Abstract
Background
Vitamin D toxicity is rare in pediatric population. Falsely elevated levels of 25‐hydroxyvitamin D have been reported as a major challenge with immunoassay methods for quantifying vitamin D metabolites.
Case Presentation and Method
Here, we present two pediatric cases of falsely elevated 25‐hydroxyvitamin D that resulted in unnecessary further testing. We also report significant same‐day variation in the measurement of 25‐hydroxyvitamin D using the Abbott i2000SR immunoassay. Samples were spun twice and their values were confirmed with the gold standard liquid chromatography‐tandem mass spectrometry (LC–MS/MS) method for confirmation.
Conclusion
The addition of a centrifugation step prior to sample testing resolved the variation observed in the measurement of 25‐hydroxyvitamin D levels. The patient samples were confirmed with instruments from a different vendor and LC–MS/MS. Re‐centrifugation of samples resolved the variation in the 25‐hydroxyvitamin D values.
Keywords: 25‐hydroxyvitamin D, architect i2000SR, immunoassays, liquid chromatography–tandem mass spectrometry, vitamin D assays
1. BACKGROUND
Vitamin D deficiency remains a global health challenge. It plays a major and important role in calcium and bone metabolism but has also been reported to be involved in a plethora of other physiological functions. Vitamin D deficiency is linked to an increased risk of cancer and autoimmune disease; and is known to be worsened by other risk factors such as pregnancy, obesity, advanced age, malabsorption (such as in chronic inflammatory bowel disease), and dark skin pigmentation. For these reasons, the demand for the measurement of vitamin D metabolites has greatly increased. The most frequently measured metabolites are 25(OH)D2 and 25(OH)D3 due to their longer half‐life in plasma. 1 Here, we report two cases of asymptomatic pediatric patients with falsely elevated vitamin D values.
2. CASE PRESENTATION
2.1. Patient 1
A 10‐year‐old boy with benign childhood epilepsy with centrotemporal spikes (BECTS), attention deficit hyperactivity disorder (ADHD), and mild intermittent asthma without complication presented for a routine follow‐up in February 2022. His usual medications were methylphenidate, clobazam, diazepam, cetirizine, budesonide, fluticasone and melatonin gummies. The physician ordered a total of 25‐hydroxy vitamin D (25(OH)D) by immunoassay and a complete metabolic panel from an external laboratory. The patient's 25(OH)D concentration was reported as >150 ng/mL (375 nmol/L) (Atellica, Siemens Healthineers), and other results were unremarkable. The patient was instructed to stop his vitamin D supplements and decrease dairy consumption. A total 25(OH)D by immunoassay was repeated twice on separate specimens by the external laboratory in addition to calcium, phosphate and parathyroid hormone. The observed 25(OH)D concentration remained at >150 ng/mL (375 nmol/L) (Atellica, Siemens Healthineers), with no abnormalities in the other analytes. The patient was referred for specialist care, and underwent further extensive endocrinology and renal workup, both of which were unremarkable. His endocrinologist ultimately collected two serum samples at the same time and sent them to two different laboratories: our laboratory, and the original outside performing laboratory. Our laboratory reported 25(OH)D of 46.9 ng/mL (117.3 nmol/L), while the external lab value remained at >150 ng/mL (375 nmol/L). The endocrinologist consulted with the chemistry laboratory for advice on the discrepancy in results. The sample tested in our laboratory was sent for confirmation by LC–MS/MS, a few days later, which yielded a value of 37 ng/mL (136.9 nmol/L).
2.2. Patient 2
During the investigation of discrepant 25(OH)D measurements, another suspected case of pseudohypervitaminosis D was identified. An 8‐week‐old boy with high gamma‐glutamyl transferase (GGT) infantile cholestasis presented to the gastroenterology department for a post‐admission follow‐up, and serum 25(OH)D was reported as >150 ng/mL (375 nmol/L) (Atellica, Siemens Healthineers). His endocrinologist instructed his caregiver to stop vitamin D supplementation until further notice due to potential Vitamin D toxicity. The clinical chemistry team reached out to the physician to inform them of the potential inaccuracy of the result. The physician was advised to submit paired samples for 25(OH)D by immunoassay and LC–MS/MS. From these samples, the immunoassay value was reported as 52 ng/mL (130 nmol/L) from our laboratory and the LC–MS/MS value was 59 ng/L (147.5 nmol/L).
3. DISCUSSION AND CONCLUSIONS
Although the analytical reference method is isotope dilution liquid chromatography‐tandem mass spectrometry (ID LC–MS/MS), immunoassays are widely used in clinical laboratories because they can be run on automated analyzers and easily integrated into the routine laboratory workflow. Most immunoassays measure the total amount of 25(OH)D in a given specimen, but may variably detect vitamin D, its metabolites and respective epimers, which can cause overestimation or underestimation of results. Immunoassays also cannot distinguish between these forms of vitamin D and are subject to cross‐reactivity and interference. 2 , 3 , 4
The first patient was brought to the attention of the clinical chemistry team during a vitamin D stability verification process in our lab. During the stability verification process, we evaluated the stability of 25(OH)D serum samples and discovered discrepancies between pre‐storage and post‐storage values in some samples, with the observed concentration of 25(OH)D levels decreasing after retrieval from storage. We described a result as discrepant if the bias exceeds the CAP total allowable error of ±5 ng/mL (12.5 nmol/L).
We further reviewed the stability of 25(OH)D in 179 residual patient samples over a period of 2 months (June 2022 to August 2022). We examined samples that had been in storage for <5 days at 2–6°C (85 samples) and samples that were being processed in the lab on the same day (94 samples). Ninety‐five percent of 25(OH)D specimens were plasma and 5% were serum samples. The 25(OH)D measurements were performed using the newly formulated version of the Abbott Architect i2000SR Vitamin D assay, which is standardized against the NIST SRM 2972 (National Institute of Standards & Technology Reference Material). The AMR for the assay is 3.4–155.9 ng/mL. There was no correlation between the observed discrepancies and other variables such as time between collection and processing, type of collection tube, collection department, patient's clinical history, and vitamin D supplementation. Over a period of 6 days, the percentage of results with discrepancies ranged from 11% to 43%.
We subsequently introduced an additional centrifugation for 3 min at 1000 RCF (3300 RPM) before analysis. This intervention appeared to eliminate the possible interference because the 25(OH)D concentration remained consistent and did not have a significant change at 24‐h post‐centrifugation (Figure 1).
FIGURE 1.
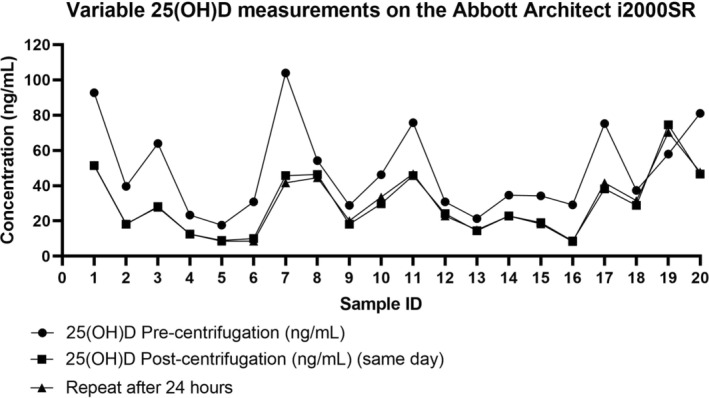
Vitamin D data for 20 specimens analyzed pre‐centrifugation, post‐centrifugation and 24 h after the last centrifugation was carried out.
We sent three random samples to an external laboratory for 25(OH)D quantification by LC–MS/MS for confirmation. The initial 25(OH)D concentrations reported on the Architect i2000SR analyzer were 44.2–66.7 ng/mL (110.5–116.8 nmol/L), but retesting following an additional centrifugation later yielded lower values (20.3–30.5 ng/mL) (50.8–76.3 nmol/L). The samples were reanalyzed with LC–MS/MS to confirm the 25(OH)D levels. The values ranged from 19.6 to 29.9 ng/mL (49.0–74.8 nmol/L) (Table 1). We confirmed the values on another Abbott Architect platform to rule out possible interference with our assay/instrument.
TABLE 1.
Comparison of 25(OH)D level on different platforms.
| Sample no | 25(OH)D Architect i2000SR (pre centrifugation) | 25(OH)D Architect i2000SR (post centrifugation) | 25(OH)D Architect ci8200 (post cetrifugation) | 25(OH) total by LC–MS/MS |
|---|---|---|---|---|
| ng/mL (nmol/L) | ng/mL (nmol/L) | ng/mL (nmol/L) | ng/mL (nmol/L) | |
| 1 | 66.7 (166.8) | 29.9 (74.8) | 27.9 (69.8) | 25 (62.5) |
| 2 | 44.2 (110.5) | 19.6 (49.0) | 20.3 (50.8) | 21 (52.5) |
| 3 | 47.7 (119.3) | 28.6 (71.5) | 30.5 (76.3) | 29 (72.5) |
Vitamin D toxicity is very rare in children and there is sparse literature on spurious vitamin D levels in this population. A previous report by Hernandez et al. identified false elevations in 25‐hydroxyvitamin D values in lithium heparin plasma samples when analyzed with the reformulated Abbott Architect i2000 vitamin D assay. The authors attributed the spurious values to the resuspension of particulate matter found in plasma samples during transportation. 5 On the contrary, we observed discrepant vitamin D values in a few serum samples analyzed in our study. Of note, there is limited data on potential interferences with the Chemiluminescent vitamin D assay on the Atellica analyzer (Siemens Healthineers), therefore we are unable to comment on the comparison of the method with other assays on the market. Interference due to sample integrity was ruled out for these two patients due to the significantly different values observed by the two assays. At this time, it is unclear why centrifugation of the samples resolved the fluctuation in 25(OH)D concentrations, however, there could be some form of interference from residual fibrin. Yet, this does not fully explain why the fluctuation occurs in only a proportion (<50%) of samples. Studies have described the stability of vitamin D as “solid as a rock” in serum and plasma samples without detectable degradation under several storage conditions and for several years. 6 , 7 Our initial centrifugation parameter was to centrifuge samples for 3 min at 1000 RCF (3300 RPM) just as we do for all other testing on the Architect analyzer and run them directly on the analyzer. Our revised process due to the discrepant values is to carry out initial centrifugation, batch the samples, and centrifuge them again prior to analyzing them during the second shift. Since this resolved our challenge, we are inclined to hypothesize that the observed variation could be due to a change in reagent versions for the newly formulated assay and the possibility of micro particle interference.
Vitamin D supplementation is particularly important in infants. The American Academy of Pediatrics strongly recommends that infants receive 400 IU of vitamin D daily for the prevention of nutritional rickets. 8 Infants with cholestasis are prone to vitamin D deficiency due to impaired absorption of fat‐soluble vitamins, therefore the accuracy of 25(OH)D concentration is very important in our pediatric patient population. 9 Similarly, patients with ADHD are more likely to have vitamin D deficiency compared to healthy populations. These patients are often prescribed methylphenidate as the first line of therapy. Vitamin D supplementation as an adjunctive therapy to methylphenidate has been reported to improve ADHD symptoms. 10 The limitation of this study was the inability to further verify stability by resuspension of the sample and retesting to rule out the interference by fibrinogen. This was due to an insufficient sample in our patient population. Although, the variation in the measurement was also seen in a few serum samples.
These serendipitous findings emphasize the importance of continuous collaboration between clinicians and laboratory specialists, to prevent unnecessary investigations and harmful consequences to patients due to discrepant laboratory test values. We have no clear explanation for this phenomenon, but our observations imply that specimens for 25(OH)D by immunoassay may require a second centrifugation step prior to measurement on the analyzer. We would encourage other clinical laboratories to review their immunoassay platforms for 25(OH)D stability.
CONFLICT OF INTEREST STATEMENT
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent‐licensing arrangements), or non‐financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Olayinka L, Poventud‐Fuentes I, Garnett E, Devaraj S. Pediatric vitamin D: Pseudo‐hypervitaminosis. J Clin Lab Anal. 2023;37:e24950. doi: 10.1002/jcla.24950
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Makris K, Bhattoa HP, Cavalier E, et al. Recommendations on the measurement and the clinical use of vitamin D metabolites and vitamin D binding protein – a position paper from the IFCC committee on bone metabolism. Clin Chim Acta. 2021;512:171‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wauthier L, Plebani M, Favresse J. Interferences in immunoassays: review and practical algorithm. Clin Chem Lab Med. 2022;60(6):808‐820. [DOI] [PubMed] [Google Scholar]
- 3. Alonso N, Zelzer S, Elbinger G, Hermann M. Vitamin D metabolites: analytical challenges and clinical relevance. Calcif Tissue Int. 2022;3:1‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alexandridou A, Volmer DA. Sample preparation techniques for extraction of vitamin D metabolites from non‐conventional biological sample matrices prior to LC–MS/MS analysis. Anal Bioanal Chem. 2022;414:4613‐4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hernandez JA, Stanford JE, Savoie CV, Nichols JH, Colby JM. Spuriously elevated 25‐hydroxyvitamin D in lithium heparin plasma samples transported by courier. J Appl Lab Med. 2018;2(5):809‐811. [DOI] [PubMed] [Google Scholar]
- 6. Cavalier E. Long‐term stability of 25‐hydroxyvitamin D: importance of the analytical method and of the patient matrix. Clin Chem Lab Med. 2021;59(10):e389‐e391. [DOI] [PubMed] [Google Scholar]
- 7. Wielders JP, Wijnberg FA. Preanalytical stability of 25(OH)‐vitamin D3 in human blood or serum at room temperature: solid as a rock. Clin Chem. 2009;55(8):1584‐1585. [DOI] [PubMed] [Google Scholar]
- 8. Casey CF, Slawson DC, Neal LR. Vitamin D supplementation in infants, children, and adolescents. Am Fam Physician. 2010;81(6):745‐748. [PubMed] [Google Scholar]
- 9. Best C, Gourley GR. Management of neonatal cholestasis. Therapy. 2009;6(1):75‐81. [Google Scholar]
- 10. Gan J, Galer P, Ma D, Chen C, Xiong T. The effect of vitamin D supplementation on attention‐deficit/hyperactivity disorder: a systematic review and meta‐analysis of randomized controlled trials. J Child Adolesc Psychopharmacol. 2019;29(9):670‐687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


