Abstract
Background
We previously demonstrated that retinal ischemic perivascular lesions (RIPLs), which are indicative of ischemia in the middle retina, may be a biomarker of ischemic cardiovascular disease. In this study, we sought to determine the relationship between RIPLs and atrial fibrillation, a common source of cardiac emboli.
Methods and Results
In this case‐control study, we identified individuals between the ages of 50 and 90 years who had undergone macular spectral domain optical coherence tomography imaging. Individuals with atrial fibrillation were identified, and age‐ and sex‐matched individuals from the same pool, but without a diagnosis of atrial fibrillation, were selected as controls. Spectral domain optical coherence tomography scans were reviewed by 3 independent and masked observers for presence of RIPLs. The relationship between RIPLs and atrial fibrillation was analyzed using multivariable logistic regression models. There were 106 and 91 subjects with and without atrial fibrillation, respectively. The percentage of subjects with RIPLs was higher in the atrial fibrillation group compared with the control group (57.5% versus 37.4%; P=0.005). After adjusting for age, sex, smoking history, hypertension, diabetes, coronary artery disease, carotid stenosis, stroke, and myocardial infarction, the presence of RIPLs was significantly associated with atrial fibrillation, with an odds ratio of 1.91 (95% CI, 1.01–3.59).
Conclusions
RIPLs are significantly associated with atrial fibrillation, independent of underlying ischemic heart disease or cardiovascular risk factors. This association may inform the diagnostic cardiovascular workup for individuals with RIPLs incidentally detected on optical coherence tomography scan of the macula.
Keywords: atrial fibrillation, optical coherence tomography, retina, retinal ischemic perivascular lesions
Subject Categories: Atrial Fibrillation, Optical Coherence Tomography (OCT)
Nonstandard Abbreviations and Acronyms
- PAMM
paracentral acute middle maculopathy
- RIPL
retinal ischemic perivascular lesion
- SD‐OCT
spectral domain optical coherence tomography
Clinical Perspective.
What Is New?
In this case‐control study, the presence of retinal ischemic perivascular lesions was significantly associated with atrial fibrillation, independent of underlying ischemic heart disease and other cardiovascular risk factors.
What Are the Clinical Implications?
This may help inform the cardiovascular workup of patients incidentally found to have retinal ischemic perivascular lesions on optical coherence tomography scans.
Future prospective studies are needed to confirm this association and determine the timing of development of retinal ischemic perivascular lesions in patients with atrial fibrillation.
Retinal ischemic perivascular lesions, or RIPLs, represent focal atrophy of the middle retina or inner nuclear layer. These lesions are a legacy of paracentral acute middle maculopathy (PAMM), which is thought to occur because of the preferential susceptibility of the inner nuclear layer, at the level of the deep capillary plexus, to ischemia. 1 , 2 Retinal hypoperfusion may occur secondary to a decrease in afferent blood flow into the retinal capillary plexus in patients with various disorders, such as low ejection fraction, carotid artery stenosis, thrombi or emboli formation, or vascular stasis, all of which have been associated with PAMM. 2 , 3 , 4 , 5 Therefore, the eye may manifest the earliest evidence of ischemia because of systemic disease at the level of the middle retina, which is exquisitely sensitive to subtle changes in blood flow.
Although both PAMM and RIPLs can be readily visualized in a noninvasive manner, using spectral domain optical coherence tomography (SD‐OCT) scans, only RIPLs are permanent. Hyperreflective PAMM lesions are transient and usually resolve within a 6‐week period but leave a legacy of inner nuclear layer thinning, with a compensatory expansion of the Henle fiber layer. Therefore, RIPLs represent an actionable imaging biomarker that can be harnessed to detect ischemia in the retina. Optical coherence tomography (OCT) is a ubiquitous tool with fast image acquisition. The detection of RIPLs with OCT is a simple process that might have ramifications beyond the eye clinic. RIPL detection may provide an entry point for general medical practice in the management of cardiovascular health and may help identify those who would benefit from additional and targeted diagnostic testing. For instance, in a case series, 8 of 11 subjects (72.7%) with incidental RIPLs were newly diagnosed with cardiovascular disease on further evaluation by their primary care provider. 6 This was prompted by RIPL detection. As such, it is important to identify the different systemic cardiovascular conditions that are associated with RIPLs.
In a case‐control study, RIPLs were significantly associated with ischemic heart disease, even after adjustment for important confounders. 7 Coronary artery disease may coexist with other cardiovascular conditions, which can also lead to retinal hypoperfusion. One common comorbidity is atrial fibrillation, as between 17% and 46% of patients with atrial fibrillation also have coronary artery disease. 8 Atrial fibrillation is a disorder of the heart rhythm and may lead to blood stasis and emboli formation. 9 Individuals with atrial fibrillation are at 5 times greater risk for stroke, compared with the general population, a risk that can be mitigated using anticoagulation. 10 Therefore, prompt detection of atrial fibrillation, before the development of disease sequelae, is important. In the present study, we sought to determine whether RIPLs are associated with atrial fibrillation, independent of underlying ischemic heart disease. Identifying whether this association exists is important, as it may help inform practitioners on the appropriate medical evaluation needed for a patient who presents to the ophthalmology clinic and is incidentally found to have RIPLs on OCT. 6
METHODS
This was a case‐control study that adhered to the tenets of the Declaration of Helsinki and was conducted in accordance with the regulations of the Health Insurance Portability and Accountability Act. Institutional review board approval for retrospective medical record review was obtained from the University of California San Diego Health System (institutional review board number 191 994). The need for informed consent was waived because of the retrospective nature of the study. The data that support the findings of this study are available from the corresponding author on reasonable request.
We identified individuals who were between 50 and 90 years of age and who underwent macular SD‐OCT scanning between January 1, 2018 and December 31, 2020 for various clinical indications. At our institution, the vast majority of patients evaluated in ophthalmology clinics have an OCT scan performed as part of the routine clinical evaluation and workflow. Exclusion criteria included any evidence of retinal vascular occlusion (including branch and central retinal artery or vein occlusion), and any history of retinal laser, intravitreal injection, and/or pars plana vitrectomy. We then identified subjects with atrial fibrillation, based on the International Classification of Diseases, Tenth Revision (ICD‐10), diagnostic code I48, who also had a cardiac rhythm monitor (Current Procedural Terminology code 93224‐9 or 33285‐6) recorded. These subjects were selected as cases. Frequency matching was used to select controls. Controls included individuals who were age and sex matched from the same pool, but without a diagnosis of atrial fibrillation. The medical records of both the atrial fibrillation and control groups were individually reviewed to confirm the presence or absence of atrial fibrillation. In addition, using electronic health record review, baseline characteristics were recorded, including age, sex, smoking status, diagnosis of hypertension, diabetes, coronary artery disease, carotid stenosis, stroke, and myocardial infarction. The macular SD‐OCT scans were reviewed, and subjects with any macular pathologic condition, other than RIPLs, were excluded. Poor quality scans, in which the individual layers of the retina were indiscernible because of media opacity, were also excluded.
RIPL Grading
All SD‐OCT scans that were included and reviewed in this study were acquired on a Spectralis SD‐OCT machine (Heidelberg Engineering, Heidelberg, Germany) as part of the routine retinal examination. RIPLs were identified using the previously published criteria. 7 Briefly, individual b‐scans were reviewed by 3 independent and masked observers, and an RIPL was defined by the presence of focal atrophy or thinning of the inner nuclear layer with a compensatory expansion of the outer nuclear layer (Figure). A consensus between all 3 observers was required to identify an RIPL. Subjects were classified into 2 groups, those with and without RIPLs.
Figure . Spectral domain optical coherence tomography b‐scan demonstrating a retinal ischemic perivascular lesion (RIPL) in an individual with atrial fibrillation.
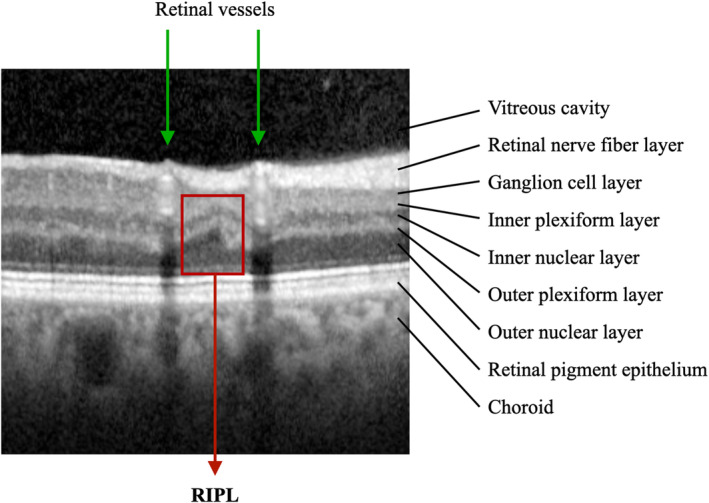
The RIPL (red box) is present between 2 retinal vessels (green arrows).
Statistical Analysis
Baseline characteristics of subjects are described using mean and SD for continuous variables and frequency with percentage for categorical variables. Demographics were compared for cases and controls using the Pearson χ2 test for categorical variables and the Student t test for continuous variables. We used univariable and multivariable logistic regression models to evaluate the relationship between RIPLs and atrial fibrillation. Covariates included age, sex, smoking status, hypertension, diabetes, coronary artery disease, carotid stenosis, stroke, and myocardial infarction. All statistical analyses were performed using SPSS version 28.0 (SPSS Inc, Armonk, NY).
RESULTS
We identified 106 subjects with atrial fibrillation and 91 subjects without atrial fibrillation as controls. The number of controls was lower than cases, as 15 controls were excluded because of either other macular pathologic findings or poor quality OCT scan. Baseline characteristics for cases and controls are shown in Table 1. The mean age was 72.5 (SD, 7.8) years and 70.6 (SD, 7.4) years for cases and controls, respectively (Table 1). Women comprised 47.2% and 48.4% of the case and control groups, respectively (Table 1). There was no difference between both groups in terms of age and sex distribution (Table 1). A significantly greater proportion of cases had hypertension (77.4%) and stroke (12.3%) compared with controls (66.2% and 1.1%, respectively) (Table 1). In addition, 37.7% of cases had coronary artery disease, compared with 19.8% of controls (Table 1). There was no difference in prevalence of diabetes between the 2 groups. The percentage of subjects with RIPLs was higher in the atrial fibrillation group compared with the control group (57.5% versus 37.4%; P=0.005).
Table 1.
Baseline Characteristics of the Study Cohort
| Characteristic | Control group (n=91) | Atrial fibrillation group (n=106) | P value |
|---|---|---|---|
| Age, y | 70.6 (7.4) | 72.5 (7.8) | 0.08 |
| Female sex | 44 (48.4) | 50 (47.2) | 0.90 |
| Race | <0.001* | ||
| White | 64 (70.3) | 78 (73.6) | |
| Asian | 15 (16.5) | 11 (10.4) | |
| Black | 1 (1.1) | 3 (2.8) | |
| Multiracial | 6 (6.6) | 12 (11.3) | |
| Other† | 5 (5.5) | 2 (1.9) | |
| Hypertension | 57 (62.6) | 82 (77.4) | 0.02* |
| Diabetes | 29 (31.9) | 42 (39.6) | 0.30 |
| Coronary artery disease | 18 (19.8) | 40 (37.7) | 0.006* |
| Positive smoking history (n=191) | 40 (47.1) | 37 (34.9) | 0.09 |
| Carotid stenosis | 7 (7.7) | 12 (11.3) | 0.39 |
| Stroke | 1 (1.1) | 13 (12.3) | 0.002* |
| Myocardial infarction | 3 (3.3) | 8 (7.5) | 0.20 |
| RIPLs | 34 (37.4) | 61 (57.5) | 0.005* |
Mean (SD) reported for age. Frequency (percentage) reported for other variables. RIPL indicates retinal ischemic perivascular lesion.
The asterisks are to indicate the P‐values that are <0.05.
Native Hawaiian or other Pacific Islander or unknown race.
In a multivariable logistic regression model adjusted for age and sex, the presence of RIPLs was significantly associated with atrial fibrillation, with an odds ratio (OR) of 2.19 (95% CI, 1.21–3.97; P=0.009). After adjusting for additional covariates, including smoking history, hypertension, diabetes, coronary artery disease, carotid stenosis, stroke, and myocardial infarction, the presence of RIPLs continued to be associated with atrial fibrillation, with an OR of 1.91 (95% CI, 1.01–3.59; Table 2).
Table 2.
Multivariable Logistic Regression Model Evaluating the Relationship Between Presence of ≥1 RIPLs and a Diagnosis of Atrial Fibrillation
| Variable | OR (95% CI) | P value |
|---|---|---|
| RIPL(s) | 1.91 (1.01–3.59) | 0.046* |
| Age (per year) | 1.02 (0.98–1.07) | 0.36 |
| Male sex | 0.67 (0.34–1.31) | 0.24 |
| Hypertension | 1.51 (0.73–3.14) | 0.27 |
| Diabetes | 0.94 (0.47–1.87) | 0.86 |
| Coronary artery disease | 2.18 (0.97–4.88) | 0.058 |
| Positive smoking history | 0.54 (0.29–1.03) | 0.062 |
| Carotid stenosis | 1.14 (0.38–3.44) | 0.82 |
| Stroke | 7.92 (0.99–63.6) | 0.052 |
| Myocardial infarction | 1.41 (0.25–8.0) | 0.70 |
OR indicates odds ratio; and RIPL, retinal ischemic perivascular lesion.
The asterisk is to indicate the p‐values that are <0.05.
DISCUSSION
In this case‐control study, we identified a significant association between RIPLs and atrial fibrillation that was independent of underlying ischemic heart disease. Atrial fibrillation is a cardiac arrhythmia that is prevalent in the adult population and increases in prevalence with age. 9 The lifetime risk of atrial fibrillation is estimated to be ≈1 in 4 individuals. 11 , 12 Because of the increasing proportion of elderly people within the population, it is projected that the prevalence of atrial fibrillation is likely to increase 2.5‐fold over the next 50 years. 13 A study from 2011 estimated the national incremental cost of atrial fibrillation to be $26 billion, accounting for 3.2 million hospital days. 14 Individuals with atrial fibrillation are at a higher risk for developing thromboembolic events, including cerebral strokes, as well as other comorbidities, such as heart failure, cognitive decline, and decline in kidney function. 9 , 15 , 16 , 17 Approximately 0.7 million (13%) of the 5.3 million cases of atrial fibrillation in the United States may be undiagnosed. 18
In this study, we identified that a subclinical biomarker of retinal ischemia, RIPL, is significantly associated with atrial fibrillation, independent of other comorbidities. The pathogenesis of RIPL attributable to atrial fibrillation may be the result of hypoperfusion of the deep retinal capillary plexus, which is at greater risk of ischemia compared with the superficial capillary plexus. 1 Retinal capillary hypoperfusion may develop secondary to microemboli formation or reduced cardiac output. We had previously established a relationship between RIPLs and ischemic heart disease, 7 and here we establish a relationship between RIPLs and atrial fibrillation, a major source of emboli formation.
We do not suggest that the identification of this biomarker would be more feasible than screening for atrial fibrillation using ECG; however, if RIPLs are identified in an individual without known cardiovascular disease on routine examination with an ophthalmologist, our data would suggest that a broad cardiac evaluation may be warranted. This is further supported by our previous findings, which also show an independent association between RIPLs and ischemic heart disease. 7 Conversely, a patient with atrial fibrillation who is found to have RIPLs may not necessarily benefit from additional cardiovascular workup. Further studies are needed to evaluate whether the presence of RIPLs may aid in risk stratification of patients with atrial fibrillation, to help determine who may benefit from more aggressive medical management.
Our study has limitations to note. First, because of the cross‐sectional nature of our analysis, we cannot infer causality. Although we hypothesize about the pathophysiological features underlying RIPLs, we cannot determine whether RIPLs are a direct consequence of emboli formation or reduced cardiac output secondary to atrial fibrillation. Second, our study is prone to selection bias. Subjects who were selected for inclusion in our study must have had an OCT scan performed. These scans are performed routinely in an ophthalmology clinic, so a subject must have sought care at an eye clinic. However, because controls were selected from the same pool as cases, this should not affect the outcomes of our primary analyses. Third, RIPL detection as a biomarker of retinal ischemia relies on OCT, which does not provide information about vascular anatomic features. OCT angiography can provide this information. However, OCT scans are more widely used than OCT angiography scans and are a more practical and accessible imaging modality. Microvascular capillary loss in PAMM has been previously documented using OCT angiography scans. 19 , 20 Fourth, given the relatively small number of covariates in our models, there is potential residual confounding. In addition, there was a trend toward younger age in controls compared with cases, although this was not statistically significant. We adjusted for age in our models, but there remains potential for residual confounding, which may lead to an overstated association. Finally, our study is a single‐center study of modest size, with 72% of patients identifying as White race, and thus, larger studies in more robust and diverse cohorts are needed to ensure the generalizability of our findings.
In conclusion, we identified that RIPLs, which are anatomic biomarkers of subclinical ischemia, are significantly associated with atrial fibrillation, independent of other underlying cardiovascular disease. This information may help guide the medical management of patients with RIPLs incidentally detected on macular OCT. Future prospective studies are needed to confirm the association between RIPLs and atrial fibrillation and to determine whether the degree of RIPL burden may be correlated with overall cardiovascular disease risk in these patients.
Sources of Funding
Dr C. Y. Bakhoum is funded by American Heart Association Award 857722 and K23 DK129836 from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr M. F. Bakhoum is supported by National Institutes of Health Research Grant P30CA016359 from the National Cancer Institute, R21EY035090 from the National Eye Institute, and a grant from the Connecticut Lions Eye Research Foundation.
Disclosures
Dr Sarraf is a consultant for Heidelberg, Topcon, Amgen, Bayer, Iveric Bio, Novartis, and Optovue; and receives equipment for research from Heidelberg, Optovue, and Topcon. The remaining authors have no disclosures to report.
This article was sent to Neel S. Singhal, MD, PhD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 5.
Contributor Information
Christine Y. Bakhoum, Email: christine.bakhoum@yale.edu.
Mathieu F. Bakhoum, Email: mathieu.bakhoum@gmail.com.
References
- 1. Bakhoum MF, Freund KB, Dolz‐Marco R, Leong BCS, Baumal CR, Duker JS, Sarraf D. Paracentral acute middle maculopathy and the ischemic cascade associated with retinal vascular occlusion. Am J Ophthalmol. 2018;195:143–153. doi: 10.1016/j.ajo.2018.07.031 [DOI] [PubMed] [Google Scholar]
- 2. Moura‐Coelho N, Gaspar T, Ferreira JT, Dutra‐Medeiros M, Cunha JP. Paracentral acute middle maculopathy‐review of the literature. Graefes Arch Clin Exp Ophthalmol. 2020;258:2583–2596. doi: 10.1007/s00417-020-04826-1 [DOI] [PubMed] [Google Scholar]
- 3. Kılıç Müftüoğlu İ, Önder Tokuç E, Karabaş VL. Bilateral sequential paracentral acute middle maculopathy. Turk J Ophthalmol. 2021;51:403–406. doi: 10.4274/tjo.galenos.2021.50207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koçak N, Erduran B, Subaşı M, Yeter V. Paracentral acute middle maculopathy associated with branch retinal artery occlusion due to polycythemia in a patient with tetralogy of Fallot. Retin Cases Brief Rep. 2020;16:558–560. doi: 10.1097/icb.0000000000001054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burnasheva MA, Maltsev DS, Kulikov AN, Sherbakova KA, Barsukov AV. Association of chronic paracentral acute middle maculopathy lesions with hypertension. Ophthalmol Retina. 2020;4:504–509. doi: 10.1016/j.oret.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 6. Madala SAF, Lando L, Yarmohammadi A, Long CP, Bakhoum CY, Goldbaum MH, Sarraf D, DeMaria AN, Bakhoum MF. Retinal ischemic perivascular lesions, a biomarker of cardiovascular disease. Ophthalmol Retina. 2022;6:865–867. doi: 10.1016/j.oret.2022.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Long CP, Chan AX, Bakhoum CY, Toomey CB, Madala S, Garg AK, Freeman WR, Goldbaum MH, DeMaria AN, Bakhoum MF. Prevalence of subclinical retinal ischemia in patients with cardiovascular disease—a hypothesis driven study. EClinicalMedicine. 2021;33:100775. doi: 10.1016/j.eclinm.2021.100775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Michniewicz E, Mlodawska E, Lopatowska P, Tomaszuk‐Kazberuk A, Malyszko J. Patients with atrial fibrillation and coronary artery disease—double trouble. Adv Med Sci. 2018;63:30–35. doi: 10.1016/j.advms.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 9. Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11:639–654. doi: 10.1038/nrcardio.2014.118 [DOI] [PubMed] [Google Scholar]
- 10. Di Giosia P, Giorgini P, Ferri C. Considerations on stroke in atrial fibrillation despite anticoagulation. J Cardiovasc Med. 2018;19:e54–e57. doi: 10.2459/jcm.0000000000000602 [DOI] [PubMed] [Google Scholar]
- 11. Heeringa J, van der Kuip DAM, Hofman A, Kors JA, van Herpen G, Stricker BHC, Stijnen T, Lip GYH, Witteman JCM. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825 [DOI] [PubMed] [Google Scholar]
- 12. Lloyd‐Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.Cir.0000140263.20897.42 [DOI] [PubMed] [Google Scholar]
- 13. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370 [DOI] [PubMed] [Google Scholar]
- 14. Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/circoutcomes.110.958165 [DOI] [PubMed] [Google Scholar]
- 15. Madhavan M, Graff‐Radford J, Piccini JP, Gersh BJ. Cognitive dysfunction in atrial fibrillation. Nat Rev Cardiol. 2018;15:744–756. doi: 10.1038/s41569-018-0075-z [DOI] [PubMed] [Google Scholar]
- 16. Chen T‐H, Chu Y‐C, Ou S‐M, Tarng D‐C. Associations of atrial fibrillation with renal function decline in patients with chronic kidney disease. Heart. 2022;108:438–444. doi: 10.1136/heartjnl-2021-319297 [DOI] [PubMed] [Google Scholar]
- 17. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta‐analysis. BMJ. 2016;354:i4482. doi: 10.1136/bmj.i4482 [DOI] [PubMed] [Google Scholar]
- 18. Turakhia MP, Shafrin J, Bognar K, Trocio J, Abdulsattar Y, Wiederkehr D, Goldman DP. Estimated prevalence of undiagnosed atrial fibrillation in the United States. PLoS One. 2018;13:e0195088. doi: 10.1371/journal.pone.0195088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maltsev DS, Kulikov AN, Burnasheva MA, Freund KB. Vascular microanatomy of small resolved paracentral acute middle maculopathy lesions. Ophthalmol Retina. 2021;5:928–934. doi: 10.1016/j.oret.2020.12.005 [DOI] [PubMed] [Google Scholar]
- 20. Riazi‐Esfahani H, Khalili Pour E, Fadakar K, Ebrahimiadib N, Ghassemi F, Nourinia R, Khojasteh H, Attarian B, Faghihi H, Ahmadieh H. Multimodal imaging for paracentral acute maculopathy; the diagnostic role of en face OCT. Int J Retina Vitreous. 2021;7:13. doi: 10.1186/s40942-021-00283-y [DOI] [PMC free article] [PubMed] [Google Scholar]


