Abstract
Background:
Depression has a major impact on the disease burden of multiple sclerosis (MS). Analyses of overlapping MS and depression risk factors [smoking, vitamin D (25-OH-VD) and Epstein-Barr virus (EBV) infection] and sex, age, disease characteristics and neuroimaging features associated with depressive symptoms in early MS are scarce.
Objectives:
To assess an association of MS risk factors with depressive symptoms within the German NationMS cohort.
Design:
Cross-sectional analysis within a multicenter observational study.
Methods:
Baseline data of n = 781 adults with newly diagnosed clinically isolated syndrome or relapsing-remitting MS qualified for analysis. Global and region-specific magnetic resonance imaging (MRI)-volumetry parameters were available for n = 327 patients. Association of demographic factors, MS characteristics and risk factors [sex, age, smoking, disease course, presence of current relapse, expanded disability status scale (EDSS) score, fatigue (fatigue scale motor cognition), 25-OH-VD serum concentration, EBV nuclear antigen-1 IgG (EBNA1-IgG) serum levels] and depressive symptoms (Beck Depression Inventory-II, BDI-II) was tested as a primary outcome by multivariable linear regression. Non-parametric correlation and group comparison were performed for associations of MRI parameters and depressive symptoms.
Results:
Mean age was 34.3 years (95% confidence interval: 33.6–35.0). The female-to-male ratio was 2.3:1. At least minimal depressive symptoms (BDI-II > 8) were present in n = 256 (32.8%), 25-OH-VD deficiency (<20 ng/ml) in n = 398 (51.0%), n = 246 (31.5%) participants were smokers. Presence of current relapse [coefficient (c) = 1.48, p = 0.016], more severe fatigue (c = 0.26, p < 0.0001), lower 25-OH-VD (c = −0.03, p = 0.034) and smoking (c = 0.35, p = 0.008) were associated with higher BDI-II scores. Sex, age, disease course, EDSS, month of visit, EBNA1-IgG levels and brain volumes at baseline were not.
Conclusion:
Depressive symptoms need to be assessed in early MS. Patients during relapse seem especially vulnerable to depressive symptoms. Contributing factors such as fatigue, vitamin D deficiency and smoking, could specifically be targeted in future interventions and should be investigated in prospective studies.
Keywords: CIS, clinically isolated syndrome, cohort study, depression, MRI, MS, neuropsychological symptoms, relapse, sex, smoking, vitamin D
Introduction
Lifetime prevalence of depression in persons with multiple sclerosis (MS) may range between 24% and 54%, thus exceeding the prevalence of 14 to 21% within the general population. 1 Depression can be present in all stages of MS, including clinically isolated syndrome (CIS), 2 and may be observed as one of a variety of prodromal signs of MS. 3
Depressive symptoms have a major impact on well-being and quality of life in persons with MS. They reduce work productivity4,5 and impact social participation, 6 but are not necessarily associated with a more severe disease course. 7
Data on sex differences of depressive symptoms in MS have revealed contradicting results, whereas one study has reported a female preponderance and higher rates of somatic symptoms in women, another study did not detect sex differences for depression in MS representing a distinctive feature towards the general population.8,9
The interaction of fatigue and depression has been well established, but does not seem to be specific for MS.10,11 A small study assessing patients during MS relapse has identified both age and relapse severity as independent predictors of depressive symptoms measured by Beck Depression Inventory-II (BDI-II), but not the disability level per se. 12 In line with this, a recent meta-analysis did not uncover clear associations of the prevalence of depression with disability level or disease duration. However, both parameters have been defined with binary cut-offs [more or less than expanded disability status scale (EDSS) score 3.0, and more or less than 10 years disease duration, respectively] 13 which might not be sensitive enough to detect differences and especially does not assess the phase around disease onset.
Modifiable factors well-known to increase MS susceptibility and progression, such as smoking14,15 and vitamin D deficiency,16–18 also in interaction with obesity, 19 have also been attributed to the risk of developing depressive symptoms.20,21 The pathophysiological role of vitamin D deficiency in depression is described to be multifaceted: direct effects may play a role as vitamin D receptors are present in the brain, especially in the cortex, limbic system and hippocampus and as vitamin D regulates different neurotrophic factors and modulates calcium toxicity and oxidative stress. In addition, it regulates neurotransmitter homeostasis for serotonin, dopamine and norepinephrine in the brain. 22
Likewise, remote Epstein-Barr virus (EBV) infection has been associated with a higher risk of developing both MS16,23–25 and major depressive disorders. 26
Comprehensive analyses of the association of MS risk factors, including smoking, vitamin D (25-OH-VD) levels and EBV antibody levels, with depressive symptoms in patients with early MS are scarce.
Neuroanatomical correlations using different magnetic resonance imaging (MRI) techniques have been investigated for MS-related symptoms such as fatigue and depression. T2-hyperintense white matter lesions load, as a commonly used MRI parameter in MS, in varying brain regions such as the right temporal lobe,2,27 amygdala/prefrontal tracts 28 has been associated with depression in MS, but another study has not detected a specific lesion pattern for both T1- and T2-weighted sequences. 29 In functional MRI analyses, patients with MS and depression had less capacities of neurocognitive emotion regulation corroborating the involvement of amygdala and prefrontal tracts as compared to patients with MS only. 28
MRI-based volumetry has demonstrated associations of MS-related fatigue with specific subcortical gray matter areas, namely the basal ganglia, and the pons. 30 Another study reported the atrophy of several cortical gray matter areas to be associated with depression or a complex of fatigue and depression, but not fatigue alone. 29 No association of gray matter atrophy and related neurotransmitter maps has been detected for depression in a more recent analysis. 31
With the increased risk of depression in MS patients, we hypothesised that common risk factors might play a role and wanted to investigate a set of markers to potentially identify populations at risk for depressive symptoms within a well-defined early MS cohort.
The aim of this study was thus to assess the potential association of MS disease characteristics and risk factors to depressive symptoms in participants of the German NationMS cohort.
Methods
Study design and setting
The German NationMS study is an ongoing multicentre prospective, longitudinal, observational cohort study as previously described. 32 The 22 participating centres across Germany represent tertiary care centres. Participants were recruited for the study between 2010 and 2017; n = 1374 persons were included in the study. All data were entered in a centralised database, hosted by the Central Information Office Marburg, and regularly monitored for completeness and accuracy. For the present cross-sectional analysis, only data referring to the baseline visit for each participant were considered.
Participants
In- and exclusion criteria for the German NationMS study are described in detail, elsewhere. 32 In brief, adult patients with newly diagnosed CIS fulfilling 3 of 4 Barkhof criteria 33 or 2 of 4 Barkhof criteria with additional cerebrospinal fluid or visual evoked potential findings suggestive of MS or with relapsing-remitting MS (RRMS) according to McDonald criteria 2005 34 or 2010, 35 naïve for treatment with disease-modifying drugs, were eligible for the study.
All patients with the available baseline data of interest including baseline results for 25-OH-VD serum concentration 17 and EBV nuclear antigen-1 IgG (EBNA1-IgG) serum antibody levels 23 were included in this analysis.
Variables of interest for this study
The full assessment plan has been described. 32 In summary, it comprises yearly visits over the first 2 years and biannual visits thereafter. The standardised assessments include sociodemographic data (age, sex, ethnicity, education, employment status, social situation, smoking, alcohol consumption and other drugs), medical history (concomitant diseases and medications, family history), physical examination and MS-specific evaluations (MS history, relapse documentation, neurological status incl. EDSS and MS Functional Composite), a short cognitive testing (MS Inventory of Cognition), patient questionnaires [BDI-II, fatigue scale motor cognition (FSMC), PainDETECT] as well as a standardised brain MRI and collection of biomaterial.
Within this analysis, baseline data of participants were assessed for the following variables (measure):
- Sex (male, female)
- Age (years)
- Smoking behaviour (no, irregularly, daily)
- Diagnosis (CIS, RRMS)
- Current relapse at visit date (yes/no)
- Disability level (EDSS)
- Depressive symptoms (BDI-II)
- Fatigue (FSMC)
- Vitamin D level [25-OH-VD serum concentration in ng/ml, measured using a commercially available kit for liquid chromatography-mass spectrometry (LC-MS/MS) 17 ]
- EBNA1-IgG serum levels [in U/ml, measured using Liaison (DiaSorin, Saluggia, Italy) automated quantitative chemiluminescence immunoassay (CLIA) 23 ]
To address potential corticosteroid effects on depressive symptoms, time in days between last corticosteroid administration and the date of visit (available for n = 755) was additionally analysed.
MRI acquisition and morphometric analyses
Per NationMS study protocol, conventional MRI images were acquired at different 3T scanners with a 32-channel receive-only head coil, according to a standardised imaging protocol in all centres. This protocol included sagittal 3D T1-weighted magnetisation prepared rapid acquisition of gradient echo and T2-weighted fluid-attenuated inversion recovery sequences. Available morphometric data from previous NationMS studies has been re-used for this analysis. The detailed methodology can thus be found in Refs. 14 and 30.
The following MRI parameters have thus been integrated in the analysis:
- Global volumes: T2 lesion volume, gray matter volume and total brain volume (in ml).
- Region-specific volumes: thalamus volumes, hippocampus volumes and amygdala volumes as well as the total subcortical gray matter volume (in µl).
Statistical methods
Patient data were summarised descriptively, as given in Table 1. Categorical variables are listed with total number (n) and relative frequencies (%). Continuous variables are expressed with mean and 95% confidence intervals (CI). In case of single missing items within a questionnaire, these data were imputed using mean imputation. 32
Table 1.
Cohort characteristics.
| Variable | Indicator |
|---|---|
| Sex (n, %) | |
| Female | 546 (69.9) |
| Male | 235 (30.1) |
| Age (years; mean, 95% CI) | 34.3 (33.6–35.0) |
| Smoking (n, %) | |
| No | 535 (68.5) |
| Irregularly | 56 (7.2) |
| Daily | 190 (24.3) |
| Diagnosis (n, %) | |
| CIS | 327 (41.9) |
| RRMS | 454 (58.1) |
| Current relapse (n, %) | |
| No | 683 (87.5) |
| Yes | 98 (12.5) |
| EDSS (mean, 95% CI) | 1.5 (1.4–1.6) |
| BDI-II (score; mean, 95% CI) | 7.3 (6.8–7.8) |
| BDI-II score, cumulative frequencies* (n, %) | |
| ⩽8 points | 525 (67.2) |
| >8 points | 256 (32.8) |
| >13 points | 126 (16.1) |
| >20 points | 54 (6.9) |
| >29 points | 13 (1.7) |
| FSMC (total score; mean, 95% CI) | 38.2 (36.9–39.5) |
| 25-OH-VD (ng/ml; mean, 95% CI) | 22.2 (21.2–23.2) |
| 25-OH-VD deficiency (<20 ng/ml; n, %) | 398 (51.0) |
| EBNA1-IgG (U/ml; mean, 95% CI) | 1453.8 (1360.4–1547.2) |
| MRI volumetry parameters | |
| T2 lesion volume (ml; mean, 95% CI) | 131.3 (−113.9–376.4) |
| Gray matter volume (ml; mean, 95% CI) | 637.8 (629.0–646.6) |
| Total brain volume (ml; mean, 95% CI) | 1388.1 (1370.8–1405.4) |
| Thalamus left (µl; mean, 95% CI) | 7211.2 (7108.7–7313.7) |
| Thalamus right (µl; mean, 95% CI) | 6851.3 (6757.5–6945.0) |
| Hippocampus left (µl; mean, 95% CI) | 4010.7 (3962.7–4058.8) |
| Hippocampus right (µl; mean, 95% CI) | 4131.8 (4082.6–4181.0) |
| Amygdala left (µl; mean, 95% CI) | 1610.6 (1586.1–1635.1) |
| Amygdala right (µl; mean, 95% CI) | 1759.9 (1733.6–1786.3) |
| Subcortical gray matter volume (µl; mean, 95% CI) | 56,270.9 (55,722.5–56,819.3) |
⩽8 points, no depressive symptoms; >8 points, minimal; >13 points, mild; >20 points, moderate; >29 points, severe depressive symptoms.
BDI-II, Beck Depression Inventory-II; 95% CI, 95% confidence interval; CIS, clinically isolated syndrome; EBNA1-IgG, Epstein-Barr virus nuclear antigen-1 IgG; EDSS, expanded disability status scale; FSMC, fatigue scale motor cognition; MRI, magnetic resonance imaging; 25-OH-VD, vitamin D; RRMS, relapsing-remitting multiple sclerosis.
As defined in the scoring manual, a BDI-II score of >8 points was defined as ‘at least minimal depressive symptoms’. 25-OH-VD deficiency was defined as values below 20 ng/ml as described in Ref. 36.
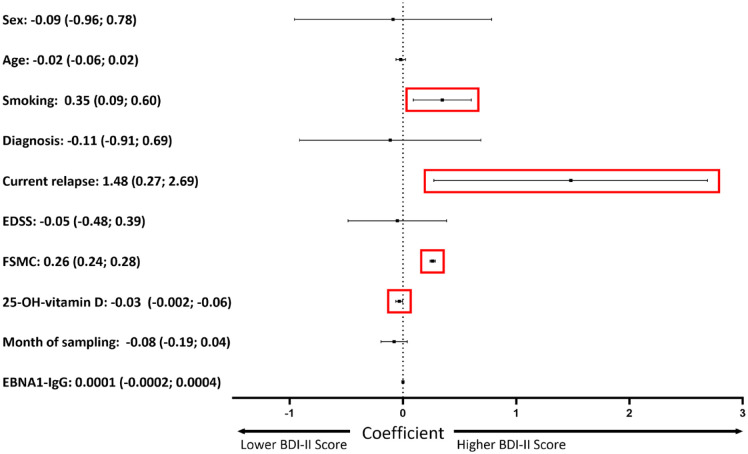
Multivariable linear regression (MLR) was performed using BDI-II score as the dependent variable and all named other factors as independent variables. Results are expressed as coefficient (c) with 95% CI (Figure 1). Corresponding p values are provided in the respective text. The complete MLR model is given in Supplemental Table 1.
Figure 1.
Factors related to depressive symptoms. Multivariable linear regression. Dependent variable: Severity of depressive symptoms as measured by BDI-II score. Regression coefficient with 95% CI given for each parameter. p values for sex, age, MS diagnosis (CIS versus RRMS), baseline EDSS, month of sampling and EBNA1-IgG levels: >0.05, for current relapse: p = 0.016, higher severity of fatigue: p < 0.0001, less 25-OH-vitamin D: p = 0.034, current smoking: p = 0.008. Linear regression model: R 2 : 0.449. Coding: sex: 0 – female, 1 – male; age – years; smoking: 0 – No, 1 – Occasionally, 2 – ⩽5 cigarettes per day, 3 – 6–10 cigarettes per day, 4 – 11–20 cigarettes per day, 5 – >20 cigarettes per day; diagnosis: 0 – CIS, 1 – RRMS; current relapse: 0 – No, 1 – Yes; EDSS – score; FSMC – total score; 25-OH-vitamin D serum concentration – ng/ml; month of sampling – month; EBNA1-IgG titer – U/ml.
BDI-II, Beck Depression Inventory-II; CIS, clinically isolated syndrome; EBNA1-IgG, EBV nuclear antigen-1 IgG; EDSS, expanded disability status scale; FSMC, fatigue scale motor cognition; RRMS, relapsing-remitting multiple sclerosis.
The expanded MLR model including the variable ‘time in days between last corticosteroid administration and date of visit’ is provided in Supplemental Table 2.
To identify potential morphometric parameters associated with the severity of depressive symptoms, a non-parametric correlation analysis (Kendall’s Tau b) and a non-parametric group comparison (Kruskal–Wallis test) using BDI-II categories as given in Table 1 were conducted. Multiple testing was accounted for using Bonferroni correction.
The reporting guideline STROBE is given as Supplemental Table 3.
Results
Of the total German NationMS cohort (n = 1374), n = 781 individuals qualified for this analysis based on the availability of all required data. With a mean age of 34.3 years (33.6–35.0) and a female-to-male ratio of 2.3:1, the study sample reflects the characteristics of the whole cohort as described earlier. 32 The study cohort characteristics are summarised in Table 1.
At least minimal depressive symptoms were present in n = 256 participants (32.8%).
Mean 25-OH-VD serum concentration was 22.2 ng/ml (21.2–23.2). 25-OH-VD deficiency, 36 was present in n = 398 persons (51.0%) of which n = 124 (15.9%) were severely deficient (<10 ng/ml).
To analyze factors relevant to depression in MS, we applied MLR. Here, we did not find associations of sex, age, MS diagnosis (CIS versus RRMS), baseline EDSS, month of sampling or EBNA1-IgG levels with severity of depressive symptoms as measured by BDI-II score. In turn, the presence of a current relapse (p = 0.016), higher severity of fatigue (p < 0.0001), lower 25-OH-VD levels (p = 0.034) and current smoking (p = 0.008) were associated with a higher BDI-II score (Figure 1, Supplemental Table 1).
To assess whether the association of a current relapse and higher depressive symptoms might be related to a more recent corticosteroid administration, time in days between the last corticosteroid administration and visit date was included in the MLR model, resulting in a reduced number of n = 755 patients, and showed no association [c 0.001 (−0.004; 0.005), p = 0.756]. The full MLR analysis including this item is given in Supplemental Table 2.
Variance inflation factors (all <1.3) were not indicative of multicollinearity in either model (Supplemental Tables 1 and 2).
To test morphological factors potentially linked to depressive mood, we analysed global and region-specific MRI-volumetry in a subgroup of n = 327 patients (Table 1).
Neither Kendall’s Tau-b correlation nor group comparison using the Kruskal–Wallis test identified global or regional MRI-markers of depressive symptoms in MS.
Discussion
Depressive symptoms were detectable in more than 30% of patients in our study cohort (32.8% with at least minimal, 16.1% with at least mild, 6.9% with at least moderate and 1.7% with severe symptoms). As reported earlier, the majority of patients in this cohort had a very short disease duration of 0.33 years (median, interquartile range 0.20–0.70). 32 In this large German network cohort, we identified a negative impact of smoking and vitamin D deficiency on the severity of depressive symptoms.
Demographic characteristics (sex and age) and EDSS were not associated with the severity of depressive symptoms, which is in line with a small study in 37 individuals. 2 With the inclusion criteria referring to older versions of the diagnostic criteria, the diagnosis of a CIS or RRMS appears to be an arbitrary distinction in our analysis. Previous studies investigating the effect of sex on depressive symptoms in MS have elicited contradictory results, pointing to a relevant gap of knowledge that should be addressed in further studies. The age range in our cohort is narrow, potentially explaining the lack of association, in contrast to a previous study that identified age as an influencing factor of depressive symptoms during relapse. 12 With the narrow age and disease duration ranges and the expected collinearity between these two parameters, we did not additionally include disease duration in our analysis.
The aforementioned study also described that not the EDSS score as such, but the relapse-associated EDSS worsening was related to depressive symptoms. 12 In that study, only patients during relapse were investigated. Based on our results and those of a small study in CIS patients (according to Poser criteria), 2 this population represents a population at-risk as the presence of a current relapse was associated with higher BDI-II scores, irrespective of a more recent corticosteroid administration. This suggests the clinical need to assess depressive symptoms specifically in patients during relapse to enable early support by pharmacological and/or psychological interventions, if necessary. In our study, we specifically assessed the early stage of MS. A limitation of the current study is the absence of a longitudinal follow-up of depressive symptoms.
As all patients of our cohort were seropositive for remote EBV infection, 23 we included EBNA1-IgG titres in our MLR model which did not show an association to depressive symptoms. For both MS and depression, remote EBV infection has been described as a risk factor.24,26 Yet, real-world cohorts revealing 100% seropositivity as here represent EBV infection in adults. 23
In contrast, in our cohort of early MS patients, lower vitamin D levels were associated with higher severity of depressive symptoms adding to the relevance of vitamin D deficiency in MS on multiple aspects.16–18,36 This effect was not related to seasonal variation of vitamin D as the month of sampling was included in the MLR model and did not show an association to depressive symptoms. As the sampling date reflects the date of visit, the missing association between the month of sampling and depressive symptoms additionally demonstrates that depressive symptoms in our early MS cohort were independent of a seasonally altered affective state, but, in line with previous data, rather represents an early or even prodromal sign of MS. 3 This is of even more importance as the presence of depression has been shown to delay the diagnosis of MS. 37
The lacking seasonality of depressive symptoms around MS onset interestingly – despite the association of fatigue and depression present in our cohort and other data 10 – represents a differentiating feature from fatigue: for MS-related fatigue, seasonality is a significant contributor to fatigue severity with more severe fatigue symptoms during summer. 38
The negative impact of smoking and vitamin D deficiency is undisputed for both MS and depression.14–18,20,21,24 Our analysis additionally underscores the negative impact of these modifiable factors for depressive symptoms in an early MS cohort. Our approach does not allow to draw causative conclusions. Yet, both factors can easily be addressed in clinical practice and individualised counselling, intervention and support might help to lower the burden of MS.
Within our dataset, we did not detect MRI-volumetric associations to the severity of depressive symptoms which is in line with another recent report. 31 To unravel the interrelations of depression in the context of MS-associated outcomes, functional MRI analyses might be more suited to identify depression with functional connectivity, temporal dynamics and neurotransmitter networks potentially paving the way for targeted drug treatment.28,39,40
As a clinical consequence of our work, depressive symptoms should be assessed in routine clinical care in early MS. Patients during relapse seem to be particularly vulnerable. Tackling modifiable contributors to depressive symptoms in early MS, such as fatigue, smoking and vitamin D deficiency, might have the potential to positively impact both the MS disease course and depression. The influence of specific interventions for these factors should further be addressed in prospective settings.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864231197309 for Factors associated with depressive mood at the onset of multiple sclerosis - an analysis of 781 patients of the German NationMS cohort by Anke Salmen, Robert Hoepner, Vinzenz Fleischer, Milena Heldt, Barbara Gisevius, Jeremias Motte, Klemens Ruprecht, Ruth Schneider, Anna Lena Fisse, Thomas Grüter, Carsten Lukas, Achim Berthele, Katrin Giglhuber, Martina Flaskamp, Mark Mühlau, Jan Kirschke, Stefan Bittner, Sergiu Groppa, Felix Lüssi, Antonios Bayas, Sven Meuth, Cristoph Heesen, Corinna Trebst, Brigitte Wildemann, Florian Then Bergh, Gisela Antony, Tania Kümpfel, Friedemann Paul, Sandra Nischwitz, Hayrettin Tumani, Uwe Zettl, Bernhard Hemmer, Heinz Wiendl, Frauke Zipp and Ralf Gold in Therapeutic Advances in Neurological Disorders
Acknowledgments
The authors express their deep gratitude to all contributors of the study, especially all patients and relatives for their participation and support, the study nurses and the data monitoring and administrative personnel of the study.
Footnotes
ORCID iDs: Anke Salmen  https://orcid.org/0000-0002-4751-299X
https://orcid.org/0000-0002-4751-299X
Robert Hoepner  https://orcid.org/0000-0002-0115-7021
https://orcid.org/0000-0002-0115-7021
Vinzenz Fleischer  https://orcid.org/0000-0002-3293-5121
https://orcid.org/0000-0002-3293-5121
Jeremias Motte  https://orcid.org/0000-0002-6624-8565
https://orcid.org/0000-0002-6624-8565
Anna Lena Fisse  https://orcid.org/0000-0003-0493-8656
https://orcid.org/0000-0003-0493-8656
Felix Lüssi  https://orcid.org/0000-0003-4334-4199
https://orcid.org/0000-0003-4334-4199
Cristoph Heesen  https://orcid.org/0000-0001-8131-9467
https://orcid.org/0000-0001-8131-9467
Hayrettin Tumani  https://orcid.org/0000-0002-1647-6201
https://orcid.org/0000-0002-1647-6201
Bernhard Hemmer  https://orcid.org/0000-0001-5985-6784
https://orcid.org/0000-0001-5985-6784
Heinz Wiendl  https://orcid.org/0000-0003-4310-3432
https://orcid.org/0000-0003-4310-3432
Ralf Gold  https://orcid.org/0000-0002-7223-3052
https://orcid.org/0000-0002-7223-3052
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Anke Salmen, Department of Neurology, St. Josef-Hospital Bochum, Ruhr-University Bochum, Gudrunstrasse 56, 44791 Bochum, Germany; Department of Neurology, Inselspital, Bern University Hospital and University of Bern, Bern, Switzerland.
Robert Hoepner, Department of Neurology, Inselspital, Bern University Hospital and University of Bern, Bern, Switzerland.
Vinzenz Fleischer, Department of Neurology, Focus Program Translational Neuroscience (FTN) and Immunotherapy (FZI), Rhine-Main Neuroscience Network (rmn), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Milena Heldt, Department of Neurology, Focus Program Translational Neuroscience (FTN) and Immunotherapy (FZI), Rhine-Main Neuroscience Network (rmn), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Barbara Gisevius, Department of Neurology, St. Josef-Hospital Bochum, Ruhr-University Bochum, Bochum, Germany.
Jeremias Motte, Department of Neurology, St. Josef-Hospital Bochum, Ruhr-University Bochum, Bochum, Germany.
Klemens Ruprecht, Department of Neurology, Charité-Universitätsmedizin Berlin, Berlin, Germany; Experimental and Clinical Research Center and NeuroCure Clinical Research Center, MaxDelbrueck Center for Molecular Medicine and Charité Universitätsmedizin Berlin, Berlin, Germany.
Ruth Schneider, Department of Neurology, St. Josef-Hospital Bochum, Ruhr-University Bochum, Bochum, Germany; Institute for Neuroradiology, St. Josef-Hospital Bochum, Ruhr-University Bochum, Bochum, Germany.
Anna Lena Fisse, Department of Neurology, St. Josef-Hospital Bochum, Ruhr-University Bochum, Bochum, Germany.
Thomas Grüter, Department of Neurology, St. Josef-Hospital Bochum, Ruhr-University Bochum, Bochum, Germany.
Carsten Lukas, Department of Neurology, St. Josef-Hospital Bochum, Ruhr-University Bochum, Bochum, Germany; Institute for Neuroradiology, St. Josef-Hospital Bochum, Ruhr-University Bochum, Bochum, Germany.
Achim Berthele, Department of Neurology, Klinikum rechtsDer Isar, Technical University of Munich, Munich, Germany.
Katrin Giglhuber, Department of Neurology, Klinikum rechtsDer Isar, Technical University of Munich, Munich, Germany.
Martina Flaskamp, Department of Neurology, Klinikum rechtsDer Isar, Technical University of Munich, Munich, Germany.
Mark Mühlau, Department of Neurology, Klinikum rechtsDer Isar, Technical University of Munich, Munich, Germany.
Jan Kirschke, Department of Neuroradiology, Klinikum rechtsDer Isar, Technical University of Munich, Munich, Germany.
Stefan Bittner, Department of Neurology, Focus Program Translational Neuroscience (FTN) and Immunotherapy (FZI), Rhine-Main Neuroscience Network (rmn), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Sergiu Groppa, Department of Neurology, Focus Program Translational Neuroscience (FTN) and Immunotherapy (FZI), Rhine-Main Neuroscience Network (rmn), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Felix Lüssi, Department of Neurology, Focus Program Translational Neuroscience (FTN) and Immunotherapy (FZI), Rhine-Main Neuroscience Network (rmn), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Antonios Bayas, Department of Neurology, Faculty of Medicine, University of Augsburg, Augsburg, Germany.
Sven Meuth, Department of Neurology, Medical Faculty, Heinrich-Heine University Düsseldorf, Germany.
Cristoph Heesen, Department of Neurology, University Hospital Hamburg-Eppendorf, Hamburg, Germany.
Corinna Trebst, Department of Neurology, Hannover Medical School, Hannover, Germany.
Brigitte Wildemann, Molecular Neuroimmunology Group, Department of Neurology, University Hospital Heidelberg, Heidelberg, Germany.
Florian Then Bergh, Department of Neurology, University of Leipzig, Leipzig, Germany.
Gisela Antony, Central Information Office German Competence Network of Multiple Sclerosis, Philipps University Marburg, Marburg, Germany.
Tania Kümpfel, Institute of Clinical Neuroimmunology, University Hospital, Ludwig-Maximilian-University Munich, Munich, Germany.
Friedemann Paul, Department of Neurology, Charité-Universitätsmedizin Berlin, Berlin, Germany; Experimental and Clinical Research Center and NeuroCure Clinical Research Center, MaxDelbrueck Center for Molecular Medicine and Charité Universitätsmedizin Berlin, Berlin, Germany.
Sandra Nischwitz, Max Planck Institute of Psychiatry, Munich, Germany.
Hayrettin Tumani, Department of Neurology, University of Ulm, Ulm, Germany.
Uwe Zettl, Department of Neurology, Neuroimmunological Section, University of Rostock, Rostock, Germany.
Bernhard Hemmer, Department of Neurology, Klinikum rechtsDer Isar, Technical University of Munich, Munich, Germany; Munich Cluster for Systems Neurology (SyNergy), Munich, Germany.
Heinz Wiendl, Department of Neurology with Institute of Translational Neurology, Medical Faculty, University Hospital, Münster, Germany.
Frauke Zipp, Department of Neurology, Focus Program Translational Neuroscience (FTN) and Immunotherapy (FZI), Rhine-Main Neuroscience Network (rmn), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Ralf Gold, Department of Neurology, St. Josef-Hospital Bochum, Ruhr-University Bochum, Bochum, Germany.
Declarations
Ethics approval and consent to participate: The cohort study German NationMS was approved by the ethics committee of Ruhr-University Bochum (registration no. 3714-10), and consecutively by all local committees of participating centers. All study participants provided written informed consent.
Consent for publication: Not applicable.
Author contributions: Anke Salmen: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Software; Visualization; Writing – original draft.
Robert Hoepner: Conceptualization; Formal analysis; Investigation; Methodology; Writing – original draft.
Vinzenz Fleischer: Investigation; Methodology; Writing – original draft.
Milena Heldt: Data curation; Investigation; Writing – review & editing.
Barbara Gisevius: Data curation; Investigation; Project administration; Writing – review & editing.
Jeremias Motte: Data curation; Investigation; Project administration; Writing – review & editing.
Klemens Ruprecht: Data curation; Methodology; Writing – review & editing.
Ruth Schneider: Data curation; Investigation; Writing – review & editing.
Anna Lena Fisse: Data curation; Investigation; Writing – review & editing.
Thomas Grüter: Data curation; Investigation; Writing – review & editing.
Carsten Lukas: Data curation; Methodology; Writing – review & editing.
Achim Berthele: Data curation; Investigation; Writing – review & editing.
Katrin Giglhuber: Data curation; Investigation; Writing – review & editing.
Martina Flaskamp: Data curation; Investigation; Writing – review & editing.
Mark Mühlau: Data curation; Investigation; Writing – review & editing.
Jan Kirschke: Data curation; Investigation; Writing – review & editing.
Stefan Bittner: Data curation; Investigation; Writing – review & editing.
Sergiu Groppa: Data curation; Investigation; Writing – review & editing.
Felix Lüssi: Data curation; Investigation; Writing – review & editing.
Antonios Bayas: Data curation; Investigation; Writing – review & editing.
Sven Meuth: Data curation; Investigation; Writing – review & editing.
Cristoph Heesen: Data curation; Investigation; Writing – review & editing.
Corinna Trebst: Data curation; Investigation; Writing – review & editing.
Brigitte Wildemann: Data curation; Investigation; Writing – review & editing.
Florian Then Bergh: Data curation; Investigation; Writing – review & editing.
Gisela Antony: Conceptualization; Data curation; Funding acquisition; Methodology; Project administration; Writing – review & editing.
Tania Kümpfel: Data curation; Investigation; Writing – review & editing.
Friedemann Paul: Data curation; Investigation; Writing – review & editing.
Sandra Nischwitz: Data curation; Investigation; Writing – review & editing.
Hayrettin Tumani: Data curation; Investigation; Writing – review & editing.
Uwe Zettl: Data curation; Investigation; Writing – review & editing.
Bernhard Hemmer: Conceptualization; Data curation; Funding acquisition; Investigation; Project administration; Writing – review & editing.
Heinz Wiendl: Conceptualization; Data curation; Funding acquisition; Investigation; Project administration; Writing – review & editing.
Frauke Zipp: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Writing – original draft.
Ralf Gold: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Resources; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The competence network Multiple Sclerosis (KKNMS) and the German NationMS cohort were supported by the German Federal Ministry for Education and Research, BMBF, grant no. 01GI0914 (Bochum), 01GI1601B (Marburg). The funders had no influence on study design or data analyses.
Competing interests: The authors declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: ABa: received personal compensation from Merck Serono, Biogen, Novartis, TEVA, Roche, Sanofi/Genzyme, Celgene/BMS, Sandoz/Hexal and Janssen; he received grants for congress travel and participation from Biogen, TEVA, Novartis, Sanofi/Genzyme, Merck Serono, Celgene and Janssen. None related to this report. ABe: reports speaker and consulting honoraria from Alexion, Biogen, Bayer Healthcare, Celgene, Merck, Novartis Pharma and Roche; all outside the submitted work. SB: received honoraria from Biogen Idec, Bristol Meyer Squibbs, Merck Serono, Novartis, Sanofi-Genzyme, Roche and Teva. His research is funded by the Deutsche Forschungsgemeinschaft (DFG) and Hertie Foundation. VF: received research support from Novartis, not related to this work. BG: received research support from Novartis, not related to this work. RG: received speaker’s and board honoraria from Baxter, Bayer Schering, Biogen Idec, CSL Behring, Genzyme, Merck Serono, Novartis, Stendhal, Talecris and Teva. His department received grant support from Bayer Schering, Biogen Idec, Genzyme, Merck Serono, Novartis and Teva. CH: received speaker honoraria and research grants from Merck, Novartis, Roche, Sanofi. BH: has served on scientific advisory boards for Novartis; he has served as DMSC member for AllergyCare, Sandoz, Polpharma, Biocon and TG therapeutics; his institution received research grants from Roche for multiple sclerosis research. He has received honoraria for counselling (Gerson Lehrmann Group). He holds part of two patents; one for the detection of antibodies against KIR4.1 in a subpopulation of patients with multiple sclerosis and one for genetic determinants of neutralising antibodies to interferon. All conflicts are not relevant to the topic of the study. RH: received speaker/advisor honorary from Merck, Novartis, Roche, Biogen, Alexion, Sanofi, Janssen, Bristol-Myers Squibb, Teva/Mepha and Almirall. He received research support within the last 5 years from Roche, Merck, Sanofi, Biogen, Chiesi and Bristol-Myers Squibb. He also received research grants from the Swiss MS Society and is a member of the Advisory Board of the Swiss and International MS Society. He also serves as an associated editor for Journal of Central Nervous System Disease. All conflicts are not related to this work. JK: received speaker honorary from Philips and Novartis. He is co-founder of Bonescreen GmbH and received grants from the ERC, DFG and BMBF. None resulted in a conflict of interest. TK: received speaker honoraria and/or personal fees for advisory boards from Novartis Pharma, Roche Pharma, Alexion/Astra Zeneca, Merck, Horizon, Chugai Pharma and Biogen. None resulted in a conflict of interest. CL: received a research grant by the German Federal Ministry for Education and Research, BMBF, German Competence Network Multiple Sclerosis (KKNMS), grant no.01GI1601I, and received consulting and speaker’s honoraria from Biogen Idec, Bayer Schering, BMS, Daiichi Sankyo, Merck Serono, Novartis, Sanofi, Genzyme and TEVA. SM: received honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen, Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS and Teva. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, German Foundation Neurology and by Almirall, Amicus Therapeutics Germany, Biogen, Diamed, Fresenius Medical Care, Genzyme, Merck Serono, Novartis, ONO Pharma, Roche and Teva. JM: received travel grants and supplies from Biogen, Novartis, Celgene (BristolMyersSquibb), Teva and Eisai, his research is funded by Klaus Tschira Foundation, Hertie Foundation and Ruhr-University, Bochum (FoRUM-program). None resulted in a conflict of interest. SN: received speaker honoraria/consultancy fees from Roche, Bristol Myers Squibb, Merck Serono, Novartis. KR: received research support from Novartis Pharma, Merck Serono, German Ministry of Education and Research, European Union (821283-2), Stiftung Charité and Arthur Arnstein Foundation, and travel grants from Guthy Jackson Charitable Foundation. He is a participant in the BIH Clinical Fellow Program funded by Stiftung Charité. AS: received speaker honoraria for activities with Bristol Myers Squibb, CSL Behring, Novartis and Roche, and research support by the Baasch Medicus Foundation, the Medical Faculty of the University of Bern and the Swiss MS Society, all not related to this work. RS: received consulting and speakers honoraria from Roche Pharma AG, Novartis Pharma, Merck KGaA and Alexion Pharma & has received research scientific grant support from Novartis Pharma, all not related to this work. CT: received honoraria for consultation and expert testimony from Alexion Pharma Germany, Chugai Pharma Germany and Roche Pharma. None of this interfered with the current report. HT: received consulting and/or speaker honoraria from Alexion, Bayer, Biogen, Celgene, GSK, Janssen, Merck, Novartis, Roche, Sanofi Genzyme and TEVA. HW: received honoraria for acting as a member of Scientific Advisory Boards for Janssen, Merck and Novartis as well as speaker honoraria and travel support from Alexion, Amicus Therapeuticus, Biogen, Biologix, Bristol Myers Squibb, Cognomed, F. Hoffmann-La Roche Ltd, Gemeinnützige Hertie-Stiftung, Medison, Merck, Novartis, Roche Pharma AG, Genzyme, Teva and WebMD Global. HW is acting as a paid consultant for Biogen, Bristol Myers Squibb, EMD Serono, Idorsia, Immunic, Novartis, Roche, Sanofi, the Swiss Multiple Sclerosis Society and UCB. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgesellschaft (DFG), Deutsche Myasthenie Gesellschaft e.V., Alexion, Amicus Therapeutics, Argenx, Biogen, CSL Behring, F. Hoffmann – La Roche, Genzyme, Merck KgaA, Novartis Pharma, Roche Pharma and UCB Biopharma. BW: received grants from the German Ministry of Education and Research, Deutsche Forschungsgemeinschaft, Dietmar Hopp Foundation and Klaus Tschira Foundation, grants and personal fees from Merck, and personal fees from Alexion, Bayer, Biogen, Teva; none related to this work. UZ: received speaking fees, travel support and financial support for research activities from Alexion, Almirall, Bayer, Biogen, Bristo-Myers-Squibb, Celgene, Janssen, Merck Serono, Novartis, Octapharma, Roche, Sanofi Genzyme, Teva as well as EU, BMBF, BMWi and DFG. None resulted in a conflict of interest. FZ: recently received research grants and/or consultation funds from Biogen, Ministry of Education and Research (BMBF), Bristol-Meyers-Squibb, Celgene, German Research Foundation (DFG), Janssen, Max-Planck-Society (MPG), Merck Serono, Novartis, Progressive MS Alliance (PMSA), Roche, Sanofi Genzyme and Sandoz. All other authors report no conflicts of interest relevant to this work.
Availability of data and materials: Anonymised data are available to every qualified researcher upon reasonable request to the corresponding author.
References
- 1. Jones CD, Motl R, Sandroff BM. Depression in multiple sclerosis: is one approach for its management enough? Mult Scler Relat Disorders 2021; 51: 102904. [DOI] [PubMed] [Google Scholar]
- 2. Di Legge S, Piattella MC, Pozzilli C, et al. Longitudinal evaluation of depression and anxiety in patients with clinically isolated syndrome at high risk of developing early multiple sclerosis. Mult Scler 2003; 9: 302–306. [DOI] [PubMed] [Google Scholar]
- 3. Disanto G, Zecca C, MacLachlan S, et al. Prodromal symptoms of multiple sclerosis in primary care. Ann Neurol 2018; 83: 1162–1173. [DOI] [PubMed] [Google Scholar]
- 4. Glanz BI, Dégano IR, Rintell DJ, et al. Work productivity in relapsing multiple sclerosis: associations with disability, depression, fatigue, anxiety, cognition, and health-related quality of life. Value Health 2012; 15: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 5. Rommer PS, Eichstadt K, Ellenberger D, et al. Symptomatology and symptomatic treatment in multiple sclerosis: results from a nationwide MS registry. Mult Scler 2019; 25: 1641–1652. [DOI] [PubMed] [Google Scholar]
- 6. Pokryszko-Dragan A, Marschollek K, Chojko A, et al. Social participation of patients with multiple sclerosis. Adv Clin Exp Med 2020; 29: 469–473. [DOI] [PubMed] [Google Scholar]
- 7. Ellenberger D, Flachenecker P, Haas J, et al. Is benign MS really benign? What a meaningful classification beyond the EDSS must take into consideration. Mult Scler Relat Disord 2020; 46: 102485. [DOI] [PubMed] [Google Scholar]
- 8. Mayo CD, Lacey C, Gawryluk JR, et al. Differences in symptoms of depression between females and males with relapsing-remitting multiple sclerosis. Mult Scler Relat Disord 2021; 51: 102884. [DOI] [PubMed] [Google Scholar]
- 9. Theaudin M, Romero K, Feinstein A. In multiple sclerosis anxiety, not depression, is related to gender. Mult Scler 2016; 22: 239–244. [DOI] [PubMed] [Google Scholar]
- 10. Penner IK, Bechtel N, Raselli C, et al. Fatigue in multiple sclerosis: relation to depression, physical impairment, personality and action control. Mult Scler 2007; 13: 1161–1167. [DOI] [PubMed] [Google Scholar]
- 11. Hasselmann H, Bellmann-Strobl J, Ricken R, et al. Characterizing the phenotype of multiple sclerosis-associated depression in comparison with idiopathic major depression. Mult Scler 2016; 22: 1476–1484. [DOI] [PubMed] [Google Scholar]
- 12. Sabanagic-Hajric S, Suljic E, Sulejmanpasic-Arslanagic G. Depression during multiple sclerosis relapse: relation to disability and relapse severity. Med Glas (Zenica) 2016; 13: 44–49. [DOI] [PubMed] [Google Scholar]
- 13. Peres DS, Rodrigues P, Viero FT, et al. Prevalence of depression and anxiety in the different clinical forms of multiple sclerosis and associations with disability: a systematic review and meta-analysis. Brain Behav Immun Health 2022; 24: 100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Graetz C, Groger A, Luessi F, et al. Association of smoking but not HLA-DRB1*15:01, APOE or body mass index with brain atrophy in early multiple sclerosis. Mult Scler 2019; 25: 661–668. [DOI] [PubMed] [Google Scholar]
- 15. Wingerchuk DM. Smoking: effects on multiple sclerosis susceptibility and disease progression. Ther Adv Neurol Disord 2012; 5: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Décard BF, von Ahsen N, Grunwald T, et al. Low vitamin D and elevated immunoreactivity against Epstein-Barr virus before first clinical manifestation of multiple sclerosis. J Neurol Neurosurg Psychiatry 2012; 83: 1170–1173. [DOI] [PubMed] [Google Scholar]
- 17. Ostkamp P, Salmen A, Pignolet B, et al. Sunlight exposure exerts immunomodulatory effects to reduce multiple sclerosis severity. Proc Natl Acad Sci U S A 2021; 118: e2018457118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martinelli V, Dalla Costa G, Colombo B, et al. Vitamin D levels and risk of multiple sclerosis in patients with clinically isolated syndromes. Mult Scler 2014; 20: 147–155. [DOI] [PubMed] [Google Scholar]
- 19. Vandebergh M, Dubois B, Goris A. Effects of Vitamin D and body mass index on disease risk and relapse hazard in multiple sclerosis: a mendelian randomization study. Neurol Neuroimmunol Neuroinflamm 2022; 9: e1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flensborg-Madsen T, von Scholten MB, Flachs EM, et al. Tobacco smoking as a risk factor for depression. A 26-year population-based follow-up study. J Psychiatr Res 2011; 45: 143–149. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Z, Yang X, Jia Y, et al. Vitamin D and the risks of depression and anxiety: an observational analysis and genome-wide environment interaction study. Nutrients 2021; 13: 3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zielinska M, Luszczki E, Deren K. Dietary nutrient deficiencies and risk of depression (Review Article 2018-2023). Nutrients 2023; 15: 2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abrahamyan S, Eberspacher B, Hoshi MM, et al. Complete Epstein-Barr virus seropositivity in a large cohort of patients with early multiple sclerosis. J Neurol Neurosurg Psychiatry 2020; 91: 681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022; 375: 296–301. [DOI] [PubMed] [Google Scholar]
- 25. Hedstrom AK, Brenner N, Butt J, et al. Overweight/obesity in young adulthood interacts with aspects of EBV infection in MS etiology. Neurol Neuroimmunol Neuroinflamm 2020; 8: e912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vindegaard N, Petersen LV, Lyng-Rasmussen BI, et al. Infectious mononucleosis as a risk factor for depression: a nationwide cohort study. Brain Behav Immun 2021; 94: 259–265. [DOI] [PubMed] [Google Scholar]
- 27. Berg D, Supprian T, Thomae J, et al. Lesion pattern in patients with multiple sclerosis and depression. Mult Scler 2000; 6: 156–162. [DOI] [PubMed] [Google Scholar]
- 28. Meyer-Arndt L, Kuchling J, Brasanac J, et al. Prefrontal-amygdala emotion regulation and depression in multiple sclerosis. Brain Commun 2022; 4: fcac152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gobbi C, Rocca MA, Riccitelli G, et al. Influence of the topography of brain damage on depression and fatigue in patients with multiple sclerosis. Mult Scler 2014; 20: 192–201. [DOI] [PubMed] [Google Scholar]
- 30. Fleischer V, Ciolac D, Gonzalez-Escamilla G, et al. Subcortical volumes as early predictors of fatigue in multiple sclerosis. Ann Neurol 2022; 91: 192–202. [DOI] [PubMed] [Google Scholar]
- 31. Fiore A, Preziosa P, Tedone N, et al. Correspondence among gray matter atrophy and atlas-based neurotransmitter maps is clinically relevant in multiple sclerosis. Mol Psychiatry 2023; 28: 1770–1782. [DOI] [PubMed] [Google Scholar]
- 32. von Bismarck O, Dankowski T, Ambrosius B, et al. Treatment choices and neuropsychological symptoms of a large cohort of early MS. Neurol Neuroimmunol Neuroinflamm 2018; 5: e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barkhof F, Filippi M, Miller DH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 1997; 120: 2059–2069. [DOI] [PubMed] [Google Scholar]
- 34. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 2005; 58: 840–846. [DOI] [PubMed] [Google Scholar]
- 35. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoepner R, Bagnoud M, Pistor M, et al. Vitamin D increases glucocorticoid efficacy via inhibition of mTORC1 in experimental models of multiple sclerosis. Acta Neuropathol 2019; 138: 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barin L, Kamm CP, Salmen A, et al. How do patients enter the healthcare system after the first onset of multiple sclerosis symptoms? The influence of setting and physician specialty on speed of diagnosis. Mult Scler 2020; 26: 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grothe M, Gross S, Susse M, et al. The seasonal fluctuation of fatigue in multiple sclerosis. Front Neurol 2022; 13: 900792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carotenuto A, Valsasina P, Preziosa P, et al. Monoaminergic network abnormalities: a marker for multiple sclerosis-related fatigue and depression. J Neurol Neurosurg Psychiatry 2023; 94: 94–101. [DOI] [PubMed] [Google Scholar]
- 40. Romanello A, Krohn S, von Schwanenflug N, et al. Functional connectivity dynamics reflect disability and multi-domain clinical impairment in patients with relapsing-remitting multiple sclerosis. Neuroimage Clin 2022; 36: 103203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864231197309 for Factors associated with depressive mood at the onset of multiple sclerosis - an analysis of 781 patients of the German NationMS cohort by Anke Salmen, Robert Hoepner, Vinzenz Fleischer, Milena Heldt, Barbara Gisevius, Jeremias Motte, Klemens Ruprecht, Ruth Schneider, Anna Lena Fisse, Thomas Grüter, Carsten Lukas, Achim Berthele, Katrin Giglhuber, Martina Flaskamp, Mark Mühlau, Jan Kirschke, Stefan Bittner, Sergiu Groppa, Felix Lüssi, Antonios Bayas, Sven Meuth, Cristoph Heesen, Corinna Trebst, Brigitte Wildemann, Florian Then Bergh, Gisela Antony, Tania Kümpfel, Friedemann Paul, Sandra Nischwitz, Hayrettin Tumani, Uwe Zettl, Bernhard Hemmer, Heinz Wiendl, Frauke Zipp and Ralf Gold in Therapeutic Advances in Neurological Disorders