Abstract
Purpose
To evaluate whether elective single embryo transfer in patients with suboptimal response to ovarian stimulation is detrimental to pregnancy rates compared to double embryo transfer.
Methods
A case–control retrospective study was performed in a cohort of couples undergoing IVF at the Infertility Unit of the ASST Lariana with ≤ 9 oocytes and at least 2 viable embryos. A total of 424 women were analyzed in the “double embryo transfer” group (n = 212) and elective “single embryo transfer” group (n = 212); they were matched 1:1 for female age, ovarian reserve and number of previous cycles. Cumulative clinical pregnancy rate per oocyte retrieval was the main outcome.
Results
The cumulative pregnancy rate per cycle, including the fresh embryo and subsequent frozen embryo transfers, was 26% and 26%, respectively. Considering the main confounding factors, a binomial logistic model indicated that the cumulative clinical pregnancy rate was not significantly affected when a single embryo transfer was performed in women recovering up to nine oocytes.
Conclusion
Live birth rate was similar between the two groups, while twin pregnancies were significantly reduced in women receiving single embryo transfer suggesting that elective single embryo transfer in patients with a limited number of embryos is not detrimental to pregnancy rates.
Keywords: Poor ovarian response, Single embryo transfer, In vitro fertilization, Twin pregnancy
Introduction
In recent years, multiple higher-order pregnancies in in vitro fertilization (IVF) programs have dropped significantly due to the evolution of embryo culture and selection techniques as well as specific legal constraints in some countries trending toward the transfer of fewer embryos; however, the incidence of twin pregnancies is currently 5–15% with well described life-threatening maternal morbidities, neonatal risks, and financial burden associated with this on the health system [1–4]. The likelihood of having multiple babies is strongly influenced by the number of embryos that are transferred during IVF treatment. Therefore, it's important to set limits on the number of embryos transferred while still maintaining the chances of success. Some analyses have suggested that replacing the transfer of two embryos with elective single embryo transfer (eSET) could be a cost-effective option allowing to reduce twin pregnancies without compromising pregnancy rates in good prognosis patients [5, 6] and also in women of advanced maternal age or those with reduced response to ovarian stimulation [7, 8].
Prolonged culture up to the blastocyst stage and the application of preimplantation genetic testing has been proposed as a “high-tech” choice to reduce the number of embryos to be transferred [9], but many routines do not involve blastocyst culture in the case of small number of embryos to limit the risk of cancelation of the cycle. In this regard, a strategy that deserves consideration may involve the transfer of a single embryo in the cleavage stage, thus reducing the risk of cancelation of the cycle, and the freezing of supernumerary embryos after extended culture at the blastocyst stage, thus limiting the use of cryopreservation to the embryos with an appropriate developmental potential. This practice, which employs the classic morphological criteria as a key element for choosing the best embryo to be transferred in the fresh cycle, has been implemented in our center since 2018 for all couples with the aim of reducing the rate of twin births. The retrospective analysis proposed here represents a pre- and post-intervention study to contribute to the lack of specific data on the efficacy of eSET in couples with small numbers of embryos. In particular, we identified double embryo transfer (DET) controls and eSET cases using a matched case–control study design within couples with a poor or suboptimal response to ovarian stimulation (≤ 9 oocytes) with the main aim to compare cumulative pregnancy rates.
Materials and Methods
This retrospective pre- and post-intervention study included data from patients who underwent IVF/ICSI cycles at the Infertility Unit of the ASST Lariana. A matched case–control study was performed in the cohort of couples with poor or suboptimal response to ovarian stimulation (defined as the recovery of ≤ 9 oocytes). After an audit to evaluate clinical outcomes, a strict policy regarding single embryo transfer was introduced in our clinic from September 2018 to reduce twin pregnancy rates independently from ovarian response or embryo quality. Accordingly, from September 2018 to March 2020, all fresh embryo transfers were performed with a single embryo on day 3 after in vitro fertilization (according to the schedule of work activities, the transfer was occasionally performed on day 2). On the contrary, in the period from September 2016 to June 2018 a double embryo transfer was performed in most women belonging to low prognosis groups based on the recovery of ≤ 9 oocytes (eSET was limited to those with previous pregnancies and were excluded from the analysis).
The clinical dataset was used to extract data after anonymization. The following inclusion criteria were applied: (1) women undergoing an IVF/ICSI non-donor cycle after ovarian stimulation recovering two to nine oocytes and receiving embryo transfer in the fresh cycle; (2) at least two viable embryos were available on the day of embryo transfer. Cases were patients undergoing elective single embryo transfer, while controls were patients undergoing double embryo transfer (DET). A matched case–control study was used to control for main confounders avoiding a large sample size [10]. The matching between cases (eSET) and controls (DET) was carried out in a 1:1 ratio based on the following characteristics: (1) female age (maximum allowable difference = 18 months); (2) first–second or third to fifth IVF cycle; (3) AMH ≥ 1.2 or < 1.2 ng/ml.
All women underwent a clinical evaluation before entering the IVF program. Ovarian stimulation for oocyte retrieval was performed by standard procedures. Morphological evaluation of embryos was performed according to ESHRE-Alpha Consensus [11] and was recorded the day of embryo transfer and at the blastocyst stage in the case of supernumerary embryos. One (in the first period) or two (in the second period) embryos were replaced two to three days after oocyte insemination. No genetic tests were performed on embryos. Embryo transfer was postponed through embryo vitrification in the presence of any risks for women's health, and this condition was an exclusion criterion for the present study. When available, supernumerary embryos were cryopreserved at the blastocyst stage on day 5 to 7 of culture. In both groups, cryopreserved embryos were always transferred individually. Clinical pregnancy per cycle was defined as the presence of at least one intrauterine gestational sac obtained with the fresh or subsequent cryopreserved embryo transfers (4 weeks after embryo transfer) deriving from the same oocyte aspiration procedure.
During the study period, no significant changes were made to the staff to the instrumentation or operative protocols. Ethical approval for this study was obtained by the Ethics Committee ATS Insubria (13/04/2021).
Statistical Analysis
Results are reported as percentage [95% confidence interval (CI)], Odds Ratios (OR) [95% CI], mean ± SD or median [Interquartile Range] according to the characteristics of the variable. Student’s t test, Wilcoxon test, Chi-square test or Fisher’s exact test were used as appropriate. A logistic regression model was run to predict dependent variables from independent variables that were found to differ between groups (p < 0.10) or known to influence the outcome; p-values < 0.05 were considered statistically significant.
The sample size calculation was based on our preliminary results: in the expected range of pregnancy rate for poor and suboptimal responders (20–23%), a sample size of 215 patients per group was foreseen to obtain a variability of the main outcome of approximately ± 5%, which was considered consistent with the main objective of the study. IBM SPSS Statistics software for Windows (version 20.0, IBM Corp. Armonk, NY) and MedCalc Statistical Software (version 19.1.3, MedCalc Software bv, Belgium) were used for statistical analysis and matching of cases (eSET) and controls (DET), respectively.
Results
A total of 463 patients with at least 2 viable embryos undergoing fresh embryo transfer after the recovery of two to nine oocytes were retrospectively evaluated, and an ad hoc dataset was created with their records as summarized in Fig. 1. They represented nearly 30% of the total IVF cycles performed in the specified period. According to matching criteria, 212 cases (eSET) and 212 matched controls (DET) were found eligible and were included for data analysis. Baseline characteristics of included cycles were similar between the two groups as shown in Table 1. Characteristics of the IVF-ICSI cycles are summarized in Table 2. After the fresh embryo transfer, clinical pregnancy rates equal to 17% (95%CI: 12–23%) and 22% (95%CI: 16–28%) were obtained in eSET and DET groups, respectively, corresponding to a crude OR = 0.78 (95%CI: 0.49–1.26), p = 0.28. The implantation rate was 17% (95%CI: 12–23%) and 13% (95%CI: 10–17%) in eSET and DET groups, respectively (p = 0.23). Since a lower number of embryos were transferred in the eSET group, a significantly higher proportion of cycles had supernumerary embryos to be cryopreserved compared to the DET group.
Fig. 1.
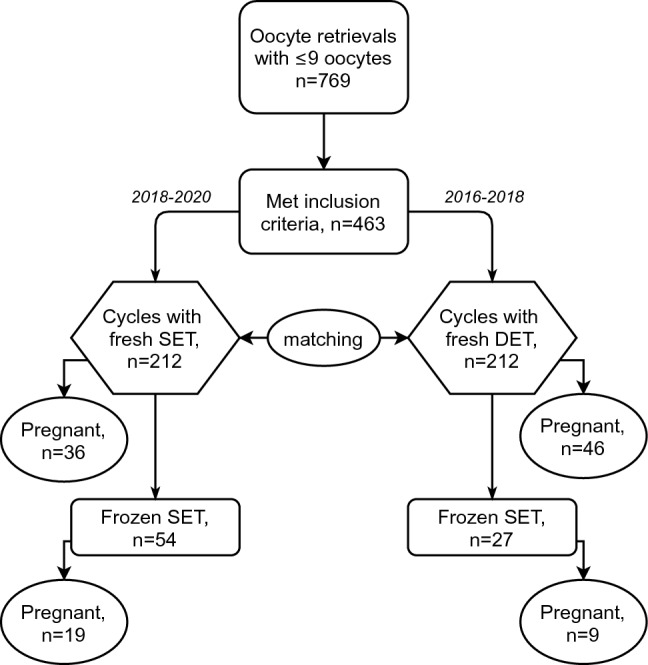
Flowchart of the study
Table 1.
Baseline characteristics of included cycles
| Characteristics | Cases (SET) n = 212 cycles | Controls (DET) n = 212 cycles | p |
|---|---|---|---|
| Female age (years) | 37.4 ± 3.7 | 38.0 ± 4.0 | 0.10 |
| < 35 years | 46 (22%) | 51 (24%) | 0.56 |
| 35–45 years | 166 (78%) | 161 (76%) | |
| Body mass index (Kg/m2) | 23.1 ± 3.6 | 23.1 ± 4.4 | 1.00 |
| Duration of infertility (years) | 4.1 ± 2.6 | 4.1 ± 1.8 | 1.00 |
| Patients with previous pregnancies | 61 (29%) | 57 (27%) | 0.67 |
| Number of previous IVF cycles | |||
| 0–1 | 169 (80%) | 169 (80%) | 1.00 |
| 2–5 | 43 (20%) | 43 (20%) | |
| AMH (ng/ml) | 1.4 [0.9–2.5] | 1.5 [0.9–2.4] | 0.90 |
| < 1.2 ng/ml | 84 (40%) | 84 (40%) | 1.00 |
| ≥ 1.2 ng/ml | 128 (60%) | 128 (60%) | |
| Sperm count (106/ml) | 36 [16–69] | 44 [19–80] | 0.10 |
| Cause of infertility | |||
| Reduced ovarian reserve/unexplained | 146 (69%) | 140 (66%) | 0.59 |
| Tubal/endometriosis | 6 (3%) | 10 (5%) | |
| Ovulatory | 7 (3%) | 4 (2%) | |
| Male factor | 94 (44%) | 87 (41%) | |
| Protocol of ovarian stimulation | |||
| Protocol with GnRH agonists | 16 (8%) | 13 (6%) | 0.56 |
| Protocol with GnRH antagonists | 196 (93%) | 199 (94%) | |
| Total amount of FSH used (IU) | 2100 [1575–2700] | 2100 [1588–2700] | 0.74 |
| Gonadotropins | |||
| FSH only | 99 (47%) | 126 (59%) | 0.009 |
| hMG or + LH | 113 (53%) | 86 (41%) | |
| Duration of stimulation (days) | 8.6 ± 2.0 | 8.5 ± 1.7 | 0.70 |
Data are reported as mean ± SD, median [IQR] or number (%) as appropriate
SET: single embryo transfer; DET: double embryo transfer
IVF: in vitro fertilization. AMH: anti-Mullerian hormone
Table 2.
IVF outcomes in cases and controls
| Characteristics | Cases (SET) n = 212 cycles | Controls (DET) n = 212 cycles | p |
|---|---|---|---|
| Number of follicles ≥ 14 mm | 5.4 ± 2.5 | 5.5 ± 2.8 | 0.74 |
| Oocyte retrieved | 5.0 ± 1.9 | 5.2 ± 2.0 | 0.19 |
| Mature oocytes | 4.1 ± 1.7 | 4.3 ± 1.8 | 0.32 |
| Insemination | |||
| Standard IVF | 66 (31%) | 74 (35%) | 0.71 |
| ICSI | 143 (68%) | 135 (64%) | |
| Both | 3 (1%) | 3 (1%) | |
| Number of inseminated oocytes | 4.3 ± 1.7 | 4.5 ± 1.8 | 0.18 |
| Fertilization rate (%) | 80 [67–100] | 75 [60–100] | 0.026 |
| Viable embryos (day of embryo transfer) | 3.3 ± 1.3 | 3.2 ± 1.4 | 0.52 |
| ≥ 2 top-quality embryos (day of embryo transfer) | 149 (70%) | 165 (78%) | 0.08 |
| No. of cryopreserved embryos | |||
| 0 | 136 (64%) | 176 (83%) | 0.001 |
| 1–2 | 68 (32%) | 35 (17%) | |
| 3–4 | 8 (4%) | 1 (1%) | |
| No. of cycles with subsequent frozen embryo transfer | 56 (26%) | 29 (14%) | 0.002 |
| No. of embryo transfers | 1 [1, 2] | 1 [1–1] | 0.001 |
| 1 (fresh only) | 156 (74%) | 183 (86%) | 0.006 |
| 2 (1 fresh + 1 frozen) | 40 (19%) | 24 (11%) | |
| 3–4 (1 fresh + 2/3 frozen) | 16 (8%) | 5 (2%) | |
| Clinical pregnancies | |||
| In the fresh cycle | 36/212 (17%) | 46/212 (22%) | 0.22 |
| With frozen embryos | 19/56 (34%) | 9/29 (31%) | 0.87 |
| Number of gestational sacs | |||
| 1 | 55 (100%) | 45 (82%) | 0.001 |
| 2 | 0 (0%) | 10 (18%) | |
| Cumulative clinical pregnancy rate per oocyte retrieval | 55 (26%) | 55 (26%) | 1.00 |
| Cumulative delivery rate per oocyte retrieval | 46 (22%) | 45 (21%) | 1.00 |
| Pregnancy follow-up | |||
| Miscarriage | 9 (16%) | 10 (18%) | 0.80 |
| Live birth | 46 (84%) | 45 (82%) | |
| Twins at birth | 0 (0%) | 10 (22%) | 0.008 |
| Major congenital abnormalities/newborns | 0/46 (0%) | 2/55 (4%)* | 0.50 |
Data are reported as mean ± SD or number (%) or median [IQR] as appropriate. SET: single embryo transfer; DET: double embryo transfer; IVF: in vitro fertilization; ICSI: intracytoplasmic sperm injection. * = Down syndrome
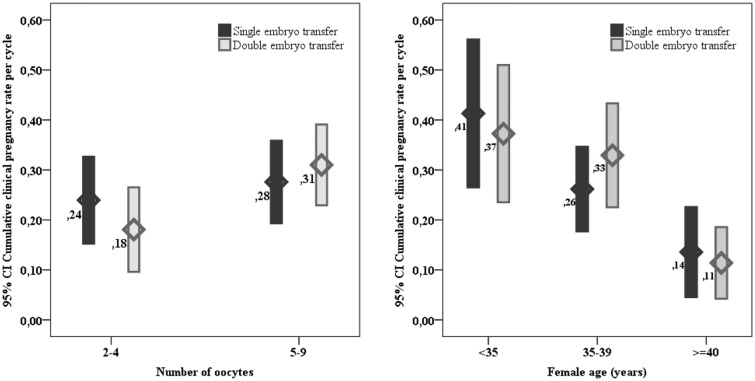
The cumulative clinical pregnancy rate per cycle was the same in the two groups (26%, 95%CI: 20–32%) with a crude OR = 1.00 (95%CI: 0.65–1.54), p = 1.00.The binomial logistic regression model on the likelihood that participants have a clinical pregnancy was statistically significant (Chi-square = 47, p = 0.001). The model correctly classified 74% of cases and explained 15% (Nagelkerke R2) of the variance in cumulative clinical pregnancy rate. Among considered predictors, age and number of top-quality embryos were added statistically significantly to the prediction (Table 3); adjusted OR for cumulative pregnancy rate in DET compared to eSET was 1.23 (95%CI: 0.77–1.97), p = 0.38. As depicted in Fig. 2, subgroup analyses based on the number of oocytes or female age showed comparable results in terms of cumulative clinical pregnancy rate between eSET and DET across different groups.
Table 3.
Summary of multiple regression analysis to predict the cumulative clinical pregnancy rate
| Variable | B | SEB | p | Odds Ratio |
|---|---|---|---|---|
| Intercept | −1.05 | 0.11 | 0.001 | |
| Controls (DET = 2) versus cases (SET = 1) | 0.21 | 0.24 | 0.37 | 1.24 |
| Female age | −0.15 | 0.03 | 0.00 | 0.86 |
| Body mass index (Kg/m2) | −0.04 | 0.03 | 0.23 | 0.96 |
| Total dose of gonadotropins | 0.00 | 0.00 | 0.73 | 1.00 |
| Number of top-quality embryos | 0.50 | 0.11 | 0.00 | 1.64 |
B = unstandardized regression coefficient; SEB = standard error of the coefficient; β = standardized coefficient. DET = double embryo transfer; SET = single embryo transfer
Fig. 2.
Cumulative clinical pregnancy rate: subgroup analysis according to the number of retrieved oocytes and female age. Differences between eSET and DET groups are not statistically different. For the number of oocytes p < 0.05 between 2 and 4 compared to 5–9 oocytes in DET group. For age, p for trend < 0.05 both for eSET and DET across the three categories
In the DET group, ten dizygotic twin pregnancies were obtained with fresh embryo transfer (18%, 95%CI: 9–31%).
In the whole cohort, all but two patients (belonging to the eSET group) among those with supernumerary cryopreserved embryos were either pregnant or used all the cryopreserved embryos. Moreover, 14 and four pregnant patients have additional cryopreserved embryos in eSET and DET groups, respectively (p = 0.027).
Discussion/Conclusion
The present study shows that in patients with poor or suboptimal response to controlled ovarian stimulation, a single embryo transfer strategy is not detrimental to cumulative pregnancy rate compared to a multiple-embryo transfer strategy, reducing to a minimum chance of twin pregnancy.
The most suitable embryo transfer strategy in poor responders has been addressed previously by several authors [12]. Two main considerations in this context are the number and developmental stage of transferred embryos. The choice of the developmental stage is generally linked to the cycle cancelation risk, which represents a major concern in all IVF programs from both an economical and emotional point of view. In cycles with a reduced number of oocytes, the risk for cycle cancelation is expected to be higher than in cycles with a normal response to ovarian stimulation mainly due to the possible developmental arrest of the embryo after the cleavage stage [13, 14].
Some studies suggest that extended culture to the blastocyst stage can improve implantation rates and reduce the risk of aneuploidy [15]. However, according to a recent Cochrane systematic meta-analysis, although there is a benefit favoring blastocyst transfer in fresh cycles in terms of implantation rate, it remains unclear whether the day of transfer impacts on cumulative live birth and pregnancy rates [16–19].
The decision of how many embryos to transfer in IVF cycles with fewer oocytes is critical.
Patients with a suboptimal response are often considered to have a poor prognosis and may have multiple embryos transferred [20], possibly due to the assumption that a reduced number of oocytes reflects a poorer developmental potential of the embryo. However, this can result in increased incidence of multiple births, especially with cleavage-stage transfer. Genetic testing for aneuploidies at the blastocyst stage can be cost-effective with an increasing number of available blastocysts [21], even in advanced maternal age patients, but is not currently offered in our unit.
It is legitimate to question the validity of the contribution of a retrospective observational study on a topic for which there are previous results from randomized studies. In this regard, however, it should be mentioned that for the specific population of women with a poor prognosis due to a suboptimal number of recovered oocytes, high-quality studies are not available. Moreover, although the transfer of multiple embryos is a common choice in poor responders, information on the rate of multiple pregnancies is often missing even in larger retrospective reports [7, 22, 23]. Jonsdottir et al. [24] reported a multiple birth rate of 9% in poor responders (four or fewer oocytes retrieved) receiving a double embryo transfer; in our study, the incidence of twin pregnancies was 18%. Altogether these evidence suggest that this specific population deserves more consideration regarding the incidence of multiple pregnancies. Results available so far suggest that any differences between eSET and DET in terms of live births, if exist, are probably numerically small: this means that any randomized studies aimed at quantifying the difference must necessarily include an exceptionally high sample size and will therefore be very difficult to conduct.
The matched experimental design is the main strength of the present study because it allows a paired comparison for the variables that are most known to influence IVF outcomes such as age, number of previous IVF cycles and ovarian reserve. Based on this experimental design, we can suggest that the transfer of a single embryo at the cleavage stage in cycles with ≤ 9 oocytes, possibly combined with subsequent frozen embryos transfer, does not involve a substantial decrease in the chances of success but a drastic reduction in the rate of twin pregnancies and a modest delay in time for pregnancy to occur.
Some limitations of the present study must be recognized. First, the study was not designed to highlight minor differences in the pregnancy rate between the two groups. Second, since our study was conducted on a specific population of women with 9 or less oocytes, our data cannot be generalized to other populations, particularly those with a better or poorer prognosis in which a blastocyst or multiple-embryo transfer strategy, respectively, may represent an advantage in terms of success rate or time for pregnancy to occur [25]. Third, the study did not use embryo morphology as a basis for selecting the number of embryos to transfer, as it is not currently precise enough, particularly at the cleavage stage [26]. While the best embryos were chosen based on their morphology within the available cohort, this did not affect the number of embryos transferred. This is actually an advantage of the study, as it provides a straightforward and transferable approach that avoids the variability associated with morphological evaluations between different centers or operators.
Our results are in line with the current literature which is, however, mainly based on couples with good prognosis [12] and confirm that despite a trend for higher rate of success after the fresh embryo transfer of two cleavage-stage embryos, cumulative results are not affected by the choice of transferring a single fresh embryo at the cleavage stage and allow a significantly reduced incidence of twins with a moderate delay in time to pregnancy.
In conclusion, we propose that in situations where there is a desire to reduce the incidence of twin pregnancies, the number of cleavage-stage embryos to be transferred can be limited to one per cycle in women with poor or non-optimal ovarian response without compromising the overall efficacy of the treatment. Larger studies are needed to provide more and detailed information on this specific topic and, in particular, on the subgroup of couples with poor prognosis requiring multiple-embryo transfer with safe and efficient results.
Funding
This work received no specific funding.
Data Availability
Data can be obtained from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical Approval
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. This study protocol was reviewed and approved by the Ethical Committee on 13/04/2021. Written informed consent was obtained from participants in order to use retrospective anonymized data.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Callahan TL, Hall JE, Ettner SL, Christiansen CL, Greene MF, Crowley WF., Jr The economic impact of multiple-gestation pregnancies and the contribution of assisted- reproduction techniques to their incidence. N Engl J Med. 1994;33:244–249. doi: 10.1056/NEJM199407283310407. [DOI] [PubMed] [Google Scholar]
- 2.European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe results generated from European registries by ESHRE. Hum Reprod Open. 2016;2020(3):hoaa032. doi: 10.1093/hropen/hoaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. https://www.cdc.gov/art/reports/2018/fertility-clinic.html. Accessed July 23, 2021.
- 4.Human Fertilisation and Embryology Authority.[cited 2021 July 23]. Available from https://www.hfea.gov.uk/about-us/publications/research-and-data/fertility-treatment-2019-trends-and-figures/#Section1.
- 5.van Heesch MM, van Asselt AD, Evers JL, et al. Cost-effectiveness of embryo transfer strategies: a decision analytic model using long-term costs and consequences of singletons and multiples born as a consequence of IVF. Hum Reprod. 2016;31:2527–2540. doi: 10.1093/humrep/dew229. [DOI] [PubMed] [Google Scholar]
- 6.Bergh C, Kamath MS, Wang R, Lensen S. Strategies to reduce multiple pregnancies during medically assisted reproduction. Fertil Steril. 2020;114:673–679. doi: 10.1016/j.fertnstert.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Leijdekkers JA, Eijkemans MJC, van Tilborg TC, et al. Cumulative live birth rates in low- prognosis women. Hum Reprod. 2019;34:1030–1041. doi: 10.1093/humrep/dez051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niinimäki M, Suikkari AM, Mäkinen S, Söderström-Anttila V, Martikainen H. Elective single-embryo transfer in women aged 40–44 years. Hum Reprod. 2013;28:331–335. doi: 10.1093/humrep/des399. [DOI] [PubMed] [Google Scholar]
- 9.Ubaldi FM, Capalbo A, Colamaria S, et al. Reduction of multiple pregnancies in the advanced maternal age population after implementation of an elective single embryo transfer policy coupled with enhanced embryo selection: pre- and post-intervention study. Hum Reprod. 2015;30:2097–2106. doi: 10.1093/humrep/dev159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Graaf MA, Jager KJ, Zoccali C, Dekker FW. Matching, an appealing method to avoid confounding? Nephron Clin Pract. 2011;118:c315–318. doi: 10.1159/000323136. [DOI] [PubMed] [Google Scholar]
- 11.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–1283. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 12.Kamath MS, Mascarenhas M, Kirubakaran R, Bhattacharya S. Number of embryos for transfer following in vitro fertilisation or intra-cytoplasmic sperm injection. Cochrane Database Syst Rev. 2020;8:CDS003416. doi: 10.1002/14651858.CD003416.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van der Auwera I, Debrock S, Spiessens C, et al. A prospective randomized study: day 2 versus day 5 embryo transfer. Hum Reprod. 2002;17:1507–1512. doi: 10.1093/humrep/17.6.1507. [DOI] [PubMed] [Google Scholar]
- 14.Grifo J, Kofinas J, Schoolcraft WB. The practice of in vitro fertilization according to the published literature. Fertil Steril. 2014;102:658–659. doi: 10.1016/j.fertnstert.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Adler A, Lee HL, McCulloh DH, et al. Blastocyst culture selects for euploid embryos: comparison of blastomere and trophectoderm biopsies. Reprod Biomed Online. 2014;28:485–491. doi: 10.1016/j.rbmo.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2016;2016:CDS002118. doi: 10.1002/14651858.CD002118.pub5. [DOI] [PubMed] [Google Scholar]
- 17.Papanikolaou EG, Camus M, Kolibianakis EM, Van Landuyt L, Van Steirteghem A, Devroey P. In vitro fertilization with single blastocyst-stage versus single cleavage-stage embryos. N Engl J Med. 2006;354:1139–1146. doi: 10.1056/NEJMoa053524. [DOI] [PubMed] [Google Scholar]
- 18.Frattarelli JL, Leondires MP, McKeeby JL, Miller BT, Segars JH. Blastocyst transfer decreases multiple pregnancy rates in in vitro fertilization cycles: a randomized controlled trial. Fertil Steril. 2003;79:228–230. doi: 10.1016/S0015-0282(02)04558-2. [DOI] [PubMed] [Google Scholar]
- 19.Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology Blastocyst culture and transfer in clinical-assisted reproduction: a committee opinion. Fertil Steril. 2013;99:667–672. doi: 10.1016/j.fertnstert.2013.01.087. [DOI] [PubMed] [Google Scholar]
- 20.Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, Esteves SC, et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril. 2016;105:1452–1453. doi: 10.1016/j.fertnstert.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Somigliana E, Busnelli A, Paffoni A, et al. Cost-effectiveness of preimplantation genetic testing for aneuploidies. Fertil Steril. 2019;111:1169–1176. doi: 10.1016/j.fertnstert.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Esteves SC, Yarali H, Vuong LN, et al. Cumulative delivery rate per aspiration IVF/ICSI cycle in POSEIDON patients: a real-world evidence study of 9073 patients. Hum Reprod. 2021;36:2157–2169. doi: 10.1093/humrep/deab152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Li X, Yang X, et al. Cumulative live birth rates in low prognosis patients according to the POSEIDON criteria: an analysis of 26,697 cycles of in vitro fertilization/intracytoplasmic sperm injection. Front Endocrinol (Lausanne) 2019;10:642. doi: 10.3389/fendo.2019.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonsdottir I, Lundin K, Bergh C. Double embryo transfer gives good pregnancy and live birth rates in poor responders with a modest increase in multiple birth rates: results from an observational study. Acta Obstet Gynecol Scand. 2011;90:761–766. doi: 10.1111/j.1600-0412.2011.01139.x. [DOI] [PubMed] [Google Scholar]
- 25.Gleicher N, Vega MV, Darmon SK, et al. Live-birth rates in very poor prognosis patients, who are defined as poor responders under the Bologna criteria, with nonelective single embryo, two-embryo, and three or more embryos transferred. Fertil Steril. 2015;104:1435–1441. doi: 10.1016/j.fertnstert.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Wong KM, Repping S, Mastenbroek S. Limitations of embryo selection methods. Semin Reprod Med. 2014;32:127–133. doi: 10.1055/s-0033-1363554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be obtained from the corresponding author upon reasonable request.