Abstract
Background
Prognostic implications of transcatheter aortic valve implantation (TAVI) in low‐gradient (LG) aortic stenosis (AS) remain controversial. The authors hypothesized that differences in cardiac functional recovery may solve this ongoing controversy. The aim was to evaluate clinical outcomes and the response of left ventricular (LV) function following TAVI in patients with LG AS.
Methods and Results
This multicenter retrospective study included 1742 patients with severe AS undergoing TAVI between January 2015 and March 2019. Patients were subdivided into low‐flow (LF) LG, normal‐flow (NF) LG, LF high‐gradient, and NF high‐gradient AS groups according to the mean gradient of the aortic valve (LG <40 mm Hg) and LV stroke volume index (LF <35 mL/m2). Outcomes and changes in echocardiographic parameters after TAVI were compared between the groups. A total of 227 patients (13%) had reduced ejection fraction, and 486 patients (28%) had LG AS (LF‐LG 143 [8%]; NF‐LG 343 [20%]). During a median follow‐up period of 747 days, 301 patients experienced a composite end point of cardiovascular death and rehospitalization for cardiovascular events, which was higher in the LF‐LG and NF‐LG groups than in the high‐gradient groups. LG AS was independently associated with the primary outcome (hazard ratio, 1.69; P<0.001). Among 1239 patients with follow‐up echocardiography, LG AS showed less improvement in the LV mass index and LV end‐diastolic volume compared with high‐gradient AS after 1 year, while LV recovery was similar between the LF AS and NF AS groups.
Conclusions
LG AS was associated with poorer outcomes and LV recovery, regardless of flow status after TAVI. Careful evaluation of AS severity may be required in LG AS to provide TAVI within the appropriate time and advanced care afterward.
Keywords: low‐flow, low‐gradient AS; low‐gradient AS; normal‐flow, low‐gradient AS; TAVI
Subject Categories: Valvular Heart Disease
Nonstandard Abbreviations and Acronyms
- AS
aortic stenosis
- AVA
aortic valve area
- HG
high‐gradient
- LF
low‐flow
- LG
low‐gradient
- LVMI
left ventricular mass index
- PG
pressure gradient
- NF
normal‐flow
- SV
stroke volume
- TAVI
transcatheter aortic valve implantation
- VTI
velocity time integral
Clinical Perspective.
What Is New?
Low‐gradient aortic stenosis (AS) was associated with an increased risk of composite outcomes of cardiovascular death and rehospitalization after transcatheter aortic valve implantation regardless of the flow state.
The longitudinal echocardiographic evaluation revealed poorer left ventricular geometry and functional recovery in the low‐gradient AS group than in the high‐gradient AS group after transcatheter aortic valve implantation.
What Are the Clinical Implications?
Patients with low‐gradient AS may gain less benefit from cardiac reverse remodeling by transcatheter aortic valve implantation, which may lower their survival benefit.
Careful management after transcatheter aortic valve implantation, including medical therapy and close follow‐up, might be warranted in both patients with low‐flow, low‐gradient AS and normal‐flow, low‐gradient AS.
Low‐gradient (LG) aortic stenosis (AS), usually defined as a mean aortic valve pressure gradient (PG) <40 mm Hg with a small aortic valve area (AVA <1.0 cm2), is an entity that can appear in particular conditions such as reduced left ventricular (LV) ejection fraction (EF) or low transaortic flow (LV stroke volume [SV] index <35 mL/m2). This inconsistency between the PG and AVA is not rare and makes proper classification of AS severity difficult. 1
The emerging application of transcatheter aortic valve implantation (TAVI) in AS has led to an increasing number of treatable cases, especially among elderly and high‐risk patients. Although previous reports have shown the advantageous impact of TAVI in the LG AS population, 2 , 3 other data have shown that LG AS, especially low‐flow (LF), LG AS, exhibited worse outcomes than high‐gradient (HG) AS, 4 making it difficult to conclude the clinical benefit of performing TAVI in the LG AS population. Moreover, LG AS can be observed even in normal transaortic flow. Although patients with normal‐flow (NF), LG AS are considered to have moderate AS according to guidelines and a conservative approach is recommended, several prior studies have shown that they still have a survival benefit from TAVI, especially in preserved EF. 2 , 5 Thus, there is an ongoing controversy regarding the clinical benefits of TAVI in different clinical scenarios according to the flow/gradient pattern.
We previously reported that the difference in the cardiac functional recovery may play an important role in the prognosis of patients who undergo TAVI. 6 Therefore, we hypothesized that prognostic differences in LG AS might be associated with poor recovery in LV function and geometry after TAVI. Comparing the survival of LG AS and HG AS along with cardiac reversibility may provide further insight into the prognostic implication of TAVI in LG AS. Thus, this study aimed to investigate the prognosis of patients with LG AS, including those with NF‐LG AS, in real‐world clinical practice. We also evaluated the strength of the beneficial impact of TAVI on functional recovery according to the gradient pattern by comparing changes in echocardiographic parameters before and after the procedure.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Statements
The study protocol was approved by the local ethics committee of each participating institution and followed the guidelines of the Declaration of Helsinki. Data were deidentified, so the requirement for informed consent was waived.
Study Design and Population
This study is an academic project of the Japanese Society of Echocardiography and was conducted as a retrospective multicenter TAVI registry that included 17 cardiovascular centers in Japan: St. Marianna Medical University Hospital, National Cerebral and Cardiovascular Center, Dokkyo Medical University Hospital, University of Tsukuba Hospital, Osaka University Hospital, Sakakibara Heart Institute, Iwate Medical University Hospital, Oita University Hospital, Kobe City Medical Center General Hospital, Hokkaido University Hospital, Nihonkai General Hospital, Asahikawa Medical University Hospital, Jichi Medical University Hospital, Hiroshima City Hiroshima Citizens Hospital, Tokushima University Hospital, Shimane University Hospital, and Japanese Red Cross Ise Hospital. All patients who underwent TAVI for severe symptomatic AS between January 1, 2015, and March 31, 2019, with preprocedural echocardiographic evaluation and follow‐up after TAVI were enrolled. Echocardiograms at baseline and 1 month and 1 year after TAVI were acquired for longitudinal data analysis. Patients without comprehensive baseline echocardiographic evaluation and follow‐up information after TAVI, as well as those with inadequate image quality, were excluded.
In the present study, LG AS was defined as a mean transvalvular gradient <40 mm Hg, whereas those with a mean gradient ≥40 mm Hg were considered HG AS. An LV SV index <35 mL/m2 was defined as LF; otherwise, it was considered NF. According to the mean gradient and SV index status, the patients were subdivided into 4 subgroups: LF‐LG, NF‐LG, LF‐HG, and NF‐HG.
Data Collection and Outcomes
Clinical and demographic data were obtained via manual extraction from the patients' electronic medical records. The presence of coronary artery disease (CAD) was defined as a history of intervention or surgical treatment or the presence of significant stenosis. Patients were followed up by chart review with the date of the last follow‐up or death recorded (last queried December 31, 2019). In addition, mortality data and hospitalization due to cardiovascular events (ie, heart failure, arrhythmia, CAD, stroke, prosthetic valve–related issue, and device implantation) were obtained from patients' medical records or available electronic databases. We used a composite of cardiovascular death and rehospitalization due to cardiovascular causes as the primary outcome. The secondary end points included the components of the primary outcome and all‐cause mortality. Follow‐up was completed in all included patients, and an outcome review committee reviewed all events to avoid ascertainment bias.
Echocardiographic Evaluation
All patients underwent comprehensive echocardiographic assessment using commercially available ultrasound systems. Experienced readers at each institution reviewed and measured all echocardiographic measurements according to current guidelines. 7 , 8 Echocardiographic parameters included the following variables: peak aortic valve velocity, peak and mean transvalvular gradient, aortic jet velocity time integral (VTI), LV outflow tract (LVOT) diameter, LVOT VTI, AVA, LVEF, LV end‐diastolic volume (LVEDV), LV end‐systolic volume, SV, and global longitudinal strain (GLS). The LVEDV, LV end‐systolic volume, and EF were evaluated using the biplane disc method in 1258 (72%) patients, while the remaining 484 patients (28%) were evaluated using the Teichholz method. The AVA was calculated using the continuity equation. The LV SV was calculated from the cross‐sectional area of the LVOT and VTI of the LVOT flow as follows: SVLVOT=cross‐sectional areaLVOT×VTILVOT. The LVOT VTI was obtained using the pulsed wave Doppler method. In 41 patients (2%) with an unreliable LVOT pulsed wave Doppler signal, we calculated LV SV using the LV volume measured by the biplane disc method.
To obtain the LV GLS at baseline, 2‐dimensional speckle‐tracking echocardiography was performed offline using commercially available vender‐independent software (Image Arena 4.6, TOMTEC Imaging Systems). Apical 4‐chamber, 2‐chamber, and long‐axis views were acquired for the analysis; the estimated peak systolic strain values from each view were averaged to obtain GLS and described in an absolute value according to American Society of Echocardiography/European Association of Cardiovascular Imaging recommendations. 7 All strain measurements were performed by a single observer blinded to the clinical and other echocardiographic data and outcomes.
Statistical Analysis
Continuous data are presented as median (interquartile range [IQR]). Categorical data are presented as absolute number and percentage. We used unpaired t test, Mann–Whitney test, and χ 2 test to compare the data between the 2 groups as appropriate. Significance values were adjusted using Bonferroni correction for multiple comparisons. Cox proportional hazards analysis was performed to assess the association between baseline characteristics and outcomes. The assumptions of proportional hazards were assessed using Schoenfeld residuals. In the multivariable model, relevant variables and possible confounding factors such as age, sex, Society of Thoracic Surgeons predicted risk of mortality, history of CAD, atrial fibrillation, cardiac surgery, New York Heart Association (NYHA) functional class, LVEF, LF, and LG, which were selected because of their known prognostic value were entered into the model. Kaplan–Meier curves were created to assess survival between the groups and were compared using the log‐rank test. The association between the PG and outcome was evaluated using Cox model and fitted by penalized smoothing splines with the hazard ratio (HR) on the y‐axis and gradient on the x‐axis. Because cardiovascular events and death due to other causes were considered competing risks, we also performed competing risk regression analysis using the Fine‐Gray proportional subhazards model 9 , 10 , 11 and subdistribution HR. We calculated 95% CIs to estimate the adjusted risk of the primary end point. We performed Gray tests in multivariable model analyses of the presence of LG AS, Society of Thoracic Surgeons predicted risk of mortality, prior cardiac surgery, atrial fibrillation, NYHA functional class, and LF AS as potential covariates. The cumulative incidence function was used to graph the mortality rates, accounting for the competing risk of cardiovascular events. To assess changes in echocardiographic parameters over time, we applied longitudinal data analysis using a mixed‐effect model with individual patients treated as random effects.
All statistical analyses were performed using SPSS software version 26.0 (SPSS Inc.) and R software version 4.2.1 (R Foundation for Statistical Computing).
RESULTS
Study Population
A total of 1742 patients who satisfied the inclusion criteria were included in the study (Figure 1). Eight patients were excluded because of inadequate image quality or insufficient evaluation of the baseline echocardiogram. In addition, 19 patients were excluded because of a lack of follow‐up data. Table 1 presents the baseline patient characteristics. Most patients underwent TAVI via a transfemoral approach (84%) with commercially available prosthetic valves: SAPIEN 3 valve (51%) (Edwards Lifesciences Corporation), SAPIEN XT (26%) (Edwards Lifesciences Corporation), Evolut R (13%) (Medtronic), CoreValve (6%) (Medtronic), and Evolut PRO (4%) (Medtronic). A total of 265 patients (15%) received a 29‐mm valve, 640 patients (37%) received a 26‐mm valve, 751 patients (43%) received a 23‐mm valve, and 86 patients (5%) received a 20‐mm valve. At baseline, 227 (13%) patients had reduced EF (LVEF <50%), and 334 (19%) had reduced LV SV index (Table 2). Among the 1742 patients, 486 (28%) had LG AS, while 1256 patients (72%) had HG AS. When we subdivided the patients according to the flow and PG state, 143 (8%) patients were classified as LF‐LG, 343 (20%) as NF‐LG, 191 (11%) as LF‐HG, and 1065 (61%) as NF‐HG (Figure 2A). When we further subdivided the patients according to the baseline LVEF and evaluated them separately, among the 227 patients (13%) with reduced LVEF, 52 patients (23%) had classical LF‐LG, 48 (21%) had NF‐LG, 51 (23%) had LF‐HG, and the remaining 76 patients (34%) had NF‐HG (Figure 2B). Among 1515 patients (87%) with preserved EF, 91 (6%) had paradoxical LF‐LG, 295 (20%) had NF‐LG, 140 (9%) had LF‐HG, and 989 (65%) had NF‐HG.
Figure 1. Study population.
TAVI indicates transcatheter aortic valve implantation.
Table 1.
Baseline Characteristics Based on the Baseline Gradient Status (N=1742)
| All patients | LG AS (n=486) | HG AS (n=1256) | P value | |
|---|---|---|---|---|
| Age, y | 84 (81–87) | 84 (80–87) | 85 (81–88) | <0.001 |
| Men, n (%) | 590 (34) | 170 (35) | 420 (33) | 0.542 |
| BSA, m2 | 1.44 (1.32–1.57) | 1.45 (1.33–1.59) | 1.43 (1.31–1.57) | 0.200 |
| NYHA class III/IV, n (%) | 591 (34) | 173 (36) | 418 (33) | 0.360 |
| CHF, n (%) | 1032/1738 (59) | 259/486 (53) | 773/1252 (62) | 0.001 |
| CAD, n (%) | 542/1741 (31) | 190/486 (39) | 352/1255 (28) | <0.001 |
| Prior PCI, n (%) | 318 (18) | 112 (23) | 206 (16) | 0.001 |
| Hypertension, n (%) | 1399 (80) | 400 (82) | 999 (80) | 0.193 |
| Diabetes, n (%) | 484 (28) | 156 (32) | 328 (26) | 0.012 |
| Dyslipidemia, n (%) | 936 (54) | 275 (57) | 661 (53) | 0.137 |
| Chronic lung disease, n (%) | 286 (16) | 84 (17) | 202 (16) | 0.544 |
| Prior MI, n (%) | 94/1740 (5) | 46/486 (10) | 48/1254 (4) | <0.001 |
| Smoking, current/quit, n (%) | 445 (26) | 142 (29) | 303 (24) | 0.029 |
| AF, n (%) | 226 (13) | 89 (18) | 137 (11) | <0.001 |
| LBBB, n (%) | 67/1732 (4) | 22/483 (5) | 45/1249 (4) | 0.357 |
| RBBB, n (%) | 242/1733 (14) | 72/484 (15) | 170/1249 (14) | 0.495 |
| STS PROM, % | 5.7 (4.1–8.3) | 5.6 (4.1–8.3) | 5.7 (4.1–8.2) | 0.954 |
| Hemoglobin, g/dL | 11.3 (10.2–12.4) | 11.4 (10.2–12.5) | 11.3 (10.1–12.4) | 0.229 |
| BNP, pg/mL | 234 (106–510) | 204 (92–418) | 244 (110–552) | 0.006 |
| NT‐proBNP, pg/mL | 1243 (555–3162) | 1074 (446–2929) | 1345 (614–3443) | 0.025 |
| TAVI procedure | ||||
| Approach | 0.077 | |||
| Transfemoral | 1459 (84) | 390 (80) | 1069 (85) | |
| Transapical | 210 (12) | 74 (15) | 136 (11) | |
| Transaortic | 45 (3) | 14 (3) | 31 (3) | |
| Others | 28 (2) | 8 (2) | 20 (2) | |
| Valve type | 0.007 | |||
| Sapien XT | 457 (26) | 145 (30) | 312 (25) | |
| Sapien 3 | 881 (51) | 253 (52) | 628 (50) | |
| CoreValve | 103 (6) | 19 (4) | 84 (7) | |
| EvoluteR | 234 (13) | 49 (10) | 185 (15) | |
| EvolutePRO | 67 (4) | 20 (4) | 47 (4) | |
| Valve size | 0.159 | |||
| 20 mm | 86 (5) | 16 (3) | 70 (6) | |
| 23 mm | 751 (43) | 222 (46) | 529 (42) | |
| 26 mm | 640 (37) | 179 (37) | 461 (37) | |
| 29 mm | 265 (15) | 69 (14) | 196 (16) | |
Continuous variables are expressed as median (interquartile range). AF indicates atrial fibrillation; AS, aortic stenosis; BNP, B‐type natriuretic peptide; BSA, body surface area; CAD, coronary artery disease; CHF, congestive heart failure; HG, high‐gradient; LBBB, left bundle branch block; LG, low‐gradient; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; RBBB, right bundle branch block; STS PROM, Society of Thoracic Surgeons predicted risk of mortality; and TAVI, transcatheter aortic valve implantation.
Table 2.
Comparison of Echocardiographic Variables Based on the Baseline Gradient Status
| No. | All patients (N=1742) | LG AS (n=486) | HG AS (n=1256) | P value | |
|---|---|---|---|---|---|
| LVEDV, mL | 1742 | 79 (64–101) | 77 (63–102) | 81 (64–101) | 0.601 |
| LVESV, mL | 1742 | 27 (20–39) | 27 (20–43) | 28 (20–38) | 0.325 |
| LVEF, % | 1742 | 64 (57–69) | 63 (52–68) | 64 (59–69) | <0.001 |
| LVEF <50% | 1742 | 227 (13) | 100 (21) | 127 (10) | <0.001 |
| LVSVi, mL/m2 | 1742 | 45 (37–55) | 41 (33–50) | 47 (39–56) | <0.001 |
| Low‐flow, n (%) | 1742 | 334 (19) | 143 (29) | 191 (15) | <0.001 |
| LVMI, g/m2 | 1742 | 118 (97–147) | 105 (84–128) | 124 (102–151) | <0.001 |
| LAD, mm | 1739 | 42 (38–47) | 43 (38–47) | 42 (38–47) | 0.564 |
| LAVi, mL/m2 | 1587 | 53 (41–67) | 52 (39–67) | 53 (41–66) | 0.083 |
| E/A | 1676 | 0.67 (0.55–0.84) | 0.68 (0.57–0.89) | 0.67 (0.54–0.83) | 0.008 |
| E/e′ | 1401 | 17.3 (13.1–22.6) | 16.0(12.2–21.7) | 17.7 (13.5–23.2) | <0.001 |
| AV V max, m/s | 1742 | 4.49 (4.07–5.04) | 3.74 (3.36–4.00) | 4.77 (4.40–5.24) | <0.001 |
| AV mean PG, mm Hg | 1742 | 48 (38–61) | 31 (26–36) | 54 (46–67) | <0.001 |
| AVA, cm2 | 1742 | 0.63 (0.51–0.75) | 0.72 (0.61–0.83) | 0.59 (0.48–0.71) | <0.001 |
| AVAi, cm2/m2 | 1742 | 0.43 (0.35–0.51) | 0.49 (0.42–0.58) | 0.41 (0.33–0.49) | <0.001 |
| TR velocity, m/s | 1607 | 2.55 (2.29–2.86) | 2.51 (2.27–2.83) | 2.55 (2.30–2.87) | 0.090 |
| TAPSE, mm | 574 | 19 (16–22) | 18 (15–22) | 19 (17–22) | 0.014 |
| S′, cm/s | 349 | 11 (9–13) | 10 (8–12) | 11 (9–13) | 0.119 |
| FAC, % | 258 | 43 (38–49) | 41 (36–46) | 44 (40–50) | 0.004 |
| LV GLS, % | 993 | 12.1 (9.0–15.0) | 12.0 (7.8–15.8) | 12.2 (9.0–15.0) | 0.763 |
| AR≥moderate, n (%) | 1742 | 220 (13) | 60 (12) | 160 (13) | 0.825 |
| MR≥moderate, n (%) | 1742 | 203 (12) | 78 (16) | 125 (10) | <0.001 |
| TR≥moderate, n (%) | 1742 | 173 (10) | 62 (13) | 111 (9) | 0.014 |
Continuous variables are expressed as median (interquartile range). AR indicates aortic regurgitation; AS, aortic stenosis; AV, aortic valve; AVA, aortic valve area; AVAi, aortic valve area index; FAC, fractional area change; GLS, global longitudinal strain; HG, high‐gradient; LAD, left atrial dimension; LAVi, left atrial volume index; LG, low‐gradient; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; LVMI, left ventricular mass index; LVSVi, left ventricular stroke volume index; MR, mitral regurgitation; PG, pressure gradient; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; and V max, peak aortic valve velocity.
Figure 2. Flow and gradient classification.
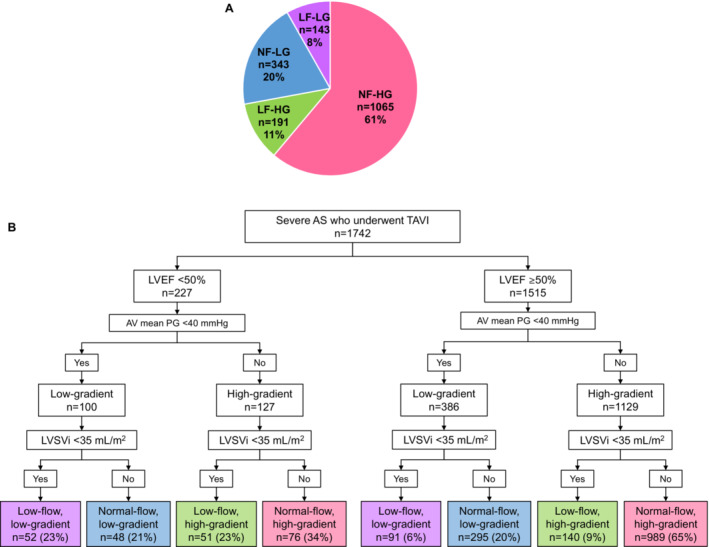
Subgroups by flow/gradient classification in the entire population (A) and in the preserved and reduced EF population (B). Patients with a mean transvalvular gradient <40 mm Hg were classified as having LG AS, whereas those with a mean gradient ≥40 mm Hg were considered to have HG AS. An LVSVi <35 mL/m2 was defined as LF AS, otherwise it was considered NF AS. According to the mean gradient and LVSVi status, the patients were subdivided into 4 subgroups: LF‐LG, NF‐LG, LF‐HG, and NF‐HG. When we subdivided the patients according to the flow and PG state, 143 (8%) were classified as LF‐LG, 343 (20%) as NF‐LG, 191 (11%) as LF‐HG, and 1065 (61%) as NF‐HG. AS indicates aortic stenosis; AV, aortic valve; EF, ejection fraction; HG, high‐gradient; LF, low‐flow; LG, low‐gradient; LVEF, left ventricular ejection fraction; LVSVi, left ventricular stroke volume index; NF, normal‐flow; PG, pressure gradient; and TAVI, transcatheter aortic valve implantation.
Echocardiographic Characteristics According to the Flow and Gradient Subgroups
We compared echocardiographic characteristics among 4 flow‐gradient subtypes and discovered that B‐type natriuretic peptide levels were lower in the NF‐LG group than in the other groups (Table 3). The prevalence of CAD was higher in the LF‐LG and NF‐LG groups than in the NF‐HG group. The LF‐LG group also had a lower LVEF than the NF‐HG group. LV mass index (LVMI) was significantly lower in the LF‐LG and NF‐LG groups than in the HG AS group. In the reduced EF population, B‐type natriuretic peptide levels were lower in the NF‐LG group than in the other groups (Table S1). The prevalence of CAD was higher in the LF‐LG and NF‐LG groups than in the HG AS group. The LF‐LG group also had a lower LVEF than the HG AS group, whereas other echocardiographic parameters were similar among the 4 groups. Similarly, in patients with preserved EF, the NF‐LG group exhibited lower B‐type natriuretic peptide levels, a lower prevalence of congestive heart failure, and higher AVA than the other 3 subgroups (Table S2). The LVMI was significantly lower in the LF‐LG and NF‐LG groups than in the HG AS group.
Table 3.
Characteristics and Echocardiographic Variables Based on the Flow and Gradient Status (N=1742)
| LF‐LG (n=143) (8%) | NF‐LG (n=343) (20%) | LF‐HG (n=191) (11%) | NF‐HG (n=1065) (61%) | P value | |
|---|---|---|---|---|---|
| Age, y | 84(80–87) | 84 (80–87) | 85 (82–88)* | 85 (81–88)* | <0.001 |
| Men, n (%) | 60 (42) | 110 (32) | 59 (31) | 361 (34) | 0.143 |
| BSA, cm2 | 1.48 (1.35–1.59) | 1.43 (1.31–1.58) | 1.44 (1.32–1.58) | 1.43 (1.31–1.56) | 0.206 |
| STS PROM, % | 6.3 (4.6–9.6)* | 5.4 (3.9–7.9) | 6.0 (4.3–9.0) | 5.7 (4.1–8.1)† | 0.006 |
| NYHA class III/IV, n (%) | 67 (47)* | 106 (31) | 91 (48)* | 327 (31) † , ‡ | <0.001 |
| Hemoglobin, g/dL | 11.5 (10.5–13.0) | 11.3 (10.2–12.4) | 11.7 (10.5–12.8) | 11.2 (10.1–12.3) † , ‡ | 0.002 |
| BNP, pg/mL | 388 (170–738)* | 172 (75–359) | 380 (163–753)* | 228 (105–514)*, † , ‡ | <0.001 |
| AF, n (%) | 48 (34)* | 41 (12) | 49 (26)* | 88 (8) † , ‡ | <0.001 |
| CHF, n (%) | 100/143 (70)* | 159/343 (46) | 136/191 (71)* | 637/1061 (60)*, ‡ | <0.001 |
| CAD, n (%) | 61/143 (43) | 129/343 (38) | 56/191 (29) | 296/1064 (28)*, † | <0.001 |
| Prior PCI, n (%) | 40 (28) | 72 (21) | 39 (20) | 167 (16)† | 0.001 |
| Prior MI, n (%) | 21/143 (15) | 25/343 (7) | 6/191 (3)† | 42/1063 (4)† | <0.001 |
| LVEDV, mL | 80 (60–110) | 77 (63–100) | 75 (57–95) | 82 (65–101)‡ | 0.010 |
| LVESV, mL | 34 (21–63)* | 26 (20–38) | 29 (19–44) | 27 (20–38)† | 0.001 |
| LVEF, % | 55 (40–64)* | 65 (57–69) | 61 (48–66)* | 64 (60–69) † , ‡ | <0.001 |
| LVSVi, mL/m2 | 30 (26–33)* | 46 (40–53) | 31 (27–33)* | 50 (43–58)*, † , ‡ | <0.001 |
| LVMI, g/m2 | 107 (83–134) | 104 (84–126) | 125 (104–148)*, † | 124 (102–151)*, † | <0.001 |
| LAD, mm | 45 (41–48)* | 42 (37–46) | 43 (38–48) | 42 (38–47)† | <0.001 |
| LAVi, mL/m2 | 54 (39–75) | 50 (39–64) | 57 (40–76) | 53 (42–65) | 0.050 |
| E/A | 0.68 (0.57–1.14) | 0.68 (0.57–0.86) | 0.65 (0.52–0.83) | 0.67 (0.54–0.83) | 0.063 |
| E/e′ | 16 (13–21) | 16 (12–22) | 18 (13–24) | 18 (14–23)* | 0.005 |
| AV V max, m/s | 3.47 (3.10–3.84) | 3.81 (3.50–4.04) | 4.60 (4.30–5.10)*, † | 4.80 (4.40–5.28)*, † | <0.001 |
| AV mean PG, mm Hg | 28 (22–33) | 33 (27–37) | 51 (45–65)*, † | 55 (46–68)*, † | <0.001 |
| AVA, cm2 | 0.61 (0.52–0.70)* | 0.76 (0.66–0.86) | 0.42 (0.35–0.52)*, † | 0.61 (0.51–0.73)*, ‡ | <0.001 |
| AVAi, cm2/m2 | 0.40 (0.36–0.49)* | 0.51 (0.45–0.60) | 0.30 (0.25–0.35)*, † | 0.43 (0.36–0.50)*, ‡ | <0.001 |
| TR velocity, m/s | 2.60 (2.25–2.96) | 2.50 (2.28–2.78) | 2.55 (2.29–3.05) | 2.55 (2.32–2.83) | 0.143 |
| TAPSE, mm | 15 (12–19)* | 20 (17–23) | 16 (14–20)* | 20 (17–22) † , ‡ | <0.001 |
| S′, cm/s | 9 (7–11) | 11 (9–12) | 9 (7–12) | 11 (9–13) † , ‡ | <0.001 |
| FAC, % | 36 (27–41)* | 43 (38–49) | 40 (33–48) | 44 (40–50)† | <0.001 |
| LV GLS, % | 7.9 (5.4–13.4)* | 13.4 (9.5–16.1) | 9.5 (6.1–12.8)* | 12.5 (9.6–15.1) † , ‡ | <0.001 |
| AR≥moderate, n (%) | 21 (15) | 39 (11) | 17 (9) | 143 (13) | 0.255 |
| MR≥moderate, n (%) | 34 (24)* | 44 (13) | 36 (19) | 89 (8) † , ‡ | <0.001 |
| TR≥moderate, n (%) | 26 (18) | 36 (11) | 29 (15) | 82 (8) † , ‡ | <0.001 |
Continuous variables are expressed as median (interquartile range). AF indicates atrial fibrillation; AR, aortic regurgitation; AV, aortic valve; AVA, aortic valve area; AVAi, aortic valve area index; BNP, B‐type natriuretic peptide; BSA, body surface area; CAD, coronary artery disease; CHF, congestive heart failure; FAC, fractional area change; GLS, global longitudinal strain; HG, high‐gradient; LAD, left atrial dimension; LAVi, left atrial volume index; LF, low‐flow; LG, low‐gradient; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; LVMI, left ventricular mass index; LVSVi, left ventricular stroke volume index; MI, myocardial infarction; MR, mitral regurgitation; NF, normal‐flow; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PG, pressure gradient; STS PROM, Society of Thoracic Surgeons predicted risk of mortality; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; and V max, peak aortic valve velocity.
P<0.008 for NFLG.
P<0.008 for LFLG.
P<0.008 for LFHG.
Among the study population, 993 patients had echocardiographic data available for GLS evaluation. The median GLS values in LG AS and HG AS were 12.0% (IQR, 7.8%–15.8%) and 12.2% (IQR, 9.0%–15.0%), respectively, indicating significant deterioration at baseline. When we compared the baseline GLS level among the flow‐gradient subgroups in preserved EF, the NF‐LG group exhibited higher LV GLS than the other 3 subgroups, while the LF‐HG group showed the lowest GLS. In the reduced EF population, the LF‐LG and LF‐HG groups showed a lower GLS than the NF‐HG group.
Clinical Outcome After TAVI
Immediately after TAVI, 103 patients (6%) exhibited complete or advanced atrioventricular block and required permanent pacemaker implantation before discharge. Echocardiographic evaluation within 30 days revealed greater than or equal to moderate paravalvular leak in 50 patients.
During a median follow‐up period of 747 days (IQR, 389–1115 days), 301 patients exhibited primary events (86 cardiovascular deaths and 254 rehospitalizations for cardiovascular causes). Of note, 39 patients who were rehospitalized due to cardiovascular events eventually died of cardiovascular disease. The details of the primary outcomes are presented in Table S3. Noncardiovascular death was observed in 204 patients.
In the entire study population, 3‐year event‐free rates for primary outcome were 71.6% (95% CI, 62.9–81.5) in the LF‐LG group, 70.4% (95% CI, 64.8–76.5) in the NF‐LG group, 80.2% (95% CI, 72.3–88.9) in the LF‐HG group, and 83.8% (95% CI, 81.1–86.6) in the NF‐HG group. Kaplan–Meier curves showed that the LF‐LG and NF‐LG groups had lower event‐free survival than the LF‐HG and NF‐HG groups (P<0.001; Figure 3). Similar elevations in risk in the LF‐LG and NF‐LG groups were observed in secondary outcomes, including cardiac death (P=0.011) and rehospitalization for cardiovascular disease (P<0.001), but not in all‐cause death (P=0.09; Figure S1A through S1C).
Figure 3. Survival curves for cumulative cardiovascular death and rehospitalization (N=1742).
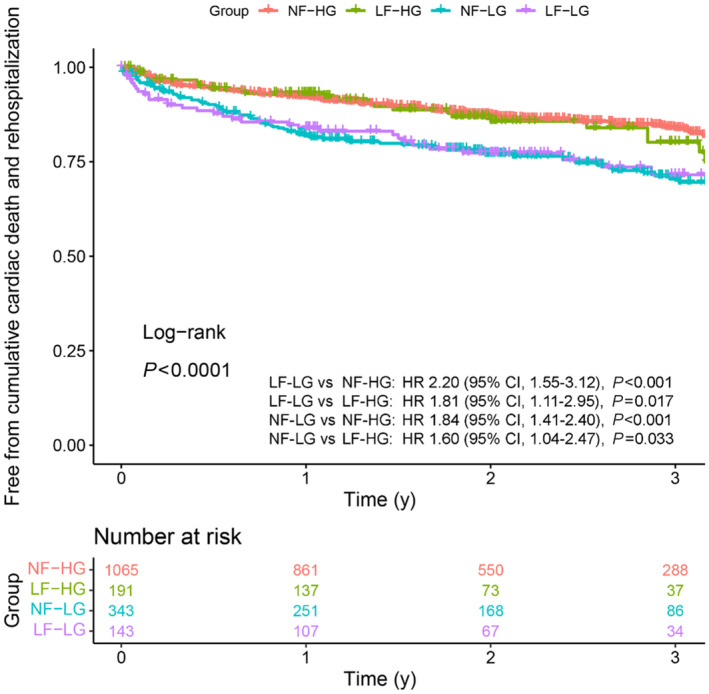
Kaplan–Meier curves demonstrate freedom from cumulative cardiovascular death and rehospitalization for cardiovascular events in our study population stratified by gradient‐flow status. HF indicates high‐flow; HG, high‐gradient; HR, hazard ratio; LF, low‐flow; LG, low‐gradient; and NF, normal‐flow.
We further constructed survival curves separately for patients with preserved and reduced EF. Patients with LF‐LG and those with NF‐LG showed a higher incidence of composite cardiovascular death or rehospitalization than patients with LF‐HG and those with NF‐HG with reduced EF (Figure 4A). In preserved EF, patients with LF‐LG and NF‐LG showed poorer event‐free survival for the primary outcome than patients with NF‐HG (Figure 4B). As for the secondary outcome, patients with LF‐LG and NF‐LG showed worse prognosis for cardiovascular death among those with reduced EF but not among those with preserved EF (Figure S2A and S2B). Patients with LF‐LG and NF‐LG also showed a higher incidence of rehospitalization than patients with LF‐HG among those with reduced EF (Figure S2C and S2D). In patients with preserved EF, the incidence of rehospitalization was lower than that in patients with reduced EF, and patients with NF‐LG showed the lowest event‐free survival. A similar increase in mortality in patients with NF‐LG was observed among those with reduced EF, but survival was similar among the 4 subgroups with preserved EF (Figure S2E and S2F).
Figure 4. Survival curves in reduced and preserved EF.
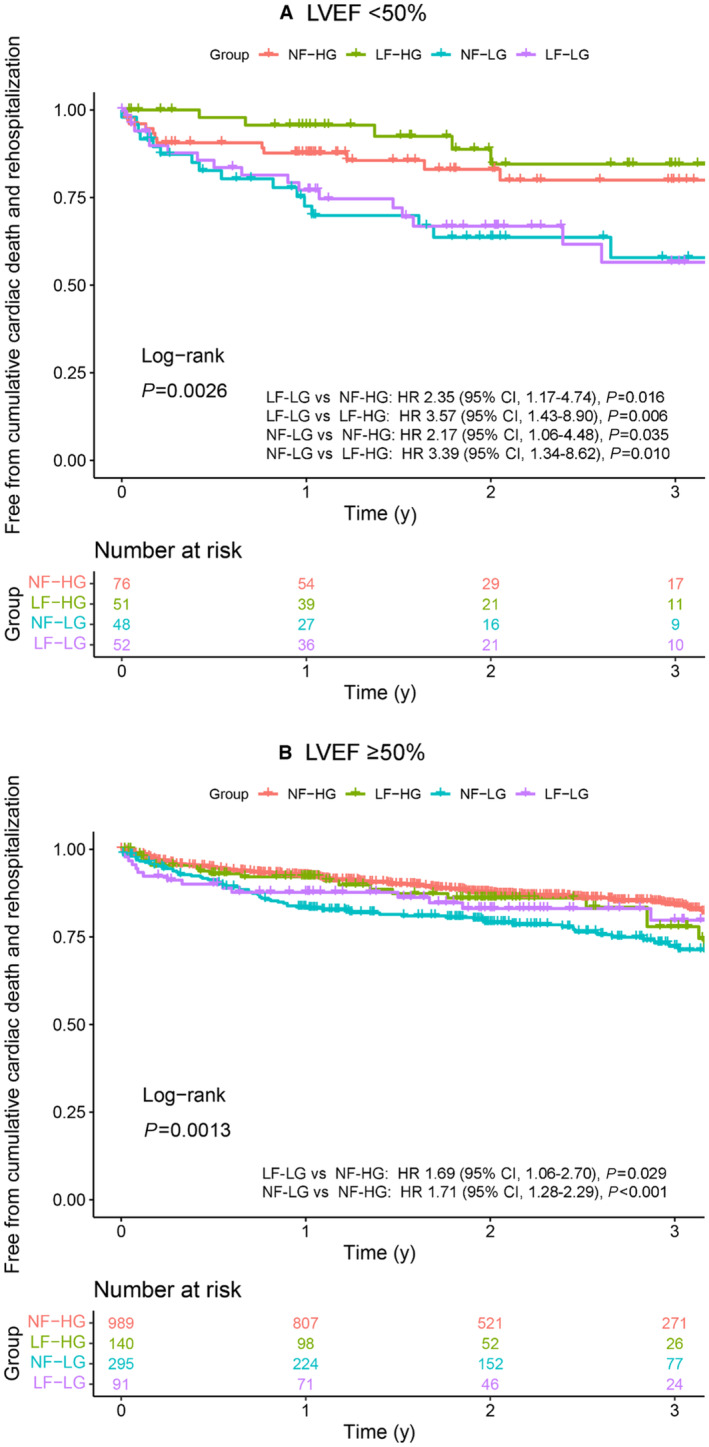
Survival analysis was performed separately for reduced (n=227; A) and preserved EF (n=1515; B). Kaplan–Meier curves demonstrate freedom from cumulative composite primary end points (cardiovascular death and rehospitalization for cardiovascular events) in our study population stratified by the gradient‐flow status. EF indicates ejection fraction; HF, high‐gradient; HR, hazard ratio; LF, low‐flow; LG, low‐gradient; LVEF, left ventricular ejection fraction; and NF, normal‐flow.
In a multivariable Cox proportional hazards model, LG AS (HR, 1.69 [95% CI, 1.33–2.15]; P<0.001), prior cardiac surgery (HR, 1.76 [95% CI, 1.25–2.49]; P=0.001), presence of atrial fibrillation (HR, 1.93 [95% CI, 1.45–2.56]; P<0.001), NYHA functional class III/IV (HR, 1.44 [95% CI, 1.13–1.83]; P=0.004), and higher Society of Thoracic Surgeons predicted risk of mortality (HR, 1.02 [95% CI, 1.004–1.04]; P=0.016) were independently associated with an increased risk of the primary outcome (Table 4). When we modeled the PG as a continuous variable, the association between the PG and risk of the primary outcomes was similar. The risk of primary events increased when the PG fell below 40 mm Hg (Figure 5). When analyzed for secondary outcomes, LG AS was also independently associated with a higher incidence of cardiovascular death (HR, 1.77 [95% CI, 1.13–2.76]; P=0.013), rehospitalization due to cardiovascular events (HR, 1.75 [95% CI, 1.35–2.27], P<0.001), and all‐cause death (HR, 1.28 [95% CI, 1.00–1.66]; P=0.050; Table 5).
Table 4.
Cox Model for the Composite Outcome of Cardiovascular Death and Rehospitalization for Cardiovascular Event (N=1742)
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, y | 1.01 (0.98–1.03) | 0.62 | 1.01 (0.99–1.03) | 0.473 |
| Men | 1.31 (1.03–1.65) | 0.025 | 1.24 (0.98–1.58) | 0.078 |
| Previous CAD | 1.22 (0.97–1.55) | 0.092 | 1.03 (0.80–1.32) | 0.817 |
| Previous CHF | 1.18 (0.93–1.48) | 0.17 | ||
| Prior cardiac surgery | 2.24 (1.62–3.10) | <0.001 | 1.76 (1.25–2.49) | 0.001 |
| Diabetes | 1.17 (0.91–1.49) | 0.21 | ||
| Hypertension | 1.05 (0.78–1.41) | 0.74 | ||
| Dyslipidemia | 0.90 (0.72–1.13) | 0.38 | ||
| Chronic lung disease | 1.38 (1.04–1.83) | 0.024 | ||
| AF | 2.15 (1.64–2.82) | <0.001 | 1.93 (1.45–2.56) | <0.001 |
| NYHA class III/IV | 1.67 (1.33–2.10) | <0.001 | 1.44 (1.13–1.83) | 0.004 |
| STS PROM, % | 1.03 (1.02–1.05) | <0.001 | 1.02 (1.004–1.04) | 0.016 |
| AVAi, cm2/m2 | 1.83 (0.74–4.54) | 0.19 | ||
| AV mean PG, per 1 mm Hg | 0.98 (0.98–0.99) | <0.001 | ||
| LVEDV, mL | 1.01 (1.003–1.01) | <0.001 | ||
| LVSVi, mL/m2 | 0.99 (0.8–0.995) | 0.002 | ||
| LVEF <50% | 1.73 (1.29–2.31) | <0.001 | 1.22 (0.87–1.70) | 0.247 |
| Low‐flow: LVSVi <35 mL/m2 | 1.35 (1.03–1.78) | 0.029 | 0.87 (0.64–1.16) | 0.338 |
|
Low‐gradient mean PG <40 mm Hg |
1.90 (1.51–2.38) | <0.001 | 1.69 (1.33–2.15) | <0.001 |
AF indicates atrial fibrillation; AV, aortic valve; AVAi, aortic valve area index; CAD, coronary artery disease; CHF, congestive heart failure; HR, hazard ratio; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVSVi, left ventricular stroke volume index; NYHA, New York Heart Association; PG, pressure gradient; and STS PROM, Society of Thoracic Surgeons predicted risk of mortality.
Figure 5. Association between the mean gradient and risk of primary outcome.
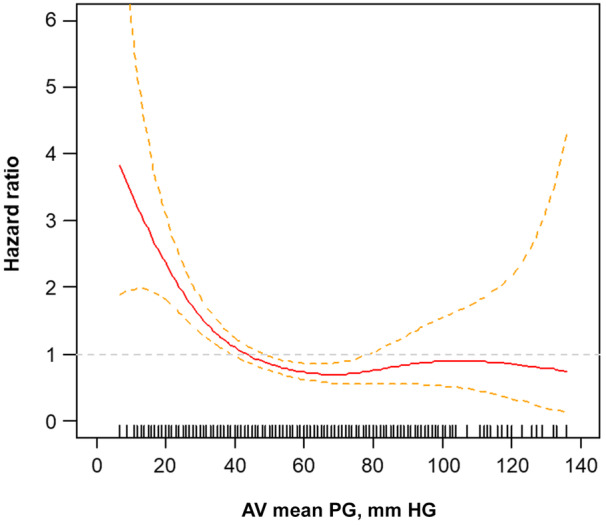
The relationship between the PG and estimated risk of primary composite events (cardiovascular death and rehospitalization) is described by fitted splines (solid line) and 95% confidence intervals (dotted lines), with HRs on the y‐axis and gradient on the x‐axis. The HR was estimated using a Cox proportional univariate model. AV indicates aortic valve; HR, hazard ratio, and PG, pressure gradient.
Table 5.
Risks of Secondary Outcomes in LG AS (N=1742)
| Unadjusted | Adjusted* | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Cardiovascular death | 1.94 (1.26–2.96) | 0.002 | 1.77 (1.13–2.76) | 0.013 |
| Rehospitalization for cardiovascular events | 1.92 (1.50–2.46) | <0.001 | 1.75 (1.35–2.27) | <0.001 |
| All‐cause death | 1.29 (1.01–1.64) | 0.041 | 1.28 (1.00–1.66) | 0.050 |
AS indicates aortic stenosis; HR, hazard ratio; and LG, low‐gradient.
A multivariable model was constructed with age, sex, Society of Thoracic Surgeons predicted risk of mortality, history of coronary artery disease, atrial fibrillation, cardiac surgery, New York Heart Association functional class, left ventricular ejection fraction, low‐flow, and LG AS.
Given the possibility of competing risks between the primary end point (composite of cardiovascular death and rehospitalization for cardiovascular events) and death due to other causes, we further performed competing risk regression analysis. In a multivariable model using Society of Thoracic Surgeons predicted risk of mortality, prior cardiac surgery, atrial fibrillation, NYHA functional class III/IV, and LF AS as potential covariates, the presence of LG AS was independently associated with a higher risk of the primary end point (subdistribution HR, 1.71 [95% CI, 1.34–2.17]; P<0.001). Univariable survival curves constructed using competing risk assumptions showed that the presence of LG AS was associated with a higher risk of the primary end point (log‐rank, P<0.001), whereas HG AS was not (Figure S3). The risk of noncardiac mortality was similar between the 2 groups. This finding was also observed when we analyzed LF AS and NF AS separately (Figure S4A and S4B).
When we repeated a similar analysis in the subgroup of patients with GLS (n=993), a lower absolute value of LV GLS was associated with the primary outcome independent of LG AS (HR, 0.95 [95% CI, 0.92–0.99]; P=0.019; Table S4). Furthermore, LV GLS was independently associated with poorer secondary outcomes, including cardiovascular death (HR, 0.91 [95% CI, 0.85–0.97]; P=0.003) and all‐cause death (HR, 0.93 [95% CI, 0.90–0.97]; P<0.001).
Changes in Echocardiographic Parameters After TAVI
Among the study population, 1239 patients underwent echocardiographic evaluation at the 1‐year follow‐up. In addition to aortic valve hemodynamics, LV geometry and function parameters such as the LVEDV, LV end‐systolic volume, LVEF, and LVMI were significantly improved over time (Table 6). Figure 6 shows the comparison of changes in LV and aortic valve echocardiographic parameters after TAVI according to the PG and flow status. When we compared changes in LV function and aortic valve parameters after TAVI between the LG AS and HG AS groups, the HG AS group showed prominent improvement in the LVEDV, LVEF, and LVMI over time compared with the LG AS group. Changes in the PG and AVA were also more prominent in the HG AS group than in the LG AS group. Notably, when we compared changes in echocardiographic parameters between the LF AS and NF AS groups, recovery in the LVEDV, LVMI, PG, and AVA in both groups was similar, while LVEF was significantly improved in the LF AS group compared with the NF AS group.
Table 6.
Comparison of Echocardiographic Parameters After TAVI (N=1239)
| Baseline | 1‐year after TAVI | P value | |
|---|---|---|---|
| LVEDV, mL | 79 (64–101) | 75 (59–96) | <0.001 |
| LVESV, mL | 27 (20–39) | 25 (19–35) | <0.001 |
| LVEF, % | 64 (57–69) | 65 (60–69) | <0.001 |
| LV mass index, g/m2 | 118 (97–147) | 102 (83–121) | <0.001 |
| AV V max, m/s | 4.49 (4.07–5.04) | 2.20 (1.86–2.51) | <0.001 |
| AV mean PG, mm Hg | 48 (38–61) | 10 (7–13) | <0.001 |
| AVA, cm2 | 0.63 (0.51–0.75) | 1.51 (1.25–1.90) | <0.001 |
| AVAi, cm2/m2 | 0.43 (0.35–0.51) | 1.06 (0.88–1.30) | <0.001 |
Continuous variables are expressed as median (interquartile range). AV indicates aortic valve; AVA, aortic valve area; AVAi, aortic valve area index; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; PG, pressure gradient; TAVI, transcatheter aortic valve implantation; and V max, peak aortic valve velocity.
Figure 6. Changes in echocardiographic parameters after TAVI according to the PG and flow status (N=1239).
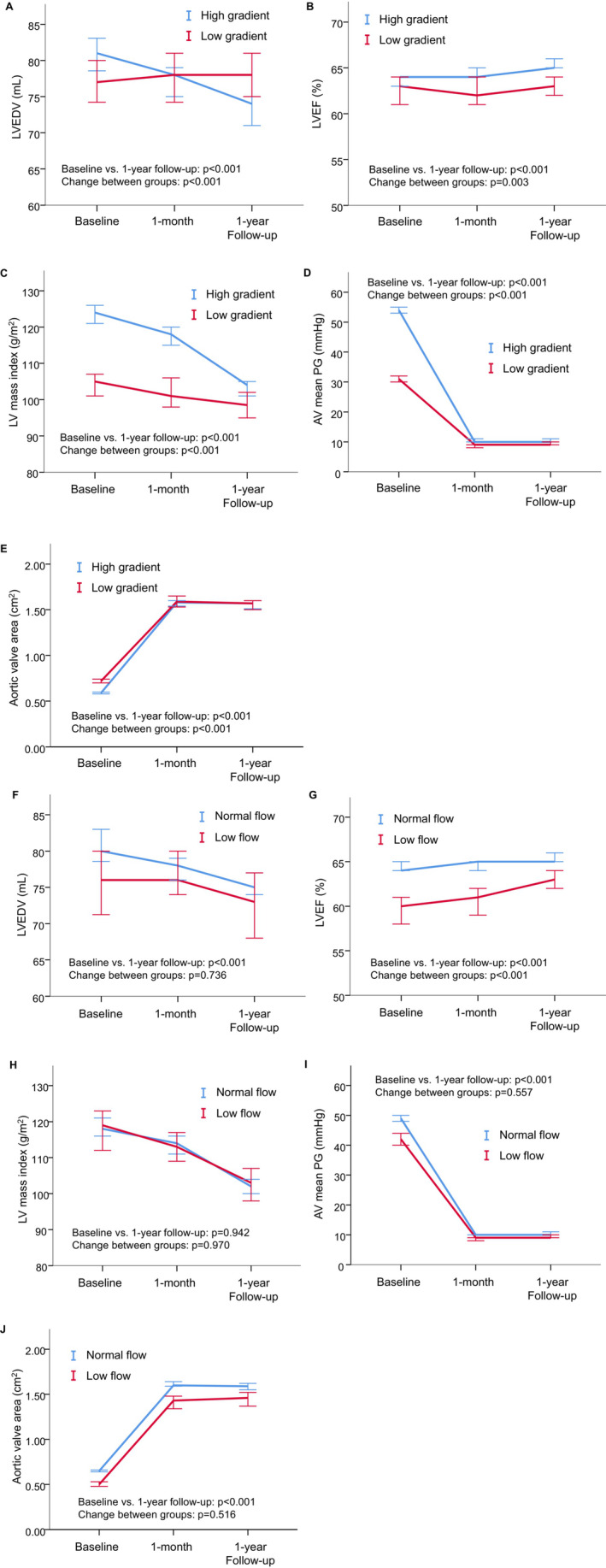
Changes in the LVEDV, LVEF, LVMI, AV mean PG, and aortic valve area between low‐gradient and high‐gradient AS (A–E), as well as between low‐flow and normal‐flow AS (F–J), were compared using a mixed effect model. Markers represent the median of the observed data obtained before TAVI and at 1‐month and at 1‐year follow‐up. Error bars represent 95% CIs. The mixed model was constructed with patient groups, changes over time, and interactions between groups and changes. P values for changes over time and differences in the magnitude of change between groups are shown as baseline vs 1‐year follow‐up and change between groups, respectively. Low‐gradient AS show significantly less improvement in the LVEDV, LVEF, LVMI, AV mean PG, and AV area compared with high‐gradient AS, while low‐flow and normal‐flow AS show similar recovery after TAVI except for LVEF. AS indicates aortic stenosis; AV, aortic valve; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; PG, pressure gradient; and TAVI, transcatheter aortic valve implantation.
When we further compared the changes in LV function and aortic valve parameters over time according to the PG and LVEF at baseline, the PG and AVA were significantly improved in both the LG AS and HG AS groups, regardless of baseline EF (Figure S5). In the reduced EF group, the LVEDV, LVEF, and LVMI showed significant improvement. When comparing the improvement in LV parameters between the LG AS and HG AS groups in the reduced EF population, the HG AS group showed prominent improvements in the LVEF and the LVMI over time compared with the LG AS group. The LVEDV improved similarly in both the LG AS and HG AS groups. In preserved EF population, the LVEDV and LVEF did not improve over time, while the LVMI showed a significant decrease over time; however, the HG AS group showed prominent improvement in the LVMI compared with the LG AS group.
DISCUSSION
In this study with a large number of octogenarians, we showed that LG AS was associated with an increased risk of primary composite outcomes of cardiovascular death and rehospitalization after TAVI regardless of flow status. Both LF‐LG and NF‐LG were associated with poorer event‐free survival after TAVI than HG AS. LG AS was also associated with an increased risk of rehospitalization for cardiovascular events, cardiovascular death, and all‐cause death. Furthermore, the longitudinal echocardiographic evaluation revealed significant differences in LV geometry and functional recovery between the LG AS and HG AS groups. While comparing recovery between the LF AS and NF AS groups, we found no significant difference except for LVEF. Although TAVI improved aortic valve hemodynamics dramatically and led to improvement in LV function afterward, the follow‐up echocardiographic evaluation showed poorer improvement in the LV geometry in the LG AS group than in the HG AS group, suggesting that LG AS may gain less benefit from cardiac reverse remodeling by restoration of the stenotic valve.
Gradient Matters Rather Than Flow Status: Differences in Cardiac Reversibility
Our data show that the gradient may play a causal role in survival after TAVI, while the LV SV index and flow status were not associated with prognosis after TAVI, which is in line with the results of previous studies. 12 , 13 Transaortic flow status is considered an important factor for the accurate determination of AS severity, and the prognostic value of the transvalvular flow rate has been proposed. Although flow might play an important role in detecting true severe AS, our data indicate that flow status might not be necessary for predicting outcomes and LV recovery after TAVI.
To our knowledge, this is the first study to compare cardiac reversibility according to flow/gradient patterns. As we showed that cardiac reverse remodeling after the TAVI procedure was observed less in the LG AS group compared with the HG AS group, limited cardiac reverse remodeling might be one explanation for the worse outcome in the LG AS group than in the HG AS group. Since LV deterioration in AS is mainly associated with pressure overload, TAVI leads to an immediate improvement in LV structural and functional changes, mainly by removal of the afterload, followed by delayed structural changes that further improve cardiac function over time. 6 Thus, one can expect that patients with LG AS may benefit less from afterload unloading by TAVI, which could explain the limited cardiac reverse remodeling in patients with LG AS compared with patients with HG AS, especially among those with reduced EF.
Another possible reason for the lack of LV reverse remodeling in LG AS might be the interference of underlying cardiac comorbidities other than AS. Patients with LG AS and reduced EF had a significantly higher incidence of underlying CAD than those with HG AS; these underlying comorbidities may interfere with myocardial recovery in the late phase, independent of pressure overload.
Impact of TAVI on Patients With LG AS
In the current study, the LG AS group comprised 28% of the study population, including 20% of patients with NF‐LG and 8% of patients with LF‐LG. We showed that LG AS, regardless of the flow status, was associated with worse outcomes. Patients with LG AS, especially those with LF‐LG AS, have been known as a high‐risk patient population in the surgical aortic valve replacement (AVR) era. 14 , 15 , 16 Several studies have also confirmed that the outcome of LF‐LG AS, even after successful TAVI, is still considerably worse than that of patients with HG AS. 4 , 12 , 17 , 18 Although poor prognosis in patients with LF‐LG AS was mainly associated with a deteriorated cardiac condition due to AS, several studies have suggested that the poor prognosis is attributable to a higher baseline cardiovascular risk in patients. 17 , 18 Nonetheless, in a propensity‐matched cohort, Fischer‐Rasokat et al showed that the mortality of patients with classical LF‐LG AS was twice as high as that of patients with HG AS, and patients with paradoxical LF‐LG AS might benefit from TAVI to the same extent as patients with HG AS. 12 These data suggest that the poor prognosis seen in patients with classical LF‐LG AS was not solely associated with baseline characteristics of patients, although this was true in the paradoxical LF‐LG AS population. Our data confirm the findings of Fischer‐Rasokat et al by showing that patients with classical LF‐LG AS had a significantly worse prognosis in the reduced EF population, but the prognosis of patients with paradoxical LF‐LG AS was comparable to that of patients with HG AS in the preserved EF population. Notably, in the reduced EF population, LF‐LG AS showed the lowest LVEF and LV GLS compared with NF‐HG AS in the present study, which indicates the existence of more advanced cardiac damage. Therefore, the poor prognosis of patients with LF‐LG AS, especially classical LF‐LG AS, might reflect their intrinsic cardiac deterioration and lack of recovery.
Impact of TAVI on NF‐LG AS
NF‐LG AS is a subjective characteristic that raises suspicion of AS severity and is often considered moderate AS. Nonetheless, prior studies have shown that NF‐LG AS could exist because of an inherent discrepancy in echocardiographic parameters used for the diagnosis of severe AS in the current guidelines. 1 While several prior studies showed that patients with NF‐LG AS demonstrated similar outcomes compared with those who had moderate AS with no survival benefit after AVR, 19 , 20 , 21 other studies demonstrated that survival of patients with NF‐LG AS was comparable to that of patients with severe HG AS, revealing a significant prognostic benefit after AVR 5 , 22 ; thus, performing TAVI for NF‐LG AS remains controversial. Interestingly, our data showed that the NF‐LG AS with reduced EF group had worse prognosis than the HG AS group but a comparable prognosis to the LF‐LG AS group, indicating that the NF‐LG AS group, especially among the reduced EF population, may also represent a subset of patients who need aortic valve treatment to improve their prognosis. This finding supports prior data that showed the survival benefit of AVR in the NF‐LG AS population. 5 , 22 In clinical situations, difficulty in determining the treatment benefit for those patient populations could cause a delay in performing TAVI for NF‐LG AS, which might lead to a poor prognosis compared with LF‐LG AS. Further prospective studies are required to validate our present findings; however, our data suggest that patients with LG AS who have undergone TAVI may require further management, including heart failure medication and close follow‐up.
Limitations
Although this was a multicenter study, its retrospective nature and the fact that most of the participating institutions were tertiary referral centers raise the possibility of selection bias. AS management planning was performed using a heart team approach; however, the decision‐making process may have varied at each institution. This study only included patients who underwent TAVI, and our data limited the assessment of the effect of TAVI compared with other treatments, such as surgical AVR or conservative treatment, on each hemodynamic subtype. Furthermore, we have confirmed survival benefits using multiple statistical approaches and controlled for various risk factors; however, other risk factors that were not accounted for might have hidden effects that might not have been eliminated. Moreover, the Teicholz method was used to perform LV quantification in 28% of the population because the biplane disc method was unavailable; this may have caused hidden bias. The retrospective study design also made it difficult for us to adjust the condition of the echocardiographic evaluation at each institution. In this study, hemodynamic data, dobutamine stress echocardiographic data, and computed tomography calcium score were not obtained to confirm patients' hemodynamic subtype and AS severity. Therefore, further prospective studies are required to confirm the effect of flow and gradient status on outcomes after TAVI.
CONCLUSIONS
LG AS was associated with poorer outcomes and LV function improvement over time, regardless of the flow state. Poorer LV reversibility in LG AS indicates that patients with LG AS may gain less benefit from cardiac reverse remodeling by restoration of the stenotic valve and lower survival benefit, which may result in them requiring advanced care after TAVI. Careful evaluation of AS severity might be required in both patients with LF‐LG AS and NF‐LG AS to provide the treatment within an appropriate time and proper follow‐up afterward.
Sources of Funding
This study was approved as an academic project of the Japanese Society of Echocardiography and was supported by a grant from the Japanese Society of Echocardiography.
Disclosures
Dr Sato belongs to an endowed department funded by Medtronic and DVX. Drs Izumi C. and Ishizu received speaker fees from Edwards Lifesciences. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S4
Figures S1–S5
Acknowledgments
Yuki Irie (Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center, Suita, Japan) and Tomonori Takahashi (Department of Cardiovascular Medicine, Tokushima University Hospital, Tokushima, Japan) contributed to this project.
This article was sent to Amgad Mentias, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.029717
For Sources of Funding and Disclosures, see page 15.
References
- 1. Minners J, Allgeier M, Gohlke‐Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur Heart J. 2008;29:1043–1048. doi: 10.1093/eurheartj/ehm543 [DOI] [PubMed] [Google Scholar]
- 2. Ueyama H, Kuno T, Harrington M, Takagi H, Krishnamoorthy P, Sharma SK, Kini A, Lerakis S. Impact of surgical and transcatheter aortic valve replacement in low‐gradient aortic stenosis: a meta‐analysis. JACC Cardiovasc Interv. 2021;14:1481–1492. doi: 10.1016/j.jcin.2021.04.038 [DOI] [PubMed] [Google Scholar]
- 3. Sato K, Sankaramangalam K, Kandregula K, Bullen JA, Kapadia SR, Krishnaswamy A, Mick S, Rodriguez LL, Grimm RA, Menon V, et al. Contemporary outcomes in low‐gradient aortic stenosis patients who underwent dobutamine stress echocardiography. J Am Heart Assoc. 2019;8:e011168. doi: 10.1161/jaha.118.011168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kataoka A, Watanabe Y, Kozuma K, Nara Y, Nagura F, Kawashima H, Hioki H, Nakashima M, Yamamoto M, Takagi K, et al. Prognostic impact of low‐flow severe aortic stenosis in small‐body patients undergoing TAVR: the OCEAN‐TAVI registry. JACC Cardiovasc Imaging. 2018;11:659–669. doi: 10.1016/j.jcmg.2016.12.028 [DOI] [PubMed] [Google Scholar]
- 5. Saeed S, Vamvakidou A, Seifert R, Khattar R, Li W, Senior R. The impact of aortic valve replacement on survival in patients with normal flow low gradient severe aortic stenosis: a propensity‐matched comparison. Eur Heart J Cardiovasc Imaging. 2019;20:1094–1101. doi: 10.1093/ehjci/jez191 [DOI] [PubMed] [Google Scholar]
- 6. Sato K, Kumar A, Jones BM, Mick SL, Krishnaswamy A, Grimm RA, Desai MY, Griffin BP, Rodriguez LL, Kapadia SR, et al. Reversibility of cardiac function predicts outcome after transcatheter aortic valve replacement in patients with severe aortic stenosis. J Am Heart Assoc. 2017;6:6. doi: 10.1161/jaha.117.005798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 8. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23; quiz 101–102. doi: 10.1016/j.echo.2008.11.029 [DOI] [PubMed] [Google Scholar]
- 9. Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant. 2010;45:1388–1395. doi: 10.1038/bmt.2009.359 [DOI] [PubMed] [Google Scholar]
- 10. Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–387. doi: 10.1038/sj.bmt.1705727 [DOI] [PubMed] [Google Scholar]
- 11. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.2307/2670170 [DOI] [Google Scholar]
- 12. Fischer‐Rasokat U, Renker M, Liebetrau C, van Linden A, Arsalan M, Weferling M, Rolf A, Doss M, Möllmann H, Walther T, et al. 1‐year survival after TAVR of patients with low‐flow, low‐gradient and high‐gradient aortic valve stenosis in matched study populations. JACC Cardiovasc Interv. 2019;12:752–763. doi: 10.1016/j.jcin.2019.01.233 [DOI] [PubMed] [Google Scholar]
- 13. Chetcuti SJ, Deeb GM, Popma JJ, Yakubov SJ, Grossman PM, Patel HJ, Casale A, Dauerman HL, Resar JR, Boulware MJ, et al. Self‐expanding transcatheter aortic valve replacement in patients with low‐gradient aortic stenosis. JACC Cardiovasc Imaging. 2019;12:67–80. doi: 10.1016/j.jcmg.2018.07.028 [DOI] [PubMed] [Google Scholar]
- 14. Monin JL, Quéré JP, Monchi M, Petit H, Baleynaud S, Chauvel C, Pop C, Ohlmann P, Lelguen C, Dehant P, et al. Low‐gradient aortic stenosis: operative risk stratification and predictors for long‐term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation. 2003;108:319–324. doi: 10.1161/01.Cir.0000079171.43055.46 [DOI] [PubMed] [Google Scholar]
- 15. Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J. 2010;31:281–289. doi: 10.1093/eurheartj/ehp361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lancellotti P, Magne J, Donal E, Davin L, O'Connor K, Rosca M, Szymanski C, Cosyns B, Piérard LA. Clinical outcome in asymptomatic severe aortic stenosis: insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol. 2012;59:235–243. doi: 10.1016/j.jacc.2011.08.072 [DOI] [PubMed] [Google Scholar]
- 17. Conrotto F, D'Ascenzo F, D'Amico M, Moretti C, Pavani M, Scacciatella P, Omedè P, Montefusco A, Biondi‐Zoccai G, Gaita F, et al. Outcomes of patients with low‐pressure aortic gradient undergoing transcatheter aortic valve implantation: a meta‐analysis. Catheter Cardiovasc Interv. 2017;89:1100–1106. doi: 10.1002/ccd.26839 [DOI] [PubMed] [Google Scholar]
- 18. Lauten A, Figulla HR, Möllmann H, Holzhey D, Kötting J, Beckmann A, Veit C, Cremer J, Kuck KH, Lange R, et al. TAVI for low‐flow, low‐gradient severe aortic stenosis with preserved or reduced ejection fraction: a subgroup analysis from the German aortic valve registry (GARY). EuroIntervention. 2014;10:850–859. doi: 10.4244/eijv10i7a145 [DOI] [PubMed] [Google Scholar]
- 19. Eleid MF, Sorajja P, Michelena HI, Malouf JF, Scott CG, Pellikka PA. Flow‐gradient patterns in severe aortic stenosis with preserved ejection fraction: clinical characteristics and predictors of survival. Circulation. 2013;128:1781–1789. doi: 10.1161/circulationaha.113.003695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tribouilloy C, Rusinaru D, Maréchaux S, Castel AL, Debry N, Maizel J, Mentaverri R, Kamel S, Slama M, Lévy F. Low‐gradient, low‐flow severe aortic stenosis with preserved left ventricular ejection fraction: characteristics, outcome, and implications for surgery. J Am Coll Cardiol. 2015;65:55–66. doi: 10.1016/j.jacc.2014.09.080 [DOI] [PubMed] [Google Scholar]
- 21. Salaun E, Clavel MA, Hahn RT, Jaber WA, Asch FM, Rodriguez L, Weissman NJ, Gertz ZM, Herrmann HC, Dahou A, et al. Outcome of flow‐gradient patterns of aortic stenosis after aortic valve replacement: an analysis of the PARTNER 2 trial and registry. Circ Cardiovasc Interv. 2020;13:e008792. doi: 10.1161/circinterventions.119.008792 [DOI] [PubMed] [Google Scholar]
- 22. Ozkan A, Hachamovitch R, Kapadia SR, Tuzcu EM, Marwick TH. Impact of aortic valve replacement on outcome of symptomatic patients with severe aortic stenosis with low gradient and preserved left ventricular ejection fraction. Circulation. 2013;128:622–631. doi: 10.1161/circulationaha.112.001094 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S5


