Abstract
Background
Aneurysm size is an imperfect risk assessment tool for those with thoracic aortic aneurysm (TAA). Assessing arterial age may help TAA risk stratification, as it better reflects aortic health. We sought to evaluate arterial age as a predictor of faster TAA growth, independently of chronological age.
Methods and Results
We examined 137 patients with TAA. Arterial age was estimated according to validated equations, using patients' blood pressure and carotid‐femoral pulse wave velocity. Aneurysm growth was determined prospectively from available imaging studies. Multivariable linear regression assessed the association of chronological age and arterial age with TAA growth, and multivariable logistic regression assessed associations of chronological and arterial age with the presence of accelerated aneurysm growth (defined as growth>median in the sample). Mean±SD chronological and arterial ages were 62.2±11.3 and 54.2±24.5 years, respectively. Mean baseline TAA size and follow‐up time were 45.9±4.0 mm and 4.5±1.9 years, respectively. Median (interquartile range) TAA growth was 0.31 (0.14–0.52) mm/year. Older arterial age (ß±SE for 1 year: 0.004±0.001, P<0.0001) was independently associated with faster TAA growth, while chronological age was not (P=0.083). In logistic regression, each 5‐year increase in arterial age was associated with a 23% increase in the odds of accelerated TAA growth (95% CI, 1.085–1.394; P=0.001).
Conclusions
Arterial age is independently associated with accelerated aneurysm expansion, while chronological age is not. Our results highlight that a noninvasive and inexpensive assessment of arterial age can potentially be useful for TAA risk stratification and disease monitoring as compared with the current clinical standard (chronological age).
Keywords: aortic aneurysm, aortic stiffness, arterial age, arterial stiffness, early vascular aging, hemodynamics, thoracic aortic aneurysm
Subject Categories: Aneurysm, Vascular Disease, Hypertension
Nonstandard Abbreviations and Acronyms
- cfPWV
carotid‐femoral pulse wave velocity
- EVA
early vascular aging
Clinical Perspective.
What Is New?
Arterial age measured by noninvasive methods is independently associated with faster thoracic aortic aneurysm expansion in the future, while chronological age is not.
Arterial age had the strongest association with future aneurysm growth as compared with other variables presently used in clinical assessment of patients with thoracic aortic aneurysm.
What Are Clinical Implications?
Arterial age and early vascular aging may provide a more individualized prediction of thoracic aortic aneurysm disease progression as compared with currently available methods for risk stratification.
Arterial age can be measured noninvasively and inexpensively in the outpatient setting and may potentially be used for individualized disease monitoring in patients with thoracic aortic aneurysm.
Thoracic aortic aneurysms (TAAs) are associated with high morbidity and mortality. Data from large‐scale studies show that thoracic aorta disease affects nearly 0.16% of the population, but true prevalence of TAA is likely much higher given its indolent behavior and their discovery being incidental in about 1% of imaging studies. 1 , 2 Patients who experience acute aortic syndromes often have poor outcomes, with an approximate 22% prehospital mortality rate. 1 , 3 TAAs increase the risk of acute aortic syndrome (AAS) by a factor of thousands. 4 For this reason, current guidelines rely on aneurysm size to determine timing of elective surgical repair to prevent AAS. 4 , 5 , 6 However, evaluating TAA size alone is an imperfect risk strategy. 7 , 8 , 9 Specifically, the IRAD (International Registry of Acute Aortic Dissection) showed that 59% of patients presenting with type A aortic dissections had aortic diameters smaller than the usual 5.5 cm size cut off used for elective surgical repair of the aortic root and ascending aorta, and 40% of patients dissected with aortic root or ascending aorta diameters <5.0 cm. 7 Therefore, there is a critical need to identify novel predictors of TAA risk to help improve clinical management of this disease.
Previous studies have shown that the majority of patients with TAA (≈60%) have degenerative forms of aortopathy (degenerative TAA) and have identified older age, hypertension, and TAA growth rate as independent predictors of AAS. 7 , 10 , 11 In a healthy aorta, the media‐rich layer of organized elastic fibers allows for a buffering process in response to the high pressures generated by the left ventricle with each heartbeat. However, in aortic aneurysms, there is a breakdown of elastic fibers, disorganization of vascular smooth muscle cells, and media fibrosis. 12 Interestingly, this same process is known to happen in the natural aging of the aorta, and as such it has been suggested that TAA may represent a focal, accelerated form of aortic aging. 12 , 13 Arterial age is predominantly determined by aortic stiffness and has implications for the pressure‐buffering function of the aorta and the resulting pulsatile arterial load. 14 Given the similar histopathological abnormalities present in natural aortic aging and in the wall of a TAA, in this study we sought to evaluate the role of arterial age on future TAA expansion, as compared with the current standard of care in clinical evaluation of patients with TAA (chronological age).
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Participants and Baseline Assessment
One hundred and fifty participants with TAA were recruited from the University of Ottawa Heart Institute's Aorta Clinic and prospectively followed. One participant was lost to follow‐up, and 12 had aortic surgery or events before a second imaging study to calculate growth, leaving 137 unoperated participants with longitudinal follow‐up in our study. Details about inclusion and exclusion criteria, and the assessment of baseline characteristics, are outlined in Data S1. In short, we included participants of at least 18 years of age, with a documented aneurysm of the thoracic portion of the aorta (≥40 mm), and no previous history of aortic surgery or AAS. Participants with all causes of TAA were included in the study, simulating real‐world clinical practice. TAA cause was broadly categorized as heritable TAA if associated with bicuspid aortic valve, Marfan syndrome, Ehlers‐Danlos syndrome, Loeys‐Dietz syndrome, familial thoracic aortic aneurysms and dissections, or any genetic syndromes known to be associated with aortopathy. TAA cause was classified as degenerative TAA if associated with chronological age ≥50 years and hypertension or other cardiovascular risk factors, in the absence of heritable TAA causes as above. Informed written consent was received from all participants at the time of enrollment. Research ethics approval was received from the University of Ottawa Heart Institute's Research Ethics Board.
Aneurysm Size and Growth Rate Assessment
Imaging studies to assess aneurysm size included transthoracic echocardiogram, computed tomography, and magnetic resonance imaging, all of which are recommended in guidelines for TAA surveillance. 5 Further details of our imaging protocol are described in Data S1. If a participant had the same imaging modality at baseline and follow‐up, concordant imaging was achieved. If the baseline imaging modality was not subsequently repeated and a different imaging modality was used in follow‐up, this was defined as discordant imaging. Aneurysm size was measured at the maximal diameter during diastole, by an imaging cardiologist (T.C.) who was blinded to arterial age. We have previously demonstrated excellent repeatability and intra‐observer agreement for TAA measurements across all imaging modalities within our group. 15 Aneurysm growth rate, our dependent variable in the linear regression models, was computed by dividing the absolute change in TAA size by total follow‐up time, and reported as millimeters per year. “Accelerated TAA growth,” our dependent variable in the logistic regression models, was defined as TAA growth greater than the sex‐specific median in the sample.
Estimation of Arterial Age
Arterial age was estimated using published validated equations based on carotid‐femoral pulse wave velocity (cfPWV) and mean arterial pressure. 14 Brachial blood pressure was measured using an electronic sphygmomanometer and a microphone over the right brachial artery to enhance accuracy (Non‐Invasive Hemodynamics, NIHem—Cardiovascular Engineering Inc., Norwood, MA). 16 Blood pressure was measured 3 times, 2 minutes apart, and their average was used for analyses. Mean arterial pressure was calculated as one‐third of the systolic plus two‐thirds of the diastolic brachial blood pressure. 17 , 18 cfPWV is the gold standard noninvasive measure of aortic stiffness 19 and was assessed with arterial applanation tonometry according to the protocol suggested by the Expert Consensus Document on Arterial Stiffness 20 (NIHem—Cardiovascular Engineering Inc.). In summary, transit distances from the suprasternal notch and the carotid and femoral pulse sites were used to estimate aortic path length (D) according to the subtraction method. 20 Aortic transit time (t) was estimated as the time between the onset of carotid and femoral waveforms, using the QRS as the fiducial point. cfPWV was calculated as aortic path length (D)/aortic transit time (t), in meters per second. Details of the validated equations used to calculate arterial age can be found in Data S1. For the purposes of our study, early vascular aging (EVA) was defined as having an estimated arterial age older than chronological age.
Statistical Analysis
For participants whose cfPWV was too low to be applied to the formula (n=4), the minimum value for arterial age in the sample was inputted. For participants with missing blood pressure data (n=3), mean blood pressure values in the sample were inputted. Spearman correlation coefficients were used to assess the correlation of chronological age and arterial age with TAA expansion rate. Then, TAA growth was log‐transformed after adding 1, to reduce skewness, and we performed multivariable linear regression models using log (TAA growth+1) as the dependent variable. Chronological age and arterial age were first assessed in separate models as the independent variables of interest, then together in the same model to assess the association of arterial age with TAA growth once chronological age was accounted for. Standardized β coefficients (β*) were also calculated to directly compare strength of association among variables in the model. Next, we conducted multivariable logistic regression to predict accelerated aneurysm growth, adjusted for the same variables as in the linear regression models above, and assessing chronological age and arterial age first in separate models, then in the same model. Model performance was assessed with the c‐statistic, initially for a base model that included all clinical covariates above but no chronological or arterial age, then for the base model with added chronological age, arterial age, or both.
Linear and logistic regression model covariates included clinically relevant parameters: sex, body surface area, mean arterial pressure, aneurysm location, cause, and baseline size, time between studies, concordant/discordant nature of imaging modalities, and previous history of hypertension, diabetes, hyperlipidemia, and smoking. In sensitivity analyses, we performed variable selection for the linear and logistic regression models using a stepwise approach with criteria of P≤0.25 to enter, and P≤0.10 to stay in the models. We then compared the results to the fully adjusted models.
Statistical analyses were conducted using JMP versus 13 (SAS Institute Inc, Cary, NC), and a 2‐sided P value ≤0.05 was considered statistically significant.
RESULTS
Table 1 presents the participants’ baseline characteristics. The mean±SD for chronological and arterial age were 62.2±11.3 and 54.2±24.5 years, respectively. Thirty‐one percent of study participants were women. EVA was present in 29.2% of participants. The mean±SD size of aneurysm at time of enrollment and at final follow‐up was 45.9±4.0 and 47.3±3.7 mm, respectively, with an average follow‐up time of 4.5±1.9 years. The median (interquartile range) TAA growth rate was 0.31 (0.14, 0.52) mm/year.
Table 1.
Baseline Characteristics of the Participants (n=137)
| Variable | Mean±SD or n (%) |
|---|---|
| Age, y | 62.2±11.3 |
| Arterial age, y | 54.2±24.5 |
| Early vascular aging, n (%) | 40 (29.2%) |
| Male sex, n (%) | 94 (68.6%) |
| Height, cm | 173.8±8.7 |
| Weight, kg | 86.2±16.9 |
| BMI, kg/m2 | 28.5±4.9 |
| Hypertension, n (%) | 62 (45.2%) |
| Diabetes, n (%) | 5 (3.7%) |
| Hyperlipidemia, n (%) | 58 (42.3%) |
| Ever smoking, n (%) | 69 (50.4) |
| Brachial blood pressure | |
| Systolic blood pressure, mm Hg | 125.1±16.1 |
| Diastolic blood pressure, mm Hg | 67.0±9.5 |
| Mean arterial pressure, mm Hg | 89.4±9.6 |
| Aneurysm characteristics | |
| Degenerative/heritable | 74/63 |
| Baseline aneurysm size, mm | 45.9±4.0 |
| Largest aneurysm site (root/ascending/descending) | 33/103/1 |
| Imaging | |
| MRI/CT/echocardiography | 63/31/43 |
| Concordant/discordant imaging modalities | 80/57 |
| Follow‐up, y | 4.5±1.9 |
| Last aneurysm size, mm | 47.3±3.7 |
| TAA growth rate, median (IQR) | 0.31 (0.14,0.52) |
BMI indicates body mass index; CT, computed tomography; ECHO, echocardiogram; IQR, interquartile range; MRI, magnetic resonance imaging; and TAA, thoracic aortic aneurysm.
The Spearman ρ for chronological age and TAA growth was not statistically significant (ρ=0.13, P=0.122), while arterial age was significantly correlated with future TAA growth (ρ=0.35, P<0.0001). There was a moderate correlation between chronological age and arterial age (ρ=0.65, P<0.0001).
Linear regression assumptions were met. Table 2 shows the multivariable linear regression models to predict TAA growth rate, expressed as log (growth rate+1) in fully adjusted models. In summary, older arterial age was independently associated with faster TAA growth, while chronological age was not. The association of arterial age with TAA growth was also independent of chronological age, when both age variables were added to the model. Importantly, when standardized beta coefficients were compared among covariates, we observed that arterial age had the strongest association with aneurysm growth as compared to other clinical covariates used in practice, including TAA size. When models were additionally adjusted for antihypertensive and lipid‐lowering medication use, inferences remained unchanged (analysis not shown). In sensitivity analyses, after stepwise variable selection, the following variables were included in the model: sex, time between studies, and aneurysm size and location. In this model, arterial age remained independently associated with future, faster aneurysm expansion, with similar coefficients as in the fully adjusted models (β±SE: 0.003±0.0007, P<0.0001. Model R 2: 0.38).
Table 2.
Multivariable Linear Regression Models to Assess Future TAA Growth Rate [log(TAA growth+1)]
| Model | Chronological age alone (Model's adjusted R 2=0.268) | Arterial age alone (Model's adjusted R 2=0.343) | Chronological age+arterial age (Model's adjusted R 2=0.354) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | β±SE | β* | P value | β±SE | β* | P value | β±SE | β* | P value |
| Chronological age, 1 y | 0.0007±0.002 | 0.033 | 0.763 | … | … | … | −0.004±0.003 | −0.206 | 0.083 |
| Arterial age, 1 y | … | … | … | 0.003±0.0009 | 0.333 | 0.0003 | 0.004±0.001 | 0.420 | <0.0001 |
| Male sex (vs female sex) | −0.043±0.024 | −0.163 | 0.080 | −0.043±0.023 | −0.165 | 0.063 | −0.041±0.023 | −0.156 | 0.076 |
| BSA, 1 m2 | −0.005±0.103 | −0.005 | 0.958 | −0.010±0.092 | −0.009 | 0.918 | −0.068±0.098 | −0.064 | 0.487 |
| dTAA (vs hTAA) | 0.029±0.025 | 0.118 | 0.249 | 0.002±0.022 | 0.009 | 0.926 | 0.015±0.023 | 0.056 | 0.474 |
| Baseline TAA size, 1 mm | 0.010±0.005 | 0.165 | 0.053 | 0.005±0.005 | 0.263 | 0.091 | 0.007±0.005 | 0.108 | 0.184 |
| Time between studies, 1 y | −0.022±0.010 | 0.025 | −0.178 | −0.027±0.009 | −0.217 | 0.004 | −0.027±0.009 | −0.214 | 0.005 |
| Ascending aneurysm location (vs root location) | −0.376±0.075 | −1.334 | <0.0001 | −0.367±0.070 | −1.302 | <0.0001 | −0.349±0.070 | −1.239 | <0.0001 |
| Descending aneurysm location (vs root location) | 0.752±0.147 | 1.371 | <0.0001 | 0.736±0.139 | 1.342 | <0.0001 | 0.730±0.138 | 1.332 | <0.0001 |
| Concordant imaging modalities (vs discordant) | −0.005±0.019 | −0.019 | 0.800 | −0.013±0.018 | −0.052 | 0.471 | −0.016±0.018 | −0.064 | 0.374 |
| Hypertension | −0.005±0.022 | −0.022 | 0.804 | −0.020±0.021 | −0.083 | 0.337 | −0.022±0.021 | −0.091 | 0.292 |
| Diabetes | 0.032±0.052 | 0.049 | 0.543 | 0.011±0.049 | 0.017 | 0.826 | 0.008±0.049 | 0.012 | 0.877 |
| Ever smoking | −0.007±0.019 | −0.028 | 0.723 | −0.0003±0.018 | −0.012 | 0.871 | 0.003±0.018 | 0.014 | 0.857 |
| Hyperlipidemia | 0.025±0.021 | −0.101 | 0.242 | 0.016±0.019 | 0.066 | 0.407 | 0.024±0.020 | 0.100 | 0.224 |
| MAP, 1 mm Hg | 0.0002±0.002 | 0.009 | 0.913 | 0.0008±0.002 | 0.033 | 0.667 | 0.001±0.002 | 0.056 | 0.474 |
BSA indicates body surface area; dTAA, degenerative thoracic aortic aneurysm; hTAA, hereditary thoracic aortic aneurysm; MAP, mean arterial pressure; and TAA, thoracic aortic aneurysm.
Multivariable logistic regression models to predict accelerated TAA growth are depicted in Table 3. The c‐statistic for the base model that included usual clinical variables was 0.660. Arterial age was independently associated with accelerated TAA growth and increased the c‐statistic to 0.708 when added to the base model. When both chronological age and arterial age were added to the model, arterial age remained independently associated with accelerated TAA growth, while chronological age was not. In sensitivity analyses, after stepwise variable selection, the following variables were included in the model: age, aneurysm location, time between studies, and hyperlipidemia. In this model, arterial age remained independently associated with future, faster aneurysm expansion, with similar odds ratio (OR) as in the fully adjusted model (OR, 1.180 [95% CI, 1.057–1.317]; P=0.003).
Table 3.
Multivariable Logistic Regression Models to Assess Future Accelerated TAA Growth (Growth>Sex‐Specific Median)
| Model | Chronological age alone c‐statistic: 0.660 | Arterial age alone c‐statistic: 0.708 | Chronological age+arterial age c‐statistic: 0.728 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value |
| Chronological age, 5 y | 0.985 | 0.783–1.238 | 0.895 | … | … | … | 0.769 | 0.582–1.017 | 0.066 |
| Arterial age, 5 y | … | … | … | 1.161 | 1.045–1.290 | 0.005 | 1.230 | 1.085–1.394 | 0.001 |
| Male sex (vs female sex) | 0.923 | 0.360–2.537 | 0.867 | 0.900 | 0.339–2.387 | 0.832 | 0.941 | 0.350–2.527 | 0.904 |
| BSA, 1 m2 | 0.985 | 0.130–7.461 | 0.988 | 1.110 | 0.154–8.005 | 0.917 | 0.551 | 0.065–4.649 | 0.584 |
| dTAA (vs hTAA) | 1.011 | 0.388–2.632 | 0.983 | 0.593 | 0.285–0.227 | 0.285 | 0.781 | 0.286–2.137 | 0.631 |
| Baseline TAA size, 1 mm | 1.057 | 0.956–1.040 | 0.281 | 1.015 | 0.914–1.128 | 0.778 | 1.026 | 0.923–1.140 | 0.639 |
| Time between studies, 1 y | 0.888 | 0.730–1.080 | 0.233 | 0.839 | 0.682–1.031 | 0.094 | 0.841 | 0.683–1.035 | 0.102 |
| Non‐root aneurysm location | 1.681 | 0.619–4.567 | 0.308 | 1.685 | 0.635–4.467 | 0.295 | 2.484 | 0.845–7.299 | 0.098 |
| Concordant imaging modalities (vs discordant) | 0.771 | 0.365–1.626 | 0.494 | 0662 | 0.306–1.432 | 0.295 | 0.607 | 0.276–1.334 | 0.214 |
| Hypertension | 1.009 | 0.432–2.236 | 0.983 | 0.744 | 0.301–1.835 | 0.521 | 0.713 | 0.285–1.784 | 0.470 |
| Diabetes | 0.623 | 0.082–4.752 | 0.648 | 0.493 | 0.055–4.393 | 0.526 | 0.473 | 0.051–4.377 | 0.509 |
| Ever smoking | 1.246 | 0.585–2.653 | 0.569 | 1.323 | 0.611–2.886 | 0.474 | 1.568 | 0.702–3.505 | 0.273 |
| Hyperlipidemia | 2.396 | 1.046–5.487 | 0.039 | 2.066 | 0.906–4.710 | 0.085 | 2.579 | 1.081–6.154 | 0.033 |
| MAP, 1 mm Hg | 0.999 | 0.959–1.040 | 0.959 | 1.002 | 0.961–1.044 | 0.940 | 1.008 | 0.966–1.052 | 0.704 |
BSA indicates body surface area; dTAA, degenerative thoracic aortic aneurysm; hTAA, hereditary thoracic aortic aneurysm; MAP, mean arterial pressure; OR, odds ratio; and TAA, thoracic aortic aneurysm.
DISCUSSION
To our knowledge, this is the first study to assess arterial age as a marker of disease activity in patients with TAA, demonstrating that EVA is common among patients with TAA (affecting one‐third of them) and that greater arterial age is independently associated with accelerated TAA growth. Importantly, we demonstrated that arterial age has the strongest association with future aneurysm expansion as compared with clinically available variables, including chronological age and aneurysm size, which are, presently, the greatest drivers of clinical decisions in TAA. These findings highlight the potential of arterial age in improving clinical assessment of disease activity and risk in TAA (Figure).
Figure . Independent associations of arterial age and chronological age with future thoracic aortic aneurysm expansion.
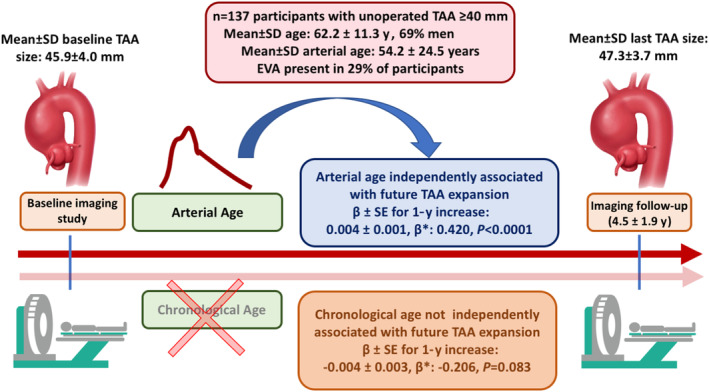
Arterial age is associated with faster thoracic aortic aneurysm growth, while chronological age is not. Further, arterial age had the strongest association with thoracic aortic aneurysm expansion as compared with all other clinical variables, including aneurysm size. EVA indicates early vascular aging, defined as arterial age>chronological age; TAA, thoracic aortic aneurysm; and β*, standardized β coefficient.
Current Pitfalls in the Clinical Management of TAAs, and the Potential Use of Arterial Age
As the most feared complication of TAAs is AAS, current guidelines suggest prophylactic surgical repair when the aneurysm reaches a critical size (5.5 cm in most patients with TAA 21 ) in hopes to avoid this ominous complication. However, according to the IRAD, >50% of dissections will occur in patients with aortic diameters smaller than 5.5 cm. 7 Thus, TAA size alone is an imperfect risk stratification tool. For a disease as deadly as TAA/AAS, it is of utmost importance that we continue refining our methods for risk stratification to improve accuracy and, by doing so, improve future outcomes. Based on our findings, the estimation of arterial age in TAA carries significant potential to improve the identification of patients at risk for accelerated aneurysm growth, which may highlight a more unstable aortic pathology.
The other important pitfall of TAA care is the lack of established nonsurgical interventions and therapeutic targets outside of Marfan aortopathy (for whom the use of beta blockers and angiotensin receptor blockers to decelerate aneurysm growth rests on randomized clinical trial data). 22 As such, clinicians and patients are limited to blood pressure control and watchful waiting until an irreversible abnormality occurs, necessitating surgical repair. This highlights the need to identify novel therapeutic targets that may be used to develop and test medical therapies aimed at containing disease activity and obviating the need for surgery. In this context, our findings suggest arterial age as a potential therapeutic target for the development of novel medical therapies for TAA.
Structural Changes of the Aging Aorta With Links to TAA
To understand the role of arterial aging in TAA, it is imperative to understand the histopathological similarities between natural aortic aging and TAA. The aortic wall is composed of 3 layers and is continually subject to pulsatile stress from the high pressures generated by the beating left ventricle. 13 Elastic fibers and fibrillar collagens represent about 60% of the normal aortic media layer, and the remaining is composed of smooth muscle cells. This structure gives the aorta its essential pressure‐buffering capacity, as well as the ability to withhold high pressures generated by the left ventricle. However, as the aorta ages naturally, over time the repetitive pulsatile stress leads to fatigue of the elastic fibers in its wall, with eventual breakdown and loss of the elastic fibers within the media layer. 12 This is accompanied by medial fibrosis (collagen deposition) and disorganization of the vascular smooth muscle cells. Resulting from this process, the aortic wall becomes stiffer over time, and as such demonstrates decreased capacity to buffer pulsatile pressure. Interestingly, these exact changes occur in the wall of a TAA regardless of chronological age, to the point that experts have suggested that a TAA represents a focal, accelerated form of aortic aging. 12 Aortic calcification is also a marker of arterial aging and contributes to aortic stiffness, albeit only modestly (r=0.39) 23 to moderately (r=0.60). 24 As such, assessing arterial aging based on aortic stiffness is preferable to evaluating aortic calcification alone. While the latter lacks age‐based normative values, the former is also the recommended method, 25 making aortic stiffness a more attractive marker for estimation of arterial age.
In this context, it is possible that assessment of arterial age provides insights into the overall structure and function of the aorta that may be further compromised by additional structural damage to the media imposed by the TAA. This framework allows us to understand how arterial age can independently associate with faster TAA expansion, serving as a noninvasive biomarker of aortic health and TAA disease activity.
Role of Arterial Age in Other Forms of Cardiovascular Disease
Arterial age and EVA can be estimated by different methodologies. 26 Although our study is the first to assess the role of arterial age in TAA, other groups have reported the use of arterial age/vascular age/EVA as predictors of adverse outcomes in other forms of cardiovascular diseases. Stein et al have described that the noninvasive measurement of carotid intima‐media thickness to determine a patient's vascular age helps reclassify patients' risk of complications from coronary artery disease using the Framingham Risk Score. 27 In this study, vascular age was determined by linear regression modeling using published nomograms of carotid intima‐media thickness percentiles according to chronological age, sex, and race. 27 Adjusted 10‐year coronary heart disease risk estimates were calculated after substituting vascular age for chronological age, leading to about 15% of subjects being reclassified to a higher risk group when vascular age was used over chronological age. 27 Thus, the atherosclerotic burden of individuals with the same chronological age and similar risk profiles can differ substantially. 28 , 29 , 30 Thus, using vascular age as substitute for chronological age in risk calculation formulas increased the 10‐year coronary heart disease risk estimates. 27 Moreover, additional studies have shown that although arteries undergo natural changes in response to aging, these same processes are accelerated in the presence of cardiovascular diseases, 31 which independently predicts future stroke and myocardial infarction. 32 In a large cohort from Sweden, vascular age was highly correlated with adverse cardiovascular events, with patients who exhibited EVA having a 2.7‐fold increase in the risk of cardiovascular events as compared with those who had normal vascular aging. 33 Additionally, EVA has a higher predictive value to determine cardiovascular events compared with single snapshot blood pressure measurements, highlighting its clinical significance. 34
As such, there is growing evidence to support the use of arterial age in cardiovascular risk prediction; our study adds to this body of literature by demonstrating the potential role of arterial age in TAA management.
Strengths and Limitations
Our study is not without limitations. Firstly, different imaging modalities were used to assess TAA size. This, however, reflects real‐world clinical practice, which increases the external validity of our study. Importantly, we have previously shown excellent intra‐observer agreement for TAA size measurement across imaging modalities 15 and adjusted our regression models for the concordant/discordant nature of imaging, demonstrating that arterial age's association with TAA expansion was independent of the nature of the imaging studies. Moreover, using AAS as the outcome variable of interest would have been ideal; however, this will require larger cohorts with longer periods of observation given the low proportion of AAS among patients with TAA undergoing careful surveillance. However, we believe our choice of TAA expansion as an outcome is still robust, as TAA growth is associated with AAS, and as such has been the outcome of choice in all clinical trials of TAA. 22 Lastly, our protocol did not include measurement of circulating biomarkers potentially associated with extracellular matrix remodeling and arterial aging, which remains amenable to testing in future studies.
CONCLUSIONS
Our findings highlight that arterial age, which conveys information about aortic health and function, is independently associated with accelerated TAA growth, while chronological age is not. Importantly, arterial age was the clinical parameter with the strongest association with TAA expansion, even when compared with the present standard of care for clinical decisions (aneurysm size). Arterial age is a parameter that can be measured noninvasively and represents a concept that is readily understood by health care providers and patients (for example, while an individual without knowledge of arterial function may not be able to readily interpret the values of cfPWV in meters per second, anyone can understand arterial age in years as compared with one's chronological age). Moreover, the simplicity and inexpensive nature of this test further increase the clinical applicability of arterial age, as it can be measured in an office setting without risks to patients. As such, arterial age carries significant potential as a novel marker of TAA disease activity that may be integrated as part of individualized risk stratification and disease monitoring algorithms for TAA.
Sources of Funding
This research was funded by an Early Research Leaders Initiative grant from the Canadian Vascular Network and the Canadian Institutes of Health Research, and a Grant‐in‐Aid from the Heart and Stroke Foundation of Canada.
Disclosures
Dr. Coutinho was supported by the chair of Women's Heart Health at the University of Ottawa Heart Institute at the time of manuscript submission. The remaining authors have no disclosures to report.
Supporting information
Data S1
References 35–37
Acknowledgments
The authors would like to acknowledge Dr Jodi Edwards for her help with statistical analyses.
This manuscript was sent to Ajay K. Gupta, MD, MSc, PhD, FRCP, FESC, Senior Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.029466
For Sources of Funding and Disclosures, see page 8.
REFERENCES
- 1. Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population‐based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611–2618. doi: 10.1161/CIRCULATIONAHA.106.630400 [DOI] [PubMed] [Google Scholar]
- 2. van Walraven C, Wong J, Morant K, Jennings A, Jetty P, Forster AJ. Incidence, follow‐up, and outcomes of incidental abdominal aortic aneurysms. J Vasc Surg. 2010;52(282–289):e1–e2. doi: 10.1016/j.jvs.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 3. Mészáros I, Mórocz J, Szlávi J, Schmidt J, Tornóci L, Nagy L, Szép L. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117:1271–1278. doi: 10.1378/chest.117.5.1271 [DOI] [PubMed] [Google Scholar]
- 4. Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74:S1877–S1880; discussion S1892–S1898. [DOI] [PubMed] [Google Scholar]
- 5. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, et al. 2010 AHA guidelines for the diagnosis and management of patients with thoracic aortic disease. A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Heart Assoc. 2010;55:e27–e129. [DOI] [PubMed] [Google Scholar]
- 6. Booher AM, Eagle KA. Diagnosis and management issues in thoracic aortic aneurysm. Am Heart J. 2011;162:38–46.e1. doi: 10.1016/j.ahj.2011.04.010 [DOI] [PubMed] [Google Scholar]
- 7. Pape LA, Tsai TT, Isselbacher EM, Oh JK, O'gara PT, Evangelista A, Fattori R, Meinhardt G, Trimarchi S, Bossone E, et al. International registry of acute aortic dissection (IRAD) investigators. Aortic diameter >or=5.5 cm is not a good predictor of type a aortic dissection: observations from the international registry of acute aortic dissection (IRAD). Circulation. 2007;116:1120–1127. doi: 10.1161/CIRCULATIONAHA.107.702720 [DOI] [PubMed] [Google Scholar]
- 8. Bum KJ, Kibeom K, Lindsay ME, Thomas MG, Isselbacher EM, Cambria RP, Sundt TM. Risk of rupture or dissection in descending thoracic aortic aneurysm. Circulation. 2015;132:1620–1629. doi: 10.1161/CIRCULATIONAHA.114.015177 [DOI] [PubMed] [Google Scholar]
- 9. Elefteriades JA, Farkas EA. Thoracic aortic aneurysm. J Am Coll Cardiol. 2010;55:841–857. doi: 10.1016/j.jacc.2009.08.084 [DOI] [PubMed] [Google Scholar]
- 10. Lobato AC, Puech‐Leão P. Predictive factors for rupture of thoracoabdominal aortic aneurysm. J Vasc Surg. 1998;27:446–453. doi: 10.1016/S0741-5214(98)70319-2 [DOI] [PubMed] [Google Scholar]
- 11. Thompson AR, Cooper JA, Ashton HA, Hafez H. Growth rates of small abdominal aortic aneurysms correlate with clinical events. Br J Surg. 2010;97:37–44. doi: 10.1002/bjs.6779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Halushka MK, Angelini A, Bartoloni G, Basso C, Batoroeva L, Bruneval P, Buja LM, Butany J, d'Amati G, Fallon JT, et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the association for European cardiovascular pathology: II. Noninflammatory degenerative diseases—nomenclature and diagnostic criteria. Cardiovasc Pathol. 2016;25:247–257. doi: 10.1016/j.carpath.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 13. Humphrey JD, Schwartz MA, Tellides G, Milewicz DM. Role of mechanotransduction in vascular biology. Circ Res. 2015;116:1448–1461. doi: 10.1161/CIRCRESAHA.114.304936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reference Values for Arterial Stiffness' Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “establishing normal and reference values.” Eur Heart J. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheung K, Boodhwani M, Chan K‐L, Beauchesne L, Dick A, Coutinho T. Thoracic aortic aneurysm growth: role of sex and aneurysm etiology. J Am Heart Assoc. 2017;6:6. doi: 10.1161/JAHA.116.003792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boczar KE, Boodhwani M, Dennie C, Chan KL, Wells GA, Coutinho T. Aortic stiffness, central blood pressure, and pulsatile arterial load predict future thoracic aortic aneurysm expansion. Hypertension. 2021;77:126–134. doi: 10.1161/HYPERTENSIONAHA.120.16249 [DOI] [PubMed] [Google Scholar]
- 17. Boczar KE, Boodhwani M, Beauchesne L, Dennie C, Chan KL, Wells GA, Coutinho T. Aortic stiffness, central blood pressure, and pulsatile arterial load predict future thoracic aortic aneurysm expansion. Hypertension. 1979;2021(77):126–134. doi: 10.1161/HYPERTENSIONAHA.120.16249 [DOI] [PubMed] [Google Scholar]
- 18. Mitchell GF, Hwang S‐J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace‐Raso FUS, Protogerou AD, et al. Artery Society, European Society of Hypertension Working Group on vascular structure and function, European network for noninvasive investigation of large arteries. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid‐femoral pulse wave velocity. J Hypertens. 2012;30:445–448. doi: 10.1097/HJH.0b013e32834fa8b0 [DOI] [PubMed] [Google Scholar]
- 20. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker‐Boudier H. European network for non‐invasive investigation of large arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254 [DOI] [PubMed] [Google Scholar]
- 21. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases|European Heart Journal|Oxford Academic. Accessed April 20, 2022. https://academic.oup.com/eurheartj/article/35/41/2873/407693. [DOI] [PubMed]
- 22. Lacro RV, Dietz HC, Sleeper LA, Yetman AT, Bradley TJ, Colan SD, Pearson GD, Selamet Tierney ES, Levine JC, Atz AM, et al. Atenolol versus losartan in children and young adults with Marfan's syndrome. N Engl J Med. 2014;371:2061–2071. doi: 10.1056/NEJMoa1404731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moon I, Jin KN, Kim HL, Suh HJ, Lim W‐H, Seo J‐B, Kim S‐H, Zo J‐H, Kim M‐A. Association of arterial stiffness with aortic calcification and tortuosity. Medicine. 2019;98:e16802. doi: 10.1097/MD.0000000000016802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McEniery CM, McDonnell BJ, So A, Aitken S, Bolton CE, Munnery M, Hickson SS, Yasmin, Maki‐Petaja KM, Cockcroft JR, et al. Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension. 2009;53:524–531. doi: 10.1161/HYPERTENSIONAHA.108.126615 [DOI] [PubMed] [Google Scholar]
- 25. Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laurent S, Marais L, Boutouyrie P. The noninvasive assessment of vascular aging. Can J Cardiol. 2016;32:669–679. doi: 10.1016/j.cjca.2016.01.039 [DOI] [PubMed] [Google Scholar]
- 27. Stein JH, Fraizer MC, Aeschlimann SE, Nelson‐Worel J, McBride PE, Douglas PS. Vascular age: integrating carotid intima‐media thickness measurements with global coronary risk assessment. Clin Cardiol. 2004;27:388–392. doi: 10.1002/clc.4960270704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol In adults (adult treatment panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 29. Grundy SM, Pasternak R, Greenland P, Smith S, Fuster V. Assessment of cardiovascular risk by use of multiple‐risk‐factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 1999;100:1481–1492. doi: 10.1161/01.CIR.100.13.1481 [DOI] [PubMed] [Google Scholar]
- 30. Grundy SM. Coronary plaque as a replacement for age as a risk factor in global risk assessment. Am J Cardiol. 2001;88:8E–11E. doi: 10.1016/S0002-9149(01)01712-X [DOI] [PubMed] [Google Scholar]
- 31. Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 1979;2005(46):454–462. doi: 10.1161/01.HYP.0000177474.06749.98 [DOI] [PubMed] [Google Scholar]
- 32. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular health study collaborative research group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103 [DOI] [PubMed] [Google Scholar]
- 33. Bruno RM, Nilsson PM, Engström G, Wadström BN, Empana J‐P, Boutouyrie P, Laurent S. Early and supernormal vascular aging. Hypertension. 2020;76:1616–1624. doi: 10.1161/HYPERTENSIONAHA.120.14971 [DOI] [PubMed] [Google Scholar]
- 34. Laurent S, Boutouyrie P, Cunha PG, Lacolley P, Nilsson PM. Concept of extremes in vascular aging. Hypertension. 2019;74:218–228. doi: 10.1161/HYPERTENSIONAHA.119.12655 [DOI] [PubMed] [Google Scholar]
- 35. Gehan EA, George SL. Estimation of human body surface area from height and weight. Cancer Chemother Rep. 1970;54:225–235. [PubMed] [Google Scholar]
- 36. Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham heart study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa [DOI] [PubMed] [Google Scholar]
- 37. Goldstein SA, Evangelista A, Abbara S, Arai A, Asch FM, Badano LP, Bolen MA, Connolly HM, Cuéllar‐Calàbria H, Czerny M, et al. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging: endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2015;28:119–182. doi: 10.1016/j.echo.2014.11.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
References 35–37


