Abstract
Obesity epidemic reached the dimensions of a real global health crisis with more than one billion people worldwide living with obesity. Multiple obesity-related mechanisms cause structural, functional, humoral, and hemodynamic alterations with cardiovascular (CV) deleterious effects. A correct assessment of the cardiovascular risk in people with obesity is critical for reducing mortality and preserving quality of life. The correct identification of the obesity status remains difficult as recent evidence suggest that different phenotypes of obesity exist, each one associated with different degrees of CV risk. Diagnosis of obesity cannot depend only on anthropometric parameters but should include a precise assessment of the metabolic status. Recently, the World Heart Federation and World Obesity Federation provided an action plan for management of obesity-related CV risk and mortality, stressing for the instauration of comprehensive structured programs encompassing multidisciplinary teams. In this review we aim at providing an updated summary regarding the different obesity phenotypes, their specific effects on CV risk and differences in clinical management.
Keywords: Obesity, Cardiovascular risk, Inflammation, Insulin resistance, Fat, Adipose tissue
Introduction to obesity and cardiovascular risk
Over the last 30 years, the epidemic of overweight and obesity has increased dramatically, reaching the dimension of a real global health crisis [1]. According to the data of the World Health Organization, more than 1 billion people worldwide are living with obesity (650 million adults, 340 million adolescents and 39 million children) accounting for about 2.8 million deaths every year [2]. Adipocytes secrete different hormones and peptides under several physiological and pathological conditions, known globally as adipokines and playing an important role in local and systemic regulation of energy homeostasis and inflammation [3–5]. Multiple obesity-related mechanisms are cause of structural, functional, humoral and hemodynamic alterations believed to underpin the development of CVD including atherothrombosis, atrial fibrillation (AF) and myocardial dysfunction [6–8]. Thus, a correct assessment of the cardiovascular (CV) risk in people with obesity is critical for reducing mortality and preserving quality of life in this class of patients. However, the correct identification of the obesity status is still tricky as recent evidence suggest that different phenotypes of obesity exist, each one associated with different degree of CV risk [9, 10]. Body mass index (BMI) has been longtime indicated as golden standard to assess adipose depots and the associated cardiovascular risk, but several limitations apply [8]. Considerable variations occur according with sex, age, and race/ethnicity. In the last decade, a shift toward a qualitative approach led to rephrase the paradigm of obesity into the concept of obesities [11]. With time, several other anthropometric measures have made their way alongside or replacing BMI: mainly waist circumference (WC) [12, 13] but also, waist-hip ratio (WHiR), waist to height ratio (WHtR), bioimpedance, 3D scanning and dual energy x-ray absorptiometry (DEXA). Such a paradigm shift takes into great account qualitative differences in adiposity as associated with different degrees of metabolic and atherogenic derangements and different responses to weight loss, lifestyle modification or medications. Recently, the World Heart Federation (WHF) and World Obesity Federation (WOF) provided an action plan for management of obesity-related CV risk and mortality, stressing for the institution of comprehensive structured programs encompassing multidisciplinary teams [14]. In this review we aim at providing an updated summary regarding the different obesity phenotypes, their specific effects on CV risk and differences in clinical management.
The journey from BMI to visceral adiposity and obesity phenotypes
The obesity paradox
Historically, the increase in adiposity depots expressed by BMI is linearly associated with growing CVD risk and mortality. Nevertheless, the first decade of this century saw the emergence of a mismatch between the awareness of excess body weight burden and its related metabolic consequences. The concept of ‘obesity paradox’ was born, and scientist stayed in this swamp for a decade further [15]. In several studies patients with obesity have indeed shown a better prognosis as compared with leaner ones [16]. Gruberg and co-workers firstly described this evidence in patients affected by coronary artery disease (CAD) undergoing percutaneous coronary intervention (PCI) [17]. Subsequently, numerous other conflicting data where published regarding the benefits of weight reduction in some high-risk CV conditions—heart failure (HF), atrial fibrillation (AF) or hypertension—as well as other non-CV conditions such as frailty, diabetes mellitus (DM), end-stage renal disease and chronic obstructive pulmonary disease [18, 19]. Notably, in patients affected by chronic HF, those losing more weight over time also showed higher mortality rate [20]. Numerous possible explanations to this phenomenon were provided. First, patients with obesity and CVD are on average younger and with better conserved systolic function than lean patients. Acute myocardial infarction (AMI) in patients with obesity has been found to be associated with less severe and complex CAD than in non-obese subjects [21]. Moreover, patients with obesity have higher levels of arterial pressure, thus they can be exposed to higher dosages of anti-ischemic and anti-remodeling medications. Nevertheless, the higher survival after AMI in this population was found to be independent of their younger age and more intensive medication treatment [22]. Other clinical features may in part explain the reduction of hospitalization time, as well as short- and long-term mortality [21, 23]. Different confounding factors (e.g. smoking, chronic illness, lung disease, cancer) as well as reverse causality were also pointed out as possible explanations for the OP. Indeed, the severity of the disease could strongly impact the weight loss trajectory. On the other hand, unintentional weight loss is often marked by relative reduction of muscle mass and peripheral fat, rather than central fat [24]. This phenomenon cannot be discriminate by the use of BMI [25]. The predictive role of WHR seems to be higher for CV risk stratification in those patients [26, 27]. In a recent study, WHR and WC better correlate with the severity of CAD in patients undergoing PCI while BMI only showed a low predictive value [28]. Markers of central fat should be considered better indicators of future risk in this context [29].
“Adiposopathy” and “diabesity”
Adipocyte hypertrophy in visceral adipose tissue and ectopic fat accumulation leads to cellular dysfunction, metabolic abnormalities and endocrine disturbances [30]. Adipose tissue dysfunction also known as “adiposopathy” is a root cause of some of the most common metabolic diseases observed in clinical practice, including DM, hypertension and dyslipidemia [31]. While classically related to the visceral fat, growing evidence suggest a role for dysfunctional stimulation of the subcutaneous adipose tissue in obesity [32]. Metabolic consequences of adiposopathy have been traditionally clustered in the general term metabolic syndrome (MetS) accounting for central obesity, hyperglycemia, hypertriglyceridemia, low levels of HDL and hypertension [33]. Shift toward visceral adipose tissue distribution, ectopic fat deposition and inflammatory/adipokines dysregulation are now considered the central tenets of adiposopathy [34]. Hypertrophic adipocytes showed an unbalanced adipokines production, promoting insulin resistance (IR), inflammation, fatty liver, increased LDL-cholesterol, oxidative stress, endothelial dysfunction and pro-thrombotic state [35]. Among adipokines, leptin levels were shown to be directly proportional to obesity and body fat levels, while its counter-hormone adiponectin resulted reduced [36]. This imbalance is thought to enhance atherogenesis, fibrosis, hyperglycemia and inflammation [37, 38]. Chemerin, a newly characterized chemoattractant released by adipocytes, is gaining more and more attention as a potential MetS biomarker being related with adipogenesis, angiogenesis and glucose metabolism [39, 40]. In humans, chemerin positively correlates with adiposity [41, 42], independently from WC or BMI [42], and strongly predicts MetS development [43]. Adipocyte hypertrophy also leads to ischemic dysfunction and hypoxia-related signaling. The surrounding microenvironment then modifies its architecture. Inflammatory cells from both innate and adaptive immunity infiltrate the dysfunctional adipose tissue and activate inflammatory pathways that further sustain such pathophysiological processes. Among the other, the upstream mediator osteopontin (OPN) seems also to be strongly associated with adiposopathy and cardiometabolic consequences. Released by macrophage within dysfunctional adipose tissue, OPN sustains adipocyte and metabolic dysregulation in both experimental and clinical studies [44–46]. Lipolysis and insulin resistance finally characterize such a dysfunctional microenvironment and reach peripheral tissues (e.g., skeletal muscle and liver) [47, 48]. Especially within the skeletal muscle, decrease in GLUT-4 translocation reduces glucose uptake and facilitates glycogenolysis [49]. In the liver, FFAs promote gluconeogenesis and lipogenesis further increasing insulin levels. Again, within pancreas islets, FFAs exert lipotoxic effect on beta cells leading to reduced insulin secretion and a failure of compensation [50]. Since adiposity and DM are strictly related, the term “diabesity” was coined to describe the superadded effects of DM and obesity on CV risk [51].
Obesity phenotypes
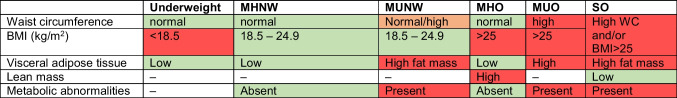
Pitfalls in the characterization of body fat distribution through the BMI and distinction of fat vs. lean tissue have provided a critical contribution to explain the non-unique subdivision of obesity phenotypes among studies. Based on current knowledge, “obesities” may be categorized across four groups: i) metabolic unhealthy normal weight (MUNW), ii) metabolically healthy overweight/obese (MHO), iii) metabolically unhealthy overweight/obese (MUO), and iv) sarcopenic obesity (SO) (Table 1). MHO and MUO are the most representative categories, including patients with a BMI > 25 kg/m [2] but very different metabolic profile [52] (Table 2). Alterations in body fat distribution is the key factor characterizing those two phenotypes. MUO encompasses the old features of MetS, which translates in a higher cardiometabolic risk [53]. In addition to age and higher WC, reduced subcutaneous fat and shift toward a visceral and dysfunctional/pro-inflammatory hypertrophic adipose tissue distribution characterize MUO. Impaired fat storage and ectopic visceral fat deposition in liver and skeletal muscle further characterize this prototypic phenotype of adiposopathy [54, 55]. Contrariwise, the healthier MHO phenotype is less common among European population, with a prevalence of 10–30% [56]. They are more often young, female, physically active people with a better nutritional status [57]. Although a definition of MHO is not standardized yet [58], this group would include people with high BMI and healthy metabolic profile: preserved insulin sensitivity, favorable lipid profile and low plasma levels of pro-inflammatory cytokines [59]. Nevertheless, even the alleged lower CV risk associated with MHO has been questioned [60]. Although CV risk did not differ from normal weight individuals, MHO had significantly higher risk to develop MetS over time and then increase by about 60% the chance of suffering major CV events in the MESA study [61, 62].
Table 1.
Summary of defining criteria of different obesity phenotypes
Normal values are in green, pathological ones are in red, and the intermediate ones in orange. Waist circumference categorized as normal (men < 102 cm and women < 88 cm) or high (men ≥ 102 cm and women ≥ 88 cm). Visceral adipose tissue and lean mass are a non-standardized measure actually. Metabolic abnormalities refer to the metabolic syndrome defining criteria
BMI body mass index, MHNW metabolically healthy normal weight, MUNW metabolically unhealthy normal weight, MHO metabolically healthy overweight/obese, MUO metabolically unhealthy overweight/obese, SO sarcopenic obese
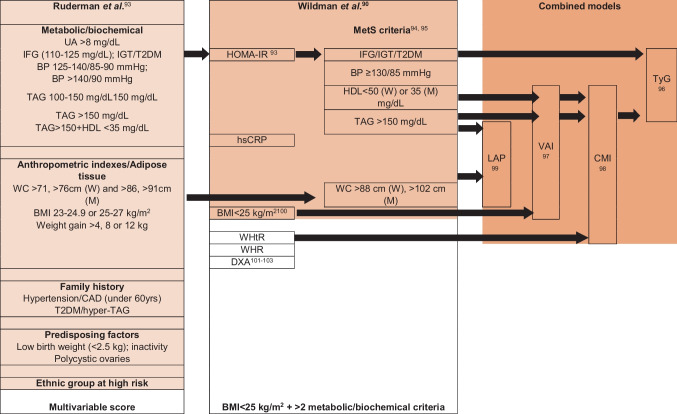
Table 2.
Over time development and controversies in definition of metabolically healthy/unhealthy overweight/obese
| Wildman et al. [90] | BioSHaRE-EU Healthy Obese Project [56] | Lavie et al. [91] | |
|---|---|---|---|
| Less strict | Stricter | ||
| IFG/IGT/T2DM | FPG > 126 mg/dL | FPG > 110 mg/dL | FPG > 100 mg/dL |
| BP ≥ 130/85 mmHg | BP > 140/90 mmHg | BP > 130/85 mmHg | BP > 130/85 mmHg |
| TAG > 150 mg/dL | |||
| HDL < 40 (W) or < 35 (M) mg/dL | |||
| WC > 88 cm (W), > 102 cm (M) | |||
| HOMA-IR [92] | |||
| hsCRP | |||
| BMI > 25 or > 30 kg/m [2] | None (MHO) or ≥ 1 (MUO) | 0–1 (MHO) or ≥ 1 (MUO) | |
IFG impaired fasting glucose, IGT impaired glucose tolerance, T2DM type 2 diabetes mellitus, BP blood pressure, TAG triglycerides, HDL high-density lipoprotein, WC waist circumference, BMI body mass index, CAD coronary artery disease, HOMA-IR homeostasis model assessment for insulin resistance, hsCRP high-sensitivity C-reactive protein, MHO metabolically healthy overweight/obese, MUO metabolically unhealthy overweight/obese
Similarly, a recent report from a UK biobank including > 380.000 people characterized MHO as at increased risk of HF (76%), respiratory diseases, all-cause mortality, and atherosclerotic CVD (20%) as compared to normal weight/MHO individuals [63]. Despite a lower baseline CV risk, MHO then develops atherosclerotic CVD risk factors earlier than lean individuals. Moreover, overweight itself is a non-negligible adverse factor that affects the natural history of several comorbidities such as respiratory, renal, and orthopedic ones [64, 65]. The MUNW group is another paradigm of the prevalent qualitative – rather than quantitative – relevance of adiposity (Table 3). They share similar CV risk factors [66] and metabolic alterations with traditionally patients with obesity, including chronic low-grade inflammation [67, 68]. MUNW has the highest rate of underdiagnoses among obesity phenotypes due to both the lack of consensus definition and the limited access to diagnostic tools for discriminating increased visceral adiposity and/or unbalanced fat/lean mass ratio [69, 70]. Its prevalence is estimated in high as 67% [71]. MUNW may or not be associated with changes in other anthropometric parameters, such as WC, WHiR, WHtR. The threshold of body fat mass applied in MUNW diagnosis varies among different studies, ranging from 19 to 32% for men and from 29 to 44% for women [72]. MUNW usually includes older and sedentary individuals [73] with generally a very low amount of gluteo-femoral fat mass compared with the visceral one [74]. Cardiometabolic risk associated with MUNW is high and high risk of CVD independently of elevated trunk fat mass as reported in lean women from Women’s Health Initiative Study [75]. MUO and MUNW phenotypes genetically differ: a variability in loci regulating food intake is reported in MUO, whereas genetic characterization of MUNW has highlighted a prevalence in genes regulating adipocyte differentiation, lipogenesis, and lipolysis (e.g. IRS1, GRB14, PPARG, LYPLAL1) [76, 77].
Table 3.
Over time development and controversies in definition of metabolically unhealthy normal weight
UA uric acid, IFG impaired fasting glucose, IGT impaired glucose tolerance, T2DM type 2 diabetes mellitus, BP blood pressure, TAG triglycerides, HDL high-density lipoprotein, WC waist circumference, BMI body mass index, CAD coronary artery disease, HOMA-IR homeostasis model assessment for insulin resistance, WHtR waist-to height ratio, WHR waist-to-hip-ratio, DXA dual-energy X-ray absorptiometry, LAP lipid accumulation product; VAI visceral adiposity index, CMI cardiometabolic index, TyG triglycerides-glucose index [90, 93–103]
As additional phenotype, SO is characterized by low skeletal muscle mass due to metabolic changes secondary to a sedentary lifestyle, adipose tissue derangements or chronic comorbidities [78]. Loss of skeletal muscle mass and function generally occurs with ageing and is commonly paralleled by relative or absolute body fat gain, favoring the potential development of SO. Adipose tissue has indeed a negative impact on muscle mass both directly through metabolic derangements (i.e. inflammation and IR) [79] and indirectly through increased prevalence of obesity-related chronic diseases with a negative impact on muscle metabolism (i.e., orthopedic disorders). Of interest, the skeletal muscle is now increasingly considered as an endocrine organ secreting a large number of factors, termed myokines, that favour the metabolic dialogue between the muscle and other organs, including the adipose tissue [80]. Although diagnostic criteria are variable among studies, SO is usually diagnosed when parameters of altered skeletal muscle strength coexist with altered body composition, in particular increased fat mass and reduced muscle mass [81]. Preclinical and clinical studies suggest the existence of a biological connection between IR, obesity and sarcopenia, mediated by the impaired function of the growth differentiation factor myostatin [82]. Such mediator, historically recognized among most important negative regulators of muscle mass, recently gains notoriety due to its role on glucose and fat metabolism including inhibition of insulin signaling, lipid oxidation and energy expenditure [83]. In addition to myostatin, sarcopenia and sarcopenic obesity are associated with a dysregulation of other myokines with important cardiometabolic functions, such as IL-6, FNDC5/irisin, fibroblast growth factor 21 or brain-derived neurotrophic factor, which play a critical role in skeletal muscle mass and function as well as metabolic homeostasis [84]. In SO, obesity and sarcopenia may therefore synergistically enhance each another with a vicious cycle facilitating weight gain and muscle loss through reduced mobility, dependency and disability [85]. As a consequence, such individuals show higher rate of adverse health consequences including falls and fractures, decreased mobility [86], poor quality of life and hospitalization [87] as compared to patients with isolated obesity or sarcopenia. Furthermore, systematic reviews and metanalysis report SO as a strongly predictor for all-cause mortality [88, 89].
How does obesity affect the heart?
Inflamm-aging and metaflammation
The term “inflamm-aging” merges two words “inflammation” and “aging” to describe the chronic, sterile, low-grade inflammation characterizing elderly individuals and playing fundamental roles in different age-dependent chronic diseases or conditions [5, 104–107]. Increased body fat composition and IR strongly associates with aging through several cellular and molecular mechanisms including cellular senescence, mitochondrial dysfunction, impaired autophagy and dysbiosis [108]. Moreover, the impaired crosstalk between adipocytes and the immune cells infiltrating the adipose tissue as well as the degeneration of self- and non-self-receptors is thought to contribute to the establishment of inflamm-aging itself [109]. Of interest, the innate immune response activates after food ingestion [110]. The so-called “postprandial inflammation” is part of the adaptive response to meals and causes the release of several pro-inflammatory mediators [111]. Therefore, the excess nutrients intake characterizing obesity associates with higher levels of inflammatory hormones (i.e. leptin) secreted by adipose tissue, leading to a metabolic reprogramming of immune cells, in particular macrophages, towards a pro-inflammatory phenotype. Such condition – known as “metaflammation” – synergistically works with accelerated inflammaging to create a dysregulated energetic environment, whose metabolic hallmarks are high levels of lipids, free fatty acids, glucose, and reactive oxygen species (ROS). Prolonged mitogenic signal induced by chronic hyperinsulinemia leads dysfunctional hypertrophic adipocytes to activate a post-mitotic cell cycle that initiate a senescent cell program. This process is associated to a pro-inflammatory secretome, which sustains and further contributes to low-grade chronic inflammation [112]. Macrophages and adipocytes demonstrate remarkable functional overlap, as both cell types secrete cytokines and can be activated by bacterial products (i.e. lipopolysaccharide) [113]. Furthermore, pre-adipocytes can transdifferentiate into macrophages. Of interest, whereas inflammation-resolving M2 macrophages dominate insulin-sensitive adipose tissue in the lean, pro-inflammatory M1 macrophages accumulate in parallel to adiposity in individuals with obesity, promoting inflammation and IR. Indeed, M1/M2 ratio indirectly correlates with both tissue-specific and whole-body insulin sensitivity [114]. Dysfunctional adipocytes induce M1 phenotype shifting by altering several intracellular pathways including IKK, JNK1, HIF and TLR signals against IL-4- and IL-13-mediated phosphorylation of STAT6 and expression of the lipid-sensing nuclear factors PPAR-γ and PPAR-δ [115, 116] M1 macrophages produce IL-1β, IL-6, TNFα and ROS further reducing insulin signaling in adipocytes. As a result, the number of M1 macrophages parallels the expansion of adipose tissue, exacerbating inflammation and IR. Many of those mediators may be clustered in the emerging concept of senescence-associated secretory patterns (SASP), increasingly considered leading driver of age-related disorders [117]. Among different molecules our research group has long time focused on the role of OPN – above described as upstream mediators of adipocyte dysfunction – is an interesting candidate bridge with cardiometabolic risk [118, 119]. Finally, gut microbiota also plays central roles in energetic homeostasis, as it can release inflammatory and anti-inflammatory products contributing to metaflammation [120]. Patients with obesity present a characteristically overgrowth of Firmicutes phyla (i.e. Lactobacillus and Faecalibacterium) and Escherichia coli against Bacteroidetes [121]. Such “obese microbiota” showed higher ability to extract calories from the diet [122] as well as being associated with increased gut permeability, leading to increased absorption of bacterial endotoxins [123]. The gut microbiota produces a wide variety of metabolites because of the anaerobic fermentation of undigested food [124]. Short-chain fatty acids (SCFAs) including acetate, propionate and butyrate are main metabolites of gut microbiota providing important anti-inflammatory effects. Studies showed that a reduction in the levels of SCFAs generate intestinal inflammation and foam cell formation, contributing to gut barrier disruption and favoring bacterial translocation including mobilization of lipopolysaccharides (LPS), trimethylamine N-oxide (TMAO) and phenylacetyl glutamine (PAGIn) which, in general circulation, induce systemic inflammation, macrophage activation and favor atherosclerosis [125].
Cardiac fibrosis
The strong association between obesity and CVD directly involves the heart, independent of the atherosclerotic process. Several stress factors are involved in substantial changes at molecular, cellular, and interstitial levels in obese hearts including dysregulated activation of different neuro-hormonal systems, hyperinsulinemia and inflammation [126]. Cardiac cells respond to such an environment eliciting the hypertrophic growth response through secretion of cytokines, growth factors (GFs), vasoactive peptides, and hormones [127]. Although considered an adaptation mechanism, such response associates with cell death, fibrosis, and microvascular dysfunction. Cardiac fibrosis plays an important role in the pathogenesis of heart disease in patients with obesity causing impaired diastolic function, altered contraction, atrial and ventricular remodeling eventually leading to heart failure with preserved ejection fraction (HFpEF), atrial and ventricular tachyarrhythmias and increased incidence of sudden death [128]. Cardiac fibroblasts are the most abundant interstitial cells in myocardium and are responsible for the formation and preservation of the matrix network [129]. Cardiac fibroblasts can influence cardiac function through direct and indirect effects on cardiomyocytes [130]. While in young individuals, cardiac fibroblasts maintain quiescence exhibiting limited inflammatory or proliferative activity, in aging hearts cardiomyocyte loss parallels the expansion of the interstitium and increased collagen content due to activation of fibroblasts [131, 132]. Documentation of cardiac fibrosis in the isolated obesity is challenging considering its common association with conditions affecting the cardiac interstitium (such as hypertension and DM). Effects of the activation of the renin–angiotensin–aldosterone system (RAAS) is consistently noted in the fibrotic myocardium of these patients. Several cellular pathways are involved in the fibrogenic program [133]. The link between an overactive TGF-β cascade and cardiac fibrosis is well-established and mediated through effects involving Smad signaling [134, 135]. TGF- β stimulates different other GFs (i.e. epidermal GF, insulin-like GF-1, growth differentiation factor-11 and CTGF) involved in the inhibition of myofibroblast apoptosis leading to a vicious circle of sustained and progressive fibrotic response [136]. The altered adipokine balance also play a role in cardiac fibrosis and dysfunction. Impaired leptin/adiponectin ratio was implicated in the pathogenesis of cardiac remodeling in obesity and metabolic dysfunction being a marker of inflammation [137]. Elevated circulating leptin levels were associated with left ventricular hypertrophy and fibrosis [138, 139]. On the contrary, adiponectin exerts anti-fibrotic and anti-inflammatory effects on cardiac fibroblasts, presumably mediated by PPAR-α activation [140, 141]. OPN has been widely associated with cardiac remodeling in both experimental and clinical studies [142, 143].
Although not listed among adipokines, neprilysin is largely expressed on the surface of mature adipocytes in people with obesity [144]. This molecule degrades endogenous natriuretic peptides increasing renal sodium reabsorption, aldosterone secretion from the adrenal gland, cardiac inflammation and fibrosis. In subjects with obesity and HFpEF soluble neprilysin levels and its inhibition decreased ventricular overload and improved LA overfilling [145].
Matricellular proteins are upregulated in remodeled hearts and regulate inflammatory, fibrotic and angiogenic responses [146]. Thrombospondins (TSP), tenascins, Cilp-1, secreted protein acidic and rich in cysteine (SPARC), osteopontin and members of the CCN family are involved in a variety of cardiac pathophysiologic conditions such as MI, cardiac hypertrophy, aging, diabetic cardiomyopathy and valvular disease. TSP-1 is the best-characterized matricellular protein in obesity, DM and MetS and it is potently induced by hyperglycemia [147]. The role of TSP-1 in cardiac remodeling was largely explored in clinical and preclinical studies, confirming its regulatory effect in fibrotic response of injured myocardium, deposition of collagen and angiogenesis [148-150]. Accordingly, such mediator is increasingly seen as a potential target for novel drugs in this context (Fig. 1).
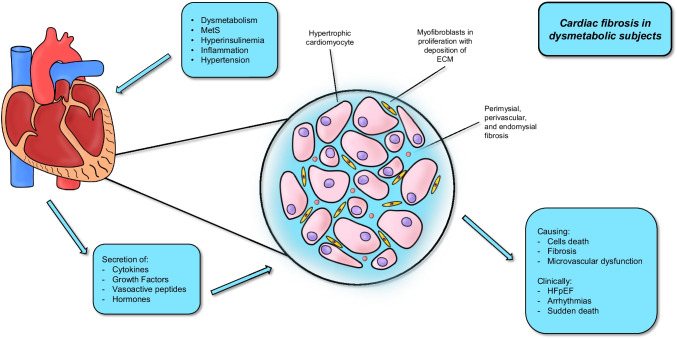
Fig. 1.
Cardiac fibrosis in dysmetabolic subjects. Patients with dysmetabolism are at higher risk of developing cardiac fibrosis. The long-term exposure to inflammatory, oxidative, and hyper-insulinemic environment causes the secretion of several molecules that concur in causing cardiac fibrosis. Microscopically, this process causes cells deaths, microvascular damages, and deposition of excessive extracellular matrix. Consequently, patients frequently experience heart failure, especially HFpEF, eventually arrhythmias, and even sudden death. HFpEF, heart failure with preserved ejection fraction; TGF-β, transforming growth factor beta; TSP-1, tronbospondin-1
Ectopic adipose tissue
Obesity-related vascular dysfunction is not only characterized by increased collagen deposition within the vascular wall and progressive arterial thickening but also by perivascular fat accumulation and inflammatory infiltrate [151]. Perivascular adipose tissue (PVAT) is located around most large blood vessels close to the vasculature and direct contact with the adventitia, providing mechanical protection and regulation of blood vessel tone via paracrine and vasocrine pathways [152, 153]. PVAT’s phenotype is heterogeneous and strongly location-dependent [154, 155]. In lean individuals, PVAT is mostly thermogenic brown and beige, located in the cervical, supraclavicular, axillary, paraspinal, renal and epicardial regions [156, 157]. Instead, the abdominal aorta and mesenteric vasculature are surrounded by white adipocytes, also found in visceral and subcutaneous adipose depots [158]. Functional PVAT secrets a number of adipokines (i.e. adiponectin and angiotensin 1–7) with antithrombotic and vasodilating effect on the vasculature [159, 160]. Moreover, PVAT is populated with different immune cells important for vascular homeostasis (i.e. regulatory T-cells) [161]. Obesity induces changes in the vasoactive factors in which the beneficial paracrine effect of PVAT is shifted to a pro-oxidant, pro-inflammatory, contractile and trophic environment [162]. Furthermore, the dysfunctional PVAT promote endothelial dysfunction, atherogenesis, vascular IR, impaired relaxation, and vascular stiffness. Quite different from PVAT, the interest to the epicardial one (EAT) has grown rapidly in the past decade after the discovery of its role in physiological and pathological modulation of coronary homeostasis. EAT is located on the surface of the myocardium in direct contact with coronaries and accounts for ≈5% to 20% of the heart weight [163]. Age, WC, ethnicity, and cardiac mass are independent determinants of EAT volume [164]. Of interest, EAT volume is a known risk factor for CAD, HFpEF and AF [165]. Specifically, EAT thickness has been correlated with the presence of high-risk/unstable coronary plaques [166] and coronary microvascular impairment [167, 168]. Similarly to PVAT, EAT releases factors (i.e. adiponectin, leptin omentin-1, nitric oxide, palmitic acid methyl ester prostacyclin) and cytokines that affect both vascular and myocardial homeostasis through paracrine and vasocrine pathways [169]. Recent studies focused on the role of EAT-released exosomes, through which EAT carries lipids, proteins, ribonucleic acids (RNAs), and microRNAs, facilitating intercellular signaling. According to these studies, EAT’s exosomes may be implicated in a number of CVD such as MI, adverse cardiac remodeling and atrial fibrillation (AF) and are currently investigated for their potential role in modulation of myocardium healing [170, 171].
Although not close to myocardial tissue, ectopic fat accumulation in the liver, skeletal muscle, and kidney belong to the central tenets of adiposopathy [172]. Macrovesicular steatosis involving more than 5% of hepatocytes is considered the cut-off point triggering a multiple-hit cascade is mainly characterized by lipotoxicity, but would also include mitochondrial dysfunction, endoplasmic reticulum stress, hypoxia. Those mechanisms would include cytokine unbalance, hypothalamic signaling modifications and changes in microbiota. Although far from myocardial tissue, non-alcoholic fatty liver disease has been associated with right ventricular dysfunction and right bundle branch block, AF and QTc prolongation [173]. Although less is known about other ectopic fat depots, increasing data are describing those within skeletal muscle. They are highly expressed in diabetic patients and associated with cardiovascular risk and poor outcome after cardiovascular events [174]. Similarly, peri-renal fat has been demonstrated an index of sub-clinical atherosclerosis.
Obesity phenotypes and cardiovascular risk
Obesity phenotypes have been shown to impact on CV diseases differently (Table 4 and Fig. 2). For coronary vascular and microvascular disease risk increases in MUO is proportional to the number of MetS defining criteria (hypertension, dyslipidemia, glucose intolerance and the degree of WC) [175, 176]. Direct negative effects of energetic dysmetabolism related to MUO and NUNW on cardiac structure are diverse and fall within the broad family of metabolic cardiomyopathies [177]. The hallmark of this condition is the development of left ventricle hypertrophy (LVH), independently related to the predominance of obesity, hypertension, and diabetes [178, 179]. The pathway from LVH to overt HF is complex and still partially unexplored, despite LVH being clearly recognized as an independent predictor of CV mortality [180], stroke, and renal outcomes [181]. Increased left ventricle stiffness and mass impairs the relaxation phase of the cardiac cycle leading to diastolic dysfunction, potentially leading to HF with preserved ejection fraction (HFpEF) [182]. To be noted, HF with reduced ejection fraction (HFrEF) is reported less frequently in patients with MUO and MUNW, and mostly associates with acute CV events (e.g., acute MI) [183]. Such negative structural and energetic remodeling is—together with inflammation and neuro-hormonal activation—a well-established substrate for arrhythmias [184]. In MUO, cardiac arrhythmias are frequent and precipitated by several co-factors including hypoxia, hypercapnia, electrolyte imbalances due to diuretic therapy, CAD and obstructive sleep apnea [185]. AF is the most common sustained cardiac arrhythmia diagnosed in individuals with obesity being an important determinant of stroke, HF, MI, dementia, and death in such population [186]. Of interest, positive correlations were found between the cumulative metabolic affliction and the risk of incident AF [187]. Of paramount, DM and hypertension are well-known independent risk factor of AF as well as criteria of the CHA2DS2-VASc-score [188]. As for the relationship between elevated TG and the risk of AF, reports remain controversial. While the Multi-Ethnic Study of Atherosclerosis (MESA) and the Framingham Heart Study (FHS) reported an association between hypertriglyceridemia and AF [189], this was not confirmed by the Niigata Preventive Medicine Study and by post-hoc analysis from the ARIC study [187, 190]. Obesity has been identified as the most common nonischemic cause of SCD [191]. Indeed, its association with SCD is well established [192] and every 5-unit increment in BMI indeed confers a 16% higher risk of SCD [193]. Cardiac fibrosis due to LVH, QRS fragmentation, QT prolongation, premature ventricular complexes, autonomic imbalance and increased EAT [194, 195] may explain the greater risk of ventricular tachycardia/ventricular fibrillation in such population [196].
Table 4.
Summary of studies linking cardiometabolic disease with different obesity phenotypes
| MUNW | MHO | MUO | SO | |
|---|---|---|---|---|
| MetS | – |
↑ risk insulin resistance ↑ risk hyper-TAG ↑ risk low HDL ↑ risk hypertension vs. normal weight lean [217] |
– | |
| Atherosclerosis |
↑ vascular inflammation [203] ↑ PWV ↑ soft plaques [201] ↑ CACS 218vs. normal weight lean |
↑ peripheral microvascular dysfunction (PMID: 28,275,071) ↑ CACS [218] vs. normal weight lean ↑ cIMT [221] vs. MUNW < 60y old |
↑ peripheral microvascular dysfunction [222] ↑ cIMT [220] ↑ CACS [218] vs. normal weight lean ↑ cIMT [221] vs. MUNW < 60y old |
↑ arterial stiffness [223] ↑ CACS [224] vs. non-sarcopenic ↑ cIMT [225] vs. non-sarcopenic elderly |
| HF |
↑ LVsD ↑ risk [228] vs. normal weight lean ↑ risk [183] vs. normal weight lean post-menopausal woman ↑ LVH [178] vs. MHO |
vs. normal weight lean ↑ LVdD [231] vs. MUNW ↑ risk [230] over time = risk than normal weight lean in post-menopausal woman (PMID: 33775111) |
↑ risk [183] vs. normal weight lean post-menopausal woman |
vs. non-sarcopenic HFrEF |
| AF |
↑ risk [228] vs. normal weight lean |
vs. normal weight lean ↓ risk [236] vs. MUO |
↑ risk [186] vs. normal weight lean |
↑ risk [225] vs. non-sarcopenic elderly |
| CV events/mortality |
↑ risk [204, 230, 237] vs. normal weight lean ↑ risk [238] vs. obese (MHO/MUO) |
↑ risk [199, 229, 237, 239, 240] vs. normal weight lean vs. MUNW |
vs. normal weight lean |
vs. non-sarcopenic HF and elderly ↑ risk [213] vs. non-sarcopenic after STEMI ↑ MI risk vs. non-sarcopenic elderly |
Waist circumference categorized as normal (men < 102 cm and women < 88 cm) or high (men ≥ 102 cm and women ≥ 88 cm). Visceral adipose tissue and lean mass are non-standardized measures. Metabolic abnormalities refer to the metabolic syndrome defining criteria
BMI body mass index, MONW metabolically obese normal weight, NOW normal weight obese, MHO metabolically healthy obese, MO metabolically obese, SO sarcopenic obese
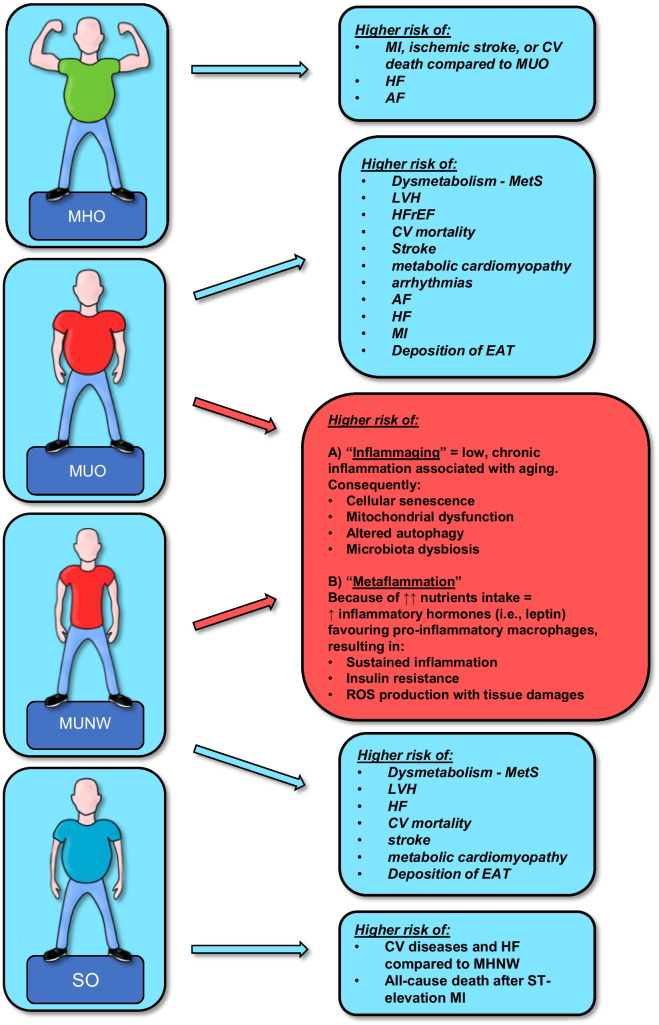
Fig. 2.
Obesity phenotypes and cardiovascular risk. This figure summarizes the close relationship between the different obesity phenotypes and the CV risk. AF, atrial fibrillation; CV, cardiovascular; EAT, epicardial adipose tissue; HF, heart failure; LVH, left ventricular hypertrophy; MetS, metabolic syndrome; MHO, metabolically healthy obese; MI, myocardial infarction; MUNW, metabolically unhealthy normal weight; MUO, metabolically unhealthy obese; ROS, reactive oxygen species; SO, sarcopenic obese
Few studies explored and compared the different mechanisms involved in the development of CV disease (CVD) in MHO and MUNW with respect to MUO and metabolically healthy individuals. Several studies suggested MHO as a pre-MUO condition with an intermediate risk of CVD between MUO and the healthy phenotype [197, 198]. However, this relationship may vary depending on the definition of MHO, the lack of adjustment for some confounding factors such as age, sex or a history of smoking, and the lack of separate analyses of the different subtypes of incident CV events. Individuals with MHO do not appear to carry a higher risk of MI, ischemic stroke, or CV death than healthy individuals. On the opposite, they show an increased risk HF and AF [199]. Similarly, in a nationwide analysis conducted in South Korea, Lee et al. reported a non-increased risk of ischemic stroke in MHO individuals [200].
On the contrary, MUNW is historically defined as a “fat mass disease” due to its higher risk of developing MetS and CVD despite normal weight [68]. Several large studies suggested the absence of correlation between normal weight and unhealthy status in patients with CV events, pointing out the possible role of other risk factors. MUNW individuals carry a higher incidence of subclinical atherosclerosis assessed by coronary computed tomography angiography as compared with healthy individuals [201]. Moreover, MUNW associates with soft atherosclerotic plaques [202] and subclinical vascular inflammation [203], known predictors of plaque rupture and ischemic events. The characterization of CV risk in such patients is far from being yet compete. Few clinical studies explored the incidence of CVD in this subgroup of obesity, reporting an increased risk of myocardial infarction in Chines [204] and Mexican American [205] populations. Of interest, a single study evaluated the incidence of HF in MUNW compared with MHO so far, reporting a twofold risk over 6 years [206]. Regarding AF, MUNW carries twofold increased risk as compared with healthy people or to MHO/MUO individuals [207].
Sarcopenia may promote atherogenesis due to relative fat mass increase in response to loss of muscle mass and replacement of myocytes by adipocytes. Hence, an even greater effect on CVD is expected for such a derangement with respect to obesity or sarcopenia alone [208]. Despite evidence on the relationships between SO and cardiovascular risk factors, its association with CVD is far from being clarified [209]. Cross-sectional studies have often yielded inconsistent results while prospective studies reported higher CV events in SO groups compared with the normal body composition groups only when SO was defined by using grip strength and WC criteria [86, 210]. In the Cardiovascular Health Study, a large prospective study of community-dwelling older men and women, SO based on WC and muscle strength was associated with the highest risk of CVD and HF over 8 years as compared with healthy subjects [211]. Few studies reported also higher incidence of myocardial infarction and AF, particularly in elderly [212]. Furthermore, patients with SO showed poor prognosis after STEMI, characterized by increased rate of all-cause death, MI, ischemic stroke, hospitalization for HF and unplanned revascularization [213]. The role of body composition in the development and progression of HF has recently received intense scrutiny [214]. In fact, in addition to cardiac dysfunction patients with HF also present abnormalities in body composition such as sarcopenia, SO and cachexia [215] with direct negative impact on their quality of life and survival. The FRAGILE-HF trial reported an high predictive role of SO in predicting mortality in adults with HF [216]. However, the lack of universally recognized diagnostic criteria remained a non-negligible factor which affects patient identification, reliable assessment of SO prevalence and outcomes. In 2022, the European Society for Clinical Nutrition and Metabolism (ESPEN) and the European Association for the Study of Obesity (EASO) provided the first consensus on SO definition, screening, diagnosis and staging [81]. Such consensus will help to uniform the selection criteria of SO patients in future studies.
Therapeutic management of obesity phenotypes
Preliminary results suggest that the different obesity phenotypes also have different responses to weight loss interventions, including diets, medications, devices, and surgery [245]. Yet, by now no randomized controlled trials on obesity treatment compared cardiometabolic outcomes among individuals with different obesity phenotypes. However, numerous studies support the need for a stratification effort in relation to the type of obesity. Weight reduction approaches are initially based on incremented on physical activity implementation and dietary strategies. In patients living with obesity, regular physical activity and aerobic exercise provide a moderately reduction of risk factors for CAD, including body fat and body mass, blood pressure, triglycerides, and improved lipoprotein profile. Furthermore, physical activity improved insulin sensitivity and endothelial function regardless of weight loss. As a result, regular physical activity associates with a sensible improvement of obesity-associated complications including CAd [246]. As for the diet, despite the scientific soundness of energy restriction approaches, the evidence shows only modest effects with high individual differences and short duration. The Mediterranean dietary pattern has been widely recognized for its protective effects on obesity, CVD and DM in addition to decreasing all-cause mortality [247]. In MUO phenotype, weight loss is the cornerstone of the clinical management. Body weight reduction together with a low glycemic index diet have several beneficial effects on serum glucose, LDL and blood pressure improving CVD risk [248]. In patients with MHO weight loss strategies should be recommended to preserve cardiometabolic risk profile and avoid MHO/MUO conversion. Several studies highlighted the importance of improving fat oxidation in patients with MHO to prevent MetS/DM [249]. Suitable approaches include increased aerobic activity, Mediterranean diet, and supplementation with catechins, capsaicin, or L-carnitin [250, 251]. Regarding SO, both dietary interventions and regular exercise are reccomended [252]. Aerobic activity, resistance training and their combination increase muscle protein synthesis in older adults despite age-related decreases in anabolic signaling [253]. Furthermore, physical activity leads to the recruitment of muscle satellite cells located between myofibers and their surrounding basal lamina [254] and downregulation of inflammatory biomarker [255]. SO patients should be advised to follow a hypocaloric high-protein diet (1.2–1.4 g/kg body weight reference/day) to preserve their muscle mass [256]. On the opposite, significant weight loss is not recommended for individuals with MUNW. These individuals have less fat mass than other phenotypes, therefore, therapeutic strategies should focus on improving metabolic health and their effects on different adipose tissue compartments and on lipid accumulation in the liver. As an example, the Mediterranean diet reduces the risk of CV events by about 30%, compared with a control diet, despite having little effect on bodyweight [257]. Anti-obesity drugs have historically faced multiple issues relating to study design, premature termination due to safety issues or failure to show CV benefit [258]. Furthermore, there is no evidence on obesity phenotype‐specific effects of such medications to date. Metabolic/bariatric surgery remains the most effective strategy to accomplish a significant (≥ 30%) and durable (at ≥ 5 years) weight loss leading to reduced all-cause and CV mortality and lower incidence of several CVD [259]. However, this approach remains strictly recommended only for patients with complicated severe obesity.
Conclusions
Guidelines from major European and American Societies highlight the importance of effective diagnosis and treatment of obesity in preventing CVD in clinical practice [8, 260, 261]. Obesity diagnosis may not be as simple as previously thought. Specifically, it cannot depend only on anthropometric parameters but should include a precise assessment of the metabolic status. Under this point of view different phenotypes of obesity have been proposed each one with specific effects on the CV system and with different responses to anti-obesity interventions. The current lack of standardized definitions reflects on a general paucity of experimental evidence impacting on the daily ability to provide personalized prescriptions to patients living with obesity. Such a complexity requires a multidisciplinary approach including specialists in obesity medicine, internal medicine, cardiology, psychology, as well as dieticians, family doctors, and bariatric surgeons. Accordingly, the therapeutic management of adiposopathy and its CV sequelae should be based on combination approaches encompassing surgery, pharmacotherapy, and lifestyle interventions.
Acknowledgements
none
Author contribution
AP and LL drafted the first version of the manuscript. FC drafted the tables. AT drafted the figures. All authors conceived, revised and approved the manuscript.
Funding
Open access funding provided by Università degli Studi di Genova within the CRUI-CARE Agreement.
Declarations
Ethical statement
Financial or non-financial interests: LL is coinventor on the International Patent WO/2020/226993 filed in April 2020. The patent relates to the use of antibodies which specifically bind IL-1α to reduce various sequelae of ischemia–reperfusion injury to the central nervous system. LL reports speaker fees outside of this work from Daichi-Sankyo.
Ethical statement
N/A.
Informed consent
N/A.
Conflict of interest
All other authors report no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Catalan V, Aviles-Olmos I, Rodriguez A, Becerril S, Fernandez-Formoso JA, Kiortsis D, et al. Time to consider the "Exposome Hypothesis" in the development of the obesity pandemic. Nutrients 2022;14(8). [DOI] [PMC free article] [PubMed]
- 2.Trends in adult body-mass index in 200 countries from 1975 to 2014. A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016;387(10026):1377–1396. [DOI] [PMC free article] [PubMed]
- 3.Zorena K, Jachimowicz-Duda O, Ślęzak D, Robakowska M, Mrugacz M. Adipokines and obesity. Potential link to metabolic disorders and chronic complications. Int J Mol Sci. 2020;21(10). [DOI] [PMC free article] [PubMed]
- 4.Lau WB, Ohashi K, Wang Y, Ogawa H, Murohara T, Ma XL, et al. Role of Adipokines in cardiovascular disease. Circ J. 2017;81(7):920–928. doi: 10.1253/circj.CJ-17-0458. [DOI] [PubMed] [Google Scholar]
- 5.Frasca D, Blomberg BB, Paganelli R. Aging, Obesity, and Inflammatory Age-Related Diseases. Front Immunol. 2017;8:1745. doi: 10.3389/fimmu.2017.01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. 2010;2010:535918. doi: 10.1155/2010/535918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 8.Powell-Wiley TM, Poirier P, Burke LE, Despres JP, Gordon-Larsen P, Lavie CJ, et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2021;143(21):e984–e1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vecchie A, Dallegri F, Carbone F, Bonaventura A, Liberale L, Portincasa P, et al. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur J Intern Med. 2018;48:6–17. doi: 10.1016/j.ejim.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Pujia R, Tarsitano MG, Arturi F, De Lorenzo A, Lenzi A, Pujia A, et al. Advances in Phenotyping Obesity and in Its Dietary and Pharmacological Treatment: A Narrative Review. Front Nutr. 2022;9:804719. doi: 10.3389/fnut.2022.804719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yarnoz-Esquiroz P, Olazaran L, Aguas-Ayesa M, Perdomo CM, Garcia-Goni M, Silva C, et al. 'Obesities': Position statement on a complex disease entity with multifaceted drivers. Eur J Clin Invest. 2022;52(7):e13811. doi: 10.1111/eci.13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16(3):177–189. doi: 10.1038/s41574-019-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Jimenez F, Almahmeed W, Bays H, Cuevas A, Di Angelantonio E, le Roux CW, et al. Obesity and cardiovascular disease: mechanistic insights and management strategies. A joint position paper by the World Heart Federation and World Obesity Federation. Eur J Prev Cardiol. 2022;29(17):2218–2237. [DOI] [PubMed]
- 14.Lopez-Jimenez F, Almahmeed W, Bays H, Cuevas A, Di Angelantonio E, le Roux CW, et al. Obesity and cardiovascular disease: mechanistic insights and management strategies. A joint position paper by the World Heart Federation and World Obesity Federation. Eur J Prev Cardiol. 2022. [DOI] [PubMed]
- 15.Carbone F. CardioMetabolic medicine, one more last step forward. Eur Heart J. 2022;43(20):1895–1896. doi: 10.1093/eurheartj/ehab713. [DOI] [PubMed] [Google Scholar]
- 16.Cornier M-A, Després J-P, Davis N, Grossniklaus DA, Klein S, Lamarche B, et al. Assessing Adiposity. Circulation. 2011;124(18):1996–2019. doi: 10.1161/CIR.0b013e318233bc6a. [DOI] [PubMed] [Google Scholar]
- 17.Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE, et al. The impact of obesity on the short-term andlong-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39(4):578–584. doi: 10.1016/S0735-1097(01)01802-2. [DOI] [PubMed] [Google Scholar]
- 18.Carbone S, Canada JM, Billingsley HE, Siddiqui MS, Elagizi A, Lavie CJ. Obesity paradox in cardiovascular disease: where do we stand? Vasc Health Risk Manag. 2019;15:89–100. doi: 10.2147/VHRM.S168946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 20.Pocock SJ, Ariti CA, McMurray JJV, Maggioni A, Køber L, Squire IB, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34(19):1404–1413. doi: 10.1093/eurheartj/ehs337. [DOI] [PubMed] [Google Scholar]
- 21.Cepeda-Valery B, Chaudhry K, Slipczuk L, Pressman GS, Figueredo VM, Lavie CJ, et al. Association between obesity and severity of coronary artery disease at the time of acute myocardial infarction: Another piece of the puzzle in the “obesity paradox”. Int J Cardiol. 2014;176(1):247–249. doi: 10.1016/j.ijcard.2014.06.061. [DOI] [PubMed] [Google Scholar]
- 22.Bucholz EM, Beckman AL, Krumholz HA, Krumholz HM. Excess weight and life expectancy after acute myocardial infarction: The obesity paradox reexamined. Am Heart J. 2016;172:173–181. doi: 10.1016/j.ahj.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan X-F, Shi J-X, Chen, Meng H. Prolonged and intensive medication use are associated with the obesity paradox after percutaneous coronary intervention: A systematic review and meta-analysis of 12 studies. BMC Cardiovascular Disorders 2016;16(1):125. [DOI] [PMC free article] [PubMed]
- 24.Li C-w, Yu K, Shyh-Chang N, Jiang Z, Liu T, Ma S, et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J Cachexia Sarcopenia Muscle 2022;13(2):781–794. [DOI] [PMC free article] [PubMed]
- 25.Sattar N, Welsh P. The obesity paradox in secondary prevention: a weighty intervention or a wait for more evidence? Eur Heart J. 2020;41(28):2678–2680. doi: 10.1093/eurheartj/ehaa398. [DOI] [PubMed] [Google Scholar]
- 26.Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk—a review of the literature. Eur J Clin Nutr. 2010;64(1):16–22. doi: 10.1038/ejcn.2009.68. [DOI] [PubMed] [Google Scholar]
- 27.Hu G, Jousilahti P, Antikainen R, Katzmarzyk PT, Tuomilehto J. Joint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio on the risk of heart failure. Circulation. 2010;121(2):237–244. doi: 10.1161/CIRCULATIONAHA.109.887893. [DOI] [PubMed] [Google Scholar]
- 28.Hayajneh AA, Alhusban IM, Rababa M. The role of traditional obesity parameters in predicting the number of stenosed coronary arteries (≥ 60%) among patients undergoing cardiac catheterization. Sci Rep. 2022;12(1):13830. doi: 10.1038/s41598-022-17517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medina-Inojosa JR, Batsis JA, Supervia M, Somers VK, Thomas RJ, Jenkins S, et al. Relation of Waist-Hip Ratio to Long-Term Cardiovascular Events in Patients With Coronary Artery Disease. Am J Cardiol. 2018;121(8):903–909. doi: 10.1016/j.amjcard.2017.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bays H. Central obesity as a clinical marker of adiposopathy; increased visceral adiposity as a surrogate marker for global fat dysfunction. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):345–351. doi: 10.1097/MED.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fruhbeck G, Busetto L, Dicker D, Yumuk V, Goossens GH, Hebebrand J, et al. The ABCD of Obesity: An EASO Position Statement on a Diagnostic Term with Clinical and Scientific Implications. Obes Facts. 2019;12(2):131–136. doi: 10.1159/000497124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez A, Becerril S, Hernandez-Pardos AW, Fruhbeck G. Adipose tissue depot differences in adipokines and effects on skeletal and cardiac muscle. Curr Opin Pharmacol. 2020;52:1–8. doi: 10.1016/j.coph.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Fahed G, Aoun L, Bou Zerdan M, Allam S, Bou Zerdan M, Bouferraa Y, et al. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int J Mol Sci. 2022;23(2). [DOI] [PMC free article] [PubMed]
- 34.Neeland IJ, Poirier P, Despres JP. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation. 2018;137(13):1391–1406. doi: 10.1161/CIRCULATIONAHA.117.029617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinonen S, Saarinen L, Naukkarinen J, Rodriguez A, Fruhbeck G, Hakkarainen A, et al. Adipocyte morphology and implications for metabolic derangements in acquired obesity. Int J Obes (Lond) 2014;38(11):1423–1431. doi: 10.1038/ijo.2014.31. [DOI] [PubMed] [Google Scholar]
- 36.Stern JH, Rutkowski JM, Scherer PE. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016;23(5):770–784. doi: 10.1016/j.cmet.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest. 2017;127(1):74–82. doi: 10.1172/JCI88883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghantous CM, Azrak Z, Hanache S, Abou-Kheir W, Zeidan A. Differential Role of Leptin and Adiponectin in Cardiovascular System. International Journal of Endocrinology. 2015;2015:534320. doi: 10.1155/2015/534320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buechler C, Feder S, Haberl EM, Aslanidis C. Chemerin Isoforms and Activity in Obesity. Int J Mol Sci. 2019;20(5). [DOI] [PMC free article] [PubMed]
- 40.Helfer G, Wu QF. Chemerin: a multifaceted adipokine involved in metabolic disorders. J Endocrinol. 2018;238(2):R79–r94. doi: 10.1530/JOE-18-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jialal I, Devaraj S, Kaur H, Adams-Huet B, Bremer AA. Increased chemerin and decreased omentin-1 in both adipose tissue and plasma in nascent metabolic syndrome. J Clin Endocrinol Metab. 2013;98(3):E514–E517. doi: 10.1210/jc.2012-3673. [DOI] [PubMed] [Google Scholar]
- 42.Wang D, Yuan GY, Wang XZ, Jia J, Di LL, Yang L, et al. Plasma chemerin level in metabolic syndrome. Genet Mol Res. 2013;12(4):5986–5991. doi: 10.4238/2013.November.26.8. [DOI] [PubMed] [Google Scholar]
- 43.Chu SH, Lee MK, Ahn KY, Im JA, Park MS, Lee DC, et al. Chemerin and adiponectin contribute reciprocally to metabolic syndrome. PLoS ONE. 2012;7(4):e34710. doi: 10.1371/journal.pone.0034710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabhi N, Desevin K, Belkina AC, Tilston-Lunel A, Varelas X, Layne MD, et al. Obesity-induced senescent macrophages activate a fibrotic transcriptional program in adipocyte progenitors. Life Sci Alliance 2022;5(5). [DOI] [PMC free article] [PubMed]
- 45.Karampatsou SI, Paltoglou G, Genitsaridi SM, Kassari P, Charmandari E. The Effect of a Comprehensive Life-Style Intervention Program of Diet and Exercise on Four Bone-Derived Proteins, FGF-23, Osteopontin, NGAL and Sclerostin, in Overweight or Obese Children and Adolescents. Nutrients 2022;14(18). [DOI] [PMC free article] [PubMed]
- 46.Carbone F, Montecucco F. Osteopontin in CardioMetabolic Medicine: A Risk Stratification Biomarker with Future Therapeutic Implication. Curr Med Chem. 2022;29(25):4314–4316. doi: 10.2174/0929867329666211228113716. [DOI] [PubMed] [Google Scholar]
- 47.Kim JI, Huh JY, Sohn JH, Choe SS, Lee YS, Lim CY, et al. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Mol Cell Biol. 2015;35(10):1686–1699. doi: 10.1128/MCB.01321-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 49.Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48(6):1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 50.Unger RH, Zhou YT. Lipotoxicity of beta-cells in obesity and in other causes of fatty acid spillover. Diabetes. 2001;50(Suppl 1):S118–S121. doi: 10.2337/diabetes.50.2007.S118. [DOI] [PubMed] [Google Scholar]
- 51.Farag YMK, Gaballa MR. Diabesity: an overview of a rising epidemic. Nephrol Dial Transplant. 2010;26(1):28–35. doi: 10.1093/ndt/gfq576. [DOI] [PubMed] [Google Scholar]
- 52.Iacobini C, Pugliese G, Blasetti Fantauzzi C, Federici M, Menini S. Metabolically healthy versus metabolically unhealthy obesity. Metabolism. 2019;92:51–60. doi: 10.1016/j.metabol.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 53.Badoud F, Perreault M, Zulyniak MA, Mutch DM. Molecular insights into the role of white adipose tissue in metabolically unhealthy normal weight and metabolically healthy obese individuals. FASEB J. 2015;29(3):748–758. doi: 10.1096/fj.14-263913. [DOI] [PubMed] [Google Scholar]
- 54.Zoghi G, Shahbazi R, Mahmoodi M, Nejatizadeh A, Kheirandish M. Prevalence of metabolically unhealthy obesity, overweight, and normal weight and the associated risk factors in a southern coastal region, Iran (the PERSIAN cohort study): A cross-sectional study. BMC Public Health. 2021;21(1):2011. doi: 10.1186/s12889-021-12107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goossens GH. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes Facts. 2017;10(3):207–215. doi: 10.1159/000471488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Vliet-Ostaptchouk JV, Nuotio ML, Slagter SN, Doiron D, Fischer K, Foco L, et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC Endocr Disord. 2014;14:9. doi: 10.1186/1472-6823-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126(10):1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 58.Stefan N, Häring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1(2):152–162. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
- 59.Naukkarinen J, Heinonen S, Hakkarainen A, Lundbom J, Vuolteenaho K, Saarinen L, et al. Characterising metabolically healthy obesity in weight-discordant monozygotic twins. Diabetologia. 2014;57(1):167–176. doi: 10.1007/s00125-013-3066-y. [DOI] [PubMed] [Google Scholar]
- 60.Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, et al. Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J Am Coll Cardiol 2017;70(12):1429–1437. [DOI] [PubMed]
- 61.Sattar N, Preiss D. Research digest: assessment and risks of obesity. Lancet Diabetes Endocrinol 2018;6(6). [DOI] [PubMed]
- 62.Mongraw-Chaffin M, Foster MC, Anderson CAM, Burke GL, Haq N, Kalyani RR, et al. Metabolically Healthy Obesity, Transition to Metabolic Syndrome, and Cardiovascular Risk. J Am Coll Cardiol. 2018;71(17):1857–1865. doi: 10.1016/j.jacc.2018.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou Z, Macpherson J, Gray SR, Gill JMR, Welsh P, Celis-Morales C, et al. Are people with metabolically healthy obesity really healthy? A prospective cohort study of 381,363 UK Biobank participants. Diabetologia. 2021;64(9):1963–1972. doi: 10.1007/s00125-021-05484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Csige I, Ujvárosy D, Szabó Z, Lőrincz I, Paragh G, Harangi M, et al. The Impact of Obesity on the Cardiovascular System. J Diabetes Res. 2018;2018:3407306. doi: 10.1155/2018/3407306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kotsis V, Martinez F, Trakatelli C, Redon J. Impact of Obesity in Kidney Diseases. Nutrients 2021;13(12). [DOI] [PMC free article] [PubMed]
- 66.Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2009;31(6):737–746. doi: 10.1093/eurheartj/ehp487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hyun YJ, Koh SJ, Chae JS, Kim JY, Kim OY, Lim HH, et al. Atherogenecity of LDL and Unfavorable Adipokine Profile in Metabolically Obese. Normal-weight Woman Obesity. 2008;16(4):784–789. doi: 10.1038/oby.2007.127. [DOI] [PubMed] [Google Scholar]
- 68.De Lorenzo A, Soldati L, Sarlo F, Calvani M, Di Lorenzo N, Di Renzo L. New obesity classification criteria as a tool for bariatric surgery indication. World J Gastroenterol. 2016;22(2):681–703. doi: 10.3748/wjg.v22.i2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. American association of clinical endocrinologists and american college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 70.Jakicic JM, Clark K, Coleman E, Donnelly JE, Foreyt J, Melanson E, et al. American college of sports medicine position stand. Appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2001;33(12):2145–56. [DOI] [PubMed]
- 71.Batsis JA, Mackenzie TA, Lopez-Jimenez F, Bartels SJ. Normal-Weight Obesity and Disability in Older Adults: Data from the National Health and Nutrition Examination Study 1999–2004. J Am Geriatr Soc. 2016;64(6):1367–1368. doi: 10.1111/jgs.14157. [DOI] [PubMed] [Google Scholar]
- 72.Wijayatunga NN, Dhurandhar EJ. Normal weight obesity and unaddressed cardiometabolic health risk—a narrative review. Int J Obes. 2021;45(10):2141–2155. doi: 10.1038/s41366-021-00858-7. [DOI] [PubMed] [Google Scholar]
- 73.Conus F, Allison DB, Rabasa-Lhoret R, St-Onge M, St-Pierre DH, Tremblay-Lebeau A, et al. Metabolic and behavioral characteristics of metabolically obese but normal-weight women. J Clin Endocrinol Metab. 2004;89(10):5013–5020. doi: 10.1210/jc.2004-0265. [DOI] [PubMed] [Google Scholar]
- 74.Stefan N, Schick F, Häring HU. Causes, Characteristics, and Consequences of Metabolically Unhealthy Normal Weight in Humans. Cell Metab. 2017;26(2):292–300. doi: 10.1016/j.cmet.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 75.Chen GC, Arthur R, Iyengar NM, Kamensky V, Xue X, Wassertheil-Smoller S, et al. Association between regional body fat and cardiovascular disease risk among postmenopausal women with normal body mass index. Eur Heart J. 2019;40(34):2849–2855. doi: 10.1093/eurheartj/ehz391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8(7):616–627. doi: 10.1016/S2213-8587(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 77.Stefan N, Häring HU, Hu FB, Schulze MB. Divergent associations of height with cardiometabolic disease and cancer: epidemiology, pathophysiology, and global implications. Lancet Diabetes Endocrinol. 2016;4(5):457–467. doi: 10.1016/S2213-8587(15)00474-X. [DOI] [PubMed] [Google Scholar]
- 78.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11(6):693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hong SH, Choi KM. Sarcopenic obesity, insulin resistance, and their implications in cardiovascular and metabolic consequences. Int J Mol Sci. 2020;21(2). [DOI] [PMC free article] [PubMed]
- 80.Febbraio MA, Pedersen BK. Who would have thought - myokines two decades on. Nat Rev Endocrinol. 2020;16(11):619–620. doi: 10.1038/s41574-020-00408-7. [DOI] [PubMed] [Google Scholar]
- 81.Donini LM, Busetto L, Bischoff SC, Cederholm T, Ballesteros-Pomar MD, Batsis JA, et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes Facts. 2022;15(3):321–335. doi: 10.1159/000521241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sakuma K, Yamaguchi A. Sarcopenic obesity and endocrinal adaptation with age. Int J Endocrinol. 2013;2013:204164. doi: 10.1155/2013/204164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Consitt LA, Clark BC. The Vicious Cycle of Myostatin Signaling in Sarcopenic Obesity: Myostatin Role in Skeletal Muscle Growth, Insulin Signaling and Implications for Clinical Trials. J Frailty Aging. 2018;7(1):21–27. doi: 10.14283/jfa.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo A, Li K, Xiao Q. Sarcopenic obesity: Myokines as potential diagnostic biomarkers and therapeutic targets? Exp Gerontol. 2020;139:111022. doi: 10.1016/j.exger.2020.111022. [DOI] [PubMed] [Google Scholar]
- 85.Guillet C, Masgrau A, Walrand S, Boirie Y. Impaired protein metabolism: interlinks between obesity, insulin resistance and inflammation. Obes Rev. 2012;13(Suppl 2):51–57. doi: 10.1111/j.1467-789X.2012.01037.x. [DOI] [PubMed] [Google Scholar]
- 86.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12(12):1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 87.Cawthon PM, Lui LY, Taylor BC, McCulloch CE, Cauley JA, Lapidus J, et al. Clinical Definitions of Sarcopenia and Risk of Hospitalization in Community-Dwelling Older Men: The Osteoporotic Fractures in Men Study. J Gerontol A Biol Sci Med Sci. 2017;72(10):1383–1389. doi: 10.1093/gerona/glw327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tian S, Xu Y. Association of sarcopenic obesity with the risk of all-cause mortality: A meta-analysis of prospective cohort studies. Geriatr Gerontol Int. 2016;16(2):155–166. doi: 10.1111/ggi.12579. [DOI] [PubMed] [Google Scholar]
- 89.Liu P, Hao Q, Hai S, Wang H, Cao L, Dong B. Sarcopenia as a predictor of all-cause mortality among community-dwelling older people: A systematic review and meta-analysis. Maturitas. 2017;103:16–22. doi: 10.1016/j.maturitas.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 90.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168(15):1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 91.Lavie CJ, Laddu D, Arena R, Ortega FB, Alpert MA, Kushner RF. Healthy Weight and Obesity Prevention: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72(13):1506–1531. doi: 10.1016/j.jacc.2018.08.1037. [DOI] [PubMed] [Google Scholar]
- 92.Dvorak RV, DeNino WF, Ades PA, Poehlman ET. Phenotypic characteristics associated with insulin resistance in metabolically obese but normal-weight young women. Diabetes. 1999;48(11):2210–2214. doi: 10.2337/diabetes.48.11.2210. [DOI] [PubMed] [Google Scholar]
- 93.Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47(5):699–713. doi: 10.2337/diabetes.47.5.699. [DOI] [PubMed] [Google Scholar]
- 94.National cholesterol education program expert panel on detection E, treatment of high blood cholesterol in A. Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002;106(25):3143–421. [PubMed]
- 95.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 96.Lee SH, Han K, Yang HK, Kim HS, Cho JH, Kwon HS, et al. A novel criterion for identifying metabolically obese but normal weight individuals using the product of triglycerides and glucose. Nutr Diabetes. 2015;5(4):e149. doi: 10.1038/nutd.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Amato MC, Giordano C. Visceral adiposity index: an indicator of adipose tissue dysfunction. Int J Endocrinol. 2014;2014:730827. doi: 10.1155/2014/730827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wakabayashi I, Daimon T. The, "cardiometabolic index" as a new marker determined by adiposity and blood lipids for discrimination of diabetes mellitus. Clin Chim Acta. 2015;438:274–278. doi: 10.1016/j.cca.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 99.Kahn HS. The "lipid accumulation product" performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. 2005;5:26. doi: 10.1186/1471-2261-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nuttall FQ. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr Today. 2015;50(3):117–128. doi: 10.1097/NT.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Lorenzo A, Martinoli R, Vaia F, Di Renzo L. Normal weight obese (NWO) women: an evaluation of a candidate new syndrome. Nutr Metab Cardiovasc Dis. 2006;16(8):513–523. doi: 10.1016/j.numecd.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 102.Kim JY, Han SH, Yang BM. Implication of high-body-fat percentage on cardiometabolic risk in middle-aged, healthy, normal-weight adults. Obesity (Silver Spring) 2013;21(8):1571–1577. doi: 10.1002/oby.20020. [DOI] [PubMed] [Google Scholar]
- 103.Correa-Rodriguez M, Gonzalez-Ruiz K, Rincon-Pabon D, Izquierdo M, Garcia-Hermoso A, Agostinis-Sobrinho C, et al. Normal-weight obesity is associated with increased cardiometabolic risk in young adults. Nutrients 2020;12(4). [DOI] [PMC free article] [PubMed]
- 104.Liberale L, Montecucco F, Tardif JC, Libby P, Camici GG. Inflamm-ageing: the role of inflammation in age-dependent cardiovascular disease. Eur Heart J. 2020;41(31):2974–2982. doi: 10.1093/eurheartj/ehz961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liberale L, Badimon L, Montecucco F, Luscher TF, Libby P, Camici GG. Inflammation, Aging, and Cardiovascular Disease: JACC Review Topic of the Week. J Am Coll Cardiol. 2022;79(8):837–847. doi: 10.1016/j.jacc.2021.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Puspitasari YM, Ministrini S, Schwarz L, Karch C, Liberale L, Camici GG. Modern Concepts in Cardiovascular Disease: Inflamm-Aging. Front Cell Dev Biol. 2022;10:882211. doi: 10.3389/fcell.2022.882211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liberale L, Bonetti NR, Puspitasari YM, Vukolic A, Akhmedov A, Diaz-Canestro C, et al. TNF-alpha antagonism rescues the effect of ageing on stroke: Perspectives for targeting inflamm-ageing. Eur J Clin Invest. 2021;51(11):e13600. doi: 10.1111/eci.13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vitale G, Salvioli S, Franceschi C. Oxidative stress and the ageing endocrine system. Nat Rev Endocrinol. 2013;9(4):228–240. doi: 10.1038/nrendo.2013.29. [DOI] [PubMed] [Google Scholar]
- 109.Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, et al. Immunosenescence and inflamm-aging as two sides of the same coin: Friends or Foes? Front Immunol. 2018;8. [DOI] [PMC free article] [PubMed]
- 110.Meessen ECE, Warmbrunn MV, Nieuwdorp M, Soeters MR. Human postprandial nutrient metabolism and low-grade inflammation: A narrative review. Nutrients 2019;11(12). [DOI] [PMC free article] [PubMed]
- 111.Ye J, Keller JN. Regulation of energy metabolism by inflammation: a feedback response in obesity and calorie restriction. Aging (Albany NY) 2010;2(6):361–368. doi: 10.18632/aging.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li Q, Hagberg CE, Silva Cascales H, Lang S, Hyvonen MT, Salehzadeh F, et al. Obesity and hyperinsulinemia drive adipocytes to activate a cell cycle program and senesce. Nat Med. 2021;27(11):1941–1953. doi: 10.1038/s41591-021-01501-8. [DOI] [PubMed] [Google Scholar]
- 113.Chung S, Lapoint K, Martinez K, Kennedy A, Boysen Sandberg M, McIntosh MK. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology. 2006;147(11):5340–5351. doi: 10.1210/en.2006-0536. [DOI] [PubMed] [Google Scholar]
- 114.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hubler MJ, Erikson KM, Kennedy AJ, Hasty AH. MFe(hi) adipose tissue macrophages compensate for tissue iron perturbations in mice. Am J Physiol Cell Physiol. 2018;315(3):C319–c329. doi: 10.1152/ajpcell.00103.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chawla A. Control of Macrophage Activation and Function by PPARs. Circ Res. 2010;106(10):1559–1569. doi: 10.1161/CIRCRESAHA.110.216523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schafer MJ, Zhang X, Kumar A, Atkinson EJ, Zhu Y, Jachim S, et al. The senescence-associated secretome as an indicator of age and medical risk. JCI Insight 2020;5(12). [DOI] [PMC free article] [PubMed]
- 118.Caserza L, Casula M, Elia E, Bonaventura A, Liberale L, Bertolotto M, et al. Serum osteopontin predicts glycaemic profile improvement in metabolic syndrome: A pilot study. Eur J Clin Invest. 2021;51(3):e13403. doi: 10.1111/eci.13403. [DOI] [PubMed] [Google Scholar]
- 119.Carbone F, Valente A, Perego C, Bertolotto M, Pane B, Spinella G, et al. Ficolin-2 serum levels predict the occurrence of acute coronary syndrome in patients with severe carotid artery stenosis. Pharmacol Res. 2021;166:105462. doi: 10.1016/j.phrs.2021.105462. [DOI] [PubMed] [Google Scholar]
- 120.Cani PD, Van Hul M, Lefort C, Depommier C, Rastelli M, Everard A. Microbial regulation of organismal energy homeostasis. Nat Metab. 2019;1(1):34–46. doi: 10.1038/s42255-018-0017-4. [DOI] [PubMed] [Google Scholar]
- 121.Liu BN, Liu XT, Liang ZH, Wang JH. Gut microbiota in obesity. World J Gastroenterol. 2021;27(25):3837–3850. doi: 10.3748/wjg.v27.i25.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Angelakis E, Armougom F, Million M, Raoult D. The relationship between gut microbiota and weight gain in humans. Future Microbiol. 2012;7(1):91–109. doi: 10.2217/fmb.11.142. [DOI] [PubMed] [Google Scholar]
- 123.Muscogiuri G, Cantone E, Cassarano S, Tuccinardi D, Barrea L, Savastano S, et al. Gut microbiota: a new path to treat obesity. International Journal of Obesity Supplements. 2019;9(1):10–19. doi: 10.1038/s41367-019-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Warmbrunn MV, Herrema H, Aron-Wisnewsky J, Soeters MR, Van Raalte DH, Nieuwdorp M. Gut microbiota: a promising target against cardiometabolic diseases. Expert Rev Endocrinol Metab. 2020;15(1):13–27. doi: 10.1080/17446651.2020.1720511. [DOI] [PubMed] [Google Scholar]
- 125.Bastin M, Andreelli F. The gut microbiota and diabetic cardiomyopathy in humans. Diabetes Metab. 2020;46(3):197–202. doi: 10.1016/j.diabet.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 126.Ng ACT, Delgado V, Borlaug BA, Bax JJ. Diabesity: the combined burden of obesity and diabetes on heart disease and the role of imaging. Nat Rev Cardiol. 2021;18(4):291–304. doi: 10.1038/s41569-020-00465-5. [DOI] [PubMed] [Google Scholar]
- 127.Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128(4):388–400. doi: 10.1161/CIRCULATIONAHA.113.001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, et al. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107(16):2096–2101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 129.Ivey MJ, Tallquist MD. Defining the Cardiac Fibroblast. Circ J. 2016;80(11):2269–2276. doi: 10.1253/circj.CJ-16-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hall C, Gehmlich K, Denning C, Pavlovic D. Complex Relationship Between Cardiac Fibroblasts and Cardiomyocytes in Health and Disease. J Am Heart Assoc. 2021;10(5):e019338. doi: 10.1161/JAHA.120.019338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gazoti Debessa CR, Mesiano Maifrino LB, Rodrigues de Souza R. Age related changes of the collagen network of the human heart. Mech Ageing Dev 2001;122(10):1049–58. [DOI] [PubMed]
- 132.Biernacka A, Frangogiannis NG. Aging and Cardiac Fibrosis. Aging Dis. 2011;2(2):158–173. [PMC free article] [PubMed] [Google Scholar]
- 133.Spoladore R, Falasconi G, Fiore G, Di Maio S, Preda A, Slavich M, et al. Cardiac fibrosis: emerging agents in preclinical and clinical development. Expert Opin Investig Drugs. 2021;30(2):153–166. doi: 10.1080/13543784.2021.1868432. [DOI] [PubMed] [Google Scholar]
- 134.Ma ZG, Yuan YP, Wu HM, Zhang X, Tang QZ. Cardiac fibrosis: new insights into the pathogenesis. Int J Biol Sci. 2018;14(12):1645–1657. doi: 10.7150/ijbs.28103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Saadat S, Noureddini M, Mahjoubin-Tehran M, Nazemi S, Shojaie L, Aschner M, et al. Pivotal role of TGF-β/Smad signaling in cardiac fibrosis: Non-coding RNAs as effectual players. Frontiers in Cardiovascular Medicine 2021;7. [DOI] [PMC free article] [PubMed]
- 136.Kulasekaran P, Scavone CA, Rogers DS, Arenberg DA, Thannickal VJ, Horowitz JC. Endothelin-1 and transforming growth factor-beta1 independently induce fibroblast resistance to apoptosis via AKT activation. Am J Respir Cell Mol Biol. 2009;41(4):484–493. doi: 10.1165/rcmb.2008-0447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fruhbeck G, Catalan V, Rodriguez A, Ramirez B, Becerril S, Salvador J, et al. Adiponectin-leptin Ratio is a Functional Biomarker of Adipose Tissue Inflammation. Nutrients 2019;11(2). [DOI] [PMC free article] [PubMed]
- 138.Perego L, Pizzocri P, Corradi D, Maisano F, Paganelli M, Fiorina P, et al. Circulating leptin correlates with left ventricular mass in morbid (grade III) obesity before and after weight loss induced by bariatric surgery: a potential role for leptin in mediating human left ventricular hypertrophy. J Clin Endocrinol Metab. 2005;90(7):4087–4093. doi: 10.1210/jc.2004-1963. [DOI] [PubMed] [Google Scholar]
- 139.Fukui A, Takahashi N, Nakada C, Masaki T, Kume O, Shinohara T, et al. Role of leptin signaling in the pathogenesis of angiotensin II-mediated atrial fibrosis and fibrillation. Circ Arrhythm Electrophysiol. 2013;6(2):402–409. doi: 10.1161/CIRCEP.111.000104. [DOI] [PubMed] [Google Scholar]
- 140.Fujita K, Maeda N, Sonoda M, Ohashi K, Hibuse T, Nishizawa H, et al. Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-alpha. Arterioscler Thromb Vasc Biol. 2008;28(5):863–870. doi: 10.1161/ATVBAHA.107.156687. [DOI] [PubMed] [Google Scholar]
- 141.Dadson K, Chasiotis H, Wannaiampikul S, Tungtrongchitr R, Xu A, Sweeney G. Adiponectin mediated APPL1-AMPK signaling induces cell migration, MMP activation, and collagen remodeling in cardiac fibroblasts. J Cell Biochem. 2014;115(4):785–793. doi: 10.1002/jcb.24722. [DOI] [PubMed] [Google Scholar]
- 142.Herum KM, Romaine A, Wang A, Melleby AO, Strand ME, Pacheco J, et al. Syndecan-4 Protects the Heart From the Profibrotic Effects of Thrombin-Cleaved Osteopontin. J Am Heart Assoc. 2020;9(3):e013518. doi: 10.1161/JAHA.119.013518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Christersson C, Baron T, Flachskampf F, Lindhagen L, Lindahl B, Siegbahn A. Screening for biomarkers associated with left ventricular function during follow-up after acute coronary syndrome. J Cardiovasc Transl Res. 2022. [DOI] [PMC free article] [PubMed]
- 144.Standeven KF, Hess K, Carter AM, Rice GI, Cordell PA, Balmforth AJ, et al. Neprilysin, obesity and the metabolic syndrome. Int J Obes (Lond) 2011;35(8):1031–1040. doi: 10.1038/ijo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Packer M. Leptin-Aldosterone-Neprilysin Axis. Circulation. 2018;137(15):1614–1631. doi: 10.1161/CIRCULATIONAHA.117.032474. [DOI] [PubMed] [Google Scholar]
- 146.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92(2):635–688. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Raman P, Krukovets I, Marinic TE, Bornstein P, Stenina OI. Glycosylation mediates up-regulation of a potent antiangiogenic and proatherogenic protein, thrombospondin-1, by glucose in vascular smooth muscle cells. J Biol Chem. 2007;282(8):5704–5714. doi: 10.1074/jbc.M610965200. [DOI] [PubMed] [Google Scholar]
- 148.Gonzalez-Quesada C, Cavalera M, Biernacka A, Kong P, Lee D-W, Saxena A, et al. Thrombospondin-1 Induction in the Diabetic Myocardium Stabilizes the Cardiac Matrix in Addition to Promoting Vascular Rarefaction Through Angiopoietin-2 Upregulation. Circ Res. 2013;113(12):1331–1344. doi: 10.1161/CIRCRESAHA.113.302593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang K, Li M, Yin L, Fu G, Liu Z. Role of thrombospondin-1 and thrombospondin-2 in cardiovascular diseases (Review) Int J Mol Med. 2020;45(5):1275–1293. doi: 10.3892/ijmm.2020.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bao Q, Zhang B, Suo Y, Liu C, Yang Q, Zhang K, et al. Intermittent hypoxia mediated by TSP1 dependent on STAT3 induces cardiac fibroblast activation and cardiac fibrosis. Elife 2020;9. [DOI] [PMC free article] [PubMed]
- 151.Koenen M, Hill MA, Cohen P, Sowers JR. Obesity, Adipose Tissue and Vascular Dysfunction. Circ Res. 2021;128(7):951–968. doi: 10.1161/CIRCRESAHA.121.318093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Britton KA, Fox CS. Perivascular adipose tissue and vascular disease. Clin Lipidol. 2011;6(1):79–91. doi: 10.2217/clp.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chang L, Garcia-Barrio MT, Chen YE. Perivascular Adipose Tissue Regulates Vascular Function by Targeting Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol. 2020;40(5):1094–1109. doi: 10.1161/ATVBAHA.120.312464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2009;29(10):1458–1464. doi: 10.1161/ATVBAHA.109.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Padilla J, Jenkins NT, Vieira-Potter VJ, Laughlin MH. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am J Physiol Regul Integr Comp Physiol. 2013;304(7):R543–R552. doi: 10.1152/ajpregu.00567.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360(15):1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 157.Zhang F, Hao G, Shao M, Nham K, An Y, Wang Q, et al. An Adipose Tissue Atlas: An Image-Guided Identification of Human-like BAT and Beige Depots in Rodents. Cell Metab. 2018;27(1):252–262.e3. doi: 10.1016/j.cmet.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kwok KH, Lam KS, Xu A. Heterogeneity of white adipose tissue: molecular basis and clinical implications. Exp Mol Med. 2016;48(3):e215. doi: 10.1038/emm.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lynch FM, Withers SB, Yao Z, Werner ME, Edwards G, Weston AH, et al. Perivascular adipose tissue-derived adiponectin activates BK(Ca) channels to induce anticontractile responses. Am J Physiol Heart Circ Physiol. 2013;304(6):H786–H795. doi: 10.1152/ajpheart.00697.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Numaguchi R, Furuhashi M, Matsumoto M, Sato H, Yanase Y, Kuroda Y, et al. Differential Phenotypes in Perivascular Adipose Tissue Surrounding the Internal Thoracic Artery and Diseased Coronary Artery. J Am Heart Assoc. 2019;8(2):e011147. doi: 10.1161/JAHA.118.011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gil-Ortega M, Stucchi P, Guzman-Ruiz R, Cano V, Arribas S, Gonzalez MC, et al. Adaptative nitric oxide overproduction in perivascular adipose tissue during early diet-induced obesity. Endocrinology. 2010;151(7):3299–3306. doi: 10.1210/en.2009-1464. [DOI] [PubMed] [Google Scholar]
- 163.Talman AH, Psaltis PJ, Cameron JD, Meredith IT, Seneviratne SK, Wong DT. Epicardial adipose tissue: far more than a fat depot. Cardiovasc Diagn Ther. 2014;4(6):416–429. doi: 10.3978/j.issn.2223-3652.2014.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Silaghi A, Piercecchi-Marti MD, Grino M, Leonetti G, Alessi MC, Clement K, et al. Epicardial adipose tissue extent: relationship with age, body fat distribution, and coronaropathy. Obesity (Silver Spring) 2008;16(11):2424–2430. doi: 10.1038/oby.2008.379. [DOI] [PubMed] [Google Scholar]
- 165.Lechner K, McKenzie AL, Kränkel N, Von Schacky C, Worm N, Nixdorff U, et al. High-Risk Atherosclerosis and Metabolic Phenotype: The Roles of Ectopic Adiposity, Atherogenic Dyslipidemia, and Inflammation. Metab Syndr Relat Disord. 2020;18(4):176–185. doi: 10.1089/met.2019.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tachibana M, Miyoshi T, Osawa K, Toh N, Oe H, Nakamura K, et al. Measurement of epicardial fat thickness by transthoracic echocardiography for predicting high-risk coronary artery plaques. Heart Vessels. 2016;31(11):1758–1766. doi: 10.1007/s00380-016-0802-5. [DOI] [PubMed] [Google Scholar]
- 167.Mahmoud I, Dykun I, Kärner L, Hendricks S, Totzeck M, Al-Rashid F, et al. Epicardial adipose tissue differentiates in patients with and without coronary microvascular dysfunction. Int J Obes. 2021;45(9):2058–2063. doi: 10.1038/s41366-021-00875-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sade LE, Eroglu S, Bozbaş H, Ozbiçer S, Hayran M, Haberal A, et al. Relation between epicardial fat thickness and coronary flow reserve in women with chest pain and angiographically normal coronary arteries. Atherosclerosis. 2009;204(2):580–585. doi: 10.1016/j.atherosclerosis.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 169.Xia N, Li H. The role of perivascular adipose tissue in obesity-induced vascular dysfunction. Br J Pharmacol. 2017;174(20):3425–3442. doi: 10.1111/bph.13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Del Campo CV, Liaw NY, Gunadasa-Rohling M, Matthaei M, Braga L, Kennedy T, et al. Regenerative potential of epicardium-derived extracellular vesicles mediated by conserved miRNA transfer. Cardiovasc Res. 2022;118(2):597–611. doi: 10.1093/cvr/cvab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Adamiak M, Sahoo S. Exosomes in Myocardial Repair: Advances and Challenges in the Development of Next-Generation Therapeutics. Mol Ther. 2018;26(7):1635–1643. doi: 10.1016/j.ymthe.2018.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ferrara D, Montecucco F, Dallegri F, Carbone F. Impact of different ectopic fat depots on cardiovascular and metabolic diseases. J Cell Physiol. 2019;234(12):21630–21641. doi: 10.1002/jcp.28821. [DOI] [PubMed] [Google Scholar]
- 173.Mantovani A. Nonalcoholic Fatty Liver Disease (NAFLD) and Risk of Cardiac Arrhythmias: A New Aspect of the Liver-heart Axis. J Clin Transl Hepatol. 2017;5(2):134–141. doi: 10.14218/JCTH.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ballin M, Nordstrom P, Niklasson J, Nordstrom A. Associations of Visceral Adipose Tissue and Skeletal Muscle Density With Incident Stroke, Myocardial Infarction, and All-Cause Mortality in Community-Dwelling 70-Year-Old Individuals: A Prospective Cohort Study. J Am Heart Assoc. 2021;10(9):e020065. doi: 10.1161/JAHA.120.020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mandviwala T, Khalid U, Deswal A. Obesity and Cardiovascular Disease: a Risk Factor or a Risk Marker? Curr Atheroscler Rep. 2016;18(5):21. doi: 10.1007/s11883-016-0575-4. [DOI] [PubMed] [Google Scholar]
- 176.Zhang X, Shu XO, Li H, Yang G, Xiang YB, Cai Q, et al. Visceral adiposity and risk of coronary heart disease in relatively lean Chinese adults. Int J Cardiol. 2013;168(3):2141–2145. doi: 10.1016/j.ijcard.2013.01.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Guertl B, Noehammer C, Hoefler G. Metabolic cardiomyopathies. Int J Exp Pathol. 2000;81(6):349–372. doi: 10.1046/j.1365-2613.2000.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang N, Chen Y, Guo X, Sun G, Dai D, Sun Y. Metabolic Abnormalities, But Not Metabolically Healthy Obesity, Are Associated with Left Ventricular Hypertrophy. Heart Lung Circ. 2017;26(3):251–257. doi: 10.1016/j.hlc.2016.06.1212. [DOI] [PubMed] [Google Scholar]
- 179.Genovesi S, Tassistro E, Giussani M, Lieti G, Patti I, Orlando A, et al. Association of obesity phenotypes with left ventricular mass index and left ventricular hypertrophy in children and adolescents. Front Endocrinol (Lausanne) 2022;13:1006588. doi: 10.3389/fendo.2022.1006588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.de Simone G, Izzo R, Chinali M, De Marco M, Casalnuovo G, Rozza F, et al. Does information on systolic and diastolic function improve prediction of a cardiovascular event by left ventricular hypertrophy in arterial hypertension? Hypertension. 2010;56(1):99–104. doi: 10.1161/HYPERTENSIONAHA.110.150128. [DOI] [PubMed] [Google Scholar]
- 181.Tsioufis C, Kokkinos P, Macmanus C, Thomopoulos C, Faselis C, Doumas M, et al. Left ventricular hypertrophy as a determinant of renal outcome in patients with high cardiovascular risk. J Hypertens. 2010;28(11):2299–2308. doi: 10.1097/HJH.0b013e32833d95fe. [DOI] [PubMed] [Google Scholar]
- 182.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53(13):1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cordola Hsu AR, Xie B, Peterson DV, LaMonte MJ, Garcia L, Eaton CB, et al. Metabolically Healthy/Unhealthy Overweight/Obesity Associations With Incident Heart Failure in Postmenopausal Women: The Women's Health Initiative. Circ Heart Fail. 2021;14(4):e007297. doi: 10.1161/CIRCHEARTFAILURE.120.007297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Diaz-Melean CM, Somers VK, Rodriguez-Escudero JP, Singh P, Sochor O, Llano EM, et al. Mechanisms of adverse cardiometabolic consequences of obesity. Curr Atheroscler Rep. 2013;15(11):364. doi: 10.1007/s11883-013-0364-2. [DOI] [PubMed] [Google Scholar]
- 185.Adams JP, Murphy PG. Obesity in anaesthesia and intensive care. Br J Anaesth. 2000;85(1):91–108. doi: 10.1093/bja/85.1.91. [DOI] [PubMed] [Google Scholar]
- 186.Feng T, Vegard M, Strand LB, Laugsand LE, Morkedal B, Aune D, et al. Metabolically Healthy Obesity and Risk for Atrial Fibrillation: The HUNT Study. Obesity (Silver Spring) 2019;27(2):332–338. doi: 10.1002/oby.22377. [DOI] [PubMed] [Google Scholar]
- 187.Watanabe H, Tanabe N, Watanabe T, Darbar D, Roden DM, Sasaki S, et al. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation. 2008;117(10):1255–1260. doi: 10.1161/CIRCULATIONAHA.107.744466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang A, Green JB, Halperin JL, Piccini JP., Sr Atrial Fibrillation and Diabetes Mellitus: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74(8):1107–1115. doi: 10.1016/j.jacc.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 189.Alonso A, Yin X, Roetker NS, Magnani JW, Kronmal RA, Ellinor PT, et al. Blood lipids and the incidence of atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis and the Framingham Heart Study. J Am Heart Assoc. 2014;3(5):e001211. doi: 10.1161/JAHA.114.001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chamberlain AM, Agarwal SK, Ambrose M, Folsom AR, Soliman EZ, Alonso A. Metabolic syndrome and incidence of atrial fibrillation among blacks and whites in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2010;159(5):850–856. doi: 10.1016/j.ahj.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hookana E, Junttila MJ, Puurunen VP, Tikkanen JT, Kaikkonen KS, Kortelainen ML, et al. Causes of nonischemic sudden cardiac death in the current era. Heart Rhythm. 2011;8(10):1570–1575. doi: 10.1016/j.hrthm.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 192.Chiuve SE, Sun Q, Sandhu RK, Tedrow U, Cook NR, Manson JE, et al. Adiposity throughout adulthood and risk of sudden cardiac death in women. JACC Clin Electrophysiol. 2015;1(6):520–528. doi: 10.1016/j.jacep.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Aune D, Schlesinger S, Norat T, Riboli E. Body mass index, abdominal fatness, and the risk of sudden cardiac death: a systematic review and dose-response meta-analysis of prospective studies. Eur J Epidemiol. 2018;33(8):711–722. doi: 10.1007/s10654-017-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Plourde B, Sarrazin JF, Nault I, Poirier P. Sudden cardiac death and obesity. Expert Rev Cardiovasc Ther. 2014;12(9):1099–1110. doi: 10.1586/14779072.2014.952283. [DOI] [PubMed] [Google Scholar]
- 195.Fuller B, Garland J, Anne S, Beh R, McNevin D, Tse R. Increased Epicardial Fat Thickness in Sudden Death From Stable Coronary Artery Atherosclerosis. Am J Forensic Med Pathol. 2017;38(2):162–166. doi: 10.1097/PAF.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 196.Sabbag A, Goldenberg I, Moss AJ, McNitt S, Glikson M, Biton Y, et al. Predictors and Risk of Ventricular Tachyarrhythmias or Death in Black and White Cardiac Patients: A MADIT-CRT Trial Substudy. JACC Clin Electrophysiol. 2016;2(4):448–455. doi: 10.1016/j.jacep.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 197.Yoon JW, Jung C-H, Kim M-K, Park HE, Park KS, Jang HC, et al. Influence of the definition of “metabolically healthy obesity” on the progression of coronary artery calcification. PLoS ONE. 2017;12(6):e0178741. doi: 10.1371/journal.pone.0178741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Opio J, Croker E, Odongo GS, Attia J, Wynne K, McEvoy M. Metabolically healthy overweight/obesity are associated with increased risk of cardiovascular disease in adults, even in the absence of metabolic risk factors: A systematic review and meta-analysis of prospective cohort studies. Obes Rev. 2020;21(12):e13127. doi: 10.1111/obr.13127. [DOI] [PubMed] [Google Scholar]
- 199.Fauchier G, Bisson A, Bodin A, Herbert J, Semaan C, Angoulvant D, et al. Metabolically healthy obesity and cardiovascular events: A nationwide cohort study. Diabetes Obes Metab. 2021;23(11):2492–2501. doi: 10.1111/dom.14492. [DOI] [PubMed] [Google Scholar]
- 200.Lee HJ, Choi EK, Lee SH, Kim YJ, Han KD, Oh S. Risk of ischemic stroke in metabolically healthy obesity: A nationwide population-based study. PLoS ONE. 2018;13(3):e0195210. doi: 10.1371/journal.pone.0195210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kim S, Kyung C, Park JS, Lee SP, Kim HK, Ahn CW, et al. Normal-weight obesity is associated with increased risk of subclinical atherosclerosis. Cardiovasc Diabetol. 2015;14:58. doi: 10.1186/s12933-015-0220-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Alexanderson E, Slomka P, Cheng V, Meave A, Saldaña Y, García-Rojas L, et al. Fusion of positron emission tomography and coronary computed tomographic angiography identifies fluorine 18 fluorodeoxyglucose uptake in the left main coronary artery soft plaque. J Nucl Cardiol. 2008;15(6):841–843. doi: 10.1007/BF03007367. [DOI] [PubMed] [Google Scholar]
- 203.Kang S, Kyung C, Park JS, Kim S, Lee SP, Kim MK, et al. Subclinical vascular inflammation in subjects with normal weight obesity and its association with body fat: an 18 F-FDG-PET/CT study. Cardiovasc Diabetol. 2014;13:70. doi: 10.1186/1475-2840-13-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xu Y, Li H, Wang A, Su Z, Yang G, Luo Y, et al. Association between the metabolically healthy obese phenotype and the risk of myocardial infarction: results from the Kailuan study. Eur J Endocrinol. 2018;179(6):343–352. doi: 10.1530/EJE-18-0356. [DOI] [PubMed] [Google Scholar]
- 205.Aung K, Lorenzo C, Hinojosa MA, Haffner SM. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. J Clin Endocrinol Metab. 2014;99:462–468. doi: 10.1210/jc.2013-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Voulgari C, Tentolouris N, Dilaveris P, Tousoulis D, Katsilambros N, Stefanadis C. Increased Heart Failure Risk in Normal-Weight People With Metabolic Syndrome Compared With Metabolically Healthy Obese Individuals. J Am Coll Cardiol. 2011;58(13):1343–1350. doi: 10.1016/j.jacc.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 207.Tsai SY, Chen HH, Hsu HY, Tsai MC, Hsu LY, Hwang LC, et al. Obesity phenotypes and their relationships with atrial fibrillation. PeerJ. 2021;9:e12342. doi: 10.7717/peerj.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Atkins JL, Wannamethee SG. The effect of sarcopenic obesity on cardiovascular disease and all-cause mortality in older people. Rev Clin Gerontol. 2015;25(2):86–97. doi: 10.1017/S0959259815000076. [DOI] [Google Scholar]
- 209.Atkins JL, Wannamathee SG. Sarcopenic obesity in ageing: cardiovascular outcomes and mortality. Br J Nutr. 2020;124(10):1102–1113. doi: 10.1017/S0007114520002172. [DOI] [PubMed] [Google Scholar]
- 210.Chin SO, Rhee SY, Chon S, Hwang YC, Jeong IK, Oh S, et al. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: The Korea national health and nutrition examination survey (KNHANES) from 2009. PLoS ONE. 2013;8(3):e60119. doi: 10.1371/journal.pone.0060119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Stephen WC, Janssen I. Sarcopenic-obesity and cardiovascular disease risk in the elderly. J Nutr Health Aging. 2009;13(5):460–466. doi: 10.1007/s12603-009-0084-z. [DOI] [PubMed] [Google Scholar]
- 212.Santana NM, Mendes RML, Silva NFD, Pinho CPS. Sarcopenia and sarcopenic obesity as prognostic predictors in hospitalized elderly patients with acute myocardial infarction. Einstein (Sao Paulo) 2019;17(4):eAO4632. [DOI] [PMC free article] [PubMed]
- 213.Sato R, Okada K, Akiyama E, Konishi M, Matsuzawa Y, Nakahashi H, et al. Impact of sarcopenic obesity on long-term clinical outcomes after ST-segment elevation myocardial infarction. Atherosclerosis. 2021;335:135–141. doi: 10.1016/j.atherosclerosis.2021.08.038. [DOI] [PubMed] [Google Scholar]
- 214.Carbone S, Lavie CJ, Arena R. Obesity and Heart Failure: Focus on the Obesity Paradox. Mayo Clin Proc. 2017;92(2):266–279. doi: 10.1016/j.mayocp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 215.Carbone S, Billingsley HE, Rodriguez-Miguelez P, Kirkman DL, Garten R, Franco RL, et al. Lean Mass Abnormalities in Heart Failure: The Role of Sarcopenia, Sarcopenic Obesity, and Cachexia. Curr Probl Cardiol. 2020;45(11):100417. doi: 10.1016/j.cpcardiol.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Saito H, Matsue Y, Kamiya K, Kagiyama N, Maeda D, Endo Y, et al. Sarcopenic obesity is associated with impaired physical function and mortality in older patients with heart failure: insight from FRAGILE-HF. BMC Geriatr. 2022;22(1):556. doi: 10.1186/s12877-022-03168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bradshaw PT, Reynolds KR, Wagenknecht LE, Ndumele CE, Stevens J. Incidence of components of metabolic syndrome in the metabolically healthy obese over 9 years follow-up: the Atherosclerosis Risk In Communities study. Int J Obes (Lond) 2018;42(3):295–301. doi: 10.1038/ijo.2017.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tsou MT, Yun CH, Lin JL, Sung KT, Tsai JP, Huang WH, et al. Visceral adiposity, pro-inflammatory signaling and vasculopathy in metabolically unhealthy non-obesity phenotype. Diagnostics (Basel) 2020;11(1). [DOI] [PMC free article] [PubMed]
- 219.Kim TJ, Shin HY, Chang Y, Kang M, Jee J, Choi YH, et al. Metabolically healthy obesity and the risk for subclinical atherosclerosis. Atherosclerosis. 2017;262:191–197. doi: 10.1016/j.atherosclerosis.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 220.Farello G, Iapadre G, Lizzi M, Gentile C, Altobelli E, Ciocca F, et al. Carotid intima media-thickness is increased in obese children metabolically healthy, metabolically unhealthy, and with metabolic syndrome, compared to the non-obese controls. Eur Rev Med Pharmacol Sci. 2021;25(1):241–249. doi: 10.26355/eurrev_202101_24390. [DOI] [PubMed] [Google Scholar]
- 221.Gutierrez-Mariscal FM, Garcia-Rios A, Gomez-Luna P, Fernandez-Gandara C, Cardelo MP, de la Cruz-Ares S, et al. Age-dependent effect of metabolic phenotypes on carotid atherosclerotic disease in coronary heart disease patients (CORDIOPREV study) BMC Geriatr. 2020;20(1):151. doi: 10.1186/s12877-020-01544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Brant LC, Wang N, Ojeda FM, LaValley M, Barreto SM, Benjamin EJ, et al. Relations of metabolically healthy and unhealthy obesity to digital vascular function in Three Community-Based Cohorts: A meta-analysis. J Am Heart Assoc 2017;6(3). [DOI] [PMC free article] [PubMed]
- 223.Kim TN, Park MS, Lim KI, Yang SJ, Yoo HJ, Kang HJ, et al. Skeletal muscle mass to visceral fat area ratio is associated with metabolic syndrome and arterial stiffness: The Korean Sarcopenic Obesity Study (KSOS) Diabetes Res Clin Pract. 2011;93(2):285–291. doi: 10.1016/j.diabres.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 224.Chung GE, Park HE, Lee H, Kim MJ, Choi SY, Yim JY, et al. Sarcopenic Obesity Is Significantly Associated With Coronary Artery Calcification. Front Med (Lausanne) 2021;8:651961. doi: 10.3389/fmed.2021.651961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Xia MF, Chen LY, Wu L, Ma H, Li XM, Li Q, et al. Sarcopenia, sarcopenic overweight/obesity and risk of cardiovascular disease and cardiac arrhythmia: A cross-sectional study. Clin Nutr. 2021;40(2):571–580. doi: 10.1016/j.clnu.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 226.Kosmala W, Jedrzejuk D, Derzhko R, Przewlocka-Kosmala M, Mysiak A, Bednarek-Tupikowska G. Left ventricular function impairment in patients with normal-weight obesity: contribution of abdominal fat deposition, profibrotic state, reduced insulin sensitivity, and proinflammatory activation. Circ Cardiovasc Imaging. 2012;5(3):349–356. doi: 10.1161/CIRCIMAGING.111.969956. [DOI] [PubMed] [Google Scholar]
- 227.Kossaify A, Nicolas N. Impact of overweight and obesity on left ventricular diastolic function and value of tissue Doppler echocardiography. Clin Med Insights Cardiol. 2013;7:43–50. doi: 10.4137/CMC.S11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ueno K, Kaneko H, Kamiya K, Itoh H, Okada A, Suzuki Y, et al. Relationship of normal-weight central obesity with the risk for heart failure and atrial fibrillation: Analysis of a nationwide health check-up and claims database. Eur Heart J Open 2022;2(3):oeac026. [DOI] [PMC free article] [PubMed]
- 229.Itoh H, Kaneko H, Kiriyama H, Kamon T, Fujiu K, Morita K, et al. Metabolically Healthy Obesity and the Risk of Cardiovascular Disease in the General Population - Analysis of a Nationwide Epidemiological Database. Circ J. 2021;85(6):914–920. doi: 10.1253/circj.CJ-20-1040. [DOI] [PubMed] [Google Scholar]
- 230.Commodore-Mensah Y, Lazo M, Tang O, Echouffo-Tcheugui JB, Ndumele CE, Nambi V, et al. High Burden of Subclinical and Cardiovascular Disease Risk in Adults With Metabolically Healthy Obesity: The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care. 2021;44(7):1657–1663. doi: 10.2337/dc20-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rozenbaum Z, Topilsky Y, Khoury S, Pereg D, Laufer-Perl M. Association of body mass index and diastolic function in metabolically healthy obese with preserved ejection fraction. Int J Cardiol. 2019;277:147–152. doi: 10.1016/j.ijcard.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 232.Sciacqua A, Cimellaro A, Mancuso L, Miceli S, Cassano V, Perticone M, et al. Different patterns of left ventricular hypertrophy in metabolically healthy and insulin-resistant obese subjects. Nutrients 2020;12(2). [DOI] [PMC free article] [PubMed]
- 233.Lin YK, Tsai KZ, Han CL, Lin YP, Lee JT, Lin GM. Obesity Phenotypes and Electrocardiographic Characteristics in Physically Active Males: CHIEF Study. Front Cardiovasc Med. 2021;8:738575. doi: 10.3389/fcvm.2021.738575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Billingsley HE, Del Buono MG, Canada JM, Kim Y, Damonte JI, Trankle CR, et al. Sarcopenic Obesity Is Associated With Reduced Cardiorespiratory Fitness Compared With Nonsarcopenic Obesity in Patients With Heart Failure With Reduced Ejection Fraction. Circ Heart Fail. 2022;15(10):e009518. doi: 10.1161/CIRCHEARTFAILURE.122.009518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lee H, Choi EK, Lee SH, Han KD, Rhee TM, Park CS, et al. Atrial fibrillation risk in metabolically healthy obesity: A nationwide population-based study. Int J Cardiol. 2017;240:221–227. doi: 10.1016/j.ijcard.2017.03.103. [DOI] [PubMed] [Google Scholar]
- 236.Wang R, Olier I, Ortega-Martorell S, Liu Y, Ye Z, Lip GY, et al. Association between metabolically healthy obesity and risk of atrial fibrillation: taking physical activity into consideration. Cardiovasc Diabetol. 2022;21(1):208. doi: 10.1186/s12933-022-01644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Aung K, Lorenzo C, Hinojosa MA, Haffner SM. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. J Clin Endocrinol Metab. 2014;99(2):462–468. doi: 10.1210/jc.2013-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kwon W, Lee SH, Yang JH, Choi KH, Park TK, Lee JM, et al. Impact of the Obesity Paradox Between Sexes on In-Hospital Mortality in Cardiogenic Shock: A Retrospective Cohort Study. J Am Heart Assoc. 2022;11(11):e024143. doi: 10.1161/JAHA.121.024143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, et al. Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J Am Coll Cardiol. 2017;70(12):1429–1437. [DOI] [PubMed]
- 240.Bi J, Song L, Wang L, Su B, Wu M, Li D, et al. Transitions in metabolic health status over time and risk of heart failure: A prospective study. Diabetes Metab. 2022;48(1):101266. doi: 10.1016/j.diabet.2021.101266. [DOI] [PubMed] [Google Scholar]
- 241.Kouvari M, Panagiotakos DB, Yannakoulia M, Georgousopoulou E, Critselis E, Chrysohoou C, et al. Transition from metabolically benign to metabolically unhealthy obesity and 10-year cardiovascular disease incidence: The ATTICA cohort study. Metabolism. 2019;93:18–24. doi: 10.1016/j.metabol.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 242.Liu Y, Douglas PS, Lip GYH, Thabane L, Li L, Ye Z, et al. Relationship between obesity severity, metabolic status and cardiovascular disease in obese adults. Eur J Clin Invest. 2022:e13912. [DOI] [PubMed]
- 243.Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68(9):1001–1007. doi: 10.1038/ejcn.2014.117. [DOI] [PubMed] [Google Scholar]
- 244.Atmis V, Yalcin A, Silay K, Ulutas S, Bahsi R, Turgut T, et al. The relationship between all-cause mortality sarcopenia and sarcopenic obesity among hospitalized older people. Aging Clin Exp Res. 2019;31(11):1563–1572. doi: 10.1007/s40520-019-01277-5. [DOI] [PubMed] [Google Scholar]
- 245.Heymsfield SB, Wadden TA. Mechanisms, Pathophysiology, and Management of Obesity. N Engl J Med. 2017;376(3):254–266. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 246.Fruh SM. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J Am Assoc Nurse Pract. 2017;29(S1):S3–S14. doi: 10.1002/2327-6924.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. 2018;378(25):e34. doi: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 248.Jenkins DJA, Dehghan M, Mente A, Bangdiwala SI, Rangarajan S, Srichaikul K, et al. Glycemic Index, Glycemic Load, and Cardiovascular Disease and Mortality. N Engl J Med. 2021;384(14):1312–1322. doi: 10.1056/NEJMoa2007123. [DOI] [PubMed] [Google Scholar]
- 249.Pujia A, Mazza E, Ferro Y, Gazzaruso C, Coppola A, Doldo P, et al. Lipid Oxidation Assessed by Indirect Calorimetry Predicts Metabolic Syndrome and Type 2 Diabetes. Front Endocrinol (Lausanne) 2018;9:806. doi: 10.3389/fendo.2018.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ryan AS, Ortmeyer HK. Insulin suppression of fatty acid skeletal muscle enzyme activity in postmenopausal women, and improvements in metabolic flexibility and lipoprotein lipase with aerobic exercise and weight loss. Int J Obes (Lond) 2019;43(2):276–284. doi: 10.1038/s41366-018-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rondanelli M, Riva A, Petrangolini G, Allegrini P, Perna S, Faliva MA, et al. Effect of acute and chronic dietary supplementation with green tea Catechins on resting metabolic rate, energy expenditure and respiratory quotient: A systematic review. Nutrients 2021;13(2). [DOI] [PMC free article] [PubMed]
- 252.Bouchonville MF, Villareal DT. Sarcopenic obesity: how do we treat it? Current opinion in endocrinology, diabetes and obesity 2013;20(5). [DOI] [PMC free article] [PubMed]
- 253.Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14(9):513–537. doi: 10.1038/s41574-018-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve. 2006;33(2):242–253. doi: 10.1002/mus.20461. [DOI] [PubMed] [Google Scholar]
- 255.Kohut ML, McCann DA, Russell DW, Konopka DN, Cunnick JE, Franke WD, et al. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun. 2006;20(3):201–209. doi: 10.1016/j.bbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 256.Sammarco R, Marra M, Di Guglielmo ML, Naccarato M, Contaldo F, Poggiogalle E, et al. Evaluation of Hypocaloric Diet With Protein Supplementation in Middle-Aged Sarcopenic Obese Women: A Pilot Study. Obes Facts. 2017;10(3):160–167. doi: 10.1159/000468153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Fitó M, Chiva-Blanch G, et al. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: a prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):e6–e17. doi: 10.1016/S2213-8587(19)30074-9. [DOI] [PubMed] [Google Scholar]
- 258.Wilding JPH, Jacob S. Cardiovascular outcome trials in obesity: A review. Obes Rev. 2021;22(1):e13112. doi: 10.1111/obr.13112. [DOI] [PubMed] [Google Scholar]
- 259.Perdomo CM, Cohen RV, Sumithran P, Clement K, Fruhbeck G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet. 2023. [DOI] [PubMed]
- 260.Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 261.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563–e595. doi: 10.1161/CIR.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]