Abstract
Objective
To analyze the clinical and genetic characteristics of zinc finger protein 408 (ZNF408)-related familial exudative vitreoretinopathy (FEVR) in a Chinese cohort.
Methods
Ninety families from Chongqing and 16 families from Xinjiang were selected according to fundus lesion characteristics. Peripheral venous blood was collected from patients and their families; genomic DNA was extracted for whole exome sequencing. Relationships between genotype and phenotype in patients with ZNF408-related FEVR were analyzed.
Results
ZNF408 variants were detected in three patients (2.83%, 3/106). ZNF408 variants in these three probands were all missense mutations at novel sites. One proband had a ZNF408 and LRP5 double-gene variant, and two probands had ZNF408 single-gene variants. Patients with double-gene variants did not display more severe clinical manifestations.
Conclusions
This study expands the spectrum of known ZNF408 variants and confirms that ZNF408 variants can cause FEVR. Most variants detected in this study have not been reported in the literature and are suspected pathogenic variants of FEVR. In patients with FEVR, phenotype and genotype do not necessarily display a direct one-to-one relationship.
Keywords: Familial exudative vitreoretinopathy, whole exome sequencing, ZNF408, clinical characteristic, missense mutation, phenotype, genotype
Introduction
Familial exudative vitreoretinopathy (FEVR) is an inherited retinal disorder characterized by incomplete vascularization and poor vascular differentiation. 1 The clinical manifestations of FEVR are diverse. Some patients have no obvious symptoms and may not exhibit peripheral retinal vascular abnormalities; other patients may develop vision-threatening retinal detachment. Thus far, 11 FEVR-associated genes have been identified: NDP, FZD4, LRP5, TSPAN12, ZNF408, KIF11, RCBTB1, CTNNB, JAG1, EVR3, and ATOH7. 2 However, variants involving these genes do not explain all cases of FEVR.
Zinc finger protein 408 (ZNF408), located on chromosome 11p11.2, encodes a protein belonging to the PR domain zinc finger protein (PRDM) family. 3 Because the pathogenic relationship between ZNF408 and FEVR was recently identified, little is known regarding the function of ZNF408 and the mechanism by which it contributes to FEVR. Furthermore, only a few ZNF408 variants have been detected in patients with FEVR.4–6 Because of the limited information available, it is difficult to determine the clinical characteristics of patients with such variants. This study analyzed genetic and clinical characteristics of ZNF408-related FEVR by analyzing the medical histories of affected patients, relevant clinical manifestations, and genetic findings in patients and their family members.
Patients and methods
This study protocol was approved by the Institutional Review Board of Chongqing Health Center for Women and Children (approval no. 2018-012). The reporting in this study adhered to the STROBE guidelines. 7 Written informed consent was obtained from the parents/guardians of all children included in this study, and all patient details were de-identified.
In this cohort study, the families of patients with FEVR (diagnosed according to fundus lesion characteristics) underwent genetic testing of blood samples collected in the Department of Ophthalmology at Chongqing Health Center for Women and Children and Urumqi Health Center for Women and Children. FEVR severity was evaluated and classified according to published grading criteria: stage 1, only peripheral retinal avascularity; stage 2, retinal neovascularization with or without exudates; stage 3, extramacular retinal detachment with or without exudates; stage 4, partial retinal detachment with or without exudates; stage 5, total retinal detachment. 8
Peripheral blood samples were collected from family members for whole exome analysis of 11 FEVR-related genes (e.g., FZD4, LRP5, NDP, TSPAN12, ZNF408, and KIF11) via high-throughput sequencing. Variants were identified using the following databases: Human Gene Mutation Database (http://www.hgmd.cf.ac.uk/ac/index.php/), Single Nucleotide Polymorphism Database (www.ncbi.nlm.nih.gov/snp/), 1000 Genomes Project database (http://www.1000genomes.org/), ExAC database (http://exac.broadinstitute.org/), and a normal population database established at our center. Missense mutation pathogenicity was predicted by mutation tolerance (SIFT, http://sift.jcvi.org/ and Polyphen2, http://genetics.bwh.harvard.Edu/pph2/). Variant sites were filtered, and the pathogenicity of all variant sites was predicted in accordance with the standards and guidelines of the American College of Medical Genetics and Genomics. 9
Results
General characteristics and mutation rate
This cohort study included the families of 90 patients with FEVR from Chongqing (210 family members), of whom 39 displayed variants. Additionally, the study included the families of 16 patients with FEVR from Xinjiang (33 family members), of whom six displayed variants, as shown in Table 1. Only three patients displayed ZNF408 variants: all were Han Chinese individuals from Chongqing (two female patients and one male patient), and the mutation rate was 2.83%.
Table 1.
Distribution of FEVR variants among patients in Chongqing and Xinjiang.
| Gene | LRP5 | CFTR | CAPN5 | FZD4 | KIF11 | SLC9A3 | ZNF408 | TSPAN12 | ADGRG2 | NDP | Unknown |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients in Chongqing | 18 | 8 | 4 | 4 | 4 | 3 | 3 | 2 | 1 | 1 | 51 |
| No. of patients in Xinjiang | 3 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 10 |
ADGRG2, adhesion G protein-coupled receptor G2; CAPN5, calpain 5; CFTR, cystic fibrosis transmembrane conductance regulator; FEVR, familial exudative vitreoretinopathy; FZD4, frizzled-4; KIF11, kinesin family member 11; LRP5, LDL receptor related protein 5; NDP, norrin cystine knot growth factor NDP; SLC9A3, solute carrier family 9 member A3; TSPAN12, tetraspanin 1; ZNF408, zinc finger protein 408.
Clinical characteristics of patients with ZNF408 variants
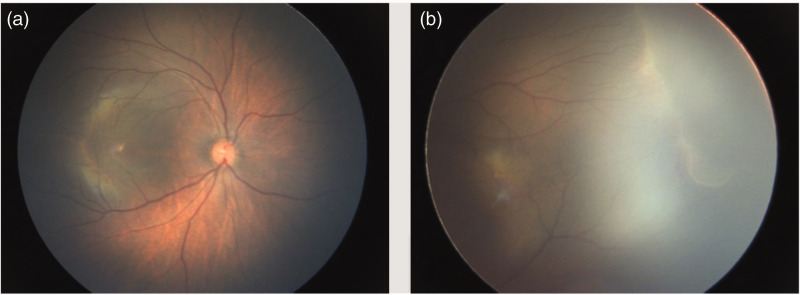
Proband 1 (FEVR-10): The proband was a female newborn with a gestational age of 39+1 weeks and birth weight of 2760 g. She was delivered by spontaneous vaginal birth, and her mother had gestational diabetes during pregnancy. Fundus examination revealed a boundary between the temporal peripheral vascular zone and the avascular zone of the retina in both eyes; the temporal retinal blood vessels displayed a willow branch-like appearance. Vessels immediately adjacent to the avascular zone were dilated and straightened, forming new blood vessels in a manner accompanied by hemorrhage and exudation. Diffuse retinal hemorrhage was present in both eyes. Right eye involvement spanned from 8 to 10 o'clock, whereas left eye involvement was limited to the 3 o'clock position. Both eyes exhibited stage 2B FEVR. The fundus conditions are shown in Figure 1, and no similar symptoms were observed in the proband’s family members.
Proband 2 (FEVR-64): The proband was a male newborn with a gestational age of 39 weeks and birth weight of 3050 g. He was delivered by spontaneous vaginal birth, and his mother had gestational diabetes during pregnancy. Fundus examination revealed a boundary between the peripheral vascular zone and the avascular zone in the temporal side of the left eye; it also showed partial retinal detachment in the temporal side of the right eye, without macular involvement. The right eye exhibited stage 3A FEVR, and the left eye exhibited stage 1 FEVR. The fundus conditions are shown in Figure 2, and no similar symptoms were observed in the proband’s family members.
Proband 3 (FEVR-295): The proband was a female newborn with a gestational age of 40+4 weeks and a birth weight of 2820 g. She was delivered by spontaneous vaginal birth, and her mother had no unusual medical conditions during pregnancy. Fundus examination revealed no obvious abnormality in the right eye. In the left eye, a boundary between the vascular zone and the avascular zone was present in the temporal peripheral retina; blood vessels adjacent to the avascular zone were dilated, forming new vessels in a manner accompanied by hemorrhage. The left eye exhibited stage 2A FEVR, spanning from 2 to 4 o'clock. The fundus conditions are shown in Figure 3.
Figure 1.
Fundus images of proband 1 (FEVR-010). a, right eye; b, left eye.
Figure 2.
Fundus images of proband 2 (FEVR-064). a, right eye; b, left eye.
Figure 3.
Fundus images of proband 3 (FEVR-295). a, right eye; b, left eye.
Clinical manifestations
The clinical manifestations of the three probands with ZNF408 variants were consistent with FEVR characteristics. Because the probands were all newborns, fundus fluorescein angiography was not performed. Each proband’s fundus examination revealed numerous, densely distributed peripheral retinal blood vessels in both eyes, with a willow branch-like morphology; inner/inferior retinal exudation was evident on the temporal side, and peripheral vascular hemorrhage was present.
ZNF408 variants in affected families
Among the three probands with ZNF408 variants, one also displayed an LRP5 variant; the others did not display additional variants. In the family of one proband with a ZNF408 single-gene variant, the mother and grandfather carried the ZNF408 variant, whereas the father did not; the variant was assumed to have a maternal origin. The patient displayed bilateral involvement, constituting stage 3A FEVR in the right eye and stage 1 FEVR in the left eye. The other patient with a ZNF408 single-gene variant exhibited unilateral stage 2A FEVR; the genetic origin in that patient could not be determined because no blood samples could be collected from family members. In the family of the proband with two variants, the mother carried the LRP5 variant and the father carried the ZNF408 variant; the respective pathogenic variants in the proband were assumed to have maternal and paternal origins. The patient exhibited bilateral stage 2B FEVR. The variant characteristics in each family are shown in Table 2.
Table 2.
Variant characteristics in three families with FEVR.
| Gene | Variant position | Nucleotide change | Amino acid change | Patient number | Family number |
|---|---|---|---|---|---|
| ZNF408: NM_024741 | chr11-46726503 | c.A1253G | p.Y418C | FEVR-010 (proband 1) | Family 1 |
| LRP5: NM_002335 | chr11-68177511 | c.A2221G | p.R741G | ||
| ZNF408: NM_024741 | chr11-46726503 | c.A1253G | p.Y418C | FEVR-09 (proband 1, father) | |
| LRP5: NM_002335 | chr11-68177511 | c.A2221G | p.R741G | FEVR-08 (proband 1, mother) | |
| ZNF408: NM_024741 | chr11-46726031 | c.G781A | p.V261M | FEVR-64 (proband 2) | Family 2 |
| No related variant was detected | FEVR-63 (proband 2, father) | ||||
| ZNF408: NM_024741 | chr11-46726031 | c.G781A | p.V261M | FEVR-62 (proband 2, mother) | |
| ZNF408: NM_024741 | chr11-46726031 | c.G781A | p.V261M | FEVR-61 (proband 2, grandfather) | |
| ZNF408: NM_024741 | chr11-46727151 | c.C1901T | p.P634L | FEVR-295 (proband 3) | Family 3 |
FEVR, familial exudative vitreoretinopathy; ZNF408, zinc finger protein 408.
ZNF408 variants in three probands
The ZNF408 variant in each proband was located in an exon, and all variants were missense mutations, as shown in Table 3.
Table 3.
ZNF408 variants in three probands.
| Patient number | Gene | Variant position | Variant position | Variant type | Nucleotide change | Amino acid change |
|---|---|---|---|---|---|---|
| FEVR-010 | ZNF408 | chr11-46726503 | Exon | Nonsynonymous SNV | c.A1253G | p.Y418C |
| LRP5 | chr11-68177511 | Exon | Nonsynonymous SNV | c.A2221G | p.R741G | |
| FEVR-064 | ZNF408 | chr11-46726031 | Exon | Nonsynonymous SNV | c.G781A | p.V261M |
| FEVR-295 | ZNF408 | chr11-46727151 | Exon | Nonsynonymous SNV | c.C1901T | p.P634L |
FEVR, familial exudative vitreoretinopathy; SNV, single nucleotide variant; ZNF408, zinc finger protein 408.
Prediction of variant pathogenicity in three probands
Analysis of proband FEVR-64 revealed that the ZNF408 variant was located on chromosome 11 at position 46726031 (c.G781A, p.V261M). The predictive values in SIFT, Polyphen2_HDIV, and Polyphen2_HVAR analyses were T (0.371), B (0.013) and B (0.002), respectively (Table 4). The changes in amino acid sequence, three-dimensional protein structure, and overall protein function caused by the variant were limited, suggesting low pathogenicity.
Table 4.
Prediction of variant pathogenicity in three probands.
| Patient number | Gene | Variant position | Nucleotide change | Amino acid change | SIFT_ pred* | Polyphen2_ HDIV_pred** | Polyphen2_ HVAR_pred*** |
|---|---|---|---|---|---|---|---|
| FEVR-010 | ZNF408 | chr11-46726503 | c.A1253G | p.Y418C | D (0) | D (0.992) | P (0.901) |
| LRP5 | chr11-68177511 | c.A2221G | p.R741G | D (0.001) | D (0.999) | D (0.985) | |
| FEVR-064 | ZNF408 | chr11-46726031 | c.G781A | p.V261M | T (0.371) | B (0.013) | B (0.002) |
| FEVR-295 | ZNF408 | chr11-46727151 | c.C1901T | p.P634L | D (0.01) | B (0.034) | B (0.007) |
: SIFT score ranges from 0 to 1; deleterious (D, score < 0.05) = predicted to be deleterious; tolerated (T, score ≥0.05) = predicted to be harmless.
: Polyphen2_HDIV (based on HumanDiv database); probably damaging (D, score ≥ 0.957), possibly damaging (P, score 0.453 ≤ 0.956), benign (B, score ≤ 0.452).
: Polyphen2_HVAR (based on HumanVar database); probably damaging (D, score ≥ 0.909), possibly damaging (P, score 0.447 ≤ 0.909), benign (B, score ≤ 0.446).
In proband FEVR-010, the respective ZNF408 and LRP5 variants were located on chromosome 11 at positions 46726503 (c.A1253G, p.Y418C) and 68177511 (c.A2221G, p.R741G). The predictive values in SIFT, Polyphen2_HDIV, and Polyphen2_HVAR analyses for ZNF408 were D (0.0), D (0.992), and P (0.901), respectively; for LRP5, they were D (0.001), D (0.999), and D (0.985) (Table 4). These results suggest that the presence of multiple variants on chromosome 11 caused likely pathogenic changes in protein structure and function.
In proband FEVR-295, the ZNF408 variant was located on chromosome 11 at position 46727151 (c.C1901T, p.P634L). The predictive values in SIFT, Polyphen2_HDIV, and Polyphen2_HVAR analyses were D (0.01), B (0.034) and B (0.007), respectively. The value of D (0.01) was predicted to be harmful according to SIFT analysis. However, the values of B (0.034 and 0.007) were predicted to be harmless according to Polyphen2_HDIV and Polyphen2_HVAR analyses (Table 4). These findings suggest that the change in amino acid sequence was not pathogenic, but the change in three-dimensional protein structure was likely to be pathogenic.
Discussion
The pathogenic relationship between ZNF408 and FEVR was recently discovered. ZNF408 contains five exons that encode a 720-amino acid transcription factor belonging to the zinc finger family of transcription factors. Zinc finger domains, multifunctional DNA recognition elements in regulatory proteins, participate in various cellular activities, including embryonic development and cell differentiation. ZNF408 is expressed in all tissues or organs, but its highest expression in adult tissues and organs occurs in the retina—almost 30-fold higher than expression levels in heart, placenta, and liver—suggesting that ZNF408 plays important roles in retinal development and homeostasis. 10 Additional research has confirmed that ZNF408 is closely associated with retinal vascular development and retinal homeostasis.11–13
Thus far, 11 FEVR-associated genes have been identified: NDP, FZD4, LRP5, TSPAN12, ZNF408, KIF11, RCBTB1, CTNNB, JAG1, EVR3, and ATOH7. Four of these genes (FZD4, LRP5, TSPAN12, and NDP) encode proteins that are important components of the Wnt/Norrin signaling pathway, which monitors retinal vascular development. Variants in these four genes cause FEVR by altering Wnt/Norrin signaling interactions. 2 It is unclear whether ZNF408 regulates retinal vascular development through the Wnt/Norrin signaling pathway; further studies are needed.
Pathogenic ZNF408 variants are the rarest FEVR-associated variants. One study of 389 patients with FEVR showed that ZNF408 variants were present in 0.77% of 389 patients with FEVR 6 ; another study revealed that ZNF408 variants were present in 1.6% of 3257 patients with FEVR. 14 In the present study, three patients with FEVR had ZNF408 variants; the mutation rate of 2.83% (3/106) presumably differed from the rates in previous studies because of regional and ethnic factors.
To our knowledge, there have been few reports of families with FEVR in which both parents carry pathogenic variants. 15 Previously, there was speculation that patients with FEVR only had a single pathogenic variant, which was consistent with the monogenic inheritance pattern. However, the increasing number of patients with FEVR and discovery of additional FEVR-associated genes have led to the detection of pathogenic patterns involving two or three variants in one or more genes. 16 , 17 Because ZNF408 variants are the rarest known FEVR-associated variants, the corresponding mutation rate is very low. Thus far, there have been no reports of double variants involving ZNF408 and other FEVR-associated genes. In the present study, three patients had ZNF408 variants; of these patients, one also had an LRP5 variant, whereas two had a ZNF408 single-gene variant. Concerning the patient with ZNF408 and LRP5 variants, the mother carried the LRP5 variant and the father carried the ZNF408 variant; the respective pathogenic variants were assumed to have maternal and paternal origins. These findings confirmed the existence of variants in two or more genes among patients with FEVR; they also suggested that ZNF408 can cause disease in a cooperative manner with other variants. Notably, in a family carrying variants in both FZD4 and LRP5, patients who displayed both variants had more severe clinical manifestations, compared with patients who only displayed an FZD4 variant. 16 There may also be a synergistic effect between variant sites in patients with two or more affected genes, such that their clinical manifestations are more severe than the manifestations in patients with single-gene variants.5,18,19 Among the three patients with ZNF408 variants in this study, proband FEVR-10 (variants in both ZNF408 and LRP5) displayed bilateral stage 2B FEVR. One patient with a ZNF408 single-gene variant (proband FEVR-64) had retinal detachment in the right eye (stage 3A FEVR) and exhibited stage 1 FEVR in the left eye. The other patient with a ZNF408 single-gene variant (proband FEVR-295) had a normal right eye and stage 2A FEVR in the left eye. Notably, the patient with variants in two genes did not show more severe clinical manifestations, compared with the other two probands. Thus, the clinical manifestations of FEVR are complex and diverse; the binocular manifestations also appear to differ among patients, suggesting that phenotype and genotype do not necessarily have a direct one-to-one relationship in patients with FEVR. The diverse manifestations among patients with the same gene variant, as well as differences between eyes in the same patient, imply that the clinical manifestations of FEVR are influenced by non-genetic factors.
This study had a few limitations. First, most detected variants have not been reported in the literature; they constitute suspected FEVR-related pathogenic variants. Functional testing is needed to determine whether each variant site can cause disease without additional contributing factors. Second, the cohort was not particularly large, and there were only three patients with ZNF408 variants; thus, the findings may not be generalizable to other populations of individuals with FEVR. Further investigations are needed to acquire sufficient statistical power to clarify the relationship between genotype and phenotype with respect to FEVR. Third, fundus fluorescein angiography examinations of the probands’ family members were not performed, and some family members did not provide blood samples for sequencing analysis. Therefore, we could not fully analyze the genotypes and phenotypes of the family members. Finally, few patients with FEVR were recruited from Xinjiang, and no patients in Xinjiang had ZNF408 variants. Therefore, we could not compare ZNF408 variants between regions and ethnic groups.
Footnotes
The authors declare that there is no conflict of interest.
Funding: This work was supported by the Key Project of Chongqing Science and Technology Commission (no. cstc2017shms-zdyfX0049), the National Key Clinical Specialty Construction Project (Obstetrics and Gynecology), and the Chongqing Research Center for Prevention & Control of Maternal and Child Diseases and Public Health.
ORCID iD: Xueying Tao https://orcid.org/0009-0000-4403-1886
References
- 1.Zhang Q, Zhao P, Cai X, et al. Clinical features of familial exudative vitreoretinopathy. Chin J Ocul Fundus Dis 2014; 30: 374–376. [Google Scholar]
- 2.Miao T. Research progress on genetics of familial exudative vitreoretinopathy. Chin J Exp Ophthalmol 2016; 34: 558–560. [Google Scholar]
- 3.Yian L, Qi Z, Peiquan Z. Zinc figure protein 408 in the pathogenesis of familial exudative vitreoretinopathy. Chin J Ocul Fundus Dis 2016; 32: 661–663. [Google Scholar]
- 4.Collin RW, Nikopoulos K, Dona M, et al. ZNF408 is mutated in familial exudative vitreoretinopathy and is crucial for the development of zebrafish retinal vasculature. Proc Natl Acad Sci USA 2013; 110: 9856–9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvo J, Lyubasyuk V, Xu M, et al. Next-generation sequencing and novel variant determination in a cohort of 92 familial exudative vitreoretinopathy patients. Invest Ophthalmol Vis Sci 2015; 56: 1937–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li JK, Li Y, Zhang X, et al. Spectrum of variants in 389 Chinese probands with familial exudative vitreoretinopathy. Invest Ophthalmol Vis Sci 2018; 59: 5368–5381. [DOI] [PubMed] [Google Scholar]
- 7.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 8.Boonstra FN, van Nouhuys CE, Schuil J, et al. Clinical and molecular evaluation of probands and family members with familial exudative vitreoretinopathy. Invest Ophthalmol Vis Sci 2009; 50: 4379–4385. [DOI] [PubMed] [Google Scholar]
- 9.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayyagari R, Mandal MN, Karoukis AJ, et al. Late-onset macular degeneration and long anterior lens zonules result from a CTRP5 gene mutation. Invest Ophthalmol Vis Sci 2005; 46: 3363–3371. [DOI] [PubMed] [Google Scholar]
- 11.Karjosukarso DW, Ali Z, Peters TA, et al. Modeling ZNF408-associated FEVR in zebrafish results in abnormal retinal vasculature. Invest Ophth Vis Sci 2020; 61: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izumi N, Helker C, Ehling M, et al. Fbxw7 controls angiogenesis by regulating endothelial Notch activity. PLoS One 2012; 7: e41116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben Shoham A, Malkinson G, Krief S, et al. S1P1 inhibits sprouting angiogenesis during vascular development. Development 2012; 139: 3859–3869. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Chen J, Xiong H, et al. Genotype-phenotype associations in familial exudative vitreoretinopathy: a systematic review and meta-analysis on more than 3200 individuals. PLoS One 2022; 17: e0271326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Peng J, Li J, et al. The characteristics of digenic familial exudative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol 2018; 256: 2149–2156. [DOI] [PubMed] [Google Scholar]
- 16.Qin M, Hayashi H, Oshima K, et al. Complexity of the genotype-phenotype correlation in familial exudative vitreoretinopathy with mutations in the LRP5 and/or FZD4 genes. Hum Mutat 2005; 26: 104–112. [DOI] [PubMed] [Google Scholar]
- 17.Chunli C, Peiquan Z, Xiaorong L. . Cohort study of genotype and clinical phenotype in 34 families with familial exudative vitreoretinopathy. Chin J Ocul Fundus Dis 2020; 36: 184–191. [Google Scholar]
- 18.Schatz P, Khan AO. Variable familial exudative vitreoretinopathy in a family harbouring variants in both FZD4 and TSNPAN12. Acta Ophthalmol 2017; 95: 705–709. [DOI] [PubMed] [Google Scholar]
- 19.Stiegel E, Say EA, Carter BC, et al. Simultaneous FZD4 and LRP5 mutation in autosomal dominant familial exudative vitreoretinopathy. Retin Cases Brief Rep 2013; 7: 26–28. [DOI] [PubMed] [Google Scholar]