Abstract
Given the increasing number of people living with obesity and related chronic metabolic disease, precision nutrition approaches are required to increase the effectiveness of prevention strategies. This review addresses these approaches in different metabolic phenotypes (metabotypes) in obesity. Although obesity is typically associated with an increased cardiometabolic disease risk, some people with obesity are relatively protected against the detrimental effects of excess adiposity on cardiometabolic health, also referred to as ‘metabolically healthy obesity’ (MHO). Underlying mechanisms, the extent to which MHO is a transient state as well as lifestyle strategies to counteract the transition from MHO to metabolically unhealthy obesity (MUO) are discussed. Based on the limited resources that are available for dietary lifestyle interventions, it may be reasonable to prioritize interventions for people with MUO, since targeting high-risk patients for specific nutritional, lifestyle or weight-loss strategies may enhance the cost-effectiveness of these interventions. Additionally, the concept of tissue insulin resistant (IR) metabotypes is discussed, representing distinct etiologies towards type 2 diabetes (T2D) as well as cardiovascular disease (CVD). Recent evidence indicates that these tissue IR metabotypes, already present in individuals with obesity with a normal glucose homeostasis, respond differentially to diet. Modulation of dietary macronutrient composition according to these metabotypes may considerably improve cardiometabolic health benefits. Thus, nutritional or lifestyle intervention may improve cardiometabolic health, even with only minor or no weight loss, which stresses the importance of focusing on a healthy lifestyle and not on weight loss only. Targeting different metabotypes towards T2D and cardiometabolic diseases may lead to more effective lifestyle prevention and treatment strategies. Age and sex-related differences in tissue metabotypes and related microbial composition and functionality (fermentation), as important drivers and/or mediators of dietary intervention response, have to be taken into account. For the implementation of these approaches, more prospective trials are required to provide the knowledge base for precision nutrition in the prevention of chronic metabolic diseases.
Keywords: Metabotype, Tissue-specific insulin resistance, Precision nutrition, Metabolically (un)healthy obesity, Cardiometabolic risk
Introduction
The prevalence of obesity, insulin resistance (IR) and related chronic metabolic diseases such as type 2 diabetes (T2D), cardiovascular diseases (CVD) and mental diseases has grown dramatically over the past decades, with far reaching consequences for individuals, society and economy [1, 2].
A healthy diet may improve cardiometabolic health, even in the presence of minor weight loss [3]. Nevertheless, although preventive strategies such as lifestyle interventions have improved over time, long-term maintenance and adherence to a healthy lifestyle remains poor [4]. It is becoming progressively evident that the concept of a universal dietary solution does not apply when considering lifestyle or dietary strategies for enhancing health, as a substantial subset of individuals does not respond to dietary interventions. It is widely recognized that only about 40% of the study population exhibit a beneficial metabolic response to generic dietary interventions, wherein the responsiveness is closely linked to distinct metabolic phenotypes, so-called metabotypes [5–8]. The usage of machine learning algorithms to improve blood glucose control has been proven successful [9–11]. In a retrospective analysis of the Tubingen Lifestyle Intervention Program, a high-risk metabotype was identified, characterized by beta-cell dysfunction and/or insulin-resistant (IR) nonalcoholic fatty liver disease with higher probability of long-term non-response to lifestyle intervention [12]. In the latter group, intensification of lifestyle intervention showed a higher improvement in glucose tolerance [13]. Additionally, we recently provided, for the first time, the proof-of-concept that isocaloric dietary macronutrient modulation according to tissue IR metabotype may considerably further improve insulin sensitivity and cardiometabolic health [14]. Combined, these data indicate that a precision-based approach to improve cardiometabolic health seems promising and may increase intervention efficacy as well as adherence to intervention.
This review will discuss the different metabotypes in obesity and their relationship with the risk of developing cardiometabolic disease, and highlight underlying (tissue-specific) metabolic disturbances. Next, our focus lies in assessing the efficacy of a precision-based approach that targets specific (tissue) metabotypes to enhance the success of nutritional or lifestyle interventions in individuals who are living with overweight or obesity. Results from recent dietary intervention studies that used a precision nutrition or lifestyle approach targeting specific metabotypes to further improve intervention effectiveness in individuals living with overweight or obesity will be discussed. Finally, future perspectives and approaches will be addressed.
Metabolically healthy versus unhealthy obesity metabotypes
Although obesity is typically related to metabolic dysfunction and an elevated cardiometabolic disease risk, expansion of adipose tissue does not always result in metabolic perturbations. There is a group of individuals with obesity that is relatively protected against the detrimental effects of excess adiposity on cardiometabolic health, also alluded to as ‘metabolically healthy obesity’ (MHO). Several lines of evidence have shown that the absolute amount of body fat is not the main factor determining the metabotype in people with obesity. For example, abdominal liposuction did not improve metabolic health, including IR, in humans [15]. Furthermore, despite an increase in fat mass, pharmacological activation of PPARγ using thiazolidinediones increased insulin sensitivity in humans [16]. Another condition showing that there fat mass is not directly associated with metabolic health is lipodystrophy. Adipose tissue deficiency in patients with (partial) lipodystrophy is accompanied by IR and an increased risk of T2D [17].
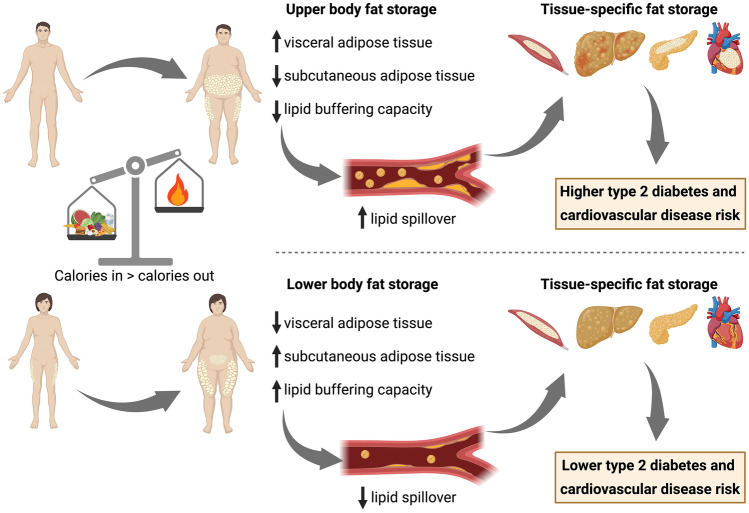
The location where lipids are stored is a stronger risk factor for metabolic and cardiovascular diseases than excess adiposity per se. Abdominal obesity (fat accumulation in the upper body) is positively associated with the development of obesity-related comorbidities and all-cause mortality, while lower body obesity (fat accumulation in the gluteofemoral region) is related to a protective lipid and glucose profile, and a lower prevalence of cardiometabolic diseases after adjustment for total fat mass [18–21] (Fig. 1). Noteworthy, deep abdominal subcutaneous adipose tissue, which refers to the fat situated below Scarpa’s fascia, dividing the superficial and deep layers of abdominal subcutaneous fat, appears to increase disproportionately compared to the superficial fat as obesity progresses. This particular expansion tendency contributes to a higher susceptibility to cardiometabolic complications and chronic diseases in men, regardless of other measures of adiposity [22]. The risk associated with a certain body fat distribution pattern seems to be explained by strikingly distinct functional properties of different fat depots, as extensively reviewed elsewhere [23]. One important factor underlying the differential cardiometabolic disease risk between people with upper versus lower body obesity is that abdominal adipose tissue has a high lipid turnover or, in other words, is able to rapidly take up and store nutrients after meal intake and release fatty acids under fasting or exercise conditions. In contrast, lower body fat stores exhibit a diminished rate of lipid turnover and retain lipids that would otherwise be directed to non-adipose tissues [23]. Thus, lower body fat seems to act as a ‘metabolic sink’ that protects from ectopic fat deposition (i.e. fat deposition in skeletal muscle, the liver and visceral adipose tissue) and, consequently, insulin resistance and cardiometabolic complications [24–26] (Fig. 1). Although visceral fat mass is a key factor in cardiometabolic disease development compared with the amount of fat stored in other adipose tissue depots, there is evidence that a (relatively) low mass of lower body fat depots may independently predict cardiometabolic disease risk, suggesting that a reduced amount of lower body fat is as important as a high visceral fat mass regarding the risk of cardiometabolic disease development, at least in people with a normal weight [27].
Fig. 1.
Body fat distribution is a key determinant of cardiometabolic disease risk. Subcutaneous adipose tissue buffers the daily influx of excess calories during a prolonged positive energy balance. Upper body fat storage in people with abdominal obesity (that is, predominant lipid storage in the abdominal region) is usually paralleled by more lipid spillover in the circulation, an increase in visceral adipose tissue and ectopic fat storage (that is, lipid accumulation in non-adipose such as skeletal muscle, liver, pancreas and heart), which instigates the development of insulin resistance and chronic cardiometabolic diseases such as type 2 diabetes and cardiovascular diseases. In contrast, predominant fat storage in the lower body as seen in people with lower body obesity limits lipid spillover in the circulation, since gluteo-femoral adipose tissue acts as a metabolic sink to buffer excess lipids. Consequently, less lipids will accumulate in visceral adipose tissue and certain non-adipose tissues, thereby reducing cardiometabolic disease risk. Premenopausal women living with obesity seem to be characterized by more lipid accumulation in skeletal muscle (smaller lipid droplets consisting of less saturated fatty acids) and similar or less lipid storage in the liver, with less detrimental effects on tissue-specific insulin sensitivity compared to men with obesity. Created with BioRender.com
In accordance with the importance of body fat distribution in cardiometabolic disease development, many studies have demonstrated that excess fat mass does not explain the distinct cardiometabolic profile between individuals with MHO and ‘metabolically healthy obesity’ (MUO). Rather, differences in the location where the excessive calories are stored seems to distinguish these obesity phenotypes. Indeed, several studies have shown that individuals with MHO have more subcutaneous adipose tissue (more gluteofemoral fat in women), less visceral fat mass, less fat accumulation in the liver and skeletal muscle, and less macrophage infiltration and inflammation in (visceral) adipose tissue than people with MUO, matched for age, sex, BMI and fat mass [27–39]. Of note, it seems that beside dietary intake the physical activity level and cardiorespiratory fitness are also important determinants of the metabotype in individuals with obesity [39, 40].
Metabolically healthy obesity: a transient state?
To date, there is no universally accepted definition of MHO. Most studies that compared characteristics of people with MHO and MUO phenotypes and/or examined the association of MHO with the risk of developing chronic diseases or mortality defined MHO as having ≤ 2 components of the metabolic syndrome, although the homeostasis model assessment of insulin resistance (HOMA-IR) was used to define MHO in other studies [41]. This means that many study participants were likely ‘misclassified’ as having MHO, while these individuals might just had fewer cardiometabolic perturbations than those classified as having MUO. The latter is also important when interpreting the results of studies that reported the prevalences of MHO in different populations.
In addition to the criteria used to define MHO, the prevalences of MHO and MUO depend on several characteristics of the study populations such as BMI, age, sex, ethnicity and the absence/presence of other chronic diseases. These factors likely explain the large variability in reported MHO prevalence, ranging from ~5% (MHO defined as no metabolic syndrome component and normal HOMA-IR) to ~50% (MHO defined as no metabolic syndrome) [41]. It has been shown that MHO is more common in individuals with a BMI below 35 kg/m2 [42], younger adults [43–47], women [43–45], and people of European ancestry compared to those from South Asia, South America and Africa [45, 48].
The risk of developing cardiovascular diseases among individuals in various BMI categories is likely influenced by their metabolic health. An key question is whether and to what extent metabolic health in people with MHO deteriorates over time and how this impacts cardiometabolic disease risk. Several prospective cohort studies and meta-analyses have demonstrated that the majority of people with MHO has an increased risk of T2D and cardiovascular disease compared to healthy people with normal body weight [49–51]. A meta-analysis demonstrated that MHO was related to a higher risk of events when only studies with 10 or more years of follow-up were considered [51]. Longitudinal studies indicate that 30–50% of MHO individuals switch to the MUO phenotype after 4–20 years of follow-up [33, 52–57]. Indeed, it was shown in the North West Adelaide Health Study that MHO is a transient phenotype in about one-third of these individuals, and that those who maintained a MHO phenotype during 5.5–10.3 years of follow-up (i.e. younger individuals with less abdominal adiposity) had a similar T2D and cardiovascular disease risk compared to metabolically healthy, normal weight participants [33]. In the Nurses’ Health Study (median follow-up of 24 years), women with MHO had a higher CVD risk than women with metabolically healthy normal weight, but the risk was considerably higher in women who converted to MUO [50]. In agreement with these observations, it was shown in a community-based population in China, in which ~45% of individuals with MHO developed MUO (follow-up period of 4.4 years), that individuals who experienced transient MHO, but not those with persistent MHO, demonstrated an elevated risk of subclinical atherosclerosis [58]. Population-based studies in Korea (Korean NHIS datasets (2002–2017, mean follow-up of 3.7 years)) and Norway (Nord-Trøndelag Health Study, mean follow-up of ~12 years) indicated that people with persistent MHO during follow-up were, however, not protected against heart failure [59, 60]. Taken together, although MHO seems a transient state in the majority of people, those with persistent MHO have a lower risk of chronic cardiometabolic diseases. One must take into account that the decline in cardiometabolic health associated with aging, the adverse metabolic consequences of prolonged excess adiposity, and the tendency to accumulate fat mass with age will influence the (lack of) stability of the MHO phenotype [41]. The development of IR and elevation of fasting glucose concentration seem major factors related to the transition from MHO to MUO [61]. In line, people with higher BMI, older age, more severe metabolic perturbations, a poor lifestyle index, and body weight gain seem to have a greater risk of transitioning from MHO to MUO [53, 62–67].
Lifestyle interventions to improve cardiometabolic health and counteract the transition from metabolically healthy to unhealthy obesity
A healthy lifestyle can reduce the risk of cardiometabolic complications and many chronic diseases significantly. Several large population studies have investigated habitual dietary intake in people with MHO and MUO. Most of these studies found that total dietary energy intake and macronutrient composition was similar between individuals with MHO and MUO [35, 68, 69]. In accordance with these observations, no difference in diet quality, appraised as the consumption of Mediterranean-style and DASH-style diets, was found between individuals with MHO and MUO in the US National Health and Nutrition Examination Survey [70]. An intriguing observation was made, indicating that women aged 19–44 years with MHO exhibited a higher total Healthy Eating Index 2005 (HEI-2005) score, which indicates better dietary quality based on the 2005 US National Dietary Guidelines, compared to women with MUO. However, for men aged 19–44 years or adults aged 45–85 years, no significant differences were found in the HEI-2005 total scores between MHO and MUO individuals [71]. Although calorie intake and dietary macronutrient composition did not differ between MHO and MUO individuals in several studies, differences between these metabolic phenotypes were found in number of daily servings of fruit and vegetables, dairy, meats, fats and high fat/sugar food and drinks in some [35, 68, 71–73] but not all [74] studies, with MHO individuals consuming less sugar, sugar-sweetened beverages, and saturated fat and more whole fruits, whole grains, and protein from vegetable sources. Importantly, these results should be interpreted with caution, because of the limitations in the assessment of dietary intake [75, 76] as well as in the definition of MHO in the current studies.
The first treatment recommended for body weight and cardiometabolic health management is lifestyle intervention [77]. To what extent are lifestyle interventions able to prevent the transition from MHO to MUO, or reverse metabolic perturbations in people with MUO? In the Tübingen Lifestyle Intervention Program, achieving ~9 kg median weight loss over 9 months through intensive lifestyle intervention consisting of diet changes and increased physical activity, was associated with improved metabolic health (i.e. conversion from MUO to a healthier metabotype [78]. Interestingly, BMI and liver fat content were independent predictors of the metabolic improvements in the latter study [78].
Would it also be possible to improve cardiometabolic health in MUO with nutritional or lifestyle interventions in the absence of marked weight loss? A very recent study investigated whether adherence to healthy diets was related to the incidence of metabolically unhealthy phenotypes in adults in various BMI categories. It was shown that high compliance with the Dietary Approaches to Stop Hypertension (DASH), Mediterranean (MeDi), and Mediterranean-DASH intervention for neurodegenerative delay (MIND) diets was related to a reduced risk of metabolically unhealthy normal weight. Furthermore, adherence to these dietary patterns was negatively associated with the incidence of MUO in those with MHO at baseline [79]. Furthermore the large Prevención con Dieta Mediterránea (PREDIMED) trial demonstrated that the Mediterranean diet lowered the risk of cardiovascular events by about 30% in comparison to the control diet, without large effects on body weight [80]. Interestingly, findings from the PREDIMED study also demonstrated that even without substantial weight loss, adherence to a Mediterranean diet can promote the transition from MUO to a healthy metabotype, and protects against worsening of metabolic health in MHO [81]. Comparable findings were seen in several other dietary intervention studies [82].
Differences in body composition, tissue-specific metabolism and insulin sensitivity between men and women not only underlies sexual dimorphism in cardiometabolic disease risk, but may also affect the response to prevention and treatment strategies in a sex-specific manner [22]. Indeed, dietary interventions have a differential effect on body weight, weight maintenance and cardiometabolic risk factors in men compared to women, as extensively reviewed elsewhere [23]. The recent PREVIEW study investigated age- and sex-specific effects of a low-energy diet (LED), followed by a 3 year lifestyle-based weight maintenance intervention in overweight adults with prediabetes [83]. A lifestyle intervention had less beneficial effects with respect to body composition and cardiometabolic health markers in older than younger adults, despite better weight maintenance, which might be due to the loss of more fat-free mass in older adults. The LED, followed by a lifestyle intervention, showed less beneficial effects on body weight and body composition in women than men [83]. The available evidence suggests that factors such as age and sex should be taken into account to optimize prevention and treatment strategies for those at risk of or already living with cardiometabolic diseases.
Tissue insulin resistance metabotype: the concept
Insulin plays a crucial role in regulating nutrient partitioning within the body. Insulin resistance (IR) encompasses impaired insulin action in various tissues, including muscle, liver, adipose tissue, gut, and brain. This dysfunction may occur before the onset of cardiometabolic diseases.
Importantly, IR may develop in different organs but the severity may vary between organs. Indeed, in individuals with prediabetes, the state of impaired glucose tolerance (IGT) or the state of impaired fasting glucose (IFG) and are characterized, among other factors by more pronounced peripheral (skeletal muscle) or more pronounced liver IR [26]. Consistent with this, our previous research demonstrated that individuals with impaired fasting glucose (IFG) did not exhibit disruptions in skeletal muscle lipid turnover, whilst in individuals with impaired glucose tolerance (IGT), there were disturbances in skeletal muscle lipid handling. These disturbances were accompanied by impaired postprandial insulin sensitivity, an increase in postprandial triglyceride (TAG) extraction, and a reduction in muscle lipid turnover [84].
Individuals with more pronounced liver insulin resistance (LIR) and individuals with more pronounced muscle insulin resistance (MIR) can already be distinguished in the overweight or obese state [85]. Using a 5 or 7-points oral glucose tolerance test, wherein insulin and glucose concentrations were measured, we calculated hepatic insulin resistance index (HIRI) and muscle insulin sensitivity index (MISI). These indices were validated against a hyperinsulinemic clamp, a well-established method for assessing insulin sensitivity [86]. Additionally, we optimized the MISI calculation by means of cubic splining [87]. Individuals with LIR have a distinct metabolome [88] and lipidome profile [89] as compared to individuals with more pronounced MIR. Furthermore, an enriched inflammatory gene expression profile was particularly present in abdominal subcutaneous adipose tissue of individuals with primarily MIR [90]. Additionally, an altered extracellular matrix remodelling gene expression profile was present in individuals with pronounced LIR [90]. In line with the former findings, in two population-based cohorts, the Cohort on Diabetes and Atherosclerosis Maastricht and The Maastricht Study, we observed that an elevated systemic low-grade inflammation profile, as indicated by the combined score of plasma markers associated with low-grade inflammation, showed a specific association with MIR but not with LIR [90]. The connection between adipose tissue inflammation and IR has been previously shown, but our data further expand on these findings by demonstrating the tissue-specific nature of this relationship. Nevertheless, only one-third of the population with overweight harbor the MIR/LIR phenotype, and it is evident that more tissue metabotypes can be identified (hypothesized around six assuming an even distribution). Recent findings show that adipose tissue IR and whole-body IR, as reflected by Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), not always coincide [91]. Adults that are discordant for adipose tissue insulin resistance and HOMA-IR had unique features related to visceral fat, plasma triglycerides and basal metabolic rate [91].
Furthermore, tissue metabotypes may also vary based on tissue fat accumulation and may vary between sexes. Body composition profiles, as assessed by whole body magnetic resonance imaging (MRI), may further our understanding of the complex interplay between muscle and liver metabolism and ectopic fat and adipose tissue fat accumulation. Indeed, distinct etiologies towards cardiometabolic health outcomes have been shown for discordant visceral and liver fat phenotypes [92] as well as discordant liver and muscle fat/mass phenotypes [93]. Finally, sex-specific differences in the accumulation of surplus lipids, mobilization of stored lipids, as well as substrate supply and utilization in critical metabolic organs (such as skeletal muscle, adipose tissue, and the liver) are linked to variations in tissue-specific insulin sensitivity and cardiometabolic risk profiles between men and women (Fig. 1). Premenopausal women have, in general, an increased liver and muscle insulin sensitivity as compared to males [23] (Fig. 1). We previously showed that in women, but not in men, LIR was positively associated with the sum of plasma diacylglycerols and triacylglycerols (TAG) [89]. The latter results remained consistent even after adjusting for body composition and body fat distribution, suggesting that factors beyond body composition play a significant role in these sex-specific differences. Furthermore, these women had lower plasma TAG and higher HDL concentrations and a reduced LIR as compared to men. In general, healthy premenopausal women appear to possess a greater capacity for fat storage without incurring detrimental cardiometabolic health risks, a phenomenon often referred to as the female advantage [23]. Our findings revealing a deterioration in blood lipid profile among women as LIR progresses are particularly noteworthy. These findings imply that women with LIR eventually "catch-up" with men in terms of CVD risk highlighting a sex-specific relationship between (L)IR and cardiometabolic risk. This is consistent with findings of Kim and Reaven [94], who showed that the female advantage is not solely explained by differences in insulin action itself. Instead, they found that the female advantage arises from an attenuation of the association between IR and CVD risk, particularly evident in younger individuals (aged below 51 years). The mechanisms behind these intruiging sex-specific metabolic differences remain to be determined. In summary, distinct tissue metabotypes can be identified in individuals with overweight and obesity which may represent different etiologies towards cardiometabolic diseases.
Precision nutrition based on tissue-IR metabotype
Post-hoc analyses in large intervention studies show responders and non-responders that feed back to tissue metabotype [5–8, 83]. Parameters associated with glucose metabolism and IR, including plasma glucose and insulin concentrations and derived indices can serve as valuable predictors of the outcome of a dietary intervention [5, 6, 11]. Post-hoc evidence from an analysis of the CORDIO-PREV-DIAB study shows an interaction between dietary macronutrient composition and tissue metabotype [8]. In the latter study, researchers compared the effects of a Mediterranean diet, rich in olive oil, to a low-fat high complex carbohydrate diet in relation to outcomes of glucose metabolism. After the study, a post hoc analysis was performed, dividing participants based on their baseline tissue-IR phenotype. The results indicated that individuals with LIR may derive greater benefits from the low-fat high complex carbohydrate diet, displaying a more pronounced increase in disposition index (a composite marker considering insulin secretion adjusted for insulin sensitivity) as compared to the MIR phenotype. On the other hand, individuals with the MIR phenotype appeared to benefit more from the Mediterranean diet, showing a higher increase in the disposition index compared to those with the LIR phenotype.
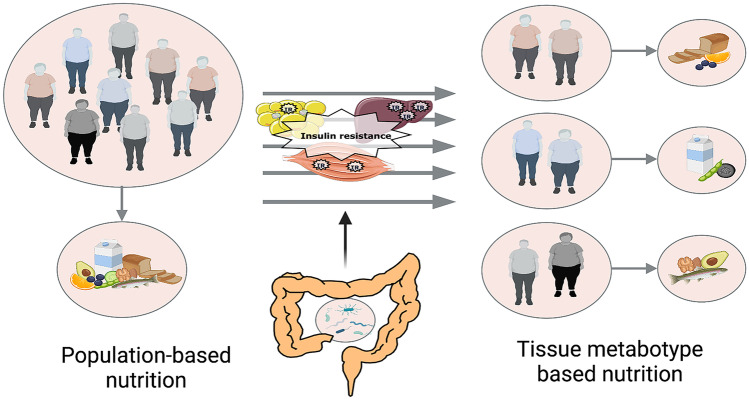
We recently provided the proof-of-concept that a precision nutrition strategy according to an individual’s tissue metabotype, within the context of healthy dietary guidelines, results in a clinically relevant further improvement in insulin sensitivity and cardiometabolic health (C-reactive protein and plasma triacylglycerol concentrations) in individuals with overweight or obesity, independent of body weight change [14]. Individuals with the LIR phenotype responded better to a diet high in mono-unsaturated fatty acids, whilst individuals with the MIR phenotype responded better to a diet low in fat and high in protein and fiber. Our data show the potential of precision nutrition based on tissue metabotypes. The latter precision nutrition concept is depicted in Fig. 2. Thus, while a diet based on existing dietary guidelines may promote general health for many individuals, it is becoming increasingly evident that precision or subgroup-based dietary approaches might be necessary for optimal dietary prevention or treatment outcomes.
Fig. 2.
The depicted precision nutrition concept is based on the definition of tissue metabotypes related to parameters of tissue-specific metabolism like MIR, LIR and adipose IR, tissue fat accumulation and microbial composition; this strategy will improve insulin sensitivity, blood glucose homeostasis and cardiometabolic risk compared to current, one-size-fits-all dietary guidelines in the population with overweight. Created with BioRender.com
Precision nutrition based on microbial phenotypes
During recent years, research has shown that a disbalance in gut microbiota communities and functionality is implicated in a large number of chronic metabolic diseases including obesity, IR, T2D and cardiometabolic risk [95]. Early studies reported an increased Firmicutes-to-Bacteroidetes ratio in both rodents and humans with obesity in comparison to lean individuals [96, 97]. However, subsequent studies have presented conflicting findings, with some reporting no significant difference in the Firmicutes-to-Bacteroidetes ratio between lean individuals and individuals with obesity, and others even showing a decreased ratio [98, 99]. Consistent findings from various studies have revealed that overweight, moderate obesity, IR, and T2D are associated with decreased microbial richness and diversity compared to lean, healthy individuals [100]. In-depth characterization of people with overweight or obesity has demonstrated that microbial gene richness is negatively associated with various metabolic parameters, including fat mass, leptin levels, fasting insulin, HOMA-IR, systemic inflammation, and TAG levels [100]. Moreover, low microbial gene richness is prevalent in severe obesity, with approximately 75% of individuals affected, while the numbers range from 23 to 40% in lean individuals or those with overweight or moderate obesity [100, 101]. Dietary interventions have been shown to improve clinical phenotypes in individuals with low microbial gene richness. However, these interventions appear less effective in improving inflammatory markers in this subgroup. As a result, low gene richness may serve as a predictive factor for the efficacy of interventions [101].
Our gut microbiota produces a large variety of health-modulating products, including short-chain fatty acids (SCFA) and branched-chain fatty acids (BCFA), by fermenting indigestible food components. It is increasingly clear that these products are essential for host health [95, 102, 103]. The major SCFA are acetate, propionate and butyrate. SCFA are produced by saccharolytic fermentation mainly in the proximal and transverse colon, with beneficial effects on metabolic health, whereas BCFA produced by proteolytic fermentation in the distal colon have general adverse effects on host health (as reviewed in 105 and 106). Interestingly, the gut microbiome of IR individuals has been shown to have an increased biosynthesis potential and decreased uptake and catabolism of branched chain amino acids (BCAAs, largely driven by Prevotella copri and B. vulgatus), which have been linked to adverse metabolic effects [104]. Additionally, metabolically compromised individuals as well as patients with T2D have an altered microbial functionality and a decreased fermentation capacity when compared with healthy individuals, characterised in particular by a lower abundance of butyrate producing bacteria [105–107]. Previous research conducted by our group revealed that acutely administering acetate directly to the distal colon led to increased levels of circulating acetate in males with overweight. This intervention resulted in elevated concentrations of the satiety-stimulating hormone peptide YY and reduced levels of the cytokine TNF-α. Notably, the acetate administration also resulted in a significant increase (25%) in fasting fat oxidation. Contrary, when acetate was administered in the proximal colon, no significant effects on the metabolic profile were observed. This suggests that the specific location of acetate administration in the colon plays a crucial role in its metabolic effects [108]. Thus, increasing the formation of SCFA in the distal colon by enhancing dietary fiber availability could be a critical factor in improving metabolic health. In particular, since a higher distal carbohydrate fermentation may help reduce detrimental proteolytic fermentation. This concept of ‘microbial substrate switch’ emphasizes the importance of dietary strategies that promote the growth and activity of beneficial gut microbes in the distal colon and might provide a novel dietary strategy for preventing and/or treating metabolic diseases (as reviewed in 105).
Interestingly, the tissue IR metabotypes, MIR and LIR are characterized by a differential microbial composition with the LIR metabotype having a higher abundance of SCFA producing genera [109]. In line, modification of microbial composition by either fecal transplant [105, 110] or by dietary maize fiber intervention [111] has been shown to affect peripheral insulin sensitivity more than LIR. Additionally, it was recently shown that microbial composition of individuals with IFG, characterized by LIR, resembles more the normal glucose tolerant state, whilst individuals with IGT, characterized by MIR, show a microbial dysbiosis resembling the T2D state with a reduced abundance of butyrate producing bacteria [107]. Combined, variation in microbial composition and functionality (fermentation) may affect (dys)metabolism in a tissue-specific manner.
Inter-individual differences in gut microbiota composition and functionality may be linked to an altered responsiveness to (dietary) interventions. Initial microbial phenotype has been shown to predict intervention outcome after dietary fiber interventions [112], after feces transplantation [110], bariatric surgery [113] or ingestion of non-caloric sweeteners [114]. Microbial responses to fiber specific interventions have also revealed responders and non-responder phenotypes related to the magnitude of production of the fiber derived SCFA [115]. We recently showed that persons living with overweight or obesity and prediabetes show changes lower postprandial insulin sensitivity in response to short-term administration of the prebiotic fiber long-chain inulin (combined with resistant starch) compared to lean individuals [116], along with reduced plasma concentrations of the SCFA butyrate. These effects were fiber-specific i.e. were not seen when administering beta-glucan and resistant starch, indicating complex structure–function relationships of dietary fibers [116]. These data suggest that the degree of saccharolytic fermentation and related SCFA production may be an important determinant of intervention outcome. Additionally, our data show a lack of response in individuals with prediabetes, which is consistent with the observation that a 4-week oral administration of butyrate altered metabolism and insulin sensitivity in lean individuals but not in individuals with obesity and IR [117]. Combined, the initial microbial composition and related SCFA production may be important determinants of dietary intervention outcome.
Future perspectives
By investigating the relationship between metabotypes and intervention outcomes, we can identify which dietary approaches are most suitable for specific individuals or subgroups at risk for chronic cardiometabolic diseases. A better understanding of the biological, psychological and socio-economic factors that may underlie the MHO and MUO phenotypes will generate important knowledge on obesity-related cardiometabolic chronic diseases that may aid in the development of more personalized interventions. Based on the limited resources that are available for lifestyle interventions, it may be reasonable to prioritize interventions to people with MUO to improve the cost-effectiveness of interventions. A study investigating the effect of phentermine/topiramate-induced weight loss on the prevention of T2D in subjects who were stratified by the Cardiometabolic Disease Staging score (very similar to the MHO/MUO concept) in those with a high or a low cardiometabolic risk provided support for this assumption [118], demonstrating that numbers needed to prevent one case of T2D over about 1 year were 120 in the low-risk group but 24 in the high-risk group [118]. Additionally, research has shown that nutritional or lifestyle interventions can significantly improve cardiometabolic health, even in the absence of substantial weight loss. This finding indicates that the focus should be on promoting a healthy lifestyle rather than focusing on weight loss as the primary goal. In this respect, a diet based on existing guidelines for healthy nutrition, which emphasizes whole foods, a variety of nutrients, and moderation in portion sizes, can indeed serve as a good foundation for promoting health in the general population. Nevertheless, these guidelines may not represent the optimal diet for all. As evidenced by the recent proof of concept in the PERSON study [14], dietary macronutrient modulation according to tissue IR metabotype within the context of healthy dietary guidelines may further improve cardiometabolic health. Thus, precision nutrition based on tissue metabotype may be more effective in cardiometabolic disease prevention as compared to general dietary guidelines. These data demonstrate that the different metabotypes towards T2D and cardiometabolic diseases have to be considered and may lead to more effective nutritional or lifestyle prevention and treatment strategies. In this consideration, age and sex-related differences in tissue metabotypes and related microbial composition and functionality, as important drivers or mediators of dietary intervention response, have to be taken into account. Overall, investing in more prospective trials focused on precision nutrition will contribute significantly to advancing the field and optimizing dietary prevention for individuals at risk for chronic cardiometabolic diseases.
Abbreviation
- BMI
Body Mass Index
- BCFA
Branched-Chain Fatty Acids
- CVD
CardioVascular Diseases
- DASH
Dietary Approaches to Stop Hypertension
- HIRI
Hepatic Insulin Resistance Index
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- IFG
Impaired Fasting Glucose
- IGT
Impaired Glucose Tolerance
- IR
Insulin Resistance
- LIR
Liver Insulin Resistance
- LED
Low-Energy Diet
- MeDi
Mediterranean
- MRI
Magnetic Resonance Imaging
- MIND
Mediterranean-DASH intervention for Neurodegenerative Delay
- MHO
Metabolically Healthy Obesity
- MUO
Metabolically Unhealthy Obesity
- metabotypes
Metabolic phenotypes
- MIR
Muscle Insulin Resistance
- MISI
Muscle Insulin Sensitivity Index
- PREDIMED
Prevención con Dieta Mediterránea
- SCFA
Short-Chain Fatty Acids
- TAG
Triacylglycerols
- T2D
Type 2 Diabetes
Author contribution
Both authors contributed to the conception of the work, literature analysis, data interpretation, writing, and editing of the article. Both authors approved the final version of the manuscript for publication.
Funding
There were no funding sources for the current work.
Data availability
N/A.
Code availability
N/A.
Declarations
Ethics approval
N/A.
Consent to participate
N/A.
Consent to publication
N/A.
Conflict of interest
The authors have nothing to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO European regional obesity report. 2022. https://apps.who.int/iris/bitstream/handle/10665/353747/9789289057738-eng.pdf.
- 2.OECD. The Heavy Burden of Obesity: The Economics of Prevention. OECD Health Policy Studies, OECD Publishing, Paris. 2019. 10.1787/67450d67-en.
- 3.Salas-Salvado J, Martinez-Gonzalez MA, Bullo M, Ros E. The role of diet in the prevention of type 2 diabetes. Nutr Metab Cardiovasc Dis. 2011;2011(21):B32–B48. doi: 10.1016/j.numecd.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 4.van Baak MA, Mariman ECM. Mechanisms of weight regain after weight loss - the role of adipose tissue. Nat Rev Endocrinol. 2019;15(5):274–287. doi: 10.1038/s41574-018-0148-4. [DOI] [PubMed] [Google Scholar]
- 5.Hjorth MF, Ritz C, Blaak EE, Saris WH, Langin D, Poulsen SK, Larsen TM, Sørensen TI, Zohar Y, Astrup A. Pretreatment fasting plasma glucose and insulin modify dietary weight loss success: Results from 3 randomized clinical trials. Am J Clin Nutr. 2017;106(2):499–505. doi: 10.3945/ajcn.117.155200. [DOI] [PubMed] [Google Scholar]
- 6.Yubero-Serrano EM, Delgado-Lista J, Tierney AC, Perez-Martinez P, Garcia-Rios A, Alcala-Diaz JF, Castaño JP, Tinahones FJ, Drevon CA, Defoort C, Blaak EE, Dembinska-Kieć A, Risérus U, Lovegrove JA, Perez-Jimenez F, Roche HM, Lopez-Miranda J. Insulin resistance determines a differential response to changes in dietary fat modification on metabolic syndrome risk factors: The LIPGENE study. Am J Clin Nutr. 2015;102(6):1509–1517. doi: 10.3945/ajcn.115.111286. [DOI] [PubMed] [Google Scholar]
- 7.Stefan N, Staiger H, Wagner R, Machann J, Schick F, Häring HU, Fritsche A. A high-risk phenotype associates with reduced improvement in glycaemia during a lifestyle intervention in prediabetes. Diabetologia. 2015;58(12):2877–2884. doi: 10.1007/s00125-015-3760-z. [DOI] [PubMed] [Google Scholar]
- 8.Blanco-Rojo R, Alcala-Diaz JF, Wopereis S, Perez-Martinez P, Quintana-Navarro GM, Marin C, Ordovas JM, van Ommen B, Perez-Jimenez F, Delgado-Lista J, Lopez-Miranda J. The insulin resistance phenotype (muscle or liver) interacts with the type of diet to determine changes in disposition index after 2 years of intervention: The CORDIOPREV-DIAB randomised clinical trial. Diabetologia. 2016;59(1):67–76. doi: 10.1007/s00125-015-3776-4. [DOI] [PubMed] [Google Scholar]
- 9.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, Suez J, Mahdi JA, Matot E, Malka G, Kosower N, Rein M, Zilberman-Schapira G, Dohnalová L, Pevsner-Fischer M, Bikovsky R, Halpern Z, Elinav E, Segal E. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079–1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Berry SE, Valdes AM, Drew DA, Asnicar F, Mazidi M, Wolf J, Capdevila J, Hadjigeorgiou G, Davies R, Al Khatib H, Bonnett C, Ganesh S, Bakker E, Hart D, Mangino M, Merino J, Linenberg I, Wyatt P, Ordovas JM, Gardner CD, Delahanty LM, Chan AT, Segata N, Franks PW, Spector TD. Human postprandial responses to food and potential for precision nutrition. Nat Med. 2020 Jun;26(6):964–73. 10.1038/s41591-020-0934-0. Epub 2020 Jun 11. Erratum in: Nat Med. 2020 Nov;26(11):1802. PMID: 32528151; PMCID: PMC8265154. [DOI] [PMC free article] [PubMed]
- 11.Rein M, Ben-Yacov O, Godneva A, Shilo S, Zmora N, Kolobkov D, Cohen-Dolev N, Wolf BC, Kosower N, Lotan-Pompan M, Weinberger A, Halpern Z, Zelber-Sagi S, Elinav E, Segal E. Effects of personalized diets by prediction of glycemic responses on glycemic control and metabolic health in newly diagnosed T2DM: a randomized dietary intervention pilot trial. BMC Med. 2022;20(1):56. doi: 10.1186/s12916-022-02254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmid V, Wagner R, Sailer C, et al. Non-alcoholic fatty liver disease and impaired proinsulin conversion as newly identified predictors of the long-term non-response to a lifestyle intervention for diabetes prevention: results from the TULIP study. Diabetologia 2017;60:2341–51. Diabetes 2021;70:2785–95. 10.2337/db21-0526. [DOI] [PubMed]
- 13.Fritsche A, Wagner R, Heni M, Kantartzis K, Machann J, Schick F, Lehmann R, Peter A, Dannecker C, Fritsche L, Valenta V, Schick R, Nawroth PP, Kopf S, Pfeiffer AFH, Kabisch S, Dambeck U, Stumvoll M, Blüher M, Birkenfeld AL, Schwarz P, Hauner H, Clavel J, Seißler J, Lechner A, Müssig K, Weber K, Laxy M, Bornstein S, Schürmann A, Roden M, de Angelis MH, Stefan N, Häring HU. Different effects of lifestyle intervention in high- and low-risk prediabetes: Results of the randomized controlled Prediabetes Lifestyle Intervention Study (PLIS) Diabetes. 2021;70(12):2785–2795. doi: 10.2337/db21-0526. [DOI] [PubMed] [Google Scholar]
- 14.Trouwborst I, Gijbels A, Jardon KM, Siebelink E, Hul GB, Wanders L, Erdos B, Péter S, Singh-Povel CM, de Vogel-van den Bosch J, Adriaens ME, Arts ICW, Thijssen DHJ, Feskens EJM, Goossens GH, Afman LA, Blaak EE. Cardiometabolic health improvements upon dietary intervention are driven by tissue-specific insulin resistance phenotype: A precision nutrition trial. Cell Metab. 2023;35(1):71–83.e5. 10.1016/j.cmet.2022.12.002. PMID: 36599304. [DOI] [PubMed]
- 15.Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350(25):2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 16.Fonseca V. Effect of thiazolidinediones on body weight in patients with diabetes mellitus. Am J Med. 2003;8(115 Suppl 8A):42S–48S. doi: 10.1016/j.amjmed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Ganda OP. Lipoatrophy, lipodystrophy, and insulin resistance. Ann Intern Med. 2000;133(4):304–306. doi: 10.7326/0003-4819-133-4-200008150-00017. [DOI] [PubMed] [Google Scholar]
- 18.Snijder MB, Zimmet PZ, Visser M, Dekker JM, Seidell JC, Shaw JE. Independent and opposite associations of waist and hip circumferences with diabetes, hypertension and dyslipidemia: The AusDiab Study. Int J Obes Relat Metab Disord. 2004;28(3):402–409. doi: 10.1038/sj.ijo.0802567. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P Jr, Razak F, Sharma AM, Anand SS, INTERHEART Study Investigators. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: A case-control study. Lancet. 2005;366(9497):1640–9. 10.1016/S0140-6736(05)67663-5. PMID: 16271645. [DOI] [PubMed]
- 20.Loos RJ. The genetics of adiposity. Curr Opin Genet Dev. 2018;50:86–95. 10.1016/j.gde.2018.02.009. Epub 2018 Mar 9. PMID: 29529423; PMCID: PMC6089650. [DOI] [PMC free article] [PubMed]
- 21.Lotta LA, Wittemans LBL, Zuber V, Stewart ID, Sharp SJ, Luan J, Day FR, Li C, Bowker N, Cai L, De Lucia RE, Khaw KT, Perry JRB, O’Rahilly S, Scott RA, Savage DB, Burgess S, Wareham NJ, Langenberg C. Association of genetic variants related to gluteofemoral vs abdominal fat distribution with type 2 diabetes, coronary disease, and cardiovascular risk factors. JAMA. 2018;320(24):2553–2563. doi: 10.1001/jama.2018.19329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marinou K, Hodson L, Vasan SK, Fielding BA, Banerjee R, Brismar K, Koutsilieris M, Clark A, Neville MJ, Karpe F. Structural and functional properties of deep abdominal subcutaneous adipose tissue explain its association with insulin resistance and cardiovascular risk in men. Diabetes Care. 2014;37(3):821–829. doi: 10.2337/dc13-1353. [DOI] [PubMed] [Google Scholar]
- 23.Goossens GH, Jocken JWE, Blaak EE. Sexual dimorphism in cardiometabolic health: The role of adipose tissue, muscle and liver. Nat Rev Endocrinol. 2021;17(1):47–66. doi: 10.1038/s41574-020-00431-8. [DOI] [PubMed] [Google Scholar]
- 24.Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94(2):206–218. doi: 10.1016/j.physbeh.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stinkens R, Goossens GH, Jocken JW, Blaak EE. Targeting fatty acid metabolism to improve glucose metabolism. Obes Rev. 2015;16(9):715–757. doi: 10.1111/obr.12298. [DOI] [PubMed] [Google Scholar]
- 27.Stefan N, Fritsche A, Schick F, Häring HU. Phenotypes of prediabetes and stratification of cardiometabolic risk. Lancet Diabetes Endocrinol. 2016;4(9):789–798. doi: 10.1016/S2213-8587(16)00082-6. [DOI] [PubMed] [Google Scholar]
- 28.Goossens GH. The metabolic phenotype in obesity: Fat mass, body fat distribution, and adipose tissue function. Obes Facts. 2017;10(3):207–15. 10.1159/000471488. Epub 2017 Jun 1. PMID: 28564650; PMCID: PMC5644968. [DOI] [PMC free article] [PubMed]
- 29.Blüher M. Are metabolically healthy obese individuals really healthy? Eur J Endocrinol. 2014;171(6):R209–R219. doi: 10.1530/EJE-14-0540. [DOI] [PubMed] [Google Scholar]
- 30.Kim LJ, Nalls MA, Eiriksdottir G, Sigurdsson S, Launer LJ, Koster A, Chaves PH, Jonsdottir B, Garcia M, Gudnason V, Harris TB, AGES-Reykjavik Study Investigators. Associations of visceral and liver fat with the metabolic syndrome across the spectrum of obesity: the AGES-Reykjavik study. Obesity (Silver Spring). 2011;19(6):1265–71. 10.1038/oby.2010.291. Epub 2010 Dec 23. PMID: 21183935; PMCID: PMC3081537. [DOI] [PMC free article] [PubMed]
- 31.Klöting N, Fasshauer M, Dietrich A, Kovacs P, Schön MR, Kern M, Stumvoll M, Blüher M. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299(3):E506–E515. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- 32.Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud’homme D, Rabasa-Lhoret R. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90(7):4145–4150. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]
- 33.Appleton SL, Seaborn CJ, Visvanathan R, Hill CL, Gill TK, Taylor AW, Adams RJ, North West Adelaide Health Study Team. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: a cohort study. Diabetes Care. 2013;36(8):2388–94. 10.2337/dc12-1971. Epub 2013 Mar 14. PMID: 23491523; PMCID: PMC3714523. [DOI] [PMC free article] [PubMed]
- 34.Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V, Sladek R, Rabasa-Lhoret R. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011;35(7):971–981. doi: 10.1038/ijo.2010.216. [DOI] [PubMed] [Google Scholar]
- 35.Chen DL, Liess C, Poljak A, Xu A, Zhang J, Thoma C, Trenell M, Milner B, Jenkins AB, Chisholm DJ, Samocha-Bonet D, Greenfield JR. Phenotypic characterization of insulin-resistant and insulin-sensitive obesity. J Clin Endocrinol Metab. 2015;100(11):4082–91. 10.1210/jc.2015-2712. Epub 2015 Sep 17. Erratum in: J Clin Endocrinol Metab. 2016;101(2):757. Erratum in: J Clin Endocrinol Metab. 2016;101(2):757. PMID: 26378474. [DOI] [PubMed]
- 36.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Häring HU. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168(15):1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 37.Jennings CL, Lambert EV, Collins M, Joffe Y, Levitt NS, Goedecke JH. Determinants of insulin-resistant phenotypes in normal-weight and obese Black African women. Obesity (Silver Spring) 2008;16(7):1602–1609. doi: 10.1038/oby.2008.233. [DOI] [PubMed] [Google Scholar]
- 38.Koster A, Stenholm S, Alley DE, Kim LJ, Simonsick EM, Kanaya AM, Visser M, Houston DK, Nicklas BJ, Tylavsky FA, Satterfield S, Goodpaster BH, Ferrucci L, Harris TB, Health ABC Study. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring). 2010;18(12):2354–61. 10.1038/oby.2010.86. Epub 2010 Apr 15. PMID: 20395951; PMCID: PMC3095947. [DOI] [PMC free article] [PubMed]
- 39.Hayes L, Pearce MS, Firbank MJ, Walker M, Taylor R, Unwin NC. Do obese but metabolically normal women differ in intra-abdominal fat and physical activity levels from those with the expected metabolic abnormalities? A cross-sectional study. BMC Public Health. 2010;24(10):723. doi: 10.1186/1471-2458-10-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavie CJ, De Schutter A, Milani RV. Healthy obese versus unhealthy lean: The obesity paradox. Nat Rev Endocrinol. 2015;11(1):55–62. doi: 10.1038/nrendo.2014.165. [DOI] [PubMed] [Google Scholar]
- 41.Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: Facts and fantasies. J Clin Invest. 2019;129(10):3978–3989. doi: 10.1172/JCI129186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goday A, Calvo E, Vázquez LA, Caveda E, Margallo T, Catalina-Romero C, Reviriego J. Prevalence and clinical characteristics of metabolically healthy obese individuals and other obese/non-obese metabolic phenotypes in a working population: Results from the Icaria study. BMC Public Health. 2016;1(16):248. doi: 10.1186/s12889-016-2921-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slagter SN, Corpeleijn E, van der Klauw MM, Sijtsma A, Swart-Busscher LG, Perenboom CWM, de Vries JHM, Feskens EJM, Wolffenbuttel BHR, Kromhout D, van Vliet-Ostaptchouk JV. Dietary patterns and physical activity in the metabolically (un)healthy obese: The Dutch Lifelines cohort study. Nutr J. 2018;17(1):18. doi: 10.1186/s12937-018-0319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Velho S, Paccaud F, Waeber G, Vollenweider P, Marques-Vidal P. Metabolically healthy obesity: different prevalences using different criteria. Eur J Clin Nutr. 2010;64(10):1043–51. 10.1038/ejcn.2010.114. Epub 2010 Jul 14. PMID: 20628408. [DOI] [PubMed]
- 45.Lin H, Zhang L, Zheng R, Zheng Y. The prevalence, metabolic risk and effects of lifestyle intervention for metabolically healthy obesity: A systematic review and meta-analysis: A PRISMA-compliant article. Medicine (Baltimore). 2017;96(47):e8838. 10.1097/MD.0000000000008838. PMID: 29381992; PMCID: PMC5708991. [DOI] [PMC free article] [PubMed]
- 46.Hinnouho GM, Czernichow S, Dugravot A, Nabi H, Brunner EJ, Kivimaki M, Singh-Manoux A. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: The Whitehall II cohort study. Eur Heart J. 2015;36(9):551–9. 10.1093/eurheartj/ehu123. Epub 2014 Mar 26. PMID: 24670711; PMCID: PMC4344958. [DOI] [PMC free article] [PubMed]
- 47.Hinnouho GM, Czernichow S, Dugravot A, Batty GD, Kivimaki M, Singh-Manoux A. Metabolically healthy obesity and risk of mortality: does the definition of metabolic health matter? Diabetes Care. 2013;36(8):2294–300. 10.2337/dc12-1654. Epub 2013 May 1. PMID: 23637352; PMCID: PMC3714476. [DOI] [PMC free article] [PubMed]
- 48.Misra A, Vikram NK. Insulin resistance syndrome (metabolic syndrome) and obesity in Asian Indians: Evidence and implications. Nutrition. 2004;20(5):482–491. doi: 10.1016/j.nut.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 49.Bell JA, Kivimaki M, Hamer M. Metabolically healthy obesity and risk of incident type 2 diabetes: a meta-analysis of prospective cohort studies. Obes Rev. 2014;15(6):504–15. 10.1111/obr.12157. Epub 2014 Mar 24. PMID: 24661566; PMCID: PMC4309497. [DOI] [PMC free article] [PubMed]
- 50.Eckel N, Meidtner K, Kalle-Uhlmann T, Stefan N, Schulze MB. Metabolically healthy obesity and cardiovascular events: A systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23(9):956–966. doi: 10.1177/2047487315623884. [DOI] [PubMed] [Google Scholar]
- 51.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions? A systematic review and meta-analysis. Ann Intern Med. 2013;159(11):758–769. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
- 52.Kouvari M, Panagiotakos DB, Yannakoulia M, Georgousopoulou E, Critselis E, Chrysohoou C, Tousoulis D, Pitsavos C, ATTICA Study Investigators. Transition from metabolically benign to metabolically unhealthy obesity and 10-year cardiovascular disease incidence: The ATTICA cohort study. Metabolism. 2019;93:18–24. 10.1016/j.metabol.2019.01.003. Epub 2019 Jan 11. PMID: 30639450. [DOI] [PubMed]
- 53.Mongraw-Chaffin M, Foster MC, Anderson CAM, Burke GL, Haq N, Kalyani RR, Ouyang P, Sibley CT, Tracy R, Woodward M, Vaidya D. Metabolically healthy obesity, transition to metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2018;71(17):1857–1865. doi: 10.1016/j.jacc.2018.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Echouffo-Tcheugui JB, Short MI, Xanthakis V, Field P, Sponholtz TR, Larson MG, Vasan RS. Natural history of obesity subphenotypes: Dynamic changes over two decades and prognosis in the framingham heart study. J Clin Endocrinol Metab. 2019;104(3):738–752. doi: 10.1210/jc.2018-01321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bell JA, Hamer M, Sabia S, Singh-Manoux A, Batty GD, Kivimaki M. The natural course of healthy obesity over 20 years. J Am Coll Cardiol. 2015;65(1):101–102. doi: 10.1016/j.jacc.2014.09.077. [DOI] [PubMed] [Google Scholar]
- 56.Achilike I, Hazuda HP, Fowler SP, Aung K, Lorenzo C. Predicting the development of the metabolically healthy obese phenotype. Int J Obes (Lond). 2015;39(2):228–34. 10.1038/ijo.2014.113. Epub 2014 Jul 2. PMID: 24984752; PMCID: PMC4351862. [DOI] [PMC free article] [PubMed]
- 57.Soriguer F, Gutiérrez-Repiso C, Rubio-Martín E, García-Fuentes E, Almaraz MC, Colomo N, Esteva de Antonio I, de Adana MS, Chaves FJ, Morcillo S, Valdés S, Rojo-Martínez G. Metabolically healthy but obese, a matter of time? Findings from the prospective Pizarra study. J Clin Endocrinol Metab. 2013;98(6):2318–25. 10.1210/jc.2012-4253. Epub 2013 Apr 4. PMID: 23559087. [DOI] [PubMed]
- 58.Lin L, Zhang J, Jiang L, Du R, Hu C, Lu J, Wang T, Li M, Zhao Z, Xu Y, Xu M, Bi Y, Ning G, Wang W, Chen Y. Transition of metabolic phenotypes and risk of subclinical atherosclerosis according to BMI: A prospective study. Diabetologia. 2020;63(7):1312–1323. doi: 10.1007/s00125-020-05116-5. [DOI] [PubMed] [Google Scholar]
- 59.Morkedal B, Vatten LJ, Romundstad PR, Laugsand LE, Janszky I. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (Nord-Tr ndelag Health Study), Norway. J Am Coll Cardiol. 2014;63:1071–1078. doi: 10.1016/j.jacc.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 60.Lee YB, Kim DH, Kim SM, Kim NH, Choi KM, Baik SH, Park YG, Han K, Yoo HJ. Hospitalization for heart failure incidence according to the transition in metabolic health and obesity status: A nationwide population-based study. Cardiovasc Diabetol. 2020;19(1):77. doi: 10.1186/s12933-020-01051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bell JA, Hamer M, Batty GD, Singh-Manoux A, Sabia S, Kivimäki M. Incidence of metabolic risk factors among healthy obese adults: 20-year follow-up. J Am Coll Cardiol. 2015;66(7):871–873. doi: 10.1016/j.jacc.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo F, Garvey WT. Cardiometabolic disease risk in metabolically healthy and unhealthy obesity: Stability of metabolic health status in adults. Obesity (Silver Spring). 2016;24(2):516–25. 10.1002/oby.21344. Epub 2015 Dec 31. PMID: 26719125; PMCID: PMC4731253. [DOI] [PMC free article] [PubMed]
- 63.Hashimoto Y, Hamaguchi M, Fukuda T, Ohbora A, Kojima T, Fukui M. Fatty liver as a risk factor for progression from metabolically healthy to metabolically abnormal in non-overweight individuals. Endocrine. 2017;57(1):89–97. doi: 10.1007/s12020-017-1313-6. [DOI] [PubMed] [Google Scholar]
- 64.Moussa O, Arhi C, Ziprin P, Darzi A, Khan O, Purkayastha S. Fate of the metabolically healthy obese-is this term a misnomer? A study from the Clinical Practice Research Datalink. Int J Obes (Lond) 2019;43(5):1093–1101. doi: 10.1038/s41366-018-0096-z. [DOI] [PubMed] [Google Scholar]
- 65.Schröder H, Ramos R, Baena-Díez JM, Mendez MA, Canal DJ, Fíto M, Sala J, Elosua R. Determinants of the transition from a cardiometabolic normal to abnormal overweight/obese phenotype in a Spanish population. Eur J Nutr. 2014;53(6):1345–1353. doi: 10.1007/s00394-013-0635-2. [DOI] [PubMed] [Google Scholar]
- 66.Cui Z, Truesdale KP, Bradshaw PT, Cai J, Stevens J. Three-year weight change and cardiometabolic risk factors in obese and normal weight adults who are metabolically healthy: The atherosclerosis risk in communities study. Int J Obes (Lond) 2015;39(8):1203–1208. doi: 10.1038/ijo.2015.56. [DOI] [PubMed] [Google Scholar]
- 67.Espinosa De Ycaza AE, Donegan D, Jensen MD. Long-term metabolic risk for the metabolically healthy overweight/obese phenotype. Int J Obes (Lond). 2018;42(3):302–9. 10.1038/ijo.2017.233. Epub 2017 Sep 25. PMID: 29064474; PMCID: PMC5867190. [DOI] [PMC free article] [PubMed]
- 68.Phillips CM, Dillon C, Harrington JM, McCarthy VJ, Kearney PM, Fitzgerald AP, Perry IJ. Defining metabolically healthy obesity: role of dietary and lifestyle factors. PLoS One. 2013;8(10):e76188. 10.1371/journal.pone.0076188. PMID: 24146838; PMCID: PMC3798285. [DOI] [PMC free article] [PubMed]
- 69.Kim HN, Song SW. Associations between macronutrient intakes and obesity/metabolic risk phenotypes: Findings of the Korean National Health and Nutrition Examination Survey. Nutrients. 2019;11(3):628. doi: 10.3390/nu11030628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park YM, Steck SE, Fung TT, Zhang J, Hazlett LJ, Han K, Lee SH, Kwon HS, Merchant AT. Mediterranean diet, Dietary Approaches to Stop Hypertension (DASH) style diet, and metabolic health in U.S. adults. Clin Nutr. 2017;36(5):1301–9. 10.1016/j.clnu.2016.08.018. Epub 2016 Sep 8. PMID: 27665232. [DOI] [PubMed]
- 71.Camhi SM, Whitney Evans E, Hayman LL, Lichtenstein AH, Must A. Healthy eating index and metabolically healthy obesity in U.S. adolescents and adults. Prev Med. 2015;77:23–7. 10.1016/j.ypmed.2015.04.023. Epub 2015 May 1. PMID: 25937589. [DOI] [PubMed]
- 72.Green AK, Jacques PF, Rogers G, Fox CS, Meigs JB, McKeown NM. Sugar-sweetened beverages and prevalence of the metabolically abnormal phenotype in the Framingham Heart Study. Obesity (Silver Spring). 2014;22(5):E157–63. 10.1002/oby.20724. Epub 2014 Mar 8. PMID: 24550031; PMCID: PMC4139414. [DOI] [PMC free article] [PubMed]
- 73.Hankinson AL, Daviglus ML, Van Horn L, Chan Q, Brown I, Holmes E, Elliott P, Stamler J. Diet composition and activity level of at risk and metabolically healthy obese American adults. Obesity (Silver Spring) 2013;21(3):637–643. doi: 10.1002/oby.20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pajunen P, Kotronen A, Korpi-Hyövälti E, Keinänen-Kiukaanniemi S, Oksa H, Niskanen L, Saaristo T, Saltevo JT, Sundvall J, Vanhala M, Uusitupa M, Peltonen M. Metabolically healthy and unhealthy obesity phenotypes in the general population: The FIN-D2D Survey. BMC Public Health. 2011;1(11):754. doi: 10.1186/1471-2458-11-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Freedhoff Y, Hall KD. Weight loss diet studies: We need help not hype. Lancet. 2016;388(10047):849–851. doi: 10.1016/S0140-6736(16)31338-1. [DOI] [PubMed] [Google Scholar]
- 76.Dhurandhar NV, Schoeller D, Brown AW, Heymsfield SB, Thomas D, Sørensen TI, Speakman JR, Jeansonne M, Allison DB, Energy Balance Measurement Working Group. Energy balance measurement: when something is not better than nothing. Int J Obes (Lond). 2015;39(7):1109–13. 10.1038/ijo.2014.199. Epub 2014 Nov 13. PMID: 25394308; PMCID: PMC4430460. [DOI] [PMC free article] [PubMed]
- 77.Perdomo CM, Cohen RV, Sumithran P, Clément K, Frühbeck G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet. 2023;401(10382):1116–1130. doi: 10.1016/S0140-6736(22)02403-5. [DOI] [PubMed] [Google Scholar]
- 78.Kantartzis K, Machann J, Schick F, Rittig K, Machicao F, Fritsche A, Häring HU, Stefan N. Effects of a lifestyle intervention in metabolically benign and malign obesity. Diabetologia. 2011;54(4):864–868. doi: 10.1007/s00125-010-2006-3. [DOI] [PubMed] [Google Scholar]
- 79.Golzarand M, Moslehi N, Mirmiran P, Azizi F. Adherence to the DASH, MeDi, and MIND diet scores and the incidence of metabolically unhealthy phenotypes. Obes Res Clin Pract. 2023:S1871–403X(23)00025-X. 10.1016/j.orcp.2023.04.001. Epub ahead of print. PMID: 37037714. [DOI] [PubMed]
- 80.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Fitó M, Gea A, Hernán MA, Martínez-González MA. PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34. 10.1056/NEJMoa1800389. Epub 2018 Jun 13. PMID: 29897866. [DOI] [PubMed]
- 81.Konieczna J, Yañez A, Moñino M, Babio N, Toledo E, Martínez-González MA, Sorlí JV, Salas-Salvadó J, Estruch R, Ros E, Alonso-Gómez A, Schröder H, Lapetra J, Serra-Majem L, Pintó X, Gutiérrez-Bedmar M, Díaz-López A, González JI, Fitó M, Forga L, Fiol M, Romaguera D. Longitudinal changes in Mediterranean diet and transition between different obesity phenotypes. Clin Nutr. 2020;39(3):966–975. doi: 10.1016/j.clnu.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 82.Schulze MB. Metabolic health in normal-weight and obese individuals. Diabetologia. 2019;62(4):558–566. doi: 10.1007/s00125-018-4787-8. [DOI] [PubMed] [Google Scholar]
- 83.Zhu R, Jalo E, Silvestre MP, Poppitt SD, Handjieva-Darlenska T, Handjiev S, Huttunen-Lenz M, Mackintosh K, Stratton G, Navas-Carretero S, Pietiläinen KH, Simpson E, Macdonald IA, Muirhead R, Brand-Miller J, Fogelholm M, Færch K, Martinez JA, Westerterp-Plantenga MS, Adam TC, Raben A. Does the effect of a 3-year lifestyle intervention on body weight and cardiometabolic health differ by prediabetes metabolic phenotype? A Post hoc analysis of the preview study. Diabetes Care. 2022;45(11):2698–2708. doi: 10.2337/dc22-0549. [DOI] [PubMed] [Google Scholar]
- 84.Goossens GH, Moors CC, Jocken JW, van der Zijl NJ, Jans A, Konings E, Diamant M, Blaak EE. Altered skeletal muscle fatty acid handling in subjects with impaired glucose tolerance as compared to impaired fasting glucose. Nutrients. 2016;8(3):164. doi: 10.3390/nu8030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blaak EE. Current metabolic perspective on malnutrition in obesity: Towards more subgroup-based nutritional approaches? Proc Nutr Soc. 2020;79(3):331–7. 10.1017/S0029665120000117. Epub 2020 Mar 3. PMID: 32122428; PMCID: PMC7663313. [DOI] [PMC free article] [PubMed]
- 86.Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care. 2007;30(1):89–94. doi: 10.2337/dc06-1519. [DOI] [PubMed] [Google Scholar]
- 87.O’Donovan SD, Lenz M, Goossens GH, van der Kallen CJH, Eussen SJMP, Stehouwer CDA, van Greevenbroek MM, Schram MT, Sep SJ, Peeters RLM, Blaak EE, van Riel NAW, de Kok TMCM, Arts ICW. Improved quantification of muscle insulin sensitivity using oral glucose tolerance test data: The MISI Calculator. Sci Rep. 2019;9(1):9388. doi: 10.1038/s41598-019-45858-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vogelzangs N, van der Kallen CJH, van Greevenbroek MMJ, van der Kolk BW, Jocken JWE, Goossens GH, Schaper NC, Henry RMA, Eussen SJPM, Valsesia A, Hankemeier T, Astrup A, Saris WHM, Stehouwer CDA, Blaak EE, Arts ICW, Diogenes consortium. Metabolic profiling of tissue-specific insulin resistance in human obesity: results from the Diogenes study and the Maastricht Study. Int J Obes (Lond). 2020;44(6):1376–86. 10.1038/s41366-020-0565-z. Epub 2020 Mar 17. PMID: 32203114. [DOI] [PubMed]
- 89.van der Kolk BW, Vogelzangs N, Jocken JWE, Valsesia A, Hankemeier T, Astrup A, Saris WHM, Arts ICW, van Greevenbroek MMJ, Blaak EE, DiOGenes consortium. Plasma lipid profiling of tissue-specific insulin resistance in human obesity. Int J Obes (Lond). 2019;43(5):989–98. 10.1038/s41366-018-0189-8. Epub 2018 Sep 21. PMID: 30242234. [DOI] [PubMed]
- 90.van der Kolk BW, Kalafati M, Adriaens M, van Greevenbroek MMJ, Vogelzangs N, Saris WHM, Astrup A, Valsesia A, Langin D, van der Kallen CJH, Eussen SJPM, Schalkwijk CG, Stehouwer CDA, Goossens GH, Arts ICW, Jocken JWE, Evelo CT, Blaak EE. Subcutaneous adipose tissue and systemic inflammation are associated with peripheral but not hepatic insulin resistance in humans. Diabetes. 2019;68(12):2247–2258. doi: 10.2337/db19-0560. [DOI] [PubMed] [Google Scholar]
- 91.Song Y, Søndergaard E, Jensen MD. Unique metabolic features of adults discordant for indices of insulin resistance. J Clin Endocrinol Metab. 2020;105(8):e2753–e2763. doi: 10.1210/clinem/dgaa265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tejani S, McCoy C, Ayers CR, Powell-Wiley TM, Després JP, Linge J, Leinhard OD, Petersson M, Borga M, Neeland IJ. Cardiometabolic health outcomes associated with discordant visceral and liver fat phenotypes: Insights from the Dallas heart study and UK Biobank. Mayo Clin Proc. 2022;97(2):225–37. 10.1016/j.mayocp.2021.08.021. Epub 2021 Sep 28. PMID: 34598789; PMCID: PMC8818017. [DOI] [PMC free article] [PubMed]
- 93.Linge J, Whitcher B, Borga M, Dahlqvist Leinhard O. Sub-phenotyping metabolic disorders using body composition: An individualized, nonparametric approach utilizing large data sets. Obesity (Silver Spring). 2019;27(7):1190–9. 10.1002/oby.22510. Epub 2019 May 16. PMID: 31094076; PMCID: PMC6617760. [DOI] [PMC free article] [PubMed]
- 94.Kim SH, Reaven G. Sex differences in insulin resistance and cardiovascular disease risk. J Clin Endocrinol Metab. 2013;98(11):E1716–21. 10.1210/jc.2013-1166. Epub 2013 Sep 24. PMID: 24064694; PMCID: PMC3816264. [DOI] [PMC free article] [PubMed]
- 95.Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mithieux G, Nauta A, Scott K, Stahl B, van Harsselaar J, van Tol R, Vaughan EE, Verbeke K. Short chain fatty acids in human gut and metabolic health. Benef Microbes. 2020;11(5):411–455. doi: 10.3920/BM2020.0057. [DOI] [PubMed] [Google Scholar]
- 96.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 97.Ley RE, Bäckhed F, Turnbaugh P, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tims S, Derom C, Jonkers DM, et al. Microbiota conservation and BMI signatures in adult monozygotic twins. Isme J. 2013;7:707–717. doi: 10.1038/ismej.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Duncan SH, Lobley GE, Holtrop G, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes. 2008;32:1720–1724. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 100.Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 101.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T; MetaHIT consortium; Bork P, Wang J, Ehrlich SD, Pedersen O. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–6. 10.1038/nature12506. PMID: 23985870. [DOI] [PubMed]
- 102.Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15(5):261–273. doi: 10.1038/s41574-019-0156-z. [DOI] [PubMed] [Google Scholar]
- 103.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 104.Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G, Le Chatelier E, Levenez F, Doré J, Mattila I, Plichta DR, Pöhö P, Hellgren LI, Arumugam M, Sunagawa S, Vieira-Silva S, Jørgensen T, Holm JB, Trošt K; MetaHIT Consortium; Kristiansen K, Brix S, Raes J, Wang J, Hansen T, Bork P, Brunak S, Oresic M, Ehrlich SD, Pedersen O. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535(7612):376–81. 10.1038/nature18646. Epub 2016 Jul 13. PMID: 27409811. [DOI] [PubMed]
- 105.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–6.e7. 10.1053/j.gastro.2012.06.031. Epub 2012 Jun 20. Erratum in: Gastroenterology. 2013 Jan;144(1):250. PMID: 22728514. [DOI] [PubMed]
- 106.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 107.Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 108.van der Beek CM, Canfora EE, Lenaerts K, Troost FJ, Damink SWMO, Holst JJ, Masclee AAM, Dejong CHC, Blaak EE. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin Sci (Lond) 2016;130(22):2073–2082. doi: 10.1042/CS20160263. [DOI] [PubMed] [Google Scholar]
- 109.Jardon KM, Umanets A, Venema K, Gijbels A, Trouwborst I, Hul GB, Afman LA, Goossens GH, Blaak EE. Gut microbiome profiling in tissue-specific insulin resistance: A cross-sectional analysis of the PERSON study. Obes Facts. 2022;15(Suppl 1):1–240. doi: 10.1159/000524469. [DOI] [Google Scholar]
- 110.Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, Bouter KE, Koopen AM, Holst JJ, Knop FK, Blaak EE, Zhao J, Smidt H, Harms AC, Hankemeijer T, Bergman JJGHM, Romijn HA, Schaap FG, Olde Damink SWM, Ackermans MT, Dallinga-Thie GM, Zoetendal E, de Vos WM, Serlie MJ, Stroes ESG, Groen AK, Nieuwdorp M. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017;26(4):611–9.e6. 10.1016/j.cmet.2017.09.008. PMID: 28978426. [DOI] [PubMed]
- 111.Robertson MD, Wright JW, Loizon E, Debard C, Vidal H, Shojaee-Moradie F, Russell-Jones D, Umpleby AM. Insulin-sensitizing effects on muscle and adipose tissue after dietary fiber intake in men and women with metabolic syndrome. J Clin Endocrinol Metab. 2012;97(9):3326–3332. doi: 10.1210/jc.2012-1513. [DOI] [PubMed] [Google Scholar]
- 112.Müller M, Hermes GDA, Emanuel E C, Holst JJ, Zoetendal EG, Smidt H, Troost F, Schaap FG, Damink SO, Jocken JWE, Lenaerts K, Masclee AAM, Blaak EE. Effect of wheat bran derived prebiotic supplementation on gastrointestinal transit, gut microbiota, and metabolic health: a randomized controlled trial in healthy adults with a slow gut transit. Gut Microbes. 2020;12(1):1704141. 10.1080/19490976.2019.1704141. Epub 2020 Jan 25. PMID: 31983281; PMCID: PMC7524158. [DOI] [PMC free article] [PubMed]
- 113.Debédat J, Le Roy T, Voland L, Belda E, Alili R, Adriouch S, Bel Lassen P, Kasahara K, Hutchison E, Genser L, Torres L, Gamblin C, Rouault C, Zucker JD, Kapel N, Poitou C, Marcelin G, Rey FE, Aron-Wisnewsky J, Clément K. The human gut microbiota contributes to type-2 diabetes non-resolution 5-years after Roux-en-Y gastric bypass. Gut Microbes. 2022;14(1):2050635. 10.1080/19490976.2022.2050635. PMID: 35435140; PMCID: PMC9037437. [DOI] [PMC free article] [PubMed]
- 114.Suez J, Cohen Y, Valdés-Mas R, Mor U, Dori-Bachash M, Federici S, Zmora N, Leshem A, Heinemann M, Linevsky R, Zur M, Ben-Zeev Brik R, Bukimer A, Eliyahu-Miller S, Metz A, Fischbein R, Sharov O, Malitsky S, Itkin M, Stettner N, Harmelin A, Shapiro H, Stein-Thoeringer CK, Segal E, Elinav E. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell. 2022;185(18):3307–3328.e19. doi: 10.1016/j.cell.2022.07.016. [DOI] [PubMed] [Google Scholar]
- 115.Salonen A, Lahti L, Salojärvi J, Holtrop G, Korpela K, Duncan SH, Date P, Farquharson F, Johnstone AM, Lobley GE, Louis P, Flint HJ, de Vos WM. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014;8(11):2218–30. 10.1038/ismej.2014.63. Epub 2014 Apr 24. PMID: 24763370; PMCID: PMC4992075. [DOI] [PMC free article] [PubMed]
- 116.Canfora EE, Muller M, Hermes G, et al. A novel fiber mixture to promote microbial fermentation in the distal colon affects substrate metabolism in lean vs prediabetic obese men. Obes Facts. 2019;12(suppl 1):191. Gut Microbes. 2022;14(1):2009297. 10.1080/19490976.2021.2009297. PMID: 34923911; PMCID: PMC8726743. [DOI] [PMC free article] [PubMed]
- 117.Bouter K, Bakker GJ, Levin E, Hartstra AV, Kootte RS, Udayappan SD, Katiraei S, Bahler L, Gilijamse PW, Tremaroli V, Stahlman M, Holleman F, van Riel NAW, Verberne HJ, Romijn JA, Dallinga-Thie GM, Serlie MJ, Ackermans MT, Kemper EM, Willems van Dijk K, Backhed F, Groen AK, Nieuwdorp M. Differential metabolic effects of oral butyrate treatment in lean versus metabolic syndrome subjects. Clin Transl Gastroenterol. 2018;9(5):155. 10.1038/s41424-018-0025-4. PMID: 29799027; PMCID: PMC5968024. [DOI] [PMC free article] [PubMed]
- 118.Guo F, Garvey WT. Cardiometabolic disease staging predicts effectiveness of weight-loss therapy to prevent type 2 diabetes: Pooled results from phase iii clinical trials assessing phentermine/topiramate extended release. Diabetes Care. 2017;40(7):856–62. 10.2337/dc17-0088. Epub 2017 Apr 28. PMID: 28455281; PMCID: PMC5481985. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A.
N/A.