Abstract
Measles, or rubeola, is a highly contagious acute febrile viral illness. Despite the availability of an effective vaccine since 1963, measles outbreaks continue worldwide. This article seeks to provide emergency physicians with the contemporary knowledge required to rapidly diagnose potential measles cases and bolster public health measures to reduce ongoing transmission.
Keywords: clinical features, diagnosis, emergency, measles infection, outbreak, special populations
1. INTRODUCTION
Measles cases have recently been on the rise in the United States and globally due to falling vaccination rates. This highly contagious illness can have serious complications and long‐term side effects, including immunosuppression and neurological complications. Emergency physicians must understand the presentation and potential complications of this disease to best treat and disposition patients, particularly patients who may be pregnant or immunocompromised. By providing necessary postexposure prophylaxis (PEP) and encouraging overall vaccinations, emergency physicians can play an important role in the public health response to measles outbreaks.
1.1. Measles resurgence in the United States and across the globe
In 2000, endemic measles was officially declared eliminated from the United States. 1 Since 2000, most US measles cases have occurred in unvaccinated patients (69%) or those with unknown vaccination status (18%). 2 Measles incidence has been highest in infants and toddlers ages 6 to 15 months. 2 In 2019, 1274 individual cases of measles were confirmed in 31 states, the largest number reported in the United States since 1992. 3 The following year (2020), cases fell precipitously to 13 but have incrementally increased over the past 2 years. 4
There were 9 million cases and 148,000 deaths from measles globally in 2021. 4 Outbreaks worldwide have been fueled by decreased vaccination rates due to COVID‐19 disruptions to routine vaccination programs, variable clinical presentations, and the potential for rapid disease spread of measles in communities. These changes are predicted to increase the likelihood of outbreaks in the United States. 5 , 6 There have been no direct correlation between COVID vaccination rates and measles cases. With additional cases still being diagnosed in 2023, emergency physicians must recognize and manage measles and its complications and engage in public health measures to reduce ongoing transmission of this highly infections illness.
1.2. Pathophysiology
The measles virus is a ribonucleic acid (RNA) virus transmitted rapidly via airborne droplets containing highly infectious virus components. 7 Measles is thought to spread to susceptible individuals through respiratory particles quickly. 8 The Centers for Disease Control and Prevention (CDC) recommend strict, protective isolation measures for a room occupied by an infectious measles patient within the prior 2 hours. 9
The pathogenesis of measles involves aggressive humoral and cellular responses. 10 Measles is an immunosuppressive infection, and individuals with measles have increased susceptibility to secondary bacterial and viral infections. This immunosuppressed state lasts several weeks to months after the initial infection, with some effects seen for up to 3 years. 11
2. CLINICAL CARE
2.1. Presentation
The incubation period of measles from exposure to onset of rash ranges from 7–21 days, with an average of 14 days. 12 Individuals infected with measles are contagious from 4 days before to 4 days after the onset of the rash. 7 The initial prodromal phase is characterized by high progressive fever and malaise lasting 2 to 4 days, followed by the 3 “Cs” of cough, conjunctivitis, and coryza (copious nasal discharge). 7
Koplik spots are pathognomonic for measles. 7 , 13 , 14 (Figure 1) They appear as small white or grey spots on an erythematous base on the buccal mucosa and typically present approximately 48 hours before the onset of the rash, lasting 12 to 72 hours. 15 Given their transient and highly localized nature, Koplik spots can be challenging to identify but can especially be found in the buccal mucosa around the second molars. 10
FIGURE 1.
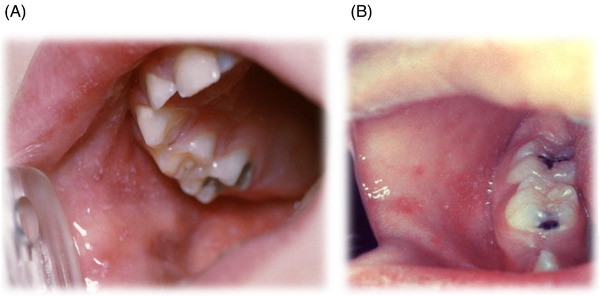
(A)–(B) Koplik spots. The presence of small white or grey‐colored “Koplik” spots, found on an erythematous base on the buccal mucosa, can be pathognomonic for a measles infection. Images used with permission from VisualDx.
A rash develops 2 to 4 days after the prodromal symptoms begin and is usually red, blotchy, and maculopapular. 7 , 10 However, the rash may have variable appearances in different skin types. (Figure 2) 13 , 14 The rash starts on the face and spreads downward and outward. 7 Initially, the lesions blanch with finger pressure but become non‐blanching by the third or fourth day of the rash. 16 After 5 to 6 days, the rash fades in the same order of appearance, from face to extremities. Fine desquamation can occur, and emergency department patients may benefit from being warned about this symptom. 7 Dermatological findings cannot definitively pinpoint the exact timing of the disease and individual cases may vary significantly from textbook descriptions. 17
FIGURE 2.
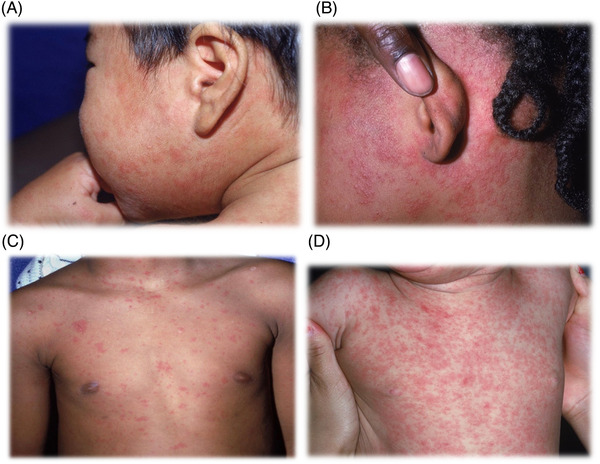
(A)–(D) Measles rash. The exanthem consists of erythematous macules and papules, having subtle differences across variable skin tones, and often fading in the order of initial appearance after the fifth day. Images used with permission from VisualDx.
2.2. Complications
Measles‐related complications have been reported to occur in up to 40% of patients across various systems. 6 , 9 , 18 The risk of complications increases for patients who are immunocompromised, pregnant, malnourished, or at extremes of age. 6 , 9 Otitis media is the most common complication of measles, possibly leading to hearing loss. 8 Pneumonia is the most common severe complication, accounting for the most measles‐associated deaths. 8 , 16 Laryngotracheobronchitis, or “measles croup,” is the second most common cause of death in children hospitalized with measles and occurs predominantly in children under 2. 16 Gastrointestinal complications include stomatitis and diarrhea, which may worsen malnutrition and hydration status. 8 , 9 , 16 Measles can also cause keratoconjunctivitis, corneal ulceration, and ultimately blindness, especially in patients deficient in vitamin A. 8 , 15 , 16
Central nervous system complications from measles are rare but devastating. Seizures occur in <1% of children in the United States and can occur with or without fever. 7 , 16 Approximately 1 in 1000 patients will develop acute measles encephalitis. 7 Symptoms are typical of meningitis/encephalitis caused by other bacterial and viral etiologies, and encephalitis is the leading cause of measles deaths in adults, with a mortality rate of 10%–15%. 7 Acute postinfectious encephalomyelitis is a demyelinating autoimmune disease that occurs within 2 weeks of the rash, characterized by the abrupt onset of new fever, seizures, altered mental status, and multifocal neurological signs or symptoms. 9 , 16 Postinfectious encephalomyelitis is frequently fatal, and survivors often have lifelong neurological sequelae, including cognitive deficits, motor deficits, and visual impairment. 16 Lastly, subacute sclerosing panencephalitis is a rare and fatal progressive degenerative brain disease 5–10 years after an initial measles infection that causes personality change, seizures, cognitive decline, and motor dysfunction. 8 , 16
2.3. Diagnosis
Patients presenting with suggestive symptoms (fever, cough, rash) in the ED should be presumed to have measles, especially if they are unvaccinated or recently traveled to an endemic area. Once measles is suspected, airborne precautions should be followed, especially when diagnostic procedures are performed. 12
Measles is a reportable disease nationwide. Suspected cases should be reported to the local health department. The diagnosis of measles is confirmed by detecting measles viral RNA by reverse transcriptase‐polymerase chain reaction (RT‐PCR), serologic testing with enzyme‐linked immunoassay (ELISA) for measles‐specific IgM or IgG, or isolation of measles virus in viral culture. 19 In the ED setting, any person with clinical features concerning for measles should be tested with combined serology testing for IgM and IgG along with RT‐PCR. Respiratory RT‐PCR (nasopharyngeal and oropharynx) specimens are preferred over urine samples. For measles PCR, swabs in viral transport media should be sent. Dry swabs are not acceptable. A combination of PCR and serology testing helps improve diagnostic accuracy, especially because viral coinfections may still occur in the ED and exhibit complex patient presentations. 17 , 20
2.4. Treatment
Emergency physicians should have a strategy for appropriately identifying, reporting, and treating suspected measles (Figure 3).There is no specific antiviral treatment for measles. Management is focused on supportive care for treating fever and dehydration, identifying and treating complications, and preventing further measles transmission. 8
FIGURE 3.
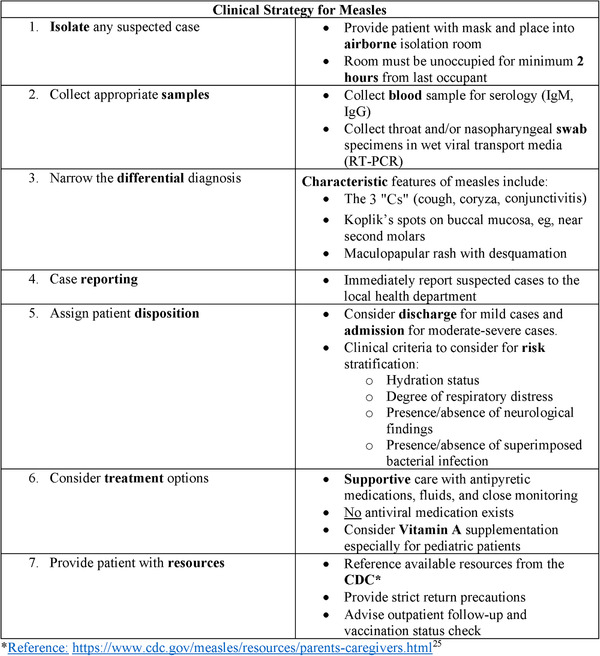
Clinical pearls. Summary of critical steps that may be used by emergency physicians to identify and treat suspected measles cases in the ED. Abbreviations: CDC, Centers for Disease Control and Prevention; ED, emergency department; IgG, immunoglobulin G; IgM, immunoglobulin M; RT‐PCR, reverse transcription polymerase chain reaction.
Vitamin A has been shown to decrease morbidity and mortality of measles infections. 21 All children with acute measles should receive vitamin A once daily for 2 days in the following doses: 200,000 IU for children 12 months or older, 100,000 IU for infants 6 through 11 months of age, and 50,000 IU for infants younger than 6 months. 9 An additional age‐specific dose should be given 2 to 4 weeks later to children with signs and symptoms of vitamin A deficiency. 8 Studies have shown an increased risk for severe measles infections in adults with vitamin A deficiency. 22 , 23
2.4.1. Disposition
Emergency physicians should notify appropriate hospital infection control staff and state public health agencies of suspected measles cases. 24 They should also assess the severity of the patient's measles infection and admit patients with severe symptoms or complications. Patients with mild cases (ie, no signs of dehydration, respiratory distress, superimposed bacterial infection, or neurologic complications) may be discharged home with proper isolation instructions and close follow‐up with their primary care physician. Infected people who are released should be isolated for the contagious period lasting 4 days after developing a rash. In an outbreak area, all people without evidence of immunity or who do not or cannot receive PEP within the appropriate time frame should be excluded from gatherings until 21 days after the onset of rash for the last case of measles. 17
Contaminated rooms should be avoided for 2 hours after the patient's departure, as the virus is infectious on surfaces and in the air until then. 7 If a discharged patient is sent to another health care facility for follow‐up while still contagious, that facility should be notified to ensure that proper isolation precautions are in place. 8 Patients discharged should receive comprehensive care instructions, such as those available from the CDC. 25
2.5. Vaccination
Vaccination against measles is recommended for all eligible susceptible children and adults. The CDC recommends that 2 doses of the live attenuated measles, mumps, and rubella (MMR) vaccine to children within their first 6 years of life (the first dose between 12 and 16 months of age, the second dose between 4 and 6 years). 26 The World Health Organization (WHO) recommends vaccination for all eligible health care workers. 27
Those with severe immunosuppression, a history of severe allergic reactions, and/or are currently pregnant are not advised to receive the immunization. For HIV‐infected individuals, the WHO, United Kingdom, and the United States offer differing recommendations based on CD4 counts and degree of immunosuppression. 22 Prevalent international outbreaks put US residents traveling abroad who are unvaccinated or undervaccinated at significant risk for acquiring measles. The CDC recommends that infants aged 6–11 months and adults who received only 1 vaccine previously be vaccinated with MMR before traveling internationally. 28
For the unvaccinated patient presenting with measles, lifelong immunity to measles will be conferred if the patient survives the initial infection. 29 However, vaccination with the MMR vaccine is still recommended after the infection resolves due to rubella and mumps protection offered by the vaccine.
Notably, routine pediatric vaccine orders and doses administered decreased significantly during the initial months of the COVID‐19 pandemic. Reasons were multifactorial: stay‐at‐home orders were in place in many states, resources were directed toward emergency response rather than primary care, vaccine hesitancy, and parental concern existed regarding COVID‐19 exposure in primary care facilities. 30 , 31
2.6. Post‐exposure prophylaxis
Given the highly infectious nature of the measles virus, close contact with a patient with measles should be considered for post‐exposure prophylaxis (PEP). Susceptible individuals should receive measles vaccination within 72 hours of exposure to reduce the risk of complications according to the CDC's Advisory Committee on Immunization Practices. 17 Vaccines may be given to infants as young as 6 months of age. 17 Vaccination should also be considered in those who have received only 1 dose of MMR, as 1 dose confers incomplete immunity. 17 PEP is not indicated for individuals of all ages who previously received 2 MMR vaccine doses.
For infants younger than 6 months, severely immunocompromised patients, and unvaccinated pregnant women, intramuscular immunoglobulin (IG) should be given within 6 days of exposure, as the vaccine is contraindicated in these groups, and IG may provide temporary protection or improve outcomes in these vulnerable populations. 7 The vaccine and IG should not be given simultaneously, as this invalidates the vaccine.
3. SPECIAL POPULATIONS
3.1. Health care workers
Health care workers are a high‐risk group for contracting measles. In previous outbreaks, unvaccinated and partially vaccinated health care workers are more likely to have severe complications from measles than children and have a 2‐fold increase in severe complications compared to the general population. 32 The CDC recommends vaccination for all eligible health care personnel. 33
3.2. Pregnant patients
Many case series and cohort studies have shown that pregnant women contracting measles have increased symptom variability, morbidity, and mortality. They were more likely to be hospitalized and to have pneumonia than nonpregnant patients. 34 Adverse pregnancy outcomes, including preterm labor, spontaneous abortions, low birth weights, and neonatal ICU admissions, have been reported to be higher in pregnant women with measles than in their comparison groups. 7 , 28
Measles can be transmitted from a pregnant woman with measles within 10 days of delivery to her neonate. Congenital measles occurs when a neonate has a rash at birth or within the first 10 days of life. This early presentation excludes the possibility of postdelivery transmission. Neonates with congenital measles are at increased risk for mortality and for subacute sclerosing panencephalitis and should be treated with pooled human IG. 35 , 36
3.3. Immunocompromised patients
Immunocompromised patients with measles have a high risk of death and severe complications, including pneumonitis and encephalitis. The patients at highest risk are those with T‐cell deficiencies, including patients with certain leukemias and lymphomas and those with acquired immunodeficiency syndrome. 7 Immunocompromised patients can have an atypical presentation, with up to 40% of patients never developing a rash. 37 These patients may have a more prolonged course, including shedding the virus for several weeks after the acute illness. 7 Additionally, many immunocompromised patients present with severe pneumonitis without the typical viral prodrome. 27
Patients infected with HIV but not severely immunocompromised should be vaccinated against measles, and transplant recipients who are not undergoing immunosuppressive treatment or do not have active chronic graft versus host disease. 13 , 38 Emergency physicians should counsel all patients to speak with their physicians about measles vaccination.
4. CONCLUSIONS
An endemic disease previously eliminated is once again a genuine threat to a growing number of people and their communities. Emergency physicians are critical in rapidly diagnosing and managing measles and its complications and engaging their patients and communities to support public health measures, including vaccinations. As a result, emergency physicians at all levels of training must be familiar with the pathophysiology, clinical presentation, diagnosis, management, complications, and prevention of measles.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose. There was no external funding for this publication. Open access journal funds were paid for by the author and reimbursed from departmental reserve funds.
ACKNOWLEDGMENTS
We wish to acknowledge contributions made by Dr William Martin, Dr Mary Bolgiano, and VisualDx for content.
Blutinger E, Schmitz G, Kang C, et al. Measles: Contemporary considerations for the emergency physician. JACEP Open. 2023;4:e13032. 10.1002/emp2.13032
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
Supervising Editor: Nicholas Johnson, MD
Meetings, Grant Support or other funding: none
REFERENCES
- 1. Berche P. History of measles. Press Medicale. 2022;51(3):104149. doi: 10.1016/j.lpm.2022.104149 [DOI] [PubMed] [Google Scholar]
- 2. Clemmons NS, Wallace GS, Patel M, Gastañaduy PA. Incidence of measles in the United States, 2001–2015. JAMA—J Am Med Assoc. 2017;318(13):1279‐1281. doi: 10.1001/jama.2017.9984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Measles Cases and Outbreaks. Accessed April 1, 2023. https://www.cdc.gov/measles/cases‐outbreaks.html
- 4. Centers for Disease Control and Prevention . Nearly 40 million children are dangerously susceptible to growing measles threat. CDC Newsroom. 2022. https://www.who.int/news/item/23‐11‐2022‐nearly‐40‐million‐children‐are‐dangerously‐susceptible‐to‐growing‐measles‐threat#:~:text=In%202021%2C%20a%20record%20high,for%20Disease%20Control%20and%20Prevention%20( [Google Scholar]
- 5. Causey K, Fullman N, Sorensen RJD, et al. Estimating global and regional disruptions to routine childhood vaccine coverage during the COVID‐19 pandemic in 2020: a modelling study. Lancet. 2021;398(10299):522‐534. doi: 10.1016/S0140-6736(21)01337-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thakur M, Zhou R, Mohan M, et al. COVID's collateral damage: likelihood of measles resurgence in the United States. BMC Infect Dis. 2022;22(1):743. doi: 10.1186/s12879-022-07703-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leung AKC, Hon KL, Leong KF, Sergi CM. Measles: a disease often forgotten but not gone. Hong Kong Med J. 2018;24(5):512‐520. doi: 10.12809/hkmj187470 [DOI] [PubMed] [Google Scholar]
- 8. Hamborsky J, Kroger A, Wolfe SE. Emiology and prevention of vaccine‐preventable diseases. Centers Dis Control Prev. 2015;17(12):51. [Google Scholar]
- 9. Centers for Disease Control and Prevention . Interim Infection Prevention and Control Recommendations for Measles in Healthcare Settings. 2019. https://www.cdc.gov/infectioncontrol/guidelines/measles/index.html
- 10. Bester JC. Measles and measles vaccination a review. JAMA Pediatr. 2016;170(12):1209‐1215. doi: 10.1001/jamapediatrics.2016.1787 [DOI] [PubMed] [Google Scholar]
- 11. Moss WJ. Measles. Lancet. 2017;390(10111):2490‐2502. doi: 10.1016/S0140-6736(17)31463-0 [DOI] [PubMed] [Google Scholar]
- 12. Abad CL, Safdar N. The reemergence of measles. Curr Infect Dis Rep. 2015;17(12):51. doi: 10.1007/s11908-015-0506-5 [DOI] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention. Photos of Measles and People with Measles. Published 2020. https://www.cdc.gov/measles/symptoms/photos.html
- 14. VisualDx . Diagnosing Measles in Light of Recent Outbreaks. 2019. https://www.visualdx.com/blog/diagnosing‐measles‐in‐light‐of‐recent‐outbreaks/
- 15. O'Donnell S, Davies F, Vardhan M, Nee P. Could this be measles? Emerg Med J. 2019;36(5):310‐314. doi: 10.1136/emermed-2019-208490 [DOI] [PubMed] [Google Scholar]
- 16. Alves Graber EM, Andrade FJ, Bost W, Gibbs MA. An update and review of measles for emergency physicians. J Emerg Med. 2020;58(4):610‐615. doi: 10.1016/j.jemermed.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 17. Battegay R, Itin C, Itin P. Dermatological signs and symptoms of measles: a prospective case series and comparison with the literature. Dermatology. 2012;224(1):1‐4. doi: 10.1159/000335091 [DOI] [PubMed] [Google Scholar]
- 18. Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis. 2004;189 Suppl 1:S4‐S16. doi: 10.1086/377712 [DOI] [PubMed] [Google Scholar]
- 19. Kimberlin DW, Brady MT, Jackson MA. 2018 Report of the committee on infectious diseases. Pediatrics AA of, ed. Red Book . 4th ed. AAP Books; 2018:537‐550. https://publications.aap.org/aapbooks/book/546/Red‐Book‐2018‐Report‐of‐the‐Committee‐on?autologincheck=redirected [Google Scholar]
- 20. Bansal J, Hameed A. Measles in pregnancy. BMJ Case Rep. 2019;12(5):e228781. doi: 10.1136/bcr-2018-228781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sudfeld CR, Navar AM, Halsey NA. Effectiveness of measles vaccination and vitamin A treatment. Int J Epidemiol. 2010;39(1):i48‐i55. doi: 10.1093/ije/dyq021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Melenotte C, Brouqui P, Botelho‐Nevers E. Severe measles, vitamin A deficiency, and the romacommunity in Europe. Emerg Infect Dis. 2012;18(9):1537‐1539. doi: 10.3201/eid1809.111701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bichon A, Aubry C, Benarous L, et al. Case report: ribavirin and vitamin A in a severe case of measles. Medicine (Baltimore). 2017;96(50):e9154. doi: 10.1097/md.0000000000009154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koenig KL, Alassaf W, Burns MJ. Identify‐Isolate‐Inform: a tool for initial detection and management of measles patients in the emergency department. West J Emerg Med. 2015;16(2):212‐219. doi: 10.5811/westjem.2015.3.25678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention. Educational Resources for Parents and Childcare Providers. Published 2020. https://www.cdc.gov/measles/resources/parents‐caregivers.html
- 26. Sanyaolu A, Okorie C, Marinkovic A, et al. Measles outbreak in unvaccinated and partially vaccinated children and adults in the United States and Canada (2018‐2019): a narrative review of cases. Inquiry. 2019;56:46958019894098. doi: 10.1177/0046958019894098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization . Measles vaccines: WHO position paper, April 2017 ‐ Recommendations. Relev Epidemiol Hebd. 2019;37(2):219‐222. doi: 10.1016/j.vaccine.2017.07.066 [DOI] [PubMed] [Google Scholar]
- 28. Patel M, Lee AD, Redd SB, et al. Increase in measles cases — United States, January 1–April 26, 2019. MMWR Morb Mortal Wkly Rep. 2019;68(17):402‐404. doi: 10.15585/mmwr.mm6817e1 [DOI] [PubMed] [Google Scholar]
- 29. Moss W. Measles in vaccinated individuals and the future of measles elimination. Clin Infect Dis. 2018;67(9):1320‐1321. doi: 10.1093/cid/ciy306 [DOI] [PubMed] [Google Scholar]
- 30. Santoli JM, Lindley MC, DeSilva MB, et al. Effects of the COVID‐19 pandemic on routine pediatric vaccine ordering and administration — United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(19):591‐593. doi: 10.15585/mmwr.mm6919e2 [DOI] [PubMed] [Google Scholar]
- 31. Bramer CA, Kimmins LM, Swanson R, et al. Decline in child vaccination coverage during the COVID‐19 pandemic — Michigan Care Improvement Registry, May 2016‐May 2020. Am J Transplant. 2020;20(7):1930‐1931. doi: 10.1111/ajt.16112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shefer A, Strikas R, Bridges CB. Updated recommendations of the advisory committee on immunization practices for healthcare personnel vaccination: a necessary foundation for the essential work that remains to build successful programs. Infect Control Hosp Epidemiol. 2012;33(1):71‐74. doi: 10.1086/662715 [DOI] [PubMed] [Google Scholar]
- 33. Rasmussen SA, Jamieson DJ. What obstetric health care providers need to know about measles and pregnancy. Obstet Gynecol. 2015;126(1):163‐170. doi: 10.1097/AOG.0000000000000903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Campbell H, Andrews N, Brown KE, Miller E. Review of the effect of measles vaccination on the epidemiology of SSPE. Int J Epidemiol. 2007;36(6):1334‐1348. doi: 10.1093/ije/dym207 [DOI] [PubMed] [Google Scholar]
- 35. Charlier C, Hourrier S, Leruez‐Ville M, et al. Polyvalent immunoglobulins in neonates after perinatal exposure to measles. Benefits and long‐term tolerance of immunoglobulins. J Infect. 2015;71(1):131‐134. doi: 10.1016/j.jinf.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 36. Kaplan LJ, Daum RS, Smaron M, McCarthy CA. Severe Measles in Immunocompromised Patients. JAMA J Am Med Assoc. 1992;267(9):1237. doi: 10.1001/jama.1992.03480090085032 [DOI] [PubMed] [Google Scholar]
- 37. Huang JG, Juh Yeoh AE, Tambyah PA, Isa Suhaila MB. The conundrum in the diagnosis and management of atypical fulminant measles in a leukemia survivor on maintenance chemotherapy. J Pediatr Hematol Oncol. 2017;39(1):e36‐e38. doi: 10.1097/MPH.0000000000000620 [DOI] [PubMed] [Google Scholar]
- 38. Ljungman P, Lonnqvist B, Bolme P, et al. Efficacy and safety of vaccination of marrow transplant recipients with a live attenuated measles, mumps, and rubella vaccine. J Infect Dis. 1989;159(4):610‐615. doi: 10.1093/infdis/159.4.610 [DOI] [PubMed] [Google Scholar]


