Abstract
Background
The prevalence of calcific aortic stenosis and amyloid transthyretin cardiomyopathy (ATTR‐CM) increase with age, and they often coexist. The objective was to determine the prevalence of ATTR‐CM in patients with severe aortic stenosis and evaluate differences in presentations and outcomes of patients with concomitant ATTR‐CM undergoing transcatheter aortic valve implantation.
Methods and Results
Prospective screening for ATTR‐CM with Technetium99‐3,3‐diphosphono‐1,2‐propanodicarboxylic acid bone scintigraphy was performed in 315 patients referred with severe aortic stenosis between August 2019 and August 2021. Myocardial Technetium99‐3,3‐diphosphono‐1,2‐propanodicarboxylic acid tracer uptake was detected in 34 patients (10.8%), leading to a diagnosis of ATTR‐CM in 30 patients (Perugini ≥2: 9.5%). Age (85.7±4.9 versus 82.8±4.5; P=0.001), male sex (82.4% versus 57.7%; P=0.005), and prior carpal tunnel surgery (17.6% versus 4.3%; P=0.007) were associated with coexisting ATTR‐CM, as were ECG (discordant QRS voltage to left ventricular wall thickness [42% versus 12%; P<0.001]), echocardiographic (left ventricular ejection fraction 48.8±12.8 versus 58.4±10.8; P<0.001; left ventricular mass index, 144.4±45.8 versus 117.2±34.4g/m2; P<0.001), and hemodynamic parameters (mean aortic valve gradient, 23.4±12.6 versus 35.5±16.6; P<0.001; mean pulmonary artery pressure, 29.5±9.7 versus 25.8±9.5; P=0.037). Periprocedural (cardiovascular death: hazard ratio [HR], 0.71 [95% CI, 0.04–12.53]; stroke: HR, 0.46 [95% CI, 0.03–7.77]; pacemaker implantation: HR, 1.54 [95% CI, 0.69–3.43]) and 1‐year clinical outcomes (cardiovascular death: HR, 1.04 [95% CI, 0.37–2.96]; stroke: HR, 0.34 [95% CI, 0.02–5.63]; pacemaker implantation: HR, 1.50 [95% CI, 0.67–3.34]) were similar between groups.
Conclusions
Coexisting ATTR‐CM was observed in every 10th elderly patient with severe aortic stenosis referred for therapy. While patients with coexisting pathologies differ in clinical presentation and echocardiographic and hemodynamic parameters, peri‐interventional risk and early clinical outcomes were comparable up to 1 year after transcatheter aortic valve implantation.
REGISTRATION
URL: https://www.clinicaltrials.gov. Unique identifier: NCT04061213.
Keywords: 99mTc‐DPD scintigraphy, aortic stenosis, cardiac amyloidosis, TAVI, transthyretin
Subject Categories: Clinical Studies, Pathophysiology
Nonstandard Abbreviations and Acronyms
- 99mTc‐DPD
Technetium99‐3,3‐diphosphono‐1,2‐propanodicarboxylic acid
- ATTR‐AS
Amyloid Transthyretin in Aortic Stenosis
- ATTR‐CM
amyloid transthyretin cardiomyopathy
- DPD
3,3‐diphosphono‐1,2‐propanodicarboxylic acid
- TAVI
transcatheter aortic valve implantation
Clinical Perspective.
What Is New?
Invasive hemodynamic data and echocardiographic features are useful to help distinguish patients with underlying amyloid transthyretin cardiomyopathy.
Perioperative adverse events occur with similar frequency among patients with coexisting pathologies and isolated aortic stenosis.
What Are the Clinical Implications?
Future studies need to evaluate whether amyloid transthyretin cardiomyopathy–targeting therapies may confer benefit in elderly patients with severe symptomatic aortic stenosis.
Mechanistic studies need to evaluate how amyloid transthyretin cardiomyopathy and aortic stenosis are linked.
Current techniques enable reliable identification of amyloid precursor proteins and provide an opportunity to determine whether amyloid transthyretin cardiomyopathy and aortic stenosis share a common pathophysiology.
The prevalence of aortic stenosis (AS) and amyloid transthyretin cardiomyopathy (ATTR‐CM) increases with age. While the global prevalence of calcific aortic valve disease is estimated to be >2% in elderly patients aged ≥70 years, 1 cardiac amyloidosis is still considered a rare entity. 2 However, screening and autopsy studies suggest that amyloid depositions are present in >10% of octogenarians and in specific patient populations. 3 , 4 As a result, coexisting cardiac amyloidosis is reported in 8% to 13% of elderly patients with symptomatic, severe AS referred for transcatheter aortic valve implantation (TAVI). 5 , 6 Owing to the availability of noninvasive bone scintigraphy, 7 ATTR‐CM is now recognized to be the most common cardiac amyloidosis type and the one typically diagnosed in patients with symptomatic severe AS. 5 , 8
Current evidence suggests that TAVI is also of therapeutic benefit in patients with both pathologies. 5 , 6 Yet it remains to be determined whether the incidental finding of ATTR‐CM in patients with symptomatic severe AS referred for TAVI confers an increased risk of adverse events during the periprocedural period as well as during the early and mid‐ to long‐term follow‐up. 5 , 6
The aim of the present study was 3‐fold: (1) to determine the prevalence and type of concomitant cardiac amyloidosis in elderly patients with symptomatic, severe AS referred for aortic valve therapy evaluation at a tertiary care center; (2) to investigate the utility of hemodynamic data to help distinguish patients with both pathologies; and (3) to compare adverse events during the periprocedural period and up to 1 year after TAVI in patients with and without coexisting ATTR‐CM.
Methods
Study Population
ATTR‐AS (Amyloid Transthyretin in Aortic Stenosis) is a single‐center cohort study conducted at Bern University Hospital between August 2019 and August 2021. Consecutive patients referred for aortic valve replacement therapy were screened and evaluated for study participation. The lone inclusion criterion was the presence of severe AS. Patients unwilling to consent or requiring emergent aortic valve intervention were excluded from study participation. Noninvasive screening for cardiac amyloidosis was performed with 99mTechnetium‐3,3‐diphosphono‐1,2‐propanodicarboxylic acid (99mTc‐DPD) bone scintigraphy and laboratory assessment for clonal immunoglobulins in addition to routine echocardiographic and invasive hemodynamic evaluation for severe AS. The design of the study was approved by the local ethics committee and conducted in accordance with the Declaration of Helsinki. Study participants provided written, informed consent for the study procedures and follow‐up. All data were prospectively collected and entered into a dedicated online database at Bern University Hospital. ATTR‐AS is registered with ClinicalTrials.gov (NCT04061213). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Hemodynamic Evaluation
Echocardiography
Routine echocardiographic assessment was performed according to current guidelines established by the American Society of Echocardiography/European Association of Cardiovascular Imaging. 9 , 10 Images were acquired using GE Vivid, Philips iE33, or Philips Epiq ultrasound equipment. Linear and 2‐dimensional measurements were used for chamber quantification. Left ventricular ejection fraction was assessed by Simpson's biplane method of disks or by visual estimation if image quality precluded the former. To determine AS severity, peak jet velocity and mean aortic valve pressure gradient were measured using continuous wave Doppler. Aortic valve area was approximated using the continuity equation. For assessment of diastolic function, mitral flow velocities, mitral annular e′ velocity, E/e′ ratio, TR jet peak velocity, and left atrial volumes were acquired. For staging, the algorithm previously described by the American Society of Echocardiography was used. 11
Invasive Hemodynamic Evaluation
Invasive assessment of AS severity and hemodynamics was performed as previously described. 12 Cardiac output was measured using the Fick principle: stroke volume calculated by dividing cardiac output by heart rate. The Gorlin formula was used to calculate aortic valve area.
Screening for Cardiac Amyloidosis
Laboratory testing for amyloidosis included serum gel electrophoresis, immunofixation, and serum free light chain assays. 13 If pathologic, hematologic consultation and, if necessary, tissue biopsy were performed to exclude light chain amyloidosis and thereby confirm transthyretin amyloidosis.
Noninvasive screening of cardiac amyloidosis was performed using 99mTc‐DPD bone scintigraphy. Three hours after intravenous injection of 700 MBq ±10% 99mTc‐DPD and peroral hydration, patients were imaged on a Siemens Intevo Bold camera. Planar whole‐body images were acquired for 15 minutes using a low energy, high‐resolution collimator with a matrix of 256 × 256. The planar scans were immediately followed by a single‐photon emission computed tomography with a low‐dose, noncontrast computed tomography scan of the heart when a pathologic myocardial uptake was observed on the planar scans. Myocardial uptake on the planar 99mTc‐DPD scan was visually categorized according to the modified Perugini Score described by Hutt et al. 14
ATTR‐CM was diagnosed in patients with moderate or strong myocardial 99mTc‐DPD uptake (Perugini grade II or III) and exclusion of light chain amyloidosis by laboratory testing and tissue biopsy, if required. 15 Genetic testing was performed in study participants only after additional written, informed consent was acquired. Peripheral blood samples were used for DNA extraction, and testing was performed using Sanger sequencing of all exons and exon‐intron boundaries of the transthyretin gene.
Follow‐Up, Periprocedural Complications, and Clinical End Points
Baseline clinical, procedural, and follow‐up data were prospectively recorded using standardized case report forms. Clinical follow‐up data were obtained through standardized interviews, documentation from referring physicians, and hospital discharge summaries. Adverse events were systematically collected and adjudicated by a dedicated clinical event committee based on the standardized Valve Academic Research Consortium‐2 criteria. 16
The primary end point of the study was the prevalence of cardiac amyloidosis in patients with symptomatic, severe AS referred for aortic valve replacement therapy evaluation. Secondary end points were evaluated in patients undergoing TAVI and encompassed all‐cause and cardiovascular death, cerebrovascular events, myocardial infarction, bleeding, access‐related complications, kidney injury, and the requirement for pacemaker implantation up to 12 months after the procedure.
Statistical Analysis
Continuous variables are presented as mean values±SD and compared between 2 groups using Student's t‐tests (ANOVAs with F‐tests were used to compare 3 groups). Categorical variables are represented as counts and percentages, and the differences between groups were tested using Fisher's exact test for every 2 × 2 comparisons and chi‐square tests if more categories were involved (eg, 2 × 3, 2 × 4, etc). Time‐to‐event curves were constructed using the Kaplan–Meier method. Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% CIs for clinical outcomes comparing the groups (adjudicated events up to 30 days and up to 1 year from TAVI). Additional analyses included patients who did not undergo TAVI, with adjudicated events being counted from the date of scintigraphy (again, up to 30 days and 1 year). Diphosphono‐1,2‐propanodicarboxylic acid (DPD) 0 is used as the reference group throughout. All statistical tests were 2‐sided, and P values <0.05 were considered significant. Statistical analyses were performed using Stata 17 (StataCorp, College Station, TX).
Results
Baseline Characteristics
Of 489 patients referred for TAVI evaluation, 444 (90.8%) were screened for study participation. A total of 315 of the 398 patients considered eligible (79.1%; 70.9% of the screened cohort, see Figure 1) agreed to participate in the study. Patient characteristics are detailed in Table 1 .
Figure 1. Study CONSORT flowchart.

AS indicates aortic stenosis; CONSORT, Consolidated Standards of Reporting Trials; DPD, 3,3‐diphosphono‐1,2‐propanodicarboxylic acid; SAVR, surgical aortic valve replacement; and TAVI, transcatheter aortic valve implantation.
Table 1.
Baseline Clinical Characteristics
| All patients | DPD 0 | DPD 1/2/3 | P value | |
|---|---|---|---|---|
| N=315 | N=281 | N=34 | ||
| Age, y | 83.1±4.6 | 82.8±4.5 | 85.7±4.9 | 0.001 |
| Sex, female | 125 (39.7) | 119 (42.3) | 6 (17.6) | 0.005 |
| Body mass index, kg/mm2 | 27.1±6.0 | 27.0±5.8 | 27.4±7.3 | 0.71 |
| Society of Thoracic Surgeons calculated risk of death | 4.3±3.5 | 4.2±3.5 | 5.1±3.6 | 0.21 |
| Clinical features | ||||
| Arterial hypertension | 278 (88.3) | 246 (87.5) | 32 (94.1) | 0.40 |
| Diabetes | 95 (30.2) | 88 (31.3) | 7 (20.6) | 0.24 |
| Dyslipidemia | 186 (69.1) | 163 (68.2) | 23 (76.7) | 0.41 |
| Chronic kidney disease (glomerular filtration rate <60 mL/min per 1.73 m2) | 193 (61.3) | 169 (60.1) | 24 (70.6) | 0.27 |
| Chronic obstructive pulmonary disease | 19 (7.1) | 18 (7.5) | 1 (3.3) | 0.71 |
| Atrial fibrillation | 116 (36.8) | 98 (34.9) | 18 (52.9) | 0.058 |
| Past medical history | ||||
| Coronary artery disease | 134 (42.5) | 117 (41.6) | 17 (50.0) | 0.36 |
| Coronary artery bypass grafting | 24 (7.6) | 21 (7.5) | 3 (8.8) | 0.73 |
| Myocardial infarction | 25 (9.3) | 22 (9.2) | 3 (10.0) | 0.75 |
| Cerebrovascular accident | 45 (14.3) | 39 (13.9) | 6 (17.6) | 0.60 |
| Peripheral arterial disease | 41 (13.0) | 36 (12.8) | 5 (14.7) | 0.79 |
| Permanent pacemaker | 24 (7.6) | 20 (7.1) | 4 (11.8) | 0.31 |
| Malignancy | 70 (22.2) | 65 (23.1) | 5 (14.7) | 0.38 |
| attr—specific characteristics | ||||
| Carpal tunnel surgery | 18 (5.7) | 12 (4.3) | 6 (17.6) | 0.007 |
| If yes, both‐sided | 10 (3.2) | 5 (1.8) | 5 (14.7) | 0.002 |
| Polyneuropathy | 12 (3.8) | 10 (3.6) | 2 (6.1) | 0.37 |
| Spinal stenosis | 23 (7.3) | 19 (6.8) | 4 (11.8) | 0.29 |
| Serum gel electrophoresis | ||||
| Performed | 45 (14.3) | 16 (5.7) | 29 (85.3) | <0.001 |
| Pathologic finding | 7 (15.6) | 3 (18.8) | 4 (13.8) | 0.69 |
| Genetic testing of transthyretin | ||||
| Performed | 24 (70.6) | |||
| Wild‐type | 23 (95.8) | |||
Depicted are means with SD or counts with percentages. P value from chi‐square test (counts) or ANOVA F‐test across all 3 groups. DPD 0: no myocardial tracer uptake in 99mTc‐DPD scintigraphy; DPD 1, 2, 3: pathologic myocardial uptake of 99mTc‐DPD according to Perugini grade I, II, or III. 99mTc‐DPD indicates Technetium99‐3,3‐diphosphono‐1,2‐propanodicarboxylic acid; ATTR, amyloid transthyretin; and DPD, 3,3‐diphosphono‐1,2‐propanodicarboxylic acid.
Prevalence of Cardiac Amyloidosis
Bone scintigraphy revealed myocardial 99mTc‐DPD uptake in 34 of 315 patients (10.8%) (Figure 2), of which 30 had moderate or strong 99mTc‐DPD uptake (Perugini ≥2: 30 of 34 [88.2%]; 30 of 315 [9.5%]). Serum assays detected a monoclonal immunoglobulin in 4 of the 34 patients with 99mTc‐DPD uptake (Perugini 1: 1 of 4 [25%]; Perugini ≥2: 3 of 30 [10%]). Bone marrow and gastrointestinal tissue biopsy was used to exclude light chain amyloidosis in 2 patients, while the other 2 declined further workup. Other types of amyloidosis were not found in the study population. Genetic testing of the transthyretin gene was performed in 24 of 34 99mTc‐DPD‐positive (DPD+) patients (70.6%). A single transthyretin mutation (Val30Met) was found in a patient with strong 99mTc‐DPD uptake, with wild‐type transthyretin being present in most patients (95.8% [n=23]).
Figure 2. Concomitant ATTR‐CM in patients with severe AS.
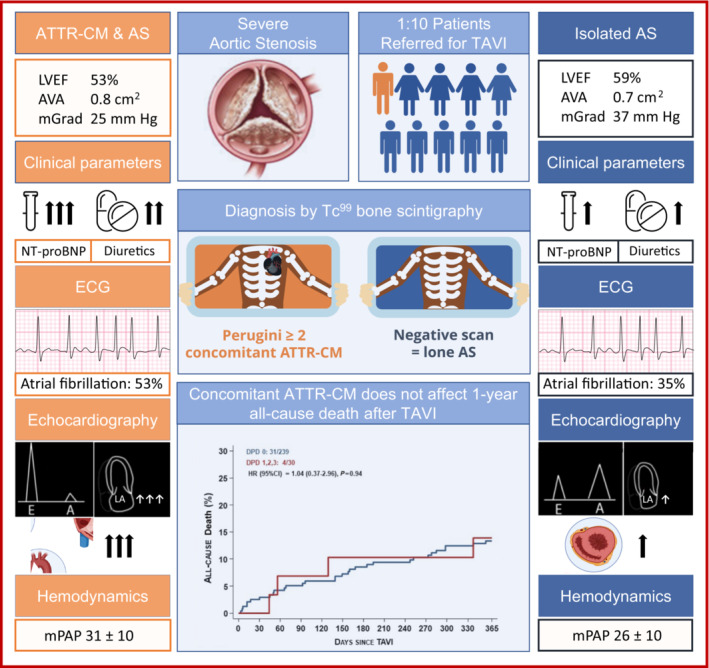
Differences in clinical presentation between patients with isolated AS and patients with concomitant ATTR‐CM referred for TAVR. AS indicates aortic stenosis; ATTR‐CM, amyloid transthyretin cardiomyopathy; AVA, aortic valve area; HR, hazard ratio; LVEF, left ventricular ejection fraction; mGrad, mean aortic valve gradient; mPAP, mean pulmonary artery pressure; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; TAVR, transcatheter aortic valve replacement; and Tc99, Technetium99.
Patients with 99mTc‐DPD uptake were older (85.7±4.9 versus 82.8±4.5 years; P=0.001) and more commonly men (82.4% versus 57.7%; P=0.005) than patients with negative bone scintigraphy. Loop diuretics were more often prescribed for patients who were DPD+ (79.4% versus 58.0%; P=0.016) (Figure 2), and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) levels were higher when patients presented for preoperative evaluation (4951.6±4452.2 pg/mL versus 2522.0±3851 pg/mL.1; P=0.001) (Figure 2, Table S1). A history of 1‐ and both‐sided carpal tunnel surgery was more frequent in patients with 99mTc‐DPD uptake (17.6% versus 4.3%; P=0.007; and 14.7% versus 1.8%; P=0.002, respectively).
Electrocardiographic, Echocardiographic, and Hemodynamic Findings
During TAVI workup, atrial fibrillation was more common in patients with 99mTc‐DPD uptake (39% versus 20%; P=0.024) (Tables S2 and S3). Left ventricular ejection fraction was lower (48.8±12.8% versus 58.4±10.8; P<0.001) and low‐flow, low‐gradient AS more prevalent (86% versus 49%; P=0.002) in patients with DPD+ bone scan. Mean transvalvular gradients measured by echocardiography were 26.6±12.5 mm Hg in patients who were DPD+ compared with 38.6±15.5 mm Hg in patients without 99mTc‐DPD uptake (P<0.001) and were 23.4±12.6 mm Hg compared with 35.5±16.6 mm Hg when assessed by invasive hemodynamics, respectively (P<0.001; Table 2 ). Noninvasive markers of concomitant cardiac amyloidosis included increased left ventricular (LV) wall thickness (15.5±3.0 mm versus 12.8±2.5 mm; P<0.001), increased LV mass index (144.4±45.8 versus 117.2±34.4 g/m2; P<0.001), presence of higher‐grade diastolic dysfunction (≥II, 71% versus 24%; P=0.001), more pronounced left atrial dilatation (57.7±16.3 versus 47.3±18.7 mL/m2; P=0.036), and increased mean pulmonary artery pressure (29.5±9.7 versus 25.8±9.5 mm Hg; P=0.037).
Table 2.
Periprocedural Characteristics
| All patients with TAVI | DPD 0 | DPD 1/2/3 | P value | |
|---|---|---|---|---|
| N=269 | N=239 | N=30 | ||
| Invasive hemodynamic data before TAVI | ||||
| Aortic valve mean gradient, mm Hg | 38.3±15.9 | 39.5±15.8 | 27.7±12.4 | <0.001 |
| Aortic valve area, cm2 | 0.7±0.3 | 0.7±0.2 | 0.8±0.4 | 0.004 |
| Stroke volume index, mL/m2 per beat | 27.1±7.3 | 27.3±7.4 | 25.3±6.6 | 0.18 |
| LV end‐diastolic pressure | 19.6±8.2 | 20.0±8.4 | 16.9±6.5 | 0.057 |
| Pulmonary artery pressure, mean | 26.5±9.7 | 26.0±9.6 | 30.5±9.7 | 0.019 |
| Echocardiographic data before TAVI | ||||
| Aortic valve area, cm2 | 0.7±0.3 | 0.7±0.3 | 0.8±0.4 | 0.002 |
| Aortic valve mean gradient, mm Hg | 36.0±15.7 | 37.3±15.6 | 25.4±12.3 | <0.001 |
| LV ejection fraction, % | 58.0±11.9 | 58.7±11.1 | 52.8±16.5 | 0.011 |
| LV mass, g | 210.7±81.3 | 204.1±78.0 | 262.9±93.5 | 0.053 |
| LV mass index, g/m2 | 115.0±36.3 | 113.7±35.5 | 126.6±43.8 | 0.35 |
| Aortic regurgitation—moderate or severe | 29 (16) | 26 (16) | 3 (16) | 1.00 |
| Mitral regurgitation—moderate or severe | 48 (22) | 42 (22) | 6 (23) | 1.00 |
| Tricuspid regurgitation—moderate or severe | 30 (16) | 25 (15) | 5 (22) | 0.38 |
| Pulmonary hypertension | 28 (19) | 22 (17) | 6 (40) | 0.077 |
| TAVI procedure | ||||
| Days between diagnosis and TAVI, median (IQR) | 23.0 (15.0–31.0) | 23.0 (15.0–31.0) | 22.0 (11.3–32.0) | 0.96 |
| General anesthesia | 19 (7) | 16 (7) | 3 (10) | 0.45 |
| Femoral main access | 265 (99) | 236 (99) | 29 (97) | 0.38 |
| Valve type | 0.101 | |||
| Balloon‐expandable | 168 (62) | 144 (60) | 24 (80) | 0.045 |
| Self‐expanding | 97 (36) | 91 (38) | 6 (20) | 0.068 |
| Mechanically expanding | 4 (1) | 4 (2) | 0 (0) | 1.00 |
| Echocardiographic data after TAVI | ||||
| Aortic valve area, cm2 | 1.7±0.5 | 1.7±0.5 | 1.8±0.5 | 0.46 |
| Aortic valve mean gradient, mm Hg | 10.3±5.0 | 10.6±5.1 | 8.2±3.3 | 0.016 |
| LV ejection fraction, % | 58.3±10.8 | 59.1±10.2 | 52.6±13.0 | 0.002 |
| LV mass, g | 201.1±79.7 | 195.2±77.3 | 248.6±84.0 | 0.001 |
| LV mass index, g/m2 | 112.0±41.6 | 109.2±41.7 | 134.1±33.2 | 0.004 |
| Aortic regurgitation—moderate or severe | 4 (1.5) | 4 (1.7) | 0 (0.0) | 1.00 |
| Mitral regurgitation—moderate or severe | 28 (10.6) | 26 (11.1) | 2 (6.7) | 0.75 |
| Tricuspid regurgitation—moderate or severe | 44 (16.6) | 38 (16.2) | 6 (20.0) | 0.60 |
| Pulmonary hypertension | 25 (13.8) | 18 (11.3) | 7 (31.8) | 0.017 |
Depicted are means with SD or counts with percentages. DPD indicates 3,3‐diphosphono‐1,2‐propanodicarboxylic acid; IQR, interquartile range; LV, left ventricular; and TAVI, transcatheter aortic valve implantation.
Treatment Strategies for Severe AS
Aortic valve replacement was performed in 31 99mTc‐DPD+ and 260 99mTc‐DPD‐negative (DPD–) study participants (91.1% versus 92.5%, baseline characteristics, see Table S4), and TAVI was the predominant treatment modality, with similar rates for patients who were DPD+ and DPD– (88.2% [n=30] versus 85.1% [n=239]). The average time between preevaluation and TAVI did not differ between the groups [DPD+: 22.0 days (interquartile range, 11.3–32.0); DPD–: 23.0 days (interquartile range, 15.0–31.0)]. One patient who was DPD+ underwent surgical aortic valve replacement, compared with 21 patients who were DPD− (2.9% versus 7.5%), while AS was managed conservatively in 2 patients who were DPD+ and 19 patients who were DPD− (5.9% versus 6.7%). Three patients died before any planned intervention (1 DPD+ [2.9%]; 2 DPD– [0.7%]).
Preoperative Hemodynamics in Patients Undergoing TAVI
Echocardiography
As seen in the overall cohort, among patients undergoing TAVI, LV ejection fraction (52.8±16.5% versus 58.7±11.1; P=0.011; Figure 2) and mean aortic valve gradient (25.4±12.3 mm Hg versus 37.3±15.6 mm Hg; P<0.001) were lower in patients who were DPD+ compared with patients who were DPD–, while aortic valve area was larger (0.8±0.4 versus 0.7±0.3 cm2; P=0.002; Figure 2). Concomitant valvular pathologies were similar in both groups (Table 2 ).
Invasive Hemodynamics
In patients who were DPD+ undergoing TAVI, mean aortic valve gradient was lower, with 27.7±12.4 mm Hg, and aortic valve area higher, at 0.8±0.4 cm2, compared with study participants who were DPD– (39.5±15.8 mm Hg; P<0.001; and 0.7±0.2 cm2 [P=0.004], respectively; Figure 2). While stroke volume index was numerically lower in patients who were DPD+, invasive measurements were not significantly different. Yet concomitant cardiac amyloidosis resulted in significantly elevated pulmonary artery pressures compared with patients with isolated AS (30.5±9.7 mm Hg versus 26.0±9.6 mm Hg; P=0.019; Table 2 ; Figure 2).
Periprocedural Characteristics and Complications
TAVI was performed under general anesthesia in 3 patients who were DPD+ and 16 patients who were DPD– (10% versus 7%; P=0.45; Table 2 ), and femoral access was employed in most patients (DPD+, 97% [n=29]; DPD–, 99% [n=236]; P=0.38). Balloon‐expandable aortic valve prostheses were more often used in patients with 99mTc‐DPD uptake (80% [n=24] versus 60% [n=144]; P=0.045), while self‐expanding valves were implanted at a higher rate in patients who were DPD– (38% versus 20%; P=0.068; Table 2 ).
Perioperative complications were rare in both groups (Table 3 ). Access site complications, paravalvular aortic regurgitation, conduction disease requiring pacemaker implantation, acute kidney injury, and rates of in‐hospital death were similar between patients who were DPD+ and patients who were DPD– (Table 3 ).
Table 3.
Adjudicated Outcomes After TAVI
| DPD 0 | DPD 1/2/3 | Hazard ratio (95% CI) | P value | |
|---|---|---|---|---|
| N=239 | N=30 | |||
| Outcomes at 30 day | ||||
| Death | 7 (2.9) | 0 (0.0) | 0.52 (0.03–8.88) | 1.00 |
| Cardiovascular death | 5 (2.1) | 0 (0.0) | 0.71 (0.04–12.53) | 1.00 |
| Cerebrovascular accident | 10 (4.2) | 0 (0.0) | 0.37 (0.02–6.16) | 0.61 |
| Stroke, any | 8 (3.4) | 0 (0.0) | 0.46 (0.03–7.77) | 0.60 |
| Disabling stroke | 5 (2.1) | 0 (0.0) | 0.71 (0.04–12.53) | 1.00 |
| Myocardial infarction | 2 (0.8) | 0 (0.0) | 1.57 (0.08–31.94) | 1.00 |
| Bleeding | 34 (14.3) | 2 (6.7) | 0.45 (0.11–1.88) | 0.28 |
| Life‐threatening or major bleeding | 30 (12.6) | 1 (3.3) | 0.26 (0.03–1.88) | 0.18 |
| Vascular access site and access‐related complications | 31 (13.0) | 2 (6.7) | 0.50 (0.12–2.09) | 0.34 |
| Major vascular complication | 24 (10.1) | 1 (3.3) | 0.32 (0.04–2.40) | 0.27 |
| Acute kidney injury | 7 (2.9) | 0 (0.0) | 0.52 (0.03–8.88) | 1.00 |
| Stage 3 | 3 (1.3) | 0 (0.0) | 1.12 (0.06–21.17) | 1.00 |
| Stage 2 | 4 (1.7) | 0 (0.0) | 0.87 (0.05–15.77) | 1.00 |
| Stage 1 | 0 (0.0) | 0 (0.0) | ||
| Pacemaker implantation | 40 (16.8) | 7 (23.3) | 1.54 (0.69–3.43) | 0.29 |
| Outcomes at 12 months | ||||
| Mortality | 31 (13.3) | 4 (13.9) | 1.04 (0.37–2.96) | 0.94 |
| Cardiovascular death | 20 (8.8) | 4 (13.9) | 1.62 (0.55–4.74) | 0.38 |
| Cerebrovascular accident | 16 (7.0) | 0 (0.0) | 0.24 (0.01–3.90) | 0.23 |
| Stroke, any | 11 (4.7) | 0 (0.0) | 0.34 (0.02–5.63) | 0.62 |
| Disabling stroke | 6 (2.5) | 0 (0.0) | 0.60 (0.03–10.39) | 1.00 |
| Myocardial infarction | 4 (1.8) | 0 (0.0) | 0.87 (0.05–15.77) | 1.00 |
| Bleeding | 43 (18.3) | 2 (6.7) | 0.35 (0.09–1.46) | 0.15 |
| Life‐threatening or major bleeding | 37 (15.7) | 1 (3.3) | 0.21 (0.03–1.50) | 0.12 |
| Vascular access site and access‐related complications | 32 (13.4) | 2 (6.7) | 0.48 (0.12–2.02) | 0.32 |
| Major vascular complication | 26 (10.9) | 1 (3.3) | 0.30 (0.04–2.20) | 0.24 |
| Permanent pacemaker implantation | 41 (17.3) | 7 (23.3) | 1.50 (0.67–3.34) | 0.32 |
Depicted are number of events counting only the first occurrence per patient (% Kaplan–Meier failure estimates). Hazard ratios (95% CIs from Cox regressions). Continuity corrected risk ratios (95% CIs) with Fisher's exact P value in case of 0 events in 1 group. DPD indicates 3,3‐diphosphono‐1,2‐propanodicarboxylic acid.
Early Procedural Success and 30‐Day Outcomes
Mean transvalvular gradients were reduced after TAVI in both groups and remained lower in patients who were DPD+ (8.2±3.3 mm Hg versus 10.6±5.1 mm Hg; P=0.016; Table 2 ), also reflecting the lower LV ejection fraction in these patients (52.6±13.0% versus 59.1±10.2; P=0.002). TAVI reduced the prevalence of mitral regurgitation irrespective of 99mTc‐DPD uptake (DPD+, from 23% [n=6] to 6.7% [n=2]; DPD–, from 22% [n=42] to 11.1% [n=26]), while pulmonary hypertension was more common in patients who were DPD+ (31.8% [n=7] versus 11.3% [n=18]; P=0.017; Table 2 ).
At 30 days, clinical event rates and the rate of post‐TAVI pacemaker requirement remained low in both groups, with similar outcomes between patients with isolated AS and patients with concomitant cardiac amyloidosis (Table 3 ).
Clinical Outcomes at 1 Year
At 1‐year follow‐up, all‐cause and cardiovascular death were not affected by 99mTc‐DPD uptake (DPD 1–3 versus DPD 0) or concomitant cardiac amyloidosis (DPD ≥2 versus DPD ≤1) (Figures 2 and 3, Table 3 ). In patients undergoing TAVI, all‐cause death at 1 year was 13.9% in patients who were DPD+ compared with 13.3% in patients who were DPD– (HR, 1.04 [95% CI, 0.37–2.96]; P=0.94), and cardiovascular death was 13.9% compared with 8.8%, respectively (HR, 1.62 [95% CI, 0.55–4.74]; P=0.38; Table 3 ). When comparing patients with cardiac transthyretin amyloidosis and patients in whom the diagnosis was excluded or not possible (DPD ≤1), all‐cause death was also not significantly different (HR, 1.25 [95% CI, 0.44–3.54]; P=0.68). Similarly, death was not affected by 99mTc‐DPD uptake when including patients managed by either surgical aortic valve replacement or optimal medical treatment only (HR, 1.12 [95% CI, 0.44–2.86]; P=0.81).
Figure 3. Kaplan–Meier curves for all‐cause death.
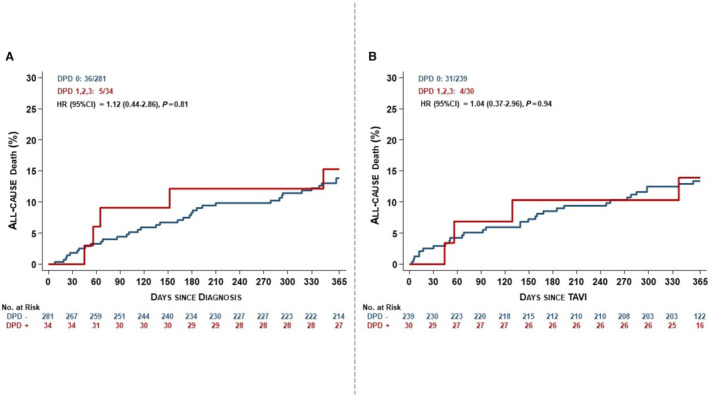
A, Overall patient cohort; B, patients undergoing TAVI. DPD indicates 3,3‐diphosphono‐1,2‐propanodicarboxylic acid; HR, hazard ratio; and TAVI, transcatheter aortic valve implantation.
Late‐onset (>30 days after TAVI), higher‐grade conduction disease was observed in a study participant who was DPD–. The requirement for pacemaker implantation was not affected by DPD– status 12 months after TAVI (23.3% [n=7] versus 17.3% [n=41]; HR, 1.5 [95% CI, 0.67–3.34]; P=0.32; Table 3 ).
Discussion
In the current study, we prospectively screened the largest single‐center cohort of elderly patients with AS for concomitant ATTR‐CM. Regarding the prevalence of 99mTc‐DPD uptake (34 of 315 patients; 10.8%, Perugini ≥2: 30 of 315 [9.5%]), our findings are in line with previous reports. 5 , 6 While ATTR‐CM is predominantly diagnosed in men, 17 , 18 some screening studies suggested a similar sex distribution in patients undergoing TAVI. 5 , 6 In our patient cohort, 4 of 5 cases of ATTR‐CM were found in men. Differences within the literature may be partly explained by the fact that men were more likely to participate in study procedures, with regional differences in the recruited study populations, and a more pronounced healthier volunteer effect in women.
Advanced age, increased NT‐proBNP levels, and the need for loop diuretics were associated with 99mTc‐DPD uptake and ATTR‐CM. Echocardiography and invasive hemodynamics further allowed to discern patients with isolated AS from those with both pathologies. Compatible with an underlying cardiomyopathy, LV ejection fraction and mean aortic valve gradients were lower in patients who were DPD+ (P<0.001 for both), while pulmonary hypertension was more common (P=0.037). Increased LV wall thickness and LV mass index (P<0.001), presence of higher‐grade diastolic dysfunction (≥II°, 71% versus 24%; P=0.001), and progressive left atrial dilatation (P=0.036) were also markers of concomitant ATTR‐CM.
Irrespective of the presence of cardiac amyloid depositions, patients with symptomatic, severe AS benefit from TAVI, 6 and a similar proportion of study participants irrespective of 99mTc‐DPD scintigraphy status underwent aortic valve replacement (DPD+, 91.1%; DPD–, 92.5%). Increased age likely contributed to a higher rate of TAVI in patients who were DPD+ (88.2% [n=30] versus 85.1% [n=239]) and lesser referral for surgical aortic valve replacement (2.9% [n=1] versus 7.5% [n=21]). All‐cause and cardiovascular death were not affected by 99mTc‐DPD uptake or concomitant ATTR‐CM at 12 months (Figure 3, Table 3 ; P>0.05 for all end points).
An important argument for identifying TAVI candidates with underlying ATTR‐CM may be the hypothetical higher risk for permanent pacemaker implantation after TAVI, 19 , 20 particularly in light of the potential for adverse LV remodeling and aggravation of heart failure owing to unphysiological right ventricular pacing. 21 Yet in our study, 99mTc‐DPD uptake was neither associated with baseline prevalence of conduction abnormalities nor rates of permanent pacemaker implantation after TAVI.
Screening of all patients undergoing TAVI for ATTR‐CM is not feasible and may also not improve outcomes in an aging patient population with comorbidities and competing risks of death. It has been proposed that patients with dual pathology have a milder or less advanced form of ATTR‐CM 22 and that aortic valve replacement also reverses LV remodeling in patients with concomitant ATTR‐CM, 23 albeit to a lesser degree. With similar rates of death up to 3 years after TAVI, 6 , 18 it remains uncertain whether ATTR‐CM–targeting therapies may confer additional benefit in elderly patients primarily referred for TAVI.
To date, it remains unknown how ATTR‐CM and AS are linked. Amyloid depositions have routinely been found in elderly patients with severe calcific AS 24 , 25 but also in younger patients with rheumatic heart disease. 26 With novel techniques to identify amyloid precursor proteins, there is an opportunity to determine whether ATTR‐CM and AS, at least in part, share a pathophysiological pathway and whether amyloid deposition in AS may contribute to the progression or calcification of valvular stenoses.
The present study needs to be interpreted in light of the following potential limitations: This study was an unblinded, single‐center, observational study, and we are unable to exclude (referral/selection) bias and healthy volunteer effect. Indeed, this study was performed during the COVID‐19 pandemic, and we were not able to recruit consecutive patients in part due to hospital restrictions. Two patients with 99mTc‐DPD uptake and presence of a monoclonal immunoglobulin declined additional testing to formally rule out light chain amyloidosis; the clinical course was favorable, suggesting ATTR‐CM. ATTR‐CM remains an underdiagnosed and, in combination with AS, rare and underreported condition. Low event rates of periprocedural complications do not allow adequately powered statistical analysis for all complications at all time points. The wide CIs should inform the readers about the high uncertainty that remains regarding periprocedural complications in this patient population. Yet low absolute event rates in patients who are DPD+ are reassuring, suggesting that TAVR may be performed with an acceptable level of risk. For future studies, Bayesian credible intervals have been added to 1‐year adjudicated outcomes (see Table S5). Concomitant ATTR‐CM may particularly influence cardiovascular hospitalizations. However, a nationwide linkage of routine health care data that allows assessment of this more subjective end point was not available, and it thus remains unclear whether dual pathology or 99mTc‐DPD uptake may increase hospitalizations in our cohort. Some baseline characteristics that may determine TAVI procedural success (eg, frailty, LV outflow tract calcification) were not routinely collected, and thus their effect on clinical outcomes cannot be assessed.
Conclusions
Concomitant ATTR‐CM is common in elderly patients with symptomatic, severe AS referred for evaluation of TAVI. Echocardiography and invasive hemodynamics contribute to identifying patients with both pathologies. Concomitant ATTR‐CM did not impact periprocedural adverse events or death throughout 1 year in patients undergoing TAVI.
Sources of Funding
This study was supported by research grant from the Bangerter‐Rhyner‐Stiftung and Pfizer.
Disclosures
Dr Dobner reports a research grant for the B‐CARE (Bern amyloidosis registry; NCT04776824) on behalf of the institution (Inselspital Bern) from Pfizer and has received a travel grant from Alnylam. Dr Windecker reports research, travel, or educational grants to the institution from Abbott, Abiomed, Amgen, Astra Zeneca, Bayer, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardinal Health, CardioValve, Corflow Therapeutics, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Guerbet, InfraRedx, Janssen‐Cilag, Johnson & Johnson, Medicure, Medtronic, Merck Sharp & Dohm, Miracor Medical, Novartis, Novo Nordisk, Organon, OrPha Suisse, Pfizer, Polares, Regeneron, Sanofi‐Aventis, Servier, Sinomed, Terumo, Vifor, and V‐Wave. Dr Windecker serves as advisory board member and member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, Astra Zeneca, Bayer, Boston Scientific, Biotronik, Bristol Myers Squibb, Edwards Lifesciences, Janssen, MedAlliance, Medtronic, Novartis, Polares, Recardio, Sinomed, Terumo, V‐Wave, and Xeltis, with payments to the institution but no personal payments. He is also a member of the steering/executive committee group of several investigator‐initiated trials that receive funding by industry without impact on his personal remuneration. Dr Stortecky reports research grants to the institution from Edwards Lifesciences, Medtronic, Boston Scientific, and Abbott, as well as personal fees from Boston Scientific, Teleflex, and BTG. Dr Pilgrim reports research grants to the institution from Biotronik, Boston Scientific, and Edwards Lifesciences; speaker fees from Biotronik and Boston Scientific; clinical event committee for study sponsored by HighLifeSAS; travel reimbursement from Medira; and proctoring for Medtronic. Dr Gräni receives funding from the Swiss National Science Foundation, InnoSuisse, Center of Artificial Intelligence UniBern, and GAMBIT Foundation. Dr Lanz reports speaker fees from Edwards Lifesciences. The remaining authors have no disclosures to report.
Supporting information
Data S1
This manuscript was sent to Amgad Mentias, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030271
For Sources of Funding and Disclosures, see page 11.
REFERENCES
- 1. Yadgir S, Johnson CO, Aboyans V, Adebayo OM, Adedoyin RA, Afarideh M, Alahdab F, Alashi A, Alipour V, Arabloo J, et al. Global, regional, and national burden of calcific aortic valve and degenerative mitral valve diseases, 1990‐2017. Circulation. 2020;141:1670–1680. doi: 10.1161/CIRCULATIONAHA.119.043391 [DOI] [PubMed] [Google Scholar]
- 2. Lauppe RE, Liseth Hansen J, Gerdeskold C, Rozenbaum MH, Strand AM, Vakevainen M, Kuusisto J, Gude E, Gustafsson F, Smith JG. Nationwide prevalence and characteristics of transthyretin amyloid cardiomyopathy in Sweden. Open Heart. 2021;8:8. doi: 10.1136/openhrt-2021-001755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tanskanen M, Peuralinna T, Polvikoski T, Notkola IL, Sulkava R, Hardy J, Singleton A, Kiuru‐Enari S, Paetau A, Tienari PJ, et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2‐macroglobulin and tau: a population‐based autopsy study. Ann Med. 2008;40:232–239. doi: 10.1080/07853890701842988 [DOI] [PubMed] [Google Scholar]
- 4. Gonzalez‐Lopez E, Gallego‐Delgado M, Guzzo‐Merello G, de Haro‐Del Moral FJ, Cobo‐Marcos M, Robles C, Bornstein B, Salas C, Lara‐Pezzi E, Alonso‐Pulpon L, et al. Wild‐type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–2594. doi: 10.1093/eurheartj/ehv338 [DOI] [PubMed] [Google Scholar]
- 5. Nitsche C, Scully PR, Patel KP, Kammerlander AA, Koschutnik M, Dona C, Wollenweber T, Ahmed N, Thornton GD, Kelion AD, et al. Prevalence and outcomes of concomitant aortic stenosis and cardiac amyloidosis. J Am Coll Cardiol. 2021;77:128–139. doi: 10.1016/j.jacc.2020.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scully PR, Patel KP, Treibel TA, Thornton GD, Hughes RK, Chadalavada S, Katsoulis M, Hartman N, Fontana M, Pugliese F, et al. Prevalence and outcome of dual aortic stenosis and cardiac amyloid pathology in patients referred for transcatheter aortic valve implantation. Eur Heart J. 2020;41:2759–2767. doi: 10.1093/eurheartj/ehaa170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perugini E, Guidalotti PL, Salvi F, Cooke RM, Pettinato C, Riva L, Leone O, Farsad M, Ciliberti P, Bacchi‐Reggiani L, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc‐3,3‐diphosphono‐1,2‐propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46:1076–1084. doi: 10.1016/j.jacc.2005.05.073 [DOI] [PubMed] [Google Scholar]
- 8. Lane T, Fontana M, Martinez‐Naharro A, Quarta CC, Whelan CJ, Petrie A, Rowczenio DM, Gilbertson JA, Hutt DF, Rezk T, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140:16–26. doi: 10.1161/CIRCULATIONAHA.118.038169 [DOI] [PubMed] [Google Scholar]
- 9. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39, e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 10. Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, Lancellotti P, LeFevre M, Miller F Jr, Otto CM. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:372–392. doi: 10.1016/j.echo.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 11. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 12. Nishimura RA, Carabello BA. Hemodynamics in the cardiac catheterization laboratory of the 21st century. Circulation. 2012;125:2138–2150. doi: 10.1161/CIRCULATIONAHA.111.060319 [DOI] [PubMed] [Google Scholar]
- 13. Witteles RM, Liedtke M. Avoiding catastrophe: understanding free light chain testing in the evaluation of ATTR amyloidosis. Circ Heart Fail. 2021;14:e008225. doi: 10.1161/CIRCHEARTFAILURE.120.008225 [DOI] [PubMed] [Google Scholar]
- 14. Hutt DF, Quigley AM, Page J, Hall ML, Burniston M, Gopaul D, Lane T, Whelan CJ, Lachmann HJ, Gillmore JD, et al. Utility and limitations of 3,3‐diphosphono‐1,2‐propanodicarboxylic acid scintigraphy in systemic amyloidosis. Eur Heart J Cardiovasc Imaging. 2014;15:1289–1298. doi: 10.1093/ehjci/jeu107 [DOI] [PubMed] [Google Scholar]
- 15. Maurer MS, Bokhari S, Damy T, Dorbala S, Drachman BM, Fontana M, Grogan M, Kristen AV, Lousada I, Nativi‐Nicolau J, et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail. 2019;12:e006075. doi: 10.1161/CIRCHEARTFAILURE.119.006075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium‐2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. doi: 10.1016/j.jacc.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 17. Ioannou A, Patel RK, Razvi Y, Porcari A, Sinagra G, Venneri L, Bandera F, Masi A, Williams GE, O'Beara S, et al. Impact of earlier diagnosis in cardiac ATTR amyloidosis over the course of 20 years. Circulation. 2022;146:1657–1670. doi: 10.1161/CIRCULATIONAHA.122.060852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosenblum H, Masri A, Narotsky DL, Goldsmith J, Hamid N, Hahn RT, Kodali S, Vahl T, Nazif T, Khalique OK, et al. Unveiling outcomes in coexisting severe aortic stenosis and transthyretin cardiac amyloidosis. Eur J Heart Fail. 2021;23:250–258. doi: 10.1002/ejhf.1974 [DOI] [PubMed] [Google Scholar]
- 19. Castano A, Bokhari S, Maurer MS. Could late enhancement and need for permanent pacemaker implantation in patients undergoing TAVR be explained by undiagnosed transthyretin cardiac amyloidosis? J Am Coll Cardiol. 2015;65:311–312. doi: 10.1016/j.jacc.2014.09.084 [DOI] [PubMed] [Google Scholar]
- 20. Siontis GC, Juni P, Pilgrim T, Stortecky S, Bullesfeld L, Meier B, Wenaweser P, Windecker S. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta‐analysis. J Am Coll Cardiol. 2014;64:129–140. doi: 10.1016/j.jacc.2014.04.033 [DOI] [PubMed] [Google Scholar]
- 21. Donnellan E, Wazni OM, Saliba WI, Baranowski B, Hanna M, Martyn M, Patel D, Trulock K, Menon V, Hussein A, et al. Cardiac devices in patients with transthyretin amyloidosis: impact on functional class, left ventricular function, mitral regurgitation, and mortality. J Cardiovasc Electrophysiol. 2019;30:2427–2432. doi: 10.1111/jce.14180 [DOI] [PubMed] [Google Scholar]
- 22. Patel KP, Scully PR, Nitsche C, Kammerlander AA, Joy G, Thornton G, Hughes R, Williams S, Tillin T, Captur G, et al. Impact of afterload and infiltration on coexisting aortic stenosis and transthyretin amyloidosis. Heart. 2022;108:67–72. doi: 10.1136/heartjnl-2021-319922 [DOI] [PubMed] [Google Scholar]
- 23. Nitsche C, Koschutnik M, Dona C, Radun R, Mascherbauer K, Kammerlander A, Heitzinger G, Dannenberg V, Spinka G, Halavina K, et al. Reverse remodeling following valve replacement in coexisting aortic stenosis and transthyretin cardiac amyloidosis. Circ Cardiovasc Imaging. 2022;15:e014115. doi: 10.1161/CIRCIMAGING.122.014115 [DOI] [PubMed] [Google Scholar]
- 24. Ladefoged C, Rohr N. Amyloid deposits in aortic and mitral valves. A clinicopathological investigation of material from 100 consecutive heart valve operations. Virchows Arch A Pathol Anat Histopathol. 1984;404:301–312. doi: 10.1007/BF00694895 [DOI] [PubMed] [Google Scholar]
- 25. Falk E, Ladefoged C, Christensen HE. Amyloid deposits in calcified aortic valves. Acta Pathol Microbiol Scand A. 1981;89:23–26. doi: 10.1111/j.1699-0463.1981.tb00182.x [DOI] [PubMed] [Google Scholar]
- 26. Cooper JH. Localized dystrophic amyloidosis of heart valves. Hum Pathol. 1983;14:649–653. doi: 10.1016/s0046-8177(83)80208-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


