Abstract
Silicon-stereogenic optically active silylboranes could potentially allow the formation of chiral silyl nucleophiles as well as the synthesis of various chiral silicon compounds. However, the synthesis of such silicon-stereogenic silylboranes has not been achieved so far. Here, we report the synthesis of silicon-stereogenic optically active silylboranes via a stereospecific Pt(PPh3)4-catalyzed Si–H borylation of chiral hydrosilanes, which are synthesized by stoichiometric and catalytic asymmetric synthesis, in high yield and very high or perfect enantiospecificity (99% es in one case, and >99% es in the others) with retention of the configuration. Furthermore, we report a practical approach to generate silicon-stereogenic silyl nucleophiles with high enantiopurity and configurational stability using MeLi activation. This protocol is suitable for the stereospecific and general synthesis of silicon-stereogenic trialkyl-, dialkylbenzyl-, dialkylaryl-, diarylalkyl-, and alkylary benzyloxy-substituted silylboranes and their corresponding silyl nucleophiles with excellent enantiospecificity (>99% es except one case of 99% es). Transition-metal-catalyzed C–Si bond-forming cross-coupling reactions and conjugate-addition reactions are also demonstrated. The mechanisms underlying the stability and reactivity of such chiral silyl anion were investigated by combining NMR spectroscopy and DFT calculations.
Subject terms: Synthetic chemistry methodology, Asymmetric synthesis, Reaction mechanisms
Silicon-stereogenic optically active silylboranes could potentially allow the formation of chiral silyl nucleophiles as well as the synthesis of various chiral silicon compounds but the synthesis of such silicon-stereogenic silylboranes remains underdeveloped. Here, the authors report the synthesis of silicon-stereogenic optically active silylboranes via a stereospecific Si–H borylation of chiral hydrosilanes in high yield enantiospecificity
Introduction
The asymmetric synthesis of optically active compounds with stereogenic carbon centers is a major topic in contemporary organic synthesis and has advanced significantly over the last several decades1,2. Compared to the synthesis of chiral carbon compounds, synthetic routes to chiral compounds with a stereogenic silicon center, which is a higher homologue of carbon, remain less explored3–67. After many pioneering studies, chiral organosilicon compounds that bear silicon stereocenters have recently shown attractive and widespread application prospects in organic synthesis5–13, materials science14–19, medicinal chemistry20–24, and polymer chemistry25,26 due to their unique electronic and physical properties. The development of synthetic methods for such silicon-stereogenic optically active compounds has also become an important research subject27–32. In addition to classical methods that employ stoichiometric chiral auxiliaries33–41, transition-metal catalyzed and organocatalyzed desymmetrization of prochiral silicon-containing molecules also represents an efficient approach for the synthesis of silicon-stereogenic chiral molecules42–67.
Despite the recent rapid ascent of chiral silicon chemistry, the development of silyl nucleophiles with stereogenic silicon centers is still not well developed. Silyllithiums are generally useful synthetic intermediates toward various organosilicon compounds, albeit that originally, the scope of silicon anions that were synthetically accessible was considerably more limited than that of carbanions, and examples of silyl anions that can be readily synthesized still remain limited68–77. Although enantiomerically pure silyllithiums that show configurational stability can be used as useful silicon-stereogenic silyl group transfer reagents78–91, synthetic access to such chiral silyllithiums remains restricted, and few successful examples have been reported to date. Sommer78, Kawakami79, and Strohman80–83 have made significant contributions in this direction, showing that the reaction of chiral disilanes and lithium metal generates chiral silyllithiums with unchanged configuration via the reduction of Si–Si or Si–Ph bonds (Fig. 1A-a). Similarly, lithium metal selectively cleaves the Si–Ge bond in optically active silylgermane to yield enantiomerically pure silyllithium without racemization (Fig. 1A-a)84. Kawakami has obtained silyllithium in a stereo-retentive manner via tin–lithium exchange of enantiomerically pure silylstannane derivative (Fig. 1A-b)79,85–87. However, these three methods generate stoichiometric quantities of undesired side products, including achiral silyllithium, phenyl lithium, trimethylgermyl lithium, and trimethylstannyl lithium, which compete in the nucleophilic reaction of the chiral silyllithium and decrease their synthetic utility for the generation of chiral organosilicon compounds. In 1976, Corriu developed a cobalt–lithium exchange system in which a chiral silyllithium was partially racemized (Fig. 1A-c)88,89. Enantiomerically pure chlorosilanes undergo significant racemization with lithium metal or di-tert-butylbiphenylide (LiDBB) due to chloride-induced racemization, as reported by Oestreich (Fig. 1A-d)79,90. In 2010, Tomooka’s group reported that chiral chlorosilanes react with an excess lithium 1-(dimethylamino)naphthalenide (LDMAN) to obtain chiral silyllithium and silacarboxylic acids in a highly stereospecific manner, being attributed to sterically bulky substituents around the silicon atom (Fig. 1A-d)91. It should also be noted here that all these known methods require a silicon center with at least one aryl group and steric demand to stabilize the silyl anion, which severely restricts their practical applications. In addition, excess amount of reductant (Li, LiDBB, LDMAN, etc) and nucleophiles (MeLi, etc) are required in many cases. While the silyl anion configuration is relatively stable, there are currently no reliable and general methods available for generating chiral silyl anions from chiral silicon compounds78–91. Therefore, the development of practical and widely applicable methods that allow the synthesis of a wide variety of silicon-stereogenic nucleophiles is highly desirable, as these have great significance for the construction of chiral organosilicon compounds.
Fig. 1. Synthetic routes to silicon-stereogenic optically active silyllithiums.
A Reported synthetic routes to silicon-stereogenic optically active silyllithiums. B Synthesis of chiral silylboranes and the generation of the corresponding chiral silyl nucleophiles.
Silylboranes have become indispensable reagents for introducing silicon and boron groups into various substrates, and silylboranes can produce silyl nucleophiles in the presence of bases92–98. We anticipated that a diverse range of optically active silyl anions could be generated by employing optically active silylboranes with a silicon stereogenic center. However, silicon-stereogenic optically active silylboranes have not yet been synthesized99–117. Our group has developed a general synthetic method for silylboranes based on the platinum- or rhodium-catalyzed borylation of hydrosilanes, which provides access to dialkylarylsilylboranes and trialkylsilylboranes with various alkyl groups (Fig. 1B)116. Moreover, we have demonstrated that the preparation of trialkylsilyllithum species from the corresponding trialkylsilylboranes is feasible. Subsequently, we developed an iridium- or nickel-catalyzed monoborylation of dihydrosilane to produce hydrosilylboronates and demonstrated the generation of dialkylhydrosilyl lithium through the activation of hydrosilylboronates with MeLi (Fig. 1B)117 (Despite numerous attempts, asymmetric monoborylation of pro-chiral dihydrosilanes has yet to be successfully achieved). Based on our previous studies, we envisioned that the general synthesis of chiral silylboranes might be realized if the transition-metal-catalyzed Si–H borylation of the corresponding chiral hydrosilanes could proceed (Fig. 1B).
Here, we report the synthesis of silicon-stereogenic optically active silylboranes and the generation of a wide range of chiral silyl nucleophiles. We found that the Pt(PPh3)4-catalyzed borylation of chiral hydrosilanes, which are synthesized by stoichiometric and catalytic asymmetric synthesis, in high yield and perfect stereoselectivity with retention of their configuration (Fig. 1B). The single-crystal X-ray diffraction analyses of these chiral silylboranes are presented. The synthesized silicon-stereogenic silylboranes are easy to handle and can be used as silicon-stereogenic silyl-group transfer reagents with high enantiomeric purity, exhibiting complete enantiospecificity in the generation of chiral silyllithiums, silicon–silicon bond-forming reactions. In addition, we demonstrate that palladium(0)-catalyzed C–Si bond-forming cross-coupling reactions and copper(I)-catalyzed silyl-conjugate-addition reactions can also be conducted with perfect stereospecificity.
Results and discussion
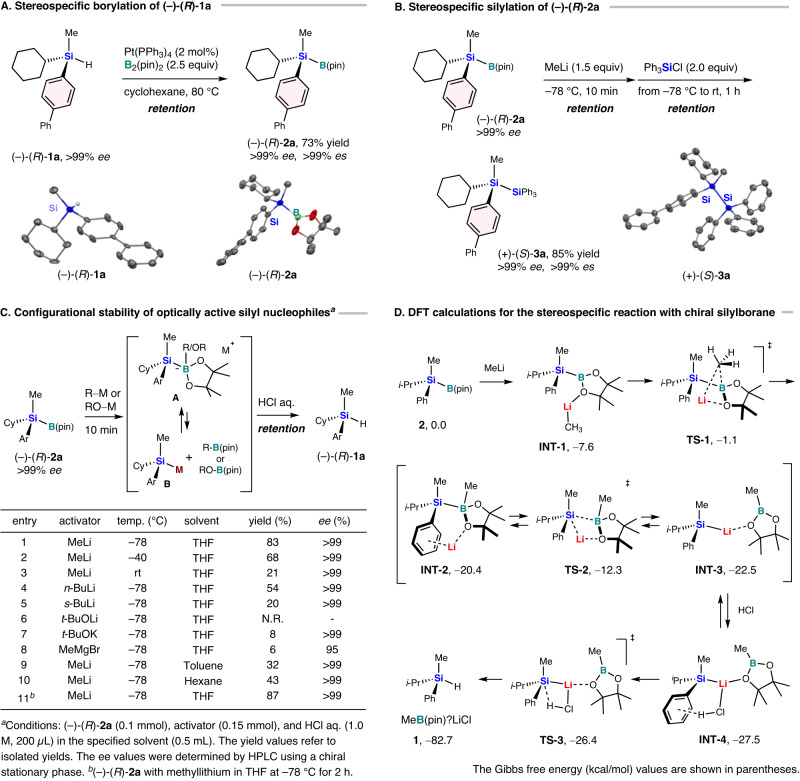
We started the investigation of the synthesis of silicon-stereogenic optically active silylboranes based on our previous research116. The most important aspect of this study is to unambiguously determine the absolute configuration of the hydrosilane, the silylborane, and the silyl anion. For that purpose, we first searched for a chiral crystalline hydrosilane that can be analyzed by single-crystal X-ray diffraction analysis. Ideally, the silylborane produced by the hydrosilane should be crystalline as well. Moreover, to determine the stereochemistry of the silyllithium, crystalline products should be obtained by trapping the silyllithium without loss of the stereochemistry. Although this was a very difficult task, we finally settled on the chiral crystalline hydrosilane (–)-(R)-[(1,1’-biphenyl)−4-yl](cyclohexyl)methylsilane [(–)-(R)−1a], which was prepared with >99% ee by resolution of the corresponding racemic hydrosilane using preparative HPLC equipped with a chiral column (Fig. 2A; for details, see the Supplementary Information)118. The absolute configuration of (–)-(R)-1a was unequivocally determined via single-crystal X-ray diffraction analysis (for details, see the Supplementary Information). After screening potential catalysts for the borylation of (–)-(R)-1a (for details, see the Supplementary Information), we found that Pt(PPh3)4 is suitable for the borylation of (–)-(R)-1a and bis(pinacolato)diboron, which affords (–)-(R)-[(1,1’-biphenyl)-4-yl](cyclohexyl)methyl(4,4,5,5-tetramethyl-1,3,2-dioxaborolan−2-yl)silane [(–)-(R)-2a] in 73% yield with perfect enantiospecificity (>99% ee; >99% es). A single-crystal X-ray diffraction analysis of the product unambiguously confirmed the reaction proceeded with retention of the configuration of the silicon-stereogenic center (Fig. 2A; for details, see the Supplementary Information).
Fig. 2. Synthesis of chiral silylboranes and the generation of the corresponding chiral silyl nucleophiles.
A Stereospecific borylation of chiral hydrosilane (–)-(R)-1a. B Stereospecific silylation of silicon-stereogenic optically active silylborane (–)-(R)-2a. C Configurational stability of silicon-stereogenic optically active silyl nucleophiles under various conditions. D DFT calculations for the stereospecific reaction between silylborane and MeLi followed by protonation. The Gibbs free energy (kcal/mol) values are shown in parentheses.
We then investigated the reaction of the chiral silylborane (–)-(R)-2a with chlorotriphenylsilane in the presence of methyl lithium as a nucleophilic activator of the silylboranes in order to observe whether the synthesized chiral silylborane could act as a silicon-stereogenic silyl-group transfer reagent (Fig. 2B). When (–)-(R)-2a (>99% ee) was treated with methyl lithium in THF at –78 °C for 10 min, it subsequently reacted with chlorotriphenylsilane to furnish (+)-(S)-1-[(1,1’-biphenyl)−4-yl]-1-cyclohexyl-1-methyl-2,2,2-triphenyldisilane [(+)-(S)-3a] in 85% yield with perfect enantiospecificity (>99% ee; >99% es). A single-crystal X-ray diffraction analysis confirmed the absolute configuration to be (+)-(S)-3a; the nucleophilic reaction, therefore proceeds with retention of the configuration (Fig. 2B; for details, see the Supplementary Information).
Subsequently, we performed in situ 11B{1H} NMR and 29Si{1H} NMR experiments to explore key intermediates in the reaction of (±)-2a with methyl lithium (for details, see the Supplementary Information). Kawachi and Tamao reported the formation of Ph3SiLi (29Si: δ –9.1) via a boron–lithium exchange reaction between triphenylsilylborane and methyl lithium119,120. Previous work by our group showed that i-Pr3SiLi (29Si: δ 14.7) is the major product in the reaction of i-Pr3Si–B(pin) and MeLi, while the i-Pr3Si–B(pin)/MeLi ate complex (11B: δ 8.2) is generated as the minor product116. Similarly, two new 11B signals appeared when (±)-2a was treated with 1.5 equiv of MeLi in THF-d8 at –78 °C. However, in contrast to our previous results, the small signal was consistent with Me–B(pin) (11B: δ 33.5), while the large signal was tentatively attributed to the (±)-2a/MeLi ate complex (11B: δ 8.5). The 29Si NMR spectrum showed only one peak (δ –15.5), which most likely corresponds to (±)-{[(1,1’-biphenyl)-4-yl](cyclohexyl)(methyl)silyl}lithium. The 29Si signal of the (±)-2a/MeLi ate complex was not observed, probably due to dynamic processes in equilibrium and the adjacent quadrupolar boron atom121. We then carried out a 29Si{1H} NMR analysis at –95 °C, which revealed a broad peak (δ –17.1) that was tentatively assigned to (±)-{[(1,1’-biphenyl)-4-yl](cyclohexyl)(methyl)silyl}lithium, albeit that Si–Li coupling was not observed. These results indicate the presence of an equilibrium between (±)-{[(1,1’-biphenyl)-4-yl](cyclohexyl)(methyl)silyl}lithium and the (±)-2a/MeLi ate complex.
We then decided to investigate the stereospecificity and configurational stability of the silicon-stereogenic optically active silyl nucleophiles generated from the chiral silylboranes. As shown in Fig. 2C, the chiral silyl nucleophile can be expected to be formed first in the equilibrium between ate complex A and silyllithium intermediate B from (–)-(R)-2a (>99% ee) via treatment with methyl lithium in THF at –78 °C. The nucleophile was then quenched with 1.0 M aqueous HCl to give the corresponding chiral hydrosilanes (–)-(R)-1a in 83% yield with >99% ee (entry 1)79. The absolute configuration of (–)-(R)-1a remains unchanged, demonstrating that all processes proceed with retention of the configuration and perfect enantiospecificity (>99% es)79,81,90. Even when the reaction temperature was increased to –40 °C, (–)-(R)-1a was obtained with >99% ee, albeit that the yield decreased to 68% (entry 2), and 26% of (–)-(R)-2a was recovered (for details, see the Supplementary Information). Further increasing the reaction temperature to room temperature resulted in a decreased yield of 21%, while the enantiomeric purity remained high (entry 3, >99% ee). Only 17% of (–)-(R)-2a was recovered, and other unidentified side products were observed (for details, see the Supplementary Information). Next, we examined the nucleophilic activators for the silylboranes. When we used n-butyl lithium instead of methyl lithium, a yield of 54% was obtained with >99% ee (entry 4). With the more reactive and basic sec-butyl lithium, the yield was further reduced to only 20%, albeit the stereospecificity of the protonation remained unchanged (>99% ee) (entry 5). Interestingly, when lithium tert-butoxide was used as the nucleophile, the reaction did not proceed, even though lithium tert-butoxide has been reported to be a good activator for other silylboranes such as Me2PhSi–B(pin) (entry 6)122. With potassium tert-butoxide, only an 8% yield was obtained, while the erosion of the enantiomeric purity (>99% ee) was not observed (entry 7). When the reaction was carried out with methylmagnesium bromide, the enantiomeric excess of (–)-(R)-1a was reduced to 95% and the yield was very low (6%; entry 8). When toluene was used as the solvent, the yield of (–)-(R)-1a was 32% with >99% ee (entry 9), whereas the use of n-hexane as the solvent resulted in 43% yield with >99% ee, suggesting that a less polar solvent affects the yield but not the stereoselectivity (entry 10). In addition, when (–)-(R)-2a was treated with methyl lithium in THF at –78 °C for a longer reaction time of 2 h, (–)-(R)-1a was obtained in 87% yield with >99% ee after quenching with 1.0 M aqueous HCl (entry 11). These results demonstrate the high stability of the chiral ate complex and the silyllithium with regard to stereochemistry.
In order to investigate the reaction mechanism for the reaction of silylborane and MeLi, we conducted DFT calculations at the B3LYP-D3/Def2-SVP level (Fig. 2D). The reaction between model silylborane 2 and MeLi first forms complex INT-1 (–7.6 kcal/mol), which spontaneously transforms to a thermodynamically stable boronate complex INT-2 (–20.4 kcal/mol) through TS-1 (–1.1 kcal/mol) with a low activation barrier (G‡ = 6.5 kcal/mol). Boronate INT-2 proceeds via transition state TS-2 (–12.3 kcal/mol) to form silyl lithium INT-3 (–22.5 kcal/mol) while retaining its stereochemistry. The low activation barriers (G‡ = 8.1 and 10.2 kcal/mol) and an energy change of only –2.1 kcal/mol suggest an equilibrium between INT-2 and INT-3, which is consistent with the results of the NMR experiments described above (vide supra). The reaction of INT-3 with HCl forms INT-4, which is rapidly converted into protonation product 1 via TS-3 with a very low activation barrier (G‡ = 1.1 kcal/mol) with retention of the configuration. We hypothesize that the high stereospecificity observed during the generation of silyl anions can be attributed to the avoidance of generating silyl radicals and chloride ions, which can lead to racemization of the silicon center90,123.
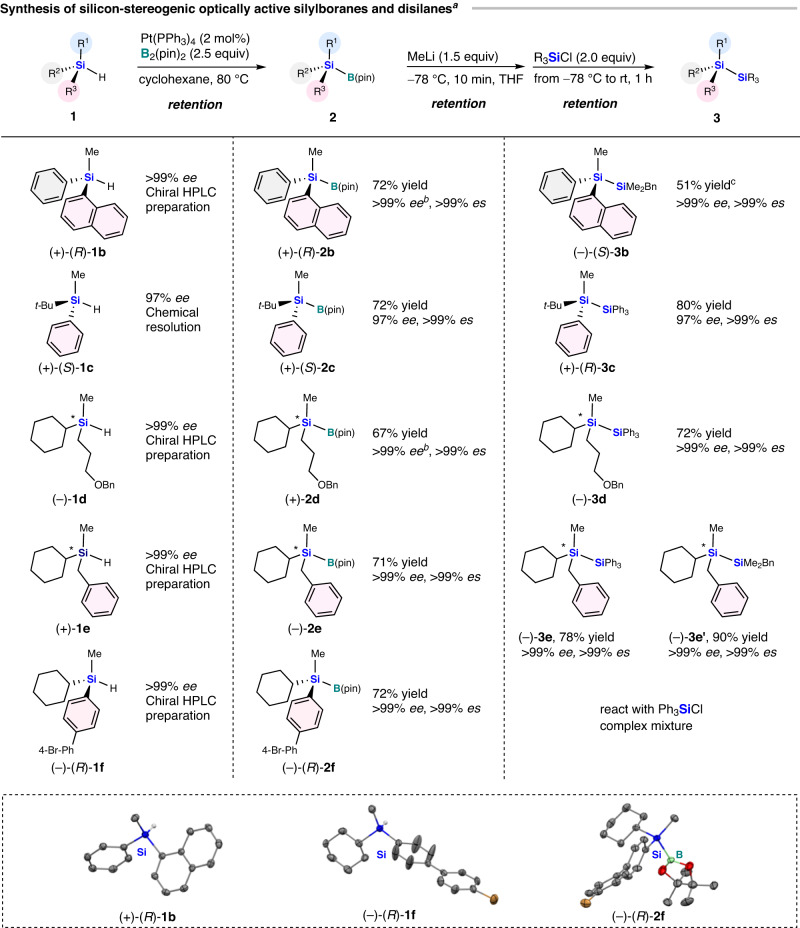
We also applied the stereospecific borylation and silylation to other silicon-stereogenic optically active hydrosilanes (Fig. 3). Most notably, ( + )-(R)-methyl(naphthalen-1-yl)phenylsilane [(+)-(R)-1b] (>99% ee), which bears two aryl groups and one alkyl group124, also reacted well in the borylation reaction to afford (+)-(R)-methyl(naphthalen-1-yl)phenyl(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)silane [(+)-(R)-2b] in 72% yield (>99% ee) (The enantiomeric purity of (+)-(R)-2b could not be evaluated on account of the poor separation on the chiral HPLC column. From (+)-(R)-1b and (–)-(S)-3b, we deduced that the enantiomeric purity of (+)-(R)-2b is >99% ee). The subsequent stereoretentive silicon–silicon bond-forming reaction of (+)-(R)-2b with benzylchlorodimethylsilane proceeded smoothly to afford (–)-(S)−1-benzyl-1,1,2-trimethyl−2-(naphthalen−1-yl)−2-phenyldisilane [(–)-(S)-3b] in moderate yield (51%) without loss of enantiomeric purity (>99% ee). The absolute configuration of (+)-(R)-1b was unambiguously confirmed by a single-crystal X-ray diffraction analysis after crystallization (for details, see the Supplementary Information), albeit the absolute configurations of (+)-(R)-2b and (–)-(S)-3b were deduced from the results for (+)-(R)-1b, because their single crystals suitable for XRD analysis were not obtained. ( + )-(S)-tert-Butyl(methyl)phenylsilane [(+)-(S)-1c], a chiral dialkylarylsilane, was prepared by the conventional optical resolution method reported by Oestreich125 (We synthesized (+)-(S)-1c according to a previously reported procedure (ref. 123) and used a different diastereomer to obtain the opposite enantiomer synthesized in ref. 123. The absolute configuration of (+)-(S)-1c was also confirmed by comparison to other reports (ref. 35)). ( + )-(S)-1c was easily converted into (+)-(S)-tert-butyl(methyl)phenyl(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)silane [(+)-(S)-2c] and (+)-(R)-1-(tert-butyl)-1-methyl-1,2,2,2-tetraphenyldisilane [(+)-(R)-3c] in high yield with perfect enantioselectivity [(+)-(S)-2c: 72% yield, 97% ee; ( + )-(R)-3c: 80% yield, 97% ee] (Similar to those of (+)-(R)-2b and (–)-(S)-3b, the absolute configuration of (+)-(S)-2c and (+)-(R)-3c was deduced from the above results due to the lack of their crystallinity). Trialkyl-substituted hydrosilane (–)-[3-(benzyloxy)propyl](cyclohexyl)methylsilane [(–)-1d] afforded the desired (+)-[3-(benzyloxy)propyl](cyclohexyl)methyl(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)silane [(+)-2d] in 67% yield with >99% ee. Subsequently, ( + )-2d was used in the silylation reaction with chlorotriphenylsilane to give (–)-1-[3-(benzyloxy)propyl]-1-cyclohexyl-1-methyl-2,2,2-triphenyldisilane [(–)-3d] in 72% yield with >99% ee, showing perfect stereospecificity of the reaction steps. Moreover, borylation of (+)-benzyl(cyclohexyl)methylsilane [(+)-1e] provided (–)-benzyl(cyclohexyl)methyl(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)silane [(–)-2e] in 71% yield with excellent enantioselectivity (>99% ee). Then, (–)-2e was treated with chlorotriphenylsilane or benzylchlorodimethylsilane to obtain (–)-1-benzyl-1-cyclohexyl-1-methyl-2,2,2-triphenyldisilane [(–)-3e] (78% yield, >99% ee) or (–)-1,2-dibenzyl-1-cyclohexyl-1,2,2-trimethyldisilane [(–)-3e’] (90% yield, >99% ee), respectively (We did not determine the absolute configuration of (–)-1d, (+)-2d, (–)-3d, (+)-1e, (–)-2e, (–)-3e, and (–)-3e’ because we could not obtain single crystals of sufficient quality for X-ray diffraction analysis). Moreover, (–)-(R)-[4’-bromo-(1,1’-biphenyl)−4-yl](cyclohexyl)methylsilane [(–)-(R)-1f] efficiently underwent the Pt(PPh3)4-catalyzed borylation to furnish (–)-(R)-[4’-bromo-(1,1’-biphenyl)-4-yl](cyclohexyl)methyl(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)silane [(–)-(R)-2f] in 72% yield with >99% ee. However, since the bromine substituent also reacts with methyl lithium to generate the aryl lithium species, the silicon–silicon bond-forming reaction of (–)-(R)-2f produced a relatively complex mixture. The absolute configuration of (–)-(R)-1f and (–)-(R)-2f was unambiguously confirmed by single-crystal X-ray diffraction analysis. These results clearly demonstrate that the Pt(PPh3)4-catalyzed borylation of various optically active hydrosilanes proceeds stereospecifically with retention of the configuration, followed by the stereospecific generation of silyl nucleophiles and their reaction with silicon electrophiles, all of which occur in a stereoretentive manner.
Fig. 3. Synthesis of silicon-stereogenic optically active silylboranes and disilanes.
aConditions for the borylation system: 1 (0.3 mmol), Pt(PPh3)4 (2 mol%), B2(pin)2 (0.75 mmol) in cyclohexane (0.3 mL) at 80 °C; conditions for the Si–Si bound formation reaction: 2 (0.1 mmol), MeLi (1.2 M in Et2O; 0.15 mmol), chlorosilane (0.2 mmol) in THF (0.5 mL). The yield values refer to isolated yields. The ee values were determined by chiral HPLC. bPresumed ee value. c(+)-(R)-2b with methyllithium in THF at –78 °C for 30 min.
The results described above clearly show that the Pt(PPh3)4-catalyzed borylation of the hydrosilanes proceeds stereoselectively to produce various chiral silylboranes. However, the synthesis of optically active starting hydrosilanes 1a–1f requires chiral HPLC separation or the use of a stoichiometric chiral source. To find a more efficient method, we examined the transition-metal-catalyzed preparation of chiral hydrosilanes and their stereoselective borylation (Fig. 4)18,59. For that purpose, we prepared chiral hydrosilane (+)-(R)-1g (94%, 97% ee; Fig. 4A) via the Rh-catalyzed enantioselective dehydrogenative Si‒O coupling reported by He and conducted the borylation to obtain silylborane (+)-(R)-2g with 95% ee18. The low yield (34%) and slight erosion of the enantiopurity can most likely be attributed to the instability of the silyl Pt intermediate with a Si‒O bond. Further reaction with MeLi and Ph3SiCl resulted in the formation of the chiral disilane (+)-(S)-3g in high yield with complete retention of configuration (83%, 95% ee). We also synthesized optically active hydrosilane (+)-(S)-1h (90%, 96% ee) from 5 using a chiral Rh catalyst according to the procedure reported by Wang59. ( + )-(S)-1h was subjected to a Pt-catalyzed borylation to give chiral silylborane (–)-(S)-2h in high enantiomeric excess (71%, 96% ee; Fig. 4B). These results show the high applicability of this Pt-catalyzed borylation for the highly efficient asymmetric synthesis of chiral silylboranes. To develop other efficient routes to chiral hydrosilanes, we also carried out a preliminary study on chiral ligand screening for platinum-, iridium-, nickel-, and rhodium-catalyzed asymmetric borylations of achiral tert-butyl(phenyl)silane with B2(pin)2, albeit that unfortunately mostly racemic products were obtained in most cases (for details, see the Supplementary Information).
Fig. 4. Synthesis and reaction of silicon-stereogenic optically active silylboranes from chiral hydrosilanes prepared by catalytic asymmetric synthesis.
A Rh-catalyzed enantioselective dehydrogenative Si‒O coupling. B Rh-catalyzed intramolecular hydrosilylation.
Subsequently, we investigated transition-metal-catalyzed silylation reactions with the silicon-stereogenic optically active silylboranes (Fig. 5A). In 2015, He’s group reported a palladium-catalyzed reaction of silylboranes with aryl bromides126. By modifying the reaction conditions, the more sterically hindered (–)-(R)-2a was made compatible with the reaction with no erosion of the enantiomeric excess, albeit the products were obtained only in moderate yield. When we conducted the reaction of (–)-(R)-2a with 1-bromonaphthalene, the corresponding (–)-(S)-(1,1’-biphenyl)−4-yl(cyclohexyl)methyl(naphthalen-1-yl)silane [(–)-(S)-6a] was obtained in 42% yield with outstanding enantiospecificity (>99% ee; >99% es). The absolute configuration of (–)-(S)-6a was confirmed by a single-crystal X-ray diffraction analysis, which revealed retention of the stereochemistry (Fig. 5A, for details, see the Supplementary Information).
Fig. 5. Transition metal-catalyzed reactions of silicon-stereogenic optically active silylboranes.
A Pd-catalyzed silylation of 1-bromonaphthalene. B Pd-catalyzed silylation of 1-(bromomethyl)naphthalene. C Cu-catalyzed silyl conjugate addition.
We further investigated transition-metal-catalyzed reactions for the introduction of a chiral silicon group. For that purpose, we performed a palladium-catalyzed silylation of primary alkyl halides with silylboranes, which was reported by Xu’s group in 2016127. The reaction between (–)-(R)-2a and 1-(bromomethyl)naphthalene proceeded effectively to afford the desired (–)-(S)-(1,1’-biphenyl)−4-yl(cyclohexyl)methyl(naphthalen-1-ylmethyl)silane [(–)-(S)-7a] in 76% yield in a completely stereoretentive manner (>99% ee; >99% es) (Fig. 5B). The absolute configuration of (–)-(S)-7a was unambiguously confirmed by a single-crystal X-ray diffraction analysis (Fig. 5B, for details, see the Supplementary Information).
We next examined the utility of silicon-stereogenic optically active silylborane in copper(I)-catalyzed conjugate addition of chiral silylborane and phenyl acrylate (Fig. 5C). In 2010, Hoveyda’s group reported the enantioselective conjugate silyl additions to unsaturated carbonyls catalyzed by a copper(I)/NHC system128. After modifying the reaction conditions, (–)-(R)-2a was successfully applied in this reaction, and (+)-phenyl {3-[(1,1’-biphenyl)−4-yl](cyclohexyl)(methyl)silyl}propanoate [(+)-8a] was obtained in 50% yield without loss of enantioselectivity (>99% ee; >99% es). It should furthermore be noted here that copper(I)-catalyzed conjugate reaction proceeded in a stereospecific manner, although it was not possible to determine the stereochemistry due to a lack of crystallinity of the obtained product.
Silicon-stereogenic optically active silylboranes were synthesized by a Pt(PPh3)4-catalyzed stereospecific borylation of chiral hydrosilanes. The corresponding chiral silyl nucleophiles generated from the chiral silylboranes are configurationally stable even at room temperature and react with chlorosilanes to yield the corresponding disilanes in a stereoretentive manner >99% enantiospecificity). The synthesized chiral silylboranes can also be used as silicon-stereogenic optically active silyl-group transfer reagents in various transition-metal-catalyzed silylation reactions. Combined with the recent catalytic asymmetric synthesis of chiral silanes, a wide range of chiral silylboranes will be possible in the future50–67. The present study can thus be expected to significantly expand the chemistry of chiral silylboranes, providing exciting opportunities to develop silicon-stereogenic optically active bioactive molecules, polymers, and optoelectronic materials.
Methods
Representative procedure for the platinum-catalyzed borylation of chiral hydrosilane
Chiral hydrosilane 1 (0.30 mmol, 1.0 equiv), bis(pinacolato)diboron (0.75 mmol, 2.5 equiv) were placed in a vial with a screw cap containing a Teflon®-coated rubber septum under air. The vial was placed in a glove box, and then Pt(PPh3)4 (0.006 mmol, 2.0 mol %) was added to the vial in the glove box under an argon atmosphere. After closing the vial, the reaction vial was removed from the glove box, and then dry cyclohexane (0.3 mL) was added to the vial via a syringe. After being stirred at 80 °C for 24 h, the reaction mixture was analyzed by GC to check the completeness of the reaction. The mixture was directly filtered through celite with Et2O as an eluent, and then the resultant solution was concentrated under reduced pressure. The crude product was purified by flash column chromatography (SiO2, hexane/Et2O, 100:0 to 99:1) to give the corresponding product 2.
Representative procedure for activation of chiral silylborane with methyl lithium followed by reaction with chlorosilane
Chiral silylborane 2 (0.10 mmol, 1.0 equiv) was placed in a vial with a screw cap containing a Teflon®-coated rubber septum. After the vial was connected to manifold with nitrogen and a vacuum line through a needle, it was evacuated and backfilled with nitrogen. This cycle was repeated three times. Dry THF (0.5 mL) was added to the vial through the rubber septum using a syringe, and the mixture was cooled to –78 °C. Then, MeLi (0.15 mmol, 1.5 equiv) was added to the vial. After the mixture was stirred at –78 °C for 10 min, chlorosilane (0.20 mmol, 2.0 equiv) was added dropwise to the vial at –78 °C. The mixture was allowed to warm to room temperature and stirred for 1 h. After that, the mixture was quenched by the addition of EtOH and filtered through a short silica-gel column with Et2O as an eluent, then the resultant solution was concentrated under reduced pressure. The crude product was purified by flash column chromatography (SiO2, hexane/Et2O, 100:0 to 99:1) and then further purified by GPC to give the corresponding product 3.
Supplementary information
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS) via KAKENHI grants 22H00318 (H.I.), 21H01926 (K.K.), 22H05328 (K.K.), and 22K18333 (H.I.) as well as by the JST via CREST grant JPMJCR19R1 (H.I.) and FOREST grant JPMJFR201I (K.K.) as well as by the Institute for Chemical Reaction Design and Discovery (ICReDD), which was established by the World Premier International Research Initiative (WPI), MEXT, Japan.
Author contributions
K.K. and H.I. conceived and designed the study. X.W., J.J., S.M., K.K., and H.I. co-wrote the paper. X.W. performed the chemical experiments and analyzed the data. C.F. measured and analyzed the single-crystal X-ray diffraction data. J.J. and S.M. conducted the theoretical study. All authors discussed the results and the manuscript.
Peer review
Peer review information
Nature Communications thanks Ming Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data reported in this paper are available in the main text or the Supplementary Information. Raw data are also available from the corresponding author on request. Materials and methods, experimental procedures, characterization data, 1H, 13C, 29Si, 11B NMR spectra, HPLC chromatograms and mass spectrometry data are available in the Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2193705, 2193706, 2193707, 2193708, 2193709, 2193710, 2193711, 2193712, and 2244288. Copies of the data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Koji Kubota, Email: kbt@eng.hokudai.ac.jp.
Hajime Ito, Email: hajito@eng.hokudai.ac.jp.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-41113-z.
References
- 1.Stephenson, G. R. Ed., Advanced Asymmetric Synthesis: State-of-the-art and Future Trends in Feature Technology (Springer Science & Business Media, B. V., 2012).
- 2.Akiyama, K., Ojima, K. I., Eds., Catalytic Asymmetric Synthesis, 4th edn (John Wiley & Sons, Inc., 2022).
- 3.Pawlenko, S., Organosilicon Chemistry (New York: De Gruyter, Berlin, 1986).
- 4.Hiyama, T., Oestreich, M., Eds., Organosilicon Chemistry: Novel Approaches and Reactions (Wiley-VCH, Weinheim, 2019).
- 5.Corriu, R. J. P., Guérin, C., Moreau J. J. E. “Stereochemistry at Silicon” in Topics in Stereochemistry (eds. Allnger, N. L., Eliel, E. L. & Wilen, S. H.) 43–198 (John Wiley & Sons, Inc., 1984).
- 6.Corriu, R. J. P., Guerin, C., Moreau, J. J. E. in Organic Silicon Compounds, (eds. Patai, S., Rappoport, Z.) 305–370 (John Wiley & Sons, Ltd, 1989).
- 7.Brook, M. A., Silicon in Organic, Organometallic, and Polymer Chemistry (John Wiley & Sons, Inc., 2000).
- 8.Hoveyda, A. H., Snapper M. L. Organosilicon Chemistry: Novel Approaches and Reactions (Wiley-VCH, Weinheim, 2019).
- 9.Igawa, K. & Tomooka, K. in Organosilicon Chemistry: Novel Approaches and Reactions (eds. Hiyama, T. & Oestreich, M.) 495–532 (Wiley-VCH, Weinheim, 2019).
- 10.Chan TH, Wang D. Chiral organosilicon compounds in asymmetric synthesis. Chem. Rev. 1992;92:995–1006. doi: 10.1021/cr00013a012. [DOI] [Google Scholar]
- 11.Oestreich M. Silicon-stereogenic silanes in asymmetric catalysis. Synlett. 2007;11:1629–1643. doi: 10.1055/s-2007-980385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weickgenannt A, Mewald M, Oestreich M. Asymmetric Si–O coupling of alcohols. Org. Biomol. Chem. 2010;8:1497–1504. doi: 10.1039/b925722e. [DOI] [PubMed] [Google Scholar]
- 13.Bauer JO, Strohmann C. Recent progress in asymmetric synthesis and application of difunctionalized silicon-stereogenic silanes. Eur. J. Inorg. Chem. 2016;2016:2868–2881. doi: 10.1002/ejic.201600100. [DOI] [Google Scholar]
- 14.Shimada M, et al. Multifunctional octamethyltetrasila[2.2]cyclophanes: conformational variations, circularly polarized luminescence, and organic electroluminescence. J. Am. Chem. Soc. 2017;139:11214–11221. doi: 10.1021/jacs.7b05671. [DOI] [PubMed] [Google Scholar]
- 15.Koga S, et al. Access to chiral silicon centers for application to circularly polarized luminescence materials. J. Org. Chem. 2017;82:6108–6117. doi: 10.1021/acs.joc.7b00583. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Yan N, Ju C-W, Zhao D. Nickel(0)-catalyzed asymmetric ring expansion toward enantioenriched silicon-stereogenic benzosiloles. Angew. Chem. Int. Ed. 2021;60:25723–25728. doi: 10.1002/anie.202111025. [DOI] [PubMed] [Google Scholar]
- 17.Guo Y, Liu M-M, Zhu X, Zhu L, He C. Catalytic asymmetric synthesis of silicon-stereogenic dihydrodibenzosilines: silicon central-to-axial chirality relay. Angew. Chem. Int. Ed. 2021;60:13887–13891. doi: 10.1002/anie.202103748. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, Chen S, He C. Catalytic enantioselective dehydrogenative Si−O coupling to access chiroptical silicon-stereogenic siloxanes and alkoxysilanes. J. Am. Chem. Soc. 2021;143:5301–5307. doi: 10.1021/jacs.1c01106. [DOI] [PubMed] [Google Scholar]
- 19.Chen S, et al. Enantioselective construction of six- and seven-membered triorgano-substituted silicon-stereogenic heterocycles. Nat. Commun. 2021;12:1249–1258. doi: 10.1038/s41467-021-21489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tacke R, et al. Syntheses and pharmacological characterization of achiral and chiral enantiopure C/Si/Ge−analogous derivatives of the muscarinic antagonist cycrimine: a study on C/Si/Ge bioisosterism. J. Organomet. Chem. 2001;640:140–165. doi: 10.1016/S0022-328X(01)01179-2. [DOI] [Google Scholar]
- 21.Tacke R, Heinrich T. Syntheses of enantiopure Si−centrochiral silicon-based muscarinic antagonists using an enantioselective enzymatic esterification as the key step. Silicon Chem. 2002;1:35–39. doi: 10.1023/A:1016095507813. [DOI] [Google Scholar]
- 22.Mutahi MW, Nittoli T, Guo L, Sieburth SM. Silicon-based metalloprotease inhibitors: synthesis and evaluation of silanol and silanediol peptide analogues as inhibitors of angiotensin-converting enzyme. J. Am. Chem. Soc. 2002;124:7363–7375. doi: 10.1021/ja026158w. [DOI] [PubMed] [Google Scholar]
- 23.Tacke R, Dörrich S. Drug design based on the carbon/silicon switch strategy. Top. Med. Chem. 2014;17:29–59. doi: 10.1007/7355_2014_55. [DOI] [Google Scholar]
- 24.Igawa K, Yoshihiro D, Abe Y, Tomooka K. Enantioselective synthesis of silacyclopentanes. Angew. Chem. Int. Ed. 2016;55:5814–5818. doi: 10.1002/anie.201511728. [DOI] [PubMed] [Google Scholar]
- 25.Kawakami Y, Li Y. Stereochemical study on polymers with stereogenic silicon atoms. J. Polym. Res. 2000;7:63–72. doi: 10.1007/s10965-006-0105-0. [DOI] [Google Scholar]
- 26.Kawakami Y, et al. Control of stereochemical structures of silicon-containing polymeric systems. Polym. Int. 2009;58:279–284. doi: 10.1002/pi.2524. [DOI] [Google Scholar]
- 27.Bauer JO, Strohmann C. From an α-functionalized silicon-stereogenic N,O-silane to a monomeric and tetracoordinate t-BuLi adduct with lithium-centered chirality. Angew. Chem. Int. Ed. 2014;53:8167–8171. doi: 10.1002/anie.201404255. [DOI] [PubMed] [Google Scholar]
- 28.Bauer JO, Strohmann C. Stereocontrol in nucleophilic substitution reactions at silicon: the role of permutation in generating silicon-centered chirality. J. Am. Chem. Soc. 2015;137:4304–4307. doi: 10.1021/jacs.5b00861. [DOI] [PubMed] [Google Scholar]
- 29.Barth ER, Krupp A, Langenohl F, Brieger L, Strohmann C. Kinetically controlled asymmetric synthesis of silicon-stereogenic methoxy silanes using a planar chiral ferrocene backbone. Chem. Commun. 2019;55:6882–6885. doi: 10.1039/C9CC03619A. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, et al. Asymmetric retro-[1,4]-brook rearrangement of 3-silyl allyloxysilanes via chirality transfer from silicon to carbon. RSC Adv. 2019;9:26209–26213. doi: 10.1039/C9RA05482K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandes A, et al. Chiral memory in silyl-pyridinium and quinolinium cations. J. Am. Chem. Soc. 2020;142:564–572. doi: 10.1021/jacs.9b11704. [DOI] [PubMed] [Google Scholar]
- 32.Zhou M, et al. Construction of tetrasubstituted silicon-stereogenic silanes via conformational isomerization and N‑heterocyclic carbene-catalyzed desymmetrization. ACS Catal. 2022;12:7781–7788. doi: 10.1021/acscatal.2c01082. [DOI] [Google Scholar]
- 33.Kipping FS. XXII.−Organic Derivatives of Silicon. Part II. The synthesis of benzylethylpropylsilicol, its sulphonation, and the resolution of the dl-sulphonic derivative into its optically active components. J. Chem. Soc. Trans. 1907;91:209–240. doi: 10.1039/CT9079100209. [DOI] [Google Scholar]
- 34.Sommer LH, Frye CLG, Parker A, Michael KW. Stereochemistry of Asymmetric Silicon. I. Relative and absolute configurations of optically active α-naphthylphenylmethylsilanes. J. Am. Chem. Soc. 1964;86:3271–3276. doi: 10.1021/ja01070a014. [DOI] [Google Scholar]
- 35.Klebe JF, Finkbeiner H. The asymmetric synthesis of an optically active difunctional silane. J. Am. Chem. Soc. 1966;88:4740–4741. doi: 10.1021/ja00972a048. [DOI] [Google Scholar]
- 36.Jankowski P, Schaumann E, Wicha J, Zarecki A, Adiwidjaja G. Facile synthesis of enantiomerically pure tert-butyl(methyl)phenylsilanes. Tetrahedron.: Asymmetry. 1999;10:519–526. doi: 10.1016/S0957-4166(99)00022-1. [DOI] [Google Scholar]
- 37.Kawachi A, Maeda H, Mitsudo K, Tamao K. Optical resolution and epimerization of fluorosilane having an optically active amino group: a new, convenient access to optically active silicon compounds. Organometallics. 1999;18:4530–4533. doi: 10.1021/om9904394. [DOI] [Google Scholar]
- 38.Xu L-W, Li L, Lai G-Q, Jiang J-X. The recent synthesis and application of silicon-stereogenic silanes: a renewed and significant challenge in asymmetric synthesis. Chem. Soc. Rev. 2011;40:1777–1790. doi: 10.1039/C0CS00037J. [DOI] [PubMed] [Google Scholar]
- 39.Tomooka K, Nakazaki A, Nakai T. A novel aryl migration from silicon to carbon: an efficient approach to asymmetric synthesis of α-aryl β-hydroxy cyclic amines and silanols. J. Am. Chem. Soc. 2000;122:408–409. doi: 10.1021/ja993295t. [DOI] [Google Scholar]
- 40.Trzoss M, Shao J, Bienz S. Preparation of a ‘Si-centered’ chiral auxiliary by resolution. Tetrahedron.: Asymmetry. 2004;15:1501–1505. doi: 10.1016/j.tetasy.2004.03.014. [DOI] [Google Scholar]
- 41.Rendler S, Auer G, Keller M, Oestreich M. Preparation of a privileged silicon-stereogenic silane: classical versus kinetic resolution. Adv. Synth. Catal. 2006;348:1171–1182. doi: 10.1002/adsc.200606071. [DOI] [Google Scholar]
- 42.Corriu RJP, Moreau JJE. Synthesis asymetriqlie d’alcoxysilanes catalysee pra des complexes du rhodium. Tetrahedron Lett. 1973;45:4469–4472. doi: 10.1016/S0040-4039(01)87251-8. [DOI] [Google Scholar]
- 43.Corriu RJP, Moreau JJE. Asymmetric hydrosilylation of ketones catalyzed by a chiral rhodium complex. J. Organomet. Chem. 1974;64:C51–C54. doi: 10.1016/S0022-328X(00)92172-7. [DOI] [Google Scholar]
- 44.Corriu RJP, Moreau JJE. Asymmetric synthesis at silicon II. alcoholysis of prochiral organosilicon compounds catalysed by rhodium complexs. J. Organomet. Chem. 1976;120:337–346. doi: 10.1016/S0022-328X(00)98043-4. [DOI] [Google Scholar]
- 45.Ohta T, Ito M, Tsuneto A, Takaya H. Asymmetric synthesis of silanes with a stereogenic centre at silicon via hydrosilylation of symmetric ketones with prochiral diary1 silanes catalysed by binap-RhI complexes. J. Chem. Soc., Chem. Commun. 1994;1994:2525–2526. doi: 10.1039/C39940002525. [DOI] [Google Scholar]
- 46.Tamao K, Nakamura K, Ishii H, Yamaguchi S, Shiro M. Axially chiral spirosilanes via catalytic asymmetric intramolecular hydrosilation. J. Am. Chem. Soc. 1996;118:12469–12470. doi: 10.1021/ja962754c. [DOI] [Google Scholar]
- 47.Schmidt DR, O’Malley SJ, Leighton J. L. catalytic asymmetric silane alcoholysis: practical access to chiral silanes. J. Am. Chem. Soc. 2003;125:1190–1191. doi: 10.1021/ja0283201. [DOI] [PubMed] [Google Scholar]
- 48.Igawa K, Takada J, Shimono T, Tomooka K. Enantioselective synthesis of silanol. J. Am. Chem. Soc. 2008;130:16132–16133. doi: 10.1021/ja805848z. [DOI] [PubMed] [Google Scholar]
- 49.Kuninobu Y, Yamauchi K, Tamura N, Seiki T, Takai K. Rhodium-catalyzed asymmetric synthesis of spirosilabifluorene derivatives. Angew. Chem. Int. Ed. 2013;52:1520–1522. doi: 10.1002/anie.201207723. [DOI] [PubMed] [Google Scholar]
- 50.Xu L-W. Desymmetrization catalyzed by transition-metal complexes: enantioselective formation of silicon-stereogenic silanes. Angew. Chem. Int. Ed. 2012;51:12932–12934. doi: 10.1002/anie.201207932. [DOI] [PubMed] [Google Scholar]
- 51.Shintani R. Recent advances in the transition-metal-catalyzed enantioselective synthesis of silicon-stereogenic organosilanes. Asian J. Org. Chem. 2015;4:510–514. doi: 10.1002/ajoc.201500066. [DOI] [Google Scholar]
- 52.Cui Y-M, Lin Y, Xu L-W. Catalytic synthesis of chiral organoheteroatom compounds of silicon, phosphorus, and sulfur via asymmetric transition metal-catalyzed C–H functionalization. Coord. Chem. Rev. 2017;330:37–52. doi: 10.1016/j.ccr.2016.09.011. [DOI] [Google Scholar]
- 53.Shintani R. Recent progress in catalytic enantioselective desymmetrization of prochiral organosilanes for the synthesis of silicon-stereogenic compounds. Synlett. 2018;29:388–396. doi: 10.1055/s-0036-1591839. [DOI] [Google Scholar]
- 54.Shintani R. Catalytic asymmetric synthesis of silicon-stereogenic compounds by enantioselective desymmetrization of prochiral tetraorganosilanes. J. Synth. Org. Chem., Jpn. 2018;76:1163–1169. doi: 10.5059/yukigoseikyokaishi.76.1163. [DOI] [Google Scholar]
- 55.Diesel J, Cramer N. Generation of heteroatom stereocenters by enantioselective C−H functionalization. ACS Catal. 2019;9:9164–9177. doi: 10.1021/acscatal.9b03194. [DOI] [Google Scholar]
- 56.Zheng L, Nie X-X, Wu Y, Wang P. Construction of Si-stereogenic silanes through C–H activation approach. Eur. J. Org. Chem. 2021;2021:6006–6014. doi: 10.1002/ejoc.202101084. [DOI] [Google Scholar]
- 57.Zhang M, et al. Asymmetric synthesis of chiral organosilicon compounds via transition metal-catalyzed stereoselective C–H activation and silylation. Chem. Commun. 2021;57:8250–8263. doi: 10.1039/D1CC02839A. [DOI] [PubMed] [Google Scholar]
- 58.Yuan W, He C. Enantioselective C–H functionalization toward silicon-stereogenic silanes. Synthesis. 2022;54:1939–1950. doi: 10.1055/a-1729-9664. [DOI] [Google Scholar]
- 59.Huang Y-H, et al. Enantioselective synthesis of silicon-stereogenic monohydrosilanes by rhodium-catalyzed intramolecular hydrosilylation. Angew. Chem. Int. Ed. 2022;61:e202113052. doi: 10.1002/anie.202113052. [DOI] [PubMed] [Google Scholar]
- 60.Lu W, Zhao Y, Meng F. Cobalt-catalyzed sequential site- and stereoselective hydrosilylation of 1,3- and 1,4-enynes. J. Am. Chem. Soc. 2022;144:5233–5240. doi: 10.1021/jacs.2c00288. [DOI] [PubMed] [Google Scholar]
- 61.An K, et al. Rhodium hydride enabled enantioselective intermolecular C–H silylation to access acyclic stereogenic Si–H. Nat. Commun. 2022;13:847–857. doi: 10.1038/s41467-022-28439-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen S, Zhu J, Ke J, Li Y, He C. Enantioselective intermolecular C−H silylation of heteroarenes for the synthesis of acyclic Si-stereogenic silanes. Angew. Chem. Int. Ed. 2022;61:e202117820. doi: 10.1002/anie.202117820. [DOI] [PubMed] [Google Scholar]
- 63.Yuan W, Zhu X, Xu Y, He C. Synthesis of Si-stereogenic silanols by catalytic asymmetric hydrolytic oxidation. Angew. Chem. Int. Ed. 2022;61:e202204912. doi: 10.1002/anie.202204912. [DOI] [PubMed] [Google Scholar]
- 64.Yang W, et al. Enantioselective hydroxylation of dihydrosilanes to Si-chiral silanols catalyzed by in situ generated copper(II) species. Angew. Chem. Int. Ed. 2022;61:e202205743. doi: 10.1002/anie.202205743. [DOI] [PubMed] [Google Scholar]
- 65.Wang L, Lu W, Zhang J, Chong Q, Meng F. Cobalt-catalyzed regio-, diastereo- and enantioselective intermolecular hydrosilylation of 1,3-dienes with prochiral silanes. Angew. Chem. Int. Ed. 2022;61:e202205624. doi: 10.1002/anie.202205624. [DOI] [PubMed] [Google Scholar]
- 66.Gao J, et al. Copper-catalyzed desymmetrization of prochiral silanediols to silicon-stereogenic silanols. ACS Catal. 2022;12:8476–8483. doi: 10.1021/acscatal.2c02482. [DOI] [Google Scholar]
- 67.Zhou H, et al. Organocatalytic asymmetric synthesis of Si-stereogenic silyl ethers. J. Am. Chem. Soc. 2022;144:10156–10161. doi: 10.1021/jacs.2c04261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lickiss PD, Smith CM. Silicon derivatives of the metals of groups 1 and 2. Coord. Chem. Rev. 1995;145:75–124. doi: 10.1016/0010-8545(95)90218-X. [DOI] [Google Scholar]
- 69.Tamao, K., Kawachi, A. in Advances in Organometallic Chemistry, (eds. Stone, F. G. A. & West, R.) 1–58 (Academic Press, 1995).
- 70.Sekiguchi A, Lee VY, Nanjo M. Lithiosilanes and their application to the synthesis of polysilane dendrimers. Coord. Chem. Rev. 2000;210:11–45. doi: 10.1016/S0010-8545(00)00315-5. [DOI] [Google Scholar]
- 71.Lerner H-W. Silicon derivatives of group 1, 2, 11 and 12 elements. Coord. Chem. Rev. 2005;249:781–798. doi: 10.1016/j.ccr.2004.08.020. [DOI] [Google Scholar]
- 72.Gilman H, Lichtenwalter GD. Cleavage of symmetrically substituted disilanes by lithium in tetrahydrofuran. J. Am. Chem. Soc. 1958;80:608–611. doi: 10.1021/ja01536a025. [DOI] [Google Scholar]
- 73.Still WC. Conjugate addition of trimethylsilyllithium. A preparation of 3-silyl ketones. J. Org. Chem. 1976;41:3063–3064. doi: 10.1021/jo00880a044. [DOI] [Google Scholar]
- 74.Tamao K, Kawachi A, Ito Y. The first stable functional silyl anions: (aminosily1)lithiums. J. Am. Chem. Soc. 1992;114:3989–3990. doi: 10.1021/ja00036a064. [DOI] [Google Scholar]
- 75.Tamao K, Kawachi A. The chemistry of silylenoids: preparation and reactivity of (alkoxysily1)lithium compounds. Angew. Chem. Int. Ed. 1995;34:818–820. doi: 10.1002/anie.199508181. [DOI] [Google Scholar]
- 76.Xue W, Shishido R, Oestreich M. Bench-stable stock solutions of silicon grignard reagents: application to iron- and cobalt-catalyzed radical C(sp3)–Si cross-coupling reactions. Angew. Chem. Int. Ed. 2018;57:12141–12145. doi: 10.1002/anie.201807640. [DOI] [PubMed] [Google Scholar]
- 77.Wang J, et al. Salt-stabilized silylzinc pivalatesfor nickel-catalyzed carbosilylation of alkenes. Angew. Chem. Int. Ed. 2022;61:e2022023. doi: 10.1002/anie.202202379. [DOI] [PubMed] [Google Scholar]
- 78.Sommer LH, Mason R. Optically active R3Si*Li from lithium metal cleavage of an optically active disilane. J. Am. Chem. Soc. 1965;87:1619–1620. doi: 10.1021/ja01085a042. [DOI] [Google Scholar]
- 79.Omote M, et al. Stereospecific formation of optically active trialkylsilyllithiums and their configurational stability. J. Organomet. Chem. 2000;611:20–25. doi: 10.1016/S0022-328X(00)00294-1. [DOI] [Google Scholar]
- 80.Strohmann C, Hörnig J, Auer D. Synthesis of a highly enantiomerically enriched silyllithium compound. Chem. Commun. 2002;2002:766–767. doi: 10.1039/b111687h. [DOI] [PubMed] [Google Scholar]
- 81.Strohmann C, Bindl M, Fraaß VC, Hörnig J. Enantiodivergence in the reactions of a highly enantiomerically enriched silyllithium compound with benzyl halides: control of inversion and retention by selection of halide. Angew. Chem. Int. Ed. 2004;43:1011–1014. doi: 10.1002/anie.200352700. [DOI] [PubMed] [Google Scholar]
- 82.Strohmann C, Däschlein C, Kellert M, Auer D. A Highly enantiomerically enriched lithiosilane by selective cleavage of a silicon–phenyl bond with lithium. Angew. Chem. Int. Ed. 2007;46:4780–4782. doi: 10.1002/anie.200604636. [DOI] [PubMed] [Google Scholar]
- 83.Däschlein C, Bauer SO, Strohmann C. Mechanistic insights into the reaction of enantiomerically pure lithiosilanes and electrophiles: Understanding the differences between aryl and alkyl halides. Eur. J. Inorg. Chem. 2011;2011:1454–1465. doi: 10.1002/ejic.201000834. [DOI] [Google Scholar]
- 84.Strohmann C, Däschlein C. Synthesis of a highly enantiomerically enriched silagermane and selective cleavage of the Si–Ge bond with lithium. Organometallics. 2008;27:2499–2504. doi: 10.1021/om700848z. [DOI] [Google Scholar]
- 85.Oh H-S, Imae I, Kawakami Y, Raj SSS, Yamane T. Synthesis, stereochemistry and chiroptical properties of naphthylphenyl-substituted optically active oligosilanes with α,ω-chiral silicon centers. J. Organomet. Chem. 2003;685:35–43. doi: 10.1016/S0022-328X(03)00169-4. [DOI] [Google Scholar]
- 86.Oh H-S, Imae I, Kawakami Y. Evaluation of absolute configuration of naphthylphenyl-substituted oligosilanes by CD exciton chirality method. Chirality. 2003;15:231–237. doi: 10.1002/chir.10192. [DOI] [PubMed] [Google Scholar]
- 87.Suzuki K, Kawakami Y, Velmurugan D, Yamane T. Stereoselective synthesis of optically active disilanes and selective functionalization by the cleavage of silicon-naphthyl bonds with bromine. J. Org. Chem. 2004;69:5383–5389. doi: 10.1021/jo049398y. [DOI] [PubMed] [Google Scholar]
- 88.Colomer E, Corriu R. Optically active silylanions. Evidence for the formation of analogues of silyl grignard reagents. J. Chem. Soc. Chem. Commun. 1976;1976:176–177. doi: 10.1039/c39760000176. [DOI] [Google Scholar]
- 89.Colomer E, Corriu RJP. On the behaviour of an optically active cobalt–silicon bond. Evidence for the formation of analogues of silyl–Grignard reagents. J. Organomet. Chem. 1977;133:159–168. doi: 10.1016/S0022-328X(00)92869-9. [DOI] [Google Scholar]
- 90.Oestreich M, Auer G, Keller M. On the mechanism of the reductive metallation of asymmetrically substituted silyl chlorides. Eur. J. Org. Chem. 2005;2005:184–195. doi: 10.1002/ejoc.200400622. [DOI] [Google Scholar]
- 91.Igawa K, Kokan N, Tomooka K. Asymmetric synthesis of chiral silacarboxylic acids and their ester derivatives. Angew. Chem. Int. Ed. 2010;49:728–731. doi: 10.1002/anie.200904922. [DOI] [PubMed] [Google Scholar]
- 92.Suginome M, Ito Y. Transition-metal-catalyzed additions of silicon−silicon and silicon−heteroatom bonds to unsaturated organic molecules. Chem. Rev. 2000;100:3221–3256. doi: 10.1021/cr9902805. [DOI] [PubMed] [Google Scholar]
- 93.Suginome M, Ito Y. Regio- and stereoselective synthesis of boryl-substituted allylsilanes via transition metal-catalyzed silaboration. J. Organomet. Chem. 2003;680:43–50. doi: 10.1016/S0022-328X(03)00441-8. [DOI] [Google Scholar]
- 94.Beletskaya I, Moberg C. Element−element additions to unsaturated carbon−carbon bonds catalyzed by transition metal complexes. Chem. Rev. 2006;106:2320–2354. doi: 10.1021/cr050530j. [DOI] [PubMed] [Google Scholar]
- 95.Ohmura T, Suginome M. Silylboranes as new tools in organic synthesis. Bull. Chem. Soc. Jpn. 2009;82:29–49. doi: 10.1246/bcsj.82.29. [DOI] [Google Scholar]
- 96.Oestreich M, Hartmann E, Mewald M. Activation of the Si−B interelement bond: mechanism, catalysis, and synthesis. Chem. Rev. 2013;113:402–441. doi: 10.1021/cr3003517. [DOI] [PubMed] [Google Scholar]
- 97.Feng J-J, Mao W, Zhang L, Oestreich M. Activation of the Si–B interelement bond related to catalysis. Chem. Soc. Rev. 2021;50:2010–2073. doi: 10.1039/D0CS00965B. [DOI] [PubMed] [Google Scholar]
- 98.Kubota. K., Ito, H. “Catalytic Generation of Silicon Nucleophiles” in Organosilicon Chemistry: Novel Approaches and Reactions, Hiyama, T., Oestreich, M. Eds. (Wiley-VCH, Weinheim, 2019)
- 99.Cowley AH, Sisler HH, Ryschkewit GE. The chemistry of borazine. III. B-silyl borazines. J. Am. Chem. Soc. 1960;82:501–502. doi: 10.1021/ja01487a069. [DOI] [Google Scholar]
- 100.Seyferth D, Kögler HP. Preparation of organosilicon-substituted borazenes. J. Inorg. Nucl. Chem. 1960;15:99–104. doi: 10.1016/0022-1902(60)80016-4. [DOI] [Google Scholar]
- 101.Nöth H, Höllerer G. Beitrage zur chemie des bors, XXXVI. organylsilylborane. Chem. Ber. 1966;99:2197–2205. doi: 10.1002/cber.19660990718. [DOI] [Google Scholar]
- 102.Jiang Q, Carroll PJ, Berry DH. Metal-mediated formation of boron-silicon bonds. synthesis and characterization of ηl- and η2-silylborohydride complexes of tantalum. Organometallics. 1993;12:177–183. doi: 10.1021/om00025a031. [DOI] [Google Scholar]
- 103.Blumenthal A, Bissinger P, Schmidbaur H. The crystal and molecular structure of tricyclohexylphosphine-(trimethylsilyl)borane Cy3P·BH2SiMe3. J. Organomet. Chem. 1993;462:107–110. doi: 10.1016/0022-328X(93)83347-X. [DOI] [Google Scholar]
- 104.Buynak JD, Geng B. Synthesis and reactivity of silylboranes. Organometallics. 1995;14:3112–3115. doi: 10.1021/om00006a064. [DOI] [Google Scholar]
- 105.Metzler N, Denk M. Synthesis of a silylene-borane adduct and its slow conversion to a silylborane. Chem. Commun. 1996;1996:2567–2568. [Google Scholar]
- 106.Suginome M, Matsuda T, Ito Y. Convenient preparation of silylboranes. Organometallics. 2000;19:4647–4649. doi: 10.1021/om000254t. [DOI] [Google Scholar]
- 107.Takeda N, Kajiwara T, Tokitoh N. Reaction of stable silylene–isocyanide complexes with boranes: synthesis and properties of the first stable silylborane–isocyanide complexes. Chem. Lett. 2001;30:1076–1077. doi: 10.1246/cl.2001.1076. [DOI] [PubMed] [Google Scholar]
- 108.Kajiwara T, Takeda N, Sasamori T, Tokitoh N. Insertion of an overcrowded silylene into hydro- and haloboranes: a novel synthesis of silylborane derivatives and their properties. Organometallics. 2004;23:4723–4734. doi: 10.1021/om049558w. [DOI] [Google Scholar]
- 109.Kajiwara T, Takeda N, Sasamori T, Tokitoh N. Unprecedented insertion reaction of a silylene into a B–B bond and generation of a novel borylsilyl anion by boron–metal exchange reaction of the resultant diborylsilane. Chem. Commun. 2004;2004:2218–2219. doi: 10.1039/B409575H. [DOI] [PubMed] [Google Scholar]
- 110.Ohmura T, Masuda K, Furukawa H, Suginome M. Synthesis of silylboronic esters functionalized on silicon. Organometallics. 2007;26:1291–1294. doi: 10.1021/om0609867. [DOI] [Google Scholar]
- 111.Boebel TA, Hartwig JF. Iridium-catalyzed preparation of silylboranes by silane borylation and their use in the catalytic borylation of arenes. Organometallics. 2008;27:6013–6019. doi: 10.1021/om800696d. [DOI] [Google Scholar]
- 112.Tsurusaki A, Yoshida K, Kyushin S. Synthesis and structures of lithium alkoxytris-(dimethylphenylsilyl)borates. Dalton Trans. 2017;46:8705–8708. doi: 10.1039/C7DT00395A. [DOI] [PubMed] [Google Scholar]
- 113.Liu Z, Cui C. Reaction of a boryl anion with silicon halides and alkoxysilanes: synthesis of borylsilanes. J. Organomet. Chem. 2020;906:121041–121047. doi: 10.1016/j.jorganchem.2019.121041. [DOI] [Google Scholar]
- 114.Kamio S, Imagawa T, Nakamoto M, Oestreich M, Yoshida H. HMPA-free generation of trialkylsilyllithium reagents and its applications to the synthesis of silylboronic esters. Synthesis. 2021;53:4678–4681. doi: 10.1055/a-1558-9135. [DOI] [Google Scholar]
- 115.Yoshida H, et al. A stable silylborane with diminished boron lewis acidity. Dalton Trans. 2022;51:6543–6546. doi: 10.1039/D2DT00962E. [DOI] [PubMed] [Google Scholar]
- 116.Shishido R, et al. General synthesis of trialkyl- and dialkylarylsilylboranes: versatile silicon nucleophiles in organic synthesis. J. Am. Chem. Soc. 2020;142:14125–14133. doi: 10.1021/jacs.0c03011. [DOI] [PubMed] [Google Scholar]
- 117.Takeuchi T, Shishido R, Kubota K, Ito H. Synthesis of hydrosilylboronates via the monoborylation of a dihydrosilane Si–H bond and their application for the generation of dialkylhydrosilyl Anions. Chem. Sci. 2021;12:11799–11804. doi: 10.1039/D1SC01440D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim SH, et al. Zirconocene complexes as catalysts for the cycloaddition of CO2 to propylene oxide. Eur. J. Inorg. Chem. 2014;2014:5107–5112. doi: 10.1002/ejic.201402782. [DOI] [Google Scholar]
- 119.Kawachi A, Minamimoto T, Tamao K. Boron−metal exchange reaction of silylboranes with organometallic reagents: a new route to aryl silyl anions. Chem. Lett. 2001;30:1216–1217. doi: 10.1246/cl.2001.1216. [DOI] [Google Scholar]
- 120.Edlund U, Lejon T. Evidence on covalency and monomeric structure of (phenylsilyl)lithiums in ethereal solutions from scalar 29Si−6(7)Li couplings. J. Am. Chem. Soc. 1985;107:6408–6409. doi: 10.1021/ja00308a051. [DOI] [Google Scholar]
- 121.Kleeberg C, Borner C. On the reactivity of silylboranes toward lewis bases: heterolytic B–Si cleavage vs. adduct formation. Eur. J. Inorg. Chem. 2013;2013:2799–2806. doi: 10.1002/ejic.201300119. [DOI] [Google Scholar]
- 122.Xue W, Qu Z-W, Grimme S, Oestreich M. Copper-catalyzed cross-coupling of silicon pronucleophiles with unactivated alkyl electrophiles coupled with radical cyclization. J. Am. Chem. Soc. 2016;138:14222–14225. doi: 10.1021/jacs.6b09596. [DOI] [PubMed] [Google Scholar]
- 123.Chatgilialoglu C. Structural and chemical properties of silyl radicals. Chem. Rev. 1995;95:1229–1251. doi: 10.1021/cr00037a005. [DOI] [Google Scholar]
- 124.Tokoro Y, Sugita K, Fukuzawa S-I. Synthesis of silaphenalenes by ruthenium-catalyzed annulation between 1-naphthylsilanes and internal alkynes through C–H bond Cleavage. Chem. Eur. J. 2015;21:13229–13232. doi: 10.1002/chem.201502746. [DOI] [PubMed] [Google Scholar]
- 125.Trepohl VT, Fröhlich R, Oestreich M. Conjugate phosphination of cyclic and acyclic acceptors using Rh(I)–phosphine or Rh(I)–carbene complexes. Probing the mechanism with chirality at the silicon atom or the phosphorus atom of the Si–P reagent. Tetrahedron. 2009;65:6510–6518. doi: 10.1016/j.tet.2009.04.038. [DOI] [Google Scholar]
- 126.Guo H, Chen X, Zhao C, He W. Suzuki-type cross coupling between aryl halides and silylboranes for the syntheses of aryl silanes. Chem. Commun. 2015;51:17410–17412. doi: 10.1039/C5CC07071F. [DOI] [PubMed] [Google Scholar]
- 127.Huang Z-D, Ding R, Wang P, Xu Y-H, Loh T-P. Palladium-catalyzed silylation reaction between benzylic halides and silylboronate. Chem. Commun. 2016;52:5609–5612. doi: 10.1039/C6CC00713A. [DOI] [PubMed] [Google Scholar]
- 128.Lee K-s, Hoveyda AH. Enantioselective conjugate silyl additions to cyclic and acyclic unsaturated carbonyls catalyzed by Cu complexes of chiral N-heterocyclic carbenes. J. Am. Chem. Soc. 2010;132:2898–2900. doi: 10.1021/ja910989n. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data reported in this paper are available in the main text or the Supplementary Information. Raw data are also available from the corresponding author on request. Materials and methods, experimental procedures, characterization data, 1H, 13C, 29Si, 11B NMR spectra, HPLC chromatograms and mass spectrometry data are available in the Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2193705, 2193706, 2193707, 2193708, 2193709, 2193710, 2193711, 2193712, and 2244288. Copies of the data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif.