, Natalia Gorbokon, Florian Viehweger, Maximilian Lennartz, Claudia Hube-Magg, Lisa Hornsteiner, Martina Kluth, Cosima Völkel, Andreas M. Luebke, Christoph Fraune, Ria Uhlig, Sarah Minner, David Dum, Doris Höflmayer, Guido Sauter, Ronald Simon, Eike Burandt, Till S. Clauditz, Patrick Lebok, Frank Jacobsen, Stefan Steurer, Till Krech, Andreas H. Marx and Christian Bernreuther in International Journal of Surgical Pathology
Supplemental material, sj-docx-2-ijs-10.1177_10668969221117243 for Pan-keratin Immunostaining in Human Tumors: A Tissue Microarray Study of 15,940 Tumors by Anne Menz, Natalia Gorbokon, Florian Viehweger, Maximilian Lennartz, Claudia Hube-Magg, Lisa Hornsteiner, Martina Kluth, Cosima Völkel, Andreas M. Luebke, Christoph Fraune, Ria Uhlig, Sarah Minner, David Dum, Doris Höflmayer, Guido Sauter, Ronald Simon, Eike Burandt, Till S. Clauditz, Patrick Lebok, Frank Jacobsen, Stefan Steurer, Till Krech, Andreas H. Marx and Christian Bernreuther in International Journal of Surgical Pathology