Abstract
Background
Immature platelets (IP) are the youngest circulating platelets, released from megakaryocytes, and demonstrating increased dimensions, significant RNA content, and enhanced activity. Immature platelet research focuses on a differential diagnostic help in patients with thrombocytopenia. The objectives of this study were to compare the variability of IP in citrate and EDTA samples, and to determine stability over time.
Methods
Fifty‐six patients were included for comparison between EDTA and citrate whole blood sample collection. Among the patients, 28 had thrombocytopenia (platelet count < 150G/L). Platelet measurement impedancemetry and fluorimetry were performed with Sysmex XN‐9000. The immature platelet fraction (IPF) and absolute immature platelet count (A‐IPC) were determined with a fluorescent method.
Results
The mean value of platelet count with fluorescence was, in EDTA sample, 215 ± 171 and, in citrate sample, 153 ± 118 G/L. No significant difference was observed between IPF between EDTA and citrate (7.74 ± 6.68% vs. 8.45 ± 7.37%, p = 0.69), respectively. With the Bland–Altman analysis, the mean difference in the EDTA sample, between 1 and 24 h, was 8.06 ± 6.96% and 8.73 ± 7.12% for IPF, whereas in the citrate sample, between 1 and 6 h, it was 8.60 ± 7.29% and 7.54 ± 6.97%, for IPF. Comparing 1 h EDTA sample with 6 h citrate sample, the variance ratio was 0.974 (95% CI: 0.864–1.084) in IPF.
Conclusions
We confirmed the potential to conduct IP measurements up to 24 h in the EDTA sample and IPF measurements in the citrate sample for up to 6 h. These results may be useful for the use of IPF, which is a promising parameter whose interest in clinical practice and standardization is not yet well defined.
Keywords: citrate, delayed analysis, EDTA, immature platelet count, immature platelet fraction
Immature platelet research focuses on a differential diagnostic help in patients with thrombocytopenia. We confirmed the possibility to perform the IP measurement until 24 h in the EDTA sample and IPF in the citrate sample until 6 h. These results may be useful for the use of IPF, which is a promising parameter whose interest in clinical practice and standardization is not yet well defined.
1. INTRODUCTION
Platelets are small, non‐nucleated blood cells that are produced in the bone marrow. Among them, immature platelets (IPs), which are the youngest circulating platelets and derived from megakaryocytes, are characterized by a larger size and a higher potential for activation. 1 IPs are equivalent to reticulocyte counts in the context of anemia and can provide valuable diagnostic information for thrombocytopenic patients. 1 Rapid assessment of IPs can differentiate between thrombocytopenia caused by bone marrow failure or imminent bone marrow recovery after cytotoxic therapy or increased peripheral platelet destruction. 2
A spurious low platelet count can be caused by ethylenediamine tetraacetic acid (EDTA), which is present in peripheral blood samples. EDTA‐induced pseudothrombocytopenia is a rare phenomenon (occurring in 0.1%–2% of hospitalized patients) that is not associated with hemorrhagic signs. 3 Platelet clumps can be checked by viewing a May Grunwald–Giemsa‐stained blood smear. 4 Sodium citrate is an alternative anticoagulant that minimizes clumping and provides results on automated analyzers. 5 However, citrate used as an anticoagulant leads to a negative bias in platelet count due to anticoagulant dilution and worsening over time. 6
Currently, available hematology analyzers use different methods, such as impedance or fluorescent methods, to count platelets. IP quantification can be performed using flow cytometers or fluorescent dyes that bind to RNA present in young platelets. 7 , 8 In daily practice, IP results can be obtained in less than 1 min on hematology automatic analyzers. 2 However, this method has limited clinical utility due to a lack of standardization and variation in reference intervals.
The aim of this study was to compare the variability of IPs in the citrate and EDTA samples and determine stability over time.
2. MATERIALS AND METHODS
2.1. Patients
This prospective study was conducted between 2019 and 2022. Included patients had laboratory measurement of whole blood count. Clinical data, biological results, treatment, and clinical outcomes were retrieved from hospital medical records. The study was performed in accordance with the Declaration of Helsinki. The institutional review board approved the study (person committee protection of Rouen University Hospital) and an anonymous data collection was declared (protocol number: E‐2023‐01).
2.2. Blood sample collection
Whole blood was collected on EDTA and citrated tubes. The anticoagulant in the used tubes were sodium citrate: 3.2%, 0.109 M (Greiner). Complete blood count was performed using XN‐9000 (Sysmex, Villepinte, France). The platelet measurement, with impedancemetry and fluorimetry was performed. The immature platelet fraction (IPF) and absolute immature platelet count (A‐IPC) was determined with a fluorescent method. This method improves the gating of platelets, using side fluorescence (reflecting RNA content), side scatter (intracellular structure), and forward scatter (cell sizer). For thrombocytopenic patients, a blood smear was performed to evaluate EDTA clumping.
The citrate sample was diluted with anticoagulant at a ratio of 1:10 and assessed using the following ratio: analysis with citrate sample/analysis with EDTA sample.
Each sample was kept at room temperature and analyzed at 30 min, 1, 2, 4, 6 and 24 h.
To evaluate no technical error with dilution effect of citrate versus EDTA, we performed haematocrit citrate/haematocrit EDTA ratio.
2.3. Statistical analysis
Data are expressed as mean ± standard deviation (SD) or median [IQR]. Deming regression and the Bland–Altman analysis were performed to assess the concordance between the citrate and EDTA sampling. Correlation, the Bland–Altman plot, and Deming regression were performed with Graphpad Prism 9.4.1. p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Comparison between citrate and EDTA
Fifty‐six patients were included for comparison between the EDTA and citrate sampling. Median result of haematocrit citrate/haematocrit EDTA ratio was 0.88 [IQR: 0.81–0.95].
Among the patients, 28 had thrombocytopenia (platelet count < 150G/L) and three thrombocytosis (platelet count>450 G/L). Among thrombocytopenia, 18 had platelet count < 100G/L and six had platelet count < 50G/L. By fluorescence, the mean value of platelet count in EDTA sample was 215 ± 171 G/L (maximal range: 14–913 G/L) and in citrate sample was 153 ± 118 G/L (maximal range 4–596 G/L) (Table 1). No significant difference was observed between IPFs in EDTA and citrate (7.74 ± 6.68% vs. 8.45 ± 7.37%, p = 0.69) respectively. Platelet impedance count was lower than platelet fluorescent count (193 ± 152 G/L vs. 215 ± 171G/L; p = 0.37).
TABLE 1.
Laboratory characteristics in the EDTA and citrate samples.
| EDTA sample | Citrate sample | |
|---|---|---|
| Red blood cells (T/L) | 3.80 ± 0.91 | 3.47 ± 0.88 |
| Hemoglobin (g/dL) | 11.29 ± 2.58 | 10.32 ± 2.46 |
| Hematocrit (%) | 34.0 ± 7.1 | 30.7 ± 7.1 |
| MCV (fL) | 90.7 ± 6.8 | 89.4 ± 6.6 |
| Platelet impedance count (G/L) | 193 ± 152 | 113 ± 90.8 |
| Platelet fluorescent count (G/L) | 215 ± 171 | 153 ± 118 |
| IPF (%) | 7.74 ± 6.88 | 8.45 ± 7.37 |
| A‐IPC (G/L) | 11.67 ± 8.52 | 9.01 ± 6.68 |
Abbreviations: A‐IPC, absolute immature platelet count; IPF, immature platelet fraction; MCV, mean corpuscular volume.
The ratio of citrate/EDTA analysis is summarized in Table 2. The ratio of platelet measurement was 0.655 ± 0.156 for fluorecent assay. The ratio of IP was 1.09 ± 0.25 and 0.692 ± 0.145 for IPF and A‐IPC, respectively.
TABLE 2.
Ratio of citrate/EDTA results.
| Ratio Citrate/EDTA | |
|---|---|
| Platelet fluorescent count (G/L) | 0.655 ± 0.156 |
| IPF (%) | 1.086 ± 0.249 |
| A‐IPC (G/L) | 0.692 ± 0.145 |
Abbreviations: A‐IPC, absolute immature platelet count; IPF, immature platelet fraction.
3.2. Stability over the time of fluorescent parameters in EDTA and citrate sample
We evaluated the possibility to perform delayed EDTA and citrate platelet measurement with Deming regression (Figure S1). Regarding EDTA sample comparison between 1 and 24 h, the variance ratio was 0.959 (95% CI: 0.885–1.034). Regarding citrate sample comparison between 1 and 6 h, the variance ratio was 0.955 (95% CI: 0.784–1.127). The variance ratio was not acceptable in citrate sample comparison between 1 and 24 h: 1.23 (95% CI: 1.039–1.42) (Figure S1C).
The mean differences ±SD in EDTA sample, between 1 and 24 h were 8.06 ± 6.96% and 8.73 ± 7.12% for IPF and 11.80 ± 8.51 G/L and 12.39 ± 8.69 G/L for A‐IPC. The concordance between 1 and 24 h was −0.66 ± 2.2% for IPF (Figure 1), and 0.60 ± 2.59 G/L for A‐IPC (Figure 2).
FIGURE 1.
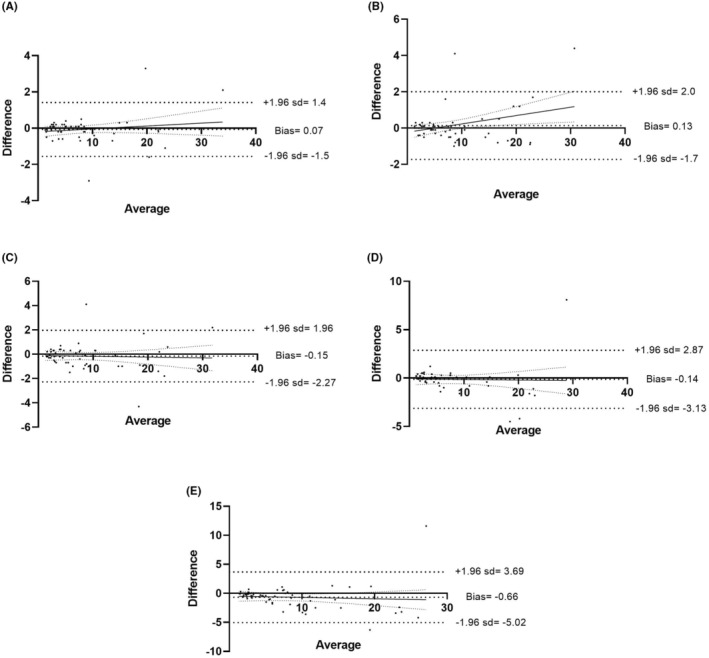
Comparison of IPF‐delayed analysis in EDTA sample. Bland–Altman comparison between 1 and 0.5 h (A), 2 h (B), 4 h (C), 6 h (D) and 24 h (E).
FIGURE 2.
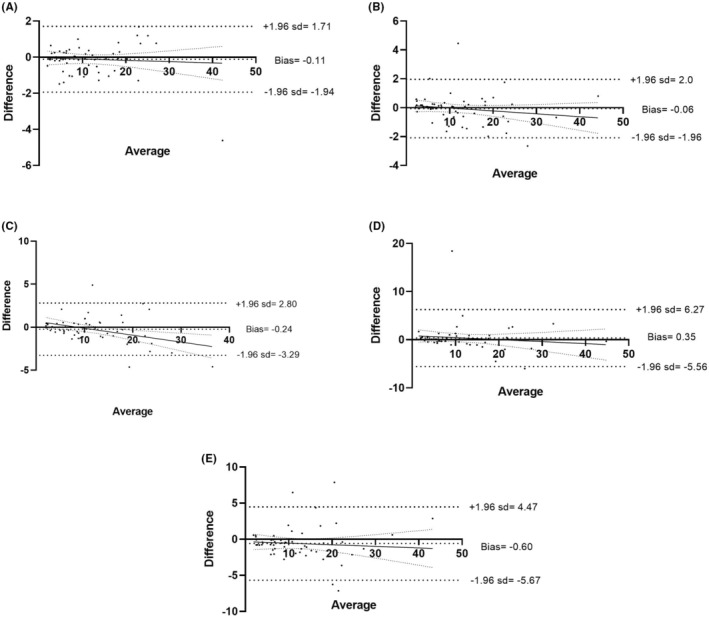
Comparison of A‐IPC‐delayed analysis in EDTA sample. Bland–Altman comparison between 1 and 0.5 h (A), 2 h (B), 4 h (C), 6 h (D) and 24 h (E).
The mean differences ± SD in citrate sample, between 1 and 6 h were 8.60 ± 7.29% and 7.54 ± 6.97%, and 8.22 ± 6.12 G/L and 6.73 ± 5.70 G/L for IPF and A‐IPC, respectively. The concordance between 1 and 6 h was 1.02 ± 2.73% for IPF (Figure S2) and 1.42 ± 2.05 G/L for A‐IPC (Figure S3). The Bland–Altman analysis demonstrated a poor concordance between 1 and 24 h IPF measurement, with four points outside the range (Figure S2E).
3.3. Comparison between EDTA and citrate sample over the time
We also evaluated the possibility to perform delayed EDTA and citrate IP measurement with Deming regression. Regarding EDTA sample comparison between 1 and 24 h, the variance ratio was 1.024 (95% CI: 0.785–1.263) and 1.023 (95% CI: 0.891–1.155) in IPF and A‐IPC, respectively (Figure 3A,B). Regarding comparison between 1 h EDTA sample and 6 h citrate sample, the variance ratio was 0.974 (95% CI: 0.864–1.084) in IPF (Figure 3C). The variance ratio was not acceptable in 1 h EDTA sample and 6 h citrate sample A‐IPC comparison (Figure 3D) and in 1 h EDTA sample and 24 h citrate sample IPF comparison (Figure 3E). Equations are in Table S1.
FIGURE 3.
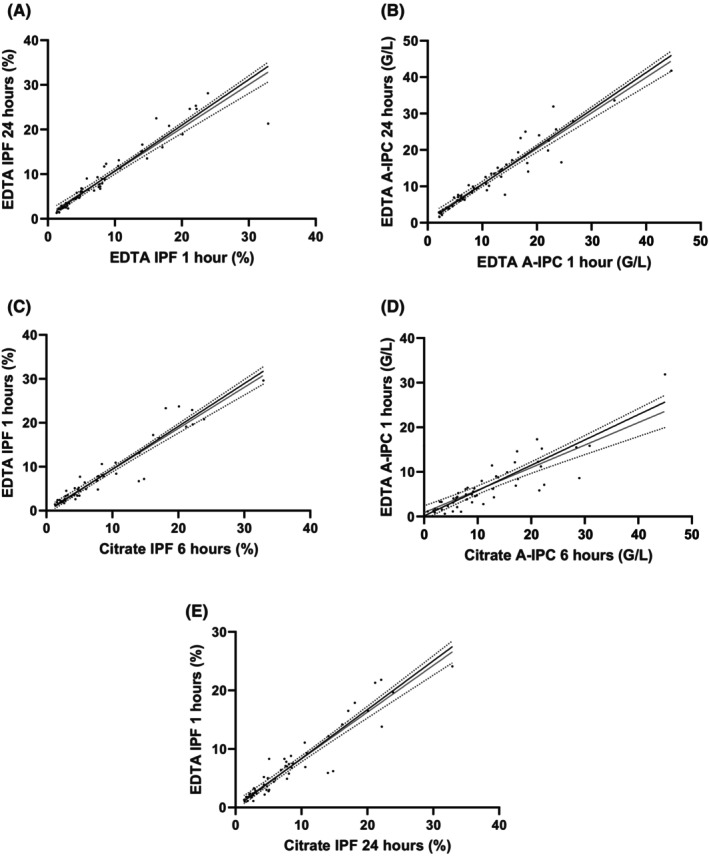
Stability over the time evaluation with Deming regression. Evaluation between 1 and 24 h in EDTA sample for IPF (A) and A‐IPC (B). Evaluation between 1 and 6 h in EDTA and citrate sample, respectively, for IPF (C) and A‐IPC (D). Evaluation between 1 and 24 h in the EDTA and citrate sample, respectively, for IPF (E).
4. DISCUSSION
Our study demonstrated the stability of IP in EDTA samples and showed that IP measurement in citrate samples could be performed up to 6 h after blood collection. Sodium citrate is the most widely used anticoagulant to prevent platelet aggregation in vitro, and it is recommended to perform the platelet count within a maximum of 3 h after blood collection. 9 Platelet activation and aggregation induced by citrate anticoagulant is much more rare than EDTA. 10 However, citrate can cause platelet activation and aggregation, and the study showed a decrease in platelet count of nearly 40% due to citrate dilution. The use of magnesium sulfate (MgSO4) was found to be more effective than citrate in removing platelet clumps, but it resulted in a decrease in the platelet fluorescent count. 11 Fluorescent measurement may be more suitable in citrate anticoagulant samples and showed better detection in low platelet ranges, especially in the presence of microcytes. 12 In patients with a congenital thrombocytopenia associated with giant platelets (MYH9 disorder), the platelet count may be underestimated by platelet measurement in red blood cells. 13
The release of platelets from megakaryocytes results in the presence of a small amount of RNA within the platelets. 14 The concentration of immature platelets has been found to correlate with megakaryocyte activity. 15 While platelets have a lifespan of 8 days in the circulation, IP are transitory with a much shorter lifespan similar to that of reticulocytes, making them a useful marker for megakaryopoietic activity in the bone marrow. 14 Despite the lack of standardization and correlation in IP measurement among different analyzers, these parameters are good indicators of thrombopoietic activity. 14 , 16 , 17 Previous studies have shown heterogeneity in the analytical performance of different hematological analyzers for IP measurement. 18 On the XN modular series, IP is measured using a specific optical fluorescence method and oxazine RNA dye. A study in 10 healthy individuals and 20 routine samples demonstrated good stability of IP measurement over 24 h in the EDTA samples, with platelet counts above 100 G/L. However, platelet counts in the thrombocytopenic range are important for assessing bleeding risk and transfusion requirements, which were not evaluated in this study.
Stability of IP has not been yet evaluated in the citrate sample. The stability of IP measurement in citrate samples, which are performed to prevent platelet clumping induced by EDTA, was evaluated and found to be stable for up to 6 h. Logically, A‐IPC was decreased, induced by anticoagulant citrated dilution. Immature platelet measurement help on a differential diagnostic in patients with thrombocytopenia. The bone marrow failure is associated with a low megakaryopoietic activity, the assumption was that consequently IP would be low in aplastic patients. In contrast, conditions with peripheral platelet destruction are characterized by accelerated megakaryopoiesis and IP increase. Many studies have confirmed that IPF are valuable in establishing the cause of thrombocytopenia: decreased production can reliably be distinguished from peripheral destruction. Immature platelet measurement is useful in differentiating the cause of thrombocytopenia and has been found to be valuable in establishing the distinction between decreased production and peripheral destruction. 2 , 19 The correlation between platelet counts and IP has not been thoroughly evaluated. In aplastic patients, there is a correlation between platelet counts and IP. However, in the context of inflammation or infection, thrombocytosis is often observed and the evaluation of immature platelet levels is not commonly performed. Furthermore, it is important to interpret platelet counts and immune parameters (IPs) in conjunction with other relevant clinical circumstances.
Immature platelet measurement is also of interest in patients undergoing chemotherapy and transplantation for hematological malignancies, as an increase in IP has been shown to precede the recovery of platelet count by 2–3 days. 20 An increase in IP precedes the recovery of platelet count by 2–3 days. Based on these results, the opportunity to defer platelet transfusions that would be given when transfusion decisions are based on platelet counts only. 21 IP has also been implicated in several pathologies, such as acute myocardial injury and sepsis, and has been found to be an early predictor of worsening in severe COVID‐19 patients. 22 , 23 , 24 The use of IP measurement, particularly with citrated anticoagulant, could help in patients with pseudothrombocytopenia induced by EDTA anticoagulant.
This study has limitations, including the use of only one hematological analyzer and the lack of checking of platelet counts obtained with the reference. 25 However, previous studies have shown good measurement using the XN analyzer. The results of this study suggest that IP measurement is a promising parameter for clinical use, although its full clinical utility and standardization are yet to be defined.
5. CONCLUSION
Our study has demonstrated the feasibility of measuring immature platelet fractions (IPFs) in the EDTA‐anticoagulated samples for up to 24 h, and in the citrate‐anticoagulated samples for up to 6 h, at room temperature. These findings are of significance as IPF is an important indicator of megakaryopoietic activity and have potential clinical utility, although the standardization and correlation between measurements obtained using different analytical instruments is still an area in need of improvement.
AUTHOR CONTRIBUTIONS
MM Noel, G. Feugray, and F. Kasonga included patients and discussed the obtained results and critically revised the manuscript. V. Barbay, G. Buchonnet, S. Daliphard, and E. Bera critically revised the manuscript and results. V. Le Cam Duchez analyzed and interpreted the data. P. Billoir designed the research, analyzed and interpreted the data and wrote the manuscript All authors read and approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
Authors declare no conflict of interest.
Supporting information
Figure S1‐S3.
Table S1.
Noel MM, Feugray G, Kasonga F, et al. Stability over time of immature platelet fraction and comparison between EDTA and citrated whole blood samples. J Clin Lab Anal. 2023;37:e24946. doi: 10.1002/jcla.24946
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Reeves HM, Maitta RW. Immature platelet dynamics in immune‐mediated thrombocytopenic states. Front Med (Lausanne). 2020;7:597734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Briggs C, Kunka S, Hart D, Oguni S, Machin SJ. Assessment of an immature platelet fraction (IPF) in peripheral thrombocytopenia. Br J Haematol. 2004;126:93‐99. [DOI] [PubMed] [Google Scholar]
- 3. Chae H, Kim M, Lim J, Oh E‐J, Kim Y, Han K. Novel method to dissociate platelet clumps in EDTA‐dependent pseudothrombocytopenia based on the pathophysiological mechanism. Clin Chem Lab Med. 2012;50:1387‐1391. [DOI] [PubMed] [Google Scholar]
- 4. Baccini V, Geneviève F, Jacqmin H, et al. Platelet counting: ugly traps and good advice. Proposals from the French‐speaking cellular hematology group (GFHC). J Clin Med. 2020;9:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zandecki M, Genevieve F, Gerard J, Godon A. Spurious counts and spurious results on haematology analysers: a review. Part I: platelets. Int J Lab Hematol. 2007;29:4‐20. [DOI] [PubMed] [Google Scholar]
- 6. Weber D, Nakashima MO. Platelet count in sodium citrate‐anticoagulated whole blood: comparison to EDTA‐anticoagulated results and stability over time. Int J Lab Hematol. 2021;43:e35‐e37. [DOI] [PubMed] [Google Scholar]
- 7. D'Souza C, Briggs C, Machin SJ. Platelets: the few, the young, and the active. Clin Lab Med. 2015;35:123‐131. [DOI] [PubMed] [Google Scholar]
- 8. Robinson MS, Mackie IJ, Khair K, et al. Flow cytometric analysis of reticulated platelets: evidence for a large proportion of non‐specific labelling of dense granules by fluorescent dyes. Br J Haematol. 1998;100:351‐357. [DOI] [PubMed] [Google Scholar]
- 9. Lardinois B, Favresse J, Chatelain B, Lippi G, Mullier F. Pseudothrombocytopenia‐A review on causes, occurrence and clinical implications. J Clin Med. 2021;10:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schrezenmeier H, Müller H, Gunsilius E, Heimpel H, Seifried E. Anticoagulant‐induced pseudothrombocytopenia and pseudoleucocytosis. Thromb Haemost. 1995;73:506‐513. [PubMed] [Google Scholar]
- 11. Soulard M, Croix P, Cohen P. Comparison of platelet count results on the Sysmex XN between citrate or MgSO4 and K2 EDTA anticoagulants. Int J Lab Hematol. 2003;45:20‐28. doi: 10.1111/ijlh.13966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanaka Y, Tanaka Y, Gondo K, et al. Performance evaluation of platelet counting by novel fluorescent dye staining in the XN‐series automated hematology analyzers. J Clin Lab Anal. 2014;28:341‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greinacher A, Pecci A, Kunishima S, et al. Diagnosis of inherited platelet disorders on a blood smear: a tool to facilitate worldwide diagnosis of platelet disorders. J Thromb Haemost. 2017;15:1511‐1521. [DOI] [PubMed] [Google Scholar]
- 14. Hoffmann JJML. Reticulated platelets: analytical aspects and clinical utility. Clin Chem Lab Med. 2014;52:1107‐1117. [DOI] [PubMed] [Google Scholar]
- 15. Stohlawetz P, Schulenburg A, Stiegler G, et al. The proportion of reticulated platelets is higher in bone marrow than in peripheral blood in haematological patients. Eur J Haematol. 1999;63:239‐244. [DOI] [PubMed] [Google Scholar]
- 16. Imperiali CE, Arbiol‐Roca A, Sanchez‐Navarro L, et al. Reference interval for immature platelet fraction on Sysmex XN haematology analyser in adult population. Biochem Med (Zagreb). 2018;28:010708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ali U, Knight G, Gibbs R, Tsitsikas DA. Reference intervals for absolute and percentage immature platelet fraction using the Sysmex XN‐10 automated haematology analyser in a UK population. Scand J Clin Lab Invest. 2017;77:658‐664. [DOI] [PubMed] [Google Scholar]
- 18. Gioia M, Da Rin G, Manenti B, et al. Multicenter evaluation of analytical performances of platelet counts and platelet parameters: carryover, precision, and stability. Int J Lab Hematol. 2020;42:552‐564. [DOI] [PubMed] [Google Scholar]
- 19. Meintker L, Haimerl M, Ringwald J, Krause SW. Measurement of immature platelets with Abbott CD‐Sapphire and Sysmex XE‐5000 in haematology and oncology patients. Clin Chem Lab Med. 2013;51:2125‐2131. [DOI] [PubMed] [Google Scholar]
- 20. Kuter DJ. Managing thrombocytopenia associated with cancer chemotherapy. Oncology (Williston Park). 2015;29:282‐294. [PubMed] [Google Scholar]
- 21. Have LWJ, Hasle H, Vestergaard EM, Kjaersgaard M. Absolute immature platelet count may predict imminent platelet recovery in thrombocytopenic children following chemotherapy. Pediatr Blood Cancer. 2013;60:1198‐1203. [DOI] [PubMed] [Google Scholar]
- 22. Yahud E, Schilo T, Nevzorov R, et al. Immature platelet fraction over time and clinical outcomes in patients with acute myocardial infarction. Int J Lab Hematol. 2021;43:966‐972. [DOI] [PubMed] [Google Scholar]
- 23. Thorup CV, Christensen S, Hvas A‐M. Immature platelets As a predictor of disease severity and mortality in sepsis and septic shock: a systematic review. Semin Thromb Hemost. 2020;46:320‐327. [DOI] [PubMed] [Google Scholar]
- 24. Billoir P, Leprêtre P, Thill C, et al. Routine and advanced laboratory tests for hemostasis disorders in COVID‐19 patients: a prospective cohort study. J Clin Med. 2022;11:1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. International Council for Standardization in Hematology (ICSH) . International council for standardization in hematology expert panel on cytometry and international society of laboratory hematology task force on platelet counting. Platelet counting by the RBC/platelet ratio method: a reference method. Am J Clin Pathol. 2001;115:460‐464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1‐S3.
Table S1.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


