Abstract
Background
Shoulder replacement is a reliable treatment for the relief of pain and improvement of function in patients with glenohumeral arthritis, rotator cuff arthropathy, osteonecrosis and fracture. Limited data is available comparing revision rates for the different types of shoulder replacement when used in younger patients. This study aims to compare the survivorship of hemi resurfacing, stemmed hemiarthroplasty, total shoulder arthroplasty and reverse total shoulder arthroplasty in younger patients using data from a large national arthroplasty registry.
Methods
Data from the Australian Orthopaedic Association National Joint Replacement Registry was obtained for the period 16 April 2004–31 December 2018. The study population included all shoulder arthroplasty patients aged <65 years. These were stratified into two groups: <55 years and 55–64 years. A total of 8742 primary shoulder arthroplasty procedures were analysed (1936 procedures in the <55 years and 6806 in the 55–64 years age group).
Results
In the <55 years age group, there was no difference in revision rate for total shoulder arthroplasty versus reverse total shoulder arthroplasty at any time point. Reverse total shoulder arthroplasty had a lower revision rate after six months when compared to hemi resurfacing (HRA) (p = 0.031). Also, reverse total shoulder arthroplasty had a higher early rate of revision in the first 12 months compared to hemiarthroplasty (p = 0.018). However, from 2 years reverse total shoulder arthroplasty had a lower revision rate overall (p = 0.029).
In the 55–64 years patient age group, reverse total shoulder arthroplasty had a lower earlier revision rate. This was statistically significant compared to hemi resurfacing (HRA) (p = 0.028), hemiarthroplasty (p = 0.049) and total shoulder arthroplasty (p < 0.001).
Conclusion
This study demonstrated that for patients aged <55 years there was no significant difference in the rate of revision when total shoulder arthroplasty and reverse total shoulder arthroplasty were compared. reverse total shoulder arthroplasty had a lower rate of revision when compared to hemi resurfacing and hemiarthroplasty after 2 years. reverse total shoulder arthroplasty had the lowest comparative revision rate in patients aged 55–64 years overall.
Keywords: Shoulder joint, arthroplasty, less than 65 years, joint registry, young patient, replacement, revision rate
Introduction
Shoulder replacement is a reliable treatment for the relief of pain and improvement of function in patients with glenohumeral arthritis, rotator cuff arthropathy, osteonecrosis and fracture. 1 The use of shoulder arthroplasty is increasing as surgeons become more familiar with its use and the indications expand.1–3 However, concern exists around shoulder arthroplasty in younger patients with higher demands expected to lead to higher revision rates. 4 Younger patients have been shown to both have greater expectations following shoulder arthroplasty and are more likely to participate in sporting activities.5,6 This has the potential to result in higher rates of revision due to glenoid wear, loosening of the components and rotator cuff failure. 4 In 2018, data from the Australian Orthopaedic Association National Joint Replacement Registry showed high rates of revision in younger patients undergoing shoulder joint arthroplasty compared to older cohorts. 7
Varying rates of osteoarthritis, post traumatic arthritis, chondrolysis, osteonecrosis, instability and rheumatoid arthritis are reported as the primary indication for shoulder arthroplasty in young patients.4,8,9 The causative pathology is an important consideration as a diagnosis other than osteoarthritis or rheumatoid arthritis has been linked with higher revision rates and the potential for early revision in younger patients.4,10
Hemi resurfacing (HRA), stemmed hemiarthroplasty (HA), TSA and reverse total shoulder arthroplasty (RTSA) are the four main categories of shoulder reconstruction for patients with end stage shoulder pathology that require surgical treatment. Hemiarthroplasty has been used for the management of non-reconstructable proximal humerus fractures with satisfactory results. 11 Studies suggest that its use in the treatment of glenohumeral arthritis has been less satisfactory compared to TSA with respect to functional outcomes and revision rate.12–14 However, along with HRA, HA remains an attractive option in younger patients as it removes some of the concern around loosening of the glenoid component which has been shown to be the primary cause of late failure in TSA.4,15 RTSA is commonly used to treat end stage arthritis in older patients with a deficient rotator cuff. The low revision rates have led to a widening of the indications for RTSA and increasing use in younger patients with successful outcomes.16–18
Shoulder arthroplasty in younger patients both with and without an intact or functional rotator cuff remains an area of controversy and a complex and difficult clinical situation for shoulder surgeons. This study aimed to compare the revision rate of shoulder arthroplasty for younger patients using data from the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Secondary aims were to compare the effect of age, revision diagnosis and type of revision between the four categories of shoulder replacement.
Materials and methods
This was a large prospective cohort study of patients who have undergone shoulder arthroplasty for all diagnosis using data from the AOANJRR. The AOANJRR began data collection on 1 September 1999 and includes data on almost 100% of the hip and knee arthroplasty procedures performed in Australia since 2002. Data collection was expanded to include shoulder arthroplasty procedures in April 2004 and has documented almost all shoulder arthroplasty procedures Australia-wide since November 2007. This data is externally validated against patient-level data provided by all Australian state and territory health departments. A sequential, multilevel matching process is used to identify any missing data which are subsequently obtained by follow-up with the relevant hospital. Each month, in addition to internal validation and data quality checks, all primary procedures are linked to any subsequent revision involving the same patient, joint and side. Data are also matched bi-annually to the Australian National Death Index data to identify patients who have died.
The study population included all primary total shoulder arthroplasty procedures undertaken for any reason and reported to the registry between 16 April 2004 and 31 December 2018. Procedures were grouped into the class of primary shoulder arthroplasty; HRA, HA, TSA or RTSA. For each type of shoulder arthroplasty, patients were grouped into age categories; <55 years, and 55–64 years. Additional analysis was undertaken to identify the reason for revision for each class of arthroplasty in each of the age groups outlined above.
Statistical analysis
Kaplan-Meier estimates of survivorship were used to report the time to revision, with censoring at the time of death and closure of the dataset at the end of December 2018. The unadjusted cumulative percent revision (CPR), with 95% confidence intervals (CI), were calculated using unadjusted point wise Greenwood estimates. Age and gender adjusted hazard ratios (HR) were calculated from Cox proportional hazard models to compare the rate of revision between groups. The assumption of proportional hazards was checked analytically for each model. If the interaction between the predictor and the log of time was statistically significant in the standard Cox model, then a time varying model was estimated. Time points were selected based on the greatest change in hazard, weighted by a function of events. Time points were iteratively chosen until the assumption of proportionality was met and HRs were calculated for each selected time-period. For the current study, if no time-period was specified, the HR was calculated over the entire follow-up period. All tests were two-tailed at 5% levels of significance. Statistical analysis was performed using SAS software version 9.4 (SAS Institute Inc., Cary, North Carolina).
Results
Age and class of shoulder replacement
A total of 8742 shoulder arthroplasty procedures were identified. These included 1936 procedures undertaken in patients aged <55 years and 6806 procedures in patients aged 55–64 years (Table 1).
Table 1.
Age, gender and follow-up of primary shoulder replacement in patients aged <65 years.
| Hemi stemmed | Hemi resurfacing | Total stemmed | Total reverse | Total | ||
|---|---|---|---|---|---|---|
| Male | Number | 663 | 542 | 2055 | 1276 | |
| Percent | 44.2 | 69.5 | 54.6 | 47.3 | ||
| Minimum age | 14 | 19 | 21 | 17 | ||
| Maximum age | 64 | 64 | 64 | 64 | ||
| Median age | 55 | 55 | 60 | 61 | ||
| Mean age | 53.0 | 53.1 | 57.9 | 59.4 | ||
| Std. dev. | 9.4 | 8.5 | 6.2 | 5.5 | ||
| Female | Number | 836 | 238 | 1712 | 1420 | |
| Percent | 55.8 | 30.5 | 45.4 | 52.7 | ||
| Minimum age | 13 | 27 | 21 | 13 | ||
| Maximum age | 64 | 64 | 64 | 64 | ||
| Median age | 59 | 58 | 60 | 61 | ||
| Mean age | 56.8 | 55.8 | 58.9 | 59.3 | ||
| Std. dev. | 7.5 | 8.0 | 5.4 | 5.7 | ||
| Total | Number | 1499 | 780 | 3767 | 2696 | |
| Percent | 100 | 100 | 100 | 100 | ||
| Minimum age | 13 | 19 | 21 | 13 | ||
| Maximum age | 64 | 64 | 64 | 64 | ||
| Median age | 58 | 56 | 60 | 61 | ||
| Mean age | 55.1 | 53.9 | 58.4 | 59.3 | ||
| Std. dev. | 8.6 | 8.4 | 5.9 | 5.6 | ||
| Follow-Up | Number | 1499 | 780 | 3767 | 2696 | 8742 |
| Minimum follow-up | 0.01 | 0.03 | 0.01 | 0.00 | 0.00 | |
| Maximum follow-up | 13.1 | 12.8 | 13.7 | 12.4 | 13.7 | |
| Median follow-up | 5.24 | 6.12 | 4.61 | 2.14 | 3.87 | |
| Mean follow-up | 5.34 | 5.80 | 5.00 | 3.00 | 4.51 | |
| Std. dev. | 3.46 | 3.50 | 3.20 | 2.74 | 3.30 |
In the <55 years age group, there were 347 (17.9%) HRA procedures, 525 (27.1%) HA, 708 (36.6%) TSA, and 356 (18.4%) RTSA procedures (Table 2). In the 55–64 years age group, 433 (5.4%) were HRA procedures, 974 (14.3%) were HA, 3059 (44.9%) were TSA, and 2340 (34.4%) were RTSA procedures. Overall, for patients aged <65 years, procedures were most often undertaken in males (51.9%) (Table 2).
Table 2.
Yearly cumulative percent revision of primary shoulder replacement by age and type of primary (all diagnoses).
| Age | Type of primary | N revised | N total | Obs. years | Revisions/100 obs. years (95% CI) |
|---|---|---|---|---|---|
| <55 | Hemi resurfacing | 44 | 347 | 1901 | 2.31 (1.68, 3.11) |
| Hemi stemmed | 70 | 525 | 2550 | 2.75 (2.14, 3.47) | |
| Total stemmed | 88 | 708 | 3448 | 2.55 (2.05, 3.14) | |
| Total reverse | 24 | 356 | 1077 | 2.23 (1.43, 3.32) | |
| 55–64 | Hemi resurfacing | 72 | 433 | 2624 | 2.74 (2.15, 3.46) |
| Hemi stemmed | 123 | 974 | 5458 | 2.25 (1.87, 2.69) | |
| Total stemmed | 297 | 3059 | 15,375 | 1.93 (1.72, 2.16) | |
| Total reverse | 130 | 2340 | 7019 | 1.85 (1.55, 2.20) |
The mean age for patients undergoing HRA was 53.9 years (SD 8.4), 55.1 years (SD 8.6) for HA, 58.4 years (SD 5.9) for TSA and 59.3 years (SD 5.7) for RTSA. The mean follow-up was 5.8 years (SD 3.5) for HRA, 5.3 years (SD 3.5) for HA, 5.0 years (SD 3.2) for TSA and 3.0 years (SD 2.7) for RTSA (Table 1).
Primary diagnosis
The class of shoulder replacement varied according to primary diagnosis. TSA was the most common arthroplasty performed for osteoarthritis in patients aged <55 years and patients aged 55–64 years accounting for 52% and 64%, respectively (Tables 3 and 4). Fracture was the main indication for HA in the <55 years group (46.9%) and the 55–64 years age group (60%).
Table 3.
Primary diagnosis of primary shoulder replacement in patients aged <55 years by type of primary.
| Hemi resurfacing | Hemi stemmed | Total stemmed | Total reverse | |||||
|---|---|---|---|---|---|---|---|---|
| Primary diagnosis | N | Col% | N | Col% | N | Col% | N | Col% |
| Osteoarthritis | 291 | 83.9 | 124 | 23.6 | 578 | 81.6 | 126 | 35.4 |
| Fracture | 5 | 1.4 | 246 | 46.9 | 17 | 2.4 | 46 | 12.9 |
| Osteonecrosis | 14 | 4.0 | 75 | 14.3 | 45 | 6.4 | 14 | 3.9 |
| Rheumatoid arthritis | 8 | 2.3 | 19 | 3.6 | 44 | 6.2 | 38 | 10.7 |
| Tumour | . | . | 46 | 8.8 | 3 | 0.4 | 48 | 13.5 |
| Rotator cuff arthropathy | 12 | 3.5 | 3 | 0.6 | 3 | 0.4 | 70 | 19.7 |
| Instability | 14 | 4.0 | 10 | 1.9 | 8 | 1.1 | 9 | 2.5 |
| Other inflammatory arthritis | 3 | 0.9 | 2 | 0.4 | 9 | 1.3 | 5 | 1.4 |
| Other | 1 | 0.1 | ||||||
| Total | 347 | 100.0 | 525 | 100.0 | 708 | 100.0 | 356 | 100.0 |
Table 4.
Primary diagnosis of primary shoulder replacement in patients aged 55–64 years by type of primary.
| Hemi resurfacing | Hemi stemmed | Total stemmed | Total reverse | |||||
|---|---|---|---|---|---|---|---|---|
| Primary diagnosis | N | Col% | N | Col% | N | Col% | N | Col% |
| Osteoarthritis | 384 | 88.7 | 256 | 26.3 | 2836 | 92.7 | 976 | 41.7 |
| Fracture | 2 | 0.5 | 593 | 60.9 | 31 | 1.0 | 390 | 16.7 |
| Rotator cuff arthropathy | 17 | 3.9 | 20 | 2.1 | 16 | 0.5 | 737 | 31.5 |
| Rheumatoid arthritis | 10 | 2.3 | 26 | 2.7 | 76 | 2.5 | 98 | 4.2 |
| Osteonecrosis | 15 | 3.5 | 36 | 3.7 | 57 | 1.9 | 57 | 2.4 |
| Tumour | . | . | 29 | 3.0 | 4 | 0.1 | 32 | 1.4 |
| Instability | 3 | 0.7 | 10 | 1.0 | 14 | 0.5 | 35 | 1.5 |
| Other inflammatory arthritis | 2 | 0.5 | 4 | 0.4 | 23 | 0.8 | 13 | 0.6 |
| Other | 2 | 0.1 | 2 | 0.1 | ||||
| Total | 433 | 100.0 | 974 | 100.0 | 3059 | 100.0 | 2340 | 100.0 |
Revision rates by age
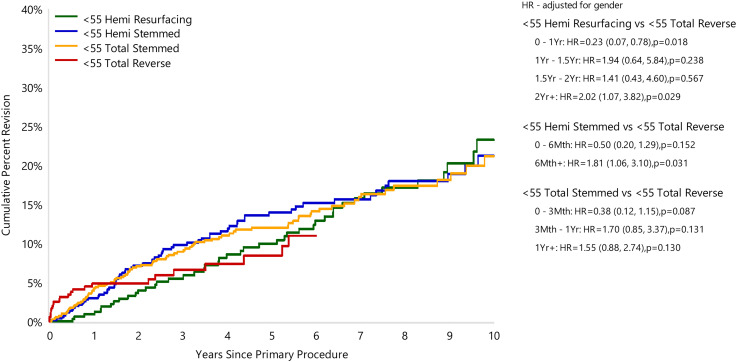
In the <55 years age group RTSA has the lowest CPR at 5 years. The 5-year CPR rate for HRA was 10.0% (95% CI 6.9, 14.3), for HA it was 13.9% (95% CI 10.8, 17.8), for TSA it was 12.0% (95% CI 9.6, 15.0) and for RTSA it was 8.4% (95% CI 5.3, 13.3). The 10-year CPR for this age group was for HRA, HA and TSA, 23.2% (95% CI 17.0, 31.2), 21.2% (95% CI 16.3, 27.3) and 21.1% (95% CI 16.5, 26.8), respectively (Figure 1) (Table 5). Insufficient data was available to calculate a CPR for RTSA at 10 years in this age group.
Figure 1.
Cumulative percent revision of primary shoulder replacement in patients aged <55 years by type of primary (all diagnoses).
Table 5.
Revision rates of primary shoulder replacement by Age and type of primary (all diagnoses).
| Age | Type of primary | 1 year | 2 years | 3 years | 4 years | 5 years | |
|---|---|---|---|---|---|---|---|
| <55 | Hemi resurfacing | 0.9 (0.3, 2.8) | 4.0 (2.3, 6.9) | 5.5 (3.4, 8.8) | 8.6 (5.8, 12.7) | 10.0 (6.9, 14.3) | |
| Hemi stemmed | 3.0 (1.8, 4.9) | 7.2 (5.1, 9.9) | 9.8 (7.3, 13.0) | 11.6 (8.8, 15.1) | 13.9 (10.8, 17.8) | ||
| Total stemmed | 4.2 (2.9, 6.1) | 7.1 (5.3, 9.3) | 8.9 (6.9, 11.5) | 11.0 (8.7, 13.9) | 12.0 (9.6, 15.0) | ||
| Total reverse | 4.8 (3.0, 7.8) | 4.8 (3.0, 7.8) | 6.6 (4.2, 10.4) | 7.4 (4.7, 11.6) | 8.4 (5.3, 13.3) | ||
| 55–64 | Hemi resurfacing | 2.4 (1.3, 4.4) | 6.1 (4.2, 8.9) | 10.4 (7.8, 13.9) | 12.5 (9.6, 16.3) | 15.6 (12.3, 19.8) | |
| Hemi stemmed | 4.9 (3.7, 6.5) | 8.6 (6.9, 10.6) | 10.9 (9.0, 13.2) | 11.6 (9.6, 13.9) | 12.5 (10.5, 14.9) | ||
| Total stemmed | 3.5 (2.9, 4.3) | 5.8 (5.0, 6.8) | 7.2 (6.3, 8.3) | 8.1 (7.1, 9.2) | 9.5 (8.4, 10.7) | ||
| Total reverse | 3.8 (3.0, 4.6) | 5.2 (4.3, 6.3) | 6.0 (5.0, 7.2) | 6.8 (5.6, 8.1) | 7.1 (5.9, 8.5) | ||
| Age | Type of primary | 6 Yrs | 7 Yrs | 8 Yrs | 9 Yrs | 10 Yrs | 11 Yrs |
| <55 | Hemi resurfacing | 12.9 (9.2, 17.9) | 15.8 (11.6, 21.3) | 17.1 (12.7, 22.9) | 20.2 (15.0, 27.0) | 23.2 (17.0, 31.2) | |
| Hemi stemmed | 15.1 (11.8, 19.2) | 15.6 (12.2, 19.8) | 17.9 (14.1, 22.7) | 18.9 (14.7, 24.0) | 21.2 (16.3, 27.3) | ||
| Total stemmed | 14.1 (11.3, 17.5) | 15.9 (12.8, 19.7) | 17.4 (14.0, 21.4) | 18.1 (14.5, 22.5) | 21.1 (16.5, 26.8) | ||
| Total reverse | 11.0 (6.8, 17.4) | ||||||
| 55–64 | Hemi resurfacing | 17.3 (13.8, 21.7) | 18.5 (14.8, 23.0) | 20.0 (16.0, 24.8) | 20.0 (16.0, 24.8) | 20.9 (16.7, 26.1) | |
| Hemi stemmed | 13.4 (11.2, 15.9) | 13.8 (11.6, 16.4) | 14.5 (12.2, 17.3) | 14.9 (12.5, 17.7) | 16.5 (13.6, 19.8) | ||
| Total stemmed | 10.7 (9.5, 12.0) | 11.4 (10.1, 12.9) | 12.4 (11.0, 14.0) | 14.0 (12.3, 15.8) | 15.9 (13.9, 18.3) | 16.6 (14.2, 19.4) | |
| Total reverse | 7.3 (6.0, 8.9) | 8.0 (6.5, 9.8) | 8.4 (6.7, 10.6) | 9.7 (7.4, 12.6) | 9.7 (7.4, 12.6) |
In the <55 years age group, HRA had a lower early CPR rate compared to RTSA in the first 12 months (HR = 0.23 (95% CI 0.07, 0.78), p = 0.018). After this time, there was no difference until two years when HRA had a higher revision rate (HR = 2.02 (95% CI 1.07, 3.82), p = 0.029) compared to RTSA (Figure 1) (Table 5). Similarly, when compared to RTSA, HA had a higher revision rate after six months (HR = 1.81 (1.06, 3.10), p = 0.031) (Figure 1). There was no difference in the revision rate for TSA compared to RTSA at any stage for patients in this age group (Figure 1).
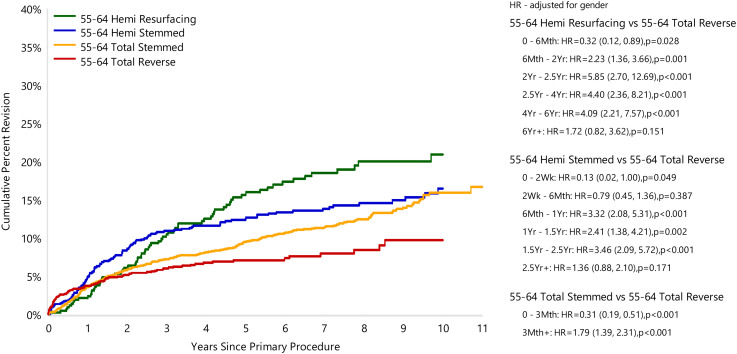
In the 55–64 years age group RTSA again had the lowest CPR at 5 years. The 5-year CPR rate for HRA was 15.6% (95% CI 12.3, 19.8), for HA it was 12.5% (95% CI 10.5, 14.9), for TSA it was 9.5% (95% CI 8.4, 10.7) and for RTSA it was 7.1% (95% CI 5.9, 8.5). The 10-year CPR rate of HRA, HA, TSA and RTSA was 20.9% (95% CI 16.7, 26.1), 16.5% (95% CI 13.6, 19.8), 15.9% (95% CI 13.9, 18.3) and 9.7% (95% CI 7.4, 12.6), respectively (Figure 2). In the 55–64 years age group, RTSA had a higher rate of early revision compared to HRA (0–6 months), HA (0–2 weeks) and TSA (0–3 months) (Figure 2). After this early period, the rate of revision switched with HRA (6 months–6 years), HA (6 months–2.5 years) and TSA (3 months+) having significantly higher rates of revision compared to RTSA, with no difference at all other time points (Figure 2).
Figure 2.
Cumulative percent revision of primary shoulder replacement in patients aged 55–64 years by type of primary (all diagnoses).
Reason for revision
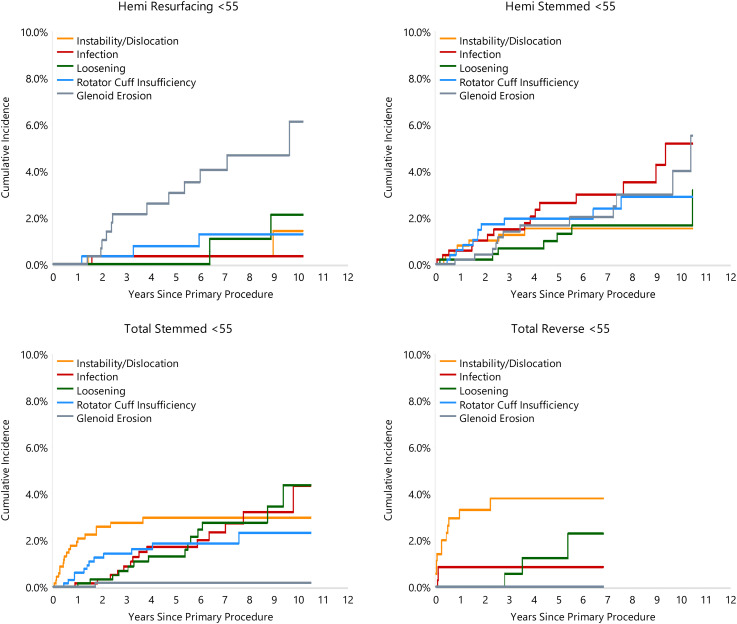
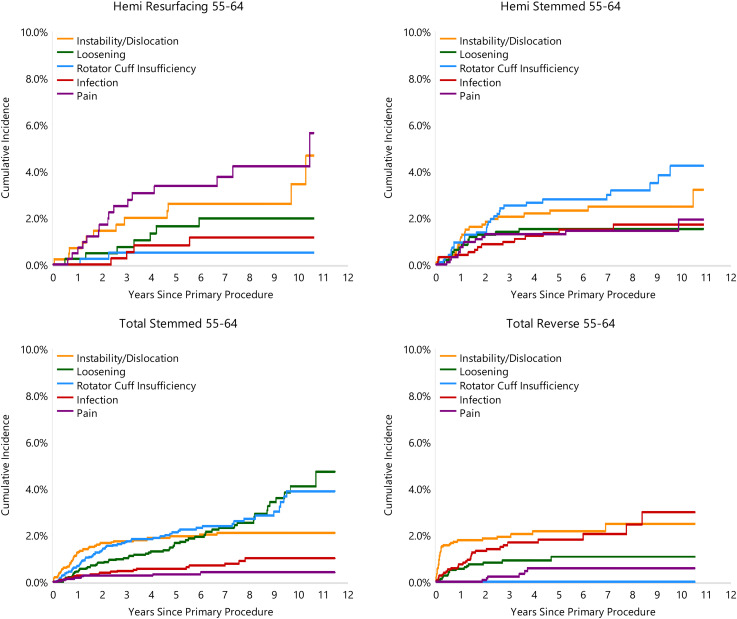
The reason for revision varied depending on the class of shoulder replacement used. For HRA, glenoid erosion and pain were the most common reasons for revision for the <55 years age group (27.3% and 25.0% of revisions, respectively) and the 55–64 years age group (27.8% and 22.2%, respectively). For HA procedures in the <55 years age group, infection was the main reason for revision (21.4%) followed by glenoid erosion (17.1%). In the 55–64 years age group for HA procedures, rotator cuff insufficiency was the main reason for revision (24.4%) (Figures 3 and 4).
Figure 3.
Cumulative incidence revision diagnosis of primary shoulder replacement by type of primary and age (all diagnoses).
Figure 4.
Cumulative incidence revision diagnosis of primary shoulder replacement by type of primary and age (all diagnoses).
For TSA instability/dislocation was the most common reason for revision in the <55 years age group (21.6%) compared to rotator cuff insufficiency in the 55–64 years age group (22.9%) (Figures 3 and 4). Implant loosening was the second most common reason for revision in the <55 year (15.9%) and 55–64 years age group (20.9%) and was the primary cause of late failure in both groups (Figures 3 and 4).
For RTSA, instability/dislocation was the most common reason for revision in both the <55 and 55–64 years age groups (50% and 34.6%, respectively). This was noted to occur frequently in the early post-operative period (Figures 3 and 4). Infection was the second most common reason for revision in both RTSA age groups (12.5 and 26.2% of revisions, respectively).
Discussion
Demographics, arthroplasty class and primary diagnosis
Overall, patients undergoing HRA were younger (53.9 years) compared to HA (55.1 years), TSA (58.4 years) and RTSA (59.3 years).
This may reflect the desire of surgeons to utilise resurfacing in younger patients to attempt to minimalize glenoid bone loss, the low prevalence of periprosthetic fractures and ease of revision to a conventional TSA. 19
Osteoarthritis was the main indication for HRA, TSA and RTSA in young patients. In HA, fracture was the primary indication. This is in contrast to previous smaller studies that indicated reasons other than osteoarthritis to account for a higher percentage of joint replacements in the younger age group.9,16,20 Saltzman in 2010 reported on 172 patients aged <50 years and found 79% had a diagnosis other than primary degenerative joint disease compared to 34% of patients aged >50 years. 9
Revision rate and survivorship in young patients
All classes of shoulder replacement had comparable CPR in patients aged <55 years. HRA had the lowest early revision rate of the group compared to RTSA which had the highest early revision rate (Figure 1). However, RTSA had a lower revision rate compared to HRA and HA but not TSA in patients aged <55 years at mid-term follow-up. CPR data for RTSA was only available out to 6 years so long-term outcome and analysis in this age group remains unknown. RTSA had the lowest CPR rate in patients aged 55–64 years when compared to all other classes of shoulder replacement however, once again showed higher early revision rates (Figure 2). To our knowledge, this is the first large scale registry based study that reports on the midterm outcomes of young arthroplasty that includes data on RTSA. In 2018, Rasmussen reported on the 10 year outcomes of TSA, HRSA and HRA from the Nordic arthroplasty registry but did not include RTSA. They found that TSA had the highest implant survival rate at 10 years for all groups including young patients under 55. 21 A separate study from the same registry evaluated the survival of 1904 RTSA at 10 years follow-up and found a low (.91) cumulative percent revision. Patient age was not associated with risk of revision. 22
Glenoid erosion in resurfacing and stemmed hemiarthroplasty
Resurfacing humeral hemiarthroplasty is often considered in young active patients to treat arthritic conditions of the shoulder where loosening or wear of the glenoid component is a concern.23,24 Our cohort included prosthesis utilising metal, pyrocarbon or ceramic as the bearing surface. Overall, glenoid erosion and pain were the most common reasons for revision in both patient age groups. Glenoid erosion was also shown to be the second most common cause of revision for HA (17.1%) in patients aged <55 years. These findings are consistent with previous studies. A multicentered cohort study of 33 shoulders treated with the Copeland shoulder resurfacing showed that 45% of patients had glenoid erosion on x-ray at 7 year follow-up. 20 Similarly, a multicentered study of 419 patients with mean age of 49 years, treated with HA using a metal head demonstrated that painful glenoid erosions accounted for 9.5% of the 11% of revisions at 10 years. 25 The cause of glenoid erosion in HRA and HA is not fully understood. Baile reviewed the outcomes of 36 patients who underwent cementless HRA for osteoarthritis and despite high satisfaction levels at two years, noted concerns over progressive glenoid erosion given that many of their younger patients chose to return to higher activity levels rather than adhere to recommended activity restriction. 26 Al-Hadithy et al. demonstrated a correlation between an oversized implant and degree of glenoid erosion in a series of 53 patients at 4.2 years follow-up for shoulder resurfacing. 27 Oversizing is thought to increase tension on the rotator cuff leading to the compressive forces that cause glenoid erosion. 20
Infection in shoulder hemiarthroplasty
Infection was the main reason for revision in HA in patients aged <55 years with an overall rate of 2.9% (Figure 3) at mean 5.2 years follow-up. The primary indication for HA was fracture as opposed to osteoarthritis for the other classes of arthroplasty in this age group. Patients that sustain proximal numerus fractures secondary to osteoporosis are more likely to have modifiable risks factors including smoking, excessive alcohol intact and poor nutrition that are known to increase the risk of infection and may contribute to the high rate of revision for infection for this subset of patients.28,29 The rate of infection in this cohort was similar to that reported in previous studies. In a meta-analysis of 810 hemiarthroplasties performed for fracture, the rate of infection was reported at 2.2% at mean follow-up of 3.7 years. 30
Instability in total shoulder arthroplasty
Instability was the main reason for revision for TSA for patients aged <55 years. The reported incidence of instability following anatomic TSA ranges from 1 to 2%.31–33 Anterior instability can be associated with subscapularis insufficiency, axillary nerve injury, component malpositioning or oversizing the humeral head. 31 To reduce the incidence of subscapularis rupture, the recommendations of several studies have advocated lesser tuberosity osteotomy over a subscapularis tenotomy due to lower failure rates of repair, improved shoulder external rotation, higher load to failure in vivo, improved strength with lift off testing and improved functional outcomes.34–36 Other studies reported no difference in strength and outcome between tenotomy, osteotomy and subscapularis peel.37–43
Regardless, the risk of subscapularis failure can be minimised by avoiding oversizing the humeral head, meticulous repair of the subscapularis and avoidance of excessive external rotation in the postoperative period. 31 Posterior shoulder instability is less common than anterior shoulder instability. 33 Excessive humeral or glenoid retroversion along with posterior capsular laxity have been implicated as the main causes. 32
Loosening in total shoulder arthroplasty
Loosening of the glenoid component was the second most common cause of revision for TSA and the most common cause of failure after 9 years in both age groups.
This is consistent with previous studies. A retrospective multicentre study with long term follow-up of patients aged <60 years who underwent stemmed total shoulder arthroplasty demonstrated that of the shoulders that underwent revision surgery, 80% were attributed to glenoid component loosening. 15 In this cohort, survivorship of TSA dropped precipitously after 10 years of follow-up, likely representing glenoid component loosening and failure. 15
Rotator cuff failure in hemiarthroplasty and total shoulder arthroplasty
Rotator cuff failure was the main reason for revision for both hemi and total stemmed shoulder replacement in patients aged 55–64 years. This highlights the importance of patient selection, ensuring rotator cuff integrity prior to proceeding with shoulder HA or TSA and the importance of intraoperative reconstruction of the subscapularis on closure. Assessment of the rotator cuff preoperatively involves both clinical examination and imaging assessment. Integrity of the rotator cuff can be inferred from plain radiography on the grashey view utilizing the Hamada-Fukuda classification. 44 MRI remains the gold standard in assessing for tears within the tendons of the cuff muscles and assessing the integrity of the cuff musculature. 45 The Goutallier grading system on CT scan has been modified by Fuchs to assess fatty infiltration of the cuff and has been shown to have good intra and interobserver reliability.46,47
Despite good patient selection, rotator cuff failure has been shown to increase over time in both this age cohort and older age cohorts as shown by the AOANJRR (7). Furthermore, previous imaging based studies have also shown that rotator cuff tearing and degeneration increases with age even in asymptomatic individuals. 48 Whilst careful patient selection remains paramount to avoid rotator cuff failure in total shoulder arthroplasty, the natural history of degenerative cuff tears with age will likely remain a contributor to revision in total shoulder arthroplasty in the future.
Instability in reverse total shoulder arthroplasty
Shoulder instability was the major reason for revision in patients aged <55 years with rates approaching 4% at three years. It was also a key reason for revision in patients aged 55–64 years. Revision for instability in RTSA can be a simpler procedure than revision of RTSA for other reasons. This may be a factor in the higher rates of revision for instability in this cohort and lower rates of revision for other causes in RTSA. The rates of dislocation for RTSA range widely in the literature from 1.5–31%.49–52 Most studies report that component malposition, inadequate tensioning of the soft tissue envelope and insufficient subscapularis for repair are contributing factors to instability post RTSA.53–56 Chalmers identified additional patient factors including a BMI >30 kg/m2, male gender, and previous surgery increased the risk of dislocation in the first 3 months post procedure. 49
Despite the higher prevalence of instability, RTSA had comparable long-term outcomes in patients aged <55 years, and patients aged 55–64 years had a lower revision rate than the other classes of joint replacements. The reason for the lower revision rates in the longer term for RTSA is not fully understood. It may reflect the design construct of a reverse prosthesis being less dependent on a functioning rotator cuff as patients age or could be confounded by the limited revision options of these prostheses.
This study has a number of limitations. Firstly, all cause revision as the primary outcome in any form of arthroplasty has bias as it does not include data on radiographic or functional outcome measures and does not capture information of the poorly performing prosthesis that is not revised. The data collection method also does not allow for multiple reasons for revision to be recorded in a hierarchical fashion. Secondly, care needs to be taken comparing revision rates as outcomes, as the revision of a HA, HRA and TSA can be an easier option than RTSA. Many surgeons view RTSA as a final surgical procedure and may be more inclined to revise an anatomic TSA to a RTSA. This may lead to bias. Furthermore, the follow-up is mid-term. In particular, despite the maximum follow for reverse total shoulder replacement being 12.4 years, the mean follow-up for this group was only 3 years. Information regarding longer term follow is not currently available for this younger age cohort. The national joint registry does, however, have a 99% capture rate and therefore almost all cases of arthroplasty have been included in this analysis.
Conclusion
This is the first large scale study that has compared the outcomes of the differing classes of shoulder arthroplasty in patients aged <65 years. Our study showed that for patients aged <55 years, all classes of shoulder replacement had similar rates of revision. In patients aged 55 to 64, reverse total shoulder replacement had higher revision rates within the first three months, however overall had the lowest rates of revision. The modes of failure of shoulder arthroplasty differed in young patients. In younger patients, glenoid erosion and pain were more frequent causes for revision for shoulder resurfacing and hemiarthroplasty. Instability accounted for a higher percentage of revision for both total shoulder arthroplasty and reverse total arthroplasty, especially in the short to medium term. Given the limitations of using revision as a primary endpoint for outcome, the results from this study should be considered carefully and with caution in clinical practice. Understanding the reasons for revision of shoulder arthroplasty in young patients will help surgeons with patient selection, prosthetic choice, refine surgical technique and lead to the development of newer materials and improved prosthetic designs.
Acknowledgements
The authors would like to acknowledge the Australian Orthopaedic Association National Joint Replacement Registry for providing accesss to the data used for the publication.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iDs: Andrew Phillip McBride https://orcid.org/0000-0002-8425-6219
References
- 1.Boileau P, Sinnerton RJ, Chuinard Cet al. et al. Arthroplasty of the shoulder. J Bone Joint Surg Br 2006; 88: 562–575. [DOI] [PubMed] [Google Scholar]
- 2.Dillon MT, Page RS, Graves SE, et al. Early revision in anatomic total shoulder arthroplasty in osteoarthritis: a cross-registry comparison. Shoulder Elbow 2020; 12: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Page RS, Navarro RA, Salomonsson B. Establishing an international shoulder arthroplasty consortium. J Shoulder Elbow Surg 2014; 23: 1081–1082. [DOI] [PubMed] [Google Scholar]
- 4.Brolin TJ, Thakar OV, Abboud JA. Outcomes after shoulder replacement surgery in the young patient: how do they do and how long can we expect them to last? Clin Sports Med 2018; 37: 593–607. [DOI] [PubMed] [Google Scholar]
- 5.Schumann K, Flury MP, Schwyzer HKet al. et al. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med 2010; 38: 2097–2105. [DOI] [PubMed] [Google Scholar]
- 6.Henn RF, 3rd, Ghomrawi H, Rutledge JRet al. et al. Preoperative patient expectations of total shoulder arthroplasty. J Bone Joint Surg Am 2011; 93: 2110–2115. [DOI] [PubMed] [Google Scholar]
- 7.Australian Orthopaedic Association National Joint Replacement Registry. 2018 Annual Report: Hip, Knee & Shoulder Arthroplasty, 2018.
- 8.Barlow JD, Abboud J. Surgical options for the young patient with glenohumeral arthritis. Int J Shoulder Surg 2016; 10: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saltzman MD, Mercer DM, Warme WJet al. et al. Comparison of patients undergoing primary shoulder arthroplasty before and after the age of fifty. J Bone Joint Surg Am 2010; 92: 42–47. [DOI] [PubMed] [Google Scholar]
- 10.Gadea F, Alami G, Pape Get al. et al. Shoulder hemiarthroplasty: outcomes and long-term survival analysis according to etiology. Orthop Traumatol Surg Res 2012; 98: 659–665. [DOI] [PubMed] [Google Scholar]
- 11.Robinson CM, Page RS, Hill RMet al. et al. Primary hemiarthroplasty for treatment of proximal humeral fractures. J Bone Joint Surg Am 2003; 85: 1215–1223. [DOI] [PubMed] [Google Scholar]
- 12.Radnay CS, Setter KJ, Chambers Let al. et al. Total shoulder replacement compared with humeral head replacement for the treatment of primary glenohumeral osteoarthritis: a systematic review. J Shoulder Elbow Surg 2007; 16: 396–402. [DOI] [PubMed] [Google Scholar]
- 13.van den Bekerom MPJ, Geervliet PC, Somford MPet al. et al. Total shoulder arthroplasty versus hemiarthroplasty for glenohumeral arthritis: a systematic review of the literature at long-term follow-up. Int J Shoulder Surg 2013; 7: 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartelt R, Sperling JW, Schleck CDet al. et al. Shoulder arthroplasty in patients aged fifty-five years or younger with osteoarthritis. J Shoulder Elbow Surg 2011; 20: 123–130. [DOI] [PubMed] [Google Scholar]
- 15.Neyton L, Kirsch JM, Collotte P, et al. Mid- to long-term follow-up of shoulder arthroplasty for primary glenohumeral osteoarthritis in patients aged 60 or under. J Shoulder Elbow Surg 2019; 28: 1666–1673. [DOI] [PubMed] [Google Scholar]
- 16.Samuelsen BT, Wagner ER, Houdek MT, et al. Primary reverse shoulder arthroplasty in patients aged 65 years or younger. J Shoulder Elbow Surg 2017; 26: e13–e17. [DOI] [PubMed] [Google Scholar]
- 17.Chelli M, Lo Cunsolo L, Gauci MO, et al. Reverse shoulder arthroplasty in patients aged 65 years or younger: a systematic review of the literature. JSES Open Access 2019; 3: 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otto RJ, Clark RE, Frankle MA. Reverse shoulder arthroplasty in patients younger than 55 years: 2- to 12-year follow-up. J Shoulder Elbow Surg 2017; 26: 792–797. [DOI] [PubMed] [Google Scholar]
- 19.Burgess DL, McGrath MS, Bonutti PMet al. et al. Shoulder resurfacing. J Bone Joint Surg Am 2009; 91: 1228–1238. [DOI] [PubMed] [Google Scholar]
- 20.Verstraelen FU, Horta LA, Schotanus MGMet al. et al. Clinical and radiological results 7 years after copeland shoulder resurfacing arthroplasty in patients with primary glenohumeral osteoarthritis: an independent multicentre retrospective study. Eur J Orthop Surg Traumatol 2018; 28: 15–22. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen JV, Hole R, Metlie T, et al. Anatomical total shoulder arthroplasty used for glenohumeral osteoarthritis has higher survival rates than hemiarthroplasty: a nordic registry-based study. Osteoarthritis Cartilage 2018; 26: 659–665. [DOI] [PubMed] [Google Scholar]
- 22.Lehtimäki K, Rasmussen JV, Mokka J, et al. Risk and risk factors for revision after primary reverse shoulder arthroplasty for cuff tear arthropathy and osteoarthritis: a nordic arthroplasty register association study. J Shoulder Elbow Surg 2018; 27: 1596–1601. [DOI] [PubMed] [Google Scholar]
- 23.Wiater JM, Fabing MH. Shoulder arthroplasty: prosthetic options and indications. J Am Acad Orthop Surg 2009; 17: 415–425. [DOI] [PubMed] [Google Scholar]
- 24.Page RS, Pai V, Eng Ket al. et al. Cementless versus cemented glenoid components in conventional total shoulder joint arthroplasty: analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Shoulder Elbow Surg 2018; 27: 1859–1865. [DOI] [PubMed] [Google Scholar]
- 25.Boileau P. Nice multicenter study 2018. Shoulder masters course 5th October 2019. Lisbon: Wright Medical, 2018. [Google Scholar]
- 26.Bailie DS, Llinas PJ, Ellenbecker TS. Cementless humeral resurfacing arthroplasty in active patients less than fifty-five years of age. J Bone Joint Surg Am 2008; 90: 110–117. [DOI] [PubMed] [Google Scholar]
- 27.Al-Hadithy N, Domos P, Sewell MDet al. et al. Cementless surface replacement arthroplasty of the shoulder for osteoarthritis: results of fifty mark III copeland prosthesis from an independent center with four-year mean follow-up. J Shoulder Elbow Surg 2012; 21: 1776–1781. [DOI] [PubMed] [Google Scholar]
- 28.Everhart JS, Bishop JY, Barlow JD. Medical comorbidities and perioperative allogeneic red blood cell transfusion are risk factors for surgical site infection after shoulder arthroplasty. J Shoulder Elbow Surg 2017; 26: 1922–1930. [DOI] [PubMed] [Google Scholar]
- 29.Yedavally-Yellayi S, Ho AM, Patalinghug EM. Update on osteoporosis. Prim Care 2019; 46: 175–190. [DOI] [PubMed] [Google Scholar]
- 30.Kontakis G, Koutras C, Tosounidis Tet al. et al. Early management of proximal humeral fractures with hemiarthroplasty: a systematic review. J Bone Joint Surg Br 2008; 90: 1407–1413. [DOI] [PubMed] [Google Scholar]
- 31.Bohsali KI, Bois AJ, Wirth MA. Complications of shoulder arthroplasty. J Bone Joint Surg Am 2017; 99: 256–269. [DOI] [PubMed] [Google Scholar]
- 32.Bohsali KI, Wirth MA, Rockwood CA, Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am 2006; 88: 2279–2292. [DOI] [PubMed] [Google Scholar]
- 33.Sperling JW, Hawkins RJ, Walch Get al. et al. Complications in total shoulder arthroplasty. J Bone Joint Surg Am 2013; 95: 563–569. [DOI] [PubMed] [Google Scholar]
- 34.Caplan JL, Whitfield B, Neviaser RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg 2009; 18: 193–196; discussion 197–198. [DOI] [PubMed] [Google Scholar]
- 35.Krishnan SG, Stewart DG, Reineck JRet al. et al. Subscapularis repair after shoulder arthroplasty: biomechanical and clinical validation of a novel technique. J Shoulder Elbow Surg 2009; 18: 184–192; discussion 197–198. [DOI] [PubMed] [Google Scholar]
- 36.Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am 2010; 92: 1627–1634. [DOI] [PubMed] [Google Scholar]
- 37.Lapner PL, Sabri E, Rakhra Ket al. et al. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am 2012; 94: 2239–2246. [DOI] [PubMed] [Google Scholar]
- 38.Shi LL, Jiang JJ, Ek ETet al. et al. Failure of the lesser tuberosity osteotomy after total shoulder arthroplasty. J Shoulder Elbow Surg 2015; 24: 203–209. [DOI] [PubMed] [Google Scholar]
- 39.Banas MP, Lewis RA. Nonunion of an olecranon epiphyseal plate stress fracture in an adolescent. Orthopedics 1995; 18: 1111–1112. [DOI] [PubMed] [Google Scholar]
- 40.Fujioka H, Tsunemi K, Takagi Yet al. et al. Treatment of stress fracture of the olecranon in throwing athletes with internal fixation through a small incision. Sports Med Arthrosc Rehabil Ther Technol 2012; 4: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moed BR, Ede DE, Brown TD. Fractures of the olecranon: an in vitro study of elbow joint stresses after tension-band wire fixation versus proximal fracture fragment excision. J Trauma 2002; 53: 1088–1093. [DOI] [PubMed] [Google Scholar]
- 42.Torg JS, Moyer RA. Non-union of a stress fracture through the olecranon epiphyseal plate observed in an adolescent baseball pitcher. A case report. J Bone Joint Surg Am 1977; 59: 264–265. [PubMed] [Google Scholar]
- 43.Wilkerson RD, Johns JC. Nonunion of an olecranon stress fracture in an adolescent gymnast. A case report. Am J Sports Med 1990; 18: 432–434. [DOI] [PubMed] [Google Scholar]
- 44.Hamada K, Fukuda H, Mikasa Met al. et al. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res 1990; 254: 92–96. [PubMed] [Google Scholar]
- 45.Dekker TJ, Steele JR, Vinson EVet al. et al. Current peri-operative imaging concepts surrounding shoulder arthroplasty. Skeletal Radiol 2019; 48: 1485–1497. [DOI] [PubMed] [Google Scholar]
- 46.Fuchs B, Weishaupt D, Zanetti Met al. et al. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg 1999; 8: 599–605. [DOI] [PubMed] [Google Scholar]
- 47.Goutallier D, Postel JM, Bernageau Jet al. et al. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res 1994; 304: 78–83. [PubMed] [Google Scholar]
- 48.Sher JS, Uribe JW, Posada Aet al. et al. Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg Am 1995; 77: 10–15. [DOI] [PubMed] [Google Scholar]
- 49.Chalmers PN, Rahman Z, Romeo AAet al. et al. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2014; 23: 737–744. [DOI] [PubMed] [Google Scholar]
- 50.Cheung E, Willis M, Walker Met al. et al. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg 2011; 19: 439–449. [PubMed] [Google Scholar]
- 51.Cheung EV, Sarkissian EJ, Sox-Harris A, et al. Instability after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2018; 27: 1946–1952. [DOI] [PubMed] [Google Scholar]
- 52.Zumstein MA, Pinedo M, Old Jet al. et al. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2011; 20: 146–157. [DOI] [PubMed] [Google Scholar]
- 53.Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect 2012; 61: 157–168. [PubMed] [Google Scholar]
- 54.Boileau P, Watkinson DJ, Hatzidakis AMet al. et al. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg 2005; 14: 147s–161s. [DOI] [PubMed] [Google Scholar]
- 55.Gallo RA, Gamradt SC, Mattern CJ, et al. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg 2011; 20: 584–590. [DOI] [PubMed] [Google Scholar]
- 56.Stephenson DR, Oh JH, McGarry MHet al. et al. Effect of humeral component version on impingement in reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2011; 20: 652–658. [DOI] [PubMed] [Google Scholar]