Abstract
Bone fragility is the determinant of the increased risk of minimal trauma fracture and must be treated with a multimodal approach that includes pharmacological therapy, physical exercise, and adequate nutrition. Pharmacological therapy, to date based on the administration of antiresorptive drugs, such as bisphosphonates and denosumab, or osteoanabolic drugs, such as teriparatide and abaloparatide, has shown to be effective in reducing the risk of fracture in osteoporotic patients. In the context of the cellular and molecular mechanisms that regulate bone metabolism, the discovery of the Wnt signaling pathway and its role in bone tissue homeostasis has allowed the identification of sclerostin as an inhibitor of osteoblastic activity and simultaneously as a stimulator of osteoclastic activity. Therefore, the use of a monoclonal antibody, romosozumab, against this protein has been tested as a potential drug with a dual action, stimulating bone neo-apposition and inhibiting bone resorption. The efficacy of romosozumab has been demonstrated in numerous clinical trials against both placebo and other drugs commonly used in the treatment of patients affected by osteoporosis. The advantages of this drug lie above all in its rapid action which makes it particularly suitable in clinical situations where it is necessary to improve bone strength very quickly due to the imminent risk of fragility fracture. Clinical studies and guidelines suggest romosozumab as an initial drug in an ideal sequential approach from osteoanabolic to antiresorptive drugs. Some aspects of cardiovascular safety remain to be fully investigated, therefore its use in osteoporotic patients at high cardiovascular risk should be avoided until further data become available.
Keywords: fragility fractures, osteoporosis, risk of falls, romosozumab, sclerostin
Introduction
Bone fragility is a pathological condition resulting from a quantitative and qualitative deterioration of the bone tissue, which typically occurs in skeletal diseases such as osteoporosis (OP). 1 This condition affects 22.1% of women and 6.6% of men aged over 50 years in Europe. 2 Population aging worldwide will be responsible for a dramatic increase in the incidence of OP, particularly in postmenopausal women. Nonetheless, a relevant gap in patients’ information and primary care physicians about the disease and limited access to diagnosis and treatment before the first fracture results in underdiagnosis and undertreatment of OP. 3
OP therapy, in addition to the supplementation of calcium and vitamin D, involves the administration of antiresorptive drugs, such as bisphosphonates (BP) and denosumab, and osteoanabolic drugs, such as teriparatide and abaloparatide. 4 The former act essentially by inhibiting the bone resorption by the osteoclasts, while the latter induce greater bone formation by stimulating the survival and activity of the osteoblasts. The latest addition to the pharmacotherapeutic options for the treatment of OP is the monoclonal antibody against sclerostin. 4
Sclerostin modulation: Historical insights and pathophysiology of bone fragility
Sclerostin is a protein produced and secreted by mature osteocytes that blocks the activation of the Wnt/β-catenin osteogenic pathway in osteoblasts by reducing bone formation. 5 Physiologically, osteocytes reduce the release of sclerostin in response to mechanical stimuli acting on bone and thus positively modulate bone mass in response to increased mechanical demands.6,7
The knowledge of the mechanisms underlying the physiological activity of sclerostin stems from the studies of genetic diseases characterized by the deficiency of the SOST gene which codes for sclerostin and which has a chromosomal localization 17q21.31. 8 This gene was identified in 2001 as the mutated gene in individuals affected by sclerosteosis, an autosomal recessive, very rare condition characterized by variable syndactyly and progressive overgrowth of the skeleton (in particular of the skull), which determines characteristic dysmorphisms (prominence of the jaw, rounded forehead, mid-facial hypoplasia), with cranial nerve entrapment and, secondarily, facial paralysis and deafness, and life-threatening increase in intracranial pressure. 9
Another disease, shortly after identified, associated with a mutation of the SOST gene is van Buchem’s disease, an autosomal recessive inherited condition, which presents phenotypically similar to and generally milder than sclerosteosis, without syndactyly, with hyperostosis of the skull, mandible, clavicles, ribs, diaphyses of the long bones and tubular bones of the hands and feet, and associated cranial nerve entrapment, with secondary paralysis of varying degrees. 10
A third pathology linked to SOST gene deficiency is craniodiaphyseal dysplasia, an autosomal dominant and very rare sclerotic bone disease with variable phenotypic expression characterized by massive, generalized hyperostosis and osteosclerosis, particularly of the skull and facial bones, which can lead to severe deformities. 11
Since SOST gene mutations are characterized almost exclusively by skeletal phenotypes, it was initially hypothesized that sclerostin was expressed only in bone. Phylogenetically, the Wnt signaling pathways, on which sclerostin acts, have been preserved during evolution because they are involved in the regulation of embryogenesis and tissue homeostasis, cell proliferation, polarity, and preservation of stem cells. 12 In bone tissue, sclerostin is secreted by mature osteocytes during the bone remodeling and it directly inhibits osteoblast precursor and bone formation and, indirectly promotes osteoclastogenesis, through inhibition of osteoprotegerin (OPG) and the induction of Receptor activator of NF-kB (RANK) ligand (RANKL) expression in osteoblasts, stimulating bone resorption. 13
The Wnt/β-catenin pathway has been identified as a modulator of bone cell mechanotransduction, through a receptor complex including a single-pass low-density lipoprotein receptor-related protein (LRP5 or LRP6) and a frizzled (FZD) heptahelic. 14 The Wnt-Lrp5/6-FZD trimer activates the intracellular machinery of the transcription factor β-catenin which promotes cell survival. 7
The canonical Wnt pathway is downregulated by several endogenously secreted inhibitors that bind LRP5/6 or Wnts, including sclerostin. The mechanical load would reduce the production of sclerostin, while its overproduction would be induced by disuse and reduced muscle contraction. 7 In mechanotransduction, many molecules, such as myokines, osteokines, and adipokines, are secreted by different tissues functionally connected by the goal of adaptation of the musculoskeletal system to mechanical needs. 15 For example, during contraction, skeletal muscles release β-aminoisobutyric acid (L-BAIBA), a metabolite that binds to the L-BAIBA Mrgprd receptor of osteocytes, prolonging their survival. 16 Several modulators of mechanical load detection (mechanosensation) and consequent anatomical-functional changes (mechanotransduction) have been recognized. In response to loading, prostaglandin production decreases secretion of negative regulators of bone formation such as sclerostin and Dkk1 and increases positive regulators of the bone formation such as various Wnts (19 Wnts have been described so far).14,17 Moreover, Wnt1 and Wnt3a, produced by osteocytes, support myogenesis and muscle function, thus supporting a role of Wnt pathways in the bone-muscle crosstalk. 18 Sclerostin and Dkk1, produced by osteocytes as inhibitors of β-catenin signaling, also affect skeletal muscles. Recently, it has been highlighted in the production of sclerostin by muscle cells in cell cultures and animal models, 19 with an inhibitory role on the bones adjacent to the secretory muscles.
Taking into account the knowledge related to genetic diseases characterized by sclerostin deficiency and to experimental studies in cultural and animal models, such as SOST-deficient mice that exhibited improved bone strength, 20 and transgenic mice expressing human SOST that showed trabecular loss, cortical thinning, and poor lamellar bone formation, 21 it was hypothesized that blocking the activity of sclerostin in humans could effectively increase, through a new mechanism of action, bone density, and bone strength. 22
Pharmacological research has therefore rapidly developed monoclonal antibodies against sclerostin and among these romosozumab, a humanized anti-sclerostin antibody was approved in 2019 for the treatment of postmenopausal OP. 22
Anti-sclerostin antibody as a drug for the management of bone fragility
As widely accepted, poor bone strength is linked to structural, architectural, or material alterations of the bone that can cause fractures even for common mechanical loads that would be well tolerated by normal bone.1,23 Low bone mineral density (BMD) is a main component of bone fragility. Therefore, an anti-osteoporotic drug is first tested on its ability to increase BMD. 1 The validity of this hypothesis is confirmed by the fact that all drugs, especially antiresorptive drugs, proportionally reduce the risk of fracture by increasing bone mass, as confirmed by a meta-analysis of RCTs which demonstrates that for each one percentage point increase in vertebral BMD, there is a 7% lower fracture risk in the same anatomical site. 24
Romosozumab has been demonstrated to significantly increase BMD compared to both antiresorptive and osteoanabolic agents currently used as anti-osteoporotic drugs.
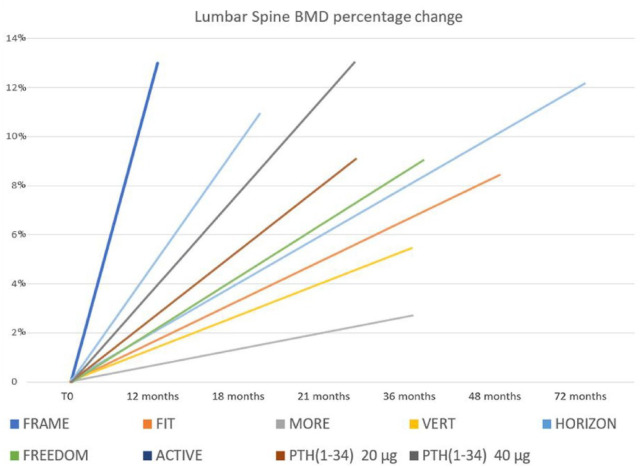
In the pivotal study, the Fracture Study in Postmenopausal Women with Osteoporosis (FRAME), 25 patients receiving romosozumab had bone mass increases of 13.3% at the vertebral level and 6.9% at the proximal femur after only 1 year of treatment (Figures 1 and 2).
Figure 1.
Lumbar spine BMD changes during treatment with anti-osteoporotic drugs at 1-year follow-up. FRAME investigated efficacy of romosozumab on fracture risk in postmenopausal osteoporosis; FIT 26 investigated efficacy of alendronate on fracture risk in postmenopausal osteoporosis; MORE 27 investigated efficacy of raloxifene on fracture risk in postmenopausal osteoporosis; VERT 28 investigated efficacy of risedronate on fracture risk in postmenopausal osteoporosis; HORIZON 29 investigated efficacy of zoledronate on fracture risk in postmenopausal osteoporosis; FREEDOM 30 investigated efficacy of denosumab on fracture risk in postmenopausal osteoporosis; ACTIVE 31 investigated efficacy of abaloparatide on fracture risk in postmenopausal osteoporosis; PTH (parathyroid hormone); FPT 32 [PTH (1–34) 20 µg, PTH (1–34) 40 µg)] investigated efficacy of alendronate on fracture risk in postmenopausal osteoporosis.
ACTIVE, Abaloparatide Comparator Trial in Vertebral Endpoints; BMD, bone mineral density; FIT, Fracture Intervention Trial; FPT, Fracture Prevention Trial; FRAME, Fracture Study in Postmenopausal Women with Osteoporosis; FREEDOM, Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months; HORIZON, Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly; MORE, Multiple Outcomes of Raloxifene Evaluation; VERT, Vertebral Efficacy with Risedronate Therapy.
Figure 2.
Femoral neck BMD changes during treatment with anti-osteoporotic drugs at 1-year follow-up. FRAME investigated efficacy of romosozumab on fracture risk in postmenopausal osteoporosis; FIT 26 investigated efficacy of alendronate on fracture risk in postmenopausal osteoporosis; MORE 27 investigated efficacy of raloxifene on fracture risk in postmenopausal osteoporosis; VERT 28 investigated efficacy of risedronate on fracture risk in postmenopausal osteoporosis; HORIZON 29 investigated efficacy of zoledronate on fracture risk in postmenopausal osteoporosis; FREEDOM 30 investigated efficacy of denosumab on fracture risk in postmenopausal osteoporosis; ACTIVE 31 investigated efficacy of abaloparatide on fracture risk in postmenopausal osteoporosis; FPT 32 [PTH (1–34) 20 µg, PTH (1–34) 40 µg)], investigated efficacy of alendronate on fracture risk in postmenopausal osteoporosis.
ACTIVE, Abaloparatide Comparator Trial in Vertebral Endpoints; BMD, bone mineral density; FIT, Fracture Intervention Trial; FPT, Fracture Prevention Trial; FRAME, Fracture Study in Postmenopausal Women with Osteoporosis; FREEDOM, Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months; HORIZON, Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly; MORE, Multiple Outcomes of Raloxifene Evaluation; VERT, Vertebral Efficacy with Risedronate Therapy.
Furthermore, drug discontinuation did not result in a rapid BMD decrease nor substantial loss of mechanical strength obtained.
The efficacy of romosozumab in improving mechanical strength is mainly due to its dual action on both bone modeling and remodeling. The structural and architectural modifications mainly related to modeling and those of the material mainly related to remodeling, have a significant impact on the bone strength. 33 The stimulation activity of the modeling results in greater deposition of osteoid both at the subperiosteal and endocortical level in the absence of greater porotization of the cortical bone as was seen with PTH analogs. 34 Poole et al. 35 analyzing lumbar 3D computed tomography (CT) scans of romosozumab-treated postmenopausal women demonstrated that this drug increases bone mass in all parts and contributes to the anatomy of the vertebra including elements of the posterior arch that showed improvements in cortical parameters.
Romosozumab therapy also modifies the composition of the bone tissue material with an increase in the degree of heterogeneity of the mineral component and the relative content of proteoglycans, both elements that improve the mechanical strength of the bone. 1
In conclusion, the monoclonal antibody against sclerostin ultimately acts by improving the mechanical strength through a triple action, by increasing bone mass, by improving structural and architectural characteristics, and by optimizing the composition of the bone tissue material.
Anti-sclerostin antibodies in fragility fractures treatment
To date, three sclerostin antibodies have been tested in clinical trials, including romosozumab, blosozumab, and setrusumab. The first two drugs have been studied in randomized controlled trials (RCTs) on osteoporotic patients, while setrusumab is currently under investigation only for the treatment of osteogenesis imperfecta and hypophosphatasia. 36
In phase I and II studies, blosozumab has shown to be well tolerated in subjects with postmenopausal OP and effective in significantly increasing both vertebral and femoral BMD compared to placebo. 37 Moreover, this drug demonstrated to have a dual mechanism of action (i.e. ‘dual action’), as well as romosozumab, being able to induce both an increase in bone formation markers and a reduction in bone resorption markers. However, data from a phase III study have not been published to date. 38
Romosozumab, therefore, remains the only monoclonal antibody against sclerostin that can be used in clinical practice for the treatment of OP.
The dual action of romosozumab is demonstrated by its ability to increase bone formation by promoting osteoblast differentiation and activity and, at the same time, to reduce osteoclast activity and bone resorption, by modulating the expression of paracrine factors, particularly by reducing the expression of RANKL and Colony-stimulating factor-1 (CSF-1) and by increasing of OPG and Wnt-1 inducible signaling pathway-1 (Wisp-1).39,40
In the phase II dose finding study, romosozumab was administered monthly at a dose of 70, 140, or 210 mg, or every 3 months at a dose of 140 or 210 mg, demonstrating significant improvement of spine BMD with all doses and similar adverse events compared to placebo, alendronate, or teriparatide. 41
The development of the clinical validation of the molecule is based on four pivotal studies, published in chronological order, including the FRAME, 25 (Fracture Study in Postmenopausal Women with Osteoporosis), the STRUCTURE, 42 (An Open-label, Randomized, Teriparatide-controlled Study to Evaluate the Effect of Treatment with Romosozumab in Postmenopausal Women With Osteoporosis Previously Treated with Bisphosphonate Therapy), the ARCH, 43 (Active-Controlled Fracture Study in Postmenopausal Women with Osteoporosis at High Risk) and the BRIDGE 44 (placeBo-contRolled study evaluatIng the efficacy anD safety of romosozumab in treatinG mEn with osteoporosis) (Table 1).
Table 1.
Pivotal studies that led to the use of romosozumab for the treatment of osteoporosis in clinical practice.
| Trial | Population | Interventions | Clinical outcomes | Instrumental outcomes | Main findings |
|---|---|---|---|---|---|
| FRAME | Postmenopausal women with a T-score of −2.5 to −3.5 at the total hip or femoral neck. | Romosozumab (210 mg) or placebo monthly for 12 months. Each group received denosumab (60 mg every 6 months) for 1 year. | Cumulative incidences of new vertebral fractures at 12 and 24 months. Clinical (a composite of nonvertebral and symptomatic vertebral) and nonvertebral fractures. Adverse events. | Areal BMD change at the total hip, femoral neck, lumbar spine. | At 1 year, 73% lower risk of new vertebral fractures, and 36% lower risk of clinical fractures with romosozumab. At 24 months, 75% lower risk of vertebral fractures. |
| STRUCTURE | Women (⩾55 to ⩽90 years) with postmenopausal osteoporosis who had taken an oral bisphosphonate for at least 3 years and alendronate the year before screening; areal BMD T-score of −2·5 or lower at the total hip, femoral neck, or lumbar spine; and a history of fracture. | Romosozumab (210 mg once monthly) or teriparatide (20 μg once daily). | Adverse events. | Areal BMD change at the total hip through month 12; Total hip cortical volumetric BMD by QCT (mg/cm3); Total hip integral volumetric BMD by QCT (mg/cm3); Hip strength under fall loading conditions estimated by finite element analysis. | Mean percentage change from baseline in total hip areal BMD was 3.2% higher with romosozumab at 1 year; significant gains with romosozumab versus teriparatide in volumetric BMC twice in cortical (+290 mg) versus trabecular (+148 mg) compartment; greater gains with romosozumab in hip strength versus teriparatide at 6-month (2.1% versus −1%) and 12-month follow-up (2.5% versus 0.7%). |
| ARCH | Postmenopausal women with osteoporosis and a fragility fracture. | Romosozumab (210 mg) or weekly oral alendronate (70 mg) for 12 months, followed by alendronate in both groups. | Cumulative incidence of new vertebral fracture at 24 months and the cumulative incidence of clinical fracture (nonvertebral and symptomatic vertebral fracture) at the time of the primary analysis (after clinical fractures had been confirmed in ⩾330 patients). Incidences of nonvertebral and hip fracture at the time of the primary analysis. Serious adverse events. | Areal BMD change at the total hip and lumbar spine. | At 24 months, a 48% lower risk of new vertebral fractures, and 27% lower risk of clinical fractures, and 19% lower risk of nonvertebral fractures, and 38% lower risk of hip fractures with romosozumab. Serious cardiovascular adverse events more often with romosozumab than with alendronate (2.5% versus 1.9%). |
| BRIDGE | Men (55–90 years) with T-score at the lumbar spine, total hip, or femoral neck of ⩽2.5 or ⩽1.5 with a history of a fragility nonvertebral or vertebral fracture. | Romosozumab 210 mg monthly or placebo for 12 months. | Adverse events. | Percentage change from baseline in lumbar spine, and total hip BMD at month 12. | At month 12, BMD was significantly greater for the romosozumab versus placebo (lumbar spine 12.1% versus 1.2%; total hip 2.5% versus 20.5%). |
BMD, bone mineral density; FRAME, Fracture Study in Postmenopausal Women with Osteoporosis; STRUCTURE, An Open-label, Randomized, Teriparatide-controlled Study to Evaluate the Effect of Treatment with Romosozumab in Postmenopausal Women With Osteoporosis Previously Treated with Bisphosphonate Therapy, ARCH, Active-Controlled Fracture Study in Postmenopausal Women with Osteoporosis at High Risk; BRIDGE, placeBo-contRolled study evaluatIng the efficacy anD safety of romosozumab in treatinG mEn with osteoporosis, QCT, quantitative computed tomography, BMC, bone mineral content.
The FRAME 25 is a controlled, double-blind study in which postmenopausal women with OP were randomized to either romosozumab or placebo therapy for 1 year, followed by an open-label extension in which patients were treated with denosumab. The romosozumab group had a significant reduction in vertebral fracture risk at 12 months (−73%), which persisted even after transitioning to denosumab therapy for an additional 12 months.
STRUCTURE 42 is a phase IIIb, randomized, open-label, active-controlled, parallel-group study to compare the efficacy of 12-month treatment with romosozumab versus teriparatide in postmenopausal women with OP with a history of treatment with BPs for at least 3 years. The primary endpoint of the study was the analysis of total hip BMD change after 1 year of treatment, while secondary endpoints were primarily the percent change from baseline in mechanical strength of the proximal femur estimated by finite element analysis, as well as the change in volumetric BMD of both cortical bone alone and integral bone measured by quantitative CT. The results demonstrated that romosozumab increases hip bone mass by about three times more than teriparatide in subjects pre-treated with antiresorptives, data also confirmed in the evaluation of the increase in mechanical strength obtained after 1 year of treatment. The authors, therefore, concluded that even under the reduced biological response of bone tissue due to previous prolonged exposure to BPs, romosozumab led to gains in BMD and hip mechanical strength significantly greater than those obtained with teriparatide. Even more interesting is the evidence of a significant difference just after 6 months of therapy with romosozumab, demonstrating the extreme rapidity of the drug’s effect on bone mass and strength.
The Active-Controlled Fracture Study in Postmenopausal Women with Osteoporosis at High Risk (ARCH) 43 aimed to compare the efficacy in reducing the risk of fragility fractures of a 12-month sequential therapy of romosozumab followed by alendronate versus alendronate alone therapy throughout the study period. Also, in this trial involving postmenopausal women with OP at high fracture risk, 12 months of romosozumab therapy followed by alendronate has been shown to reduce the fractures risk much more than alendronate alone throughout the entire study period.
The BRIDGE study 44 was designed to investigate the safety and efficacy of romosozumab also in the male population affected by OP and had as its primary endpoint the changes in lumbar spine BMD after 12 months of therapy with the drug compared to placebo. The study demonstrated that romosozumab, also in men, has comparable efficacy in bone mass increases as reported in women in previous clinical studies.
Against a substantially good safety profile, in these last two studies (ARCH and BRIDGE) higher incidence of serious cardiovascular and cerebrovascular events compared to placebo (4.9% versus 2.5% in the BRIDGE study) or alendronate (2.5% versus 1.9% in the ARCH study) was reported, resulting in the identification of a black-box warning stating that the use of this drug may ‘increase the risk of heart attack, stroke or death from cardiovascular (heart or blood vessel) problems’. Therefore, in clinical practice, the drug should not be prescribed to people with a history of myocardial infarction or stroke in the 12 months preceding the start of the therapy and in any case, its eventual use should be carefully evaluated in individuals with significant cardiovascular risk identified.
However, in the pivotal studies that led to the use of romosozumab in clinical practice, safety profile was generally comparable to placebo or active interventions, and the common adverse events reported were arthralgia, nasopharyngitis, back pain, hypersensitivity, and injection-site reaction.
In the context of sequential therapy, consisting of the use of anti-osteoporotic drugs with different mechanisms of action in a sequential administration according to the physiology of bone turnover, romosozumab might be an ideal intervention, considering that it is the only dual-action treatment available so far. 45 In particular, in case of imminent risk of fragility fractures, sequential therapy should start with bone anabolic or dual-action drugs followed by an antiresorptive treatment.
This strategy has been already investigated in the FRAME 25 (romosozumab followed by denosumab) and the ARCH 43 trials (romosozumab followed by alendronate) where BMD gains and vertebral fragility fracture risk reduction were maintained at 2 years compared to transitioning from placebo to denosumab or to continuation of alendronate, respectively.
Anti-sclerostin antibodies in reducing the risk of fall
Evidence about the role of romosozumab on fall risk reduction is not conclusive so far. A recent meta-analysis showed that 12-month treatment with romosozumab reduced the risk of falls by 16%, although not statistically significant. 46 However, a sub-analysis including only double-blind studies suggested significant fall risk reduction (Relative risk-RR: 0.80; 95% CI: 0.71–0.92) in patients treated with romosozumab. 46
The data seems to be in contrast with some experimental studies in animal models that have found that the reduction in the level of serum sclerostin is accompanied by a reduction in muscle volume. 35 A recent observational study of an elderly East Asian population also showed that serum sclerostin levels are significantly lower in sarcopenia. 47 On the other hand, some experimental studies confirm that sclerostin, as an inhibitor of the Wnt pathway also in muscle tissue would act by counteracting the mechanisms of muscle regeneration. 48
It is presumable, however, that high serum sclerostin levels act indirectly on the muscle fiber through an imbalance in favor of the RANKL of the activity of the RANK/RANKL/OPG pathway with the final effect of muscle wasting. It is also likely that the administration of a monoclonal antibody against sclerostin can rebalance this situation and promote muscle mass and function.
How much this can translate into an effective reduction in the risk of falling in patients with skeletal fragility is to be demonstrated with experimental studies with specific endpoints.
Anti-sclerostin antibody and cardiovascular risk
The romosozumab pivotal study (FRAME) 25 recruited female patients with non-severe postmenopausal OP considering that the exclusion criteria included a history of hip fractures or severe or multiple vertebral fractures. The study demonstrated that the reduction in fracture incidence was observed rapidly as early as 6 months of treatment with romosozumab, maintaining this benefit on vertebral fracture risk even after patients were switched to 1 year of denosumab.
The FRAME did not show statistically significant differences in adverse and serious events in general and in particular in serious cardiovascular events between the group treated with sequential therapy romosozumab-denosumab and that treated with the sequence placebo-denosumab.
Compared to the population recruited in the FRAME study, the population of the ARCH study 43 was at a higher risk of fracture. In the study population, a head-to-head comparison between romosozumab and alendronate was designed demonstrating that 1 year of romosozumab therapy followed by 1 year of alendronate reduced the cumulative incidence of vertebral fractures by 48% compared with the alendronate-alendronate group. FRAME and ARCH substantially complement each other in demonstrating the greater efficacy of romosozumab compared to the two most used antiresorptive drugs in the treatment of bone fragility.
As in the FRAME study, the incidence of adverse events and serious adverse events in the two study groups (romosozumab-alendronate group versus alendronate-alendronate group) was similar in the ARCH study, except for serious cardiovascular events.
The authors of the ARCH study noted that the FRAME enrolled a somewhat younger population with less advanced OP, which accounts for the higher incidence of cardiovascular events in the ARCH. Furthermore, the FRAME and the ARCH also differ in the drug against which romosozumab was compared. Alendronic acid per se has been associated with a reduction in the risk of cardiovascular disease in some studies, although a meta-analysis did not confirm this hypothesis. 49
From a biological point of view, the greater cardiovascular risk reported with the administration of romosozumab could be justified by the fact that sclerostin seems to counteract vascular calcification. 50 However, this hypothesis, already formulated by the ARCH authors themselves, did not appear to be confirmed in the experimental study considering that vascular calcifications were not reported in SOST knockout mice or patients with sclerosteosis or von Buchem’s disease. 51
Sclerostin is expressed in vascular smooth muscle cells (VSMC) and the aortic plaque. It has been hypothesized that this glycoprotein may protect against vascular inflammation, aortic aneurysm, and atherosclerosis in selected animal models.52,53 In these models, sclerostin prevented the development of vascular calcifications, presumably through the inhibition of canonical Wnt pathways and the indirect stimulation of FGF23 with consequent increase of urinary phosphate excretion, thus lowering the concentration of a calcification inducer. 50 Romosozumab administration could promote hydroxyapatite deposits in vascular and valvular tissues by reducing the level of sclerostin, thus increasing the risk of cardiovascular complications.
However, the role of Wnt pathways on pathophysiology of vascular diseases is controversial. 51 Indeed, Wnt5a is overexpressed in atherosclerotic plaques, where it enhances the secretion of proinflammatory cytokines, whereas Wnt3a seems to have anti-inflammatory effect by modulating NFκB signaling. 54 On the other hand, Wnt3a, Wnt1, and Wnt4 promote VSMC proliferation. 55
Therefore, evidence about a role of Wnt signaling in the development of vascular calcifications is controversial and vague, so data from preclinical and clinical studies do not allow us to say with certainty that the administration of romosozumab can increase cardiovascular risk.
Conclusions
The in-depth knowledge of the biological pathways that regulate the structure and function of the musculoskeletal system revealed that Wnt/β-catenin is the signaling pathway that modulates the response of this system to mechanical needs. Sclerostin is a natural inhibitor of the Wnt signaling pathway and it is overproduced during conditions characterized by a reduction in mechanical load while, on the contrary, it is downregulated during increased mechanical stimulations. Romosozumab is a monoclonal antibody against sclerostin, which therefore reduces the amount of this protein produced by osteocytes thus leading to an uncoupling between bone formation and bone resorption with considerable effects in terms of BMD and bone strength improvements. The clinical efficacy of romosozumab is proven by several clinical trials both against placebo and other drugs commonly used in the treatment of patients with OP. Its use is currently authorized in Europe for women with postmenopausal OP. Available data demonstrate its rapid action (already within 6 months) which makes it particularly suitable in clinical situations in which it is necessary to improve bone strength very quickly due to the imminent risk of a fragility fracture. Furthermore, it should be emphasized that romosozumab performs its maximum efficacy if administered before an antiresorptive drug in an ideal sequential approach from osteoanabolic to antiresorptive drugs. Romosozumab has yet to be thoroughly investigated regarding cardiovascular safety, therefore its use in osteoporotic patients at high cardiovascular risk should be avoided until further data become available.
Acknowledgments
None.
Footnotes
ORCID iDs: Giovanni Iolascon  https://orcid.org/0000-0002-0976-925X
https://orcid.org/0000-0002-0976-925X
Antimo Moretti  https://orcid.org/0000-0002-4598-2891
https://orcid.org/0000-0002-4598-2891
Contributor Information
Giovanni Iolascon, Department of Medical and Surgical Specialties and Dentistry, University of Campania “Luigi Vanvitelli”, Naples, Italy.
Sara Liguori, Department of Medical and Surgical Specialties and Dentistry, University of Campania “Luigi Vanvitelli”, via De Crecchio,n. 4, 80100, Naples, Italy.
Marco Paoletta, Department of Medical and Surgical Specialties and Dentistry, University of Campania “Luigi Vanvitelli”, Naples, Italy.
Giuseppe Toro, Department of Medical and Surgical Specialties and Dentistry, University of Campania “Luigi Vanvitelli”, Naples, Italy.
Antimo Moretti, Department of Medical and Surgical Specialties and Dentistry, University of Campania “Luigi Vanvitelli”, Naples, Italy.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Giovanni Iolascon: Conceptualization; Formal analysis; Writing – original draft; Writing – review & editing.
Sara Liguori: Formal analysis; Visualization; Writing – review & editing.
Marco Paoletta: Formal analysis; Visualization; Writing – review & editing.
Giuseppe Toro: Formal analysis; Visualization; Writing – original draft.
Antimo Moretti: Formal analysis; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Iolascon G, Paoletta M, Liguori S, et al. Bone fragility: conceptual framework, therapeutic implications, and COVID-19-related issues. Ther Adv Musculoskelet Dis 2022; 14: 1759720X221133429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kanis JA, Norton N, Harvey NC, et al. SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch Osteoporos 2021; 16: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salminen H, Piispanen P, Toth-Pal E. Primary care physicians’ views on osteoporosis management: a qualitative study. Arch Osteoporos 2019; 14: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iolascon G, Moretti A, Toro G, et al. Pharmacological therapy of osteoporosis: what’s new? Clin Interv Aging 2020; 15: 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vasiliadis ES, Evangelopoulos DS, Kaspiris A, et al. The role of sclerostin in bone diseases. J Clin Med 2022; 11: 806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tu X, Delgado-Calle J, Condon KW, et al. Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proc Natl Acad Sci U S A 2015; 112: E478-E486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galea GL, Lanyon LE, Price JS. Sclerostin’s role in bone’s adaptive response to mechanical loading. Bone 2017; 96: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sebastian A, Loots GG. Genetics of Sost/SOST in sclerosteosis and van Buchem disease animal models. Metabolism 2018; 80: 38–47. [DOI] [PubMed] [Google Scholar]
- 9. Balemans W, Ebeling M, Patel N, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 2001; 10: 537–543. [DOI] [PubMed] [Google Scholar]
- 10. Nassar K, Rachidi W, Janani S, et al. van Buchem’s disease. Joint Bone Spine 2016; 83: 737–738. [DOI] [PubMed] [Google Scholar]
- 11. Kim SJ, Bieganski T, Sohn YB, et al. Identification of signal peptide domain SOST mutations in autosomal dominant craniodiaphyseal dysplasia. Hum Genet 2011; 129: 497–502. [DOI] [PubMed] [Google Scholar]
- 12. Saito-Diaz K, Chen TW, Wang X, et al. The way Wnt works: components and mechanism. Growth Factors 2013; 31: 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delgado-Calle J, Sato AY, Bellido T. Role and mechanism of action of sclerostin in bone. Bone 2017; 96: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duan P, Bonewald LF. The role of the Wnt/β-catenin signaling pathway in formation and maintenance of bone and teeth. Int J Biochem Cell Biol 2016; 77: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kirk B, Feehan J, Lombardi G, et al. Muscle, bone, and fat crosstalk: the biological role of myokines, osteokines, and adipokines. Curr Osteoporos Rep 2020; 18: 388–400. [DOI] [PubMed] [Google Scholar]
- 16. Kitase Y, Vallejo JA, Gutheil W, et al. β-Aminoisobutyric acid, L-BAIBA, is a muscle-derived osteocyte survival factor. Cell Rep 2018; 22: 1531–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen C, Zhao M, Tian A, et al. Aberrant activation of Wnt/β-catenin signaling drives proliferation of bone sarcoma cells. Oncotarget 2015; 6: 17570–17583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rudnicki MA, Williams BO. Wnt signaling in bone and muscle. Bone 2015; 80: 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Magarò MS, Bertacchini J, Florio F, et al. Identification of sclerostin as a putative new myokine involved in the muscle-to-bone crosstalk. Biomedicines 2021; 9: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li X, Ominsky MS, Niu QT, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 2008; 23: 860–869. [DOI] [PubMed] [Google Scholar]
- 21. Winkler DG, Sutherland MK, Geoghegan JC, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 2003; 22: 6267–6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rauner M, Taipaleenmäki H, Tsourdi E, et al. Osteoporosis treatment with anti-sclerostin antibodies-mechanisms of action and clinical application. J Clin Med 2021; 10: 787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tarantino U, Cariati I, Greggi C, et al. Gaps and alternative surgical and non-surgical approaches in the bone fragility management: an updated review. Osteoporos Int 2022; 33: 2467–2478. [DOI] [PubMed] [Google Scholar]
- 24. Bouxsein ML, Eastell R, Lui LY, et al. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res 2019; 34: 632–642. [DOI] [PubMed] [Google Scholar]
- 25. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 2016; 375: 1532–1543. [DOI] [PubMed] [Google Scholar]
- 26. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348: 1535–1541. [DOI] [PubMed] [Google Scholar]
- 27. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999; 282: 637–645. Erratum in: JAMA 1999; 282: 2124. [DOI] [PubMed] [Google Scholar]
- 28. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 1999; 282: 1344–1352. [DOI] [PubMed] [Google Scholar]
- 29. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356: 1809–1022. [DOI] [PubMed] [Google Scholar]
- 30. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361: 756–765. Erratum in: N Engl J Med 2009; 361: 1914. [DOI] [PubMed] [Google Scholar]
- 31. Miller PD, Hattersley G, Riis BJ, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA 2016; 316: 722–733. Erratum in: JAMA 2017; 317: 442. [DOI] [PubMed] [Google Scholar]
- 32. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344: 1434–1441. [DOI] [PubMed] [Google Scholar]
- 33. Lim SY, Bolster MB. Profile of romosozumab and its potential in the management of osteoporosis. Drug Des Devel Ther 2017; 11: 1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tabacco G, Bilezikian JP. Osteoanabolic and dual action drugs. Br J Clin Pharmacol 2019; 85: 1084–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Poole KE, Treece GM, Pearson RA, et al. Romosozumab enhances vertebral bone structure in women with low bone density. J Bone Miner Res 2022; 37: 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fabre S, Funck-Brentano T, Cohen-Solal M. Anti-sclerostin antibodies in osteoporosis and other bone diseases. J Clin Med 2020; 9: 3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McColm J, Hu L, Womack T, et al. Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women. J Bone Miner Res 2014; 29: 935–943. [DOI] [PubMed] [Google Scholar]
- 38. McClung MR. Sclerostin antibodies in osteoporosis: latest evidence and therapeutic potential. Ther Adv Musculoskelet Dis 2017; 9: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ominsky MS, Boyce RW, Li X, et al. Effects of sclerostin antibodies in animal models of osteoporosis. Bone 2017; 96: 63–75. [DOI] [PubMed] [Google Scholar]
- 40. Stolina M, Dwyer D, Niu QT, et al. Temporal changes in systemic and local expression of bone turnover markers during six months of sclerostin antibody administration to ovariectomized rats. Bone 2014; 67: 305–313. [DOI] [PubMed] [Google Scholar]
- 41. McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 2014; 370: 412–420. [DOI] [PubMed] [Google Scholar]
- 42. Langdahl BL, Libanati C, Crittenden DB, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet 2017; 390: 1585–1594. [DOI] [PubMed] [Google Scholar]
- 43. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 2017; 377: 1417–1427. [DOI] [PubMed] [Google Scholar]
- 44. Lewiecki EM, Blicharski T, Goemaere S, et al. A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab 2018; 103: 3183–3193. [DOI] [PubMed] [Google Scholar]
- 45. Langdahl B. Treatment of postmenopausal osteoporosis with bone-forming and antiresorptive treatments: combined and sequential approaches. Bone 2020; 139: 115516. [DOI] [PubMed] [Google Scholar]
- 46. Möckel L, Bartneck M, Möckel C. Risk of falls in postmenopausal women treated with romosozumab: preliminary indices from a meta-analysis of randomized, controlled trials. Osteoporos Sarcopenia 2020; 6: 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ahn SH, Jung HW, Lee E, et al. Decreased serum level of sclerostin in older adults with sarcopenia. Endocrinol Metab (Seoul) 2022; 37: 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang J, Romero-Suarez S, Lara N, et al. Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/β-catenin pathway. JBMR Plus 2017; 1: 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim DH, Rogers JR, Fulchino LA, et al. Bisphosphonates and risk of cardiovascular events: a meta-analysis. PLoS One 2015; 10: e0122646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. De Maré A, Opdebeeck B, Neven E, et al. Sclerostin protects against vascular calcification development in mice. J Bone Miner Res 2022; 37: 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maré A, D’Haese PC, Verhulst A. The role of sclerostin in bone and ectopic calcification. Int J Mol Sci 2020; 21: 3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Golledge J, Thanigaimani S. Role of sclerostin in cardiovascular disease. Arterioscler Thromb Vasc Biol 2022; 42: e187–e202. [DOI] [PubMed] [Google Scholar]
- 53. Catalano A, Bellone F, Morabito N, et al. Sclerostin and vascular pathophysiology. Int J Mol Sci 2020; 21: 4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schaale K, Neumann J, Schneider D, et al. Wnt signaling in macrophages: augmenting and inhibiting mycobacteria-induced inflammatory responses. Eur J Cell Biol 2011; 90: 553–559. [DOI] [PubMed] [Google Scholar]
- 55. Quasnichka H, Slater SC, Beeching CA, et al. Regulation of smooth muscle cell proliferation by beta-catenin/T-cell factor signaling involves modulation of cyclin D1 and p21 expression. Circ Res 2006; 99: 1329–1337. [DOI] [PubMed] [Google Scholar]