Abstract
Epithelial-to-mesenchymal transitions (EMTs) of both endocardium and epicardium guide atrioventricular heart valve formation, but the cellular complexity and small scale of this tissue have restricted analyses. To circumvent these issues, we analyzed over 50,000 murine single-cell transcriptomes from embryonic day (E)7.75 hearts to E12.5 atrioventricular canals. We delineate mesenchymal and endocardial bifurcation during endocardial EMT, identify a distinct, transdifferentiating epicardial population during epicardial EMT, and reveal the activation of epithelial-mesenchymal plasticity during both processes. In Sox9-deficient valves, we observe increased epithelial-mesenchymal plasticity, indicating a role for SOX9 in promoting endothelial and mesenchymal cell fate decisions. Lastly, we deconvolve cell interactions guiding the initiation and progression of cardiac valve EMTs. Overall, these data reveal mechanisms of emergence of mesenchyme from endocardium or epicardium at single-cell resolution and will serve as an atlas of EMT initiation and progression with broad implications in regenerative medicine and cancer biology.
Subject terms: Epithelial-mesenchymal transition, Transdifferentiation, Epithelial-mesenchymal transition, Transcriptomics
Lotto et al. delineate cell diversity and mechanisms during heart valve development using scRNA-seq. They identify distinct cell types and states, the emergence of epithelial-mesenchymal plasticity, and cell interactions that may govern this process.
Introduction
The atrioventricular (AV) valves are unique structures composed of three extracellular matrix (ECM) layers ensheathed by valve endocardial cells (VECs) and populated by valve interstitial cells (VICs)1. VICs, which are specialized cardiac fibroblasts, are generally quiescent under homoeostatic conditions, and their activation is intimately associated with valve disease states, including calcification and regurgitation2,3. It is thought that this pathogenic, activated state may recapitulate developmental processes; however, few specific parallels have been elucidated due to our limited understanding of valvulogenesis.
During mouse embryogenesis, AV mesenchyme, which comprises the progenitor pool for VICs, is formed early in development through two epithelial-to-mesenchymal transitions (EMTs) from distinct cellular sources: endocardium and epicardium4–6 (Fig. 1a). Around E8.5, a constriction forms at the junction between the atria and ventricles, where myocardial hyaluronan and fibronectin accumulate, establishing the primitive valves, called the AV cushions4,7. Around this stage, the early heart also begins to beat, increasing the hemodynamic load exerted on the endocardium8. By E9.0, fluid shear stress from blood flow and signals from the myocardium, including BMPs, induce an endocardial-to-mesenchymal transition (EndMT) in a subset of endocardial cells lining the cushions9–13. These cells lose their endothelial cell-cell contacts, invade the ECM within the AV cushions, and adopt a mesenchymal phenotype (Fig. 1a). This transdifferentiation event is controlled by various transcription factors, like SNAI214, GATA415, and SOX916,17, which are activated in response to cues from neighbouring cell types, including the TGFβ superfamily9,10, WNT18, and NOTCH19,20. VEGF signalling is also implicated – inducing NFATc1 expression, which represses EndMT and maintains endothelial identity in non-transitioning endocardium21.
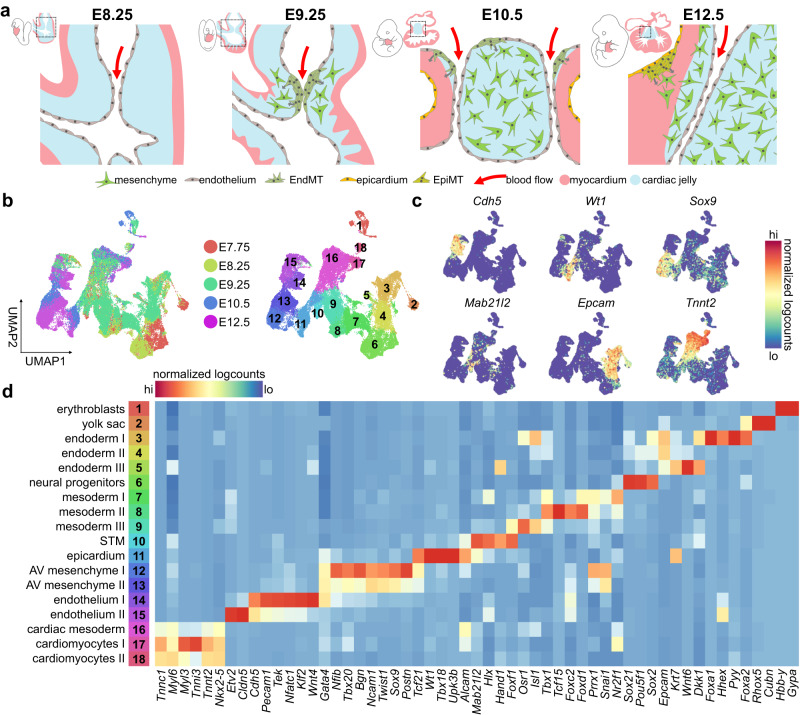
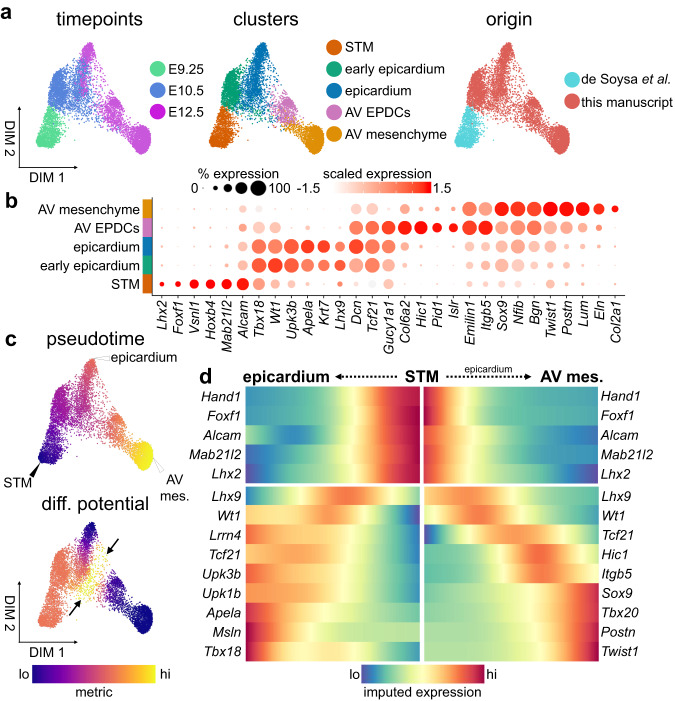
Fig. 1. Tracking the emergent lineages of the heart and early atrioventricular canal.
a Overview of murine atrioventricular development, highlighting the endocardial-to-mesenchymal transition (EndMT) at E9.25-10.5 and the epicardial-to-mesenchymal transition (EpiMT) at E12.5. b UMAP visualization of 48,822 cells from E7.75-E9.25 hearts and E10.5-E12.5 AV canals. Cell types are denoted by colour and number in legend in panel d. c UMAP visualization of select gene expression for endothelial, epicardial, mesenchymal, and myocardial biomarkers. d Select differentially expressed genes in complete dataset, showing emergence of endothelial, epicardial, myocardial, and mesenchymal populations. AV atrioventricular, STM septum transversum mesenchyme. See also Supplementary Fig. 1 and Supplementary Data 1–2. Source Data are provided as a Source Data file.
AV mesenchymal cells are also generated from the epicardium via the epicardial-to-mesenchymal transition (EpiMT) starting around E12.5 (Fig. 1a). The epicardium is the cardiac mesothelium, derived from septum transversum mesenchyme (STM), from which a subset of cells extend across the pericardial space to populate the surface of the heart22. Via EpiMT, epicardial cells contribute to smooth muscle cells, vasculature, pericytes, and fibroblasts, including those of the atrioventricular canal (AVC) and the annulus fibrosus5,6,23,24. However, the processes underlying AV EpiMT remain poorly characterized, as the few studies assessing EpiMT focus on chamber epicardium25–29.
Once EndMT and EpiMT are complete, AV mesenchymal cells drive AV septation, delamination of the cushions, and remodelling to form the three laminae of ECM. Maturation of valve cell lineages also continues postnatally30. While our understanding of atrioventricular development has improved greatly with the advent of genomics research, the details underlying many aspects of AV EMTs are incompletely characterized, mainly due to limitations related to the size of the embryonic valves. These include the cellular diversity present in this tissue during EMT; the mechanisms of initiation, progression, and resolution of these processes; and the comparison of EMT of endocardial and epicardial origin that contribute to AV mesenchyme. Mechanisms underlying the formation of the valves is pertinent to understanding the aetiology of congenital defects and disease, including cardiac fibrosis and valve disease, where EndMT genetic programmes may reactivate to drive pathogenesis31,32. Of note, defining details controlling EMT initiation and progression may further elucidate mechanisms that drive cancer metastasis as significant parallels exist between these processes33. In this work, we apply scRNA-seq to reveal mechanisms of emergence of mesenchyme from endocardium and epicardium and provide histological data to show the spatiotemporal dynamics during these processes.
Results
Single-cell transcriptomics captures early valvulogenesis
Single-cell RNA sequencing (scRNA-seq) has been successfully applied to chart transcriptional diversity within the early embryo34,35, heart36, liver37,38, and others. In the present study, we generated five scRNA-seq libraries from E10.5 and E12.5 AVCs and complemented these datasets with previously published E7.75, E8.25, and E9.25 heart datasets from the Srivastava group36 (Fig. 1b–d and Supplementary Fig. 1a–j). Our analyses cover the earliest stages of AV development, including AV endocardial specification at E8.25, EndMT induction and progression from E9.25-E10.5, and EpiMT initiation at E12.5. In addition, we generated data from endothelial-specific Sox9 conditional knockout AVCs to compare with normal development (Supplementary Fig. 1a).
A total of 53,521 single-cells from all datasets passed stringent quality control checks, with a median of 4657 genes detected per cell (Supplementary Fig. 1i, j and Supplementary Data 1). We used PhenoGraph39 clustering to identify diversity captured within the 48,822 wildtype cells and annotated 18 distinct populations, including emergent mesenchymal, myocardial, and endothelial populations (Fig. 1b–d and Supplementary Data 2). Endocardial- and epicardial-derived lineages were computationally segregated from each timepoint and analyzed independently (Supplementary Fig. 1b–f). Together, these data define the earliest stages of AV valve formation, including the acquisition and maintenance of mesenchymal, endocardial, and epicardial identities, and provide insight into the mechanisms underlying EMTs in vivo.
Activation of epithelial-mesenchymal plasticity during EndMT
Around E8.5, the accumulation of cardiac jelly at the AVC forms the AV cushions4 (Fig. 1a). Initially acellular, these structures are colonized by endocardial cells as they undergo EndMT. By E9.5, these cells lose cell-cell contacts, lose apico-basal polarity, gain migratory and invasive characteristics, and transdifferentiate to form AV mesenchyme, which is essential for establishing valve architecture1,4 (Fig. 1a). To characterize this process, we analyzed 10,354 single-cell transcriptomes from E7.75-12.5, and performed batch correction using Harmony, which approximates consecutive timepoints in a timeseries34. Clustering identified eight populations including early endothelium, endocardium, AV endocardium, two VEC progenitor populations, and three AV mesenchymal clusters (Fig. 2a, Supplementary Fig. 2a–c, and Supplementary Data 2). Some transcriptional differences across batches persist after correction, though these genes are predominantly associated with general biological processes and metabolism (Supplementary Fig. 2a and Supplementary Data 3).
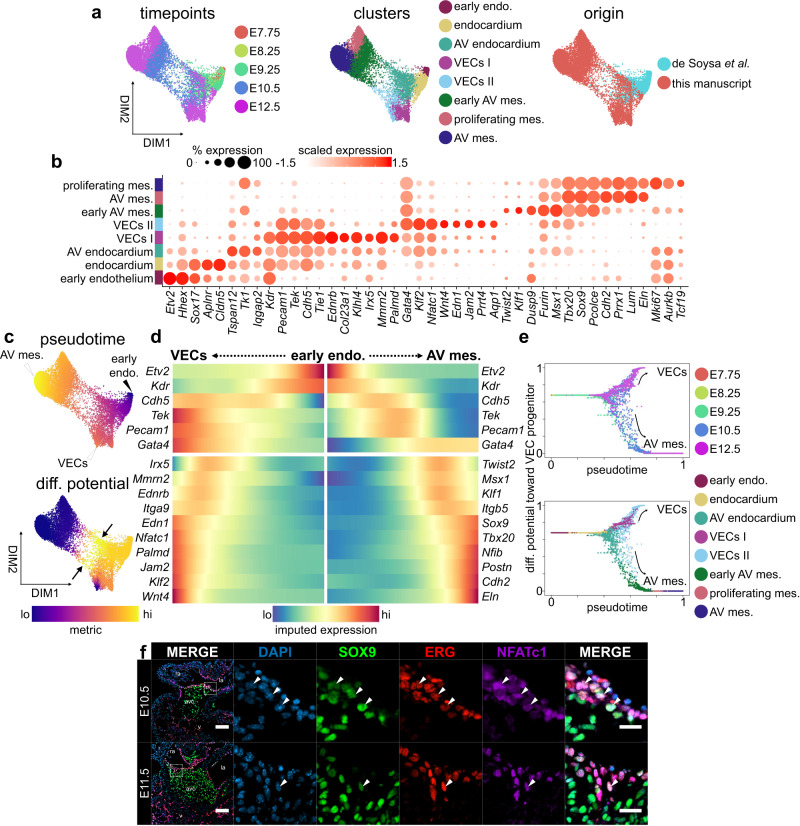
Fig. 2. Endocardial and mesenchymal cell lineage bifurcation during EndMT.
a Force-directed layouts of 10,354 endocardial and endocardial-derived cells displaying timepoint, annotated cluster, and origin. b A dot plot displaying select genes enriched within endothelial-derived clusters, including VEC populations and AV mesenchyme. Size of the dot represents proportion of the cluster that expresses each gene. Colour indicates level of expression. c Force-directed layouts of 10,354 endocardial and endocardial-derived cells displaying pseudotime and differentiation (diff.) potential. Arrows indicate high diff. potential in domains of endothelial-mesenchymal plasticity (EMP). d Gene trends along pseudotime show bifurcation between VEC and AV mesenchymal trajectories during the endocardial-to-mesenchymal transition. e Differentiation potential as a function of pseudotime for endothelial-derived lineages progressing toward a VEC terminus. Each dot represents a single-cell that is colour-coded by embryonic timepoint or cluster. f Immunostaining of AV canals for SOX9, ERG, and NFATc1 shows co-expression of on the flow side of the valves (white arrowheads) at E10.5 (n = 10) and E11.5 (n = 4). Scale bars: 100 µm, 25 µm in regions of interest (ROIs). AV atrioventricular, endo. endothelium, mes. mesenchyme. See also Supplementary Fig. 2 and Supplementary Data 2.
Cells within endocardial, AV endocardial, and VEC clusters express various endothelial biomarkers, including Pecam1, Tek, and Cdh5. The AV endocardial cluster, composed predominantly of cells from E9.25, has activated genes associated with AV identity, such as Gata4 (Fig. 2b). Endocardial Gata4 is required for EndMT initiation15, and immunostaining shows that GATA4 is enriched within the endocardial and endocardial-derived cells of the AVC compared to the ventricles from E9.5 until E12.5 (Supplementary Fig. 2d, e). This strongly supports its function in the establishment of early AV identity. Two distinct VEC populations emerge in the AVC by E12.5, including VECs I, which expresses Ednrb, Irx5, and Palmd, and VECs II, which is enriched for Wnt4, Nfatc1, and Edn1. The three AV mesenchymal populations all express regulators of mesenchymal identity, such as Sox916, Msx140, and Tbx2041, but differ in timepoint composition or proliferative status (Fig. 2b and Supplementary Fig. 2b). Researchers have previously proposed a distinct proliferative zone of AV mesenchyme exists within the cushion42,43 and our transcriptional data suggest a proliferative subset emerges by E12.5. However, immunostaining for phospho-histone H3 (pH3) or PCNA does not reveal any obvious patterns of proliferation within the cushions, suggesting proliferation is not spatially coordinated, but is activated ubiquitously in the emerging mesenchyme at E12.5 (Supplementary Fig. 2f–h). Overall, these analyses define expression signatures for distinct populations of endocardial and mesenchymal cells in early AV valves.
We next applied Palantir to infer developmental trajectories, including differentiation and cell fate choice, from our data to characterize the divergence between VEC progenitors and the AV mesenchyme during EndMT44 (Fig. 2c–e). Palantir orders cells during a process along pseudotime, then for each cell, assigns a probability for differentiating towards terminal cell states. Differentiation potential, a measure of cell plasticity, is calculated using these probabilities; multipotent cells have the highest differentiation potential and mature terminal states have the lowest potential. An early endothelial Etv2hi start cell was selected from which trajectories were calculated to either VEC progenitor or AV mesenchymal termini (Fig. 2c and Supplementary Fig. 3i–k). Differentiation potential is high among the early endothelial cluster, but highest where endocardial and mesenchymal lineages begin to bifurcate (arrows, Fig. 2c). Differentiation potential toward the AV mesenchymal terminus over pseudotime shows that a portion of cells within the AV endocardial and VEC II clusters by E9.25 and E10.5 are primed toward an AV mesenchymal identity (Fig. 2e). By E12.5, most cells have segregated into the endocardial and mesenchymal lineages as AV EndMT is complete.
Gene trends along pseudotime were calculated to characterize the divergence between these terminal states and showed the dynamic regulation of factors during this process (Fig. 2d). In the AV mesenchymal lineage, expression of Gata4 is retained, but endothelial biomarkers are repressed. Instead, we see the activation of various transcription factors, including master regulators of AV mesenchymal identity, such as Msx1, Sox9, and Tbx201, along with factors not assessed during EndMT, like Nfib and Klf1 (Fig. 2d). VEC progenitors retain expression of endothelial biomarkers and further activate expression of a variety of genes associated with AV and VEC identity, including Gata415, Irx545, Nfatc121, and Wnt446, along with genes with uncharacterized roles in AV development, like Ednrb and Palmd (Fig. 2d). Interestingly, Palmd has been identified as a susceptibility gene in calcific valve disease47. Immunostaining illustrates this cell fate decision. At E10.5, we observe NFATc1 co-expression on the flow-side of the cushions with ERG and within the VECs lining the primitive valves at E11.521,48 (Fig. 2f). Overall, these data detail bifurcation of endocardial and mesenchymal cell lineages and previously unappreciated diversity during AV EndMT.
Emergence of epithelial-mesenchymal plasticity during EndMT
Remarkably, we observe appreciable overlap of endocardial and mesenchymal factors during EndMT, including expression of Pecam1, Tek, Twist2, and Msx1 in our scRNA-seq data (Fig. 2d) and co-localization of SOX9, ERG, and NFATc1 by histology (arrowheads, Fig. 2f). To probe this shift in phenotype during EndMT, we calculated average gene expression profiles for each cell from a list of known endothelial or AV mesenchymal biomarkers (Supplementary Data 4). We further calculated a fluid shear stress response profile from genes identified to assess gene activation in response to hemodynamic forces (See Methods; Supplementary Data 4). As expected, the endothelial gene profile is highest within endocardial and VEC populations, while the mesenchymal profile is highest among emerging AV mesenchyme (Fig. 3a). However, we observe co-expression of mesenchymal and endothelial gene profiles within AV endocardial and VEC II clusters during EndMT (arrows, Fig. 3a, b and Supplementary Fig. 3a). The gene profile representing fluid shear stress response is highest among AV endocardial and VEC II clusters in the same domain where epithelial and mesenchymal gene profiles are co-expressed (Fig. 3a and Supplementary Fig. 3b). Of all characterized mechanosensitive ion channels, Piezo1 is the most highly expressed in endocardium, suggesting it acts as the major mechanosensor sensing fluid shear stress during EndMT in mouse, as is observed in zebrafish13,49 (Supplementary Fig. 3c). These findings strongly suggest the development of a cell state with epithelial-mesenchymal plasticity (EMP) during EndMT in domains of high fluid shear stress.
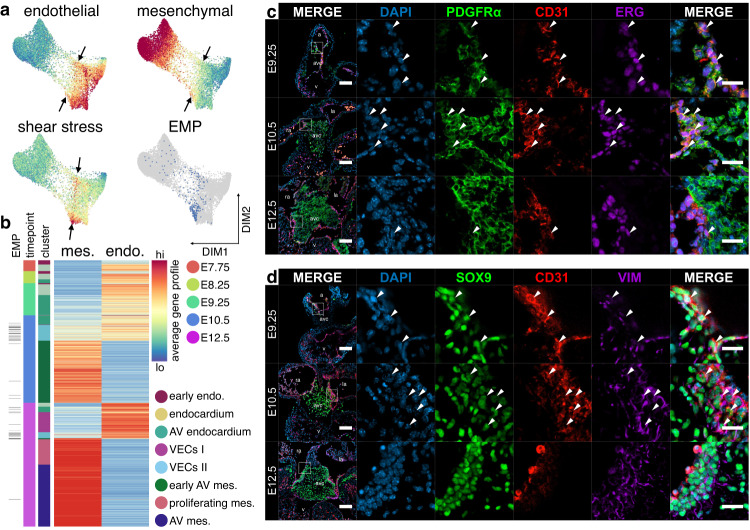
Fig. 3. Activation of epithelial-mesenchymal plasticity during EndMT.
a Force-directed layouts of 10,354 endocardial and endocardial-derived cells displaying average gene profiles denoting expression of endothelial and mesenchymal biomarkers as well as transcriptional response to shear stress, highlighting domains of EMP or high fluid shear stress response (arrows). b Heatmaps displaying average endothelial and mesenchymal gene profiles for endocardial and endocardial-derived cells from E7.75-12.5, where overlap indicates emergence of EMP. Immunostaining of AV canals for (c) PDGFRα, CD31, and ERG or (d) SOX9, CD31, and vimentin show widespread co-expression of endothelial and mesenchymal biomarkers, primarily at E9.25 and E10.5, suggesting EMP (arrowheads). Results are representative of three to nine independent experiments. Scale bars: 100 µm, 25 µm in ROIs. AV atrioventricular, endo endothelium, mes mesenchyme, VECs valve endocardial cells. See also Supplementary Fig. 3 and Supplementary Data 2 and 4.
Histology confirms the presence of an EMP hybrid cell state on the flow-side of the cushions where the hemodynamic load is highest50. Here, we see co-expression of mesenchymal PDGFRα and SOX9 with the endothelial biomarkers CD31 and ERG in cells at E9.25 and E10.5 (arrowheads, Fig. 3c, d and Supplementary Fig. 3d–f). Co-expression is significantly reduced once mesenchymal and endothelial lineages have resolved at E12.5 (Fig. 3c, d and Supplementary Fig. 3d–f). NFATc1+ cells are also found within these domains of EMP, indicating that VEC progenitors also emerge from this population (Fig. 2f). This is supported by our single-cell data, where we observe overlap between cells exhibiting EMP and high differentiation potential toward both VEC and AV mesenchymal termini, suggesting bipotency (Figs. 2c and 3a). Together, these findings indicate that cells displaying EMP emerge during early valvulogenesis, likely in response to the flow of blood. Our analyses predict that these hybrid cells exhibit high cell lineage plasticity and may be a common source for both VECs and AV mesenchyme.
Increased epithelial-mesenchymal plasticity in Sox9 cKO AVCs
SOX9 is critical for AV valve development, including in EndMT and matrix remodelling16,17,51,52, and its deregulation or mutation is associated with calcification and campomelic dysplasia, in which valve defects are often present53–55. In mouse, the role of Sox9 in AV valve development has been studied using Sox9fl/fl;Tie2-cre (Tek-cre) embryos, where Sox9 is ablated in endothelial-derived populations, including AV mesenchyme derived from EndMT56. These embryos die around E12.5 with AV developmental defects, characterized by severe cushion hypoplasia17. Despite its known roles in promoting AV valve development, surprisingly little is known about Sox9’s molecular mechanisms during valve development beyond what can be seen in the terminal phenotypes.
To address this gap in knowledge we generated scRNA-seq libraries from Sox9fl/fl;Tie2-cre (Sox9 cKO) AVCs along with Sox9fl/+;Tie2-cre or Sox9+/+;Tie2-cre normal littermate controls at E10.5 (Supplementary Fig. 1a and 4a–c). Analysis of Sox9 cKO and WT littermates shows a significant overlap between epicardial, endocardial, hematopoietic, and cardiomyocyte clusters (Supplementary Fig. 4a, c). However, pronounced differences exist in the distribution of mesenchymal clusters, where cKO and WT cells almost completely segregate (Supplementary Fig. 4a, c).
To assess shifts in cell identity between the WT and Sox9 cKO lineages, we computationally segregated endocardial lineages of the cKO library and overlaid them on the complete endocardial lineage force-directed layout to compare with our E10.5 WT EndMT data (Fig. 4a–c). Differential gene expression analysis between WT and Sox9 cKO cells in AV endocardial and VEC clusters shows modest differences in expression of endothelial biomarkers and factors (Fig. 4a). Within mesenchymal populations, however, we observe striking changes in the transcriptomes between WT and Sox9 cKO cells (Fig. 4a and Supplementary Fig. 4d; Supplementary Data 2). In WT mesenchyme we see enrichment of transcription factors, including Klf1, Scx, and Sox5, signalling components, such as Fgfr2, Notch2, Tgfbi, and Pdgfra, and ECM components, like type II and IX collagens, Fbln2, and Matn4 (Fig. 4a, Supplementary Fig. 4d, and Supplementary Data 2). Of note, we observe the enrichment of integrin receptor components in WT mesenchyme, including Itga4 and Itgb5, which are required for cell adhesion to FN1—a major cushion ECM component at this stage7,57 (Fig. 4a). Conversely, in mesenchyme sequenced from Sox9 cKO AVCs, we see stronger enrichment of a variety of master regulators of EndMT and mesenchymal development, including Twist1, Snai2, Msx2, and Zeb2 along with cell signalling components and ECM genes, including Tagln, Pdgfrb, Angpt1, Tnc, Postn, Bmp4, Flrt2, as well as type I, V, and XIII collagens (Fig. 4a, Supplementary Fig. 4d, and Supplementary Data 2). BMP signalling repressors, such as Fst and Smad7 are also retained within cKO mesenchyme, which could affect EndMT progression10,58. Gene ontology analysis of enriched genes in WT and Sox9 cKO mesenchyme shows prominent overlap between mesenchymal and developmental ontologies; however, ontologies associated with cardiac muscle development are specifically enriched within the cKO (Supplementary Fig. 4e). This is reflected in the activation of myosin and actin genes in the cKO mesenchyme, including Myh10, Myl9, Tpm1, Acta2, and Actc1, suggesting the adoption of a more myofibroblastic identity (Fig. 4a and Supplementary Fig. 4d). These findings suggest that without Sox9, we see a significant shift in mesenchymal cell identity in the AVC, specifically in cell adhesion, matrix production, and cell signalling.
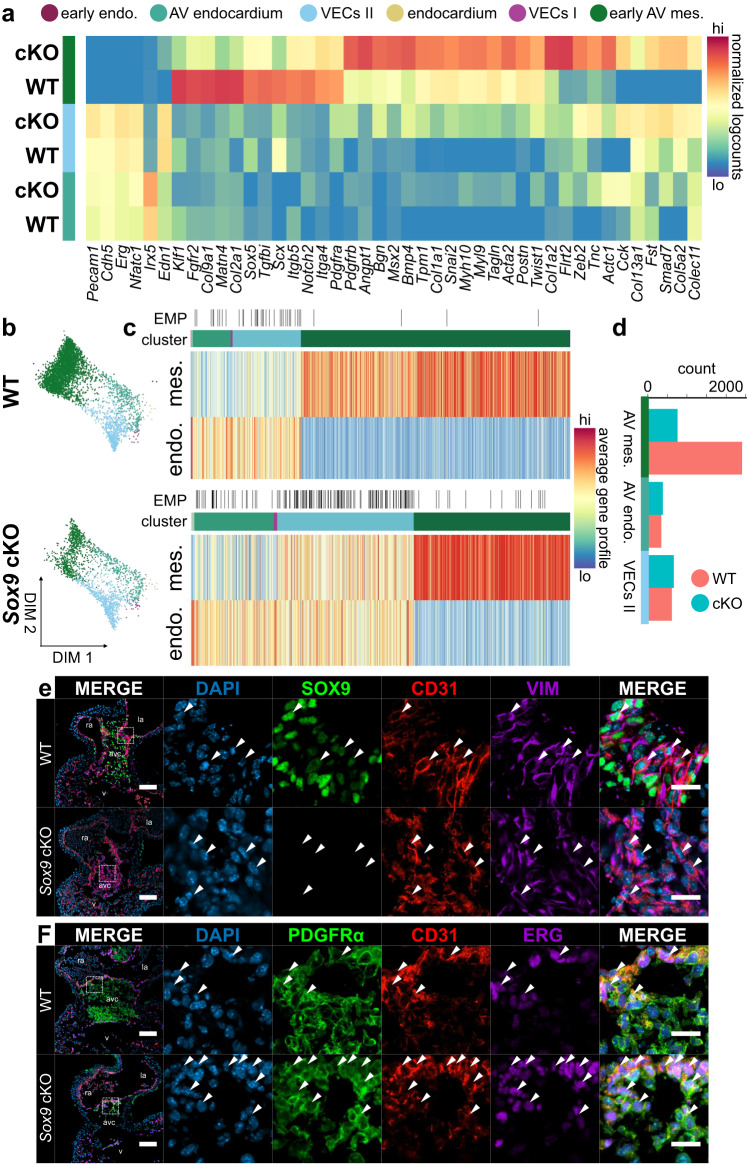
Fig. 4. Perturbations of endothelial and mesenchymal cell states in Sox9 conditional knockout AV canals.
a In the absence of Sox9, differential gene expression analysis reveals profound differences in identity between emergent mesenchymal populations as well as increased expression of mesenchymal factors in VECs. b Force-directed layouts of 3368 WT and 1810 Sox9 cKO endocardial and endocardial-derived cells from E10.5 displaying annotated cluster contribution. c Heatmaps displaying average endothelial and mesenchymal gene profiles for E10.5 WT and Sox9 cKO endocardial and endocardial-derived cells, showing a profound increase in cells exhibiting EMP in the absence of Sox9. d Cell distribution in the three main clusters captured at E10.5 in the WT and Sox9 cKO endocardial-derived lineages, showing reduced numbers of mesenchymal cells in the cKO. Comparing (e) SOX9, CD31, and vimentin or (f) PDGFRα, CD31, and ERG immunostaining in E10.5 Sox9 cKO and WT AV canals reveals developmental arrest of endocardial cells with mesenchymal features, like VIM or PDGFRα (white arrowheads). Few fully transdifferentiated mesenchymal cells lacking CD31 expression are observed in the Sox9 cKO. Results are representative of three to six independent experiments. Scale bars: 100 µm, 25 µm in ROIs. AV atrioventricular, endo. endothelium, mes mesenchyme. See also Supplementary Fig. 4 and Supplementary Data 2 and 4. Source Data are provided as a Source Data file.
Profound changes related to cell identity, including shifts in mesenchymal and endothelial phenotype, are observed among endothelial-derived cell lineages in the Sox9 cKO (Fig. 4b, c and Supplementary Fig. 4f, g). This includes increased proportions of AV endocardial and VEC II cells and reduced numbers of AV mesenchymal cells (Fig. 4b–d and Supplementary Fig. 4g). Further, we see a four-fold increase of cells that exhibit robust EMP (Fig. 4c and Supplementary Fig. 4f, g). Within the Sox9 cKO, some mesenchymal cells do emerge. Contradictorily, they display an increased mesenchymal gene profile, in part due to increased expression of EMT transcription factors, like Snai1, Twist1, and Zeb21 (Fig. 4a, c and Supplementary Fig. 4f, g). These results, including the increased proportions of cells exhibiting EMP and the upregulation of other EMT factors, reveal profound dysregulation of cell type identity in the Sox9 cKO.
To validate our findings in the Sox9 cKO, we immunostained E10.5 Sox9fl/fl;Tie2-cre hearts for several factors associated with EndMT and AV identity (Fig. 4e, f and Supplementary Fig. 4h-k). Our results show decreased proportions of mesenchymal cells within the cKO AVC, in line with decreased numbers of captured mesenchymal cells in the Sox9 cKO single-cell data (Fig. 4b–d Supplementary Fig. 4j–l). Expression of ERG, an endothelial master regulator, and CD31 (Pecam1), an endothelial cell adhesion molecule, is retained in cells at increased proportions within the AVC, indicating a failure of these cells to transdifferentiate to mesenchyme (Supplementary Fig. 4h, i). Immunostaining suggests maintenance of AV identity and promotion of VEC identity in the Sox9 cKO, where increased proportions of GATA4+ NFATc1+ endocardial cells are observed (Supplementary Fig. 4h-i). Increased numbers of cells exhibiting EMP at E10.5 are also observed by immunostaining (Fig. 4e, f and Supplementary Fig. 4l). We observe increased numbers and proportions of cells co-expressing mesenchymal PDGFRα and the endothelial biomarker CD31 at E10.5 in the Sox9 cKO (arrowheads, Fig. 4e, f and Supplementary Fig. 4l). Flow cytometry and immunostaining of sorted single-cells from individual E10.5 hearts further support increased proportions of endothelium (CD31+ PDGFRα-) and cells exhibiting EMP (CD31+ PDGFRα+) in the cKO (Supplementary Fig. 4m–o). Of note, differences in proliferation between WT and Sox9 cKO mesenchyme are observed at E9.5 and E12.5, suggesting that the phenotype observed in the Sox9 cKO may be multifactorial (Supplementary Fig. 4p–r). Overall, our data suggest that EndMT stalls without Sox9, leading to the developmental arrest of endocardium exhibiting EMP, while the few cells that do transdifferentiate to mesenchyme adopt a more myofibroblastic phenotype.
Hic1 marks a distinct epicardial cell type during EpiMT
At E12.5, EpiMT is initiated in epicardial cells lining the outside of the AVC5,59. These cells gain migratory and invasive characteristics, transdifferentiate to mesenchyme, and populate the right and left lateral cardiac cushions, along with the annulus fibrosis5,6,23,59. While chamber epicardium development has been described using single-cell transcriptomics27,60, lineage divergence of epicardial and mesenchymal populations during AV EpiMT remains uncharacterized. To detail this bifurcation, we analyzed epicardial progenitors along with a portion of the AV mesenchymal lineages captured within our datasets from E9.25 to E12.5 (Supplementary Fig. 1d–f). In total, we analyzed 4,633 single-cell transcriptomes, which correspond to five populations including septum transversum mesenchyme (STM), three epicardial populations, and AV mesenchyme (Fig. 5a and Supplementary Fig. 5a–c). Of note, epicardial lineage contribution to AV mesenchymal cells, without lineage tracing, could not be confirmed. In this analysis, the inclusion of AV mesenchyme acts as an anchor point, providing developmental context for us to investigate the process of EpiMT.
Fig. 5. The epicardial-to-mesenchymal transition progresses through a distinct cell type.
a Force-directed layouts of 4633 epicardial cells displaying timepoint, annotated cluster, and origin. b A dot plot displaying select genes enriched within epicardial clusters, including AV epicardial-derived cells (EPDCs) and mesenchyme. Size of the dot represents proportion of the cluster that expresses each gene. Colour indicates level of expression. c Force-directed layouts of 4633 epicardial cells displaying pseudotime and differentiation (diff.) potential. Arrow indicates differentiation potential maximum where AV EPDCs are specified. d Gene trends along pseudotime show bifurcation between epicardial and AV mesenchymal trajectories during the epicardial-to-mesenchymal transition. AV atrioventricular, epi epicardium, mes mesenchyme, STM septum transversum mesenchyme. See also Supplementary Fig. 5 and Supplementary Data 2.
Within the STM, we see moderate activation of characterized regulators of epicardial identity, including Tbx18, Wt1, and Lhx2 (Fig. 5b). Early epicardium and epicardium differ slightly in the expression of epicardial biomarkers, including expression of Lhx9 (Fig. 5b). We further identify an AV epicardial-derived cell (AV EPDC) population. While these cells retain expression of some epicardial transcription factors, including low Wt1 and Tbx18, and robust Tcf21, they are also enriched for genes typically associated with AV mesenchymal identity, including Postn, Itgb5, and Lum. In addition, they express a variety of genes uncharacterized in this context, including Col6a2, Pid1, Islr, and Hic1, suggesting these cells have progressed towards mesenchyme (Fig. 5b).
We applied trajectory analysis to characterize the divergence between the epicardium and the AV mesenchyme during EpiMT. An early E9.25 STM start cell was selected from which trajectories were calculated to either epicardial or AV mesenchymal termini (Fig. 5c and Supplementary Fig. 5d, e). Differentiation potential is high among the STM populations, but is highest at the interface between the epicardial and AV EPDC clusters (arrows, Fig. 5c). This suggests that AV EPDCs are specified from the epicardium and not directly from a mesodermal progenitor earlier in development. Differentiation potential toward the AV mesenchymal terminus over pseudotime shows that by E10.5 a small subset of cells is primed towards an AV mesenchymal cell fate (Supplementary Fig. 5f). In line with this observation, we see activation of Hic1 and Itgb5, markers of AV EPDCs, in some epicardial cells as early as E10.5 (Supplementary Fig. 5g). This suggests that EpiMT may initiate by E10.5, which is significantly earlier than previously understood.
Gene trends along pseudotime were calculated to better characterize the divergence between these terminal states (Fig. 5d). We see that for both epicardial and AV mesenchymal trajectories, repression of septum transversumnal biomarkers, such as Alcam, Foxf1, and Mab21l2 is coincident with the activation of transcription factors associated with the emergent epicardium, like Lhx9 and Wt1 (Fig. 5d). Cells fated toward an epicardial terminus maintain expression of various transcription factors associated with epicardial identity, including Wt1, Tbx18, and Tcf21. Biomarkers of embryonic epicardium are also activated at this stage, including Lrrn4, Msln, Apela, and the uroplakins (Fig. 5d). Conversely, in cells fated towards an AV mesenchymal terminus, factors associated with epicardial identity are maintained at low levels. Tcf21 is the sole canonical epicardial transcription factor that is not repressed as AV EPDCs emerge. Of note, in mice lacking Tcf21, chamber EpiMT is impaired61. Along with retained Tcf21, AV EPDCs express Hic1 and Itgb5 before induction of a variety of master regulators of AV mesenchymal identity, including Sox9 and Tbx20 (Fig. 5d). These data capture AV EpiMT and reveal the dynamic regulation of transcription factors, cell adhesion molecules, and signalling components. Interestingly, we also identify a distinct population of Hic1-expressing EPDCs from chamber epicardium data from Quijada et al.27. Comparing AV and chamber EPDC populations reveal transcriptional differences likely related to the fate of the transdifferentiating populations, including enriched Tagln, Acta2, and Twist1 within AV EPDCs and Igf2, Apela, and Upk3b within chamber EPDCs (Supplementary Fig. 5h, i).
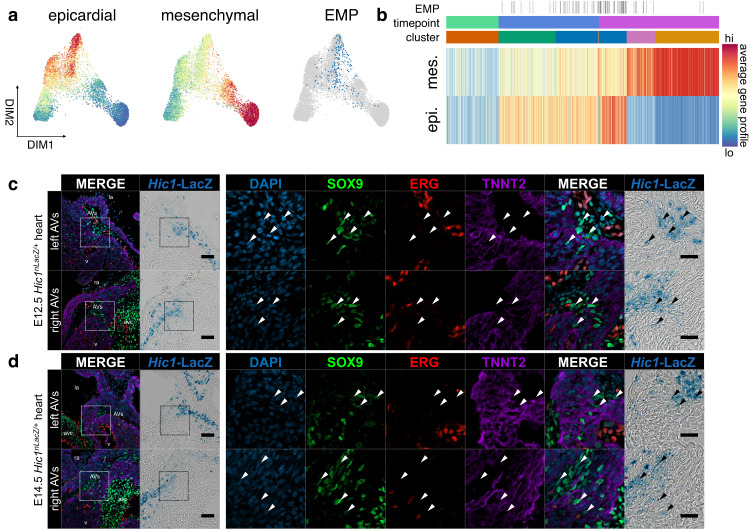
To probe shifts in phenotype during EpiMT, we calculated average gene expression profiles for each cell from a list of known epicardial or AV mesenchymal biomarkers (Supplementary Data 4). Consistent with observations during EndMT, we see induction of EMP in domains of high differentiation potential at E10.5 and E12.5 (Fig. 6a, b and Supplementary Fig. 6a). This is the result of epicardial gene activation along with moderate expression of genes associated with mesenchymal identity at the onset of EpiMT (Fig. 6a, b and Supplementary Fig. 6a). This includes Cdh2, Snai1, and Col1a1 among others (Supplementary Fig. 6b). These mesenchymal factors are further activated, and epicardial factors are further reduced, as cells transdifferentiate to AV mesenchyme via an AV EPDC intermediate during EpiMT.
Fig. 6. Epithelial-mesenchymal plasticity during EpiMT.
a Force-directed layouts and (b) a heatmap of 4633 epicardial cells displaying average epicardial and mesenchymal gene profiles. Overlap in gene profile expression suggests emergence of EMP during EpiMT. Immunostaining of Hic1nLacZ/+ AV canals for SOX9, ERG, and TNNT2 at (c) E12.5 (n = 9) and (d) E14.5 (n = 6) shows co-expression of mesenchymal SOX9 and AV EPDC Hic1nLacZ within AV sulcus, suggesting transition to mesenchyme (arrowheads). Scale bars: 100 µm, 25 µm in ROIs. AV atrioventricular, AVs AV sulcus, epi epicardium, mes mesenchyme. See also Supplementary Fig. 6 and Supplementary Data 2 and 4.
We sought to characterize the spatiotemporal emergence of AV EPDCs. However, many factors expressed within this population, including Postn, Sox9, and Nfib, are also activated during EndMT. Hic1, a transcriptional repressor, presented itself as a factor specific to EpiMT62. To characterize the spatiotemporal emergence of these cells, we combined LacZ staining on Hic1nLacZ/+ heart cryosections with fluorescence immunostaining63,64. We find that Hic1nLacZ-expressing cells are present along the outer edge of the AVC, within the AV sulcus where EpiMT occurs at E12.5 and E14.5 (Fig. 6c, d). We find that some of these cells co-express SOX9 (arrowheads, Fig. 6c, d), indicating induction of AV mesenchymal identity, and we do not observe co-expression with endothelial ERG or myocardial TNNT2. We further observe that while WT1hi Hic1nLacZ lo epicardial cells exist in a single-layered epithelium at E12.5 and E14.5 (yellow arrowheads, Supplementary Fig. 6c, d), Hic1nLacZ hi and WT1lo AV EPDCs stratify and appear to adopt a more mesenchymal phenotype as they transdifferentiate, in line with our gene profile analysis. These findings reveal that a distinct AV EPDC population, expressing Hic1, emerges during EpiMT in the AV sulcus.
Cell signalling microenvironment during EndMT and EpiMT
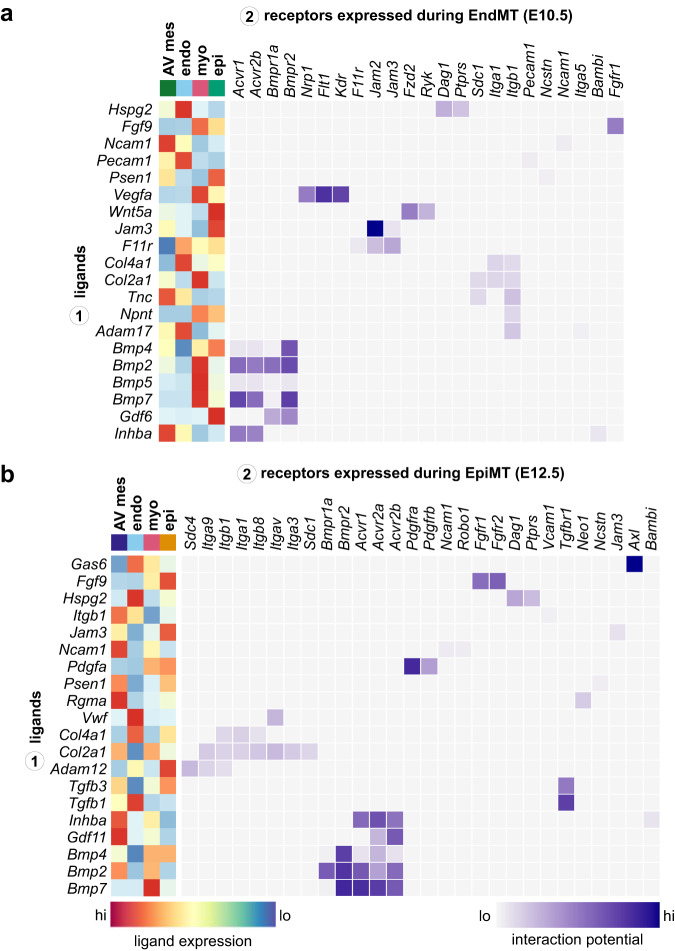
Identifying factors specific in driving EndMT and EpiMT initiation and progression is of interest, especially as the presence of endothelial and epicardial lineages in the AVC confounds interpretations of cell signalling on each of these processes individually65. We applied NicheNet to identify candidate factors that may induce and promote EndMT and EpiMT66. NicheNet shortlists candidate ligands and receptors, expressed by the defined sender and receiver cells, respectively, and ranks potential interactions along with downstream target genes using publicly available interaction databases.
We assessed which factors expressed by mesenchymal, epicardial, myocardial, and endocardial populations might signal towards endocardial cells to drive EndMT at E10.5 (Supplementary Fig. 7a and Supplementary Data 5). In our analysis, we see the activation of signalling pathways classically associated with EndMT initiation and mesenchymal development, including TGFβ superfamily9,10,67, WNT18, VEGF68,69, and FGF70 signalling. Interestingly, TGFβ1 is not predicted to influence EndMT; however, a variety of BMPs along with INHBA and GDF6 are identified as active drivers of mesenchymal identity (Fig. 7a). Cell-matrix interactions are prominent during EndMT, including endocardial receptors SDC1, DAG1, PTPRS, ITGA1, and ITGB1 interacting with NPNT, TNC, HSPG2, and type II and IV collagens (Fig. 7a). Mechanical signalling may play a role in the phenotypes observed in the hyaluronan71, FN157, and POSTN72 knockouts, for which the latter is already known to mediate atrioventricular development through integrin signalling. These findings suggest a multitude of ECM components provide cues to induce transdifferentiation through mechanical signalling73. Interactions between junctional adhesion molecules (JAMs), including F11R, JAM2, and JAM3, are identified, and while their role during AV development is unknown, they are essential drivers of EMT in the context of cancer metastasis, modulating morphology, migration, and cell polarity74.
Fig. 7. Deconvolution of signalling microenvironment during EndMT and EpiMT.
a A heatmap displaying select candidate ligand-receptor interactions signalling toward endocardial cells at E10.5 during EndMT, including scaled and log-normalized expression of ligands from sender populations. b A heatmap displaying select candidate ligand-receptor interactions signalling toward epicardial cells at E12.5 during EpiMT, including scaled and log-normalized expression of ligands from sender populations. AV atrioventricular, endo endocardium, epi epicardium, myo myocardium. See also Supplementary Fig. 7 and Supplementary Data 2 and 8.
Downstream target prediction of ligands identified in our analysis supports the role of TGFβ superfamily members in driving EndMT. BMP2, BMP4, BMP5, BMP7, INHBA, and GDF6 are all predicted to promote the expression of transcriptional regulators of mesenchymal identity along with the expression of ECM components associated with AV cushion development (Supplementary Fig. 7b). BMP2 is predicted to be especially influential, potentially inducing the expression of a variety of critical mesenchymal genes, including Sox9, Snai1, Msx1, Postn, Tagln, and Col1a1. This is in line with the Bmp2fl/fl;Nkx2-5-cre phenotype, where AV cushions and mesenchyme are absent due to the failure to initiate EndMT10,58. Of note, myocardial cues are predicted as the main drivers of EndMT (Supplementary Fig. 7b), which agrees with previous analyses that assessed AV identity induction7,58.
We next assessed which factors might signal towards epicardial cells to drive AV EpiMT at E12.5 using NicheNet (Supplementary Fig. 7c and Supplementary Data 5). As seen in EndMT, we predict prevalent TGFβ superfamily signalling in the promotion of EpiMT, including various BMPs, GDF11, and INHBA. However, we further predict active signalling through TGFβ1 and TGFβ3, which are not expected to influence EndMT (Fig. 7c). PDGFA and FGF9 are predicted to signal via PDGFRα/β and FGFR1/2, respectively. Mechanical signalling via integrins and syndecans is also featured prominently, suggesting a role for cell-matrix interactions in promoting EpiMT (Fig. 7c). Downstream target prediction of ligands identified in our analysis supports the role of TGFβ superfamily signalling in driving EpiMT and promoting a mesenchymal cell fate. Our analysis suggests that TGFβ1 is the most influential factor in promoting EpiMT and may activate a variety of regulators associated with AV EPDC or AV mesenchymal identity, including Hic1, Sox9, Snai2, Postn, Tagln, integrins, and type III and V collagens (Supplementary Fig. 7d). Other TGFβ family members are predicted to act upstream of genes associated with cell proliferation, like Junb and Myc, along with genes associated with a mesenchymal phenotype, like Acta2, Zeb2, and Mecom. Lastly, we assessed which factors may promote the proliferative pulse in AV mesenchymal cells at E12.5 (Supplementary Fig. 7e–g and Supplementary Data 5). We identify candidate signals from the endocardium, like TGFβ1 and WNT ligands, which are known mediators of valve mesenchymal proliferation46,75. We further identify candidate factors, including MDK, FGF9, and IGF1, among others, which may drive this proliferative pulse (Supplementary Fig. 7f, g).
Discussion
These data provide an in-depth look into how the early AV valves develop through the computational deconvolution of EndMT and EpiMT, revealing the molecular details underlying these processes. We define previously uncharacterized cellular heterogeneity within the AVC and propose mechanisms of lineage emergence. This includes distinct AV endocardium, VEC, and mesenchymal populations, along with a population of AV EPDCs.
Our data suggest that fluid shear stress induced by blood flow contributes to the development of EMP and endocardial cell stratification during EndMT. The early endocardium requires the maintenance of tight junctions to sustain endothelial barrier and cardiac functions. We propose that endocardial stratification during EndMT through the adoption of EMP allows for the retention of its barrier function, maintaining tight endothelial cell-cell contacts within the apical most cells in this region during the formation of the AV mesenchyme. The adoption of EMP during EndMT is closely associated with the activation of mechanoresponsive genes and high differentiation potential, indicating a role for hemodynamic forces in regulating cell fate identity. We further observe the developmental arrest of endocardial cells that exhibit EMP within the AVC when Sox9 is conditionally ablated along with mesenchymal gene dysregulation and transdifferentiation to myofibroblasts. This suggests that at least two distinct processes function to promote AV EndMT. The first is SOX9-independent, involving the adoption of EMP possibly in part due to fluid shear stress, while the second is SOX9-dependent and involves the complete transdifferentiation to AV mesenchyme. Differences in gene expression in the Sox9 cKO suggest that cues involving BMPs, FGFs, PDGFs or integrin-FN1 interactions could be responsible. The latter is supported by the reduced expression of integrins essential for FN1 adhesion in the Sox9 cKO, along with the accumulation of stratified endocardium within the Fn1 KO, which echoes the phenotype we observe in the Sox9 cKO57. Overall, this suggests a role for integrin-ECM interactions in promoting an AV mesenchymal cell fate.
Our analyses further provide insight into AV EpiMT, where we reveal the development of AV EPDCs as early as E10.5. AV EPDCs express a variety of factors associated with EMT, along with some unique genes, like Hic1, a tumour suppressor62 and biomarker of adult mesenchymal progenitors63,76. Staining of Hic1nLacZ/+ hearts shows the presence of these cells within the AV sulcus by E12.5. These cells maintain low WT1 and activate SOX9 as they transdifferentiate towards an AV mesenchymal cell state. Our cell signalling analyses suggest TGFβ superfamily, PDGF, FGF, and mechanical signalling may play a role in the activation of this cell type.
Finally, our transcriptional data indicate that EndMT and EpiMT share many features despite their distinct cellular origins. Expression of canonical EMT transcription factors, including Snai1/2, Zeb1/2, and Twist1, is observed in both contexts77. EndMT and EpiMT are both initiated within an epithelium and exist transiently in a stratified state, where the induction of EMP is associated with high cell lineage plasticity. Future research to validate the presence of EMP and potential for differentiation will likely employ dual lineage-tracing techniques, incorporating epithelial and mesenchymal drivers along with an intersectional reporter, to trace the fate of cells exhibiting EMP during valve development78. Further, the absence of endocardial and epicardial transcriptional legacies in AV mesenchyme, strongly suggest that mesenchyme generated during EndMT and EpiMT converge to an indistinguishable cell identity, though validation would likely require dual lineage tracing combined with cell sorting or scATAC-seq. However, differences also exist between these processes. This includes the presence of hemodynamic forces in promoting EndMT, differences in the cell signalling microenvironments, and process-specific expression of transcription factors, including Nfatc1 and Hic1 in EndMT and EpiMT, respectively.
In summary, these data advance our understanding of the initiation and progression of AV EMTs, providing a detailed assessment of these processes at single-cell resolution. These data will inform researchers studying cardiogenesis, congenital heart defects, in vitro models of EndMT, like ES cell differentiation of cardiac valve lineages79, as well as the recapitulation of developmental processes in disease and regenerative contexts. These data may be of special interest to cancer researchers studying EMT, during which the adoption of EMP is generally associated with more aggressive disease33.
Methods
All mice were maintained in accordance with the University of British Columbia’s Animal Care Committee’s standards under protocol numbers A20-0281, A20-0282 (principal investigator, P.A.H.), and A19-0221 (principal investigator T.M.U.) in pathogen-free conditions.
Reagents and Resources
Detailed information related to reagents and resources used in this study are available in Supplementary Data 6.
Mouse husbandry
Elite CD-1 (Charles River), Hic1nLacZ/+ 63, tdTomato (Gt(ROSA)26Sortm9(CAG-tdTomato)Hze)80, Sox9fl (B6.129S7-Sox9tm2Crm/J)56, Tie2-cre (Tg(Tek-cre)1Ywa)81 mice were used. Homozygous tdTomato females and hemizygous Tie2-cre males were bred for endothelial lineage tracing analyses, including generation of E12.5 AVC scRNA-seq libraries. Homozygous Sox9fl/fl females were bred with Sox9fl/+;Tie2-cre males to conditionally ablate Sox9 in endothelial lineages, and were used for immunostaining and generation of E10.5 Sox9 cKO AVC scRNA-seq library. Hic1nLacZ/+ embryos were used for LacZ staining and co-immunostaining of AV EPDCs. CD-1 mice were used for all other experiments. Up to five mice were housed per cage and were maintained on a regular chow diet ad libitum on a 12-h light-dark cycle. Embryos from timed matings were considered E0.5 at noon of the day a vaginal plug was observed. Embryos were collected at experimentally determined timepoints in ice-cold phosphate-buffered saline (PBS) under a Leica MZ6 dissecting scope. Embryo sex was not determined.
Primer sequences for genotyping are listed in Supplementary Data 7. Tie2-cre;tdTomato embryos were genotyped using a Zeiss AxioZoom V16 to observe tdTomato fluorescence. The presence of Hic1nLacZ transgene in embryos was done through overnight incubation of tissues in LacZ staining solution (2 mM MgCl2, 0.01% deoxycholate, 0.02% NP-40, 5 mM potassium-ferricyanide, 5 mM potassium-ferrocyanide, and 1 mg/ml X-gal in PBS) at 37 °C and visualization with a Leica MZ6 dissecting scope. Sex-specific differences were not anticipated and embryo sex was not determined.
Single-cell dissociations of atrioventricular canals
For dissociations, siliconized tips were used for all steps, and centrifugations were performed at minimum acceleration. For E10.5 AVC replicate 1 and FACS analyses related to Supplementary Fig. 4, we used a dispase/collagenase dissociation protocol, where AVCs were collected using fine dissecting forceps, ensuring the removal of the outflow tract, atria, and ventricles. The AVC, including AV cushions as well as overlying myocardium and epicardium were retained. AVCs were washed in PBS and incubated for 5 min at 37 °C in 1 ml PDEF (10 µg/ml DNAse I, 4 mM EDTA, and 2% foetal bovine serum [FBS] in PBS) with dispase/collagenase (2.5µU/ml dispase, 0.0025% collagenase type I) to dissociate tissues into single-cells. Tissues were gently triturated using a 1000 µl pipette before and after enzymatic digestion to facilitate dissociation. Single-cell suspensions were filtered through 40 µm cell strainers into 5 ml FACS tubes to remove debris and incompletely dissociated cells. The strainers were washed with PDEF to collect the remaining cells. The filtrate was subsequently centrifuged at 1000 g for 4 min to sediment single cells and the supernatant was removed.
We found that while dissociation with dispase/collagenase successfully captured endothelial- and epicardial-derived cells, myocardial cells are absent. Therefore, for all other libraries at E10.5 and E12.5, single-cell dissociations were performed with TrypLE Express as was done in36. Tie2-cre;tdTomato embryos were used for generation of E12.5 scRNA-seq libraries. Littermate Sox9fl/fl;Tie2-cre and Sox9+;Tie2-cre embryos were used for generation of E10.5 Sox9 cKO and control scRNA-seq libraries (Supplementary Fig. 1a). Tissues were collected from E10.5 and E12.5 embryonic hearts, ensuring removal of cardiac chambers and outflow tract. AVCs were washed for 5 min in PBS to remove red blood cells. PBS was removed and replaced with 250-1000 µl TrypLE Express. Tissues were gently triturated, then incubated at 37 °C for 5–15 min until the suspension was homogeneous and pieces of tissue were not visible. Cell suspensions were gently triturated part way through for longer incubations and again at the end. Single-cell suspensions were filtered through 40 µm cell strainers into 5 ml FACS tubes to remove debris and incompletely dissociated cells. The strainers were washed with PDEF to collect the remaining cells. The filtrate was subsequently centrifuged at 1000 g for 4 min to sediment single cells and the supernatant was removed. For E10.5 Sox9 cKO and littermate control libraries, AVCs were collected and left on ice for 90 min while genotyping was performed. Sox9fl/fl;Tie2-cre cKO AVCs or Sox9+/+ and Sox9fl/+;Tie2-cre AVCs were then pooled and dissociated.
Fluorescence-activated cell sorting
For single-cell library preparation, sedimented single-cells from embryonic AVCs were resuspended in PDEF and sorted using a FACSARIA III cell sorter (BD Biosciences) with a 130 µm nozzle in purity mode. All cell suspensions were stained with 1:5000 300 µM DAPI to purify live cells. Cellular debris and cell doublets were excluded based on forward and side scatter. To remove hematopoietic populations, filtered, dissociated AVC suspensions were resuspended in 2% FBS in PBS, and stained with APC-conjugated anti-Ter119 (1:200) at 4 °C for 15 min. After staining, cell suspensions were washed three times in PDEF, centrifuging at 1000 g for 4 min, and were ultimately resuspended in PDEF. APC+ fractions were excluded from scRNA-seq library construction (Supplementary Fig. 1a).
For FACS analyses related to Supplementary Fig. 4, sedimented single-cells from individual embryonic AVCs were resuspended in 2% FBS in PBS and incubated with goat anti-PDGFRα (1:100), FITC-conjugated rat anti-CD31, and APC-conjugated rat anti-Ter119 for 15 min at 4 °C. Cells were centrifuged at 1000 g for 4 min and re-suspended in 2% FBS in PBS and stained with donkey anti-goat Alexa 568 for 15 min at 4 °C. Cells were centrifuged at 1000 g for 4 min and re-suspended in PDEF with 1:5000 300 µM DAPI to identify dead cells. Cells were sorted using a FACSARIA III cell sorter (BD Biosciences) with a 130 µm nozzle in purity mode. Cellular debris and cell doublets were excluded based on forward and side scatter. CD31+ PDGFRα+ cells were collected for single-cell histology.
Single-cell RNA-seq library generation
Sorted single-cell suspensions were counted and diluted to a final concentration in low EDTA PDEF (0.1 mM EDTA) and cellular suspensions were loaded on a Chromium Controller to generate single-cell gel bead emulsions, targeting 7650–10,000 cells depending on the tissue and embryonic stage (Supplementary Data 1). For AVC scRNA-seq, three E10.5 libraries from WT and Sox9 cKO hearts and two libraries from E12.5 Tie2-cre;tdTomato AVCs were created. Single-cell 3’ RNA-seq libraries were generated according to the manufacturer’s instructions (Chromium Single Cell 3’ Reagent v2 Chemistry Kit, 10X Genomics, Inc.). Libraries were sequenced to a depth of 50-175,000 reads per cell on an Illumina Nextseq system (E10.5 and E12.5 libraries). Refer to Supplementary Data 1 for library quality metrics.
Read alignment
E7.75, E8.25, and E9.25 single-cell RNA-seq heart data from de Soysa et al. 36 was downloaded from GEO (accession number GSE126128). FASTQs from all libraries, including publicly available data, were aligned and quantified using the Cell Ranger Single Cell Software Suite version 6.0.0 (10X Genomics, Inc.) against the custom-built mm10 (GRCm38_GtC) reference genome containing tdTomato, Cre, and Gfp sequences. Cell Ranger was used to identify droplets containing cells for each library individually, identifying cells based on the number of UMIs and gene expression profiles. Aligned reads from E12.5 epicardium and EPDC single-cell RNA-seq data from Quijada et al. 27 was downloaded from GEO (accession number GSE154715).
Single-cell data processing
All data processing and analyses were performed in R and Python using standard protocols from previously published packages. The number of cells used in each of the statistical analyses is indicated in the figures or figure legends. Further quality metrics were calculated using the calculateQCMetrics in the scater package in R82, and cells were subsequently filtered based on unique features (>2000 genes) and mitochondrial RNA content (<20%).
Size-factor normalization, feature selection, dimensionality reduction, and doublet identification were performed using the scran and scater packages in R82,83. Briefly, filtered cells were loosely clustered using the quickCluster function, then size-factor normalized using the computeSumFactors function, taking both library sizes and quickCluster ID into account. Single-cell transcriptomes were subsequently log-transformed using the normalize function in scater to correct for biases between cells, considering size factors. Highly variable genes were selected based on gene expression levels and variance across all cells to highlight biological differences across datasets. Ribosomal, mitochondrial, Y-chromosome genes, and Xist were removed from highly variable genes. Doublet scores were calculated using the doubletCells function in scran, which assigns each cell a score that is defined as the ratio of the density of simulated doublets to the squared density of original cells in the neighbourhood of that cell83.
PCA dimensionality reduction and mutual-nearest neighbours84 or Harmony34 batch correction was performed to correct for non-biological confounders between replicates and timepoints. PCA was done using the RunPCA function in Seurat85. For MNN batch correction, fastMNN from the scran package was employed, and the first 100 principal components were used as input. k = 20 neighbours were used for the calculation of mutual-nearest neighbours. Harmony uses timepoint metadata to construct augmented affinity matrices, which uses mutual-nearest neighbours across consecutive timepoints to correct for batch effects. Log-normalized expression-matrices, containing data for highly variable genes only, were used as inputs. A k of 20–35 was used for the mutual nearest neighbour calculation for the EndMT and EpiMT lineage analyses. The augmented affinity matrices were subsequently used to calculate the force-directed layouts.
Dimensionality reduction, like uniform manifold approximation and projection (UMAP) was performed using the runUMAP function in scater, respectively using the first 100 principal components as inputs. Force-directed layouts to display cell type diversity were calculated using adjusted affinity matrices generated with the Harmony package in Python34. Graph-based clustering and Wilcoxon rank-sum test for differential gene expression were performed using phonograph39 and Seurat85 packages in Python and R, respectively. For phenograph clustering, a k of 150 to 300 was used and the normalized expression matrix of highly variable genes served as the input. The FindAllMarkers function in Seurat was used to identify differentially expressed genes. Globally enriched genes within a cluster were identified based on a log fold-change of 0.25 with an expression threshold of >25% within a population using two-sided Wilcoxon rank-sum test. Only positively enriched genes with a false-discovery rate (FDR) of 0.05 or lower are included. To better characterize the phenotypic identities of endocardial- and epicardial-derived lineages, we calculated an average gene profile for AV mesenchymal, endothelial, epicardial, or fluid shear stress86 identities, using annotated lists of biomarkers and master regulators (Supplementary Data 4). Gene expression profiles were calculated for each single cell in the EndMT or EpiMT datasets by calculating the average log-normalized expression of annotated lists of biomarkers and master regulators of endothelial identity, epicardial identity, mesenchymal identity, or response to fluid shear stress (Supplementary Data 4). Classification of cells to gene profile categories (e.g. exhibiting EMP), was done through determining conservative minimum thresholds in each gene profile for meeting endothelial, epicardial, or mesenchymal identity (Supplementary Figs. 3a and 6a). EMP in generally defined as the strong co-expression of factors associated with mesenchymal and epithelial identity. Cells that met the threshold of both epithelial and mesenchymal profiles were considered to be exhibiting EMP. While EMP represents a continuum, we provided these data as a binary for easy visualization. Batch-associated genes were identified based on a log fold-change of 0.5 with an expression threshold of >75% within a batch using two-sided Wilcoxon rank-sum test. Gene ontology analysis for each batch-enriched list of genes was performed and, along with the gene lists, can be found in Supplementary Data 387. Only positively enriched genes with a false-discovery rate (FDR) of 0.05 or lower are included.For visualization, gene profiles were mean-scaled for comparison and heatmaps were generated in R using pheatmap with clustering done using the ward.D2 method.
Palantir single-cell differentiation trajectories, which infer pseudotime progression, cell fate decisions, and terminal cell states from a defined ‘start cell’, were calculated using diffusion components from Harmony augmented affinity matrices discussed in the previous section34,44. ‘Start cells’ for each trajectory were selected based on current developmental paradigms, including an early endothelial progenitor for the endocardial lineages (EndMT), and a lateral plate mesoderm (LPM) progenitor for the epicardial lineages (EpiMT). 30 nearest-neighbours and 700 to 1000 waypoints were used as inputs for the generation of Palantir differentiation trajectories. For all trajectories calculated, the Pearson’s correlation of Palantir results over a range of k nearest-neighbours demonstrates that pseudotime estimation, differentiation potential, and branch probabilities to termini are robust with respect to changes in the number of nearest-neighbours, k (Supplementary Figs. 2j and 5e). Gene expression was visualized after imputation with the MAGIC algorithm to denoise the single-cell data and fill in dropouts88. Gene expression trends along pseudotime were calculated based on branch probabilities and generalized additive models, showing the stepwise transcriptional progression of endodermal and endothelial lineages along pseudotime using Palantir44.
NicheNet cell signalling analysis
The transcriptional influence of ligands during EndMT and EpiMT was predicted by applying NicheNet66. NicheNet predicts which ligands produced by a sender cell are the most active in affecting gene expression in a receiver cell, through the correlation of ligand activity with genes previously characterized as targets of their downstream pathways.
To analyze the cell signalling microenvironment during AV valve development, we applied NicheNet to predict which ligands influence transcription during EndMT and EpiMT using cells from E10.5 and E12.5, respectively. At E10.5, early endocardial and VEC II clusters were defined as receiver cells, and all populations, including endocardium, mesenchyme, epicardium, and myocardium, were used as sender populations (Supplementary Fig. 7a). At E12.5, epicardial clusters were defined as receiver cells, and all populations, including endocardium, mesenchyme, epicardium, and myocardium, were used as sender populations (Supplementary Fig. 7c). Gene sets of interest were defined based on differential gene expression analysis where the top differentially-enriched genes based on false-discovery rate were used (average log2 fold change >0.25; expressed in at least 25% of the population of interest). This includes genes specifically enriched within the mesenchyme over endocardium at E10.5 and AV EPDCs over typical epicardium at E12.5 for EndMT and EpiMT analyses, respectively (Supplementary Data 8). A background gene set includes all other genes that are highly expressed, but not differentially expressed between these populations. NicheNet’s ligand-target model was converted from human to mouse genes using the convert_human_to_mouse_symbols function. The complete results from the NicheNet analysis can be found in Supplementary Data 5.
The circlize package in R was used to separately represent the regulatory networks between the predicted ligands and their targets expressed in receiver cell types89. Ligands were grouped and annotated based on the cell of origin. The width and opacity of links were determined based on the ligand-receptor interaction weights and ligands’ activity scores, respectively.
Tissue processing for histology
Embryonic tissues for fluorescence immunohistochemistry were collected from timed matings at experimentally determined timepoints. Tissues were washed in PBS to remove blood. The heads of whole embryos and ventricles of dissected hearts were carefully punctured with dissecting forceps to allow for better washing. Tissues were incubated overnight in 4% paraformaldehyde (PFA) at 4 °C, washed with PBS, and dehydrated through a methanol gradient (25%–50%–75%–100% methanol in PBS, room temperature, 5 min each).
Immunostaining of tissue cryosections
Dehydrated tissues were rehydrated to PBS from methanol (75%–50%–25% methanol in PBS, room temperature, 5 min each), then washed in PBS for 5 min. Tissues were then washed through a sucrose gradient (15%–30%–60% sucrose in PBS, 4 °C, 1–12 h each), and overnight in 1:1 60% sucrose:optimal cutting temperature compound (OCT, TissueTek) at 4 °C. Cryoprotected tissues were embedded in OCT, flash-frozen, and stored at −80 °C.
8 µm sections were collected using a Leica CM3050S cryostat at −25 °C with SuperFrost Plus slides. Sections were circumscribed with an Elite PAP pen (Diagnostic Biosystems K039) and slides were subsequently placed in an opaque humidity chamber. Sections were re-fixed for 10 min at room temperature in 4% PFA in PBS, washed three times with PBS for 5 min, then incubated in block solution (5% bovine serum albumin, and 0.01% Triton-X in PBS) for 1 hat room temperature. Sections were subsequently incubated with primary antibodies in block solution overnight at 4 °C. These include anti-CD31 (1:50), anti-ERG (1:50), anti-GATA4 (1:50), anti-MCM-2 (1:100), anti-NFATc1 (1:15), anti-PCNA (1:100), anti-PDGFRα (1:50), anti-pH3 (1:100), anti-SOX9 (goat—1:50; rabbit—1:500), anti-TNNT2 (1:15), anti-VIM (1:50), and anti-WT1 (1:50). The next day, sections were washed with PBS three times for 5 min and incubated with species-specific Alexa Fluor 488-, 568-, 594-, or 647-conjugated secondary antibodies in block solution (all 1:500) for 1 h at room temperature. After secondary incubation, sections were washed with PBS three times for 5 min, incubated with 1:1000 300 µM DAPI to counterstain nuclei, and then washed again with PBS three times for 5 min. Slides were mounted using a minimal volume (~100 µl) 25 mg/ml DABCO in 9:1 glycerol:PBS. Coverslips were fixed in place using clear nail polish.
Images were captured using a Nikon Instruments Eclipse Ti confocal laser microscope. Image capture, processing, and quantification was done using NIS Elements 5.0 and Fiji in ImageJ90, with brightness, contrast, pseudo-colouring adjustments, and z-stack alignments applied equally across all images in a given series.
Immunostaining of FACS-sorted single-cells
Sorted cells were sedimented at 1000 g for 4 min, re-suspended in 50 µl of PDEF, and deposited onto SuperFrost Plus slides. Cells were left to settle for 30 min and then fixed with 4% PFA in PBS. Slides were placed in a humidity chamber for staining. Blocking, antibody incubation, and mounting were done as described for tissue cryosections.
Combination LacZ and immunostaining of Hic1nLacZ/+ cryosections
Hic1nLacZ/+ tissue cryopreservation and LacZ staining were done as outlined in Scott & Underhill64. Fixed Hic1nLacZ/+ hearts were washed through a sucrose gradient (15%–30%–60% sucrose in PBS, 4 °C, 1–12 h each), and overnight in 1:1 60% sucrose:OCT 4 °C. Cryoprotected tissues were embedded in OCT (TissueTek), flash-frozen, and stored at −80 °C. 8 µm sections were collected using a Leica CM3050S cryostat at −25 °C with SuperFrost Plus slides. Sections were circumscribed with an Elite PAP pen (Diagnostic Biosystems K039) and slides were subsequently placed in an opaque humidity chamber. Sections were washed with PBS three times for 10 min to remove OCT compound and incubated at 37 °C overnight in LacZ staining solution (2 mM MgCl2, 0.01% deoxycholate, 0.02% NP-40, 5 mM potassium-ferricyanide, 5 mM potassium-ferrocyanide, and 1 mg/ml X-gal in PBS). The next day, slides were washed in distilled H2O and washed three times for 30 min with PBS. Autofluorescence was quenched for 45 min with sodium borohydride (10 mg/ml in PBS), washed again with PBS, and then incubated in block solution (5% bovine serum albumin, and 0.01% Triton-X in PBS) for 1 h at room temperature. Sections were subsequently incubated with primary antibodies in block solution overnight at 4 °C. These include anti-CD31 (1:50), anti-ERG (1:50), anti-SOX9 (goat—1:50), anti-TNNT2 (1:15), and anti-WT1 (1:50). The next day, sections were washed with PBS three times for 5 min and incubated with species-specific Alexa Fluor 488-, 568-, 594-, or 647-conjugated secondary antibodies in block solution (all 1:500) for 1 h at room temperature. After secondary incubation, sections were washed with PBS three times for 5 min, incubated with 1:1000 300 µM DAPI to counterstain nuclei, and then washed again with PBS three times for 5 min. Slides were mounted using a minimal volume (~100 µl) 25 mg/ml DABCO in 9:1 glycerol:PBS. Coverslips were fixed in place using clear nail polish.
Images were captured using a Nikon Instruments Eclipse Ti confocal laser microscope, capturing transmitted and reflected light. Image capture or processing was done using NIS Elements 5.0 and Fiji in ImageJ90, with brightness, contrast, pseudo-colouring adjustments, and z-stack alignments applied equally across all images in a given series. To image stained Hic1nLacZ/+ tissue, transmitted light was captured using the blue, green, and red lasers, merged to form an RGB image, then shade-corrected and white balanced using ImageJ.
Statistics and reproducibility
No statistical method was used to predetermine sample size. The experiments were not randomized and no data was excluded from analysis. Researchers were not blinded during data acquisition and analysis. All statistical analyses were performed and graphs generated using R and Python. scRNA-seq analyses were performed as described above. The number of cells used in each of the statistical analyses is indicated in the figures or figure legends.
For single-cell RNA-seq data processing, the Cell Ranger Single Cell Software Suite version 5.0 (10X Genomics, Inc.) was used for alignment, de-multiplexing, and UMI counting using the default parameters. Sequencing metrics are presented in Supplementary Data 1. From 14 samples, 53,521 single cells were analyzed, ranging from 1313 to 11,166 cells collected per sample. Median reads per cell ranged from 46,691 to 175,383 with median UMI of 14,012 to 36,267 per cell. Cells with <1000 expressed genes or with mitochondrial gene expression accounting for >20% of total RNA content were considered of low quality and excluded from downstream analyses.
After single-cell data normalization, clustering, and dimensionality reduction, marker genes for each cluster were identified using the FindAllMarkers or FindMarkers functions in Seurat using two-sided unpaired Wilcoxon rank-sum test. Genes with an FDR < 0.05 were considered statistically enriched within a cluster.
Pearson’s correlation coefficients of a ligand’s target gene predictions and the observed transcriptional response in the target population was used to define ligand activity for NicheNet ligand-target gene analysis. The top quartile of scores of interactions were visualized and the entire interactome for both EndMT and EpiMT can be visualized in Supplementary Data 4.
Pearson’s correlation of Palantir results over a range of k nearest-neighbours demonstrates that pseudotime estimation, differentiation potential, and branch probabilities to terminal states are robust with respect to changes in the number of nearest-neighbours, k.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
We thank F. Lynn, F. Rossi, and T. Stephan for constructive discussions as well as A. Shanks and P. Stirling for antibodies. This work was supported by the Canadian Institutes of Health Research (PJT-159512 to P.A.H.; PJT-149026 to T.M.U.), the Canadian Foundation for Innovation, and the British Columbia Knowledge Development Fund. This research was enabled in part by the Digital Research Alliance of Canada (www.alliancecan.ca). J.L. is the recipient of an NSERC PGSD scholarship and CIHR Postdoctoral Fellowship. S.D. is the recipient of a CIHR CGSM scholarship.
Author contributions
J.L., R.C., V.C.G., and P.A.H. conceived the research. J.L., R.C., V.C.G., B.M.F., and A.T. performed preliminary analyses. J.L., R.C., and M.A. collected and dissected embryos. J.L. performed cell dissociations, FACS experiments, and histology. J.L. and M.C.-R. quantified histological data. J.L. and S.D. performed computational analysis of sequenced data. J.L and P.A.H. analyzed and interpreted the data, and wrote the manuscript with input from all authors. T.M.U. and P.A.H. performed supervisory roles and acquired funding.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The single-cell RNA-sequencing data generated during this study have been deposited in the ArrayExpress database at EMBL-EBI (www.ebi.ac.uk/arrayexpress) under the accession number E-MTAB-11795. The processed data can also be explored at the Broad Single-cell Portal under the accession number SCP1897. Source data are provided with this paper.
Code availability
Only publicly available tools were used in data analysis as described wherever relevant in the Methods.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-41279-6.
References
- 1.O’Donnell A, Yutzey KE. Mechanisms of heart valve development and disease. Development. 2020;147:dev183020. doi: 10.1242/dev.183020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheek JD, Wirrig EE, Alfieri CM, James JF, Yutzey KE. Differential activation of valvulogenic, chondrogenic, and osteogenic pathways in mouse models of myxomatous and calcific aortic valve disease. J. Mol. Cell Cardiol. 2012;52:689–700. doi: 10.1016/j.yjmcc.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tao G, Kotick JD, Lincoln J. Heart valve development, maintenance, and disease. Curr. Top. Dev. Biol. 2012;100:203–232. doi: 10.1016/B978-0-12-387786-4.00006-3. [DOI] [PubMed] [Google Scholar]
- 4.Markwald RR, Fitzharris TP, Manasek FJ. Structural development of endocardial cushions. Am. J. Anat. 1977;148:85–119. doi: 10.1002/aja.1001480108. [DOI] [PubMed] [Google Scholar]
- 5.Wessels A, et al. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev. Biol. 2012;366:111–124. doi: 10.1016/j.ydbio.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gittenberger-de Groot AC, Vrancken Peeters MPFM, Mentink MMT, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ. Res. 1998;82:1043–1052. doi: 10.1161/01.RES.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 7.Mjaatvedt CH, Lepera RC, Markwald RR. Myocardial specificity for initiating endothelial-mesenchymal cell transition in embryonic chick heart correlates with a particulate distribution of fibronectin. Dev. Biol. 1987;119:59–67. doi: 10.1016/0012-1606(87)90206-5. [DOI] [PubMed] [Google Scholar]
- 8.Nishii K, Shibata Y. Mode and determination of the initial contraction stage in the mouse embryo heart. Anat. Embryol. (Berl.) 2006;211:95–100. doi: 10.1007/s00429-005-0065-x. [DOI] [PubMed] [Google Scholar]
- 9.Jiao K, et al. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma L, Lu M-F, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- 11.Granados-Riveron JT, Brook JD. The impact of mechanical forces in heart morphogenesis. Circ. Cardiovasc Genet. 2012;5:132–142. doi: 10.1161/CIRCGENETICS.111.961086. [DOI] [PubMed] [Google Scholar]
- 12.Goddard LM, et al. Hemodynamic forces sculpt developing heart valves through a KLF2-WNT9B paracrine signaling axis. Dev. Cell. 2017;43:274–289.e5. doi: 10.1016/j.devcel.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukui H, et al. Bioelectric signaling and the control of cardiac cell identity in response to mechanical forces. Science. 2021;374:351–354. doi: 10.1126/science.abc6229. [DOI] [PubMed] [Google Scholar]
- 14.Niessen K, et al. Slug is a direct Notch target required for initiation of cardiac cushion cellularization. J. Cell Biol. 2008;182:315–325. doi: 10.1083/jcb.200710067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivera-Feliciano J, et al. Development of heart valves requires Gata4 expression in endothelial-derived cells. Development. 2006;133:3607–3618. doi: 10.1242/dev.02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garside VC, et al. SOX9 modulates the expression of key transcription factors required for heart valve development. Development. 2015;142:4340–4350. doi: 10.1242/dev.125252. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama H, et al. Essential role of Sox9 in the pathway that controls formation of cardiac valves and septa. Proc. Natl Acad. Sci. USA. 2004;101:6502–6507. doi: 10.1073/pnas.0401711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liebner S, et al. β-Catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J. Cell Biol. 2004;166:359–367. doi: 10.1083/jcb.200403050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luna-Zurita L, et al. Integration of a Notch-dependent mesenchymal gene program and Bmp2-driven cell invasiveness regulates murine cardiac valve formation. J. Clin. Invest. 2010;120:3493–3507. doi: 10.1172/JCI42666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timmerman LA, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu B, et al. Nfatc1 coordinates valve endocardial cell lineage development required for heart valve formation. Circ. Res. 2011;109:183–192. doi: 10.1161/CIRCRESAHA.111.245035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodgers LS, Lalani S, Runyan RB, Camenisch TD. Differential growth and multicellular villi direct proepicardial translocation to the developing mouse heart. Dev. Dyn. 2008;237:145–152. doi: 10.1002/dvdy.21378. [DOI] [PubMed] [Google Scholar]
- 23.Zhou B, von Gise A, Ma Q, Hu YW, Pu WT. Genetic fate mapping demonstrates contribution of epicardium-derived cells to the annulus fibrosis of the mammalian heart. Dev. Biol. 2010;338:251–261. doi: 10.1016/j.ydbio.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou B, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith CL, Baek ST, Sung CY, Tallquist MD. Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ. Res. 2011;108:e15–e26. doi: 10.1161/CIRCRESAHA.110.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pennisi DJ, Mikawa T. FGFR-1 is required by epicardium-derived cells for myocardial invasion and correct coronary vascular lineage differentiation. Dev. Biol. 2009;328:148–159. doi: 10.1016/j.ydbio.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quijada P, et al. Coordination of endothelial cell positioning and fate specification by the epicardium. Nat. Commun. 2021;12:1–18. doi: 10.1038/s41467-021-24414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gise Avon, et al. WT1 regulates epicardial epithelial to mesenchymal transition through β-catenin and retinoic acid signaling pathways. Dev. Biol. 2011;356:421. doi: 10.1016/j.ydbio.2011.05.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Compton LA, Potash DA, Mundell NA, Barnett JV. Transforming growth factor-β induces loss of epithelial character and smooth muscle cell differentiation in epicardial cells. Dev. Dyn. 2006;235:82–93. doi: 10.1002/dvdy.20629. [DOI] [PubMed] [Google Scholar]
- 30.Hulin A, et al. Maturation of heart valve cell populations during postnatal remodeling. Development. 2019;146:dev173047. doi: 10.1242/dev.173047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeisberg EM, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 32.Wylie-Sears J, Aikawa E, Levine RA, Yang JH, Bischoff J. Mitral valve endothelial cells with osteogenic differentiation potential. Arterioscler Thromb. Vasc. Biol. 2011;31:598–607. doi: 10.1161/ATVBAHA.110.216184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35:645–654. doi: 10.1007/s10555-016-9648-7. [DOI] [PubMed] [Google Scholar]
- 34.Nowotschin S, et al. The emergent landscape of the mouse gut endoderm at single-cell resolution. Nature. 2019;569:361–367. doi: 10.1038/s41586-019-1127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pijuan-Sala, B. et al. A single-cell molecular map of mouse gastrulation and early organogenesis. Nature566, 490–495 (2019). [DOI] [PMC free article] [PubMed]
- 36.de Soysa TY, et al. Single-cell analysis of cardiogenesis reveals basis for organ-level developmental defects. Nature. 2019;572:120–124. doi: 10.1038/s41586-019-1414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lotto J, et al. Single-cell transcriptomics reveals early emergence of liver parenchymal and non-parenchymal cell lineages. Cell. 2020;183:702–716.e14. doi: 10.1016/j.cell.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, et al. Comparative analysis of cell lineage differentiation during hepatogenesis in humans and mice at the single-cell transcriptome level. Cell Res. 2020;30:1109–1126. doi: 10.1038/s41422-020-0378-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine JH, et al. Data-driven phenotypic dissection of AML reveals progenitor-like cells that correlate with prognosis. Cell. 2015;162:184–197. doi: 10.1016/j.cell.2015.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen YH, Ishii M, Sucov HM, Maxson RE. Msx1 and Msx2 are required for endothelial-mesenchymal transformation of the atrioventricular cushions and patterning of the atrioventricular myocardium. BMC Dev. Biol. 2008;8:75. doi: 10.1186/1471-213X-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boogerd CJ, et al. Probing chromatin landscape reveals roles of endocardial TBX20 in septation. J. Clin. Investig. 2016;126:3023–3035. doi: 10.1172/JCI85350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de la Cruz, M. V. & Markwald, R. R. Embryological development of the ventricular inlets. Septation and atrioventricular valve apparatus. Liv. Morphogen. Heart10.1007/978-1-4612-1788-6_6 (1998).
- 43.de Vlaming A, et al. Atrioventricular valve development: vew perspectives on an old theme. Differentiation. 2012;84:103–116. doi: 10.1016/j.diff.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Setty M, et al. Characterization of cell fate probabilities in single-cell data with Palantir. Nat. Biotechnol. 2019;37:451–460. doi: 10.1038/s41587-019-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaborit N, et al. Cooperative and antagonistic roles for Irx3 and Irx5 in cardiac morphogenesis and postnatal physiology. Development. 2012;139:4007–4019. doi: 10.1242/dev.081703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alfieri CM, Cheek J, Chakraborty S, Yutzey KE. Wnt signaling in heart valve development and osteogenic gene induction. Dev. Biol. 2010;338:127–135. doi: 10.1016/j.ydbio.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z, et al. Phenome-wide analyses establish a specific association between aortic valve PALMD expression and calcific aortic valve stenosis. Commun. Biol. 2020;3:1–8. doi: 10.1038/s42003-020-01210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang CP, et al. A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis. Cell. 2004;118:649–663. doi: 10.1016/j.cell.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Duchemin AL, Vignes H, Vermot J. Mechanically activated Piezo channels modulate outflow tract valve development through the yap1 and KLF2-notch signaling axis. Elife. 2019;8:e44706. doi: 10.7554/eLife.44706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yalcin HC, Shekhar A, McQuinn TC, Butcher JT. Hemodynamic patterning of the avian atrioventricular valve. Dev. Dyn. 2011;240:23–35. doi: 10.1002/dvdy.22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lincoln J, Kist R, Scherer G, Yutzey KE. Sox9 is required for precursor cell expansion and extracellular matrix organization during mouse heart valve development. Dev. Biol. 2007;305:120–132. doi: 10.1016/j.ydbio.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuglerud BM, et al. SOX9 reprograms endothelial cells by altering the chromatin landscape. Nucleic Acids Res. 2022;50:8547–8565. doi: 10.1093/nar/gkac652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner T, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 54.Peacock JD, Levay AK, Gillaspie DB, Tao G, Lincoln J. Reduced Sox9 function promotes heart valve calcification phenotypes in vivo. Circ. Res. 2010;106:712–719. doi: 10.1161/CIRCRESAHA.109.213702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mansour S, Hall CM, Pembrey ME, Young ID. A clinical and genetic study of campomelic dysplasia. J. Med. Genet. 1995;32:415–420. doi: 10.1136/jmg.32.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Astrof S, Crowley D, Hynes RO. Multiple cardiovascular defects caused by the absence of alternatively spliced segments of fibronectin. Dev. Biol. 2007;311:11–24. doi: 10.1016/j.ydbio.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prados B, et al. Myocardial Bmp2 gain causes ectopic EMT and promotes cardiomyocyte proliferation and immaturity. Cell Death Dis. 2018;9:1–15. doi: 10.1038/s41419-018-0442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu K, et al. A dual genetic tracing system identifies diverse and dynamic origins of cardiac valve mesenchyme. Development. 2018;145:dev167775. doi: 10.1242/dev.167775. [DOI] [PubMed] [Google Scholar]
- 60.Lupu IE, Redpath AN, Smart N. Spatiotemporal analysis reveals overlap of key proepicardial markers in the developing murine heart. Stem Cell Rep. 2020;14:770–787. doi: 10.1016/j.stemcr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Acharya A, et al. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development. 2012;139:2139–2149. doi: 10.1242/dev.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wales MM, et al. p53 activates expression of HIC-1, a new candidate tumour suppressor gene on 17p13.3. Nat. Med. 1995;1:570–577. doi: 10.1038/nm0695-570. [DOI] [PubMed] [Google Scholar]
- 63.Scott RW, Arostegui M, Schweitzer R, Rossi FMV, Underhill TM. Hic1 defines quiescent mesenchymal progenitor subpopulations with distinct functions and fates in skeletal muscle regeneration. Cell Stem Cell. 2019;25:797–813.e9. doi: 10.1016/j.stem.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scott RW, Underhill TM. Methods and strategies for lineage tracing of mesenchymal progenitor cells. Methods Mol. Biol. 2016;1416:171–203. doi: 10.1007/978-1-4939-3584-0_10. [DOI] [PubMed] [Google Scholar]
- 65.Criem N, Zwijsen A. The epicardium obscures interpretations on endothelial-to-mesenchymal transition in the mouse atrioventricular canal explant assay. Sci. Rep. 2018;8:1–9. doi: 10.1038/s41598-018-22971-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Browaeys R, Saelens W, Saeys Y. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat. Methods. 2020;17:159–162. doi: 10.1038/s41592-019-0667-5. [DOI] [PubMed] [Google Scholar]
- 67.Camenisch TD, et al. Temporal and distinct TGFβ ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev. Biol. 2002;248:170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- 68.Dor Y, et al. A novel role for VEGF in endocardial cushion formation and its potential contribution to congenital heart defects. Development. 2001;128:1531–1538. doi: 10.1242/dev.128.9.1531. [DOI] [PubMed] [Google Scholar]
- 69.Combs MD, Yutzey KE. VEGF and RANKL regulation of NFATc1 in heart valve development. Circ. Res. 2009;105:565–574. doi: 10.1161/CIRCRESAHA.109.196469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vega-Hernández M, Kovacs A, De Langhe S, De, Ornitz DM. FGF10/FGFR2b signaling is essential for cardiac fibroblast development and growth of the myocardium. Development. 2011;138:3331–3340. doi: 10.1242/dev.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Camenisch TD, et al. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J. Clin. Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Butcher JT, Norris RA, Hoffman S, Mjaatvedt CH, Markwald RR. Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Dev. Biol. 2007;302:256–266. doi: 10.1016/j.ydbio.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwartz, M. A. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harbor Perspectives in Biol.2, a005066 (2010). [DOI] [PMC free article] [PubMed]
- 74.Lauko A, Mu Z, Gutmann DH, Naik UP, Lathia JD. Junctional adhesion molecules in cancer: a paradigm for the diverse functions of cell-cell interactions in tumor progression. Cancer Res. 2020;80:4878–4885. doi: 10.1158/0008-5472.CAN-20-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garside VC, Chang AC, Karsan A, Hoodless PA. Co-ordinating Notch, BMP, and TGF-β signaling during heart valve development. Cell Mol. Life Sci. 2013;70:2899–2917. doi: 10.1007/s00018-012-1197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soliman H, et al. Pathogenic potential of Hic1-expressing cardiac stromal progenitors. Cell Stem Cell. 2020;26:205–220.e8. doi: 10.1016/j.stem.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 77.Yang, J. et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol.21, 341–352 (2020). [DOI] [PMC free article] [PubMed]
- 78.Liu K, Jin H, Zhou B. Genetic lineage tracing with multiple DNA recombinases: a user’s guide for conducting more precise cell fate mapping studies. J. Biol. Chem. 2020;295:6413–6424. doi: 10.1074/jbc.REV120.011631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neri T, et al. Human pre-valvular endocardial cells derived from pluripotent stem cells recapitulate cardiac pathophysiological valvulogenesis. Nat. Commun. 2019;10:1–14. doi: 10.1038/s41467-019-09459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kisanuki YY, et al. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 82.McCarthy DJ, Campbell KR, Lun ATL, Wills QF. Scater: pre-processing, quality control, normalization and visualization of single-cell RNA-seq data in R. Bioinformatics. 2017;33:1179–1186. doi: 10.1093/bioinformatics/btw777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lun ATL, McCarthy DJ, Marioni JC. A step-by-step workflow for low-level analysis of single-cell RNA-seq data with bioconductor. F1000Res. 2016;5:2122. doi: 10.12688/f1000research.9501.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haghverdi L, Lun ATL, Morgan MD, Marioni JC. Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nat. Biotechnol. 2018;36:421–427. doi: 10.1038/nbt.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018;36:411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kumar S, Kim CW, Son DJ, Ni CW, Jo H. Flow-dependent regulation of genome-wide mRNA and microRNA expression in endothelial cells in vivo. Sci. Data. 2014;1:1–11. doi: 10.1038/sdata.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ashburner M, et al. Gene ontology: tool for the unification of biology. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Dijk D, et al. Recovering gene interactions from single-cell data using data diffusion. Cell. 2018;174:716–729.e27. doi: 10.1016/j.cell.2018.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gu Z, Gu L, Eils R, Schlesner M, Brors B. Circlize implements and enhances circular visualization in R. Bioinformatics. 2014;30:2811–2812. doi: 10.1093/bioinformatics/btu393. [DOI] [PubMed] [Google Scholar]
- 90.Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods9, 671–675 (2012). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The single-cell RNA-sequencing data generated during this study have been deposited in the ArrayExpress database at EMBL-EBI (www.ebi.ac.uk/arrayexpress) under the accession number E-MTAB-11795. The processed data can also be explored at the Broad Single-cell Portal under the accession number SCP1897. Source data are provided with this paper.
Only publicly available tools were used in data analysis as described wherever relevant in the Methods.